Introduction
Malignant melanoma has recently been reported to
have one of the highest incidence rates among all types of cancer,
with an increasing number of melanoma-related deaths each year
(1). The principal cause of death
in melanoma patients is attributed to widespread metastases to the
lymphatic system and other organs (2). Following traditional therapy, the
average survival time of patients with metastatic melanoma is only
6–12 months and the 5-year survival rate is consistently <10% in
most cases (3). However, tremendous
progress in both immunotherapy and molecular-targeted therapy has
revolutionized the standard of care for terminal melanoma patients.
In the meantime, some new challenges for clinicians have also
surfaced (4). One such example is
molecular-targeted therapy which often leads to fast-acting and
significant responses in most patients with the targeted mutation,
while the clinical benefit is usually transient due to the rapid
emergence of drug resistance. Consequently, it is urgent to develop
efficient agents that may be applied for melanoma treatment.
Apigenin, a natural plant flavonoid
(4′,5,7-trihydroxyflavone), is widespread in common fruits and
vegetables. According to the Biopharmaceutics Classification
System, apigenin is categorized as a class II drug with poor
solubility and high intestinal membrane permeability (5). The oral bioavailability of apigenin is
relatively low due to its low solubility in water (~2.16 µg/ml)
(5) and in high hydrophilic or
nonpolar solvents (0.001–1.63 mg/ml) (6), which has extremely hampered its
clinical development. Several formulation strategies have been
investigated to improve the bioavailability for application,
including liposome (7) and
nanocrystals fabricated by high pressure homogenization (8).
Apigenin has been shown to have marked
anti-inflammatory, antioxidant and anticarcinogenic properties
(9). Recently researchers have
demonstrated that apigenin has an anti-proliferative effect on a
variety of cancer cells, such as bladder, ovarian, breast and
prostate cancer (10–15), including melanoma (16,17).
Apoptosis plays a critical role in controlling cell proliferation
and thus is pivotal for the prevention of cancer progression and
oncogenesis (18). Extracellular
signal-regulated kinase (ERK) is a crucial signaling molecule that
regulates cell survival and proliferation. The ERK signaling
pathway controls various pro- and anti-apoptotic mechanisms that
determine cell viability (19). AKT
serves as an anti-apoptotic signaling molecule and inhibits
apoptosis through mitochondrial pathways (20). Consequently, in the present study,
we investigated the effects of apigenin on the viability, migration
and invasion potential, dendrite morphology, cell cycle
distribution, apoptosis, ERK expression and the AKT/mTOR signaling
pathway.
Materials and methods
Chemicals and reagents
Apigenin (no. A0113, CAS: 520-36-5, purity ≥98%) was
purchased from Chengdu Must Bio-Technology Co., Ltd. (Chengdu,
China). Dulbecco's modified Eagle's medium (DMEM), trypsin and
fetal bovine serum (FBS) were purchased from Gibco BRL (Grand
Island, NY, USA). Dimethyl sulfoxide (DMSO),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
Triton X-100 and anti-β-actin antibody were purchased from Sigma
Chemical Co. (St. Louis, MO, USA). Trypsin free of
ethylenediaminetetraacetic acid (EDTA) was purchased from Hyclone
Co. (Logan, UT, USA). Matrigel was purchased from BD Biosciences
(Franklin Lakes, NJ, USA). FITC-Annexin V kit was obtained from
Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Propidium iodide
(PI) and RNase were purchased from Takara Bio, Inc. (Otsu, Shiga,
Japan). Antibodies for ERK1/2 and phosphorylated (p)-ERK1/2 were
purchased from Promega Corp. (Madison, WI, USA). The antibodies for
poly(ADP-ribose) polymerase (PARP), caspase-3, AKT, p-AKT (Ser473),
mTOR and p-mTOR (Ser2448) were purchased from Cell Signaling
Technology Inc. (Beverly, MA, USA).
Cell culture and apigenin
treatment
Human malignant melanoma A375 and C8161 cell lines
were obtained from Peking Union Cell Resource Center (Beijing,
China). The cells were grown at 37°C in a humidified atmosphere
containing 5% CO2. The cells were cultured and
maintained in DMEM supplemented with 1% penicillin-streptomycin and
10% FBS. A375 and C8161 cells were treated with different
concentrations of apigenin (dissolved in DMSO) whereas the control
cells were treated with an equivalent volume of DMSO.
MTT assays
For cell proliferation assays, the A375 and C8161
cells were seeded in 96-well plates at a concentration of
1×104 cells/well. Cells were allowed to adhere for 24 h
and subsequently exposed to different concentrations of apigenin
(40, 80, 120, 160, 200, 240 and 280 µM) and incubated at 37°C for
24, 48, 72 and 96 h. MTT solution was added to each well at the
specified time-point and incubated for an additional 4 h. The
culture medium in each well was discarded and replaced with DMSO to
dissolve the formazan crystals which were formed from the MTT. The
absorbance value was evaluated using an automatic microplate reader
(T17108U; PerkinElmer, Inc., Waltham, MA, USA) at 490 nm.
Cell migration assays in vitro
Cell migration was performed using the wound healing
assay. A375 and C8161 cells were seeded in a 24-well plate at a
concentration of 5×105 cells/well and allowed to form a
confluent monolayer for 24 h. The monolayer was scratched with a
sterile pipette tip (10 µl) then washed with serum-free medium to
remove the floating and detached cells. After treatment with 40 and
80 µM of apigenin or DMSO, the cells were observed and photographed
(time 0 h and 24 h) using an inverted microscope (Olympus
Corporation, Tokyo, Japan). Moreover, the number of cells migrating
to the wound was assessed. Data were obtained from three
independent experiments.
Cell invasion assay
Cell culture inserts (24-well, pore size 8 µm; BD
Biosciences) were seeded with 1×106 cells/ml in 100 µl
of serum-free medium with 40 µM apigenin, or DMSO. Inserts were
precoated with 10 µl of Matrigel (3 mg/ml; Becton-Dickinson,
Mountain View, CA, USA). Medium with 10% FBS (500 µl) was added to
the lower chamber and served as a chemotactic agent. After
incubation for 72 h, non-invasive cells were wiped from the upper
surface of the membrane. Cells on the lower side were fixed with
chilled methanol, stained with crystal violet (dissolved in
methanol) and counted using an inverted microscope. Each individual
experiment had triplicate inserts and five random, non-overlapping
fields at a magnification of ×200 were counted per insert.
Scanning electron microscopy
A375 and C8161 cells were plated at a concentration
of 2×104 cells/well into a 60-mm culture dish. After
treatment with 100 µM of apigenin or DMSO for 24 h, the cells were
harvested, washed with PBS and fixed with 2.5% glutaraldehyde and
1% osmium tetraoxide, followed by an increasing gradient
dehydration step using ethanol solutions of 50, 70, 95 and 100%.
Samples were sputter-coated with platinum and palladium before
being observed under a scanning electron microscope (Quanta 200F;
FEI, Hillsboro, OR, USA).
Cell apoptosis
Cells were placed in 6-well culture plates
(5×104 cells/ml) and allowed to attach for 8 h. A375 and
C8161 cells were treated with apigenin (40 and 100 µM,
respectively) or DMSO for 24 h. Following the manufacturers
instructions, the cells were harvested by trypsinization free of
EDTA, washed in cold PBS and resuspended in binding buffer at a
concentration of 1×106 cells/ml. FITC-conjugated Annexin
V (BioVision, Inc., Milpitas, CA, USA) and PI (5 µl each)
(Becton-Dickinson) were added to the cells, gently mixed and then
incubated for 15 min at room temperature in the dark. Afterwards
binding buffer was added and the cells were analyzed by flow
cytometry.
