Introduction
Lung cancer is a common malignancy in China. In
2015, the incidence was approximately 733 per 100,000 people and
the age-standardized mortality rate was approximately 610 per
100,000. Lung cancer is one of the top five most diagnosed cancers
in both genders, and there is a significant upward trend in the
age-standardized incidence rates in females (1). Xuanwei, Yunnan is well known for its
high incidence of lung cancer (2).
Domestic combustion of bituminous coal (referred to as ‘smoky’
coal) is the most important risk factor for lung cancer, rather
than tobacco exposure (3). The
highest incidence of lung cancer in Xuanwei occurs among farmers,
and the most common histological type is adenocarcinoma. Women
living in this area are also more susceptible to lung cancer
(4–6). Therefore, lung adenoma patients in
Xuanwei appear to differ from those in other areas.
Platinum-based chemotherapy has been standard
treatment for lung cancer for decades. However, the efficacy of
platinum therapies can be seriously hindered by drug resistance,
especially to cisplatin (7,8). Cisplatin binds to DNA to create
platinum-DNA adducts that can induce covalent cross-linking between
DNA strands. In turn, this activates the apoptotic pathway,
resulting in cell death (9).
Mismatch repair and nucleotide excision repair (NER) are
particularly important mechanisms in the effects of platinum-based
agents. NER is a highly conserved DNA repair pathway that acts by
altering the helical structure of the DNA molecule and excising
damaged DNA fragments. This pathway involves three important steps:
recognition of DNA damage, formation of a complex that unwinds and
excises the damaged portion, and finally, resynthesis of the
damaged sequence and ligation (10). The excision repair
cross-complementation group 1 (ERCC1) enzyme, which recognizes and
removes cisplatin-induced DNA adducts (11–13),
plays a rate-limiting role in the NER pathway. High ERCC1
expression in solid tumors predicts poor prognosis and suggests a
higher possibility of resistance to platinum treatments (14–20).
This is also true for lung cancer patients, and low ERCC1
expression is beneficial in non-small cell lung cancer patients
(8,21–24).
In our previous study (25), we showed that ERCC1 expression is
significantly higher in Xuanwei lung adenocarcinoma patients before
treatment than patients from other areas. However, it is not known
how the high ERCC1 levels affect the prognosis of this patient
population. Herein, we hypothesize that high ERCC1 expression may
be related to the response to platinum-based chemotherapy and may
predict poor prognosis in lung adenoma patients from Xuanwei.
Materials and methods
Cell lines and cell culture
The established human lung adenocarcinoma cell line
XWLC05 was obtained from No. 1 Affiliated Hospital of Kunming
Medical University. Cells were maintained in RPMI-1640 medium
(HyClone, Thermo Scientific, Waltham, MA, USA) supplemented with
10% fetal bovine serum (Gibco Life Technologies, Carlsbad, CA,
USA), 100 U/ml penicillin (Biowest, Nuaillé, France), and 100 U/ml
streptomycin (Biowest) and incubated at 37°C in a humidified 5%
CO2 atmosphere.
Viral infection
A lentiviral vector carrying ERCC1 shRNA was
designed and constructed by GeneChem Co., Ltd. (Shanghai, China).
Cells were infected with the lentivirus twice for 2 days each and
positive clones were selected with puromycin (200 ng/ml) for 7–10
days. Control cell lines were generated by infecting with viruses
containing the empty vector, following the same protocol.
Real-time PCR
For each cell line, total RNA from 3×106
cells was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA). RNAs were then reverse transcribed into cDNAs for Real-time
PCR analysis using an ExScript RT-PCR kit (Takara, Shiga, Japan).
Oligonucleotide primers for ERCC1 were 5′-CGTGCTGTACCTCTCGC-3′
(forward primer) and 5′-CTGAGGAACGGTTCCTG-3′ (reverse primer), and
primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were
5′-GGCCTCCAAGGAGTAAGACC-3′ (forward primer) and
5′-CAAGGGGTCTACATGGCAAC-3′ (reverse primer). Amplification and
detection were carried out in the Applied Biosystems Prism 7900
system (Applied Biosystems, Foster City, CA, USA) using an ExScript
SYBR Green QPCR kit (Takara) and the following conditions: 95°C for
10 sec; 40 cycles of 95°C for 5 sec, 62°C for 31 sec; followed by a
30-min melting curve analysis to verify the primer dimers.
Statistical analysis was performed using the 2−∆∆CT
relative quantification method.
CCK-8 proliferation assay
Cell proliferation was measured using a CCK-8 assay
kit (Dojindo, Japan) according to the manufacturer's protocol.
Cells were seeded in 96-well plates (2×103 cells/well)
and 10 µl CCK-8 solution was added to each well on days 0, 1, 2, 3,
4, and 5. The plates were incubated at 37°C in 5% CO2
for 1 h, and the absorbance of each sample at 450 nm was measured
using a microplate reader. Three independent experiments were
required for this assay.
Colony-forming assay
A total of 500 cells were seeded at single-cell
density in 6-well plates with fresh medium with or without
cisplatin and allowed to grow for at least a week. Colonies with
more than 50 cells were then stained with gentian violet (Solarbio)
and counted.
Western blot analysis
Western blot analysis was performed to determine
protein expression levels. The cells were harvested, washed with
cold phosphate-buffered saline (PBS), lysed with RIPA lysis buffer
(Beyotime) for 30 min on ice, and then centrifuged at 12,000 × g
for 15 min at 4°C. The total protein concentration in the
supernatant was determined using a BCA protein assay kit
(Beyotime). Equal amounts (30 µg per lane) of protein were
separated by SDS-PAGE and transferred to polyvinylidene fluoride
membranes (Millipore). The membranes were blocked with 10% non-fat
milk, incubated with primary antibodies, and then incubated with
secondary antibodies conjugated to horseradish peroxidase. The
protein bands were revealed by incubation with chemiluminescent
reagents (Millipore). Antibodies to ERCC1 were from Proteintech.
The antibody to β-actin was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Three independent protein samples were harvested
and tested to make sure that ERCC1 gene has been successfully
silenced.
