Introduction
Colorectal cancer (CRC) is the third most common
form of cancer in both men and women worldwide (1). The incidence of CRC has increased in a
number of Asian countries, including China, during the past few
decades (2,3). Alarmingly, increasing numbers of
reported cases of colon cancer in recent years has made this form
of cancer a major health concern (4). The prognosis for CRC remains poor, as
few exclusively effective agents have been developed for the
treatment of CRC (5). The most
effective treatment for CRC is surgery, yet, even after curative
resection, the recurrence rate is high. Patients with CRC after
surgery are treated with chemotherapy or radiation therapy.
However, the effects of these adjuvant therapies are limited due to
adverse side-effects. Therefore, the development of novel
anticancer agents for patients with CRC is urgently required to
increase the survival rate.
Several mechanisms of cell death are well known, as
determined by morphological characteristics, including apoptosis
(type I cell death), autophagy (type II cell death) and necrosis
(type III cell death). Autophagy is involved in a conserved
membrane trafficking pathway in all eukaryotic cells and mediates
the transport of cytosolic proteins and intracellular aged
organelles to lysosomes for degradation. The physiologic process of
autophagy has been observed in many pathologic conditions,
including infectious disease and cancer (6). Although the essential role of
autophagy in cancer is not clearly identified, its role in cell
death is conflicting depending on the type and stage of
tumorigenesis (7). Apoptosis is
distinguished from necrosis in the domain of morphological
characteristics and biological function (8). Apoptosis is a self-destructive process
(9), which is activated by two
major pathways: the receptor-mediated extrinsic and
mitochondrial-mediated intrinsic pathways (10). The extrinsic apoptotic pathway is
induced by the binding of extracellular ligands (for example, FasL)
to transmembrane death receptors (for example, Fas) on the cell
surface leading to activation of caspase-8 (11). In contrast, the intrinsic apoptotic
pathway is induced by changing the permeability of the outer
mitochondrial membrane, reducing mitochondrial membrane potential,
and releasing mitochondrial pro-apoptotic factors including
cytochrome c and Apaf-1 into the cytoplasm leading to
activation of caspase-9 (12).
Caspase-8 of the extrinsic pathway and caspase-9 of the intrinsic
pathway activate caspase-3 and cleave poly(ADP ribose) polymerase
(PARP), thus, resulting in apoptosis (13). Aberrant regulation of apoptosis can
lead to inappropriate cell loss, which eventually results in
various cell disorders (14).
It is generally believed that autophagy and
apoptosis often occur in the same cell and that autophagy mostly
precedes apoptosis (15).
Furthermore, various antitumor agents that are known to trigger
apoptosis also induce autophagy (16). Conversely, evident suggests that
inhibition of autophagy appears to enhance the sensitivity of
cancer cells toward anticancer drugs. Based on these findings, it
would be useful to develop a new anticancer agent that
simultaneously induces both autophagy and apoptosis.
Cycloartane triterpenoids are the major constituents
of Cimicifuga plants. The antitumor activities of the
extracts from Cimicifuga and their major constituent
cycloartane triterpenoids have been discovered and are drawing
increased attention (17,18). Cycloartane triterpenoids have been
shown to inhibit the proliferation of cells via induction of
apoptosis and cell cycle arrest in human cancer cell lines
(19,20). Cimigenol (KY17) is a novel
cycloartane triterpenoid from Cimicifuga, and its anticancer
effect and mechanisms are still unknown.
The purpose of the present study was to investigate
whether KY17 induces apoptosis and autophagy in HT-29 cells.
Materials and methods
Plant materials and the extraction of
compounds
Plant materials and the methods of compound
extraction were described previously (21). In detail, Cimicifuga dahurica
was collected from Yangla Town, Sichuang, China, in 2008. The
plants were identified by Professor Ming-Hua Qiu, Kunming Institute
of Botany, Chinese Academy of Science. Voucher specimens (KUN no.
200809007) have been deposited at the State Key Laboratory of
Photochemistry and Plant Resources in West China, Kunming Institute
of Botany, Chinese Academy of Sciences (Kunming, China). The dried
and milled roots of Cimicifuga dahurica (0.9 kg) were
extracted by MeOH (3 × 3 l × 24 h) at room temperature to give a
residue (106 g) after evaporation in a vacuum at 50̊C. The extract
was subjected to silica gel column chromatography (cc) (2 kg,
10×150 cm) and eluted with CHCl3-MeOH [100:0 (2 l), 50:1
(4 l), 20:1 (5 l), 10:1 (4 l), 0:100 (3 l)] to afford fractions A
(21.5 g), B (13.1 g), C (14.5 g), D (16.8 g) and E (16.2 g).
Fraction B (13.1 g) was divided into five sub-fractions (B.1-B.5)
after performing RP-18 cc (180 g, 5×25 cm), eluting with
MeOH-H2O (gradient from 60:40 to 100:0, 10 l). Fraction
B.3 (1.5 g) was subjected to repeated silica gel cc (40 g, 4×40 cm)
eluted with CHCl3-Me2CO (gradient from 20:1
to 10:1, 4 l) and then to repeated semi-preparative HPLC (eluted
with CH3CN-H2O, gradient from 60:40 to 85:15)
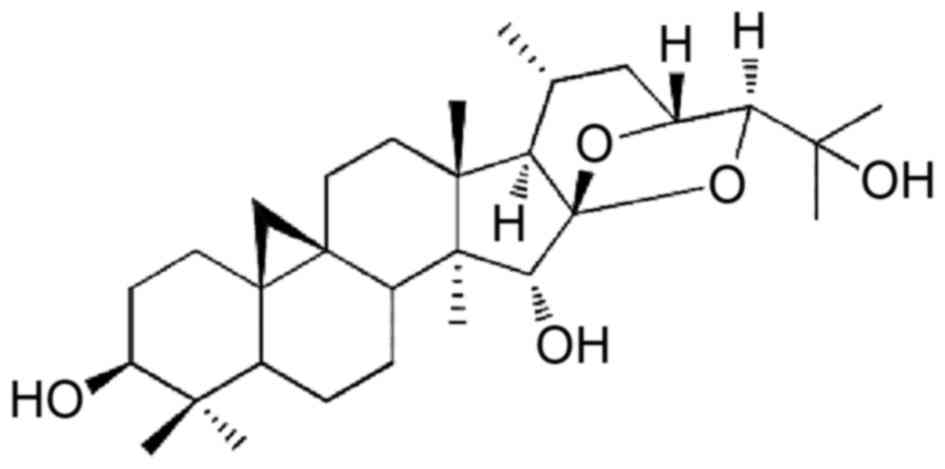
to yield KY17 (4.0 mg). The chemical structure of KY17 that was
used in the present study is shown in Fig. 1.
