Introduction
Rhein
(4,5-dihydroxy-9,10-dioxoanthracene-2-carboxylic acid) is a primary
anthraquinone (Fig. 1A) found in
the roots of rhubarb, a traditional Chinese herb, and has been
shown to exhibit various anticancer effects (1–3).
Recently, in vitro and in vivo studies have shown
that rhein inhibits the growth of many types of cancer cells, such
as those found in human oral cancer (4–6),
adenocarcinoma (7), lung cancer
(8), breast cancer (8), hepatocellular carcinoma (9), and cervical cancer (10). Major compounds of therapeutic
importance in rhubarb are derivatives of anthraquinone, and were
found to inhibit multiple signal transduction pathways, including
activator protein 1 (AP-1) and nuclear factor-κB (NF-κB) (11,12).
Although little is known in regards to the effect of rhein on
tumorigenesis, suppression of these signals is believed to
contribute to its anticancer activities as these pathways are
critical in carcinogenesis.
The peptidyl-prolyl cis/trans isomerase Pin1
catalyzes cis/trans isomerization in peptide bonds of
phosphorylated serine/threonine-proline (Ser/Thr-Pro) motifs
(13,14). Pin1 binds specifically to a wide
variety of proteins that harbor WW domains [one of the smallest
protein modules, composed of only 40 amino acids, which mediates
specific protein-protein interactions with short proline-rich or
proline-containing motifs (15)].
Thus, Pin1 plays a central role as a post-phosphorylation regulator
in fine-tuning protein functions (16–18).
It isomerizes the peptide bond of specific phosphorylated serine or
threonine residues that precede proline in several of the proteins
involved in various cellular events, including mitosis,
transcription, differentiation, and the response to DNA damage
(14,19). The Ser/Thr-Pro motifs appear to be
exclusive phosphorylation sites for many key protein kinases
involved in the control of cell growth and transformation (20,21).
Pin1 is most likely required for the full activation of multiple
signal transduction pathways, including AP-1 and NF-κB (14,22,23).
Furthermore, Pin1 plays a key role in oncogenic signaling pathways
(24,25) and is abundant in various tumors
(23,26). Notably, inhibition of Pin1 in cancer
cells triggers apoptosis or suppresses the transformed phenotype
(27,28). Rhein was reported to inhibit the
activation of HER-2/Neu and its downstream signaling pathway in
human breast cancer cells (29).
Pin1 is also a downstream effector of oncogenic Neu/Ras signaling
(30).
Therefore, we hypothesized that rhein may directly
target Pin1 to suppress critical oncogenic signaling pathways and
neoplastic cell transformation.
Materials and methods
Reagents and antibodies
The checkmate mammalian two-hybrid system, including
expression vectors and the luciferase reporter vector, was obtained
from Promega (Madison, WI, USA). Cell culture media and other
supplements were purchased from Life Technologies (Rockville, MD,
USA). Specific antibodies for use in the immunoblotting,
immunoprecipitation, and immunocytochemical analyses were obtained
from Cell Signaling Technology, Inc. (Beverly, MA, USA), Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA), Upstate Biotechnology
(Boston, MA, USA), and GE Healthcare (Piscataway, NJ, USA). For the
transfection experiments, jetPEI transfection reagent was purchased
from Qbiogene (Carlsbad, CA, USA). Rhein was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Cell viability assay
Pin1+/+, Pin1−/−, Neu/Pin1
knockout (KO) and Neu/Pin1 wild-type (WT) mouse embryonic
fibroblast (MEF) cells, which were originally developed by Fujimori
et al (31) and Ryo et
al (27), were obtained from Dr
Kun Ping Lu (Harvard Medical School, Boston, MA, USA). K1735 C10
and K1735 M2 melanoma cells were kindly provided by Zigang Dong
(University of Minnesota, Austin, MN, USA). JB6 CI41 and human
embryonic kidney 293T cells were purchased from American Type
Culture Collection (ATCC, Manassas, VA, USA). Cells from the mouse
epidermal cell line JB6 CI41 were seeded in a 96-well microtiter
plate (2×103/100 µl) and were incubated for 24 or 48 h
with rhein (10–60 µM). Viability was estimated using the CellTiter
96 AQueous One Solution Cell Proliferation Assay kit (Promega)
according to the manufacturer's instructions. The assay solution
was added to each well, and absorbance (492 nm, 690 nm background)
was read using a 96-well plate reader.
siRNA Pin1 plasmid construction
The mU6Pro-Pin1 plasmid was constructed using the
mU6Pro vector (32). The inserted
sequence was:
5′-TTTGGTCAGGAGAGGAAGACTTTTTCAAGAGAAAAGTCTTCCTCTCCTGACTTTTT-3′. The
siRNA sequence targeted against mRNA Pin1 was
GUCAGGAGAGGAAGACUUU.
Construction of wild-type (WT) or
mutant Pin1 expression vectors, and establishing stable cells
The coding sequences of Pin1 were obtained after
polymerase chain reaction (PCR) amplification (30 cycles, with
annealing temperature at 65°C) of cDNA derived from reverse
transcription of the total RNA of Neu/Pin1 in WT MEFs. The
amplified PCR products were ligated into the pcDNA4.0-HisMaxC
plasmid (Invitrogen, Grand Island, NY, USA) to produce an
expression vector for 6x-His-tagged and Xpress-tagged Pin1.
Transformants of Pin1 were obtained by transfecting 1 µg of
pcDNA4.0-HisMaxC, the pcDNA4.0-HisMaxC/Pin1 vector into Neu/Pin1 KO
and K1735 M2 cells using jetPEI and were then maintained by adding
Zeocin to the culture medium. Either the pU6pro vector control or
pU6pro vector containing siPin1 was transfected into the JB6 CI41
cells.
