Introduction
Colorectal adenocarcinoma is one of the most common
malignant neoplasms (1). Although
developments in the diagnosis and surgical treatment techniques in
combination with neoadjuvant radiochemotherapy have improved the
outcome of colorectal adenocarcinoma patients with a 5-year
survival rate of 90%, the 5-year survival rate is only 12% for
patients with metastatic colorectal adenocarcinoma (2). Recent studies provide increasing
evidence that the cause of colorectal adenocarcinoma involves
genetic and epigenetic alterations, morever, colorectal
adenocarcinoma cells acquire invasive and metastatic capacities
through the tumor microenvironment (3). Furthermore, investigations indicate
that the tumor microenvironment not only enhances cancer
metastasis, but also confers resistance to chemotherapy (4). Thus, it is imperative to elucidate the
roles of proteases in the tumor microenvironment in order to
achieve effective therapy, particularly against advanced and
metastatic colorectal adenocarcinoma.
Poly(ADP-ribose) metabolism has an important
biological function that mediates post-translational protein
modification in the process of poly-ADP-glycosylation (5,6). It
can maintain genomic stability, regulate the transcriptional level
and energy metabolism, and modify cell cycle progression and cell
death (7). Poly(ADP-ribose)
polymerases (PARPs) are a family of proteases, including PARP1-4,
PARP5α, PARP5β and PARP6-16 (8).
They play important roles in physiological and pathophysiological
processes. Recently, it was shown that PARP6 likely encodes tumor
suppressors, and an increase in its methylation was associated with
a poor prognosis of various cancer, including hepatoblastoma,
colorectal adenocarcinoma (9),
breast (10), pancreatic (11), breast (12) and colorectal cancer (13). Among the PARPs, PARP1 is the member
which has been studied most extensively. and was found to promote
tumor angiogenesis (7). However,
the roles of the new member PARP6 in cancer progressions and
metastasis remain unelucidated.
PARP6 is located on chromosome 15q23 (8). It was demonstrated that overexpression
of PARP6 suppressed the cell growth of HeLa cells (14). PARP6 was found to be negatively
correlated with the Ki-67 proliferation index, and may be a marker
for better prognosis in colorectal adenocarcinoma (13,14). A
recent study demonstrated that PAPR6 inhibited colony formation,
invasion and cell proliferation in human colorectal adenocarcinoma
cell line SW480 (13), and
indicated an interaction between the overexpression of PARP6 and
the downregulation of survivin. The authors reported an inverse
correlation between PARP6 and survivin expression in colorectal
adenocarcinoma tissues as confirmed by immunohistochemistry using
tumor tissue and normal colonic mucosa (13).
Survivin, a member of the family of inhibitor of
apoptosis (IAP) proteins, takes part in the inhibition of cell
apoptosis, which is also related to the poor prognosis of various
human cancers, including colorectal adenocarcinoma (13,15–19).
The survivin mRNA-circulating tumor cells are associated with
prostate cancer metastasis (20).
Suppression of survivin induces mitochondrial-mediated apoptosis in
gastric cancer cells (21,22). In addition, survivin is involved in
the cell cycle arrest of colorectal cancer cells (23). Increased apoptosis by a survivin
inhibitor is considered as an effective treatment for colon cancer
(24). However, limited evidence
exists regarding the interaction between PARP6 and survivin in cell
proliferation, the cell cycle and cell apoptosis.
In the present study, we used paired samples from 20
patients with colorectal adenocarcinoma. Expression of PARP6 and
survivin in both tumor tissues and adjacent normal colorectal
mucosa was assessed. In addition, their interaction and roles in
cell viability, cell cycle and cell apoptosis were further
investigated.
Materials and methods
Patients and cell culture
Twenty patients with colorectal cancer were
selected. Patients who had received chemotherapy or a family
history of polypus or IBD before surgery were excluded. All samples
were fixed in neutral buffered formalin 10% and paraffin blocked.
All cut sections from both tumor and adjacent normal colorectal
mucosa were selected, and immunohistochemical staining was
performed for expression analyses. All patients who participated in
the present study provided written informed consent. The
experimental protocol for human subjects was approved by the Ethics
Committee of The Second Affiliated Hospital of Guilin Medical
University.
The human colorectal adenocarcinoma SW620 cells and
normal colon cell line FHC were incubated in complete Leibovitz's
L-15 medium with tetracycline-free 10% fetal bovine serum (FBS)
(Gibco, Thermo Fisher Scientific), 100 U/ml penicillin and 100
µg/ml streptomycin. SW620 cells were transfected with PARP6 siRNA
or survivin siRNA using DharmaFECT reagent (Life Technologies,
Grand Island, NY, USA) according to the manufacturer's
instructions. The siRNA sequences are listed in Table I.
 | Table I.The siRNA sequences. |
Table I.