Chemicals
A stock solution of KY17 (10 mol/l) was prepared in
dimethyl sulfoxide (DMSO) and diluted with fresh complete medium
immediately before use. The DMSO and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were obtained from Amresco LLC (Solon, OH, USA). Propidium iodide
(PI), acridine orange, bafilomycin-A1 (Baf-A1), cisplatin (DDP) and
doxorubicin (DOX) were purchased from Sigma-Aldrich Co. LLC (St.
Louis, MO, USA). Antibodies that were specific for caspase-3,
poly(ADP-ribose) polymerase (PARP) and microtubule-associated
protein 1 light chain 3 (LC3) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The antibody against caspase-8
and β-actin were respectively purchased from Becton-Dickinson (BD)
and Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture and cell viability
assay
The human colon cancer cell line (HT-29) was
cultured in RPMI-1640 medium (GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 2.0 g/l sodium bicarbonate
(Sigma-Aldrich), 10% (v/v) fetal bovine serum (GE Healthcare Life
Sciences) at 37°C in a humidified 5% CO2. Cell
proliferation was assessed using an MTT assay. In detail, HT-29
cells were seeded onto a 96-well culture plate, cultured overnight
in growth media, and then treated with or without KY17 at different
concentrations for 72 h. The cells were incubated in the dark with
0.5 mg/ml MTT at 37°C for 4 h. The formazan granules that were
generated by the live cells were dissolved in DMSO, and the
absorbance at 490 nm was monitored with a multiwell reader (Thermo
Fisher Scientific Inc., Vantaa, Finland).
Assessment of cell cycle distribution
and apoptotic cell death by flow cytometry
Cell cycle distribution was determined by propidium
iodide (PI) staining. For cell cycle analysis, 5×105
cells were seeded in a 6-well culture plate and grown for 12 h.
Following treatment with different concentrations of KY17 for 24,
48 and 72 h, the cells were trypsinized, washed with cold
phosphate-buffered saline (PBS) and fixed with cold 70% ethanol at
4̊C overnight. Then, the cells were washed with PBS and incubated
with 10 mg/ml RNase A, 400 mg/ml PI and 0.1% Triton-X in PBS at
room temperature (RT) for 30 min. The stained cells were then
analyzed by flow cytometry. Apoptotic cell death was determined
using Annexin V/PI staining analyses. For the Annexin V/PI
staining, the cells were treated with different conditions of KY17
for 24 and 48 h, subsequently harvested, trypsinized, washed once
with cold PBS, and then suspended in 1X binding buffer (BD
Biosciences, San Jose, CA, USA). The cells were stained in Annexin
V-fluorescein isothiocyanate (FITC) solution (FITC Annexin V
apoptosis detection kit; BD Pharmingen, Franklin Lakes, NJ, USA)
and PI at RT for 15 min in the dark. The stained cells were
analyzed by flow cytometry within 1 h. Flow cytometric analysis was
performed on Accuri C6 (BD Biosciences).
Western blot analysis
The total cells were lysed in lysis buffer [150 mM
NaCl, 10 mM Tris (pH 7.2), 5 mM EDTA, 0.1% Triton X-100, 5%
glycerol and 2% SDS]. Equal amounts of the protein extracts were
denatured by boiling at 100°C for 5 min in sample buffer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The total proteins were
separated by SDS-PAGE electrophoresis and transferred to a
polyvinylidene fluoride (PVDF) membrane. After blocking for 1 h at
RT using PBST containing 10% dried fat-free milk with gentle
shaking, the membranes were incubated with primary antibodies
(1:1,000) overnight at 4̊C with gentle shaking, incubated with
horseradish peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.), and then visualized with the enhanced
chemiluminescence (ECL) detection system (GE Healthcare,
Piscataway, NJ, USA).
Autophagy detection with acridine
vesicular orange
As a marker of autophagy, the volume of the cellular
acidic compartment was visualized by acridine orange staining.
Cells were seeded into 6-well plates and treated as described above
for the cell viability assay for 24 and 48 h. The cells were then
stained with acridine orange (3 µM) for 15 min, trypsinized, and
then washed with PBS. The stained cell were photographed and images
were captured using fluorescent micrographs. The intensity of
acridine orange fluorescence (red) reflects the degree of cellular
acidity, which positively correlates with autophagy levels. The
numbers of red dots in 200 cells were counted for each slide.
GFP-LC3 assay
Autophagy was confirmed by the formation of punctate
LC3-positive structures, which are essential for the dynamic
process of autophagosome formation. When autophagy is induced, LC3
aggregates in the autophagosome membrane, thus, an increase in
puntuate GFP-LC3 is a specific indicator of autophagy. GFP-LC3
plasmid is specifically used to detect autophagy in cultured cells
through direct fluorescence microscopy. HT-29 cells were seeded in
6-well plates with coverslips at a density of 1×105
cells/well overnight. Cells were treated with KY17, and then
transfected with 8 µg GFP-LC3 plasmid using Lipofectamine 2000
(Invitrogen Corp.) according to the manufacturer's instructions.
