Introduction
Hepatocellular carcinoma (HCC) is a common tumor
type with high metastatic ability and recurrence rate (1,2). HCC
has a higher incidence in developing countries compared with that
in developed countries and the highest incidence rates are found in
China (3). Worlwide, HCC is the
fifth most commonly diagnosed cancer, but the second most leading
cause of cancer-related death (3).
Some patients are deprived of the opportunity of curative therapy
due to advanced stage disease, and systemic chemotherapy has shown
little benefit on the survival rate of patients (4,5).
Therefore, the underlying mechanisms of cancer cell migration,
invasion and proliferation must be thoroughly elucidated to provide
critical signaling effectors for effective molecularly targeted
therapy.
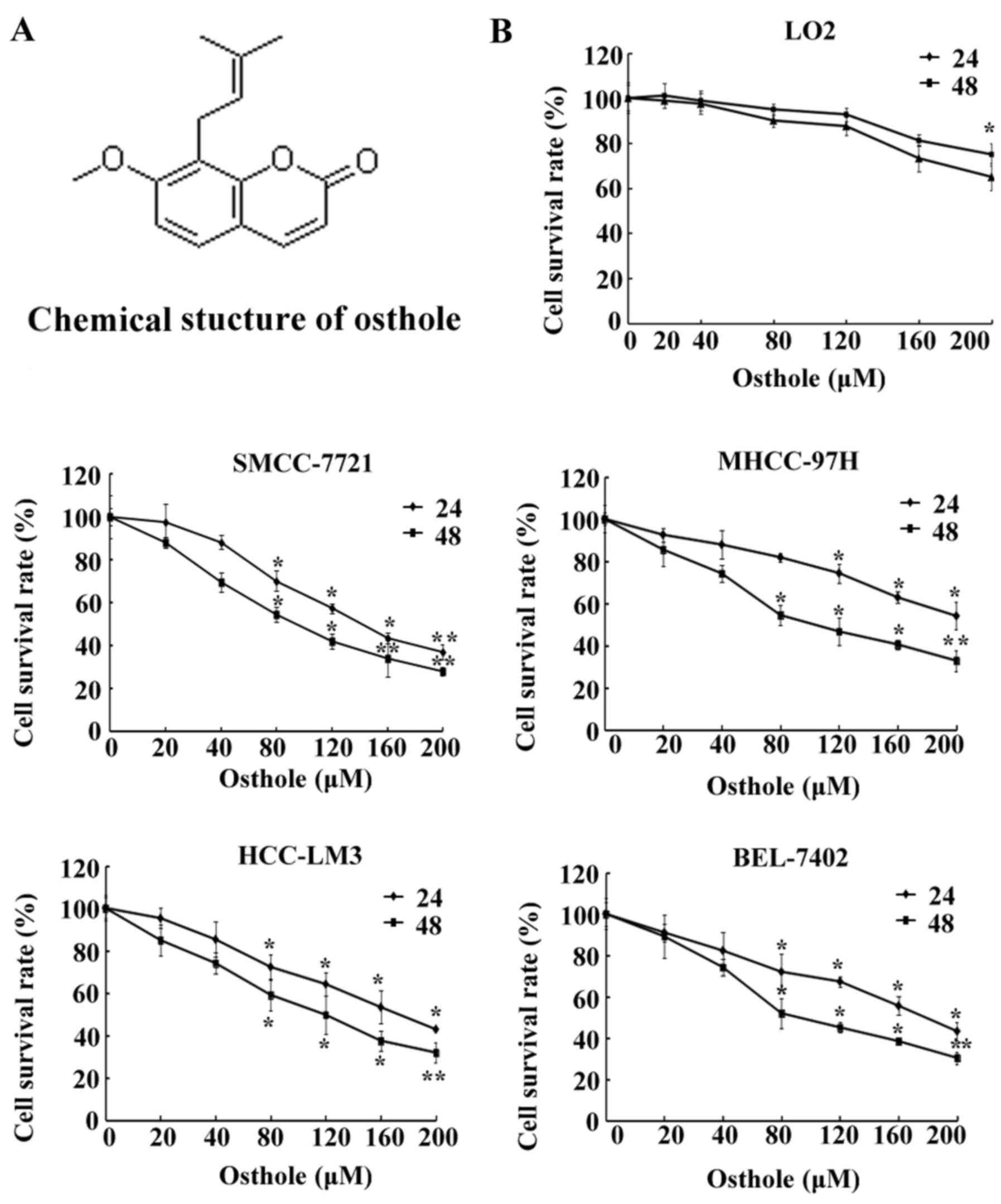
Osthole (Fig. 1A), a
herbal medicine, is a simple bioactive
[7-methoxy-8-(3methy-2-butenyl)] coumarin derivative. It has been
proven to exhibit various pharmacological functions. It is an
anticonvulsant (6), prevents
ischemia-reperfusion injury (7),
has hepatocellular protective properties (8) and has anti-allergic function (9). Research on the effect of osthole on
HCC is lacking, yet a few studies have reported that osthole
possesses anticancer potential, and was found to inhibit the growth
of human glioma (10), induce
apoptosis in human lung cancer and human osteosarcoma (11,12),
and inhibit metastasis in human glioblastoma and human lung cancer
(13,14). In HCC, osthole was reported to play
a crucial role in growth inhibition and induction of apoptosis
(15), but it is unclear whether
osthole has influence on HCC migration and invasion as well as
critical signaling pathways.
In the present study, we demonstrated for the first
time the pharmacological function of osthole in inducing cell cycle
arrest through downregulation of cycle B1 and Cdc2 levels and in
triggering DNA damage through upregulation of ERCC1 and we further
elucidated its effects on HCC cell migration associated with a
decrease in MMP-9 and MMP-2 expression and modulating the
epithelial-mesencheymal transition (EMT) signaling pathway.
Materials and methods
Materials, reagents and chemicals
Antibodies against MMP-2, MMP-9, Cdc2, cyclin B1,
β-catenin, E-cadherin, vimentin and β-actin were obtained from
ProteinTech Group, Inc. (Chicago, IL, USA), and the antibody for
N-cadherin was obtained from Cell Signaling Technology, Boston, MA,
USA). An enhanced chemiluminescence (ECL) kit was purchased from
Amersham Life Sciences, Inc. (Piscataway, NJ, USA). Cell cycle
detection and comet assay kits were purchased from Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, China). Transwells were obtained from
BD Biosciences (San Jose, CA, USA).
3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical Co.
(St. Louis, MO, USA). Osthole powder was purchased from Chengdu
Must Bio-Technology Co., Ltd. (Chengdu, China). It contained ~98%
proanthocyanidins and is stable for at least two years at 4°C.