Cell cycle analysis
Cells were seeded in 60-mm culture dishes. After
attachment, the cells were treated with 100 µM apigenin or DMSO for
24 h. Then cells were harvested and fixed with ice-cold 75%
ethanol. The cell pellets were resuspended in binding buffer
consisting of 480 µl PBS, 5 µl PI (5 mg/ml), 5 µl RNase (10 mg/ml)
and 10 µl Triton X-100 (10%). After 30 min of incubation at room
temperature in the dark, the DNA content of the cells was examined
using a flow cytometer (Accuri C6; Becton-Dickinson) for cell cycle
phase distribution.
Western blot analysis
Cells were plated in 6-well culture plates at
concentrations determined to yield 60–70% confluence within 24 h.
Next, the cells were left untreated or treated with 100 µM apigenin
for 24 h. After preparing appropriate protein concentrations of 25
µg, sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) was performed. Proteins were separated by
electrophoresis and transferred onto nitrocellulose membranes and
afterwards blocked for 1 h with 5% non-fat dry milk in TBS-T. The
membranes were incubated with respective primary antibodies at
appropriate concentrations overnight at 4°C. After being washed to
remove unbound primary antibodies, they were incubated with the
corresponding secondary antibodies. Proteins were visualized by
image scanning and the optical density for each band was assessed
using Image Lab software (version 4.0; Bio-Rad, Hercules, CA, USA)
after data were normalized to β-actin as an internal reference.
Statistical analysis
All the experiments were carried out in triplicate
and the values are expressed as the mean ± standard deviation (SD).
SPSS v17.0 software (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis. The repeated experiments used analysis of
variance, Dunnetts test and Student's t-test for the assessment of
differences between groups. A probability value of ≤0.05 was deemed
statistically significant. *P<0.05, **P<0.01 and
***P<0.001 as indicated in the figures, are relative to the
controls.
Results
Apigenin inhibits A375 and C8161 cell
proliferation
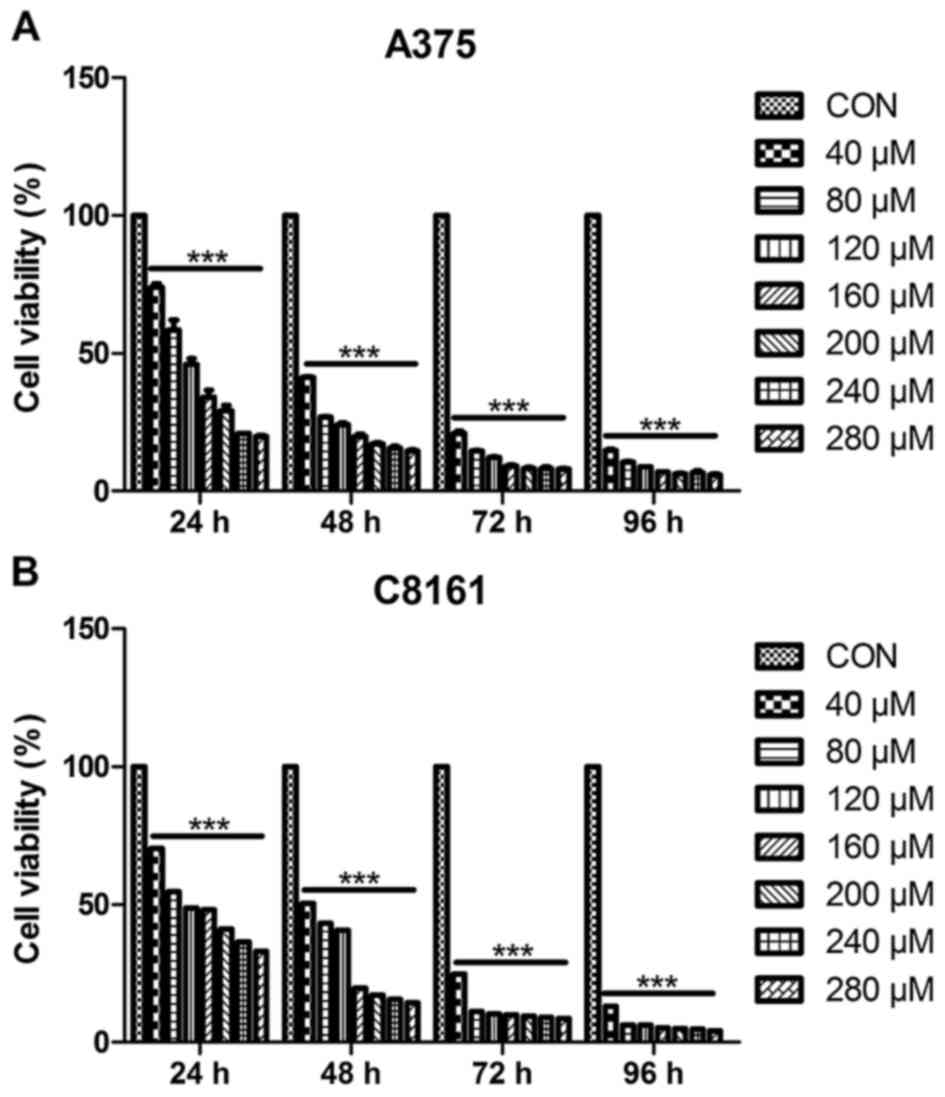
To investigate the growth inhibitory effect of
apigenin, A375 and C8161 cells were treated with different
concentrations (40, 80, 120, 160, 200, 240 and 280 µM) of apigenin
for different periods of time (24, 48, 72 and 96 h). The cell
viability was assessed by MTT assay. As shown in Fig. 1, cell growth inhibition caused by
apigenin was relatively marked in a dose-dependent, as well as a
time-dependent manner (ranging from 40–160 µM within 48 h). The
IC50 value at 24 h was estimated to be 100 µM.
