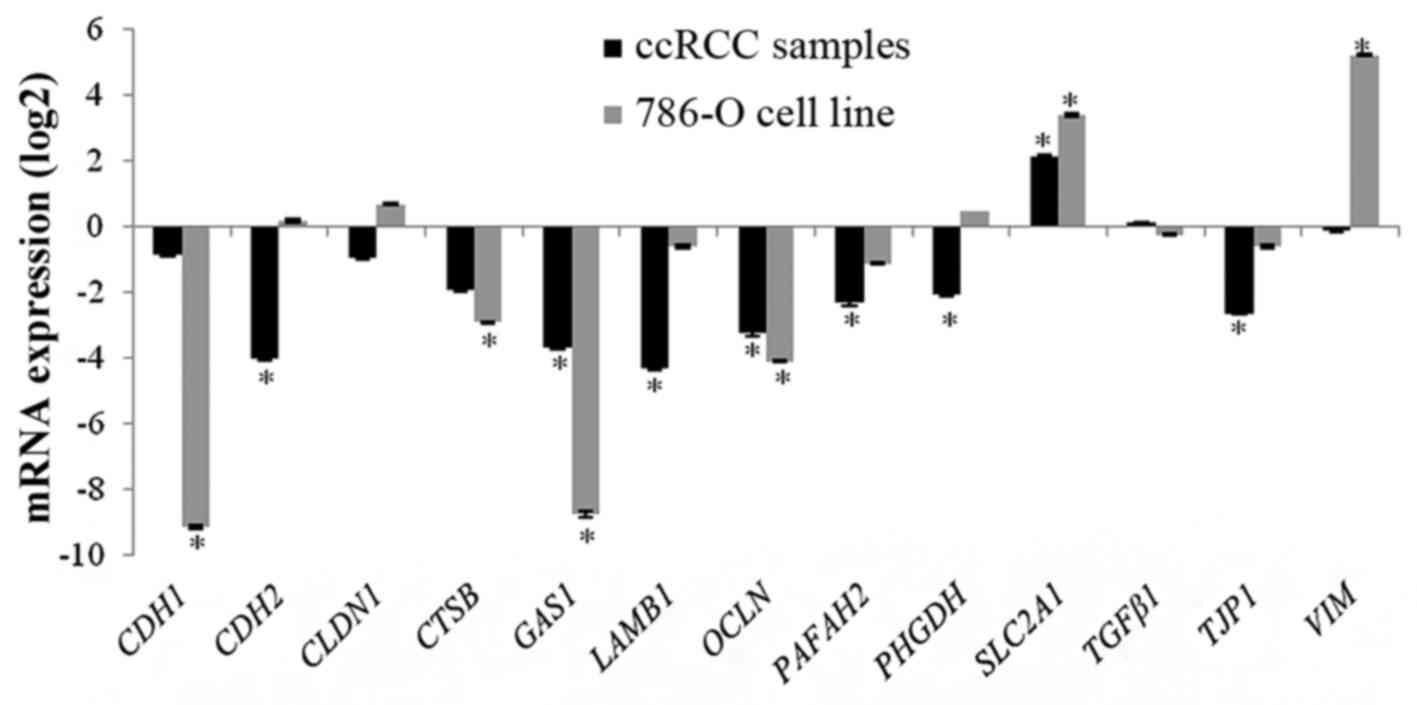
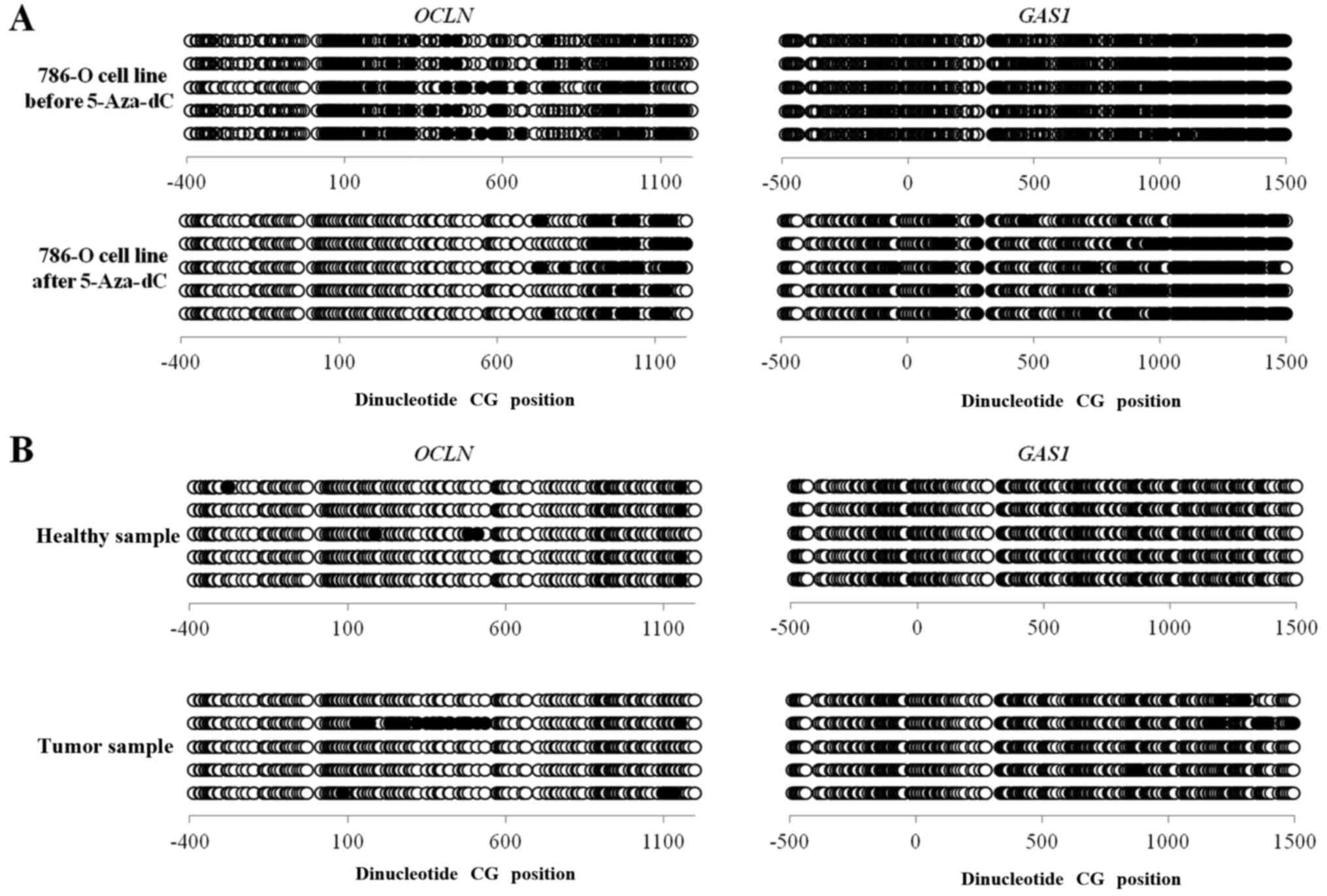
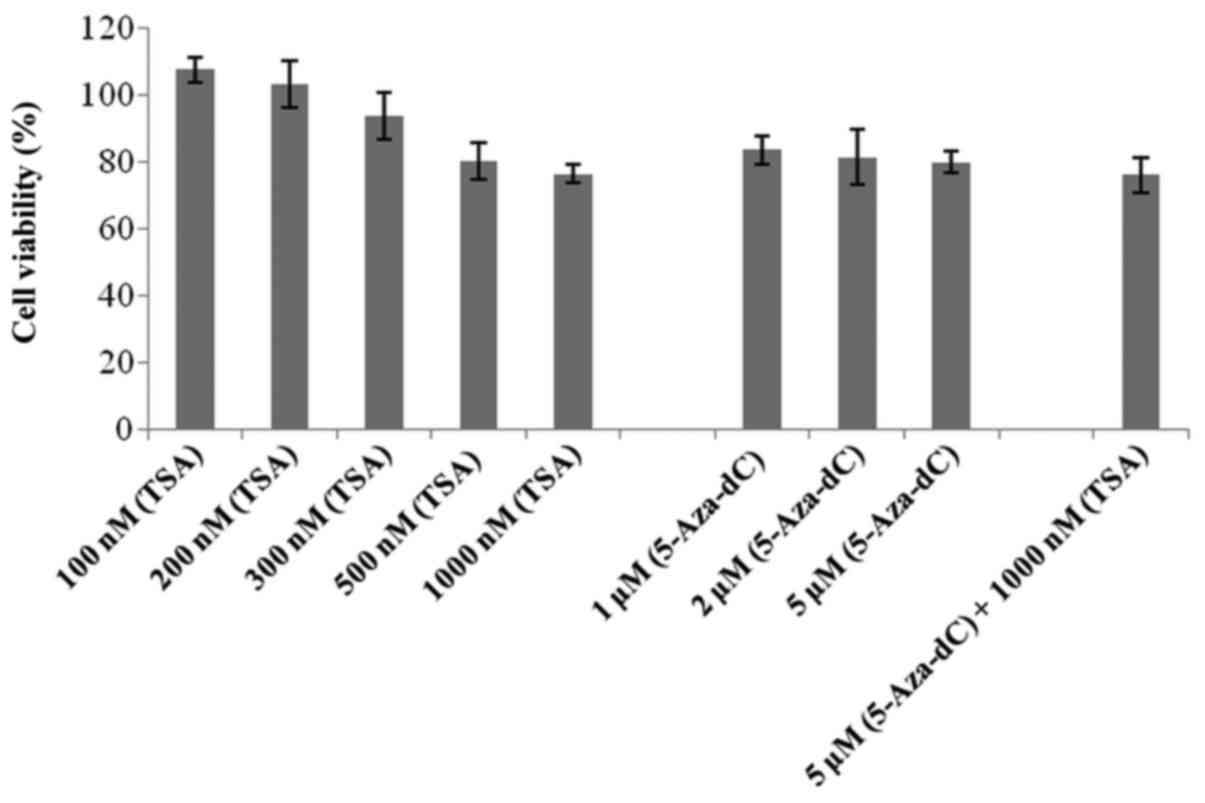
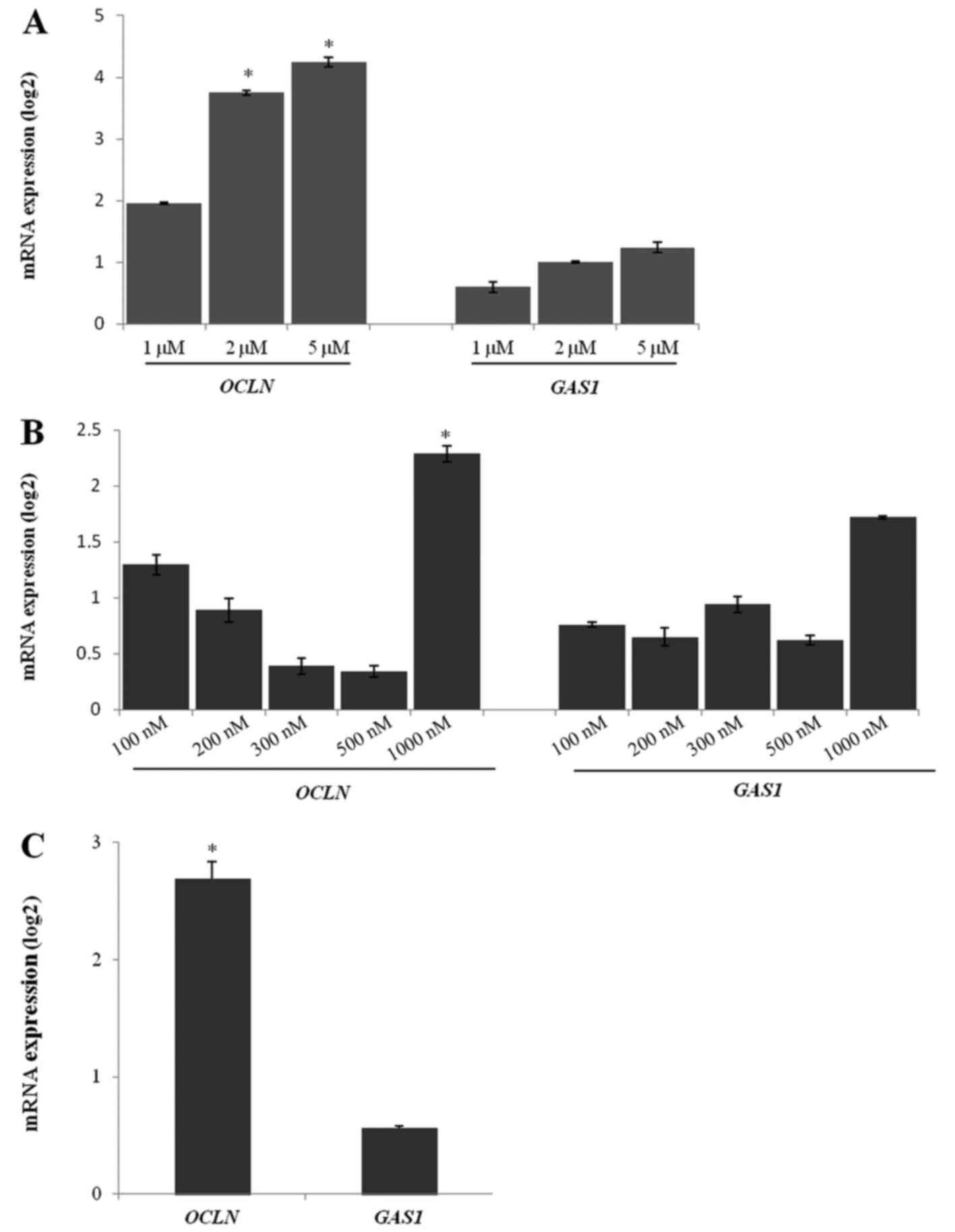
|
1
|
Shuch B, Amin A, Armstrong AJ, Eble JN,
Ficarra V, Lopez-Beltran A, Martignoni G, Rini BI and Kutikov A:
Understanding pathologic variants of renal cell carcinoma:
Distilling therapeutic opportunities from biologic complexity. Eur
Urol. 67:85–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuroda N, Hosokawa T, Michal M, Hes O,
Sima R, Ohe C and Lee GH: Clear cell renal cell carcinoma with