The siRNA sequences.
|
| Position |
| Sequences |
|---|
| PARP6 | 1808–1830 | S5′ |
GGCGAUGCCAACAUUAAUAdTdT |
| siRNA |
| mRNA |
TGGGCGATGCCAACATTAATACT |
|
|
| AS 3′ |
TdTdCCGCUACGGUUGUAAUUAU |
| Survivin | 268–290 | S5′ |
GAAGCAGUUUGAAGAAUUAdTdT |
| siRNA |
| mRNA |
AAGAAGCAGTTTGAAGAATTAAC |
|
|
| AS 3′ |
TdTdCUUCGUCAAACUUCUUAAU |
Quantitative real-time polymerase
chain reaction (qRT-PCR)
After six days of transfection, total RNA was
isolated and the transcription levels of PARP6 and survivin were
determined by real-time reverse transcriptase polymerase chain
reaction (25). The primer pairs
were: TTTGAGCCTTATCCC TCTGTG (sense) and TGGGTCATCTCCCGAATAGA
(antisense) for PARP6; TTTGAGGAAACTGCGGAGAA (sense) and
GGTGGCACCAGGGAATAAA (antisense) for survivin; and primers for
β-actin ATCGTGCGTGACATTAAGG AGAAG (sense) and
AGGAAGGAAGGCTGGAAGAGTG (antisense). The final result was expressed
as log2 of 2−ΔCT calculation, and the levels of mRNA
were normalized to β-actin (25).
Western blotting
Western blotting was performed six days
post-transfection. Proteins were extracted from the cells, and then
a total of 30 µg proteins was separated using 10% SDS-PAGE and
transferred to polyvinylidene difluoride (PVDF) membranes. The
membranes were incubated with PARP6 (1:1,000) and survivin
antibodies (1:1,000) (both from Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) for 1 h at room temperature (RT) followed by
incubation with the secondary antibody for 1 h. Visualization of
the bands was performed using an ECL kit (Beyotime, Shanghai,
China). Quantification of protein bands was performed using Gel-Pro
Analyzer software (Media Cybernetics, Inc., Rockville, MD,
USA).
Co-immunoprecipitation (Co-IP) of
PARP6 and survivin
Co-IP of PARP6 and survivin was performed. Briefly,
the cells were homogenized, supernatants collected and aliquots
were separated for protein determination and immunoblotting.
Survivin antibody or IgG was coupled to SiezeX beads following the
kit protocol (Pierce, Rockford, IL, USA). Proteins were eluted,
separated on 12–15% SDS-PAGE, and then transferred to
nitrocellulose for PARP6 immunoblotting.
Immunohistochemistry and
immunofluorescence staining
Immunohistochemistry (IHC) for the detection of
PARP6 and survivin was carried out using antibodies for PARP6
(1:1,000) and survivin (1:1,000) and the EnVision detection kit
(Dako, Carpinteria, CA, USA). The standard procedures for IHC were
performed as described in a previous study (26).
Expression and distribution of PARP6 and survivin in
cells were determined by indirect immunofluorescence microscopy, as
previously described (27).
Briefly, the cells were fixed with 3.5% PFA in phosphate-buffered
saline (PBS) for 10 min at RT and permeated and blocked with 0.1%
Triton X-100 and 5% FCS in PBS for 30 min. The fixed cells were
washed and incubated for 1 h with PARP6 or survivin antibody, and
then washed and incubated with secondary antibodies for 30 min, and
observed under a fluorescence microscopy.
CCK-8 assay
Cells were seeded in 96-well plates at a density of
3×104 cells/0.1 ml and were cultured for various days.
Then, 10 µl of Cell Counting Kit-8 (CCK-8) solution (KeyGen
Biotech, Nanjing, China) was added to each well of the plate. After
a 4-h incubation, the plates were analyzed at 450 nm. All values
are expressed as the means ± SD of at least three wells and at
least three independent experiments.
Flow cytometry
After six days of transfection, cell apoptosis, and
cell cycle distribution were analyzed by flow cytometry. Cell
apoptosis was detected using FITC-conjugated Annexin V and
propidium iodide (PI; Beyotime). Cell cycle distribution was
detected using the Cell Cycle Staining kit (Multi Sciences,
Hangzhou, China) according to the manufacturer's instructions.
Cell invasion assay
After six days of transfection, cell invasion was
examined using a reconstituted extracellular matrix membrane (BD
Biosciences, San Jose, CA, USA). Cells suspended in serum-free
media at a concentration of 3×104 cells/0.5 ml were
placed in the upper chambers, and complete medium containing 10%
FBS and 1% FBS was added to the lower chambers. After 18–24 h, the
chambers were fixed with methanol for 30 min and stained with
crystal violet for an additional 30 min. The migrated cells were
imaged.
Statistical analysis
All experiments were at least repeated in
triplicate. All data were analyzed using SPSS by the Student's
t-test and expressed as the mean ± SD. A p-value <0.05 was
considered as statistically significant.