Drugs
Cisplatin was purchased from Sigma-Aldrich and
stored at a concentration of 5 mM in dimethyl sulfoxide (DMSO).
XWLC05 cells were incubated with cisplatin at a concentration of 1
µM unless otherwise specified.
Cell apoptosis assay
Cells were incubated with various concentrations of
cisplatin or DMSO for 48 h and then harvested, washed twice with
cold PBS, and resuspended in 200 µl binding buffer at a density of
1×105 cells/ml. The cells were stained with 5 µl Annexin
V and propidium iodide (PI) (BD Biosciences) for 15 min in the dark
at room temperature and then analyzed by flow cytometry (Cytomics
FC 500 MPL, Beckman Coulter). Early- and late-stage apoptosis was
determined by the percentage of Annexin
V+/PI− and Annexin
V+/PI+ cells, respectively. The results are
expressed as the mean values from three independent
determinations.
Animal experiments
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Kunming Medical
University and were performed following Institutional guidelines
and protocols. XWL05 cells stably expressing ERCC1 shRNA were
generated by retroviral infection. Tumors were induced by injecting
5×106 cells of each cell line subcutaneously into 4- to
6-week-old BALB/c female athymic nude mice (Department of
Laboratory Animals, Kunming Medical University). Each cell type was
injected into seven mice, respectively. Mice were treated with 5
mg/kg cisplatin by intraperitoneal injection every 3 days for 2
weeks (five injections) starting immediately after injection of
tumor cells. Control mice received injections of PBS. One week
after the last cisplatin injection, the mice were sacrificed and
the tumors were weighed. The longest diameter ‘a’ and the shortest
diameter ‘b’ of the tumors were measured and the tumor volumes were
calculated using the following formula: volume (in mm3)
= a × b2 × 0.52, where 0.52 is a constant to calculate
the volume of an ellipsoid. Three tumors per cell line were
excised, fixed in 10% formalin overnight, and subjected to routine
histological examination by investigators who were blinded to the
tumor status. Animal experiments were repeated twice.
Immunohistochemical staining
Samples from the mouse tumor xenografts and lung
tumor tissue from patients were subjected to immunohistochemical
(IHC) staining to detect ERCC1 expression. Antibodies to ERCC1 were
from Maixin (Fuzhou, China, mouse clone 8F1). Paraffin-embedded
sections were pre-treated and stained with antibodies as previously
reported (26). Secondary
antibodies against mouse or rabbit IgG were supplied in an IHC kit
(#CW2069) from Beijing Cowin Bioscience Co. Ltd (Beijing,
China).
IHC scoring
Differentiated degree of these tissue samples
collected from our patients were classified into well
differentiated or poor differentiated according to the pathological
evaluation system of our hospital. The stained tissue sections were
scored separately by two pathologists blinded to the clinical
parameters. The staining intensity was scored as 0 (negative), 1
(weak), 2 (medium), or 3 (strong). The staining extent was scored
as the percentage of positively stained area compared with the
entire carcinoma area and was scored as 0 (<5%), 1 (5–25%), 2
(26–50%), 3 (51–75%), and 4 (>75%). Scores for the staining
intensity and extent were multiplied to generate an
immunoreactivity score for each sample. Tissues with a total
immunoreactivity score of <4, 4, 6, and ≥8 were expressed as -,
+, ++, and +++, respectively. In our research, IHC score ≥6 was
defined as high ERCC1 expression and IHC score ≤4 was low
expression.
Patient information
Sections of lung adenoma tissue were obtained from
106 operable patients who were staged based on the 7th edition of
the AJCC Cancer Staging Manual and graded based on WHO criteria.
The patients had undergone radical surgery at the Department of
Thoracic Surgery of No. 3 Affiliated Hospital of Kunming Medical
University (Yunnan Provincial Tumor Hospital) between January 2009
and September 2011. Each patient provided written informed consent,
and the study was approved by the ethics committee of No. 3
Affiliated Hospital of Kunming Medical University (Yunnan
Provincial Tumor Hospital). We explored the relationship between
ERCC1 expression levels and patient survival by IHC staining of
ERCC1 in sections from the 106 patients, who had received
cisplatin, carboplatin, gemcitabine, or vinorelbine as first-line
chemotherapy between December 2008 and April 2012 in No. 3
Affiliated Hospital of Kunming Medical University (Yunnan
Provincial Tumor Hospital). Complete follow-up information was
available.
Statistical analysis
SPSS software (version 18.0) was used for
statistical analysis. Student's t-test or ANOVA were used to
compare quantitative data and Chi-square or Kruskal-Wallis tests
were used to assess qualitative data. A P-value of <0.05 (two
tailed) was considered statistically significant. The median
progression-free survival (PFS) and overall survival (OS) were
estimated by the Kaplan-Meier method and compared using a log-rank
test.
