Introduction
Head and neck squamous cell carcinoma (HNSCC) has
the sixth highest incidence rate worldwide among all common cancers
(1). Despite the advances in
oncology and multidisciplinary treatment, the recurrence rate and
mortality of HNSCC have not obviously declined. One important
reason is that more than 50% of HNSCC patients present with
advanced stage at the time of diagnosis (2,3).
Therefore there is a critical need to find effective pathways by
which to predict tumor genesis and development. Recently, various
proteins have been found to be closely correlated with the
occurrence and progression of HNSCC (4,5).
However, new molecules which possess diagnostic value for clinical
application are still needed to be discovered.
Leucine-rich-α-2-glycoprotein 1 (LRG1), a member of
the leucine-rich repeat (LRR) family proteins, was initially
isolated from human serum by Haupt and Baudner in 1977 (6). The LRR family proteins are classified
as secreted proteins, characterized by the presence of leucine-rich
repeats in amino acid residues, and have 20–29-residue sequence
motifs containing a conserved 11-residue segment (7). These proteins were reported to be
involved in several important biological processes, such as
hormone-receptor interactions, enzyme inhibition, cell adhesion and
cellular trafficking (8). LRG1 is
considered as a membrane-associated protein, consisting of 312
amino acid residues, 66 of which are leucines. To date, the
biological function of LRG1 has not been fully elicidated. It has
been reported to be an inflammatory protein involved in
inflammatory responses such as active ulcerative colitis (9), acute appendicitis (10) and active rheumatoid arthritis
(11). Elevated LRG1 expression may
be induced by proinflammatory cytokines such as interleukin-6
(IL-6) (9). Evidence has also shown
that LRG1 participates in the regulatory mechanism of aberrant
angiogenesis by modulating endothelial TGF-β signaling (12). Aberrant neovascularization
contributes to tumor growth, and LRG1 was found to be a biomarker
and is upregulated in various types of carcinomas, such as
hepatocellular carcinoma (13),
ovarian cancer (14), endometrial
carcinoma (15), gastric cancer
(16), leukemia (17), colorectal (18), pancreatic (19) and bladder cancer (20). In addition, LRG1 is involved in
tumor development, progression and metastasis and is regarded as an
indicator of tumor prognosis. However, the role of LRG1 in the
tumorigenesis and progression of HNSCC is not yet clear, and
warrants elucidation.
In the present study, we first investigated the LRG1
gene expression pattern in HNSCC by analysis of the data obtained
from Gene Expression Omnibus (GEO) datasets, and its relationships
with degree of malignancy, tumor stage and regional lymph node
metastasis were explored, respectively. Secondly, tissue microassay
and clinical samples were investigated for further confirmation of
the findings.
Materials and methods
Gene expression profiles
LRG1 expression data were retrieved from Gene
Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/). Six datasets were
obtained for the analysis including GSE51985 [10 primary laryngeal
squamous cell carcinoma (LSCC) and corresponding adjacent
non-neoplastic tissues were analyzed], GSE33205 (analysis of 44
cases of HNSCC tumors and 25 cases of uvupopalatopharyngoplasty
patients as control), GSE59102 (29 LSCC samples and 13 marginal
samples were collected for microarray analysis), GSE58911 (15
paired normal and HNSCC samples from individual patients were
analyzed), GSE13399 (16 paired HNSCC tumor samples and normal
tonsil samples were collected) and GSE39366 (a total of 163 samples
were considered and a total of 138 tumor arrays remained after
removing low-quality and duplicate arrays, and arrays from
non-HNSCC samples). More details of the series data are listed in
Table I. The expression values of
the LRG1 gene were transformed to the relative expression.
 | Table I.Detailed information of the GEO
datasets in the present study. |
Table I.
Detailed information of the GEO
datasets in the present study.
| Series accession | Organism | Type | Platform |
|---|
| GSE51985 | Homo
sapiens | Expression profiling
by array | GPL10588 Illumina
HumanHT-12 v4.0 expression BeadChip |
| GSE33205 | Homo
sapiens | Expression profiling
by array | GPL5175 Affymetrix
Human Exon 1.0 ST Array |
| GSE59102 | Homo
sapiens | Expression profiling
by array | GPL6480
Agilent-014850 Whole Human Genome Microarray 4X44K G4112F |
| GSE58911 | Homo
sapiens | Expression profiling
by array | GPL6244 Affymetrix
Human Gene 1.0 ST Array |
| GSE13399 | Homo
sapiens | Expression profiling
by array | GPL7540
Agilent-scanner-UNC-custom-4X44K-without-Virus |
| GSE39366 | Homo
sapiens | Expression
profiling by array | GPL9053
Agilent-UNC-custom-4X44K |
Tissue microassay and clinical
samples
The HNSCC tissue microassay (TMA) was procured from
US Biomax Co. (Rockville, MD, USA) (production no. HN803b). Eleven
cases of normal tissue, 31 cases of tongue carcinoma, 31 larynx
carcinoma cases and 7 nose carcinoma cases were contained in the
TMA, including 62 men and 18 women. The mean age was 53.4 years
(range 18–90 years). More details are listed in Table II.
 | Table II.Immunohistochemistry staining of LRG1
and its correlation with clinicopathological features of the HNSCC
cases. |
Table II.
Immunohistochemistry staining of LRG1
and its correlation with clinicopathological features of the HNSCC
cases.
| Clinicopathological
parameters | No. of pts.