Cell culture and transfection
Pin1−/− and Pin1+/+ MEFs and
293T cells were maintained in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS). JB6 CI41
cells were grown in 5% FBS-MEM. Cells were cultured at 37°C in a 5%
CO2 incubator. Either the pU6pro vector control or
pU6pro vector containing siPin1 was transfected into the JB6 CI41
cells. The resulting stable transfectants were isolated by growing
them in the selective MEM containing G418. The JB6 CI41 cells
engineered to express Pin1 at low levels are designated as JB6
CI41/siPin1.
Anchorage-independent transformation
assay
Using soft agar assays, we evaluated the functional
activity of Pin1 in cells stimulated with
12-O-tetradecanoylphorbol-13-acetate (TPA): JB6 CI41, JB6
CI41/siMock, K1735 C10/siMock, K1735 C10/siPin1, K1735 M2/Mock and
K1735 M2/siPin1. The cells at a density of 8×103 cells
were treated with TPA (20 ng/ml) in 1 ml of 0.3% basal medium
Eagle's (BME) agar over 3 ml of 0.5% BME agar containing 10% FBS.
The cultures were maintained in a 5% CO2 incubator at
37°C for 14 days, and the cell colonies were counted using a
microscope and Image-Pro Plus computer software (Media Cybernetics,
Warrendale, PA, USA).
GST/Pin1 pull-down assay
To investigate the interaction of Pin1 and c-Jun, a
glutathione S-transferase (GST) pull-down assay was
performed, as described elsewhere (33). The pcDNA3.1/c-Jun plasmid was kindly
provided by Dr Michael J. Birrer (National Cancer Institute,
Bethesda, MD, USA). For expression of c-Jun, the c-Jun plasmid
(pcDNA3.1/c-Jun) was transfected into 293T cells. For the GST/Pin1
pull-down assay, 4 mg of GST and GST/Pin1 full-length proteins were
collected on glutathione-sepharose beads (Amersham Biosciences,
Little Chalfont, UK) and were treated with rhein (0, 20, 40, 60 and
80 µM). The bound proteins were denatured in sample buffer,
separated, and analyzed by immunoblotting with anti-phospho-c-Jun
(pc-Jun) (Ser73).
Luciferase assay
Transient transfection was conducted using jetPEI,
and activity of the firefly and Renilla luciferases was
measured according to the manufacturer's instructions (Promega).
Pin1 WT and Pin1 KO MEFs (1×104 cells/well) were
cotransfected with the AP-1 or NF-κB reporter plasmid, cultured for
24 h, and then treated with rhein (10–60 µM). The cells were
incubated for 1 h before TPA was added and were then cultured for
another 24 h. Firefly and Renilla luciferase activities were
measured using the Luminoskan Ascent plate reader (1450 MicroBeta
TriLux; Perkin-Elmer, Waltham, MA, USA). Relative luciferase
activity was calculated as a percentage of the activity of
TPA-induced AP-1 or NF-κB luciferase, as compared with untreated
controls, and transfection efficiency was normalized against
Renilla luciferase activity.
Cell cycle and apoptosis analyses
Cell cycle distribution was analyzed by flow
cytometry, as previously described (34). After treatment with rhein for 24 h,
the cells were analyzed with a Beckman Coulter Epics XL-MCL flow
cytometer (Beckman Coulter, Miami, FL, USA) using MultiCycle
software (Phoenix Flow Systems, San Diego, CA, USA), which
indicated the proportions of cells in the
G1/G0, S, and G2/M phases. Rates
of apoptosis were determined using Annexin V-based
immunofluorescence. Cells (1×105) were plated in 60-mm
culture dishes at concentrations determined to yield
G1/G0, S and G2/M within 24 h and
were treated with either DMSO or rhein (0, 30 and 60 µM) for 24 h.
The cells were then double-labeled with Annexin V and propidium
iodide (PI) and analyzed by flow cytometry.
Immunoblotting
Cell lysates including proteins were resolved onto
SDS-PAGE, and the protein of interest was visualized as a sharp
band by means of a specific antibody raised against antigen. In
brief, the protein samples were subjected to SDS-PAGE and were
subsequently transferred to membranes, which were blocked with 5%
skim milk and then probed with the indicated antibodies (1:1,000).
After the incubation of blots with horseradish peroxidase
(HRP)-conjugated secondary antibody (1:5,000), the bands of
interest were visualized using a chemiluminescence detection kit
according to the manufacturer's instructions (GE Healthcare,
Piscataway, NJ, USA).
Statistical analysis
Results are presented as mean ± SD of at least 3
independent experiments performed in triplicates. Data were
analyzed for statistical significance using a one-way analysis of
variance. A P-value <0.05 was considered to indicate a
statistically significant result.