Apigenin inhibits A375 and C8161 cell
migration potential
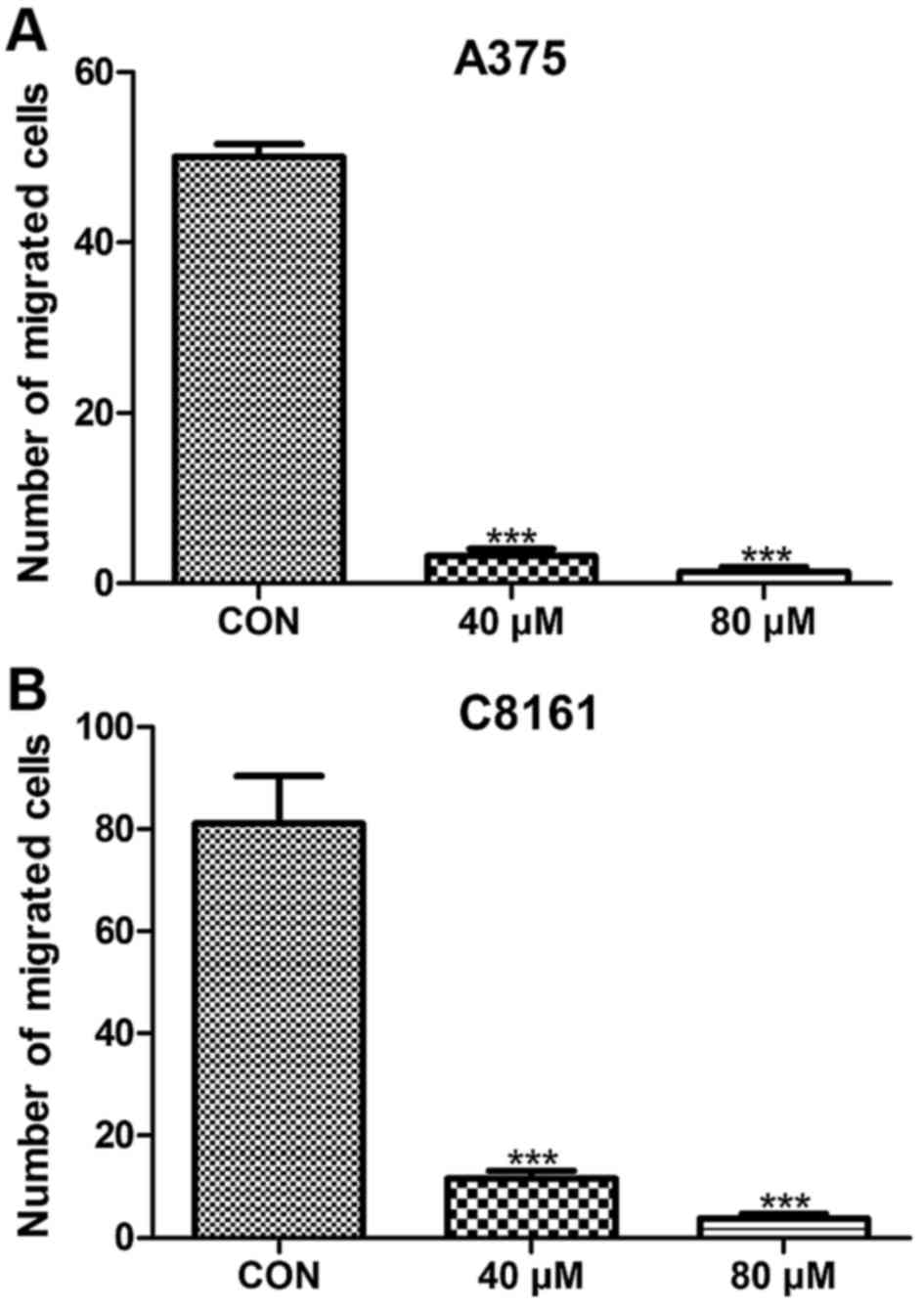
To assess whether or not apigenin has an effect on
the metastasis of A375 and C8161 cell lines, we examined the number
of migratory cells using a wound-healing approach. Migration was
significantly inhibited in the A375 and C8161 cell lines after
treatment with apigenin (40 and 80 µM) for 24 h (P<0.001)
(Fig. 2). When the cells were
exposed to 100 µM of apigenin for 24 h, no migrating cells were
observed (data not shown).
Apigenin suppresses the invasion of
A375 and C8161 cells
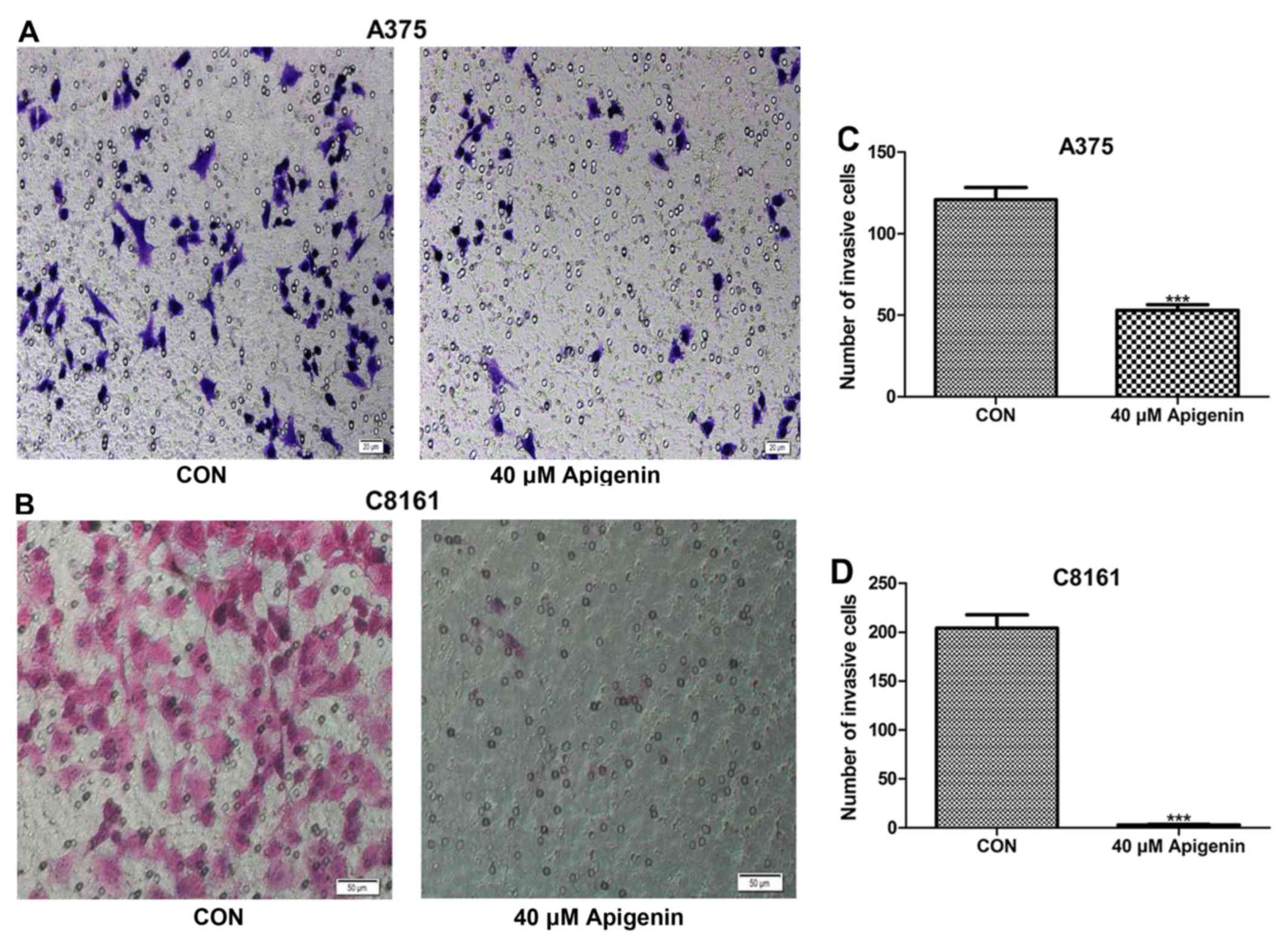
We further investigated cell motility by invasion
assays. Both the density of the invasive cells on the membrane and
the number of invasive cells/field are shown in Fig. 3. Treatment with 40 µM of apigenin
for 72 h significantly decreased the invasive ability of the A375
and C8161 melanoma cells compared with the control cells
(P<0.001). Following treatment with 80 µM of apigenin, no
invading cells were observed (data not shown). These findings
demonstrated that apigenin decreased the invasion of melanoma cells
in vitro.