Cell lines and cell culture
The HCC, SMCC-7721, MHCC-97H, HCC-LM3 and BEL-7402
cell lines were obtained from the Cell Bank of the Chinese Academy
of Sciences (Shanghai, China), and were cultured in Dulbeccos
modified Eagles medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) and 100 U/ml penicillin (all from Gibco-BRL, Grand
Island, NY, USA) at 37°C with 5% CO2 in a humidified
incubator.
Drug preparation
Osthole was dissolved in 100% DMSO at a
concentration of 1 M as a stock solution and stored at 4°C. It was
diluted with DMEM before each experiment. The final concentrations
of DMSO were <0.1% in all osthole treatment groups.
Cell viability assay
The effect of osthole on cell viability was detected
using the MTT assay. The cells (1×104/well) were seeded
into a 96-well plate and incubated for 24 h. After treatment with
osthole (20, 40, 80, 120, 160 or 200 µM) and the negative control
for 24 and 48 h, the viability of the cancer cells was detected
with MTT. Twenty microliters of MTT solution [5 mg/ml in
phosphate-buffered saline (PBS)] was added to each well, and the
mixtures were incubated for 4 h at 37°C. Then, the MTT solution was
removed and 150 µl of DMSO was added to the wells. The absorbance
was measured using a Multiskan Ascent plate reader at a 540 nm
wavelength.
Cell cycle analysis by flow
cytometry
Cell cycle progression was assayed by measuring DNA
content with propidium iodide (PI) staining. The cells were treated
with osthole (0, 80, 120 or 160 µM) for 24 h, washed twice with PBS
and fixed with 70% ethanol overnight at 4°C. Following fixation,
the DNA fragments were stained in PBS containing PI and RNase for 1
h at 37°C. The DNA content was evaluated using an Accuri C6 flow
cytometer (BD Biosciences). The data were analyzed using ModFit LT
V4.1.
Wound healing assay
SMCC-7721 and MHCC-97H cells were seeded into
24-well plates and scraped with the end of a 200-µl pipette tip.
The plates were washed with PBS to remove detached cells, and then
the cells were incubated with complete growth medium containing 0,
20 and 40 µM osthole solution for 24 h. Cell migration was observed
under a phase-contrast microscope at a magnification of ×100 field
at 0 and 24 h post-induction of injury. Migrated cells into the
denuded area in each of six random fields were measured and
quantified with computer-assisted microscope.
Transwell migration assay
Cell migration and invasion were quantified by the
Transwell assay. HCC cells were treated with 0, 20 and 40 µM
osthole for 24 h and harvested. Cells (2×104) in
serum-free DMEM were added to each upper chamber and DMEM with 10%
FBS was added to the lower chamber as a chemoattractant. After a
24-h incubation at 37°C, the cells remaining on the upper surface
of the membrane were removed, and the cells that had migrated
through the membrane were stained with 0.1% crystal violet for 10
min. Six random fields of each Transwell membrane were assessed
under a light microscope at a magnification of ×200.
Comet assay for the analysis of DNA
damage
DNA damage induced by osthole in the HCC cells was
determined using a comet assay according to the manufacturer's
protocol. Briefly, cells were treated with osthole (80 and 120 µM)
for 48 h in complete medium, and then the cells were harvested and
re-suspended in ice-cold PBS buffer. Approximately 1×104
cells in a volume of 75 µl of 0.5% (w/v) low-melting-point agarose
were pipetted into a frosted glass slide coated with a thin layer
of 1.0% (w/v) agarose, covered with a coverslip, and allowed to set
on ice for 10 min. Coverslips were removed, and the slides were
immersed in ice-cold lysis buffer. After 2 h at 4°C, the slides
were placed into a horizontal electrophoresis tank filled with
electrophoresis buffer and subjected to electrophoresis for 30 min
at 30 V at 4°C. Cells were stained with 2.5 µg/ml PI for 5 min and
visualized under a microscope at a magnification of ×200 field.
Tail lengths of a minimum of 10 cells were quantified as the
distance from the center of the cell nucleus to the tip of the
tail.
Western blot analysis
Protein was extracted using RIPA lysis buffer
(Beyotime, Shanghai, China) and protease inhibitor (Biocolors,
Shanghai, China) was added in a proportion of 1:100. Equal amount
of protein was loaded on a 10% SDS-PAGE gel. The lysates were
resolved by electrophoresis (80 V for 30 min and 120 V for 1.5 h)
and transferred onto polyvinylidene difluoride (PVDF) membranes.
The membranes were blocked in 5% non-fat milk for 1 h at room
temperature and incubated with the primary antibody overnight at
4°C, followed by incubation with relevant secondary antibodies for
1 h at room temperature. The protein bands were visualized using
the chemiluminescent ECL assay kit (Amersham Life Sciences, Inc.)
and images were captured using Bio-Rad ChemiDoc XRS. Protein
expression was quantitatively determined using ImageJ software
(National Institutes of Health, Bethesda, MD, USA). β-actin was
used as an internal reference for protein expression in all
cells.
Statistical analysis
Data were analyzed using SPSS 15.0 software and are
presented as means ± SD of three independent experiments.
Statistical differences between two groups were analyzed using a
Student's t-test. A difference was considered to be statistically
significant at P<0.05.
Results
Effect of osthole on the proliferation
of HCC cells
Human HCC cell lines SMCC-7721, BEL-7402, MHCC-97H
and HCC-LM3, and a normal cell line LO2 were incubated with
different concentrations (0, 20, 40, 80, 120, 160 and 200 µM) of
osthole for 24 and 48 h (Fig. 1B),
and then the cell viability was determined by MTT assay. As shown
in Fig. 1B, except for the MHCC-97H
cell line, incubation with osthole at 80 µM for 24 h significantly
inhibited HCC cell proliferation, and following 48 h of treatment
with osthole at 80 µM marked effects were noted on the inhibiton of
cell growth. However, a concentration of 120 µM oshole had a marked
significant effect on the inhibition of cell growth. Additionally,
HCC cell lines following osthole treatment demonstrated a
significant decrease in cell viability in a dose- and
time-dependent manner (P<0.05), while the same concentrations
did not significantly affect the viability of the normal LO2
cells.