After 24 and 48 h, the cells were fixed with 4% paraformaldehyde at
4̊C for 30 min, and the GFP-LC3 dots were examined using
fluorescence microscopes (Nikon Eclipse 80i). Two hundred cells
were counted for each slide and the numbers of GFP-LC3 dots in the
cells were calculated.
Statistical analyses
The results are expressed as mean ± standard
deviation (SD). Statistical differences were evaluated using the
two-tailed Student's t-test and analysis of variance (ANOVA)
followed by q-test, and were considered significant at p<0.05,
p<0.01 or p<0.001.
Results
Ky17 exerts potent antiproliferative
activity
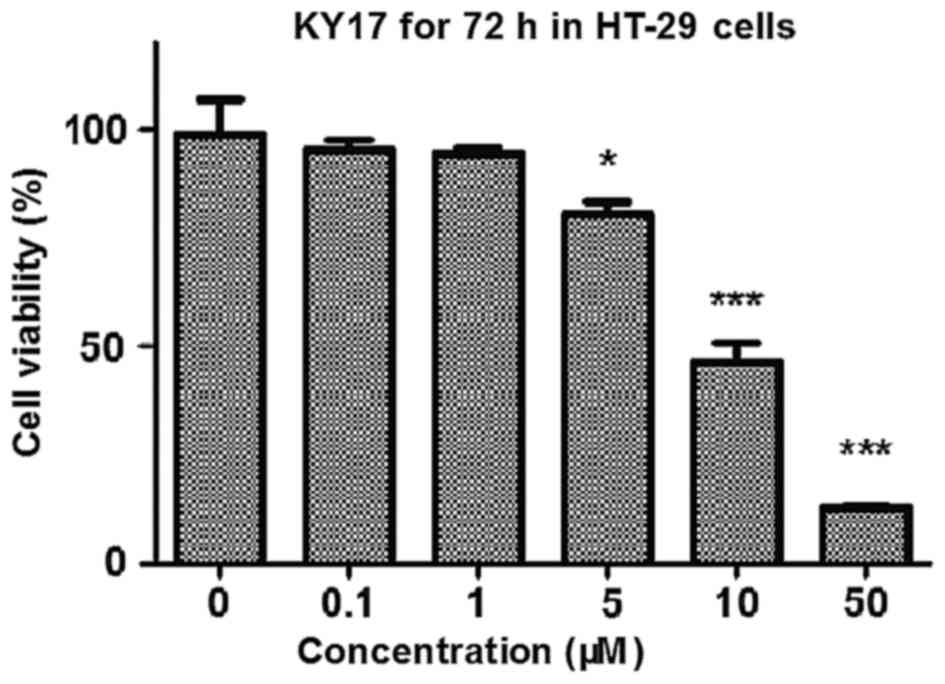
To investigate whether KY17 inhibits the growth and
proliferation of HT-29 cells in vitro, we treated the cells
for 72 h with increasing concentrations of KY17. The KY17-mediated
growth inhibition was measured by MTT assay. As shown in Fig. 2, KY17 significantly suppressed the
cell growth of HT-29 cells in a concentration-dependent manner, and
the IC50 value of KY17 was 8.84±1.1 µM. The result
suggests that KY17 suppressed the growth of HT-29 cells in a
concentration-dependent manner.
Ky17 induces G2/M phase arrest and
apoptosis in HT-29 cells
In order to study the mechanisms underlying the
effects of KY17 on the suppression of proliferation of HT-29 cells,