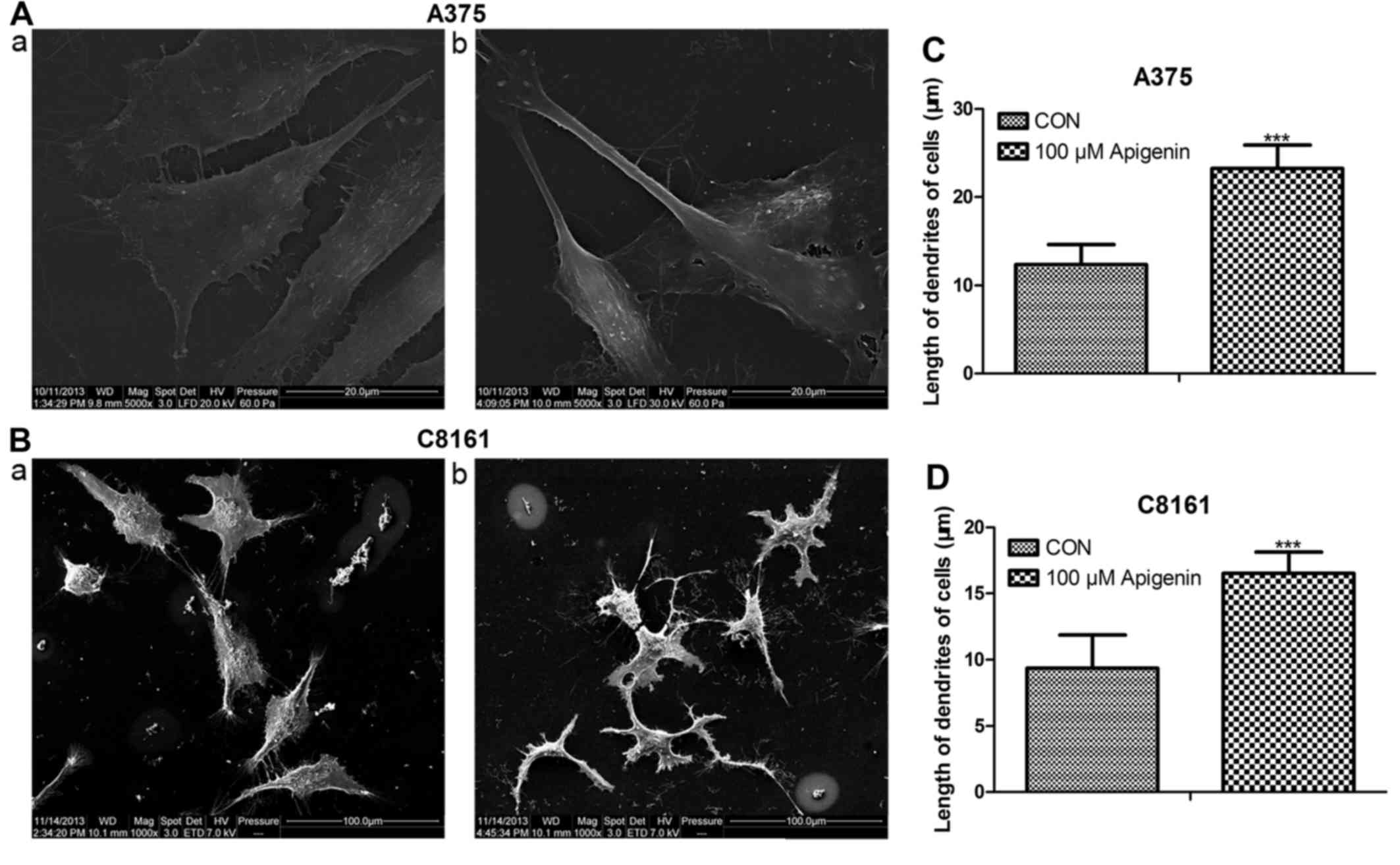
Apigenin affects the dendrite
morphology of A375 and C8161 cells
Following treatment with 100 µM of apigenin for 24
h, both the A375 and C8161 cells changed their cellular morphology
as visualized using scanning electron microscopy. The dendrites
became thinner and longer compared to those noted in the untreated
control cells (P<0.001) (Fig.
4).
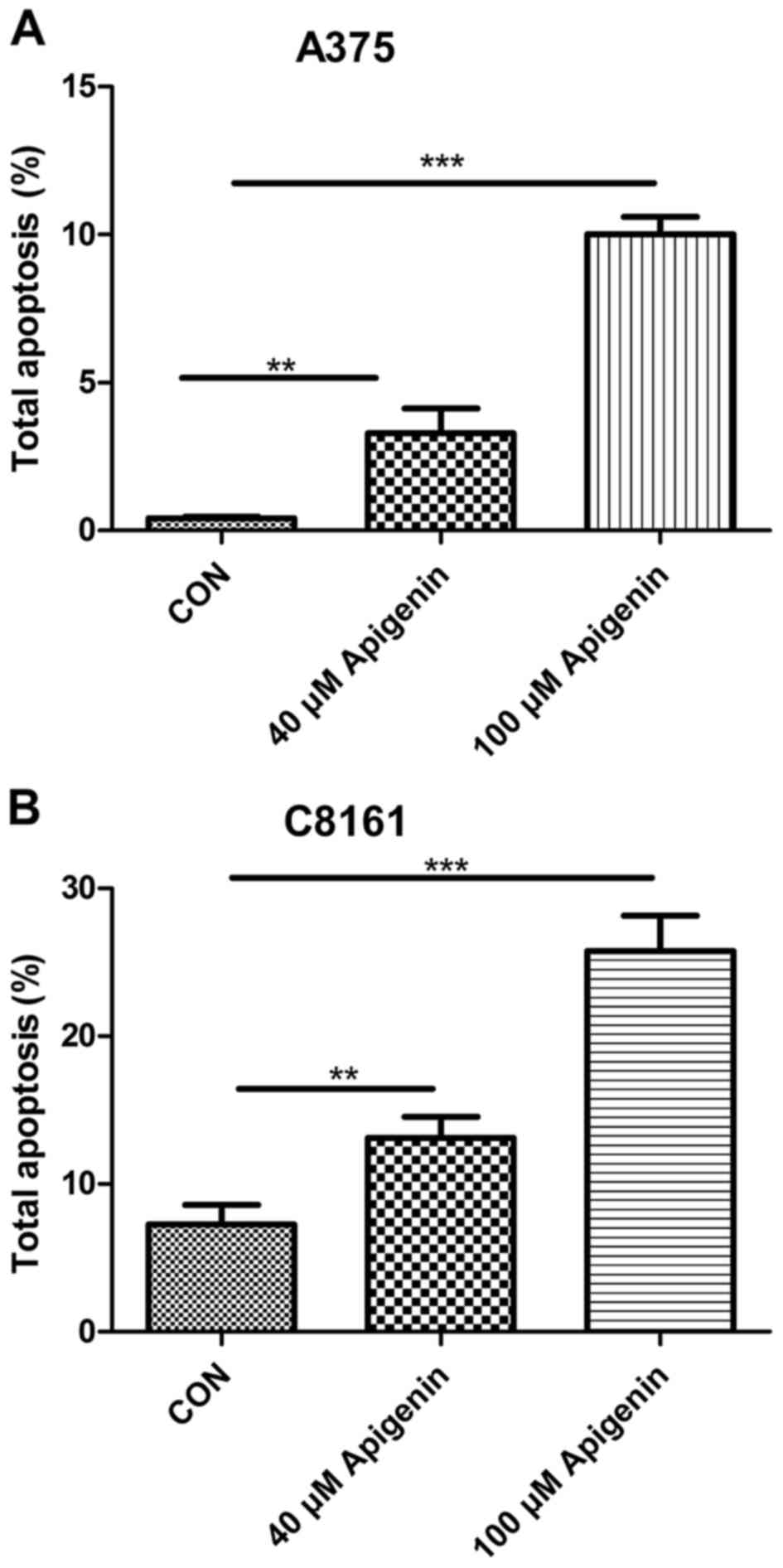
Apigenin promotes the apoptosis of
A375 and C8161 cells
To ascertain the underlying mechanism which leads to
apigenin-induced inhibition of cell proliferation, we observed the
effects of apigenin on the A375 and C8161 cells by detecting their
apoptotic rates. Significant apoptosis was found in both the A375
and C8161 cell lines. Treatment with apigenin (40 and 100 µM) for
24 h, resulted in higher apoptosis rates of the A375 and C8161
cells than the rates noted in the untreated control cells, and the
effects occurred in a dose-dependent manner (P<0.01, P<0.001)
(Fig. 5).