Results
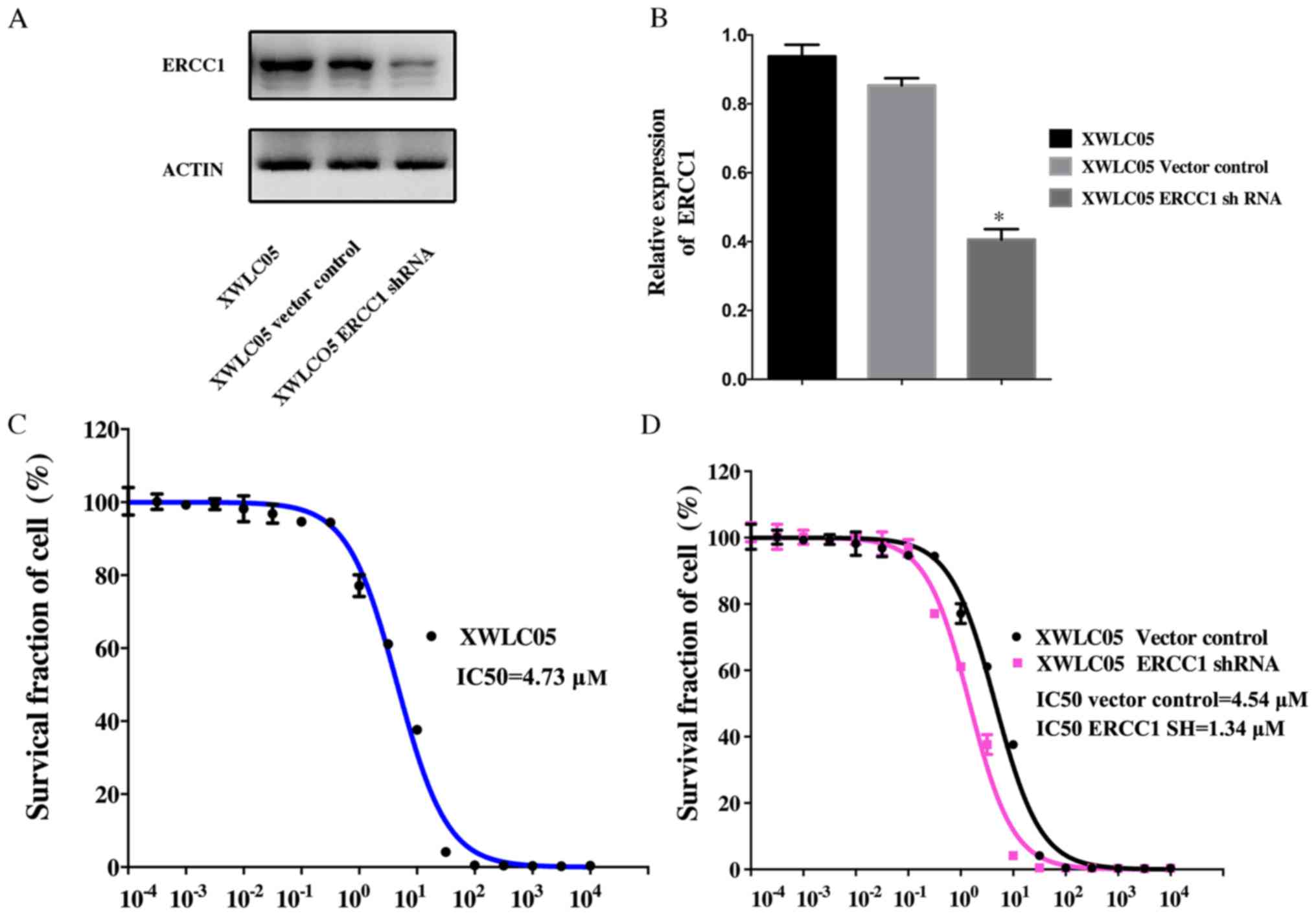
ERCC1 silencing reverses XWLC05 cell
cisplatin resistance
Expression of ERCC1 was examined in cultured
unmanipulated XWLC05 cells and cells expressing empty vector
(Vector Control) or ERCC1 shRNA (Fig.
1A and B). The level of ERCC1 in XWLC05-ERCC1-shRNA cells was
significantly lower than that in XWLC05 and Vector Control
cells.
To evaluate the anticancer effect of cisplatin,
XWLC05, Vector Control, and XWLC05-ERCC1-shRNA cells were treated
with concentrations of cisplatin ranging from 1×10−4 to
1×104 µM. A dose-dependent cytotoxic effect of cisplatin
was observed in these cell lines (Fig.
1C and D). The half-maximal inhibitory concentration
(IC50) for XWLC05, Vector Control, and
XWLC05-ERCC1-shRNA cells was 4.73, 4.54, and 1.31 µM, respectively
(P<0.05).
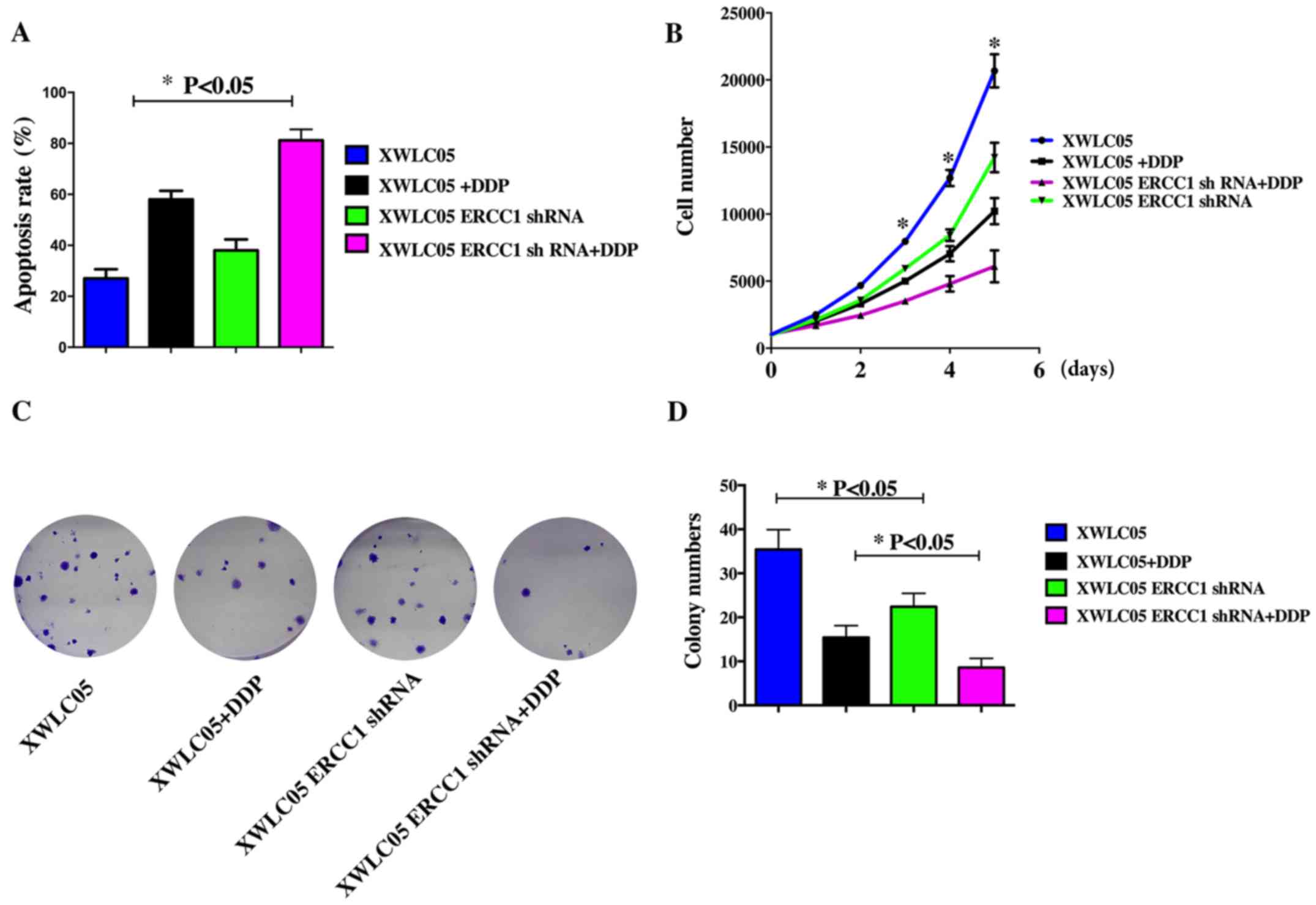
ERCC1 silencing increases apoptosis
and inhibits proliferation of cisplatin-treated cancer cells in
vitro
We studied the effect of cisplatin treatment on
apoptosis in the cell lines. After 24 h treatment with cisplatin at
1 µM, XWLC05, Vector Control, and XWLC05-ERCC1-shRNA cells were
double-stained with Annexin V and PI and subjected to flow
cytometry to quantify apoptosis (Fig.