focal renal angiomyoadenomatous tumor-like area. Ann Diagn Pathol.
15:202–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagata M, Sakurai-Yageta M, Yamada D, Goto
A, Ito A, Fukuhara H, Kume H, Morikawa T, Fukayama M, Homma Y, et
al: Aberrations of a cell adhesion molecule CADM4 in renal clear
cell carcinoma. Int J Cancer. 130:1329–1337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baylin SB: DNA methylation and gene
silencing in cancer. Nat Clin Pract Oncol. 2 Suppl 1:S4–S11. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang J and Zhuang S: Epigenetics in acute
kidney injury. Curr Opin Nephrol Hypertens. 24:351–358.
2015.PubMed/NCBI
|
|
7
|
Baylin SB and Ohm JE: Epigenetic gene
silencing in cancer - a mechanism for early oncogenic pathway
addiction? Nat Rev Cancer. 6:107–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feinberg AP and Tycko B: The history of
cancer epigenetics. Nat Rev Cancer. 4:143–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benard A, Goossens-Beumer IJ, van Hoesel
AQ, De Graaf W, Horati H, Putter H, Zeestraten EC, van de Velde CJ
and Kuppen PJ: Histone trimethylation at H3K4, H3K9 and H4K20
correlates with patient survival and tumor recurrence in
early-stage colon cancer. BMC Cancer. 14:5312014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khakpour G, Pooladi A, Izadi P, Noruzinia
M and Bazzaz J Tavakkoly: DNA methylation as a promising landscape:
A simple blood test for breast cancer prediction. Tumour Biol.
36:4905–4912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J and Mansmann UR: A microRNA molecular
modeling extension for prediction of colorectal cancer treatment.