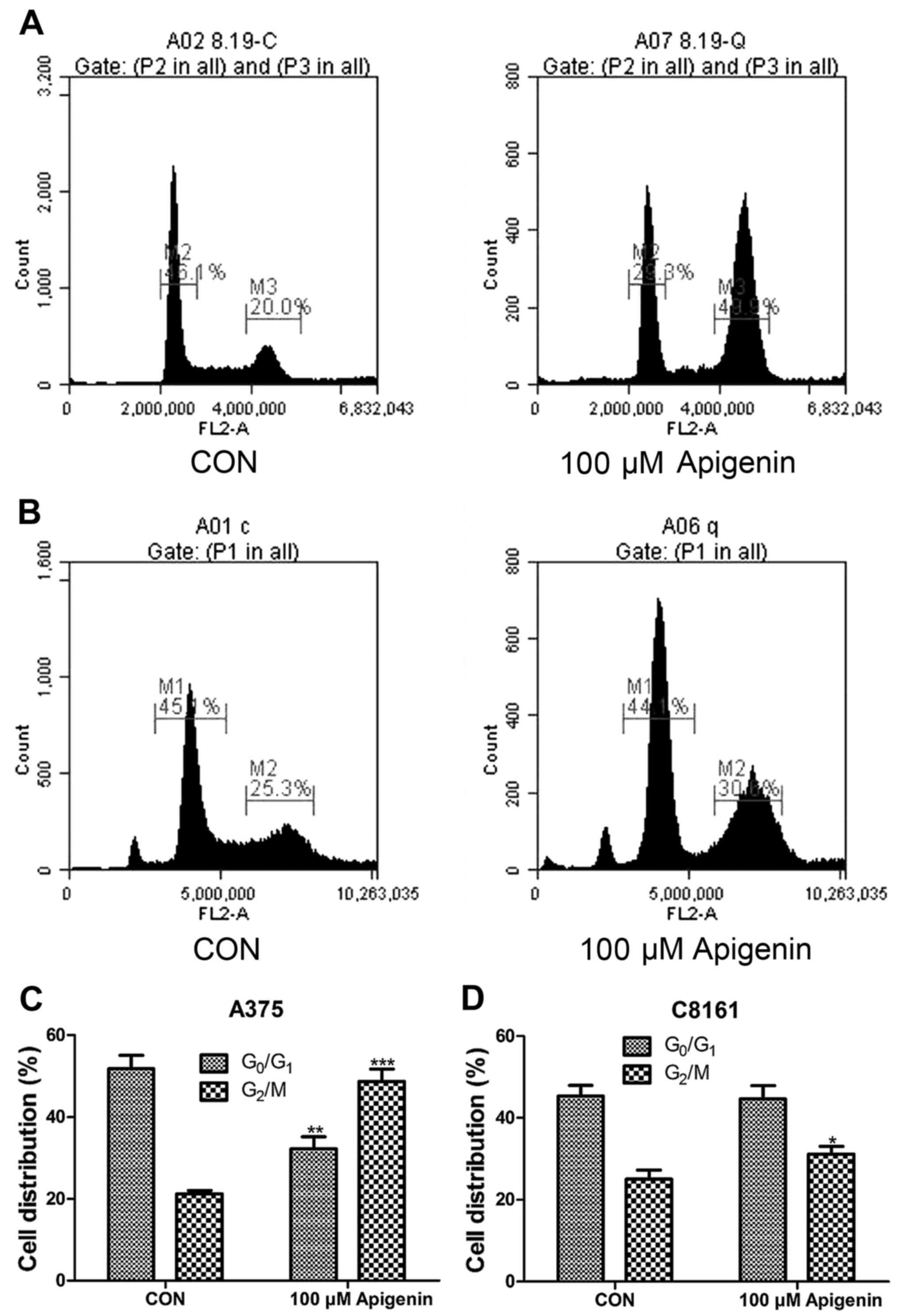
Apigenin arrests A375 and C8161 cells
at the G2/M phase of the cell cycle
Cell cycle analysis was also performed by flow
cytometry. A375 and C8161 cells were treated with 100 µM of
apigenin for 24 h. As shown in Fig.
6, apigenin treatment resulted in a noticeable accumulation of
cells in the G2/M phase with a decrease in the
G0/G1 phase during the cell cycle (P<0.05,
P<0.01, P<0.001). This blockage of cell progression may be
one of the mechanisms by which apigenin exerts its
anti-proliferative effect on melanoma cell lines.
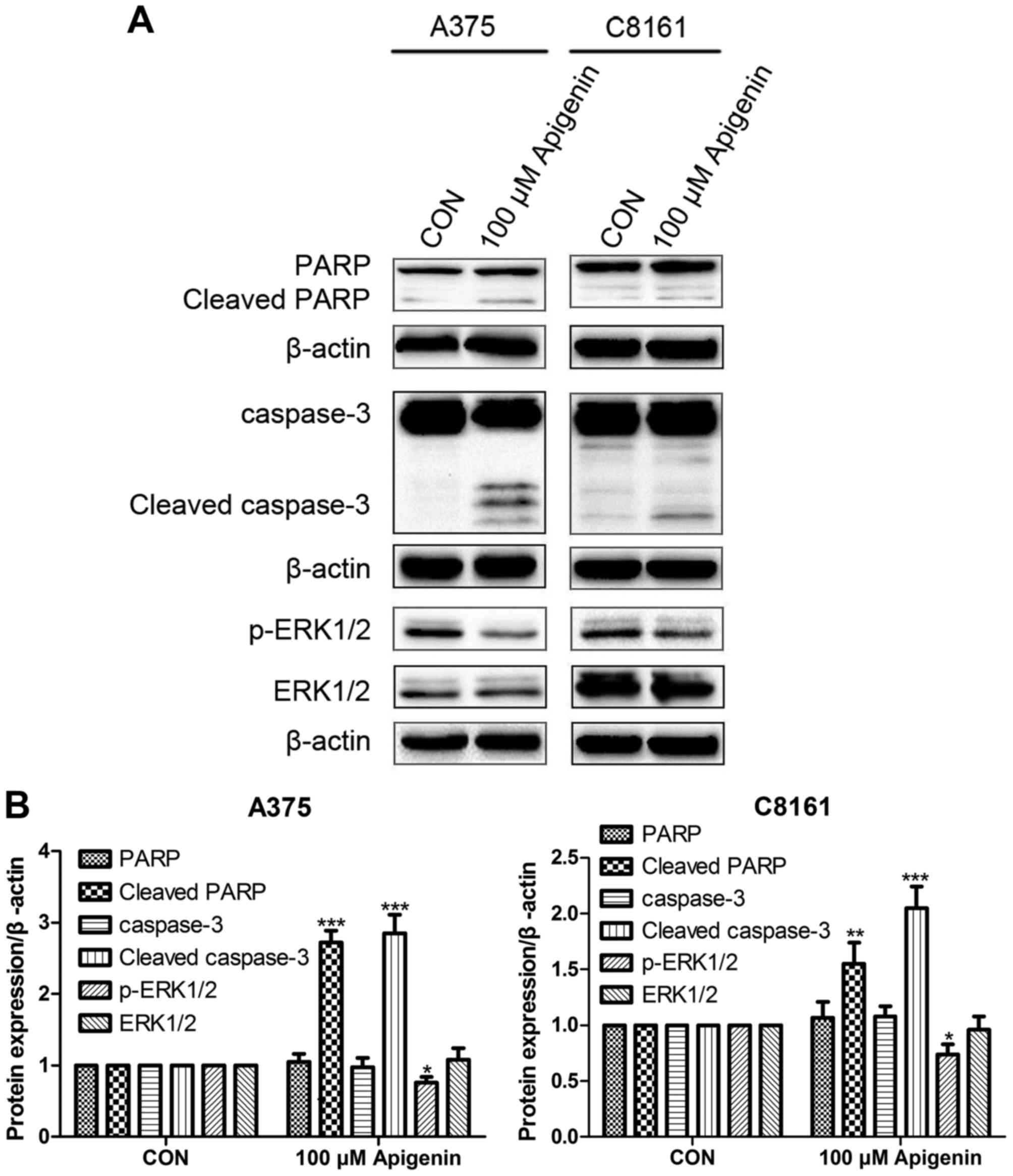
Apigenin alters ERK protein
expression
Western blot analysis showed that treatment with 100
µM of apigenin significantly increased the expression of cleaved
caspase-3 and cleaved PARP in the A375 and C8161 cells, while it
decreased the expression of p-ERK1/2 but did not alter the total
ERK1/2 level as compared to the DMSO controls (P<0.05,
P<0.01, P<0.001) (Fig.