2A). Cisplatin induced apoptosis in all cell lines; however,
XWLC05-ERCC1-shRNA cells showed a higher apoptotic rate than Vector
Control cells after incubation with or without cisplatin.
The CCK-8 assay showed that cell proliferation was
significantly suppressed in XWLC05-ERCC1-shRNA cells compared with
control cells treated with the same cisplatin concentration
(Fig. 2B). Moreover, ERCC1
silencing also significantly inhibited the colony-forming ability
of XWLC05 cells (Fig. 2C and
D).
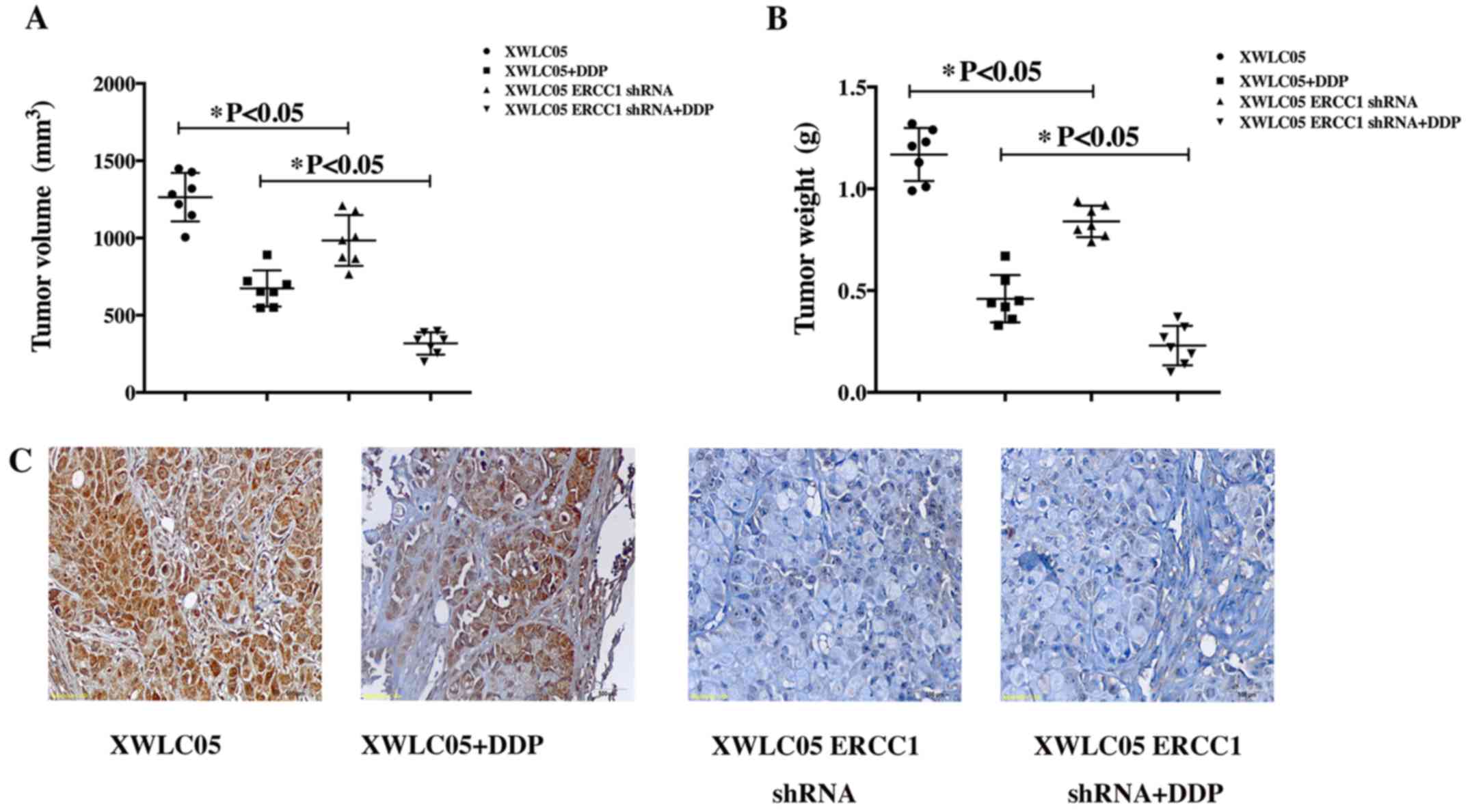
ERCC1 silencing inhibits proliferation
and increases cisplatin sensitivity of XWLC05 cells in vivo
The effect of ERCC1 on XWLC05 cells in vivo
was investigated by injecting XWLC05-ERCC1-shRNA cells and the
corresponding Vector Control cells subcutaneously into nude mice
and then treating the mice with vehicle or cisplatin (5 mg/kg) for
2 weeks. Tumor sizes were measured every 7 days. The mean volumes
of tumors formed by XWL05 control cells and XWLC05-ERCC1-shRNA
cells at day 35 were 1264 and 984 mm3, respectively
(Fig. 3A, P<0.05). After
cisplatin treatment, the mean tumor volumes at day 35 were 674 and
317 mm3, respectively (Fig.