the effects of KY17 on cell cycle distribution and apoptosis were
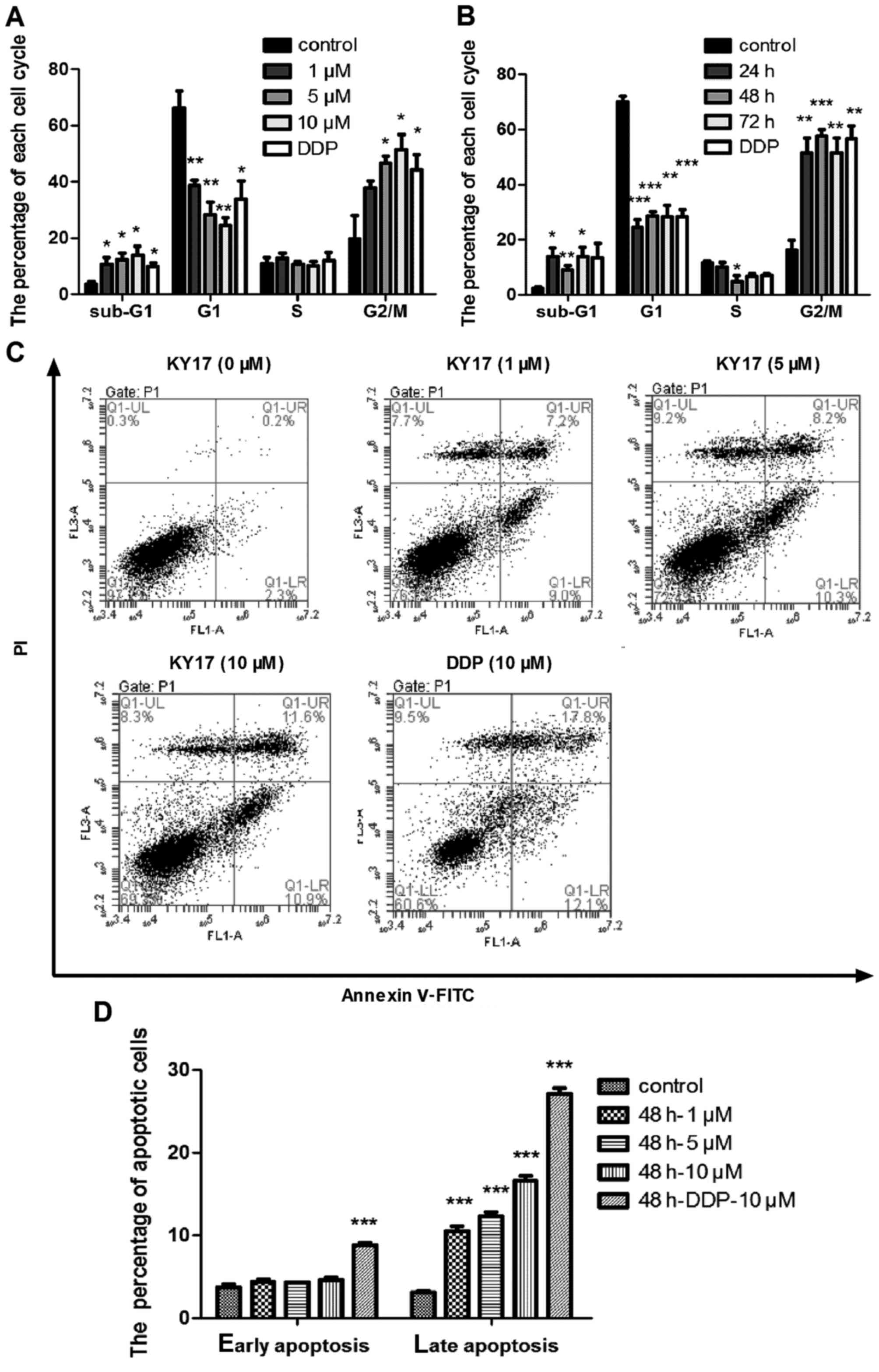
examined by flow cytometry. We found that KY17 induced G2/M phase
arrest and increased the percentage of cells in the sub-G1 phase in
a concentration- and time-dependent manner (Fig. 3A and B). Consistently, Annexin V
assays also indicated that KY17 increased cellular apoptosis in the
HT-29 cells. As shown in Fig. 3C and
D, there was a prominent increase in the percentage of late
apoptotic cells (11.6%) following 10 µM KY17 treatment for 48 h
compared with the untreated controls (0.2%). During the apoptosis
process, several caspases are active, which is induced by various
apoptotic stimuli (22). To examine
the mechanism underlying the KY17-mediated cell growth inhibition
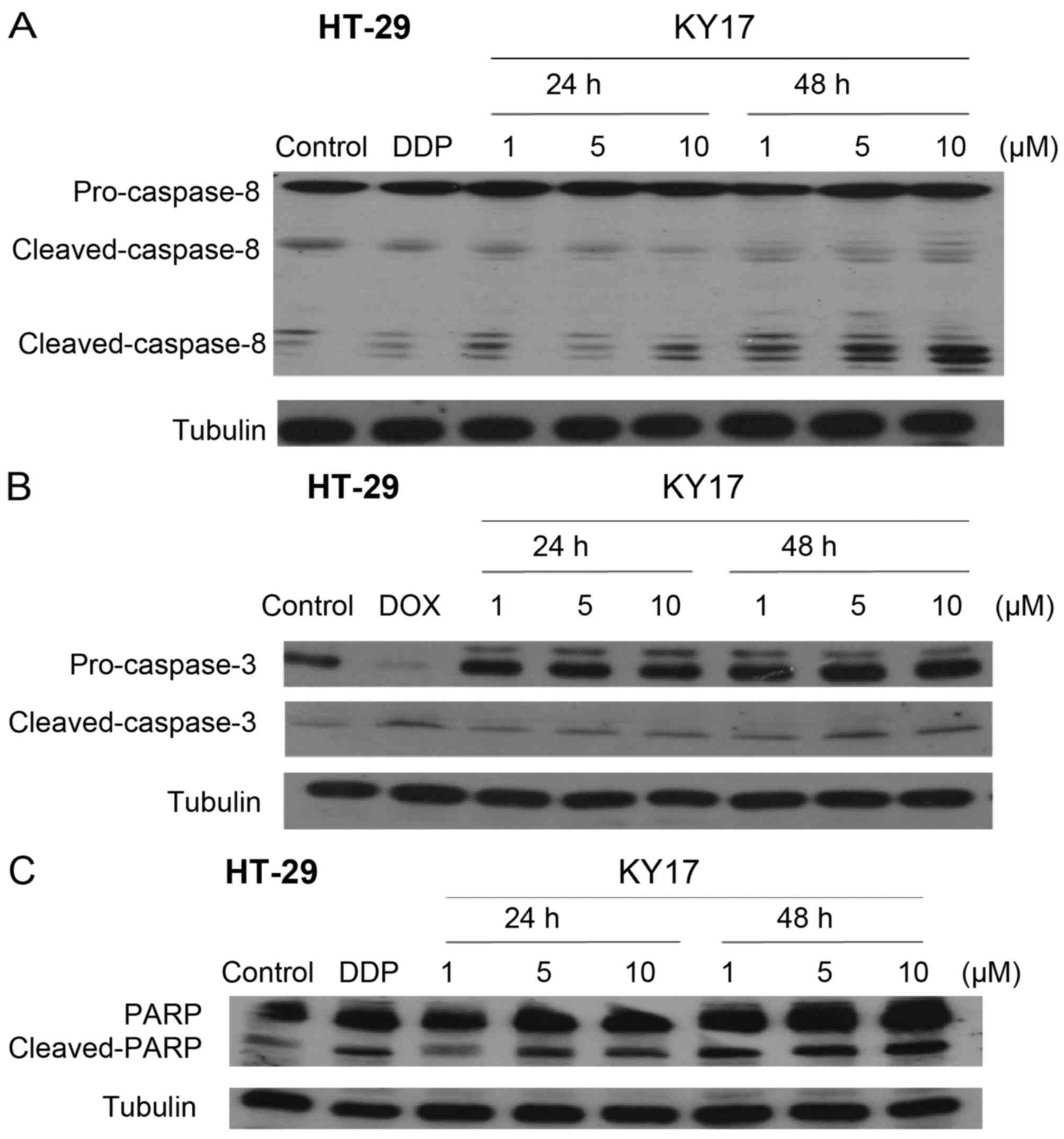
and death, caspase involvement was evaluated using western blot
analysis. The exposure of HT-29 cells to KY17 (1, 5 and 10 µM) at
24 and 48 h markedly increased the protein levels of
cleaved-caspase-8 and −3 in a concentration- and time-dependent
manner (Fig. 4A and B). In
addition, after treatment of KY17 the full length form of the PARP
protein (a selective substrate for caspase-3) was degraded to the
cleaved form (Fig. 4C). These
results further demonstrated that KY17 promoted cell death in the
HT-29 cells through apoptosis.