7).
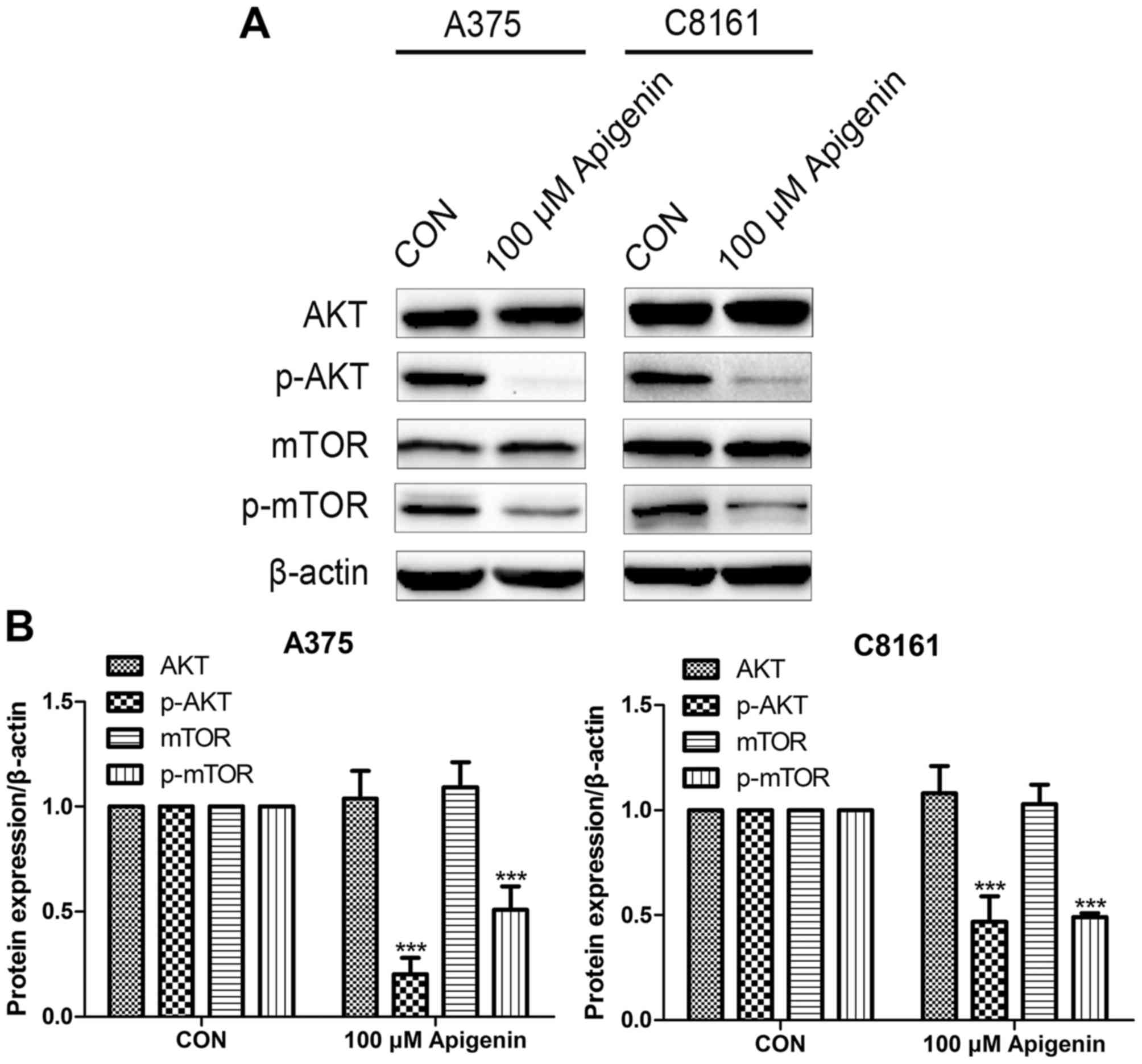
Apigenin inhibits the AKT signaling
pathway
Western blot analysis revealed that the expression
levels of phosphorylated AKT (p-AKT) and p-mTOR were decreased
after treatment with 100 µM of apigenin, whereas no significant
difference was observed in total AKT and mTOR, in comparison to the
DMSO controls (P<0.001) (Fig.
8).
Discussion
Melanoma is one of the most malignant cancers with a
propensity for metastases. The well-established conventional
treatments for melanoma, such as cryotherapy, surgery, and
chemotherapy (21) and some
nonsurgical treatments are usually limited to adjuvant therapies.
Therefore, increasing interest has focused on the search for
natural dietary phytochemicals both safe and effective against
melanoma. It is generally known that many compounds from natural
plants possess chemopreventative and chemotherapeutic efficacy in
human cancers including melanoma (22). Apigenin, a flavonoid belonging to
the flavone subgroup, is present in various vegetables, herbs,
fruits and Chinese traditional medications (9,23) and
has been shown to suppress tumor growth through inhibition of cell
proliferation (24).
In the present study, we investigated the
chemotherapeutic capacity of apigenin against human melanoma. We
selected the human melanoma A375 and C8161 cell lines which have a
different BRAF mutation status. A375 cells harbor the
BRAFV600E mutation while C8161 cells contain the
BRAF wild-type gene. Apigenin, as shown in this study,
significantly suppressed the growth of A375 and C8161 cells. These
data suggest that apigenin possesses chemotherapeutic potential
against human melanoma.
Dysregulation of the cell cycle is a hallmark of
tumorigenesis. The cell cycle is controlled at multiple
checkpoints. The G2/M checkpoint inhibits cells from
entering mitosis when DNA is impaired, enabling cell repair.
Pathways that result in apoptosis may be activated when the damage
is irreparable (25). The
G2/M checkpoint is controlled by Cdc2/cyclin B, as well
as their negative regulators including p21Cip1 and p27
(26). Regulating these
G2/M checkpoint proteins may enhance the sensitivity of
cancer cells to radiotherapy and chemotherapy (27). Therefore, the G2/M
checkpoint is a potential target for cancer therapy. It has been
reported that apigenin arrested human colon cancer HCT116 cells in
the G2/M phase. Moreover, it suppressed the expression
of both cyclin B1 and its activating partners, Cdc2 and Cdc25c
(28). In addition, apigenin
treatment led to a significant accumulation of cells in the
G2/M phase via the downregulation of Cdc25c expression
in human papillary thyroid carcinoma BCPAP cells (29). We found that apigenin suppressed the
growth of human melanoma A375 and C8161 cells by inducing
G2/M phase arrest of the cell cycle. Furthermore,
apigenin treatment decreased the expression of p-AKT and p-mTOR.
Previous studies have indicated that the AKT/mTOR pathway could
influence the progression of G2 to the mitosis phase
through the regulation of the expression of G2/M-related
proteins (30). Expression of the
active form of AKT increases Cdk1 both at the protein and mRNA
level, while its predominant negative mutation suppresses cell
proliferation by inducing G2/M arrest (31). Consequently, apigenin may inhibit
proliferation of A375 and C8161 cells by arresting G2/M
transition in the cell cycle probably via the AKT/mTOR pathway.
Invasion and metastasis are major concerns in the
prognosis and progression of cancer. The AKT/mTOR pathway is
pivotal in modulating the invasion and migration of tumor cells
(32). This pathway promotes
resistance to chemotherapy-induced apoptosis in a variety of
cancers including melanoma (33).