Results
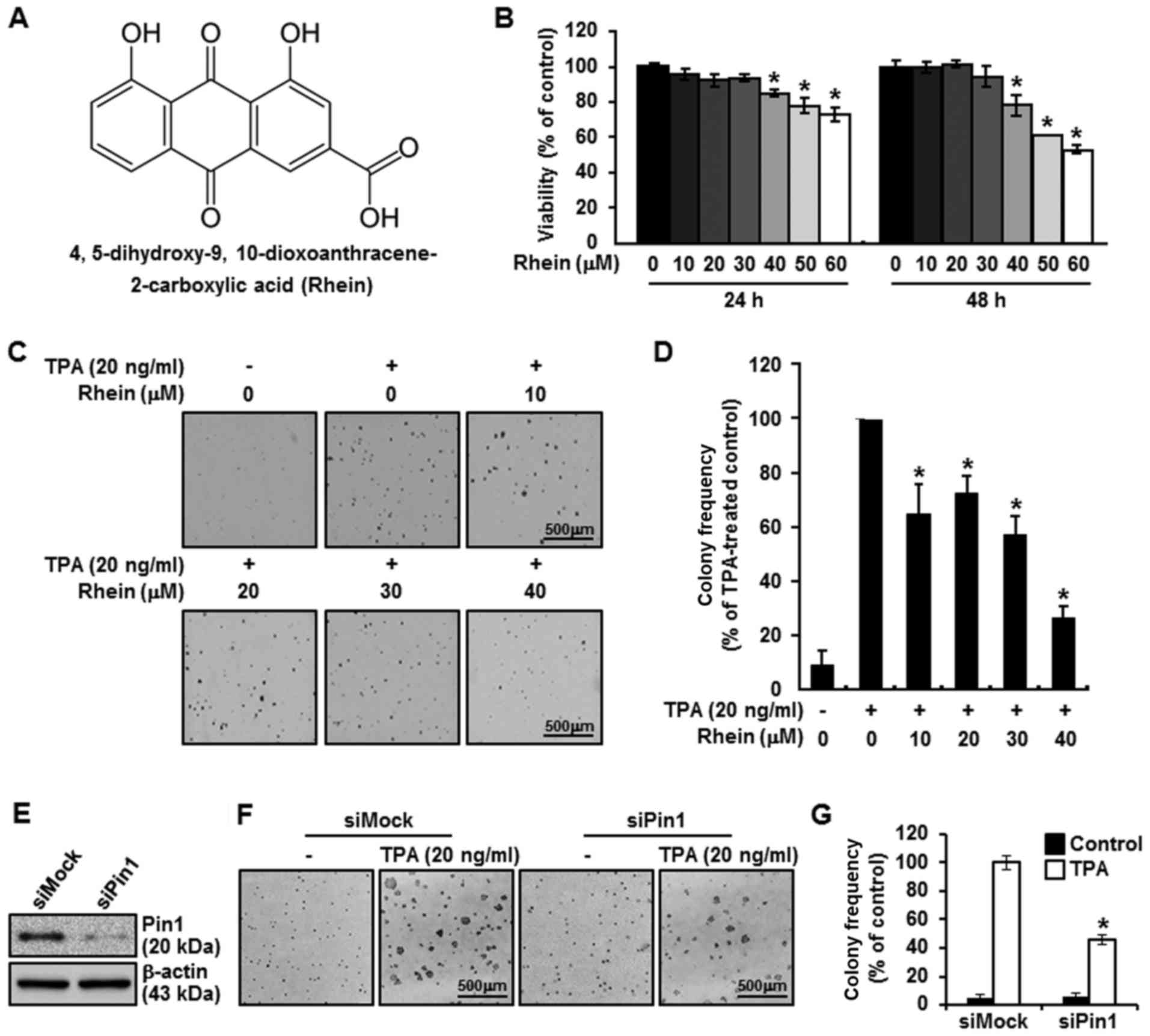
Rhein suppresses the viability of JB6
CI41 cells
The effects of rhein on the viability of JB6 CI41
cells were verified by results of the proliferation assay (Fig. 1B). First, we confirmed that the
concentration of rhein required to significantly inhibit viability
was reached in the JB6 CI41 cells and ranged from 40 to 60 µM.
Fig. 1B shows that the viability of
the JB6 CI41 cells was decreased at 24 and 48 h in a dose-dependent
as well as a time-dependent manner.
Biological function of rhein and Pin1
in mediating tumorigenic effects of TPA
We investigated whether rhein affected colony
formation via Pin1 in an anchorage-independent cell transformation
assay. Rhein exhibited an inhibitory effect on JB6 CI41 cell
transformation in a dose-dependent manner (Fig. 1C and D). Next, to gain insight into
how Pin1 expression levels are associated with tumorigenesis, a
soft agar assay was performed using cell lines that were either
subjected or not subjected to siRNA silencing. The siRNAs were
utilized to effectively diminish Pin1 expression, and siPin1 cells
exhibited decreased Pin1 expression of more than 50%, as compared
with the decrease achieved in cells with mock treatment (Fig. 1E). Subsequently, stimulation with
TPA was used to induce tumorigenesis in the siRNA-transfected JB6
CI41 cells. Mock-transfected cells produced more foci in the soft
agar assay than did the Pin1 siRNA-transfected cells, thus
expressing quantitatively that anchorage-independent potency in the
Pin1-silenced JB6 CI41 cells declined to ~46.1% when compared with
the mock-treated group (Fig. 1F).
The decrease in the number of colonies of JB6 CI41 cells subjected
to Pin1 silencing was statistically significant compared with that
in the mock group (Fig. 1G).