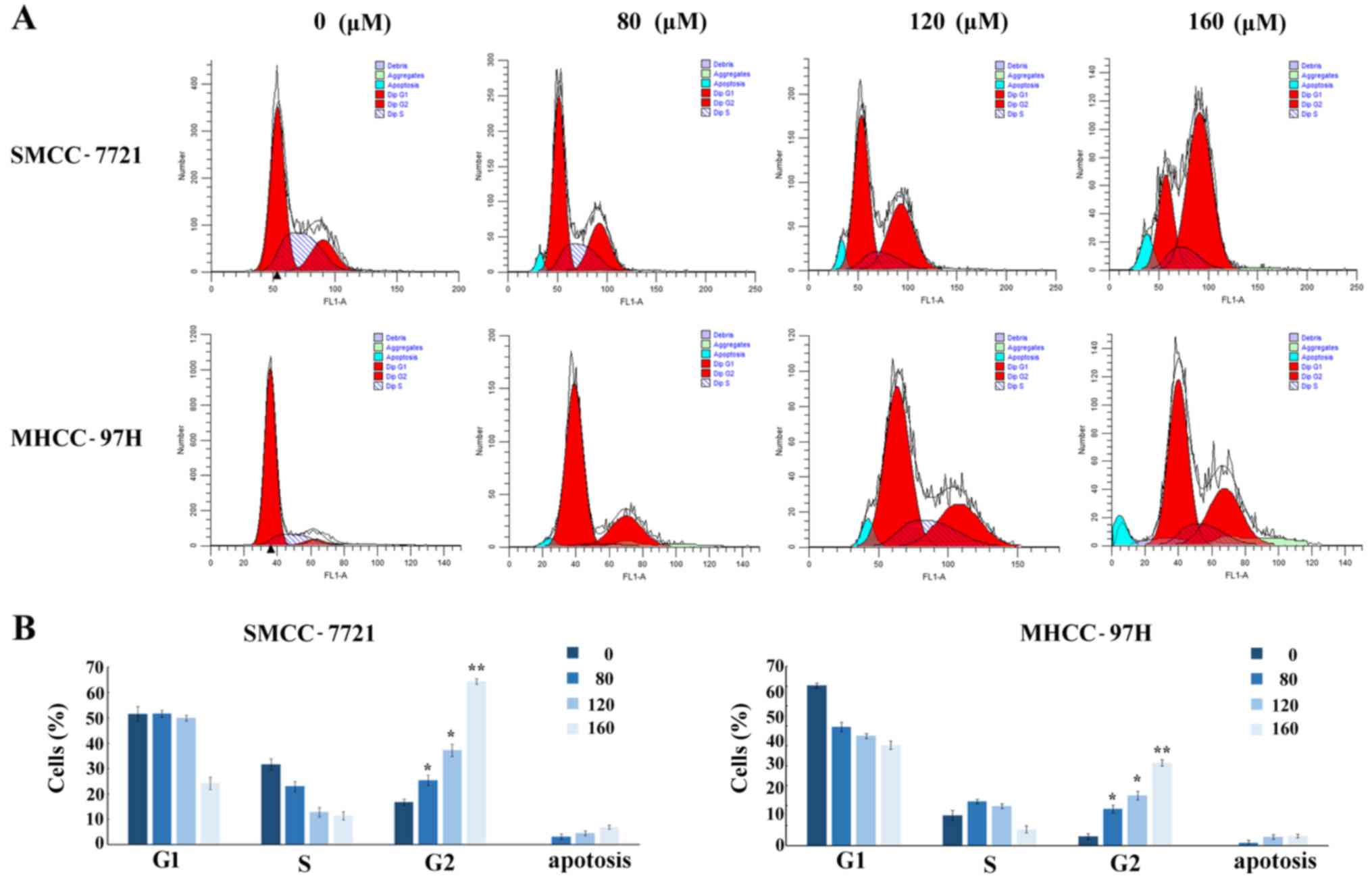
Osthole induces cell G2/M phase arrest
in HCC cell lines
To examine whether osthole contributes to the
induction of cell cycle arrest, the effect on the cell cycle was
measured by flow cytometry. HCC cell lines were treated with
osthole at different concentrations (0, 80, 120 and 160 µM) for 24
h and the SMCC-7721 and MHCC-97H cell lines exhibited significantly
increased accumulation of cells in the G2/M phase after osthole
incubation for 24 h, whereas osthole did not significantly affect
the S phase (Fig. 2A). Along with
increasing concentrations, the percentages of cells in the G2/M
phase were markedly increased from 16.8% (control group) to 64.32%
in the SMCC-7721 cells, and from 4.52% (control group) to 41.48% in
the MHCC-97H cells (Fig. 2B),
indicating that osthole was able to induce cell cycle arrest in the
G2/M phase in a dose-dependent manner. The proportion of apoptotic
cells increased with increasing concentrations of osthole (Fig. 2B).