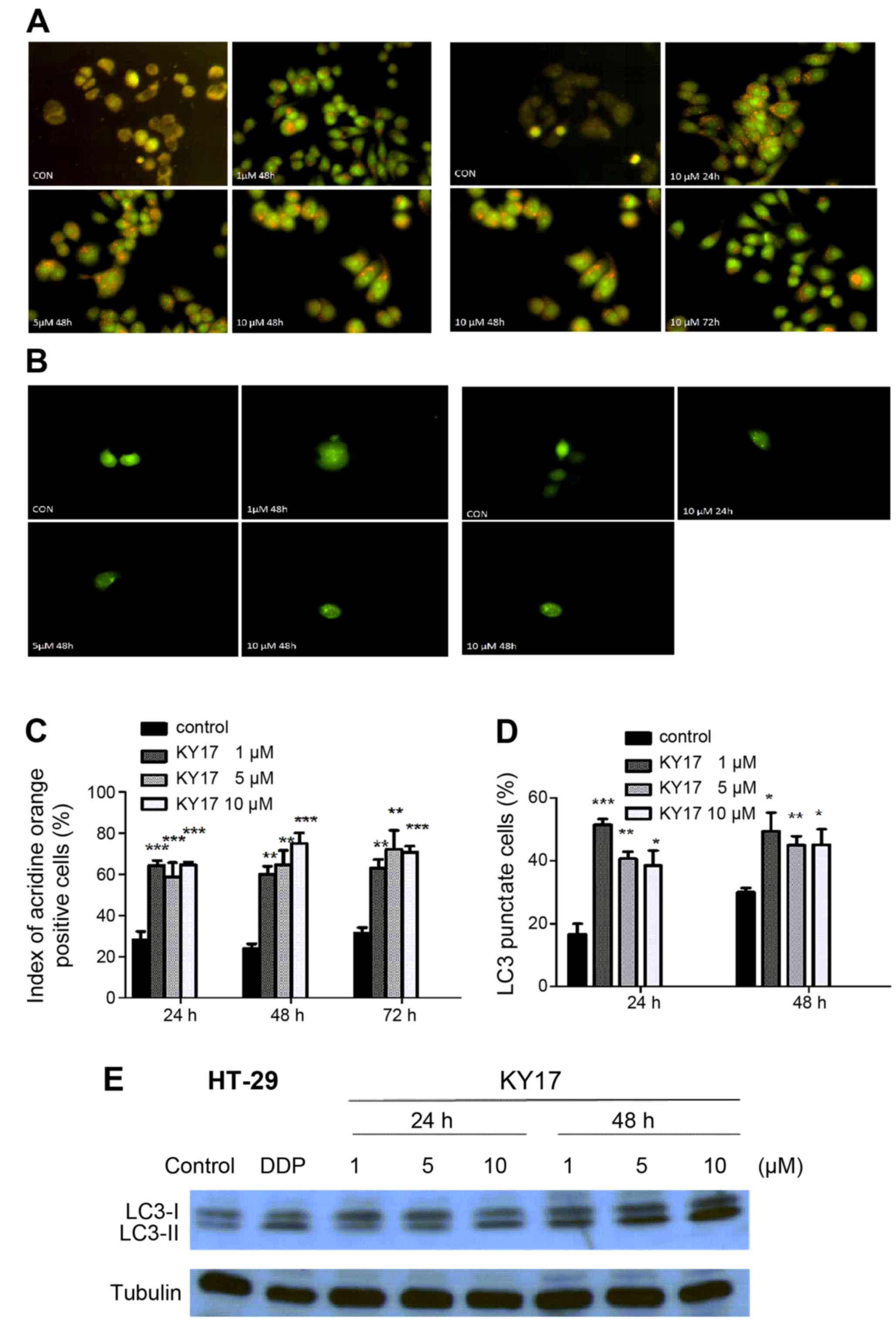
Ky17 induces cell autophagy in HT-29
cells
In addition to apoptosis, many modes of cell death
have been identified such as autophagy and necroptosis (23). In the present study, we determined
whether KY17 induces autophagy in HT-29 cells. Since the formation
of cytosolic acidic vesicular organelles (AVOs) is one of the
typical features of autophagy, fluorescence microscopy was
performed after staining the cells with acridine orange for the
quantification of the AVOs. The number of AVOs in the KY17-treated
cells was clearly increased (Fig. 5A
and C).
The conversion of microtubule association protein
LC3 is a special autophagic marker. Therefore, we examined the LC3
distribution in the KY17-treated HT-29 cells with fluorescence
microscopy. As shown in Fig. 5B and
D, compared with the untreated control cells, green fluorescent
protein (GFP)-tagged-LC3 (GFP-LC3) formed cytoplasmic puncta in the
cells that were treated with KY17 at different times and
concentrations. In order to further confirm that autophagy is
induced by KY17, a western blot analysis of LC3 was detected. The
results showed that LC3 underwent conversion from LC3-I (the
soluble form) to LC3-II (the lipidized form) in the KY17-treated
cells, thus confirming induction of autophagy (Fig. 5E).
