Introduction
The drug regorafenib is an orally available
multi-kinase inhibitor that has shown survival benefits in
metastatic colorectal cancer (mCRC) patients who had been
exacerbated after all standard therapies (1,2). The
CORRECT trial involving regorafenib demonstrated significant
survival benefits regarding progression-free survival (PFS) and
overall survival (OS) in mCRC patients after the failure of
standard therapy (1). The CONCUR
trial in 2015 also showed the survival benefits of regorafenib in
the Asian population (1). Although
significant survival benefits, together with safety in drug
management, have been reported in clinical trials, regorafenib is
not likely to be used in the clinic because of difficulty in its
management. Patients treated with regorafenib have displayed severe
adverse events (AEs) such as liver dysfunction and intolerable or
hardly manageable fatigue, resulting in discontinuation of the
treatment (3–6). These severe AEs would be unlikely to
occur in a restricted population, including patients with better
performance status in clinical trials, but could happen in clinical
practice. Therefore, it is crucial to exclude patients who are
likely to exhibit severe AEs and to select patients more likely to
benefit from regorafenib monotherapy. For this purpose, clinical
and molecular assessment was attempted for the selection of
candidates for regorafenib treatment.
The morphologic changes in tumors on enhanced CT
images were reported for the first time in 2009 in colorectal
cancer patients treated with bevacizumab (7); this drug blocked the activity of
vascular endothelial growth factor (VEGF) signaling concerning
tumor angiogenesis. Regorafenib also blocks this VEGF signaling via
inhibition of the activity in VEGFR1, VEGFR2 and VEGFR3, suggesting
that morphologic change could also be induced by regorafenib.
Consequently, it could be useful for assessing tumor response and
predicting treatment outcome. However, no study has attempted to
determine morphologic changes in tumors on CT images during
regorafenib monotherapy.
In addition, the dynamic change of genomic profiles
was monitored using liquid biopsy in this study. Liquid biopsy is a
blood-based technology platform that tracks circulating tumor DNA,
allowing multiple testing over time, monitoring of real-time
changes within the tumor and evaluation of therapeutic response.
There have been several studies that have reported the significance
of monitoring of genomic profiles such as EGFR in lung cancer
(8) and KRAS in colorectal
cancer patients (9–11). In the present study, we monitored
KRAS status in circulating tumor DNA (ctDNA), and evaluated
its significance concerning molecular assessment for the prediction
of treatment response and in aiding decision making in treatment
strategy.
Materials and methods
Patients
Twenty mCRC patients were recruited in the present
retrospective study. They underwent regorafenib monotherapy from
August in 2013 to January in 2016 at the Saitama Medical Center,
Jichi Medical University, Japan. Patients were aged >18 years,
and their Eastern Cooperative Oncology Group performance status
(PS) was 0, 1 or 2. This study was approved by the Research Ethics
Committee at Jichi Medical University. Written informed consent was
obtained from each study participant.
Assessment
Treatment response, incidence of AEs, PFS, OS and
tumor morphologic response on CT images were evaluated. PFS was
defined as the time from the start date of this therapy to the
first radiological or clinical observation (including elevation of
carcinoembryonic antigen) of disease progression (9–11). AEs
were graded according to the Common Terminology Criteria for
Adverse Events, version 4.0 (CTCAE v4.0). The tumor response or
progression was assessed every 3 months using CT or PET-CT using
the response evaluation criteria in solid tumors (RECIST) version
1.1, as well as the change in tumor morphology. Tumor morphology
was assessed using enhanced CT and characterized according to the
criteria previously described (9–11):
group 1, homogeneous low attenuation with a thin, sharply defined
tumor-liver interface; group 3, heterogeneous attenuation with a
thick, poorly defined tumor-liver interface; and group 2,
intermediate morphology that could be rated as either group 1 or 3
(Table I). A change in morphology
from group 3 or 2 to group 1 was defined as an optimal response
(Fig. 1), and a group 3 to group 2
change was defined as an incomplete response. The absence of marked
changes in tumor morphology was defined as no response. In patients
with multiple tumors, morphologic response was assigned based on
the changes observed in the majority of tumors. Response to
chemotherapy was also determined using RECIST.
 | Table I.Morphologic criteria. |
Table I.