We found that 40 µM of apigenin significantly inhibited cell
migration and invasion. Furthermore, western blot analysis
demonstrated that the expression levels of p-AKT and p-mTOR were
decreased after apigenin treatment, while no significant difference
was observed in total AKT and mTOR. These results indicate that the
AKT/mTOR pathway plays an important role in the apigenin-induced
inhibition of migration and invasion of A375 and C8161 cells.
Erdogan et al (34) also
showed that apigenin reduced prostate cancer CD44+ stem
cell survival and migration through PI3K/AKT/NF-κB signaling.
Apoptosis, a type of programmed cell death, is a
physiological process essential for normal tissue development
(35). In mammals, there are two
primary apoptotic pathways: the extrinsic pathway (death
receptor-mediated pathway) and the intrinsic pathway
(mitochondrial-mediated pathway) (36). Caspase-3 is a key executioner
caspase and its activation leads to the cleavage of PARP during
cell death (37). Cleavage of PARP
is regarded as a central indicator of apoptosis. The ERK and AKT
signaling pathways are related to cell biological functions and
cancer malignancies which could also play an important role in the
proliferation and apoptosis of cancers. In our study, we confirmed
that apigenin treatment resulted in higher apoptosis rates of the
A375 and C8161 cells compared to the control cells in a
dose-dependent manner and significantly increased the expression of
cleaved caspase-3 and cleaved PARP, while it decreased the
expression of p-ERK1/2. The cell death occurring in the A375 and
C8161 cells treated with apigenin was probably induced by
apoptosis, which was possibly involved with ERK phosphorylation.
Pretreatment of A375 and C8161 cells with ERK inhibitors is
necessary to reveal whether apigenin-induced apoptosis is dependent
on ERK activity and we will investigate the relevant mechanism in
future research. Seo et al demonstrated that apigenin
induced apoptosis via the extrinsic pathway, increasing p53 and
inhibiting STAT3 and NF-κB signaling in HER2-overexpressing breast
cancer cells (38). Shukla et
al observed that apigenin induced apoptosis by targeting
inhibitor of apoptosis proteins and Ku70-Bax interaction in
prostate cancer (39). Das et
al (40) found that apigenin
induced apoptosis in A375 and A549 cells through selective action
and dysfunction of mitochondria, suggesting the activation of the
intrinsic apoptosis pathway. However, we did not ascertain whether
apigenin induced cell apoptosis through the intrinsic or extrinsic
pathway and this relevant research will be carried out in the
future. In addition, our western blot analysis showed that the
expression of p-AKT and p-mTOR was decreased after apigenin
treatment, while the expression of total AKT and mTOR was not
altered. This indicates that the AKT/mTOR pathway plays a vital
role in the apigenin-induced apoptosis observed in A375 and C8161
cells. Zhu et al (30)
demonstrated that apigenin induced apoptosis via the PI3K/AKT
pathway, the regulation of the Bcl-2 family and activation of
caspase-3 and PARP.
Recent studies have implicated glutamate signaling
in the development of melanoma (41–43).
The antagonists of ionotropic glutamate receptors (iGluRs) have
been demonstrated to cause a rapid and reversible change in
melanocyte morphology. Metabotropic glutamate 1 (mGlu1) receptor
has been proposed as a target for metastatic melanoma therapy
(44). It is expressed aberrantly
in over half of human melanoma cell lines and biopsies (45). In our previous study (46), we found that the antagonists mGlu1
receptor and N-methyl-D-aspartate (NMDA) receptor increased
dendritic branching and inhibited the motility, migration and
proliferation of the human metastatic melanoma cell line WM451. We
also demonstrated that the invasion and motility effects were
significantly inhibited by the combination of increased
microtubule-associated protein (MAP)2a (MAP2a) expression and
either an mGlu1 receptor or NMDA receptor antagonist. One plausible
explanation for this phenomenon is that the blockade of the
glutamate-mediated signaling pathway via the ERK1/2 pathway
suppresses cell motility and invasion through a tubulin-dependent
mechanism (47). In the present
study, we found that the main cytodendrites of human melanoma A375
and C8161 cells following treatment with apigenin became thinner
and longer than those of the controls. Moreover, treatment with
apigenin significantly suppressed cell invasion and migration. We
deduced that the aforementioned effects of apigenin may be induced
by blocking the glutamate-mediated signaling pathway, leading to
cytoskeletal protein reorganization and tumor cell differentiation.
These results suggest that the blockade of glutamate signaling is a
promising novel therapy for the treatment of melanoma.
In conclusion, apigenin is a potent suppressor of
cell viability, migration and invasion. Concomitantly, it induces
apoptosis in human melanoma A375 and C8161 cells, via activation of
caspase-3 and PARP, inhibition of ERK phosphorylation and the
AKT/mTOR pathway. Furthermore, it affects the dendrite morphology
of the A375 and C8161 cells, which might be involved with the
blockade of the glutamate signaling pathway. These findings need to
be supported by further experimental evidence. Consequently,
apigenin exhibits effective antineoplastic potency and provides a
hopeful treatment paradigm for melanoma.
Acknowledgements
We would like to thank Quentin Liu for guidance in
our study. The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81472865 and
81171491) and the Natural Science Foundation of Liaoning Province
(no. 201102056).
References
|
1
|
Liu J, Gu J, Feng Z, Yang Y, Zhu N, Lu W
and Qi F: Both HDAC5 and HDAC6 are required for the proliferation
and metastasis of melanoma cells. J Transl Med. 14:72016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agarwala SS: Current systemic therapy for
metastatic melanoma. Expert Rev Anticancer Ther. 9:587–595. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC Melanoma Staging and
Classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Z, Liu W and Gotlieb V: The rapidly
evolving therapies for advanced melanoma - Towards immunotherapy,
molecular targeted therapy, and beyond. Crit Rev Oncol Hematol.
99:91–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, Liu D, Huang Y, Gao Y and Qian S:
Biopharmaceutics classification and intestinal absorption study of
apigenin. Int J Pharm. 436:311–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding SM, Zhang ZH, Song J, Cheng XD, Jiang
J and Jia XB: Enhanced bioavailability of apigenin via preparation
of a carbon nanopowder solid dispersion. Int J Nanomedicine.
9:2327–2333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arsić I, Tadić V, Vlaović D, Homšek I,
Vesić S, Isailović G and Vuleta G: Preparation of novel
apigenin-enriched, liposomal and non-liposomal, antiinflammatory
topical formulations as substitutes for corticosteroid therapy.
Phytother Res. 25:228–233. 2011.PubMed/NCBI
|
|
8
|
Al Shaal L, Shegokar R and Müller RH:
Production and characterization of antioxidant apigenin
nanocrystals as a novel UV skin protective formulation. Int J
Pharm. 420:133–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel D, Shukla S and Gupta S: Apigenin
and cancer chemoprevention: Progress, potential and promise
(Review). Int J Oncol. 30:233–245. 2007.PubMed/NCBI
|
|
10
|
Shi MD, Shiao CK, Lee YC and Shih YW:
Apigenin, a dietary flavonoid, inhibits proliferation of human
bladder cancer T-24 cells via blocking cell cycle progression and
inducing apoptosis. Cancer Cell Int. 15:332015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suh YA, Jo SY, Lee HY and Lee C:
Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor
tyrosine kinases by apigenin circumvent taxol resistance in ovarian
cancer cells. Int J Oncol. 46:1405–1411. 2015.PubMed/NCBI
|
|
12
|
Shukla S, Bhaskaran N, Babcook MA, Fu P,
Maclennan GT and Gupta S: Apigenin inhibits prostate cancer
progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway.
Carcinogenesis. 35:452–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shukla S, Kanwal R, Shankar E, Datt M,
Chance MR, Fu P, MacLennan GT and Gupta S: Apigenin blocks IKKα
activation and suppresses prostate cancer progression. Oncotarget.