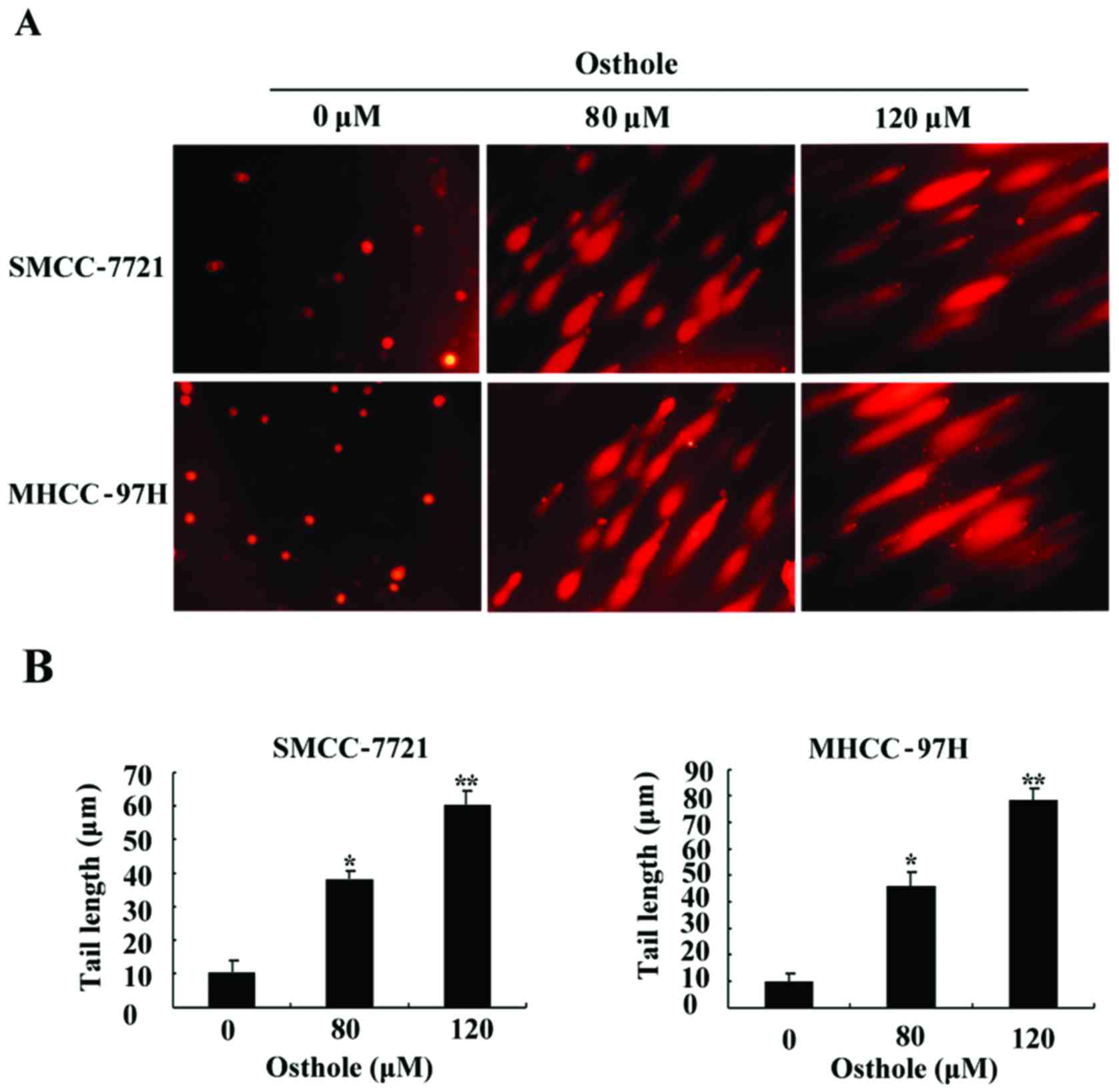
DNA damage by osthole in HCC cell
lines
The degree of DNA damage was evaluated by the comet
assay after the HCC SMCC-7721 and MHCC-97H cell lines were exposed
to osthole at various concentrations (0, 80 and 120 µM) for 24 h,
respectively. The representative images of DNA damage acquired from
the comet assay are presented in Fig.
3, which shows that the comet tail was significantly extended
compared to the control group and DNA damage was increased with the
increasing concentration of osthole, suggesting that osthole
triggered DNA damage in a dose-dependent manner.
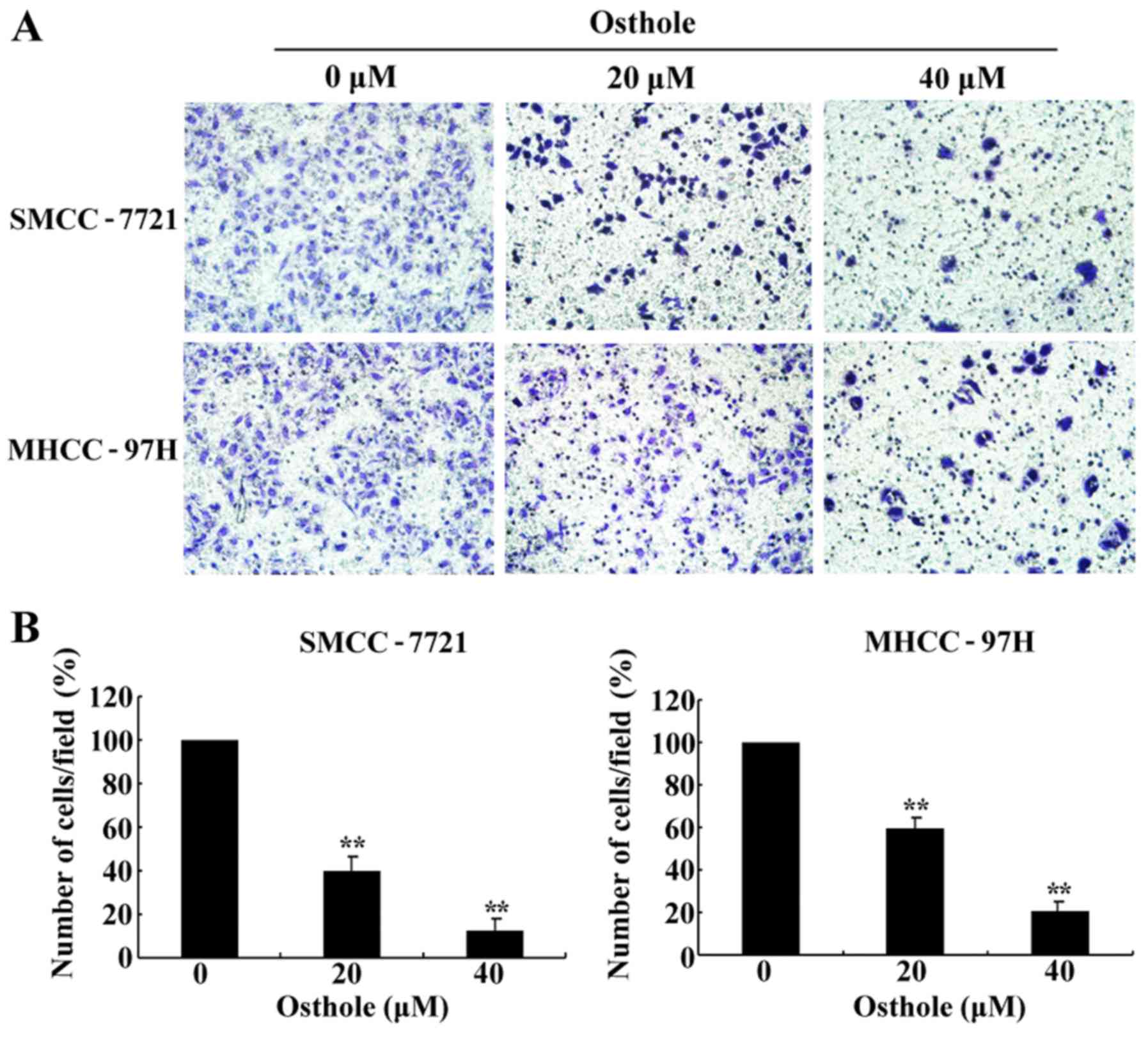
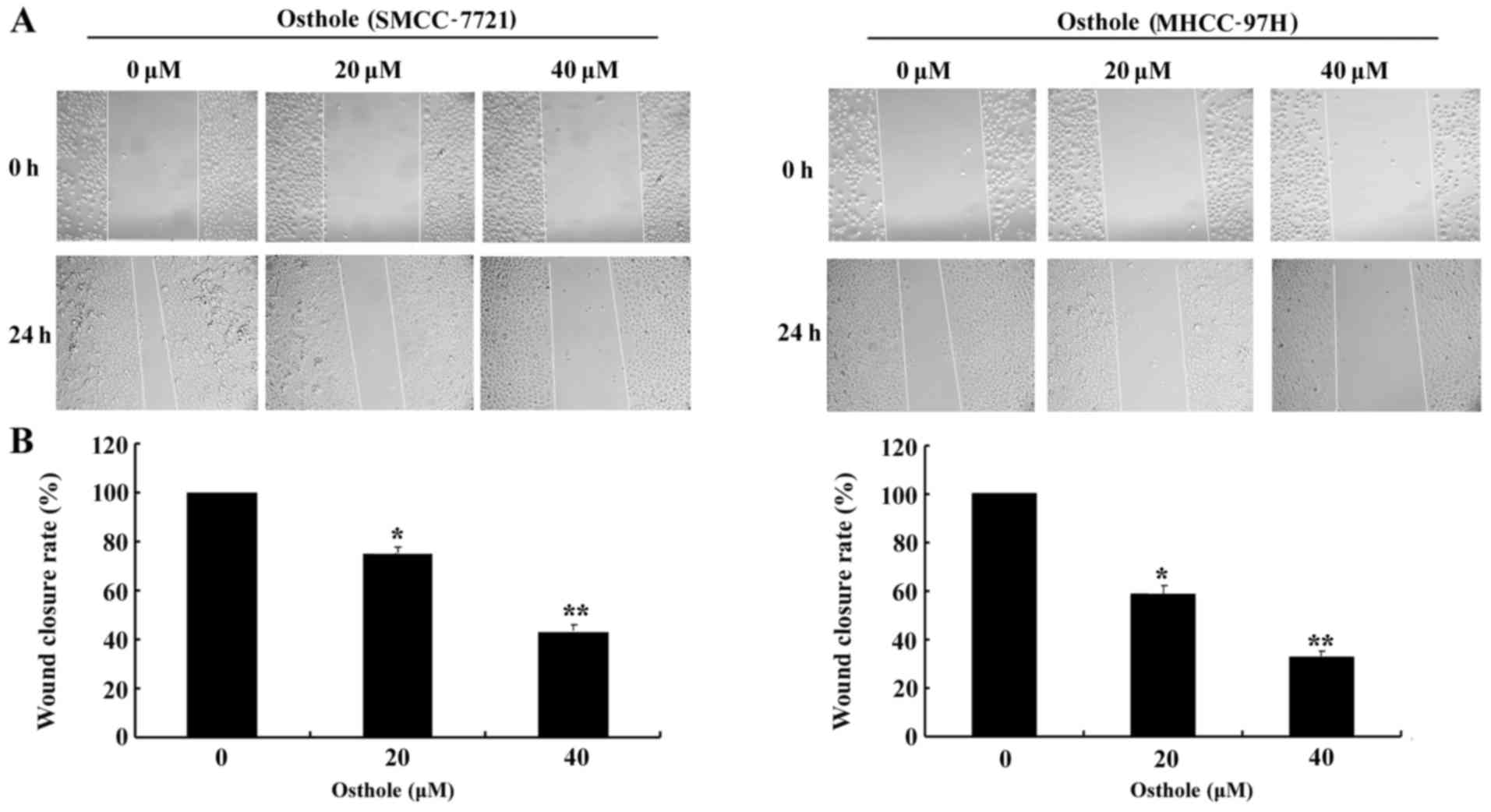
Osthole inhibits the migration of HCC
cells
The effect of osthole on HCC cell migration was
evaluated by Transwell and wound healing assays in the SMCC-7721
and MHCC-97H cell lines following treatment with different