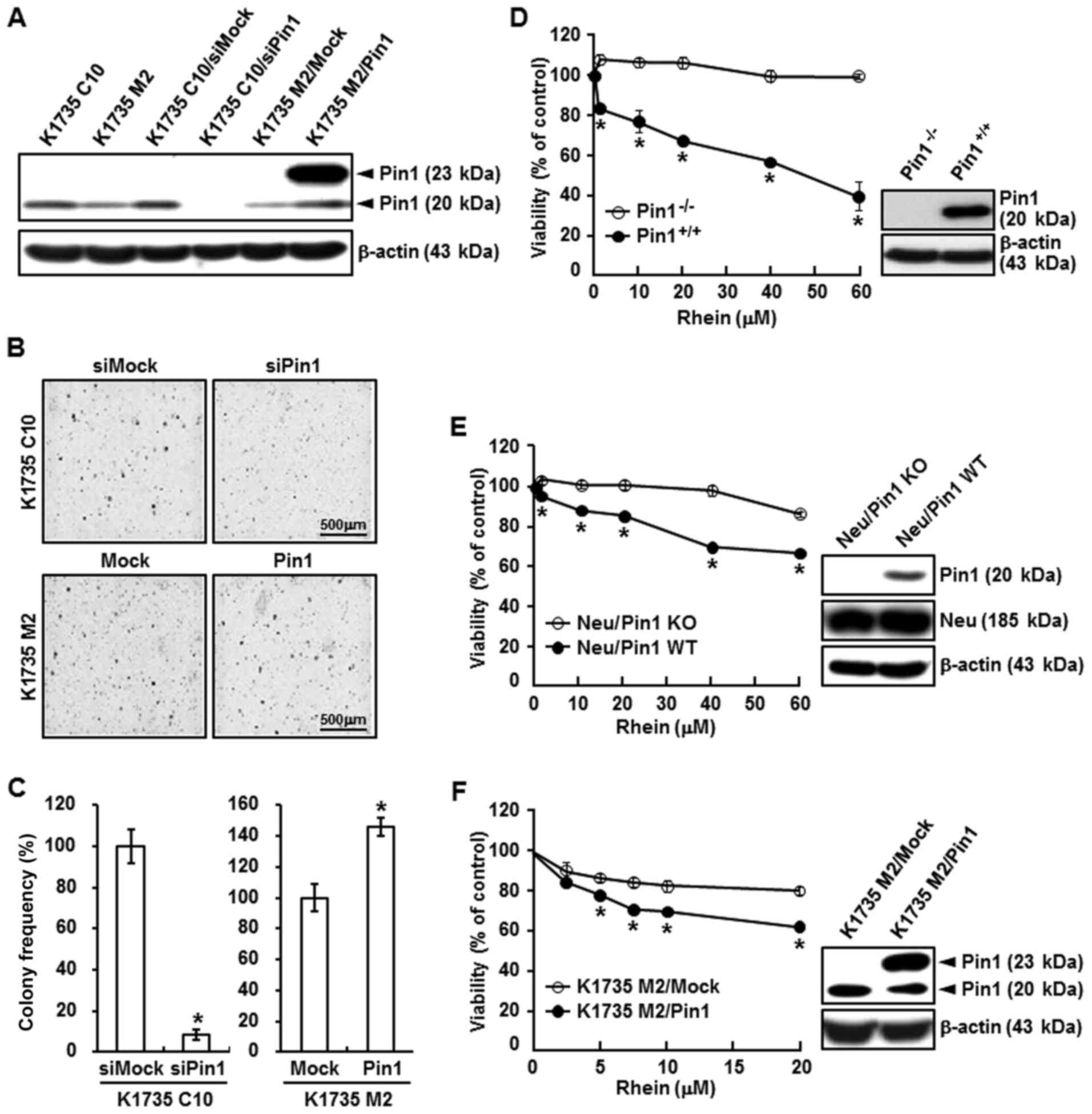
Impact of rhein on Pin1 activity
First, we showed that Pin1 is required for
tumorigenesis in the K1735 mouse melanoma cell lines derived from
the same parental lines: a non-metastatic cell line (K1735 C10) and
a metastatic cell line (K1735 M2). In the first set of experiments,
expression of Pin1 was evaluated by western blot assays in lysates
of K1735 C10, K1735 M2, K1735 C10/siMock, K1735 C10/siPin1, K1735
M2/Mock, and K1735 M2/Pin1 cells (Fig.
2A). Pin1 had an inhibitory effect on K1735 C10/siPin1 cell
transformation but enhanced K1735 M2/Pin1 cell transformation
(Fig. 2B and C). Next, to study the
effect of rhein on cell proliferation, we treated Pin1 WT
(Pin1+/+) and Pin1 KO (Pin1−/−) MEFs
(Fig. 2D, right) with rhein. Rhein
was administered at various concentrations in Neu/Pin1 KO MEFs
(overexpression of Neu oncogene but not Pin1), Neu/Pin1 WT MEFs
(overexpression of Neu oncogene and Pin1) (Fig. 2E, right), and K1735 M2/Mock, and
K1735 M2/Pin1 (overexpression of Pin1) cells (Fig. 2F, right). Proliferation was assessed
using a cell viability assay, as described in Materials and
methods. Treatment of these cells with rhein inhibited the
proliferation of the Pin1+/+ MEFs, Neu/Pin1 WT MEFs, and
K1735 M2 Pin1 MEFs but had no effect on Pin1−/− MEFs,
Neu/Pin1 KO MEFs, and K1735 M2/Mock cells (Fig. 2D-F, left). These results showed that
the Pin1-deficient cells were resistent to rhein treatment and Pin1
is essential for tumorigenesis.