(%) | LRG1 expression
score (CES): mean ± SD | P-value |
|---|
| Gender |
|
| 0.2027 |
|
Male | 57 (82.6) | 4.54±2.89 |
|
|
Female | 12 (17.4) | 3.12±2.80 |
|
| Age (years) |
|
| 0.3387 |
|
≤60 | 42 (60.9) | 4.63±2.79 |
|
|
>60 | 27 (39.1) | 3.86±3.07 |
|
| Location |
|
| 0.6628 |
|
Larynx | 31 (44.9) | 4.75±2.51 |
|
|
Nose | 7 (10.1) | 4.00±3.10 |
|
|
Tongue | 31 (44.9) | 4.04±3.23 |
|
| Histological
gradea |
|
| 0.0613 |
|
Well | 10 (15.6) | 3.00±2.14 |
|
|
Moderate | 34 (53.1) | 4.45±2.56 |
|
|
Poor | 20 (31.5) | 5.60±2.40 |
|
| T stage |
|
| 0.2470 |
| T1 | 5 (8.2) | 3.75±1.71 |
|
| T2 | 33 (54.1) | 4.00±2.54 |
|
| T3 | 14 (23.0) | 5.78±2.11 |
|
| T4 | 9 (14.8) | 3.88±2.64 |
|
| N stage |
|
| 0.1935 |
| N0 | 43 (70.5) | 3.89±2.82 |
|
| N1 | 15 (24.6) | 4.17±2.33 |
|
| N2 | 3 (4.9) | 7.50±2.12 |
|
| Clinical stage |
|
| 0.1710 |
| Early
I–II | 28 (45.9) | 3.56±2.83 |
|
|
Advanced III–IV | 33 (54.1) | 4.62±2.59 |
|
|
Metastasisb | 8 (10) | 6.80±3.63 | NA |
| Normal | 11 (13.8) | 7.10±3.10 | NA |
Twenty pairs of HNSCC tissues including 18 cases of
larynx carcinoma and 2 cases of hypopharyngeal carcinoma and their
corresponding para-carcinoma normal tissues were collected between
2012 and 2013 from the Renmin Hospital of Wuhan University.
Baseline clinical features are described in detail in Table III. All tissues were frozen at
−125°C until proteins were extracted for western blotting.
 | Table III.Clinicopathological features of the
HNSCC patients employed in western blotting. |
Table III.
Clinicopathological features of the
HNSCC patients employed in western blotting.
| Clinicopathological
parameters | No. of
patients |
|---|
| Gender
(male:female) | 20 (19:1) |
| Age, years, mean ±
SD | 59.8±7.8 |
| Tobacco
smoking |
|
|
Yes | 11 |
| No | 9 |
| Alcohol
consumption |
|
|
Yes | 6 |
| No | 14 |
| Location |
|
|
Larynx | 17 |
|
Hypopharynx | 3 |
| Occurrence |
|
|
Primary | 16 |
|
Recurrence | 4 |
| Histological
grade |
|
|
Well/moderate | 16 |
|
Poor | 4 |
| T stage |
|
| T1 | 3 |
| T2 | 7 |
| T3 | 7 |
|
T4a | 3 |
| Clinical N
stage |
|
|
cN0 | 7 |
|
cN1 | 2 |
|
cN2 | 11 |
| Pathological N
stage |
|
|
pN0 | 12 |
|
pN1 | 2 |
|
pN2 | 6 |
| Distant
metastasis | 0 |
| Clinical stage |
|
| Early
I–II | 6 |
|
Advanced III–IV | 14 |
| Neck
dissection |
|
|
Yes | 13 |
| No | 7 |
| Postoperative
radiotherapy | 20 |
The present study was approved by the appropriate
Ethics Committees related to the institutions in which it was
performed. The authors assert that all procedures contributing to
the present study comply with the ethical standards of the relevant
national and institutional guidelines.
Immunohistochemical staining
To analyze LRG1 expression by immunohistochemistry
(IHC), the TMA was deparaffined in xylene for 15 min three times at
room temperature, hydrated in a series of 100, 95, 90, 80, 70 and
60% ethanol solutions, and washed in phosphate-buffered saline
(PBS). The antigen was recovered in boiling citrate buffer (10
mmol/l, pH 6.0) for 15 min and then the sections were cooled down
to room temperature. To quench endogenous peroxidase activity, the
sections were incubated with 0.3% hydrogen peroxide
phosphate-citrate buffer for 10 min and then rinsed extensively in
PBS. The sections were incubated with primary rabbit anti-LRG1
monoclonal antibody (dilution 1:100; Sigma-Aldrich, St. Louis, MO,
USA) overnight at 4°C. After washing with PBS, the slides were
incubated with poly-HRP goat anti-rabbit (Maixin-Bio, Fuzhou,
China) for 30 min. Diaminobenzidine was used to dye the slides for
5 min and hematoxylin for counterstaining the nuclei. The sections
were then dehydrated in ethanol and cleared in xylene. Coverslips
were placed on the slides. Images of sections were captured using
an Olympus BX40 microscope and CC-12 Soft-Imaging system (Olympus,
Tokyo, Japan).
Evaluation of immunohistochemical
staining
LRG1-positive cells displayed brownish yellow
granules on the cytoplasm and/or the membrane. Evaluation of LRG1
staining included the intensity of staining (scored as: 0, no
staining; 1, weak staining; 2, moderate staining; and 3, strong
staining) and the percentage of positive tumor cells (scored as: 0,
<5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%). To
facilitate the statistical analysis, LRG1 staining intensity and
frequency were transformed into a Composite Expression Score (CES)
utilizing the formula: CES = Intensity × Frequency. The range of
CES was from 0 to 12. The CES was scored as: 0, negative; 1–4, weak
positive; 5–8, positive; 9–12, strong positive. These scores were
independently determined by two senior pathologists.
Protein extraction
Frozen HNSCC and corresponding para-carcinoma
tissues were cut into 50–100 mg fragments and ground using a
homogenizer containing liquid nitrogen, then lysed in ice-cold RIPA
buffer [50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.25%
deoxycholate (DOC), 1.5 mM MgCl2, 1 mM EGTA, 1 mM
phenylmethyl sulfonylfluoride (PMSF), 10 mM NaF, 10 mM pervanadate,
10 µg/ml leupeptin and 10 µg/ml aprotinin], and incubated on ice
with intermittent vortexing for 30 min. Insoluble cellular
components were removed by centrifugation for 30 min at 25,000 ×
g/min. The protein concentration of the supernatant lysate was
determined by the BCA method. Lysate aliquots were mixed with 4X
sample buffer containing 2-mercaptoethanol, and heated at 70°C for
10 min for western blotting.