BMC Cancer. 15:4722015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Wever O, Pauwels P, De Craene B, Sabbah
M, Emami S, Redeuilh G, Gespach C, Bracke M and Berx G: Molecular
and pathological signatures of epithelial-mesenchymal transitions
at the cancer invasion front. Histochem Cell Biol. 130:481–494.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pierucci-Alves F, Yi S and Schultz BD:
Transforming growth factor beta 1 induces tight junction
disruptions and loss of transepithelial resistance across porcine
vas deferens epithelial cells. Biol Reprod. 86:362012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katoh Y and Katoh M: Hedgehog signaling,
epithelial-to-mesenchymal transition and miRNA (Review). Int J Mol
Med. 22:271–275. 2008.PubMed/NCBI
|
|
15
|
Kunkel M, Reichert TE, Benz P, Lehr HA,
Jeong JH, Wieand S, Bartenstein P, Wagner W and Whiteside TL:
Overexpression of Glut-1 and increased glucose metabolism in tumors
are associated with a poor prognosis in patients with oral squamous
cell carcinoma. Cancer. 97:1015–1024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun L, Zeng X, Yan C, Sun X, Gong X, Rao Y
and Yan N: Crystal structure of a bacterial homologue of glucose
transporters GLUT1-4. Nature. 490:361–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasaki H, Shitara M, Yokota K, Hikosaka Y,
Moriyama S, Yano M and Fujii Y: Overexpression of GLUT1 correlates
with Kras mutations in lung carcinomas. Mol Med Rep. 5:599–602.
2012.PubMed/NCBI
|
|
18
|
Li W, Wei Z, Liu Y, Li H, Ren R and Tang
Y: Increased 18F-FDG uptake and expression of Glut1 in the EMT
transformed breast cancer cells induced by TGF-beta. Neoplasma.
57:234–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fisseler-Eckhoff A: New TNM classification
of malignant lung tumors 2009 from a pathology perspective.
Pathologe. 30 Suppl 2:S193–S199. 2009.(In German). View Article : Google Scholar
|
|
20
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calmon MF, Colombo J, Carvalho F, Souza
FP, Filho JF, Fukuyama EE, Camargo AA, Caballero OL, Tajara EH,
Cordeiro JA, et al: Methylation profile of genes CDKN2Ap14 and
p16), DAPK1CDH1, and ADAM23 in head and neck cancer. Cancer Genet
Cytogenet. 173:31–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyen CT, Weisenberger DJ, Velicescu M,
Gonzales FA, Lin JC, Liang G and Jones PA: Histone H3-lysine 9
methylation is associated with aberrant gene silencing in cancer
cells and is rapidly reversed by 5-aza-2′-deoxycytidine. Cancer
Res. 62:6456–6461. 2002.PubMed/NCBI
|
|
23
|
Zigeuner R, Hutterer G, Chromecki T,
Imamovic A, Kampel-Kettner K, Rehak P, Langner C and Pummer K:
External validation of the Mayo Clinic stage, size, grade, and
necrosis (SSIGN) score for clear-cell renal cell carcinoma in a
single European centre applying routine pathology. Eur Urol.
57:102–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gulati S, Martinez P, Joshi T, Birkbak NJ,
Santos CR, Rowan AJ, Pickering L, Gore M, Larkin J, Szallasi Z, et
al: Systematic evaluation of the prognostic impact and intratumour
heterogeneity of clear cell renal cell carcinoma biomarkers. Eur
Urol. 66:936–948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fisel P, Kruck S, Winter S, Bedke J,
Hennenlotter J, Nies AT, Scharpf M, Fend F, Stenzl A, Schwab M, et
al: DNA methylation of the SLC16A3 promoter regulates expression of
the human lactate transporter MCT4 in renal cancer with
consequences for clinical outcome. Clin Cancer Res. 19:5170–5181.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furuse M, Hirase T, Itoh M, Nagafuchi A,
Yonemura S and Tsukita S and Tsukita S: Occludin: A novel integral
membrane protein localizing at tight junctions. J Cell Biol.
123:1777–1788. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balda MS, Whitney JA, Flores C, González
S, Cereijido M and Matter K: Functional dissociation of
paracellular permeability and transepithelial electrical resistance
and disruption of the apical-basolateral intramembrane diffusion
barrier by expression of a mutant tight junction membrane protein.
J Cell Biol. 134:1031–1049. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balda MS, Flores-Maldonado C, Cereijido M
and Matter K: Multiple domains of occludin are involved in the
regulation of paracellular permeability. J Cell Biochem. 78:85–96.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farquhar MG and Palade GE: Junctional
complexes in various epithelia. J Cell Biol. 17:375–412. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orbán E, Szabó E, Lotz G, Kupcsulik P,
Páska C, Schaff Z and Kiss A: Different expression of occludin and
ZO-1 in primary and metastatic liver tumors. Pathol Oncol Res.