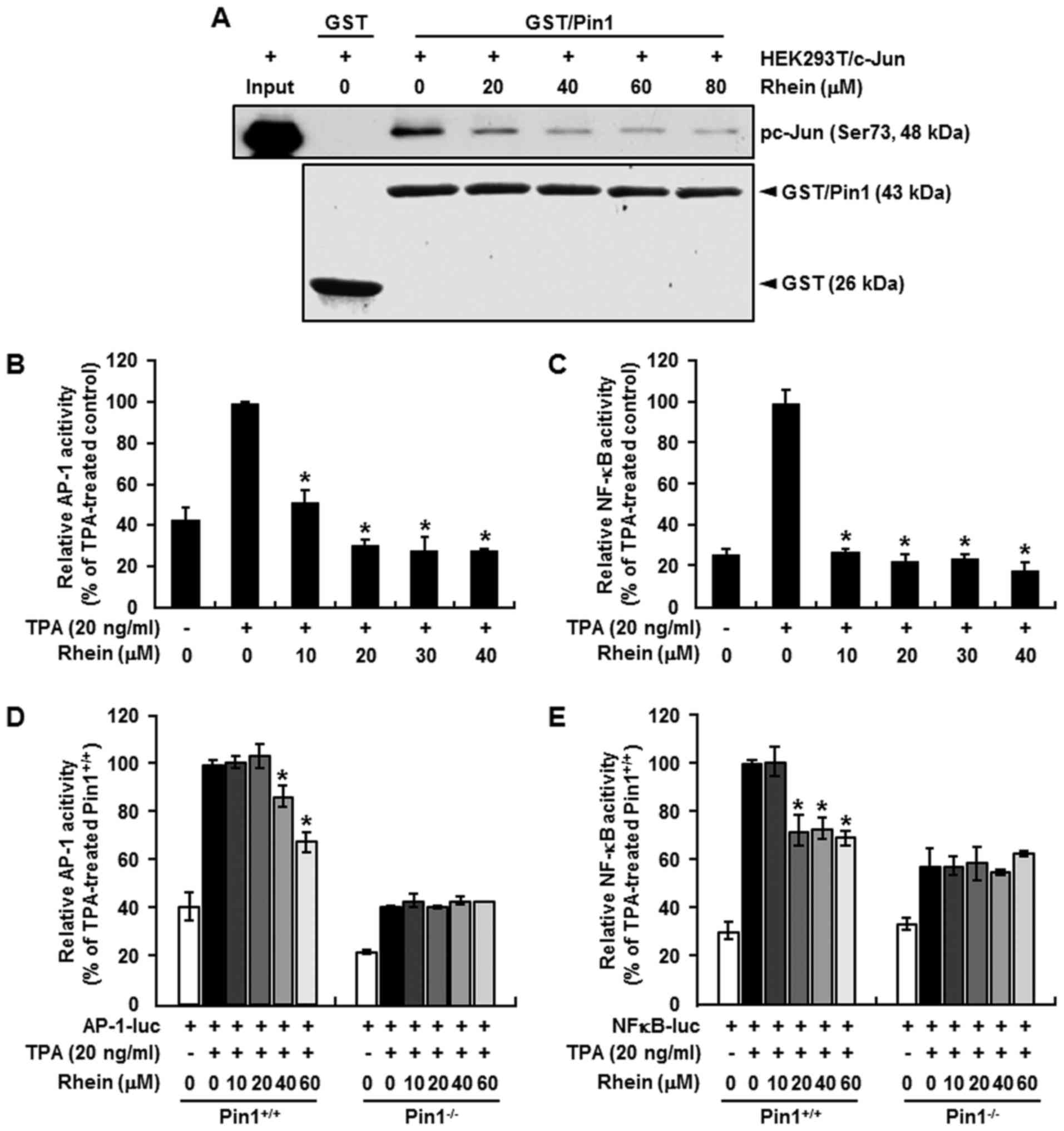
Inhibitory effect of rhein on binding
between Pin1 and c-Jun
Formation of the Pin1/c-Jun complex is an important
regulator of c-Jun stabilization, and Pin1 has been shown to bind
phosphorylated c-Jun and potentiate its transcriptional activity
toward cyclin D1 (35). We
therefore used a GST/Pin1 pull-down assay to determine the effect
of increasing concentrations of rhein on the formation of the
Pin1/c-Jun complex in cell lysates prepared from 293T cells that
were transfected with c-Jun. Pin1 was phosphorylated on Ser73, as
indicated by western blot analysis. No binding was observed between
GST alone and pc-Jun (Ser73), but binding did occur between pc-Jun
(Ser73) and the GST/Pin1. Most importantly, the binding of pc-Jun
(Ser73) with Pin1 was markedly decreased by rhein (Fig. 3A).
Inhibitory effect of rhein on AP-1 and
NF-κB promoter activities
Rhein has been reported to inhibit AP-1 and NF-κB
activity (12,36,37),
and Pin1 is required for the full activation of signal transduction
pathways, including AP-1 and NF-κB (18,22).
Therefore, we hypothesized that the inhibitory effect of rhein may
effectively suppress AP-1 and NF-κB activity through Pin1. We
examined the effect of rhein on AP-1 and NF-κB activity or AP-1 and
NF-κB promoter activity in Pin1−/− and
Pin1+/+ MEFs. The results indicated that rhein
significantly suppressed AP-1 (Fig. 3B
and D) and NF-κB (Fig. 3C and
E) reporter activity in Pin1+/+ cells but had little
effect on these activities in the Pin1−/− cells.