Results
Expression of PARP6 and survivin in
human colorectal cancer tissues
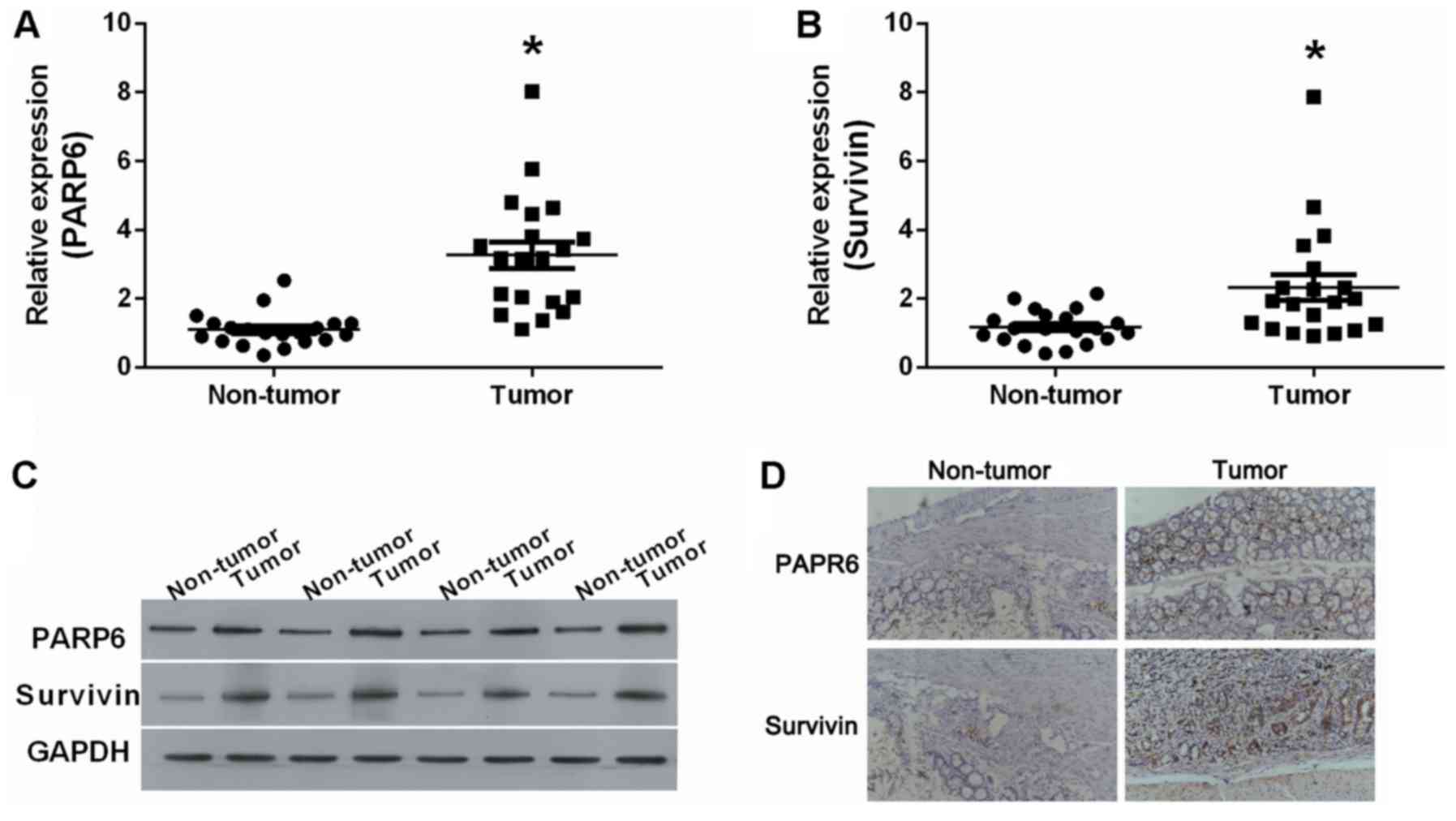
We examined the expression levels of PARP6 and
survivin in human colorectal cancer (tumor) and adjacent normal
tissue (non-tumor) (Fig. 1). The
results showed that PARP6 mRNA (Fig.
1A) and survivin mRNA (Fig. 1B)
were significantly upregulated in the tumor tissues. At the protein
level, PARP6 and survivin were upregulated in the tumor tissues
(Fig. 1C), compared to these levels
in the non-tumor tissues. The upregulation of PARP6 and survivin
was also validated by IHC (Fig.
1D). PARP6 was mostly expressed closed to the nucleus, while
survivin was mostly expressed in the cytoplasm.
Expression of PARP6 and survivin in
FHC and SW620 cells
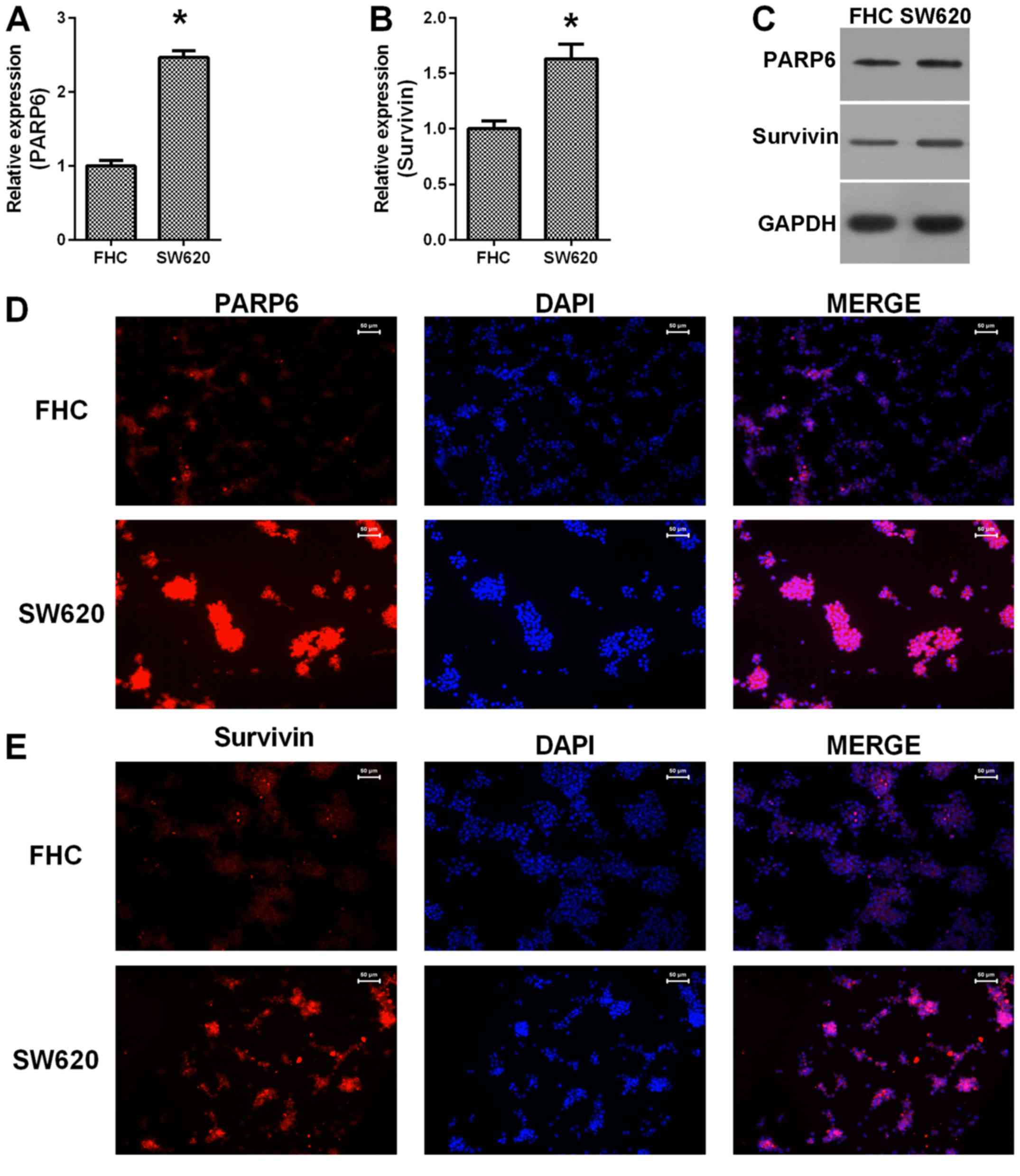