Western blotting
For western blot analysis, protein samples were
loaded on prefabricated 10% NuPAGE Bris-Tris gel (Life
Technologies, Carlsbad, CA, USA). Following separation, proteins
were transferred to a nitrocellulose membrane in 90 min for LRG1
and GAPDH. After blocking with 5% (w/v) BSA in Tris-buffer,
membrane strips were incubated with a different dilution of
different antibodies according to their descriptions. Anti-LRG1 and
anti-GAPDH primary antibodies (Sigma-Aldrich) were respectively
diluted 1:200 and 1:1,000 in 1X TBS, 0.1% Tween-20 with 5% BSA.
After three washes with TBS with 0.1% Tween-20, the blots were
incubated 1:2,000 with horseradish peroxidase-conjugated secondary
antibodies and detection was performed using enhanced
chemiluminescence.
Statistical analysis
Data and figures were mainly processed using
GraphPad Prism 6.0 software. Values are expressed as the mean ± SD
except for the intensity values in western blotting presented as
the mean. Comparisons of LRG1 expression between groups were
performed with independent samples t-test, or using one-way of
ANOVA and Bonferroni's multiple comparison tests. A probability
value <0.05 was considered to indicate a statistically
significant result.
Results
Analysis of the GEO database
To understand the expression pattern of LRG1 in
HNSCC, we first utilized GSE51985 which included 10 paired LSCC
samples and corresponding adjacent non-neoplastic samples. As shown
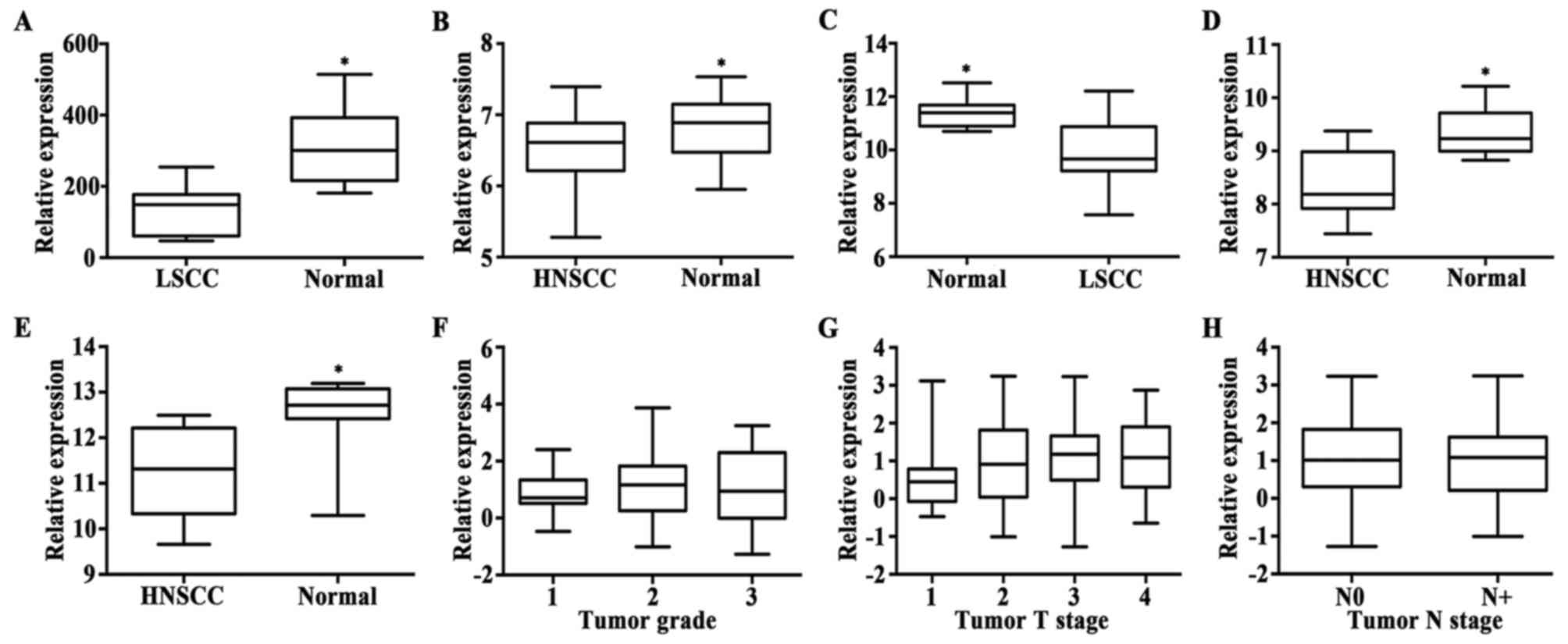
in Fig. 1A, LRG1 expression was
lower in the cancer tissues compared to that in the adjacent normal
tissues (P=0.0006). Data of other five series, containing more
samples, were also analyzed, and the results showed a similar trend
(all P-values <0.01). Details are shown in Fig. 1B-E. These data indicated that the
LRG1 gene is downregulated in HNSCC. We further investigated
whether the expression of LRG1 was related to the grade and stage
of HNSCC with series GSE39366. According to the analysis of data
extraction from series GSE39366, the expression rate of LRG1 was
not statistically different between tumor grade (P>0.05)
(Fig. 1F). In addition, there was
no statistical significance between each two T stage groups
(P>0.05) (Fig. 1G) or between
the two N stage groups (P>0.05) (Fig. 1H). Taken together, the LRG1
expression status showed no correlation with HNSCC tumor
differentiation and progression.
Clinicopathological characteristics of
the HNSCC patients
Sixty-nine HNSCCs were included in the TMA. The
location of the HNSCCs was the larynx (31 cases), the tongue (31
cases) and the nose (7 cases). The patient ages ranged from 32 to
90 years with a mean of 57.3 years. Fifty-seven were male,
occupying a predominant position and the rest were female. Tumors
categorized as T2 were the most commonly found in the TMA. Most
patients were found without local lymph node metastasis (N0). The
number of patients in the early stage was nearly equal to that in
the advanced stage. Other details of the clinicopathological
characteristics are shown in Table
II.