3A, P<0.05). Thus, the growth of XWLC05-ERCC1-shRNA cells
was most significantly inhibited by cisplatin in vivo. The
tumor weights from the four mouse groups showed similar results
(Fig. 3B). The mean weight of
tumors formed by XWL05 control cells and XWLC05-ERCC1-shRNA cells
at day 35 were 1.16 g and 0.84 g, respectively (P<0.05). After
cisplatin treatment, the mean tumor weight at day 35 were 0.46 g
and 0.23 g, respectively. We confirmed that expression of ERCC1 was
efficiently inhibited in the xenograft mouse model by performing
IHC analysis of tumor sections from each experimental group
(Fig. 3C).
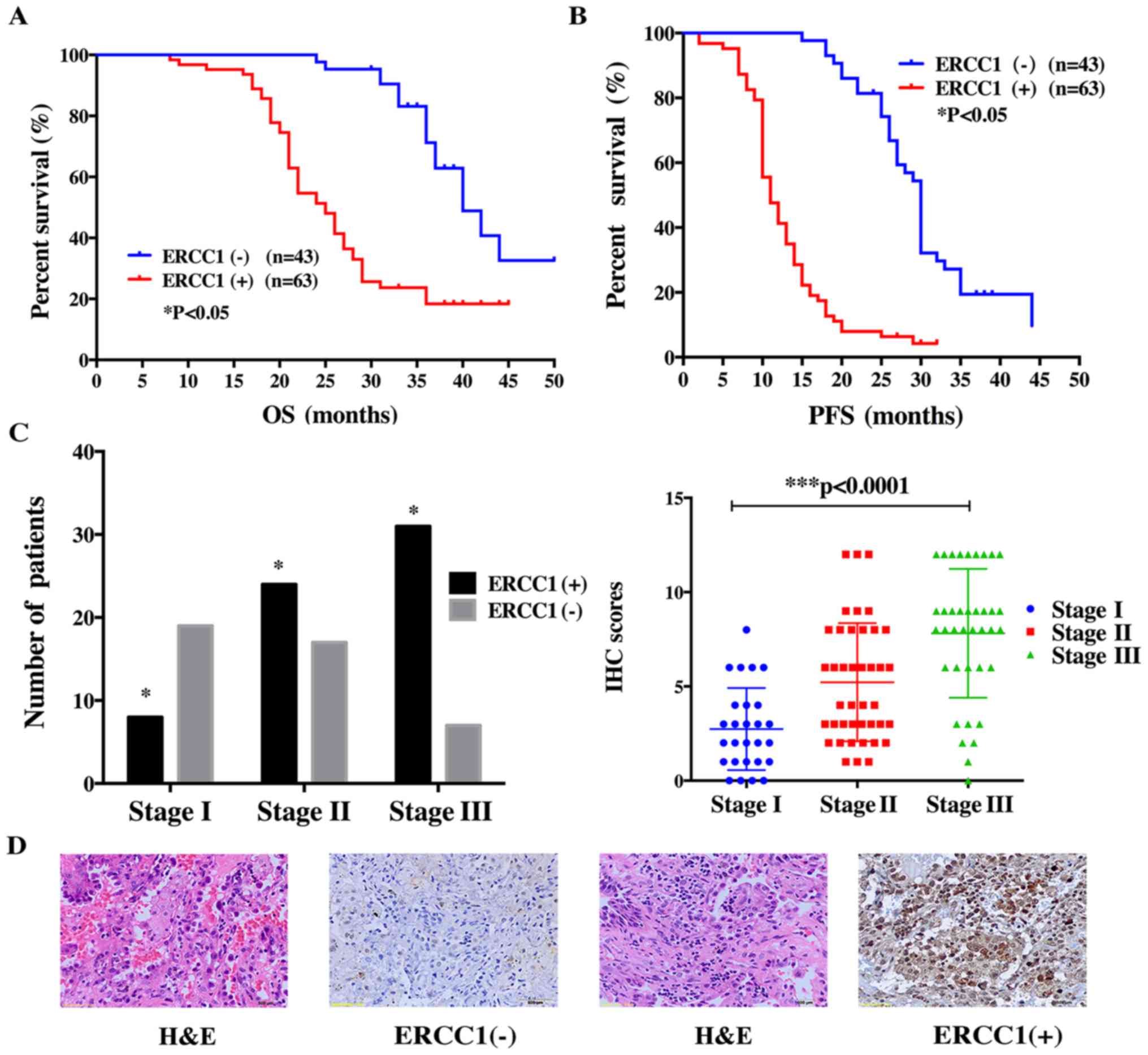
ERCC1 expression predicts poor
prognosis in lung adenoma patients
The mean age of the 106 patients in the study was
62.7 years (range 30–79 years) (Table
I). The surgical disease stage was 27 (25.4%) stage I, 41
(38.8%) stage II, and 38 (35.8%) stage III. There were 31 (29.2%)
patients with well-differentiated (low-grade) tumors and 75 (70.8%)
patients with poorly differentiated (high-grade) tumors. Among the
106 patients, 90 (84.9%) received adjuvant chemotherapy and 40
(37.7%) received radiotherapy. The mean follow-up interval was 30
months (range 2–55 months). At the last follow-up, 93 (87.7%)
patients had progressive disease after first-line chemotherapy and
64 (60.4%) patients had died. The median PFS was 17 months (range,
2–44 months) and the median OS was 30 months (range, 8–50
months).
 | Table I.Clinical characteristics of this
cohort. |
Table I.