We examined the expression of PARP6 and survivin in
colorectal adenocarcinoma cell line SW620 and a normal colon cell
line FHC (Fig. 2). The results
showed that both PARP6 mRNA (Fig.
2A) and survivin mRNA (Fig. 2B)
were upregulated in the SW620 cells, compared with the mRNA levels
in the FHC cells. Similar changes were also observed at the protein
levels (Fig. 2C) and the
immunostaining showed higher expression of PARP6 (Fig. 2D) and survivin (Fig. 2E) in the SW620 cells, compared with
the levels in the FHC cells. Thus, PARP6 and survivin were
upregulated in colorectal adenocarcinoma cells.
Correlation between PARP6 and survivin
in colorectal adenocarcinoma
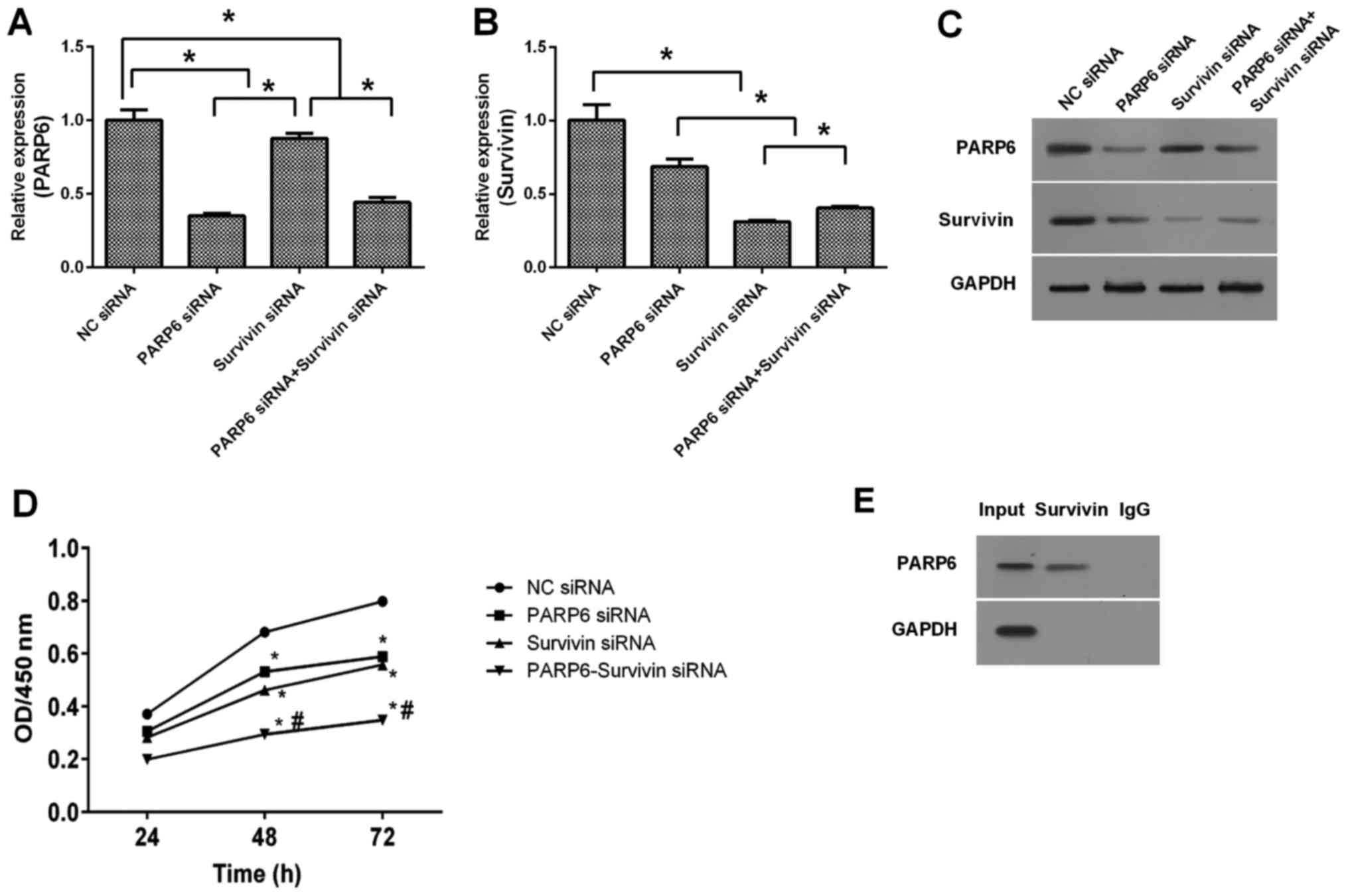
Silencing of PARP6 inhibited the expression of PARP6
mRNA, and silencing of survivin only slightly inhibited the
expression of PARP6 (Fig. 3A).