There were 20 cases of HNSCC employed in the western
blotting, including 17 cases of larynx carcinoma and 3 cases of
hypopharynx carcinoma. The patient ages ranged from 41 to 72 years
with a mean of 59.8 years. Nineteen patients were male, only one
case was female. Half of the number had a history of smoking. T2
and T3 were frequently seen in this cohort. More than half of
patients were lymph node-negative and were in the advanced stage.
All patients underwent surgical therapy and most were performed
with neck dissection. Most of the clinicopathological
characteristics are listed in Table
III.
LRG1 expression is downregulated in
the HNSCC TMA
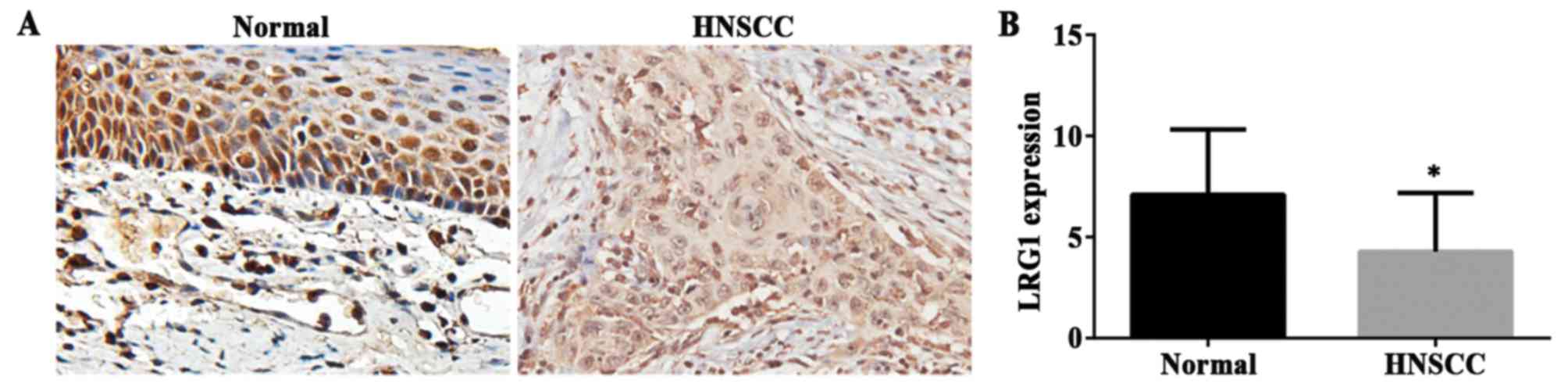
Decreased LRG1 gene expression in the HNSCC cases
from the GEO dataset was found as described above. Then, we
examined LRG1 protein expression in an independent cohort of 80
cases including three types of HNSCC (tongue, larynx and nose) and
normal tissues on a TMA by IHC staining. CES was used for the
measurement. The staining density of LRG1 in the non-cancerous
tissues had a more intense coloring and broader distribution than
that observed in the HNSCC tissues. Representative images of LRG1
in the tumor and normal tissues are shown in Fig. 2A. Accordingly, a significantly
decrease in CES was present in the tumor tissues compared to that
in the normal tissues (Fig. 2B)
(P<0.01). These results were consistent with the analysis of the
GEO dataset.
LRG1 expression is not related to T
and N stage, or differentiation of HNSCC
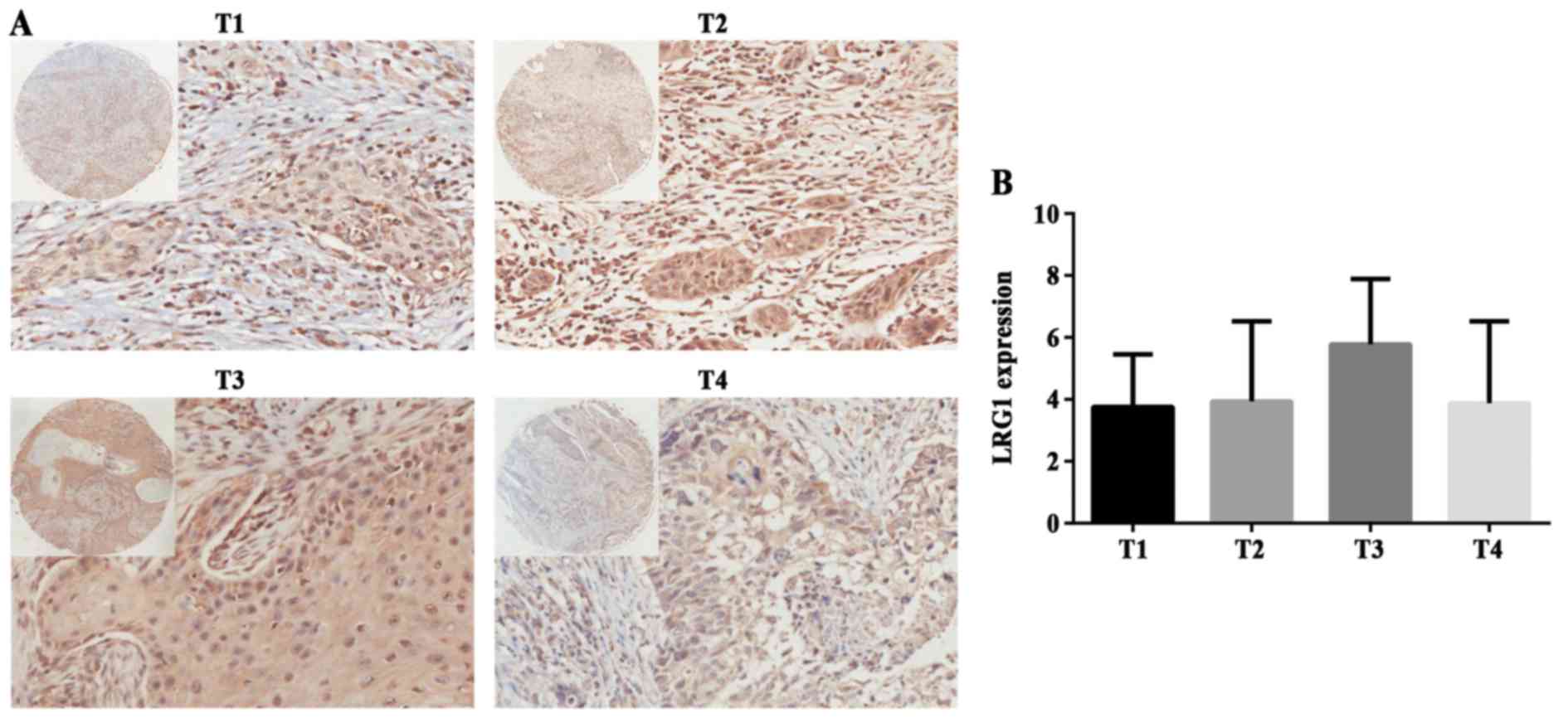
To further understand the correlation between LRG1
protein expression and different stages or grades, we also used IHC