Morphologic criteria.
| Morphology group | Overall
attenuation | Tumor-liver
interface | Peripheral rim of
enhancement |
|---|
| 3 | Heterogeneous | Ill defined | May be present |
| 2 | Mixed | Variable | If initially present,
partially resolved |
| 1 | Homogeneous and
hypoattenuating | Sharp | If initially present,
completely resolved |
Monitoring of circulating tumor DNA in
the blood
During treatment with regorafenib, 122 plasma
samples taken from 16 patients were available for the monitoring of
ctDNA in the blood. The KRAS status in ctDNA was determined
using the droplet digital polymerase chain reaction (ddPCR); this
technology provided absolute quantification of DNA with high
sensitivity. Seven targets of KRAS mutation including G12D,
G12V, G12C, G12R, G12A, G12S and G13D were assessed. Generation of
droplets was performed to partition the ddPCR reaction mix into
thousands of nanoliter-sized droplets. After PCR, droplets from
each sample were analyzed individually and read on a well by well
basis. The PCR-positive and PCR-negative droplets were counted to
provide absolute quantification of the target DNA in digital form.
A level of <0.1% of positive KRAS mutant circulating
tumor DNA was estimated as negative. Amplified products were
extracted from the droplets following PCR for Sanger sequencing. To
verify the mutation in ctDNA, amplified products were extracted
from the droplets following PCR for Sanger sequencing.
Statistical analysis
Fishers exact test was used to examine the
relationship between two categorical variables. Continuous
comparisons of variables between two groups were performed.
Student's t-test was used to evaluate those variables that followed
a normal distribution, and the non-parametric Mann-Whitney-Wilcoxon
test was used for those variables that did not follow a normal
distribution. The level of statistical significance was set at
P<0.05. Values are shown as the median and range. PFS and OS
data were plotted as Kaplan-Meier curves, and the differences among
the groups were compared using the log-rank test.
Results
Patient characteristics
The characteristics of the 20 patients, including 13
males and 7 females, are detailed in Table II. Patient median age was 67.5
(range, 49–76) years. Two patients had a PS of 2, and the remaining
patients had a PS of 0 or 1. Nine patients had colon cancer, and 11
patients had rectal cancer. KRAS analysis of tumor tissue
detected no mutation in 11 patients and mutation in 9 patients
including 2 patients with mutation of G13D. The median period from
diagnosis of metastases before regorafenib monotherapy was 20.8
(range, 4.6–43.0) months. All patients had various previous
treatments; single treatment was administered in 1 patient (5%),
two sequential treatments in 8 (40%) and three sequential
treatments in 11 (55%). Regarding previous treatment,
5-fluorouracil and irinotecan were used in all patients.
Oxaliplatin was used in 19 patients (95%), anti-VEGF antibody and
bevacizumab were administered in 18 (90%), and anti-EGFR
antibodies, panitumumab or cetuximab were used in 12 (60%)
(Table III). Ten patients (50%)
received follow on therapy with TAS102.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| Patients
background | Median (range)/n |
|---|
| Age (years) | 67.5 (49–76) |
| Gender |
|
| Male | 13 |
|
Female | 7 |
| ECOG PS |
|
|
<1 | 18 |
| 2 | 2 |
| Primary site of
disease |
|
|
Colon | 9 |
|
Rectum | 11 |
| KRAS status in
tumor tissue |
|
|
Wild-type | 11 |
|
Mutant | 9 |
| Number of previous
therapies |
|
| 1 | 1 |
| 2 | 8 |
| 3 | 11 |
| Time from diagnosis
of metastases (months) | 20.8 (5–43) |
 | Table III.Previous treatments. |
Table III.
Previous treatments.