Silencing of survivin inhibited the expression of survivin mRNA,
and silencing of PARP6 only slightly inhibited the expression of
survivin (Fig. 3B). Similar results
were shown at the protein levels (Fig.
3C). The combination of PARP6 siRNA and survivin siRNA
inhibited the expression of PARP6 and survivin at both the mRNA and
protein levels. We also examined the cell survival by CCK-8 assay
(Fig. 3D). Silencing of PARP6 or
survivin inhibited the cell survival of the SW620 cells. In
addition, the combined treatment of PARP6 siRNA and survivin siRNA
further inhibited the cell survival in the SW620 cells, suggesting
that PARP6 and survivin play roles in the cell survival in
different pathways. In order to detect the correlation between
PARP6 and survivin in SW620 cells, Co-IP was used to identify the
interactive protein (Fig. 3E). The
results revealed that PAPR6 was present in the input and in the
final homogenate collected after Co-IP, but no signal for PAPR6 was
present in the IgG Co-IP lane. This confirmed that PARP6 interacts
with survivin.
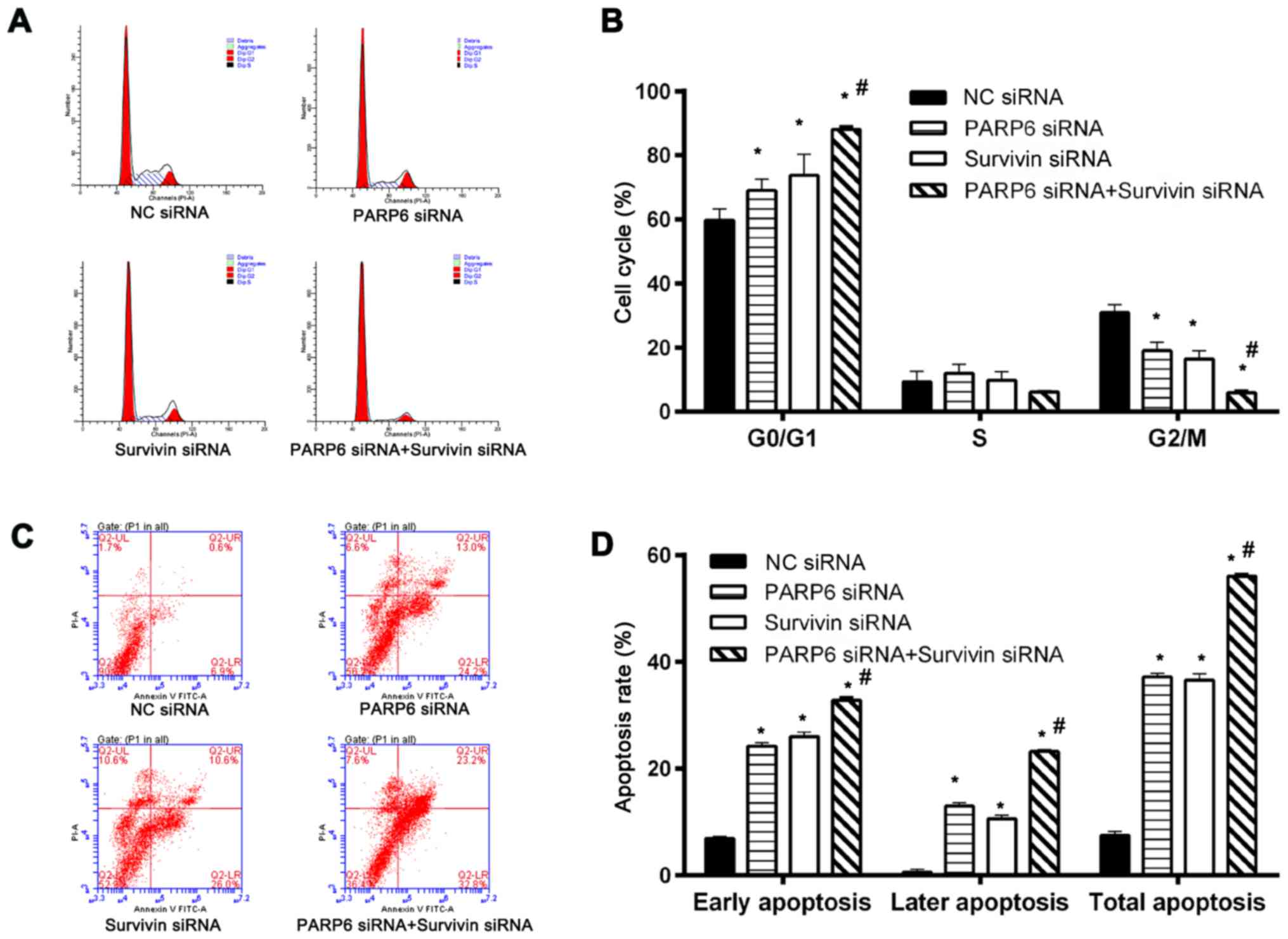
Cell cycle and cell apoptosis
Cell cycle and cell apoptosis were further assessed
(Fig. 4). Silencing of PARP6 or
survivin increased the percentage of cells in the G0/G1 phases and
decreased the percentage of cells in the G2/M phase (Fig. 4A and B). In addition, treatment with
the combination of PARP6 siRNA and survivin siRNA further increased
the percentage of cells in the G0/G1 phases and decreased the cells
in the G2/M phase. The cell cycle arrest in the G0/G1 phase was
associated with changes in cell survival (Fig. 3D) and apoptosis (Fig. 4C and D). Silencing of PARP6 or
survivin increased both early and late apoptosis. In addition, the
silencing of PARP6 and survivin further enhanced the rate of early
and late cell apoptosis, suggesting that PARP6 and survivin play
roles in cell cycle and apoptosis in different pathways.