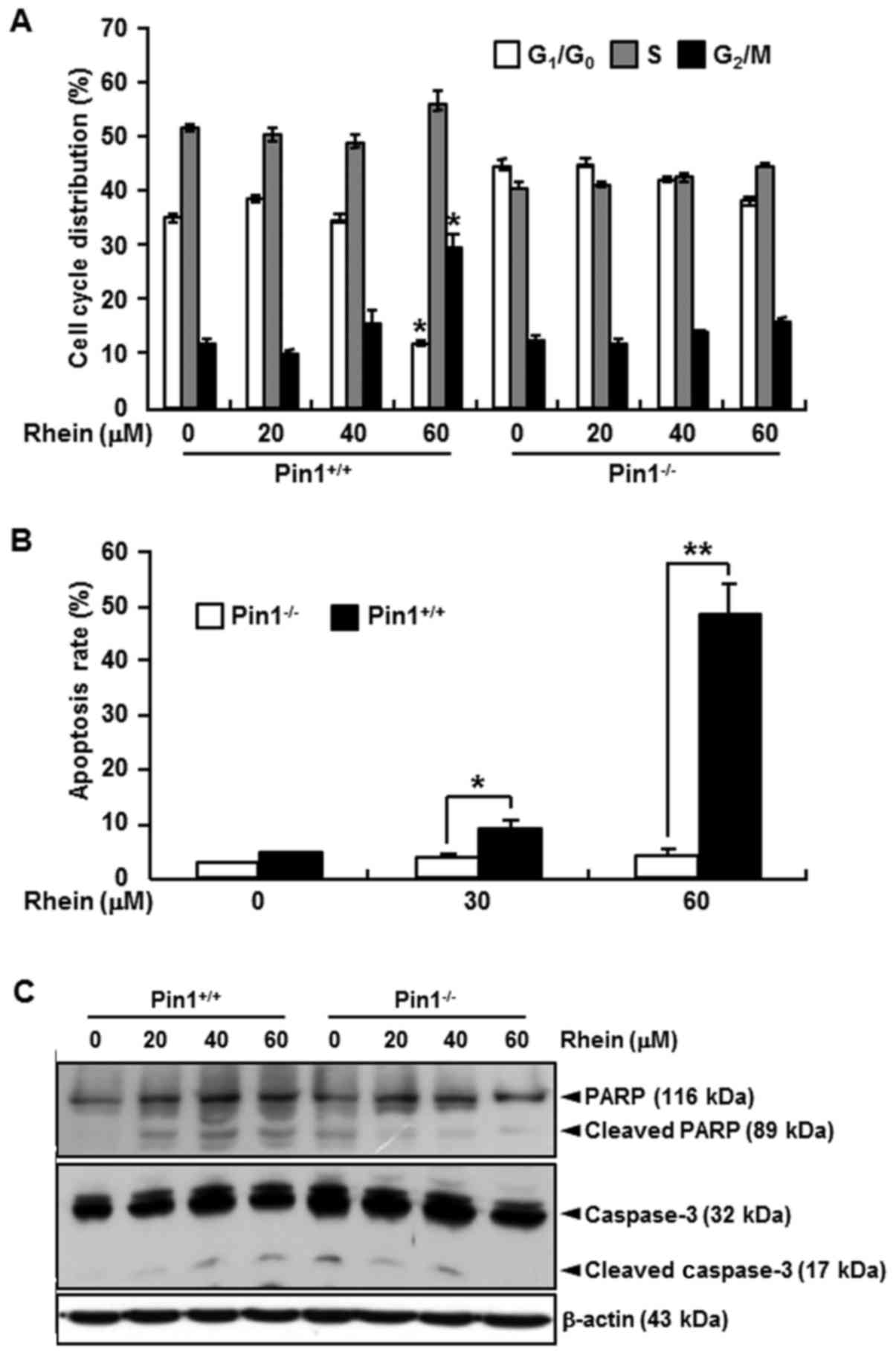
Effect of rhein on cell cycle and
apoptosis
We compared the effects of rhein on the cell cycle
and apoptosis in Pin1+/+ and Pin1−/− MEFs.
Rhein induced the accumulation of Pin1+/+ cells in the
G2/M-phase and induced apoptosis (Fig. 4A and B) and also regulated several
pro- and anti-apoptotic marker genes (Fig. 4C). Rhein induced a higher rate of
apoptosis in the Pin1+/+ cells than that in the
Pin1−/− cells (Fig.
4B).
Discussion
Pin1 can promote tumorigenesis by activating or
stabilizing numerous oncoproteins and also by inactivating or
destabilizing a number of tumor-suppressor genes (38,39).
Since the phosphorylation of proteins on Ser/Thr-Pro is a key
regulatory mechanism for the control of cell proliferation and
transformation, effective inhibitors of Pin1 activation could offer
a novel clinical strategy for the prevention or treatment of
cancer. In a previous study, we demonstrated the biological effects
of Pin1 on oncogenic signaling pathways and neoplastic cell
transformation (32), and other
studies have shown that inhibition of Pin1 in cancer cells triggers
apoptosis or suppresses the transformed phenotype (27,28).
Therefore, Pin1 has become an attractive molecule in cancer
research, and many inhibitors of Pin1 have been discovered,
including several classes of designed inhibitors and natural
products (40).
Natural anthraquinones are distinctive in having
considerable structural variety, a wide range of biological
activities, and low levels of toxicity. Some of the naturally
occurring anthraquinones currently identified include emodin,
physcion, cascarin, catenarin and rhein (41). In this study, we demonstrated that
the antitumorigenic effect of rhein occurs as a result of its
interference in the interaction between Pin1 and c-Jun, suggesting
a use for this anthraquinone in suppressing the tumor-promoting
effects of Pin1. We showed that the rhein/Pin1 association plays a
regulatory role in cell proliferation and neoplastic cell
transformation (Figs. 1 and
2).
Pin1 has many reported protein targets, and an
important Pin1 substrate is the phospho-Ser63/73-Pro motif in
c-Jun. Consistent with our data, the binding of phosphorylated
c-Jun (Ser73) with Pin1 was markedly decreased by rhein (Fig. 3A). It is possible that rhein also
regulates Pin1-mediated tumorigenesis through other substrates,
such as c-Jun.
Rhein has been reported to inhibit tumor
promoter-induced cell transformation mediated by AP-1 and NF-κB
activation (36,42,43).
Pin1 has also been shown to regulate NF-κB DNA binding and reporter
activity after Pin1 recognition of its phosphorylated p65/RelA
subunit (44). We found that rhein
effectively inhibited AP-1 and NF-κB reporter activity in
Pin1+/+ MEFs but not in Pin1−/− MEFs
(Fig. 3B-E). These data also
support the hypothesis that rhein directly targets Pin1 and that
this association can inhibit Pin1 tumor promoter activity.
Next, we compared the effects of rhein on the cell
cycle and apoptosis in Pin1+/+ and Pin1−/−
MEFs. We found that rhein induced the accumulation of
Pin1+/+ MEFs in the G2/M phase of the cell
cycle (Fig. 4A), and apoptosis was
markedly induced following rhein treatment in the
Pin1+/+ MEFs as compared with the Pin1−/−
MEFs (Fig. 4B). Moreover,
rhein-induced changes in the levels of various
pro-apoptosis-associated and anti-apoptosis-associated proteins
were more apparent in the Pin1+/+ MEFs (Fig. 4C).