6:31216–31232. 2015.PubMed/NCBI
|
|
14
|
Scherbakov AM and Andreeva OE: Apigenin
inhibits growth of breast cancer cells: The role of ERα and
HER2/neu. Acta naturae. 7:133–139. 2015.PubMed/NCBI
|
|
15
|
Seo HS, Ku JM, Choi HS, Woo JK, Jang BH,
Go H, Shin YC and Ko SG: Apigenin induces caspase-dependent
apoptosis by inhibiting signal transducer and activator of
transcription 3 signaling in HER2-overexpressing SKBR3 breast
cancer cells. Mol Med Rep. 12:2977–2984. 2015.PubMed/NCBI
|
|
16
|
Caltagirone S, Rossi C, Poggi A,
Ranelletti FO, Natali PG, Brunetti M, Aiello FB and Piantelli M:
Flavonoids apigenin and quercetin inhibit melanoma growth and
metastatic potential. Int J Cancer. 87:595–600. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye Y, Chou GX, Wang H, Chu JH and Yu ZL:
Flavonoids, apigenin and icariin exert potent melanogenic
activities in murine B16 melanoma cells. Phytomedicine. 18:32–35.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pang W, Leng X, Lu H, Yang H, Song N, Tan
L, Jiang Y and Guo C: Depletion of intracellular zinc induces
apoptosis of cultured hippocampal neurons through suppression of
ERK signaling pathway and activation of caspase-3. Neurosci Lett.
552:140–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan TO, Rittenhouse SE and Tsichlis PN:
AKT/PKB and other D3 phosphoinositide-regulated kinases: Kinase
activation by phosphoinositide-dependent phosphorylation. Annu Rev
Biochem. 68:965–1014. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lopez RF, Lange N, Guy R and Bentley MV:
Photodynamic therapy of skin cancer: Controlled drug delivery of
5-ALA and its esters. Adv Drug Deliv Rev. 56:77–94. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eggler AL, Gay KA and Mesecar AD:
Molecular mechanisms of natural products in chemoprevention:
Induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res.
52:(Suppl 1). S84–S94. 2008.PubMed/NCBI
|
|
23
|
Shukla S and Gupta S: Apigenin: A
promising molecule for cancer prevention. Pharm Res. 27:962–978.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim MA, Kang K, Lee HJ, Kim M, Kim CY and
Nho CW: Apigenin isolated from Daphne genkwa Siebold et
Zucc. inhibits 3T3-L1 preadipocyte differentiation through a
modulation of mitotic clonal expansion. Life Sci. 101:64–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Ji P, Liu J, Broaddus RR, Xue F
and Zhang W: Centrosome-associated regulators of the
G2/M checkpoint as targets for cancer therapy. Mol
Cancer. 8:82009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dash BC and El-Deiry WS: Phosphorylation
of p21 in G2/M promotes cyclin B-Cdc2 kinase activity.
Mol Cell Biol. 25:3364–3387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stewart ZA, Westfall MD and Pietenpol JA:
Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol
Sci. 24:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee Y, Sung B, Kang YJ, Kim DH, Jang JY,
Hwang SY, Kim M, Lim HS, Yoon JH, Chung HY, et al: Apigenin-induced
apoptosis is enhanced by inhibition of autophagy formation in
HCT116 human colon cancer cells. Int J Oncol. 44:1599–1606.
2014.PubMed/NCBI
|
|
29
|
Zhang L, Cheng X, Gao Y, Zheng J, Xu Q,
Sun Y, Guan H, Yu H and Sun Z: Apigenin induces autophagic cell
death in human papillary thyroid carcinoma BCPAP cells. Food Funct.
6:3464–3472. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu Y, Mao Y, Chen H, Lin Y, Hu Z, Wu J,
Xu X, Xu X, Qin J and Xie L: Apigenin promotes apoptosis, inhibits
invasion and induces cell cycle arrest of T24 human bladder cancer
cells. Cancer Cell Int. 13:542013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SR, Park JH, Park EK, Chung CH, Kang
SS and Bang OS: Akt-induced promotion of cell-cycle progression at
G2/M phase involves upregulation of NF-Y binding
activity in PC12 cells. J Cell Physiol. 205:270–277. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin HP, Jiang SS and Chuu CP: Caffeic acid
phenethyl ester causes p21Cip1 induction, Akt signaling
reduction, and growth inhibition in PC-3 human prostate cancer
cells. PLoS One. 7:e312862012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Erdogan S, Doganlar O, Doganlar ZB,
Serttas R, Turkekul K, Dibirdik I and Bilir A: The flavonoid
apigenin reduces prostate cancer CD44+ stem cell
survival and migration through PI3K-Akt/NF-κB signaling. Life Sci.
162:77–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Renehan AG, Booth C and Potten CS: What is
apoptosis, and why is it important? BMJ. 322:1536–1538. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hassen S, Ali N and Chowdhury P: Molecular
signaling mechanisms of apoptosis in hereditary non-polyposis
colorectal cancer. World J Gastrointest Pathophysiol. 3:71–79.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seo HS, Choi HS, Kim SR, Choi YK, Woo SM,
Shin I, Woo JK, Park SY, Shin YC and Ko SG: Apigenin induces
apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3
and NFκB signaling in HER2-overexpressing breast cancer cells. Mol
Cell Biochem. 366:319–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shukla S, Fu P and Gupta S: Apigenin
induces apoptosis by targeting inhibitor of apoptosis proteins and
Ku70-Bax interaction in prostate cancer. Apoptosis. 19:883–894.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Das S, Das J, Samadder A, Boujedaini N and
Khuda-Bukhsh AR: Apigenin-induced apoptosis in A375 and A549 cells
through selective action and dysfunction of mitochondria. Exp Biol
Med (Maywood). 237:1433–1448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi KY, Chang K, Pickel JM, Badger JD II
and Roche KW: Expression of the metabotropic glutamate receptor 5
(mGluR5) induces melanoma in transgenic mice. Proc Natl Acad Sci
USA. 108:15219–15224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Khan AJ, Wall B, Ahlawat S, Green C,
Schiff D, Mehnert JM, Goydos JS, Chen S and Haffty BG: Riluzole
enhances ionizing radiation-induced cytotoxicity in human melanoma
cells that ectopically express metabotropic glutamate receptor 1 in
vitro and in vivo. Clin Cancer Res. 17:1807–1814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee HJ, Wall BA, Wangari-Talbot J, Shin
SS, Rosenberg S, Chan JLK, Namkoong Jin, Goydos JS and Chen S:
Glutamatergic pathway targeting in melanoma: Single-agent and
combinatorial therapies. Clin Cancer Res. 17:7080–7092. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gelb T, Pshenichkin S, Hathaway HA,
Grajkowska E, Dalley CB, Wolfe BB and Wroblewski JT: Atypical
signaling of metabotropic glutamate receptor 1 in human melanoma
cells. Biochem Pharmacol. 98:182–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee HJ, Wall BA, Wangari-Talbot J and Chen
S: Regulation of mGluR1 expression in human melanocytes and
melanoma cells. Biochim Biophys Acta. 1819:1123–1131. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Song Z, He CD, Liu J, Sun C, Lu P, Li L,
Gao L, Zhang Y, Xu Y, Shan L, et al: Blocking glutamate-mediated
signalling inhibits human melanoma growth and migration. Exp
Dermatol. 21:926–931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tanimura S, Uchiyama A, Watanabe K,
Yasunaga M, Inada Y, Kawabata T, Iwashita K, Noda S, Ozaki K and
Kohno M: Blockade of constitutively activated ERK signaling
enhances cytotoxicity of microtubule-destabilizing agents in tumor
cells. Biochem Biophys Res Commun. 378:650–655. 2009. View Article : Google Scholar : PubMed/NCBI
|