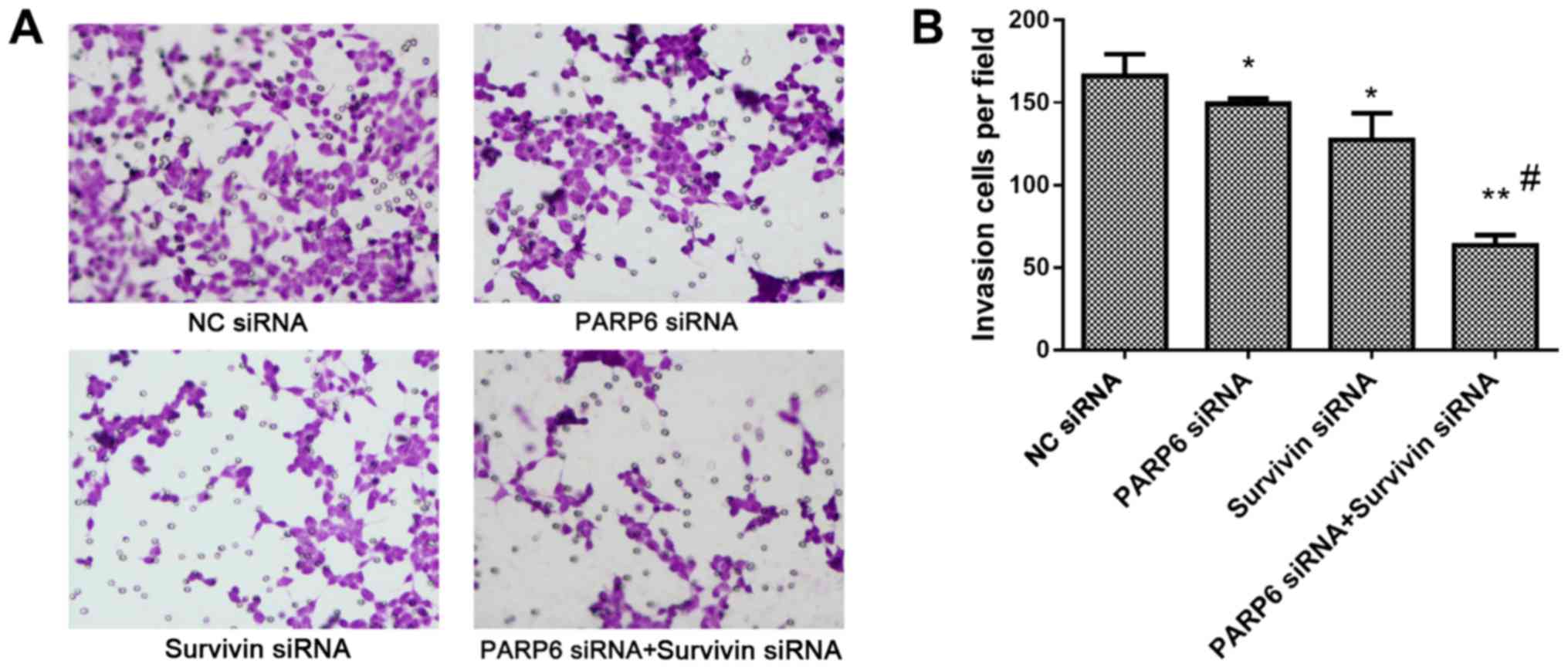
Cell invasion
Silencing of PARP6 or survivin decreased the
invasion of the SW620 cells, and this was further attenuated by the
combined silencing of PARP6 and survivin (Fig. 5), also suggesting that PARP6 and
survivin play roles in cell invasion in different pathways.
Discussion
We demonstrated a correlation between PARP6 and
survivin. These findings differ from a previous report (13). This is possible due to the different
stage of the samples collected and the different cell model used.
In the present study, we found that sole treatment of PARP6 siRNA
or survivin siRNA partially inhibited cell survival and invasion,
induced cell G0/G1 arrest, and cell apoptosis in the early and late
stages. The combined treatment of PARP6 siRNA and survivin siRNA
further suppressed the cell survival, further induced cell cycle
G0/G1 arrest, and cell apoptosis in the early and late stages. All
of these results indicate that PARP6 and survivin play an important
role in colorectal adenocarcinoma through a distinct pathway, which
should be further investigated in the future.