Clinical characteristics of this
cohort.
|
Characteristics | N (%) |
|---|
| Age at diagnosis
(years) |
|
| Mean ±
SD | 62.7±11.3 |
| Gender |
|
|
Male | 44 (41.5) |
|
Female | 62 (58.5) |
| Smoking status |
|
|
Yes | 50 (47.2) |
| No | 56 (52.8) |
| TNM stage |
|
| I | 27 (25.5) |
| II | 41 (38.7) |
|
III | 38 (35.8) |
| Pathology
grading |
|
|
Well-differentiated | 33 (31.1) |
|
Poor-differentiated | 73 (68.9) |
| Receive
chemotherapy |
|
|
Yes | 92 (86.8) |
| No | 14 (13.2) |
| Receive
radiotherapy |
|
|
Yes | 55 (51.9) |
| No | 51 (48.1) |
To confirm the relationship between the expression
level of ERCC1 and the survival of lung adenoma patients, we
performed IHC in the 106 patients with operable stage disease
(Ia-IIIa). The results showed that positive ERCC1 staining was
significantly associated with poor OS and PFS (Fig. 4A and B). In this patient cohort, we
found that ERCC1 positivity correlated with disease progression.
Lung adenoma samples were positive for ERCC1 in only 8 of 27 stage
I patients but in 31 of 38 patients with stage III disease
(Fig. 4C). This trend was also
observed in the IHC score (Fig.
4D). For stage I patients, the IHC score was 2.74±0.42,
increasing significantly to 5.22±0.48 in stage II patients and to
7.62±0.62 in stage III patients. These data suggest that as the
disease progresses, lung adenoma tissues become increasingly
positive for ERCC1.
Discussion
In this study, we constructed a lentiviral vector
encoding ERCC1-specific short hairpin RNA (shRNA) and transfected
it into the lung adenocarcinoma cell line XWLC05, established from
a patient from Xuanwei. Western blotting and quantitative PCR
analysis confirmed the silencing efficiency of the ERCC1 shRNA
construct. ERCC1 silencing increased XWL05 sensitivity to
cisplatin, as reflected by the significant decrease in cisplatin
IC50 values. Flow cytometric analysis showed that ERCC1
shRNA-infected cancer cells treated with cisplatin had a
significantly higher rate of apoptosis compared with control cells.
In vivo, the volume of tumors derived from
XWLC05-ERCC1-shRNA cells was significantly lower after cisplatin
treatment compared with tumors grown from control cells, which was
consistent with the data from in vitro experiments. In a
clinical cohort study of Xuanwei patients, we evaluated ERCC1
expression by IHC staining of tissue sections from 106 patients
with operable lung adenoma. ERCC1 expression was predictive of
worse prognosis, as reflected by the higher number of
ERCC1-positive patients with advanced disease. This may be related
to the active rate of cancer cell division in the advanced stage of
disease, since DNA damage repair pathways are critical for cell
division.
Drug resistance is associated with worse prognosis
in cancer. Given the mechanism of platinum drug-induced killing of
cancer cells and the involvement of ERCC1 in DNA damage repair, it
is reasonable to assume that higher ERCC1 levels would be
associated with worse prognosis. Our research confirms previous
studies in this regard (27–32).
Higher ERCC1 expression results in a lower mutational load in lung
cancer patients (33) since ERCC1
repairs mismatch during DNA replication. Lower mutational load is
related to the poor response to immunotherapy and body immunity of
cancer patients, likely because it reduces the number of antigenic
tumor epitopes available for detection by the immune system
(34,35). Our research confirms the ERCC1
expression status of Xuanwei lung adenoma patients and suggests
that ERCC1 could be a predictive marker for identifying patients
suitable for immunotherapy. Thus, positive ERCC1 expression may
predict a poor response to immunotherapy. Further studies will be
needed to establish the role of ERCC1 in the immune response of
Xuanwei adenoma patients.
Previous studies (36,37)
suggested that lower ERCC1 RNA expression might be related to
shorter survival of lung cancer patients and commercial ERCC1 IHC
test was not practical in the prospective evaluation of
chemotherapy resistance of lung cancer patients, which contrasts
with the results observed here. One explanation for this
discrepancy is that many factors are involved in platinum
resistance. In addition to ERCC1, many other genes, such as YAP
(38) and NEAT1 (39), play important roles in the
complicated biological events underlying the resistance process.
ERCC1 expression level and function can be affected by
post-transcriptional modifications.
Many other aspects of treatment, including the
platinum dose, subsequent radiotherapy, and combination therapy
with agents such as tyrosine kinase inhibitors (TKI) for patients
with mutated epidermal growth factor receptor (EGFR), may also be
related to the OS and PFS of lung cancer patients. As for ERCC1 IHC
commercial tests, it could be a much more complicated problem.
Different antibody with different clone number from different
company may lead to confused conclusion of lung cancer patients
with ERCC1 expression, so more studies are urgently needed. There
is an urgent need for a more appropriate antibody, better and more
mature detecting methods instead of totally declining the
possibility to predict chemotherapy response of lung cancer
patients. We should emphasize that the clone number of antibody we
used in our research was the same as that in IALT Bio study
(27) (mouse, 8F1).
In this study, negative ERCC1 expression did benefit
lung cancer patients who were administered with cisplatin based
chemotherapy. Thus, we can make some deduction: positive ERCC1
expression may be a negative factor for lung cancer patients who
were administered with cisplatin based chemotherapy. In addition,
accumulating evidence indicates ERCC1 is a good marker for cellular
or clinical resistance to cisplatin, carboplatin, and oxaliplatin
(7,40,41).
ERCC1 is still a good predictive marker for chemotherapy.
Our study enrolled 106 patients from Xuanwei with
operable cancer. These patients were positive for ERCC1 before
administration of platinum-based chemotherapy, and the proportion
of ERCC1-positive patients increased as the disease progressed.