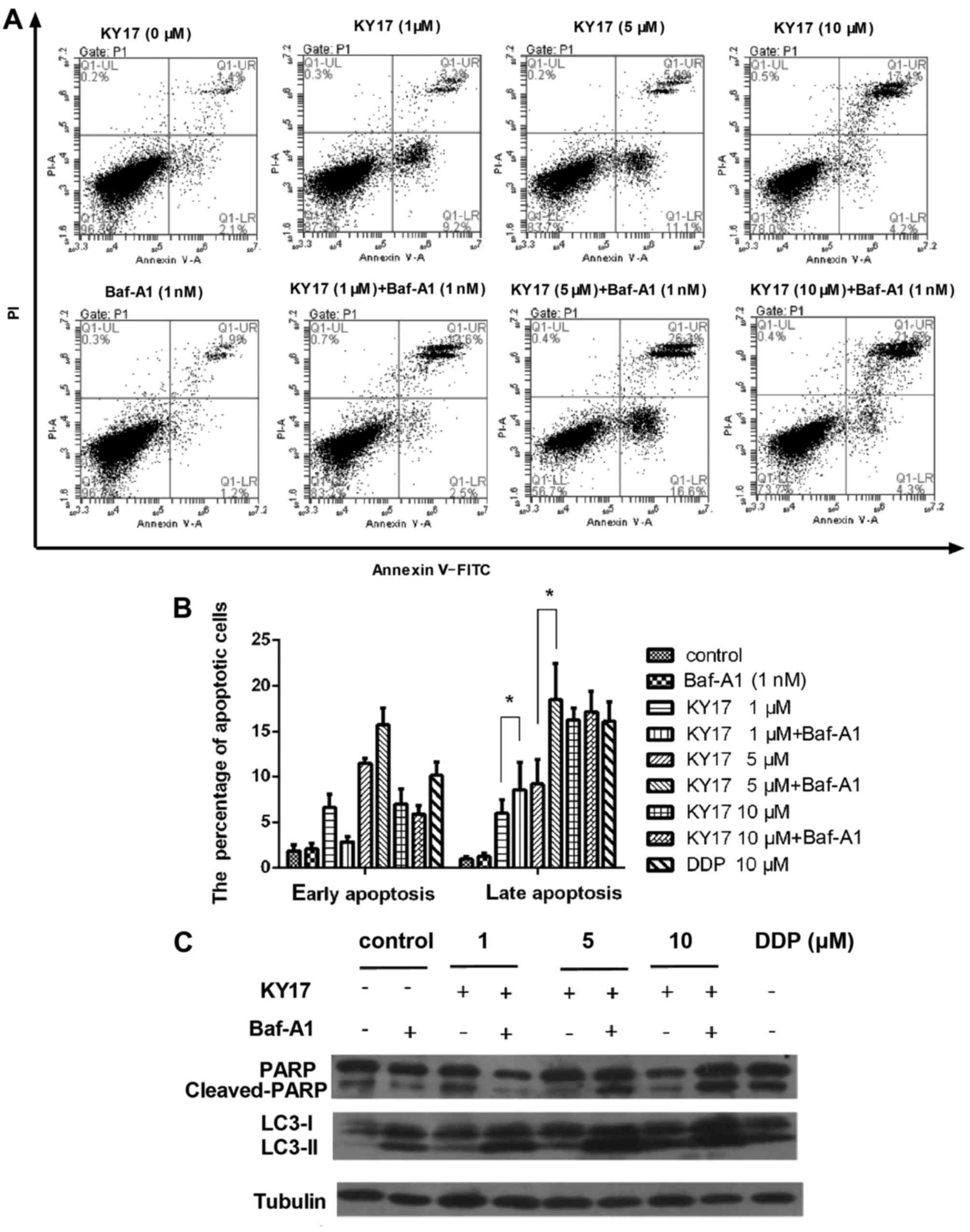
Suppression of autophagy increases
KY17-induced apoptotic cell death in HT-29 cells
Accumulating research has shown that the suppression
of autophagy may enhance the chemosensitization in human cancer
cells (24). Thus, we analyzed the
role of autophagy in KY17-induced cell death by examining the
effects of pharmacological autophagy inhibitors. We first treated
cells with Baf-A1 which is a vacuolar-type H+-ATP
inhibitor to prevent the maturation of autophagic vacuoles for 1 h,
and then incubated them with KY17 for another 48 h. Next, we used
Annexin V/PI staining to evaluate cell death. As shown in Fig. 6A and B, compared with KY17 treatment
the combination of the two agents effectively increased the
percentage of late apoptotic cells. In addition, western blot
analysis showed that KY17 significantly increased the cleaved form
of the PARP protein combined with Baf-A1 compared with KY17
treatment alone (Fig. 6C). These
data indicate that the inhibition of autophagy with autophagy
inhibitors, such as Baf-A1, enhanced the KY17-induced cell death in
the HT-29 cells.
Discussion
Colorectal cancer (CRC) is the third most lethal
malignancy worldwide, and surgery is the most common therapy for
patients with CRC, and advanced CRC also needs treatment with
chemotherapy or radiation therapy. However, the effects of these
adjuvant therapies are limited due to adverse side-effects. In the
present study, we investigated the anticancer mechanisms of KY17,
which is a novel cycloartane triterpenoid from plants from the
genus Cimicifuga. Our results indicated that KY17 induced
both apoptosis and autophagy in HT-29 cells. Furthermore, we found
that inhibition of autophagy resulted in higher levels of apoptotic
cell death in response to KY17 treatment. Collectively, our
findings suggest that inhibition of autophagy during cancer
treatment with KY17 may augment the therapeutic effects.