PARPs are DNA-dependent nuclear enzymes. They
regulate the interactions between protein and protein, and protein
and DNA, by transferring negatively charged ADP-ribose moieties to
protein substrates (28), playing
important roles in physiological and pathophysiological processes.
A total of 17 members of the PARP family have been identified
including PARP1-4, PARP5α, PARP5β and PARP6-16 (8). Among the PARPs, PARP1 was found to
promote tumor angiogenesis (7).
PARP6 located on chromosome 15q23 (8), may be a marker for better prognosis of
hepatoblastoma (9), breast
(10,12), pancreatic (11), and colorectal cancer (13). Overexpression of PARP6 was found to
suppress the cell growth of HeLa cells, and is correlated with a
decrease in the Ki-67 proliferation index (13,14).
In human colorectal adenocarcinoma cell line SW480, PAPR6 inhibited
colony formation, invasion and cell proliferation (13). However, PARP6 was found to be
downregulated in cases of colorectal cancer (13). This is inconsistent with our finding
that PARP6 was overexpression and is located in the region close to
the nuclear membrane in colorectal adenocarcinoma cells, compared
to adjacent normal colorectal mucosa.
Survivin is also correlated with the poor prognosis
of colorectal adenocarcinoma patients (13,15–19).
Survivin takes part in the cell apoptosis of gastric cancer cells
(21,22), and is involved in cell cycle arrest
in colorectal cancer (23).
Increased apoptosis by a survivin inhibitor is considered as an
effective treatment for colon cancer (24). In the present study, we also
demonstrated that survivin is overexpressed in colorectal
adenocarcinoma, and is mostly present in the cytoplasm. Thus, PARP6
and survivin are located in distinct regions, and the CO-IP assay
showed a significant correlation between PARP6 and survivin in the
SW620 cells and colorectal adenocarcinoma tissues.
Our further investigation on cell survival, cell
cycle and cell apoptosis further supported these findings.
Knockdown of PARP6 inhibited the expression of survivin in part
while knockdown of survivin only inhibited the expression of PARP6
in part. Sole knockdown of PARP6 or survivin partially inhibited
the cell survival, induced cell cycle G0/G1 arrest, and cell
apoptosis in the early and late stages. When both PARP6 and
survivin were knocked down, the cell survival and cell invasion
were further suppressed, and the cell cycle G0/G1 arrest and cell
apoptosis in the early and later stage were further enhanced. All
of these results indicate that PARP6 and survivin play an important
role in colorectal adenocarcinoma through a distinct pathway. This
is inconsistent with the literature demonstrating there is an
inverse correlation between PARP6 and survivin expression in
colorectal adenocarcinoma tissues (13).
In conclusion, knockdown of PARP6 or survivin
promoted cell apoptosis and inhibited cell invasion in colorectal
adenocarcinoma. A significant correlation exists between PARP6 and
survivin. They are promising targets for the development of new
strategies for the diagnosis and treatment of advanced or
metastatic colorectal adenocarcinoma.
Acknowledgements
The present study was supported by the China Guilin
Scientific Research and Technological Development Project (no.
20140505-1).
References
|
1
|
Robbins AS, Siegel RL and Jemal A: Racial
disparities in stage-specific colorectal cancer mortality rates
from 1985 to 2008. J Clin Oncol. 30:401–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bultman SJ: Interplay between diet, gut
microbiota, epigenetic events, and colorectal cancer. Mol Nutr Food
Res. May 3–2016.(Epub ahead of print). doi: 10.1002/mnfr.201500902.
PubMed/NCBI
|
|
3
|
Itatani Y, Kawada K, Inamoto S, Yamamoto
T, Ogawa R, Taketo MM and Sakai Y: The role of chemokines in
promoting colorectal cancer invasion/metastasis. Int J Mol Sci.
17:pii: E643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meads MB, Gatenby RA and Dalton WS:
Environment-mediated drug resistance: A major contributor to
minimal residual disease. Nat Rev Cancer. 9:665–674. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kraus WL: Transcriptional control by
PARP-1: Chromatin modulation, enhancer-binding, coregulation, and
insulation. Curr Opin Cell Biol. 20:294–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clayton C and Hotz HR:
Post-transcriptional control of PARP gene expression. Mol Biochem
Parasitol. 77:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hassa PO and Hottiger MO: The diverse
biological roles of mammalian PARPS, a small but powerful family of
poly-ADP-ribose polymerases. Front Biosci. 13:3046–3082. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hakmé A, Wong HK, Dantzer F and Schreiber
V: The expanding field of poly(ADP-ribosyl)ation reactions.
‘Protein Modifications: Beyond the Usual Suspects’ Review Series.