concentrations (0, 20 and 40 µM) of osthole for 24 h, which were
not apoptotic. As shown in Fig. 4A and
B, the mobility ratio of HCC cells from the upper to the lower
chamber gradually declined along with increased concentrations of
osthole. Then, we performed a wound healing assay (Fig. 5). The wound closure rate of the
osthole-incubated cells was lower than that of the control group
(Fig. 5B). The results revealed
that osthole significantly inhibited HCC cell migration in a
dose-dependent manner, suggesting a critical role for osthole in
the inhibition of HCC cell metastasis.
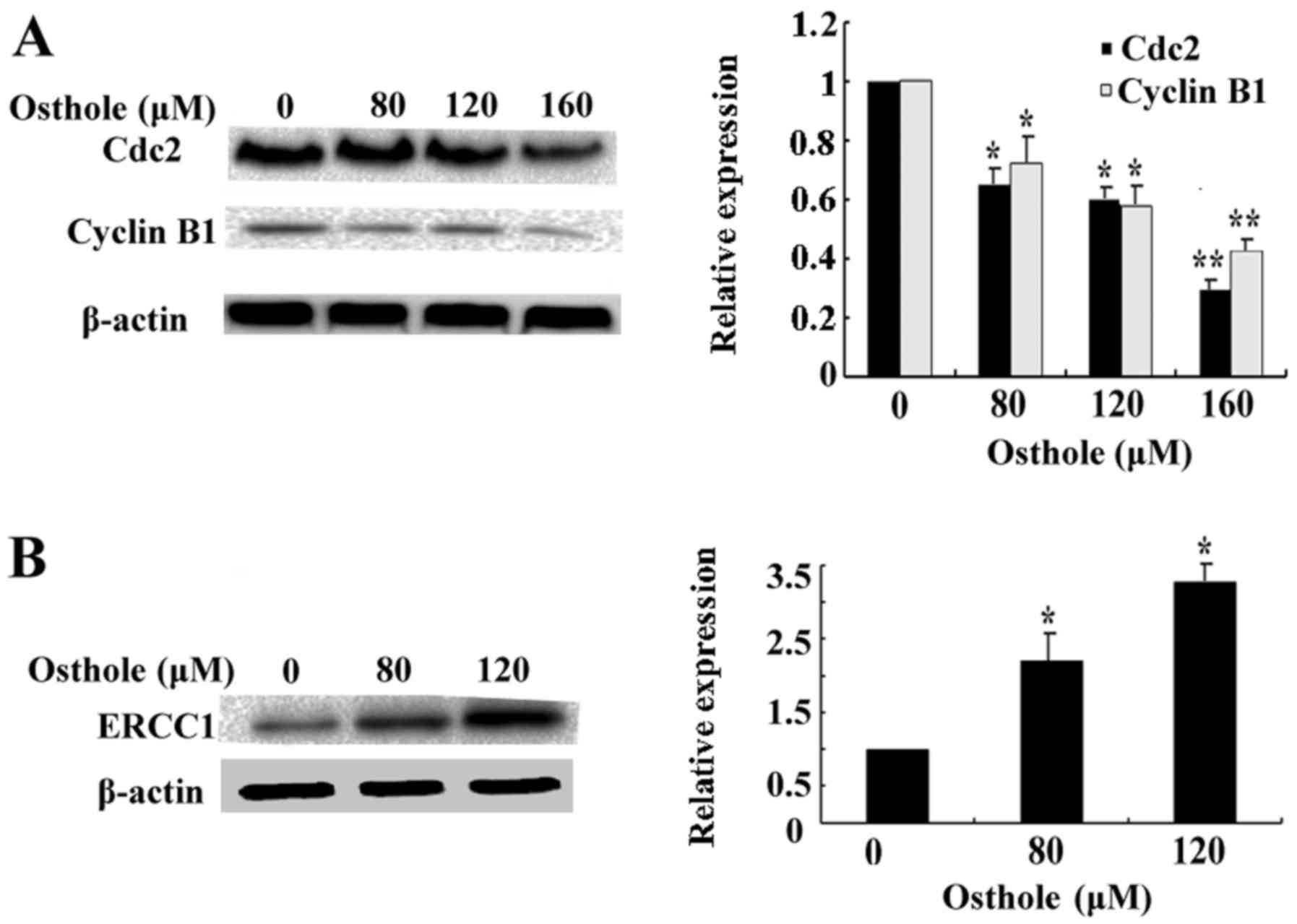
Effects of osthole on cell cycle-, DNA
damage-, EMT- and migration-related protein expression
To further confirm the underlying molecular
mechanisms of the induction of G2/M phase arrest by osthole, we
examined the expression of cell cycle marker proteins Cdc2 and
cyclin B1. As shown in Fig. 6A, the
expression levels of Cdc2 and cyclin B1 were decreased following