14:299–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martin TA, Mansel RE and Jiang WG: Loss of
occludin leads to the progression of human breast cancer. Int J Mol
Med. 26:723–734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tobioka H, Isomura H, Kokai Y, Tokunaga Y,
Yamaguchi J and Sawada N: Occludin expression decreases with the
progression of human endometrial carcinoma. Hum Pathol. 35:159–164.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tobioka H, Tokunaga Y, Isomura H, Kokai Y,
Yamaguchi J and Sawada N: Expression of occludin, a
tight-junction-associated protein, in human lung carcinomas.
Virchows Arch. 445:472–476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rachow S, Zorn-Kruppa M, Ohnemus U,
Kirschner N, Vidal-y-Sy S, von den Driesch P, Börnchen C, Eberle J,
Mildner M, Vettorazzi E, et al: Occludin is involved in adhesion,
apoptosis, differentiation and Ca2+-homeostasis of human
keratinocytes: Implications for tumorigenesis. PLoS One.
8:e551162013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Osanai M, Murata M, Nishikiori N, Chiba H,
Kojima T and Sawada N: Epigenetic silencing of occludin promotes
tumorigenic and metastatic properties of cancer cells via
modulations of unique sets of apoptosis-associated genes. Cancer
Res. 66:9125–9133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ellinger J, Kahl P, Mertens C, Rogenhofer
S, Hauser S, Hartmann W, Bastian PJ, Büttner R, Müller SC and von
Ruecker A: Prognostic relevance of global histone H3 lysine 4
(H3K4) methylation in renal cell carcinoma. Int J Cancer.
127:2360–2366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ye D, Guo S, Al-Sadi R and Ma TY: MicroRNA
regulation of intestinal epithelial tight junction permeability.
Gastroenterology. 141:1323–1333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Munari E, Marchionni L, Chitre A, Hayashi
M, Martignoni G, Brunelli M, Gobbo S, Argani P, Allaf M, Hoque MO,
et al: Clear cell papillary renal cell carcinoma: micro-RNA
expression profiling and comparison with clear cell renal cell
carcinoma and papillary renal cell carcinoma. Hum Pathol.
45:1130–1138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
White NM, Bao TT, Grigull J, Youssef YM,
Girgis A, Diamandis M, Fatoohi E, Metias M, Honey RJ, Stewart R, et
al: miRNA profiling for clear cell renal cell carcinoma: Biomarker
discovery and identification of potential controls and consequences
of miRNA dysregulation. J Urol. 186:1077–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou L, Chen J, Li Z, Li X, Hu X, Huang Y,
Zhao X, Liang C, Wang Y, Sun L, et al: Integrated profiling of
microRNAs and mRNAs: microRNAs located on Xq27.3 associate with
clear cell renal cell carcinoma. PLoS One. 5:e152242010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fong MY, Zhou W, Liu L, Alontaga AY,
Chandra M, Ashby J, Chow A, O'Connor ST, Li S, Chin AR, et al:
Breast-cancer-secreted miR-122 reprograms glucose metabolism in
premetastatic niche to promote metastasis. Nat Cell Biol.
17:183–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ruaro ME, Stebel M, Vatta P, Marzinotto S
and Schneider C: Analysis of the domain requirement in Gas1 growth
suppressing activity. FEBS Lett. 481:159–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stebel M, Vatta P, Ruaro ME, Del Sal G,
Parton RG and Schneider C: The growth suppressing gas1 product is a
GPI-linked protein. FEBS Lett. 481:152–158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Del Sal G, Ruaro ME, Philipson L and
Schneider C: The growth arrest-specific gene, gas1, is involved in
growth suppression. Cell. 70:595–607. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Beachy PA, Karhadkar SS and Berman DM:
Tissue repair and stem cell renewal in carcinogenesis. Nature.
432:324–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Allen BL, Tenzen T and McMahon AP: The
Hedgehog-binding proteins Gas1 and Cdo cooperate to positively
regulate Shh signaling during mouse development. Genes Dev.