to analyze the specimens of the tissue chips. Representative images
of LRG1 in different T staged tissues are shown in Fig. 3A. There was no statistical
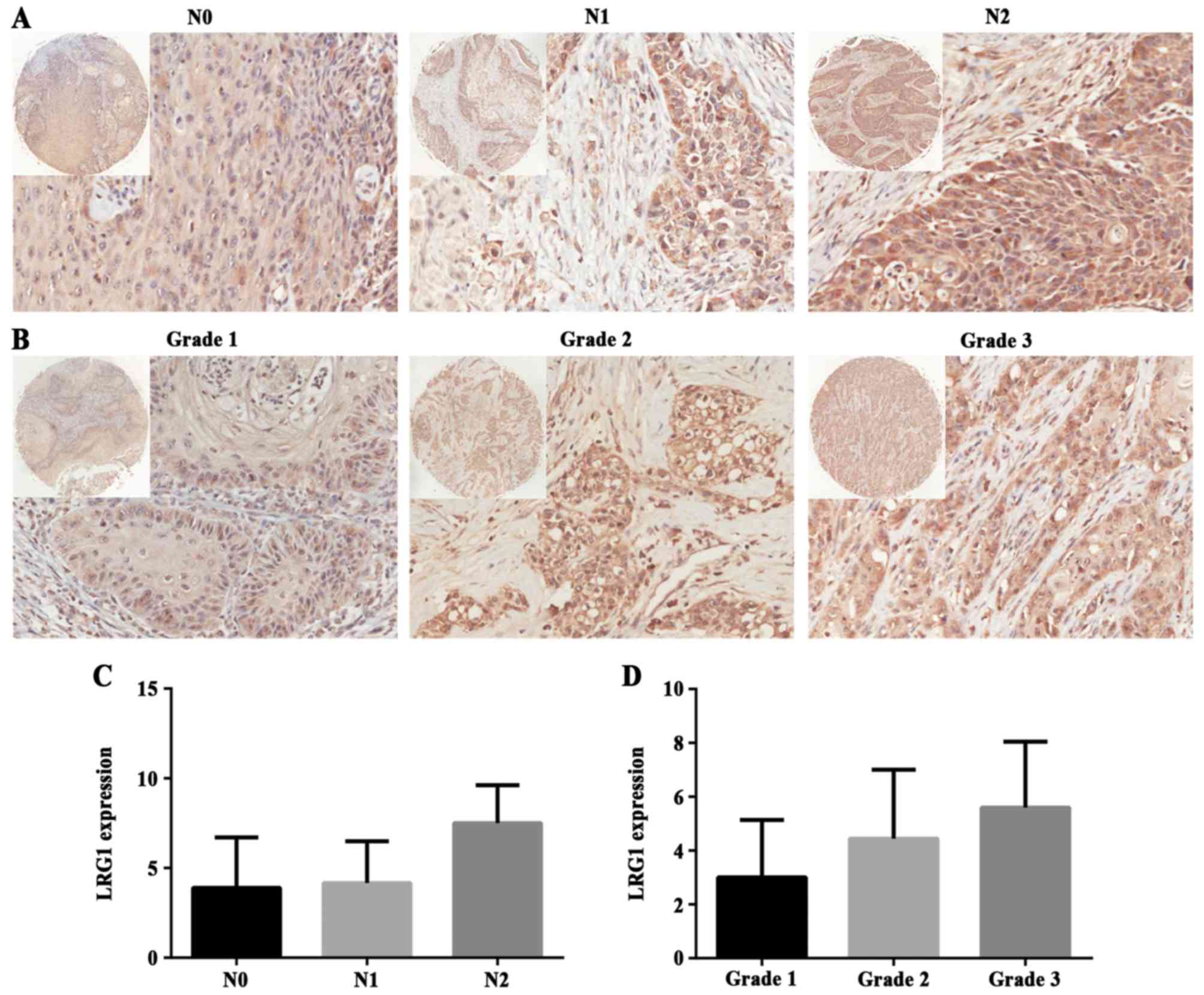
significance between T stage groups (P>0.05) (Fig. 3B). In addition, we compared three N
stage groups: N0, N1 and N2. Typical images are shown in Fig. 4A, and there was no statistical
difference among the groups (P>0.05) (Fig. 4C). These data above indicate that
LRG1 protein is not involved in the development of HNSCC.
Finally, LRG1 protein expression was analyzed in
different grades of tumor malignancy. Malignancy grade 1, 2 and 3
represent respectively well, moderate and poor differentiation.
LRG1 staining is shown in Fig. 4B,
and there was no significant difference among the groups
(P>0.05) (Fig. 4D). This result
reflected that LRG1 had no correlation with tumor grade, which was
consistent with the outcome of the GEO dataset analysis.
Association of LRG1 expression and
other clinicopathological characteristics in HNSCC
To further determine the clinical significance of
LRG1 in HNSCC, the relationship between expression of LRG1 and
other clinicopathological parameters was analyzed. In the cohort
analyzed by IHC and scored by CES, there were no significant
associations between LRG1 protein expression and
clinicopathological parameters such as gender (P=0.2027), age
(P=0.3387) and location (P=0.6628). In addition, in regards to the
clinical stages, LRG1 expression in the early stages was negatively
different from that in the advanced stages (P=0.1710). The results
of the analysis are summarized in Table II.