In conclusion, our findings and the accompanying
biochemical data showed that rhein directly inhibits the
tumor-promoting activity of Pin1 and may therefore have practical
implications for cancer prevention or therapy.
Acknowledgements
This research was supported by the Basic Science
Research Program through the National Research Foundation Korea
(NRF) Funded by the Ministry of Education, Science and Technology
(NRF-2016R1D1A1B03930116) and the Next-Generation BioGreen 21
Program (PJ01116401) from the Rural Development Administration,
Republic of Korea.
References
|
1
|
Li Y, Xu Y, Lei B, Wang W, Ge X and Li J:
Rhein induces apoptosis of human gastric cancer SGC-7901 cells via
an intrinsic mitochondrial pathway. Braz J Med Biol Res.
45:1052–1059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsang SW and Bian ZX: Anti-fibrotic and
anti-tumorigenic effects of rhein, a natural anthraquinone
derivative, in mammalian stellate and carcinoma cells. Phytother
Res. 29:407–414. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsang SW, Zhang H, Lin C, Xiao H, Wong M,
Shang H, Yang ZJ, Lu A, Yung KK and Bian Z: Rhein, a natural
anthraquinone derivative, attenuates the activation of pancreatic
stellate cells and ameliorates pancreatic fibrosis in mice with
experimental chronic pancreatitis. PLoS One. 8:e822012013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lai WW, Yang JS, Lai KC, Kuo CL, Hsu CK,
Wang CK, Chang CY, Lin JJ, Tang NY, Chen PY, et al: Rhein induced
apoptosis through the endoplasmic reticulum stress, caspase- and
mitochondria-dependent pathways in SCC-4 human tongue squamous
cancer cells. In Vivo. 23:309–316. 2009.PubMed/NCBI
|
|
5
|
Chen YY, Chiang SY, Lin JG, Yang JS, Ma
YS, Liao CL, Lai TY, Tang NY and Chung JG: Emodin, aloe-emodin and
rhein induced DNA damage and inhibited DNA repair gene expression
in SCC-4 human tongue cancer cells. Anticancer Res. 30:945–951.
2010.PubMed/NCBI
|
|
6
|
Chen YY, Chiang SY, Lin JG, Ma YS, Liao
CL, Weng SW, Lai TY and Chung JG: Emodin, aloe-emodin and rhein
inhibit migration and invasion in human tongue cancer SCC-4 cells
through the inhibition of gene expression of matrix
metalloproteinase-9. Int J Oncol. 36:1113–1120. 2010.PubMed/NCBI
|
|
7
|
Aviello G, Rowland I, Gill CI, Acquaviva
AM, Capasso F, McCann M, Capasso R, Izzo AA and Borrelli F:
Anti-proliferative effect of rhein, an anthraquinone isolated from
Cassia species, on Caco-2 human adenocarcinoma cells. J Cell Mol
Med. 14:2006–2014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin YJ and Zhen YS: Rhein lysinate
suppresses the growth of breast cancer cells and potentiates the
inhibitory effect of Taxol in athymic mice. Anticancer Drugs.
20:65–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi P, Huang Z and Chen G: Rhein induces
apoptosis and cell cycle arrest in human hepatocellular carcinoma
BEL-7402 cells. Am J Chin Med. 36:805–813. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ip SW, Weng YS, Lin SY, Mei-Dueyang Tang
NY, Su CC and Chung JG: The role of Ca+2 on rhein-induced apoptosis
in human cervical cancer Ca Ski cells. Anticancer Res. 27:379–389.
2007.PubMed/NCBI
|
|
11
|
Shrimali D, Shanmugam MK, Kumar AP, Zhang
J, Tan BK, Ahn KS and Sethi G: Targeted abrogation of diverse
signal transduction cascades by emodin for the treatment of
inflammatory disorders and cancer. Cancer Lett. 341:139–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Domagala F, Martin G, Bogdanowicz P,
Ficheux H and Pujol JP: Inhibition of interleukin-1beta-induced
activation of MEK/ERK pathway and DNA binding of NF-kappaB and
AP-1: Potential mechanism for Diacerein effects in osteoarthritis.
Biorheology. 43:577–587. 2006.PubMed/NCBI
|
|
13
|
Wulf GM, Liou YC, Ryo A, Lee SW and Lu KP:
Role of Pin1 in the regulation of p53 stability and p21
transactivation, and cell cycle checkpoints in response to DNA
damage. J Biol Chem. 277:47976–47979. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou XZ, Kops O, Werner A, Lu PJ, Shen M,
Stoller G, Küllertz G, Stark M, Fischer G and Lu KP: Pin1-dependent
prolyl isomerization regulates dephosphorylation of Cdc25C and tau
proteins. Mol Cell. 6:873–883. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen HI and Sudol M: The WW domain of
Yes-associated protein binds a proline-rich ligand that differs
from the consensus established for Src homology 3-binding modules.
Proc Natl Acad Sci USA. 92:7819–7823. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu PJ, Zhou XZ, Shen M and Lu KP: Function
of WW domains as phosphoserine- or phosphothreonine-binding
modules. Science. 283:1325–1328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yaffe MB, Schutkowski M, Shen M, Zhou XZ,
Stukenberg PT, Rahfeld JU, Xu J, Kuang J, Kirschner MW, Fischer G,
et al: Sequence-specific and phosphorylation-dependent proline
isomerization: A potential mitotic regulatory mechanism. Science.