treatment with increasing concentrations of osthole. Then, we
investigated the level of DNA damage marker protein ERCC1. As shown
in Fig. 6B, the level of ERCC1 was
significantly upregulated after treatment with osthole in a
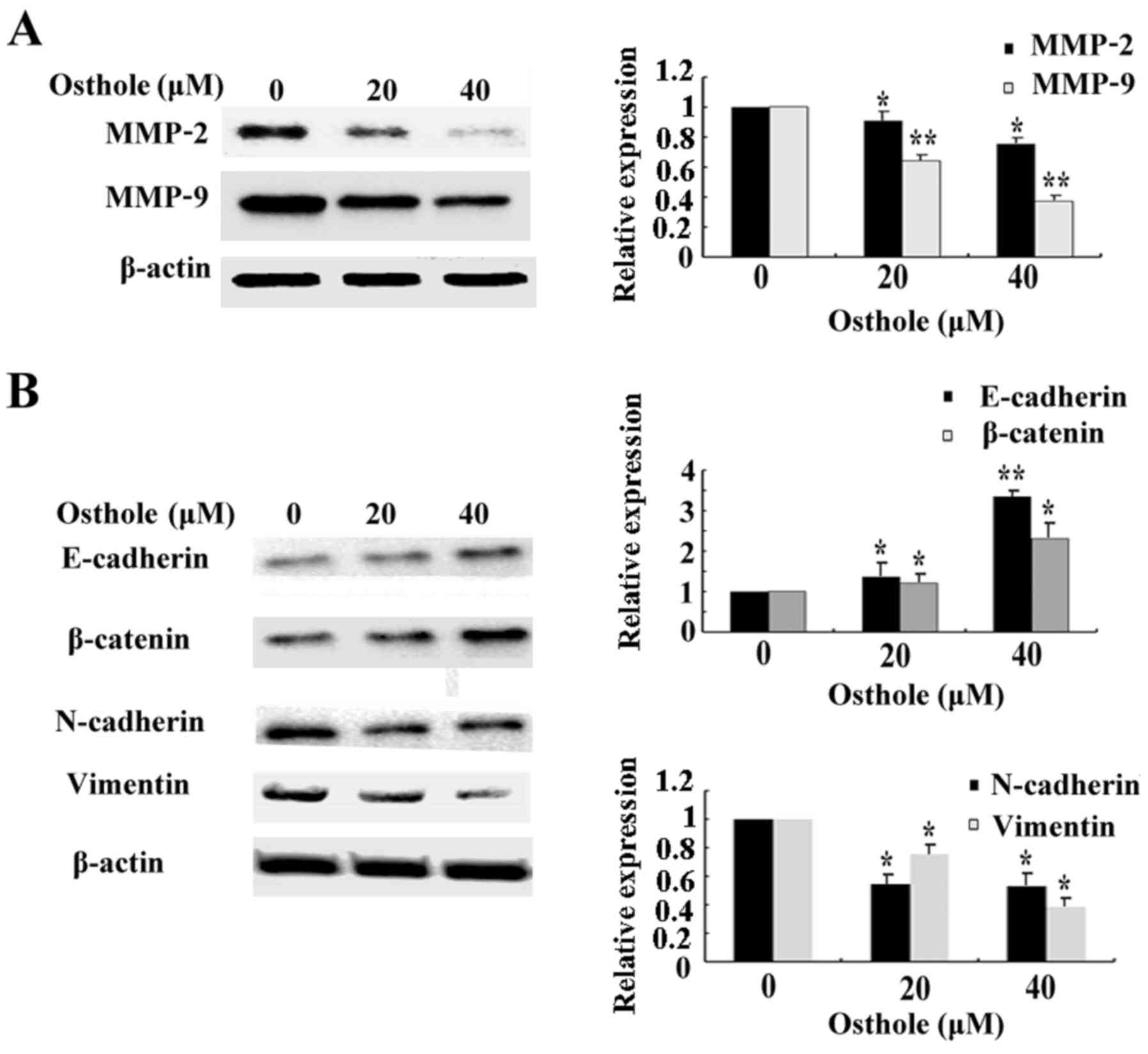
dose-dependent manner. Finally, we assessed the expression of
migration-related proteins MMP-2 and MMP-9 after cells were exposed
to osthole. As shown in Fig. 7A,
along with increasing concentrations of osthole, the expression of
MMP-2 and MMP-9 was gradually decreased. Osthole also increased the
expression of epithelial markers, E-cadherin and β-catenin, and
significantly decreased expression of mesenchymal markers,
N-cadherin and vimentin (Fig. 7B).
These results indicate the potential link between osthole and HCC
cell G2/M phase arrest, DNA damage, EMT and migration-related
protein expression, and also confirmed our previous results on the
pharmacological functions of osthole in HCC cells.