Regulation of apoptosis and cell cycle is an
important process to preserve cell homeostasis between cell death
and cell proliferation (25), which
means that the induction of apoptosis and suppression of cell cycle
progression is an advantageous strategy for cancer therapy.
Autophagy is also a crucial component of the cellular stress
adaptation response that maintains mammalian homeostasis (26). In the present study, we found that
KY17 effectively inhibited the proliferation of HT-29 cells through
induction of apoptosis and cell cycle arrest in a concentration-
and time-dependent manner. Moreover, KY17 also induced cell
autophagy in the HT-29 cells. These results suggest that
Cimicifuga may have beneficial effects for the reduction of
colon cancer growth.
In addition, we found that KY17 mediated anticancer
activity by modulating the expression of genes that are involved in
apoptosis. Death receptor-mediated apoptosis (extrinsic apoptotic
pathway) is activated by the interaction of pro-apoptotic and
pro-inflammatory cytokines, such as tumor-necrosis factor-α (TNF-α)
and their receptors (27). Caspases
are major components of the apoptotic system, which is associated
with proteolytic processes (23).
The interplay between ligands and receptors induces the formation
of intracellular death-induced signaling complexes (DISCs), which
activate caspase-8 and release DISC into the cytoplasm (28). Caspase-8 can directly activate
caspase-3. In particular, caspase-3 plays a pivotal role in
apoptosis, which may be controlled through death receptors or
mitochondria. Our data showed that KY17 markedly increased the
protein levels of cleaved-caspase-8 and −3 in HT-29 cells and the
full length form of the PARP protein was degraded to the cleaved
form suggesting that KY17 has a promoting effect on apoptosis in
HT-29 cells.
As with many other anticancer therapies, we observed
an increase in the number of AVOs and LC3 puncta expression in the
KY17-treated cells. This indicated that KY17 induced autophagy, and
autophagy appears to act as a cytoprotective mechanism (29). Numerous data have also shown that
the suppression of autophagy enhances the chemosensitization in
human cancer cells (24). Thus, we
investigated the role of autophagy in KY17-induced cell death by
examining the effects of pharmacological autophagy inhibitors. Our
data showed that the lysosomal H+-ATPase inhibitor
Baf-A1, which interrupted autophagolysosome formation, successfully
enhanced KY17-mediated apoptosis. These results indicated that the
disruption of autophagolysosome formation effectively sensitized
cells to KY17 apoptosis. However the molecular mechanisms of
autophagy in the KY17 anticancer effects warrant further
investigation. Moreover, clinically relevant animal models also
need to be explored.
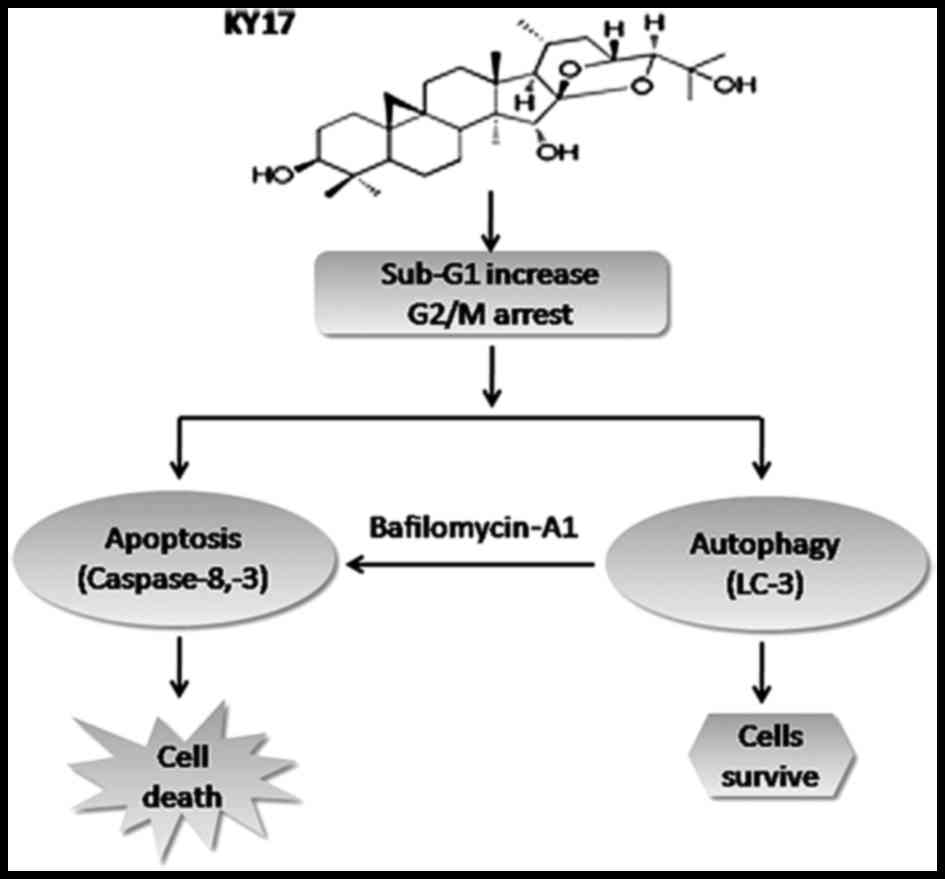
Altogether, the present study suggested that the
novel cycloartane triterpenoid KY17 from Cimicifuga
possesses anticancer activity by induction of apoptosis and
autophagy in HT-29 cells (Fig. 7).
The results from the present study provide evidence of KY17-induced
autophagy, and autophagy inhibition by Baf-A1 significantly
increased the apoptotic cell death induced by KY17 in HT-29 cells.
Our findings suggest that KY17 in combination with Baf-A1 may be a
useful candidate for the chemoprevention or treatment of colon
cancer. However, the potential of this fascinating molecule in the
treatment of other types of cancer remains unknown. Thus,
evaluation of the application of this compound in other cancer cell
lines will be carried out in future research.