21:1244–1257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang H, Zhou X, Zhang Y, Zhu H, Zhao L,
Fan L, Wang Y, Gang Y, Wu K, Liu Z, et al: Growth arrest-specific
gene 1 is downregulated and inhibits tumor growth in gastric
cancer. FEBS J. 279:3652–3664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang Z, Xu Y and Cai S: Down-regulated
GAS1 expression correlates with recurrence in stage II and III
colorectal cancer. Hum Pathol. 42:361–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gobeil S, Zhu X, Doillon CJ and Green MR:
A genome-wide shRNA screen identifies GAS1 as a novel melanoma
metastasis suppressor gene. Genes Dev. 22:2932–2940. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Scaltriti M, Brausi M, Amorosi A, Caporali
A, DArca D, Astancolle S, Corti A and Bettuzzi S: Clusterin (SGP-2,
ApoJ) expression is downregulated in low- and high-grade human
prostate cancer. Int J Cancer. 108:23–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sacilotto N, Espert A, Castillo J, Franco
L and López-Rodas G: Epigenetic transcriptional regulation of the
growth arrest-specific gene 1Gas1) in hepatic cell proliferation at
mononucleosomal resolution. PLoS One. 6:e233182011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ma Y, Qin H and Cui Y: MiR-34a targets
GAS1 to promote cell proliferation and inhibit apoptosis in
papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem
Biophys Res Commun. 441:958–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang L, He S, Guo S, Xie W, Xin R, Yu H,
Yang F, Qiu J, Zhang D, Zhou S, et al: Down-regulation of miR-34a
alleviates mesangial proliferation in vitro and glomerular
hypertrophy in early diabetic nephropathy mice by targeting GAS1. J
Diabetes Complications. 28:259–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu H, Brannon AR, Reddy AR, Alexe G,
Seiler MW, Arreola A, Oza JH, Yao M, Juan D, Liou LS, et al:
Identifying mRNA targets of microRNA dysregulated in cancer: With
application to clear cell renal cell carcinoma. BMC Syst Biol.
4:512010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu G, Li H, Wang J, Gumireddy K, Li A, Yao
W, Tang K, Xiao W, Hu J, Xiao H, et al: miRNA-34a suppresses cell
proliferation and metastasis by targeting CD44 in human renal
carcinoma cells. J Urol. 192:1229–1237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dutta KK, Zhong Y, Liu YT, Yamada T,
Akatsuka S, Hu Q, Yoshihara M, Ohara H, Takehashi M, Shinohara T,
et al: Association of microRNA-34a overexpression with
proliferation is cell type-dependent. Cancer Sci. 98:1845–1852.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yan S, Wang Y, Chen M, Li G and Fan J:
Deregulated SLC2A1 promotes tumor cell proliferation and metastasis
in gastric cancer. Int J Mol Sci. 16:16144–16157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Amann T, Maegdefrau U, Hartmann A, Agaimy
A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Schölmerich
J, Oefner PJ, et al: GLUT1 expression is increased in
hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol.
174:1544–1552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Krzeslak A, Wojcik-Krowiranda K, Forma E,
Jozwiak P, Romanowicz H, Bienkiewicz A and Brys M: Expression of
GLUT1 and GLUT3 glucose transporters in endometrial and breast
cancers. Pathol Oncol Res. 18:721–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Manel N, Kim FJ, Kinet S, Taylor N, Sitbon
M and Battini JL: The ubiquitous glucose transporter GLUT-1 is a
receptor for HTLV. Cell. 115:449–459. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Augustin R: The protein family of glucose
transport facilitators: It's not only about glucose after all.
IUBMB Life. 62:315–333. 2010.PubMed/NCBI
|
|
64
|
Cifuentes M, García MA, Arrabal PM,
Martínez F, Yañez MJ, Jara N, Weil B, Domínguez D, Medina RA and
Nualart F: Insulin regulates GLUT1-mediated glucose transport in
MG-63 human osteosarcoma cells. J Cell Physiol. 226:1425–1432.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Vermeulen JF, van Brussel AS, van der
Groep P, Morsink FH, Bult P, van der Wall E and van Diest PJ:
Immunophenotyping invasive breast cancer: Paving the road for
molecular imaging. BMC Cancer. 12:2402012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vermeulen JF, van der Wall E, Witkamp AJ
and van Diest PJ: Analysis of expression of membrane-bound tumor
markers in ductal carcinoma in situ of the breast: Paving the way
for molecular imaging. Cell Oncol. 36:333–340. 2013. View Article : Google Scholar
|