Discussion
Frequent patient diagnosis at advanced stage disease
and limited effective treatment options contribute to the high
mortality of HCC. In-depth knowledge of the multistep process
leading to hepatocarcinogenesis, and development of novel
chemotherapy targeting crucial signaling pathways may effectively
improve the overall survival of patients with HCC across different
stages (4). Osthole has been widely
investigated due to its varied pharmacological functions (6,8,15–20).
It has been reported that osthole inhibited ovarian cancer cells
in vitro (19). We used
concentrations from 0 to 200 µM to detect its effect on inhibition
of cell proliferation. Our data confirmed that osthole induced cell
cycle G2/M phase arrest and triggered DNA damage in HCC cell lines.
Moreover, osthole significantly inhibited cell migration in HCC
cells and we clarified the underlying mechanisms of these
functions.
Dysregulated cell cycle and DNA damage response are
currently two principal mechanisms of anticancer drugs (21,22).
Cell cycle arrest as a critical target to inhibit cell
proliferation has been widely investigated in recent years. Cdc2
and cyclin B1 are key regulators that modulate the G2/M checkpoint
for cancer therapy (11,23–25).
Furthermore, our results showed that osthole induced G2/M phase
arrest in SMCC-7721 and MHCC-97H cells. Then, we demonstrated that
treatment with osthole led to the downregulation of Cdc2 and cyclin
B1 levels. Several studies have illustrated that osthole inhibited
cancer cell proliferation and growth by cell cycle arrest (11,26).
Suggesting that the underlying mechanism of osthole-induced HCC
cell G2/M arrest may occur by attenuating the expression of Cdc2
and cyclin B1, this result is consistent with a previous study in
lung cancer (11). In contrast,
after incubation with osthole, DNA damage was significantly
detected. Excision cross-complementation group 1 (ERCC1) has been
found to be involved in DNA damage repair (27,28),
and we found that ERCC1 was markedly upregulated in the experiment.
Thus, we can conclude that osthole triggers DNA damage in HCC cell
lines.
A great number of studies have focused on cancer
cell metastasis due to its advanced threat to cancer patients.
Increased expression of matrix metalloproteinases (MMPs)
contributes to cancer cell migration, invasion and angiogenesis.
MMP-2 and MMP-9 belong to the family of MMPs that have been widely
studied (29–31). The EMT signaling pathway is also
associated with cancer metastasis (32–34),
and inhibition of EMT could be a potential therapeutic target to
fight cancer metastasis. Previous research revealed that osthole
inhibited cancer cell migration and invasion, in osteosarcoma,
breast and lung cancer (26,35,36).
In the present study, we demonstrated that osthole inhibited HCC
cell migration in a dose-dependent manner, and we also further
elucidated the underlying mechanisms by which osthole inhibits HCC
cell migration by downregulating MMP-2 and MMP-9 expression and
suppressing EMT by increasing the expression of epithelial markers,
E-cadherin and β-catenin while decreasing mesenchymal markers,
N-cadherin and vimentin.
Taken together, osthole possessed potential
anticancer effects on HCC cells by inhibiting cell growth, inducing
cell G2/M phase arrest, triggering DNA damage and suppressing
migration in vitro. The underlying mechanisms of these
functions were associated with the dysregulated expression of
multiple proteins including Cdc2, cyclin B1, ERCC1, MMP-2, MMP-9,
E-cadherin, β-catenin, N-cadherin and vimentin. We confirmed the
potential pharmacological functions of osthole against HCC and
elucidated the various mechanisms underlying these effects,
suggesting that osthole may have high application value in HCC
therapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China Research grant (no. 30870719 to
Z.W.).
References
|
1
|
Li H, Ge C, Zhao F, Yan M, Hu C, Jia D,
Tian H, Zhu M, Chen T, Jiang G, et al: Hypoxia-inducible factor 1
alpha-activated angiopoietin-like protein 4 contributes to tumor
metastasis via vascular cell adhesion molecule-1/integrin β1
signaling in human hepatocellular carcinoma. Hepatology.
54:910–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang S, Yue M, Shu R, Cheng H and Hu P:
Recent advances in the management of hepatocellular carcinoma. J
BUON. 21:307–311. 2016.PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu CY, Chen KF and Chen PJ: Treatment of
Liver Cancer. Cold Spring Harb Perspect Med. 5:a0215352015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verslype C, Van Cutsem E, Dicato M, Arber
N, Berlin JD, Cunningham D, De Gramont A, Diaz-Rubio E, Ducreux M,
Gruenberger T, et al: The management of hepatocellular carcinoma.
Current expert opinion and recommendations derived from the 10th
World Congress on Gastrointestinal Cancer, Barcelona, 2008. Ann
Oncol. 20 Suppl 7:vii1–vii6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luszczki JJ, Andres-Mach M, Cisowski W,
Mazol I, Glowniak K and Czuczwar SJ: Osthole suppresses seizures in
the mouse maximal electroshock seizure model. Eur J Pharmacol.
607:107–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie DQ, Sun GY, Zhang XG and Gan H:
Osthole preconditioning protects rats against renal
ischemia-reperfusion injury. Transplant Proc. 47:1620–1626. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu HP, Liu FC, Tsai YF and Hwang TL:
Osthole attenuates hepatic injury in a rodent model of
trauma-hemorrhage. PLoS One. 8:e659162013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuda H, Tomohiro N, Ido Y and Kubo M:
Anti-allergic effects of Cnidii monnieri fructus (dried fruits of
Cnidium monnieri) and its major component, osthol. Biol Pharm Bull.
25:809–812. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin K, Gao Z, Shang B, Sui S and Fu Q:
Osthole suppresses the proliferation and accelerates the apoptosis
of human glioma cells via the upregulation of microRNA-16 and
downregulation of MMP-9. Mol Med Rep. 12:4592–4597. 2015.PubMed/NCBI
|
|
11
|
Xu X, Zhang Y, Qu D, Jiang T and Li S:
Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells
by modulating PI3K/Akt pathway. J Exp Clin Cancer Res:. 30:332011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding Y, Lu X, Hu X, Ma J and Ding H:
Osthole inhibits proliferation and induces apoptosis in human
osteosarcoma cells. Int J Clin Pharmacol Ther. 52:112–117. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsai CF, Yeh WL, Chen JH, Lin C, Huang SS
and Lu DY: Osthole suppresses the migratory ability of human
glioblastoma multiforme cells via inhibition of focal adhesion
kinase-mediated matrix metalloproteinase-13 expression. Int J Mol
Sci. 15:3889–3903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu XM, Zhang Y, Qu D, Feng XW, Chen Y and
Zhao L: Osthole suppresses migration and invasion of A549 human
lung cancer cells through inhibition of matrix metalloproteinase-2
and matrix metallopeptidase-9 in vitro. Mol Med Rep. 6:1018–1022.