Acknowledgements
The present study was financially supported by the
Natural Science Foundation of China (nos. 81260501, 81560601,
81302670 and U1202221) and the Nature Science Foundation of Yunnan
Province (no. KKSY201460043).
References
|
1
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hyodo I, Suzuki H, Takahashi K, Saito Y,
Tanaka S, Chiu HM, Kim NK, Li J, Lim R, Villalon A, et al: Present
status and perspectives of colorectal cancer in Asia: Colorectal
Cancer Working Group report in 30th Asia-Pacific Cancer Conference.
Jpn J Clin Oncol. 40:(Suppl 1). i38–i43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiong F, Wu C, Bi X, Yu D, Huang L, Xu J,
Zhang T, Zhai K, Chang J, Tan W, et al: Risk of genome-wide
association study-identified genetic variants for colorectal cancer
in a Chinese population. Cancer Epidemiol Biomarkers Prev.
19:1855–1861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levin B, Lieberman DA, McFarland B, Smith
RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR,
et al: American Cancer Society Colorectal Cancer Advisory Group; US
Multi-Society Task Force; American College of Radiology Colon
Cancer Committee: Screening and surveillance for the early
detection of colorectal cancer and adenomatous polyps, 2008: A
joint guideline from the American Cancer Society, the US
Multi-Society Task Force on Colorectal Cancer, and the American
College of Radiology. CA Cancer J Clin. 58:130–160. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laubert T, Habermann JK, Hemmelmann C,
Kleemann M, Oevermann E, Bouchard R, Hildebrand P, Jungbluth T,
Bürk C, Esnaashari H, et al: Metachronous metastasis- and
survival-analysis show prognostic importance of lymphadenectomy for
colon carcinomas. BMC Gastroenterol. 12:242012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hetz CA, Torres V and Quest AF: Beyond
apoptosis: Nonapoptotic cell death in physiology and disease.
Biochem Cell Biol. 83:579–588. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mattson MP: Apoptosis in neurodegenerative
disorders. Nat Rev Mol Cell Biol. 1:120–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Konopleva M, Zhao S, Xie Z, Segall H,
Younes A, Claxton DF, Estrov Z, Kornblau SM and Andreeff M:
Apoptosis. Molecules and mechanisms. Adv Exp Med Biol. 457:217–236.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Waring P and Müllbacher A: Cell death
induced by the Fas/Fas ligand pathway and its role in pathology.
Immunol Cell Biol. 77:312–317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
13
|
Gupta S: Molecular signaling in death
receptor and mitochondrial pathways of apoptosis (Review). Int J
Oncol. 22:15–20. 2003.PubMed/NCBI
|
|
14
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Radogna F, Dicato M and Diederich M:
Cancer-type-specific crosstalk between autophagy, necroptosis and
apoptosis as a pharmacological target. Biochem Pharmacol. 94:1–11.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X and Fan Z: The epidermal growth
factor receptor antibody cetuximab induces autophagy in cancer
cells by downregulating HIF-1alpha and Bcl-2 and activating the
beclin 1/hVps34 complex. Cancer Res. 70:5942–5952. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu L, Chen JC, Li Y, Qing C, Wang YY, Nian
Y and Qiu MH: Studies on the constituents of Cimicifuga
foetida collected in Guizhou Province and their cytotoxic
activities. Chem Pharm Bull. 60:571–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang ZZ, Nian Y, Li W, Wu JJ, Ge GB, Dong
PP, Zhang YY, Qiu MH, Liu L and Yang L: Cycloartane triterpenoids
from Cimicifuga yunnanensis induce apoptosis of breast
cancer cells (MCF7) via p53-dependent mitochondrial signaling
pathway. Phytother Res. 25:17–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li GH, Zhong QQ and Shen T:
Antiproliferative effect of cycloartane-type triterpenoid from
myrrh against human prostate cancer cells. Zhong Yao Cai.
36:1640–1643. 2013.(In Chinese). PubMed/NCBI
|
|
20
|
Tian Z, Xu L, Chen S, Zhou L, Yang M, Chen
S, Xiao P and Wu E: Cytotoxic activity of schisandrolic and
isoschisandrolic acids involves induction of apoptosis.
Chemotherapy. 53:257–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun LR, Yan J, Pei SJ and Qiu MH: A new
cycloartane triterpenoid from the rhizome of Cimicifuga
foetida collected in Dali. Acta Bot Yunn. 27:331–336. 2005.
|
|
22
|
Kumar S: Caspase function in programmed
cell death. Cell Death Differ. 14:32–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Degterev A and Yuan J: Expansion and
evolution of cell death programmes. Nat Rev Mol Cell Biol.
9:378–390. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wan XM, Zheng F, Zhang L, Miao YY, Man N
and Wen LP: Autophagy-mediated chemosensitization by cysteamine in
cancer cells. Int J Cancer. 129:1087–1095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
White E, Karp C, Strohecker AM, Guo Y and
Mathew R: Role of autophagy in suppression of inflammation and
cancer. Curr Opin Cell Biol. 22:212–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schulze-Osthoff K, Ferrari D, Los M,
Wesselborg S and Peter ME: Apoptosis signaling by death receptors.
Eur J Biochem. 254:439–459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Micheau O and Tschopp J: Induction of TNF
receptor I-mediated apoptosis via two sequential signaling
complexes. Cell. 114:181–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El-Khoury V, Pierson S, Szwarcbart E,
Brons NH, Roland O, Cherrier-De Wilde S, Plawny L, Van Dyck E and
Berchem G: Disruption of autophagy by the histone deacetylase
inhibitor MGCD0103 and its therapeutic implication in B-cell
chronic lymphocytic leukemia. Leukemia. 28:1636–1646. 2014.
View Article : Google Scholar : PubMed/NCBI
|