EMBO Rep. 9:1094–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honda S, Minato M, Suzuki H, Fujiyoshi M,
Miyagi H, Haruta M, Kaneko Y, Hatanaka KC, Hiyama E, Kamijo T, et
al: Clinical prognostic value of DNA methylation in hepatoblastoma:
Four novel tumor suppressor candidates. Cancer Sci. 107:812–819.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonçalves A, Sabatier R, Charafe-Jauffret
E, Gilabert M, Provansal M, Tarpin C, Extra JM, Viens P and
Bertucci F: Triple-negative breast cancer: Histoclinical and
molecular features, therapeutic management and perspectives. Bull
Cancer. 100:453–464. 2013.(In French). PubMed/NCBI
|
|
11
|
Porcelli L, Quatrale AE, Mantuano P, Leo
MG, Silvestris N, Rolland JF, Carioggia E, Lioce M, Paradiso A and
Azzariti A: Optimize radiochemotherapy in pancreatic cancer: PARP
inhibitors a new therapeutic opportunity. Mol Oncol. 7:308–322.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salemi M, Galia A, Fraggetta F, La Corte
C, Pepe P, La Vignera S, Improta G, Bosco P and Calogero AE: Poly
(ADP-ribose) polymerase 1 protein expression in normal and
neoplastic prostatic tissue. Eur J Histochem. 57:e132013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi G, Kudo Y, Tang B, Liu T, Jin S, Liu J,
Zuo X, Mi S, Shao W, Ma X, et al: PARP6 acts as a tumor suppressor
via downregulating Survivin expression in colorectal cancer.
Oncotarget. 7:18812–18824. 2016.PubMed/NCBI
|
|
14
|
Tuncel H, Tanaka S, Oka S, Nakai S,
Fukutomi R, Okamoto M, Ota T, Kaneko H, Tatsuka M and Shimamoto F:
PARP6, a mono(ADP-ribosyl) transferase and a negative regulator of
cell proliferation, is involved in colorectal cancer development.
Int J Oncol. 41:2079–2086. 2012.PubMed/NCBI
|
|
15
|
Fragni M, Bonini SA, Stabile A, Bodei S,
Cristinelli L, Simeone C, Zani D, Spano PF, Berruti A, Memo M, et
al: Inhibition of survivin is associated with zoledronic
acid-induced apoptosis of prostate cancer cells. Anticancer Res.
36:913–920. 2016.PubMed/NCBI
|
|
16
|
Wu J, Zhao S, Zhang J, Qu X, Jiang S,
Zhong Z, Zhang F, Wong Y and Chen H: Over-expression of survivin is
a factor responsible for differential responses of ovarian cancer
cells to S-allylmercaptocysteine (SAMC). Exp Mol Pathol.
100:294–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang W, Mao Y, Zhan Y, Huang J, Wang X,
Luo P, Li LI, Mo D, Liu Q, Xu H, et al: Prognostic implications of
survivin and lung resistance protein in advanced non-small cell
lung cancer treated with platinum-based chemotherapy. Oncol Lett.
11:723–730. 2016.PubMed/NCBI
|
|
18
|
Ma WH, Liu YC, Xue ML, Zheng Z and Ge YL:
Downregulation of survivin expression exerts antitumoral effects on
mouse breast cancer cells in vitro and in vivo. Oncol
Lett. 11:159–167. 2016.PubMed/NCBI
|
|
19
|
Zhu J, Sun C, Wang L, Xu M, Zang Y, Zhou
Y, Liu X, Tao W, Xue B, Shan Y, et al: Targeting survivin using a
combination of miR 494 and survivin shRNA has synergistic effects
on the suppression of prostate cancer growth. Mol Med Rep.
13:1602–1610. 2016.PubMed/NCBI
|
|
20
|
Wang H, Yang M, Xu J, Zou B, Zhou Q, Bian
J and Wang X: Survivin mRNA-circulating tumor cells are associated
with prostate cancer metastasis. Tumour Biol. 37:723–727. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang B, Huang J, Liu H, Guo W and Li G:
miR-335 directly, while miR-34a indirectly modulate survivin
expression and regulate growth, apoptosis, and invasion of gastric
cancer cells. Tumour Biol. 37:1771–1779. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang TA, Zhang XD, Guo XY, Xian SL and Lu
YF: 3-Bromopyruvate and sodium citrate target glycolysis, suppress
survivin, and induce mitochondrial-mediated apoptosis in gastric
cancer cells and inhibit gastric orthotopic transplantation tumor
growth. Oncol Rep. 35:1287–1296. 2016.PubMed/NCBI
|
|
23
|
Zhang B, Leng C, Wu C, Zhang Z, Dou L, Luo
X, Zhang B and Chen X: Smad4 sensitizes colorectal cancer to
5-fluorouracil through cell cycle arrest by inhibiting the
PI3K/Akt/CDC2/survivin cascade. Oncol Rep. 35:1807–1815.
2016.PubMed/NCBI
|
|
24
|
Li WL, Lee MR and Cho MY: The small
molecule survivin inhibitor YM155 may be an effective treatment
modality for colon cancer through increasing apoptosis. Biochem
Biophys Res Commun. 471:309–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steponaitis G, Skiriutė D, Kazlauskas A,
Golubickaitė I, Stakaitis R, Tamašauskas A and Vaitkienė P: High
CHI3L1 expression is associated with glioma patient
survival. Diagn Pathol. 11:422016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu Y, Gu C, Wang H, Chang K, Yang X, Zhou
X, Dai B, Zhu Y, Shi G, Zhang H, et al: Diagnosis of adults Xp11.2
translocation renal cell carcinoma by immunohistochemistry and FISH
assays: Clinicopathological data from ethnic Chinese population.
Sci Rep. 6:216772016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee WK and Kang JS: Modulation of
apoptosis and differentiation by the treatment of sulfasalazine in
rabbit articular chondrocytes. Toxicol Res. 32:115–121. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schreiber V, Dantzer F, Ame JC and de
Murcia G: Poly(ADP-ribose): Novel functions for an old molecule.
Nat Rev Mol Cell Biol. 7:517–528. 2006. View Article : Google Scholar : PubMed/NCBI
|