| Previously used
drugs | n |
|---|
| 5-Fluorouracil | 20 |
| Oxaliplatin | 20 |
| Irinotecan | 20 |
| Bevacizumab | 18 |
|
Panitumumab/cetuximab | 12a,b |
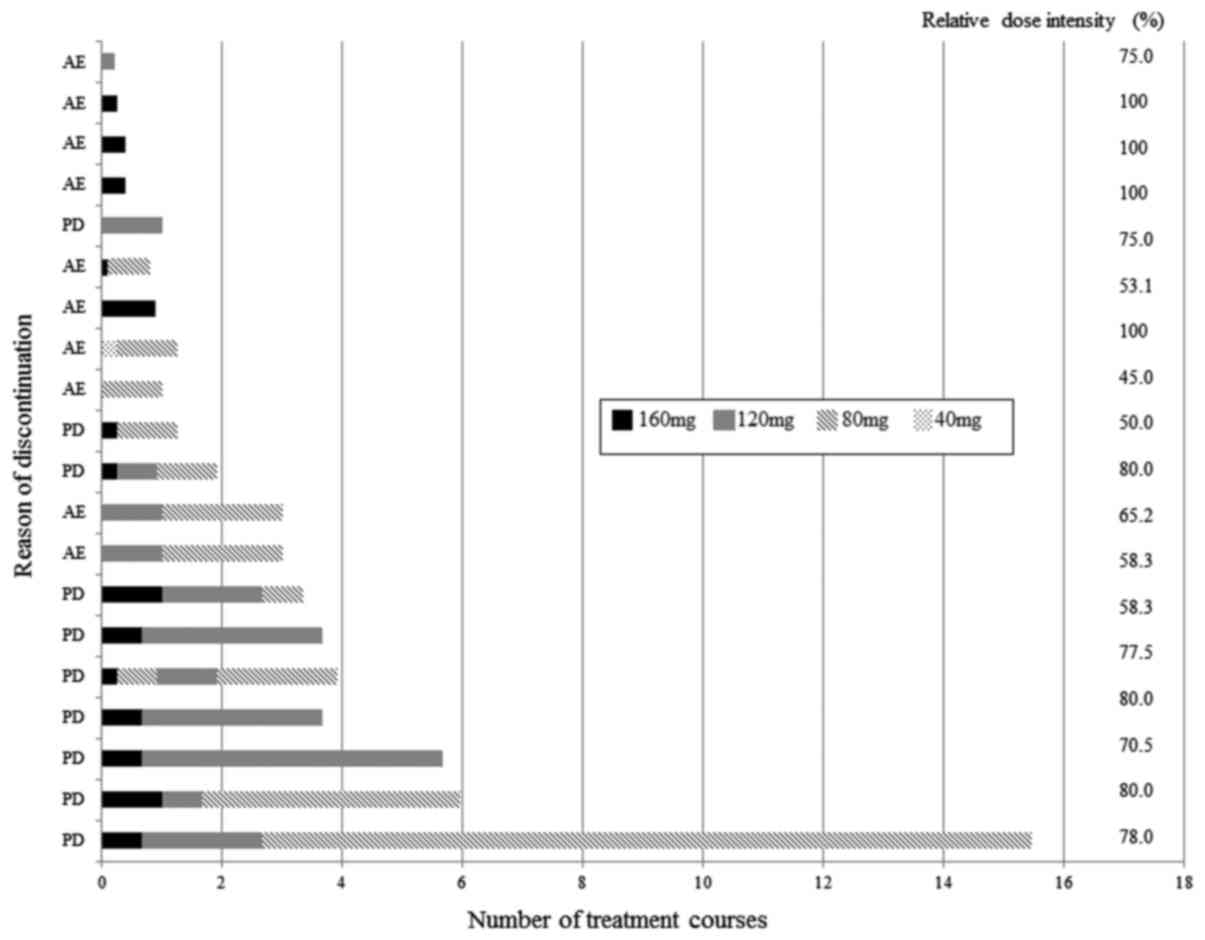
Treatment exposure
An initial dose of 160 mg was administered in 13
patients. Dose modification in the initial treatment was applied as
a result of poor PS or liver damage. Seven patients started with
modified initial doses, including 5 with 120 mg, 1 with 80 mg and 1
with 40 mg. Dose modification during treatment was performed
according to the grade of AE. The median relative dose intensity
was 76.3%. Dose modification of regorafenib during treatment is
shown in Fig. 2.
Efficacy and adverse events
There were no patients with a complete response or a
partial response, whereas 3 patients (15%) showed stable disease.
The median PFS and OS times were 2.5 months (range, 0.4–14.2) and
5.9 months (range, 1.1–20.9), respectively (Table III). Treatments were discontinued
in all patients. The most common AEs (≥30%) of any grade were
fatigue, hand-foot skin reaction, diarrhea, anorexia,
hyperbilirubinemia, anemia and dyspnea. The most common grade 3 or
higher AE (≥20%) was fatigue (Table
IV).
 | Table IV.Clinical outcome. |
Table IV.
Clinical outcome.
| Survival | Median
(months) | Range (months) |
|---|
| Progression-free
survival | 2.5 | 0.4–14.2 |
| Overall
survival | 5.9 | 1.1–20.9 |
Reasons for treatment
discontinuation
The reasons for treatment discontinuation and the
duration of administration of regorafenib are shown in Fig. 1. Ten patients (50%) had progressive
disease (PD) and 10 (50%) displayed AEs; the reasons for treatment
discontinuation were: ten patients who experienced AEs included 2
with hyperbilirubinemia, 5 with severe fatigue, and 3 with skin
rash. Two patients with hyperbilirubinemia had bilateral multiple
liver metastases before regorafenib treatment. Two of 5 patients
with intolerable or hardly manageable severe fatigue had a PS of 2
before regorafenib treatment. Successful sequential modification
was achieved in patients who did not show severe AEs; they had
longer treatment duration until discontinuation as a result of PD,
while adequate modification failed in patients with severe AEs.