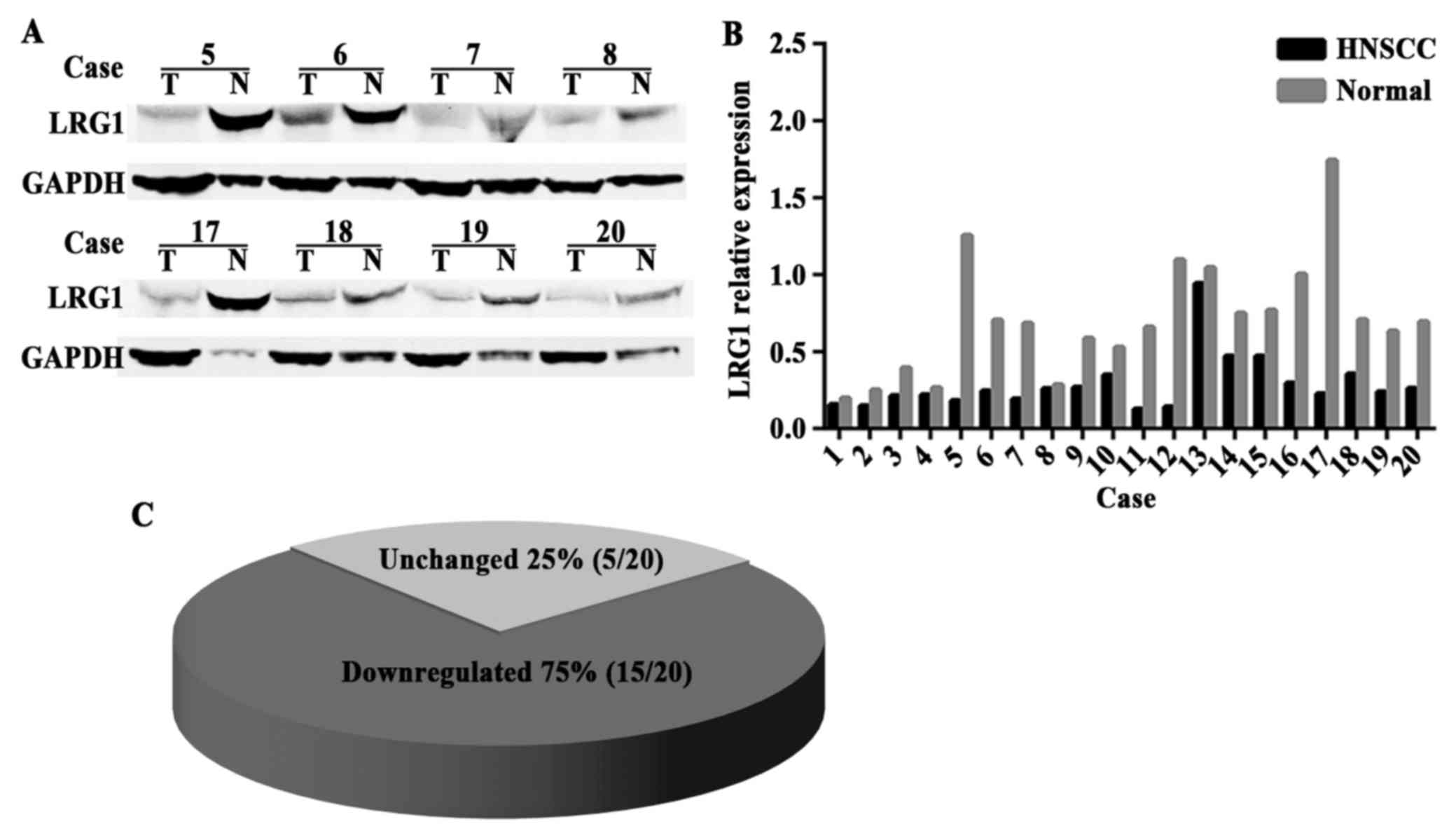
LRG1 expression is reduced in the
HNSCC tissues
The above results were confirmed by IHC staining. In
addition, we examined the level of LRG1 protein in 20 pairs of
HNSCC and adjacent non-cancerous tissues. The LRG1 protein level
was downregulated in the HNSCC tissues compared with that in the
para-carcinoma tissues, and a representative portion of the western
blot findings are shown in Fig. 5A.
Transformed pillar and pie charts are presented in Fig. 5B and C, showing a significant
decrease in LRG1 expression in 75% (15/20) of the HNSCC
patients.
Discussion
It has been reported that the expression of LRG1 is
dysregulated in various types of malignant tumors, yet its role in
HNSCC has not been addressed. The present study found that the LRG1
gene was downregulated in HNSCC tissues first based on analysis of
the GEO database. Similar results were demonstrated at the protein
level from evaluation of the tissues collected and the TMA. In
addition, the expression level of LRG1 was negatively associated
with several clinicopathological features. These findings uncovered
that LRG1 may play a role in HNSCC tumorigenesis and potentially
offers clinical value in early diagnosis.
The term head and neck carcinoma covers all
malignant tumors arising in the nasal and oral cavities, pharynx,
larynx and the paranasal sinuses. Most are squamous cell
carcinomas. Compared with other cancers such as breast, cervix and
colorectal, patients with HNSCC have relatively poor prognosis
after diagnosis (3,21). The reason is related to a failure in
identifying effective measurements for diagnosis at the early
stage. According to tumor-node-metastasis (TNM) classification and
clinical staging, treatment procedures differ substantially between
early- and late-stage HNSCC. Early-stage HNSCC patients receive
minimally invasive surgery or irradiation alone due to the small
tumor size with least lymphatic and hematogenous spread, and
benefit from removal of the neoplasia. However, lack of appropriate
screening biomarkers leads to difficulty in early diagnosis.
Biomarkers with promising potential reflecting physical changes are
in urgent need for clinical application.
LRG1 is considered as a candidate biomarker of tumor
detection as it was found aberrantly (particularly elevated)
expressed in different types of cancers or other body fluids vs.
normal unmutated cells. Wu et al (22) found that expression of LRG1 was
elevated in serum and other histological subtypes of early and late
stage epithelial ovarian cancer cases. Thus, LRG1 is a potential
biomarker alone or in combination with CA125 for the diagnosis of
ovarian cancer. LRG1 was also found to be expressed at higher
levels in urinary exosomes and lung tissue of non-small cell lung
cancer patients (23).