278:1957–1960. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu KP and Zhou XZ: The prolyl isomerase
PIN1: A pivotal new twist in phosphorylation signalling and
disease. Nat Rev Mol Cell Biol. 8:904–916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yeh ES and Means AR: PIN1, the cell cycle
and cancer. Nat Rev Cancer. 7:381–388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu KP: Pinning down cell signaling, cancer
and Alzheimer's disease. Trends Biochem Sci. 29:200–209. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu KP, Liou YC and Zhou XZ: Pinning down
proline-directed phosphorylation signaling. Trends Cell Biol.
12:164–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wulf G, Finn G, Suizu F and Lu KP:
Phosphorylation-specific prolyl isomerization: Is there an
underlying theme? Nat Cell Biol. 7:435–441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ryo A, Nakamura M, Wulf G, Liou YC and Lu
KP: Pin1 regulates turnover and subcellular localization of
beta-catenin by inhibiting its interaction with APC. Nat Cell Biol.
3:793–801. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dominguez-Sola D and Dalla-Favera R:
PINning down the c-Myc oncoprotein. Nat Cell Biol. 6:288–289. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sears RC: The life cycle of C-myc: From
synthesis to degradation. Cell Cycle. 3:1133–1137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bao L, Kimzey A, Sauter G, Sowadski JM, Lu
KP and Wang DG: Prevalent overexpression of prolyl isomerase Pin1
in human cancers. Am J Pathol. 164:1727–1737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryo A, Liou YC, Wulf G, Nakamura M, Lee SW
and Lu KP: PIN1 is an E2F target gene essential for Neu/Ras-induced
transformation of mammary epithelial cells. Mol Cell Biol.
22:5281–5295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu KP, Hanes SD and Hunter T: A human
peptidyl-prolyl isomerase essential for regulation of mitosis.
Nature. 380:544–547. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang CY, Chan HL, Lin HY, Way TD, Kao MC,
Song MZ, Lin YJ and Lin CW: Rhein induces apoptosis in human breast
cancer cells. Evid Based Complement Alternat Med. 2012:9525042012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wulf G, Garg P, Liou YC, Iglehart D and Lu
KP: Modeling breast cancer in vivo and ex vivo reveals an essential
role of Pin1 in tumorigenesis. EMBO J. 23:3397–3407. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujimori F, Takahashi K, Uchida C and
Uchida T: Mice lacking Pin1 develop normally, but are defective in
entering cell cycle from G(0) arrest. Biochem Biophys Res Commun.
265:658–663. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cho YS, Park SY, Kim DJ, Lee SH, Woo KM,
Lee KA, Lee YJ, Cho YY and Shim JH: TPA-induced cell transformation
provokes a complex formation between Pin1 and 90 kDa ribosomal
protein S6 kinase 2. Mol Cell Biochem. 367:85–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Urusova DV, Shim JH, Kim DJ, Jung SK,
Zykova TA, Carper A, Bode AM and Dong Z: Epigallocatechin-gallate
suppresses tumorigenesis by directly targeting Pin1. Cancer Prev
Res (Phila). 4:1366–1377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ryo A, Suizu F, Yoshida Y, Perrem K, Liou
YC, Wulf G, Rottapel R, Yamaoka S and Lu KP: Regulation of
NF-kappaB signaling by Pin1-dependent prolyl isomerization and
ubiquitin-mediated proteolysis of p65/RelA. Mol Cell. 12:1413–1426.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T,
Petkova V and Lu KP: Pin1 is overexpressed in breast cancer and
cooperates with Ras signaling in increasing the transcriptional
activity of c-Jun towards cyclin D1. EMBO J. 20:3459–3472. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martin G, Bogdanowicz P, Domagala F,
Ficheux H and Pujol JP: Rhein inhibits interleukin-1 beta-induced
activation of MEK/ERK pathway and DNA binding of NF-kappa B and
AP-1 in chondrocytes cultured in hypoxia: A potential mechanism for
its disease-modifying effect in osteoarthritis. Inflammation.
27:233–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Legendre F, Bogdanowicz P, Martin G,
Domagala F, Leclercq S, Pujol JP and Ficheux H: Rhein, a
diacerhein-derived metabolite, modulates the expression of matrix
degrading enzymes and the cell proliferation of articular
chondrocytes by inhibiting ERK and JNK-AP-1 dependent pathways.
Clin Exp Rheumatol. 25:546–555. 2007.PubMed/NCBI
|
|
38
|
Lin FC, Lee YC, Goan YG, Tsai CH, Yao YC,
Cheng HC, Lai WW, Wang YC, Sheu BS and Lu PJ: Pin1 positively
affects tumorigenesis of esophageal squamous cell carcinoma and
correlates with poor survival of patients. J Biomed Sci. 21:752014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liou YC, Zhou XZ and Lu KP: Prolyl
isomerase Pin1 as a molecular switch to determine the fate of
phosphoproteins. Trends Biochem Sci. 36:501–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu KP: Prolyl isomerase Pin1 as a
molecular target for cancer diagnostics and therapeutics. Cancer
Cell. 4:175–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chien SC, Wu YC, Chen ZW and Yang WC:
Naturally occurring anthraquinones: Chemistry and therapeutic
potential in autoimmune diabetes. Evid Based Complement Alternat
Med. 2015:3573572015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou YX, Xia W, Yue W, Peng C, Rahman K
and Zhang H: Rhein: A review of pharmacological activities. Evid
Based Complement Alternat Med. 2015:5781072015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bayer E, Goettsch S, Mueller JW, Griewel
B, Guiberman E, Mayr LM and Bayer P: Structural analysis of the
mitotic regulator hPin1 in solution: Insights into domain
architecture and substrate binding. J Biol Chem. 278:26183–26193.
2003. View Article : Google Scholar : PubMed/NCBI
|