2012.PubMed/NCBI
|
|
15
|
Zhang L, Jiang G, Yao F, He Y, Liang G,
Zhang Y, Hu B, Wu Y, Li Y and Liu H: Growth inhibition and
apoptosis induced by osthole, a natural coumarin, in hepatocellular
carcinoma. PLoS One. 7:e378652012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ko FN, Wu TS, Liou MJ, Huang TF and Teng
CM: Vasorelaxation of rat thoracic aorta caused by osthole isolated
from Angelica pubescens. Eur J Pharmacol. 219:29–34. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiao Y, Kong L, Yao Y, Li S, Tao Z, Yan Y
and Yang J: Osthole decreases beta amyloid levels through
up-regulation of miR-107 in Alzheimer's disease. Neuropharmacology.
108:332–344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wen YC, Lee WJ, Tan P, Yang SF, Hsiao M,
Lee LM and Chien MH: By inhibiting snail signaling and miR-23a-3p,
osthole suppresses the EMT-mediated metastatic ability in prostate
cancer. Oncotarget. 6:21120–21136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu Y, Wen Q, Liang W, Kang T, Ren L, Zhang
N, Zhao D, Sun D and Yang J: Osthole reverses beta-amyloid peptide
cytotoxicity on neural cells by enhancing cyclic AMP response
element-binding protein phosphorylation. Biol Pharm Bull.
36:1950–1958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin VC, Chou CH, Lin YC, Lin JN, Yu CC,
Tang CH, Lin HY and Way TD: Osthole suppresses fatty acid synthase
expression in HER2-overexpressing breast cancer cells through
modulating Akt/mTOR pathway. J Agric Food Chem. 58:4786–4793. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Curtin NJ: Inhibiting the DNA damage
response as a therapeutic manoeuvre in cancer. Br J Pharmacol.
169:1745–1765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Ji P, Liu J, Broaddus RR, Xue F
and Zhang W: Centrosome-associated regulators of the G2/M
checkpoint as targets for cancer therapy. Mol Cancer. 8:82009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dash BC and El-Deiry WS: Phosphorylation
of p21 in G2/M promotes cyclin B-Cdc2 kinase activity. Mol Cell
Biol. 25:3364–3387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cortese K, Daga A, Monticone M, Tavella S,
Stefanelli A, Aiello C, Bisio A, Bellese G and Castagnola P:
Carnosic acid induces proteasomal degradation of Cyclin B1, RB and
SOX2 along with cell growth arrest and apoptosis in GBM cells.
Phytomedicine. 23:679–685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao XX, Chang JJ, Wang QL, Lu R, Li LJ,
Sun X, Xie WD and Li X: 5,6-Dihydroxy-3,7,4′-trimethoxyflavonol
induces G2/M cell cycle arrest and apoptosis in human
hepatocellular carcinoma cells. J Asian Nat Prod Res. 18:1079–1090.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Yang L, Lu Y, Chen Y, Liu T, Peng
Y, Zhou Y, Cao Y, Bi Z, Liu T, et al: Osthole induces cell cycle
arrest and inhibits migration and invasion via PTEN/Akt pathways in
osteosarcoma. Cell Physiol Biochem. 38:2173–2182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsunaga T, Mu D, Park CH, Reardon JT and
Sancar A: Human DNA repair excision nuclease. Analysis of the roles
of the subunits involved in dual incisions by using anti-XPG and
anti-ERCC1 antibodies. J Biol Chem. 270:20862–20869. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Evans E, Moggs JG, Hwang JR, Egly JM and
Wood RD: Mechanism of open complex and dual incision formation by
human nucleotide excision repair factors. EMBO J. 16:6559–6573.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Frich L, Bjørnland K, Pettersen S, Clausen
OP and Gladhaug IP: Increased activity of matrix metalloproteinase
2 and 9 after hepatic radiofrequency ablation. J Surg Res.
135:297–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu B, Cui J, Sun J, Li J, Han X, Guo J,
Yi M, Amizuka N, Xu X and Li M: Immunolocalization of MMP9 and MMP2
in osteolytic metastasis originating from MDA-MB-231 human breast
cancer cells. Mol Med Rep. 14:1099–1106. 2016.PubMed/NCBI
|
|
31
|
Pietruszewska W, Bojanowska-Poźniak K and
Kobos J: Matrix metalloproteinases MMP1, MMP2, MMP9 and their
tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: An
immunohistochemical study. Otolaryngol Pol. 70:32–43. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gugnoni M, Sancisi V, Gandolfi G, Manzotti
G, Ragazzi M, Giordano D, Tamagnini I, Tigano M, Frasoldati A,
Piana S, et al: Cadherin-6 promotes EMT and cancer metastasis by
restraining autophagy. Oncogene. Jul 4–2016.(Epub ahead of print).
doi: 10.1038/onc.2016.237. PubMed/NCBI
|
|
33
|
Ji S, Zhang W, Zhang X, Hao C, Hao A, Gao
Q, Zhang H, Sun J and Hao J: Sohlh2 suppresses epithelial to
mesenchymal transition in breast cancer via downregulation of IL-8.
Oncotarget. Jun 30–2016.(Epub ahead of print). doi:
10.18632/oncotarget.10355.
|
|
34
|
Sun X, Zhao S, Li H, Chang H, Huang Z,
Ding Z, Dong L, Chen J, Zang Y and Zhang J: MicroRNA-30b suppresses
epithelial-mesenchymal transition and metastasis of hepatoma cells.
J Cell Physiol. Jun 23. 2016.(Epub ahead of print). doi:
10.1002/jcp.25466.
|
|
35
|
Hung CM, Kuo DH, Chou CH, Su YC, Ho CT and
Way TD: Osthole suppresses hepatocyte growth factor (HGF)-induced
epithelial-mesenchymal transition via repression of the
c-Met/Akt/mTOR pathway in human breast cancer cells. J Agric Food
Chem. 59:9683–9690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kao SJ, Su JL, Chen CK, Yu MC, Bai KJ,
Chang JH, Bien MY, Yang SF and Chien MH: Osthole inhibits the
invasive ability of human lung adenocarcinoma cells via suppression
of NF-κB-mediated matrix metalloproteinase-9 expression. Toxicol
Appl Pharmacol. 261:105–115. 2012. View Article : Google Scholar : PubMed/NCBI
|