Overexpression of LRG1 was interpreted as its auxo-action on
angiogenesis, cell migration and invasion (12,24,25).
It was found to bind to the accessory receptor endoglin, causing a
switch in TGF-β signaling in endothelial cells via the
ALK-1/Smad1/5/8 pathway, which led to the promotion of
neovascularization (12). Moreover,
LRG activated TGF-β signaling by upregulating TGF-β1 and promoting
the phosphorylation of its downstream smad proteins, which was
associated with enhanced migration and invasion of glioma cells
(26).
However, contrary to the above mentioned studies, in
our research LRG1 expression was decreased at both the gene and
protein level tested in the GEO database analysis, IHC and
immunoblotting in HNSCC tissues, implying LRG1 as a tumor
suppressor. Similar results were found in hepatocellular carcinoma,
endometrial carcinoma and Lewis lung carcinoma cell lines (15,27,28).
We may attribute the discrepancy of LRG1 function in certain types
of cancers to its tissue specificity, while the underlying
mechanism needs to be understood. A recent study showed that LRG1
enhanced TGF-β1-smad2-induced growth inhibition and apoptosis in
Lewis lung carcinoma and Hep3B cells which lacked endoglin
(28). However, the function of
LRG1 in the tumorigenesis of HNSCC needs further investigation.
The degree of tumor malignancy is determined by
several factors such as proliferation, cell cycle and microvessels
in neoplasms (29–31). It has been reported that LRG1 is
expressed higher in grade IV glioma cell lines than that in grade
III glioma cells and LRG1 silencing was found to lead to cell cycle
arrest with the accumulation of cells in the G0/G1 phase and
reduced cell numbers in the S and G2/M phases (32). Moreover, downregulation of cell
cycle genes including cyclin B, D1 and E by LRG1 silencing was
observed, implying that LRG1 may regulate the cell cycle of
glioblastoma cells through these cyclins. However, in the present
study, we found that LRG1 expression had no significant difference
among the three grades of HNSCC by GEO database analysis. Then we
observed the LRG1 protein staining in different tumor grades, and
the same trend was noted. Low expression of LRG1 was observed in
HNSCC due to the fact that its tissue characteristics are different
from glioblastoma as referred above. However, it is perplexing that
lower LRG1 expression was not present in a higher grade of HNSCC.
This is a focus in our following experiments.
It has been referred in various studies that LRG is
involved in the suppression of tumor invasion and migration. In
hepatocellular carcinoma cells, overexpression and knockdown of
LRG1, respectively, weakened and strengthened the capability of
tumor cell migration and invasion (27), which can also explain the
downregulated expression of LRG1 in HNSCC in the present study.
However, the LRG1 expression level was negatively associated with
HNSCC stage and lymphatic metastasis. In our observation, GEO
database analysis and IHC staining showed that there was no
significant difference in LRG1 expression between T or N stages.
This implies that LRG1 in HNSCC is involved in tumorigenesis only,
not in progression.
In conclusion, our findings first demonstrated that
LRG1 expression is downregulated in HNSCC, and this suppressed
expression was negatively associated with tumor differentiation,
tumor stage and local lymph node metastasis, implying that LRG1 is
involved in tumorigenesis, but not in the development of HNSCC.
These findings may provide potential clinical value for the early
diagnosis of HNSCC.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81372880), and
the Natural Science Foundation of Hubei Province (no.
2012FFA045).
References
|
1
|
Ota I, Okamoto N, Yane K, Takahashi A,
Masui T, Hosoi H and Ohnishi T: Therapeutic strategies for head and
neck cancer based on p53 status. Exp Ther Med. 3:585–591.
2012.PubMed/NCBI
|
|
2
|
Ozdek A, Sarac S, Akyol MU, Unal OF and
Sungur A: Histopathological predictors of occult lymph node
metastases in supraglottic squamous cell carcinomas. Eur Arch
Otorhinolaryngol. 257:389–392. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Chen C, Li F, Hua Q, Chen S, Xiao
B, Dai M, Li M, Zheng A, Yu D, et al: Down-regulation of neutrophil
gelatinase-associated lipocalin in head and neck squamous cell
carcinoma correlated with tumorigenesis, not with metastasis. Int J
Clin Exp Pathol. 8:8857–8868. 2015.PubMed/NCBI
|
|
5
|
Koole K, van Kempen PM, Swartz JE, Peeters
T, van Diest PJ, Koole R, van Es RJ and Willems SM: Fibroblast
growth factor receptor 3 protein is overexpressed in oral and
oropharyngeal squamous cell carcinoma. Cancer Med. 5:275–284. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haupt H and Baudner S: Isolation and
characterization of an unknown, leucine-rich
3.1-S-alpha2-glycoprotein from human serum (author's transl). Hoppe
Seylers Z Physiol Chem. 358:639–646. 1977.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobe B and Kajava AV: The leucine-rich
repeat as a protein recognition motif. Curr Opin Struct Biol.
11:725–732. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buchanan SG and Gay NJ: Structural and
functional diversity in the leucine-rich repeat family of proteins.
Prog Biophys Mol Biol. 65:1–44. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Serada S, Fujimoto M, Terabe F, Iijima H,
Shinzaki S, Matsuzaki S, Ohkawara T, Nezu R, Nakajima S, Kobayashi
T, et al: Serum leucine-rich alpha-2 glycoprotein is a disease
activity biomarker in ulcerative colitis. Inflamm Bowel Dis.
18:2169–2179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kharbanda AB, Rai AJ, Cosme Y, Liu K and
Dayan PS: Novel serum and urine markers for pediatric appendicitis.