ERCC1 expression has been shown to predict the response to
platinum-based therapy in advanced NSCLC patients (42). These observations serve as reminders
of the significance of surgery in managing Xuanwei lung adenoma
patients. Radical resection of lesions can reduce the
ERCC1-positive tumor load in these patients, which is important for
post-operative adjuvant therapy. Xuanwei lung adenoma patients
diagnosed at an early disease stage may benefit from relatively
complete resection of ERCC1-positive tumor tissue, thus ensuring a
better response to adjuvant platinum-based chemotherapy.
There are some limitations to our study. All of the
samples analyzed here were from Xuanwei lung adenoma patients. Most
patients in this cohort did not undergo gene analysis for detection
of EGFR mutant status and did not accept TKI treatment for economic
reasons. The potential effects of these clinical factors on our
study are unknown. In addition, patients with advanced lung adenoma
(stage IIIb-IV) were not included in this study. We should also pay
attention to the in vitro experiment because only one cell
line was applied. In animal experiments, XWLC05-ERCC1-shRNA cells
and control cells were injected subcutaneously, and the
microenvironment in this location is quite different from that in
human lung.
This study is the first to investigate ERCC1
expression in residents of Western China, which has a high
incidence of lung adenoma. We examined ERCC1 gene-modified XWLC05
cells, originating from a Xuanwei lung adenoma patient, in
vitro and in vivo. Our findings indicate that ERCC1 may
play a critical role in the treatment of Xuanwei patients with
platinum-based therapy and may have a significant effect on
prognosis. Our research could thus be a good reference for further
study of Xuanwei lung adenoma patients.
Acknowledgements
This study was supported by Yunnan Provincial
Science and Technology Agency/Kunming Medical University Joint
Project (2013FB164, 201501UH00557) to W.W.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Downward GS, Hu W, Large D, Veld H, Xu J,
Reiss B, Wu G, Wei F, Chapman RS, Rothman N, et al: Heterogeneity
in coal composition and implications for lung cancer risk in
Xuanwei and Fuyuan counties, China. Environ Int. 68:94–104. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu ZY, He XZ and Chapman RS: Smoking and
other risk factors for lung cancer in Xuanwei, China. Int J
Epidemiol. 20:26–31. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen M, Chapman RS, He X, Liu LZ, Lai H,
Chen W and Lan Q: Dietary factors, food contamination and lung
cancer risk in Xuanwei, China. Lung Cancer. 61:275–282. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lan Q, Chapman RS, Schreinemachers DM,
Tian L and He X: Household stove improvement and risk of lung
cancer in Xuanwei, China. J Natl Cancer Inst. 94:826–835. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim C, Chapman RS, Hu W, He X, Hosgood HD,
Liu LZ, Lai H, Chen W, Silverman DT, Vermeulen R, et al: Smoky
coal, tobacco smoking, and lung cancer risk in Xuanwei, China. Lung
Cancer. 84:31–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reed E: ERCC1 and clinical resistance to
platinum-based therapy. Clin Cancer Res. 11:6100–6102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sodja E, Knez L, Kern I, Ovčariček T,
Sadikov A and Cufer T: Impact of ERCC1 expression on treatment
outcome in small-cell lung cancer patients treated with
platinum-based chemotherapy. Eur J Cancer. 48:3378–3385. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: The role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zamble DB, Mu D, Reardon JT, Sancar A and
Lippard SJ: Repair of cisplatin - DNA adducts by the mammalian
excision nuclease. Biochemistry. 35:10004–10013. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Selby CP and Sancar A: Mechanisms of
transcription-repair coupling and mutation frequency decline.
Microbiol Rev. 58:317–329. 1994.PubMed/NCBI
|
|
13
|
Mu D, Hsu DS and Sancar A: Reaction
mechanism of human DNA repair excision nuclease. J Biol Chem.
271:8285–8294. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith S, Su D, de la Rigault Longrais IA,
Schwartz P, Puopolo M, Rutherford TJ, Mor G, Yu H and Katsaros D:
ERCC1 genotype and phenotype in epithelial ovarian cancer identify
patients likely to benefit from paclitaxel treatment in addition to
platinum-based therapy. J Clin Oncol. 25:5172–5179. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krivak TC, Darcy KM, Tian C, Armstrong D,
Baysal BE, Gallion H, Ambrosone CB and DeLoia JA: Gynecologic
Oncology Group Phase III Trial: Relationship between ERCC1
polymorphisms, disease progression, and survival in the Gynecologic
Oncology Group Phase III Trial of intraperitoneal versus
intravenous cisplatin and paclitaxel for stage III epithelial
ovarian cancer. J Clin Oncol. 26:3598–3606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamada Y, Boku N, Nishina T, Yamaguchi K,
Denda T, Tsuji A, Hamamoto Y, Konishi K, Tsuji Y, Amagai K, et al:
Impact of excision repair cross-complementing gene 1 (ERCC1) on the
outcomes of patients with advanced gastric cancer: Correlative
study in Japan Clinical Oncology Group Trial JCOG9912. Ann Oncol.
24:2560–2565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Squires MH III, Fisher SB, Fisher KE,
Patel SH, Kooby DA, El-Rayes BF, Staley CA III, Farris AB III and
Maithel SK: Differential expression and prognostic value of ERCC1
and thymidylate synthase in resected gastric adenocarcinoma.
Cancer. 119:3242–3250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuhlmann JD, Wimberger P, Bankfalvi A,
Keller T, Schöler S, Aktas B, Buderath P, Hauch S, Otterbach F,
Kimmig R, et al: ERCC1-positive circulating tumor cells in the
blood of ovarian cancer patients as a predictive biomarker for
platinum resistance. Clin Chem. 60:1282–1289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hatch SB, Swift LP, Caporali S, Carter R,
Hill EJ, MacGregor TP, D'Atri S, Middleton MR, McHugh PJ and Sharma
RA: XPF protein levels determine sensitivity of malignant melanoma
cells to oxaliplatin chemotherapy: Suitability as a biomarker for
patient selection. Int J Cancer. 134:1495–1503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bauman JE, Austin MC, Schmidt R, Kurland
BF, Vaezi A, Hayes DN, Mendez E, Parvathaneni U, Chai X, Sampath S,
et al: ERCC1 is a prognostic biomarker in locally advanced head and
neck cancer: Results from a randomised, phase II trial. Br J
Cancer. 109:2096–2105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cecere F, Bria E and Rosell R: DNA repair
by ERCC1 in non-small-cell lung cancer. N Engl J Med.
355:2590–2591; author reply 2591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bepler G, Williams C, Schell MJ, Chen W,
Zheng Z, Simon G, Gadgeel S, Zhao X, Schreiber F, Brahmer J, et al:
Randomized international phase III trial of ERCC1 and RRM1
expression-based chemotherapy versus gemcitabine/carboplatin in
advanced non-small-cell lung cancer. J Clin Oncol. 31:2404–2412.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soria JC: ERCC1-tailored chemotherapy in
lung cancer: The first prospective randomized trial. J Clin Oncol.
25:2648–2649. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shiraishi K, Kohno T, Tanai C, Goto Y,
Kuchiba A, Yamamoto S, Tsuta K, Nokihara H, Yamamoto N, Sekine I,
et al: Association of DNA repair gene polymorphisms with response
to platinum-based doublet chemotherapy in patients with
non-small-cell lung cancer. J Clin Oncol. 28:4945–4952. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weiwei W, Zaoxiu H, Deguang W and Yong Z:
Comparison of ERCC1 expression in lung adenoma tissue from Xuanwei
of Yunnan province and other areas. Shangdong Med. 22:67–68.
2016.
|
|
26
|
Rubatt JM, Darcy KM, Tian C, Muggia F,
Dhir R, Armstrong DK, Bookman MA, Niedernhofer LJ, Deloia J, Birrer
M, et al: Pre-treatment tumor expression of ERCC1 in women with
advanced stage epithelial ovarian cancer is not predictive of
clinical outcomes: A Gynecologic Oncology Group study. Gynecol
Oncol. 125:421–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olaussen KA, Dunant A, Fouret P, Brambilla
E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH,
et al: IALT Bio Investigators: DNA repair by ERCC1 in
non-small-cell lung cancer and cisplatin-based adjuvant
chemotherapy. N Engl J Med. 355:983–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Zhao J, Yang L, Mao L, An T, Bai
H, Wang S, Liu X, Feng G and Wang J: Positive expression of ERCC1
predicts a poorer platinum-based treatment outcome in Chinese
patients with advanced non-small-cell lung cancer. Med Oncol.
27:484–490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ota S, Ishii G, Goto K, Kubota K, Kim YH,
Kojika M, Murata Y, Yamazaki M, Nishiwaki Y, Eguchi K, et al:
Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen
predicts survival in advanced non-small-cell lung cancer treated
with cisplatin-based chemotherapy. Lung Cancer. 64:98–104. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HW, Choi YW, Han JH, Kim JH, Jung JH,
Jeong SH, Kang SY, Choi JH, Oh YT, Park KJ, et al: Expression of
excision repair cross-complementation group 1 protein predicts poor
outcome in advanced non-small cell lung cancer patients treated
with platinum-based doublet chemotherapy. Lung Cancer. 65:377–382.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holm B, Mellemgaard A, Skov T and Skov BG:
Different impact of excision repair cross-complementation group 1
on survival in male and female patients with inoperable
non-small-cell lung cancer treated with carboplatin and
gemcitabine. J Clin Oncol. 27:4254–4259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Azuma K, Komohara Y, Sasada T, Terazaki Y,
Ikeda J, Hoshino T, Itoh K, Yamada A and Aizawa H: Excision repair
cross-complementation group 1 predicts progression-free and overall
survival in non-small cell lung cancer patients treated with
platinum-based chemotherapy. Cancer Sci. 98:1336–1343. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu D, Zhang X, Liu J, Yuan P, Tan W, Guo
Y, Sun T, Zhao D, Yang M, Liu J, et al: Characterization of
functional excision repair cross-complementation group 1 variants
and their association with lung cancer risk and prognosis. Clin
Cancer Res. 14:2878–2886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Snyder A, Makarov V, Merghoub T, Yuan J,
Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et
al: Genetic basis for clinical response to CTLA-4 blockade in
melanoma. N Engl J Med. 371:2189–2199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Simon GR, Sharma S, Cantor A, Smith P and
Bepler G: ERCC1 expression is a predictor of survival in resected
patients with non-small cell lung cancer. Chest. 127:978–983. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schneider JG, Farhadfar N, Sivapiragasam
A, Geller M, Islam S and Selbs E: Commercial laboratory testing of
excision repair cross-complementation group 1 expression in
non-small cell lung cancer. Oncologist. 19:459–465. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng H, Zhang Z, Rodriguez-Barrueco R,
Borczuk A, Liu H, Yu J, Silva JM, Cheng SK, Perez-Soler R and
Halmos B: Functional genomics screen identifies YAP1 as a key
determinant to enhance treatment sensitivity in lung cancer cells.
Oncotarget. 7:28976–28988. 2016.PubMed/NCBI
|
|
39
|
Jiang P, Wu X, Wang X, Huang W and Feng Q:
NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin
sensitivity in lung cancer cells. Oncotarget. 7:43337–43351.
2016.PubMed/NCBI
|
|
40
|
Reed E: Nucleotide excision repair and
anti-cancer chemotherapy. Cytotechnology. 27:187–201. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Olaussen KA and Postel-Vinay S: Predictors
of chemotherapy efficacy in non-small-cell lung cancer: A
challenging landscape. Ann Oncol. 27:2004–2016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cobo M, Isla D, Massuti B, Montes A,
Sanchez JM, Provencio M, Viñolas N, Paz-Ares L, Lopez-Vivanco G,
Muñoz MA, et al: Customizing cisplatin based on quantitative
excision repair cross-complementing 1 mRNA expression: A phase III
trial in non-small-cell lung cancer. J Clin Oncol. 25:2747–2754.
2007. View Article : Google Scholar : PubMed/NCBI
|