Acad Emerg Med. 19:56–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Serada S, Fujimoto M, Ogata A, Terabe F,
Hirano T, Iijima H, Shinzaki S, Nishikawa T, Ohkawara T, Iwahori K,
et al: iTRAQ-based proteomic identification of leucine-rich alpha-2
glycoprotein as a novel inflammatory biomarker in autoimmune
diseases. Ann Rheum Dis. 69:770–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Abraham S, McKenzie JA, Jeffs N,
Swire M, Tripathi VB, Luhmann UF, Lange CA, Zhai Z, Arthur HM, et
al: LRG1 promotes angiogenesis by modulating endothelial TGF-β
signalling. Nature. 499:306–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He X, Wang Y, Zhang W, Li H, Luo R, Zhou
Y, Li C, Liao M, Huang H, Lv X, et al: Screening differential
expression of serum proteins in AFP-negative HBV-related
hepatocellular carcinoma using iTRAQ -MALDI-MS/MS. Neoplasma. Sep
20–2013.(Epub ahead of print). doi: 10.4149/neo_2014_001.
|
|
14
|
Wu J, Xie X, Nie S, Buckanovich RJ and
Lubman DM: Altered expression of sialylated glycoproteins in
ovarian cancer sera using lectin-based ELISA assay and quantitative
glycoproteomics analysis. J Proteome Res. 12:3342–3352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wen SY, Zhang LN, Yang XM, Zhang YL, Ma L,
Ge QL, Jiang SH, Zhu XL, Xu W, Ding WJ, et al: LRG1 is an
independent prognostic factor for endometrial carcinoma. Tumour
Biol. 35:7125–7133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uen YH, Lin KY, Sun DP, Liao CC, Hsieh MS,
Huang YK, Chen YW, Huang PH, Chen WJ, Tai CC, et al: Comparative
proteomics, network analysis and post-translational modification
identification reveal differential profiles of plasma Con A-bound
glycoprotein biomarkers in gastric cancer. J Proteomics.
83:197–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu RS, Yu CS, Liu KC, Huang HY, Ip SW, Lin
JP, Chueh FS, Yang JS and Chung JG: Citosol (thiamylal sodium)
triggers apoptosis and affects gene expressions of murine leukemia
RAW 264.7 cells. Hum Exp Toxicol. 31:771–779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ladd JJ, Busald T, Johnson MM, Zhang Q,
Pitteri SJ, Wang H, Brenner DE, Lampe PD, Kucherlapati R, Feng Z,
et al: Increased plasma levels of the APC-interacting protein
MAPRE1, LRG1, and IGFBP2 preceding a diagnosis of colorectal cancer
in women. Cancer Prev Res. 5:655–664. 2012. View Article : Google Scholar
|
|
19
|
Kakisaka T, Kondo T, Okano T, Fujii K,
Honda K, Endo M, Tsuchida A, Aoki T, Itoi T, Moriyasu F, et al:
Plasma proteomics of pancreatic cancer patients by
multi-dimensional liquid chromatography and two-dimensional
difference gel electrophoresis (2D-DIGE): Up-regulation of
leucine-rich alpha-2-glycoprotein in pancreatic cancer. J
Chromatogr B Analyt Technol Biomed Life Sci. 852:257–267. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lindén M, Segersten U, Runeson M, Wester
K, Busch C, Pettersson U, Lind SB and Malmström PU: Tumour
expression of bladder cancer-associated urinary proteins. BJU Int.
112:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jemal A, Thun MJ, Ries LA, Howe HL, Weir
HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, et al: Annual
report to the nation on the status of cancer, 1975–2005, featuring
trends in lung cancer, tobacco use, and tobacco control. J Natl
Cancer Inst. 100:1672–1694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu J, Yin H, Zhu J, Buckanovich RJ, Thorpe
JD, Dai J, Urban N and Lubman DM: Validation of LRG1 as a potential
biomarker for detection of epithelial ovarian cancer by a blinded
study. PLoS One. 10:e01211122015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Zhang Y, Qiu F and Qiu Z: Proteomic
identification of exosomal LRG1: A potential urinary biomarker for
detecting NSCLC. Electrophoresis. 32:1976–1983. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lynch J, Meehan MH, Crean J, Copeland J,
Stallings RL and Bray IM: Metastasis suppressor microRNA-335
targets the formin family of actin nucleators. PLoS One.
8:e784282013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lynch J, Fay J, Meehan M, Bryan K, Watters
KM, Murphy DM and Stallings RL: MiRNA-335 suppresses neuroblastoma
cell invasiveness by direct targeting of multiple genes from the
non-canonical TGF-β signalling pathway. Carcinogenesis. 33:976–985.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhong D, He G, Zhao S, Li J, Lang Y, Ye W,
Li Y, Jiang C and Li X: LRG1 modulates invasion and migration of
glioma cell lines through TGF-β signaling pathway. Acta Histochem.
117:551–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Luo Q, Wang N, Hu F, Jin H, Ge T,
Wang C and Qin W: LRG1 suppresses the migration and invasion of
hepatocellular carcinoma cells. Med Oncol. 32:1462015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takemoto N, Serada S, Fujimoto M, Honda H,
Ohkawara T, Takahashi T, Nomura S, Inohara H and Naka T:
Leucine-rich α-2-glycoprotein promotes TGFβ1-mediated growth
suppression in the Lewis lung carcinoma cell lines. Oncotarget.
6:11009–11022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schonberg DL, Lubelski D, Miller TE and
Rich JN: Brain tumor stem cells: Molecular characteristics and
their impact on therapy. Mol Aspects Med. 39:82–101. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cavazza A, Corradini C, Marini M, Roda LG
and Valenti A: Capillary electrophoresis coupled with mass
spectrometry for the evaluation of substance P enzymatic
degradation by SaOS-2 human osteosarcoma. J Chromatogr B Analyt
Technol Biomed Life Sci. 879:2501–2506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sulzbacher I, Birner P, Dominkus M,
Pichlhofer B and Mazal PR: Expression of platelet-derived growth
factor-alpha receptor in human osteosarcoma is not a predictor of
outcome. Pathology. 42:664–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhong D, Zhao S, He G, Li J, Lang Y, Ye W,
Li Y, Jiang C and Li X: Stable knockdown of LRG1 by RNA
interference inhibits growth and promotes apoptosis of glioblastoma
cells in vitro and in vivo. Tumour Biol. 36:4271–4278. 2015.
View Article : Google Scholar : PubMed/NCBI
|