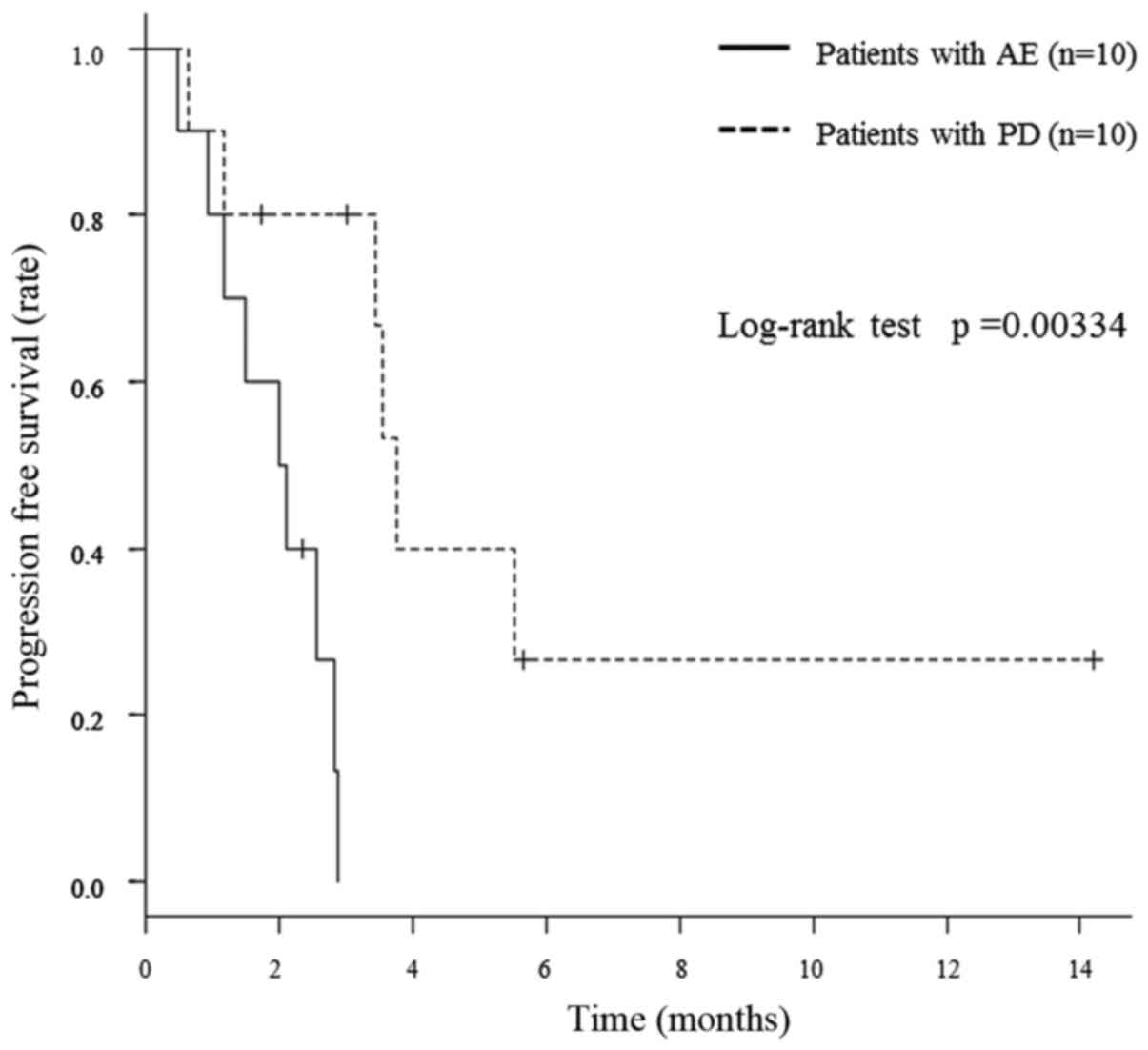
Consequently, patients with treatment discontinuation because of PD
achieved a significantly better PFS than those with treatment
discontinuation as a result of AEs (median PFS of 3.5 and 2.1
months, respectively; P=0.00334), in comparing PFS in terms of
discontinuation. Fig. 3 shows the
comparison of PFS between patients with treatment discontinuation
as a result of PD and those as a result of AEs.
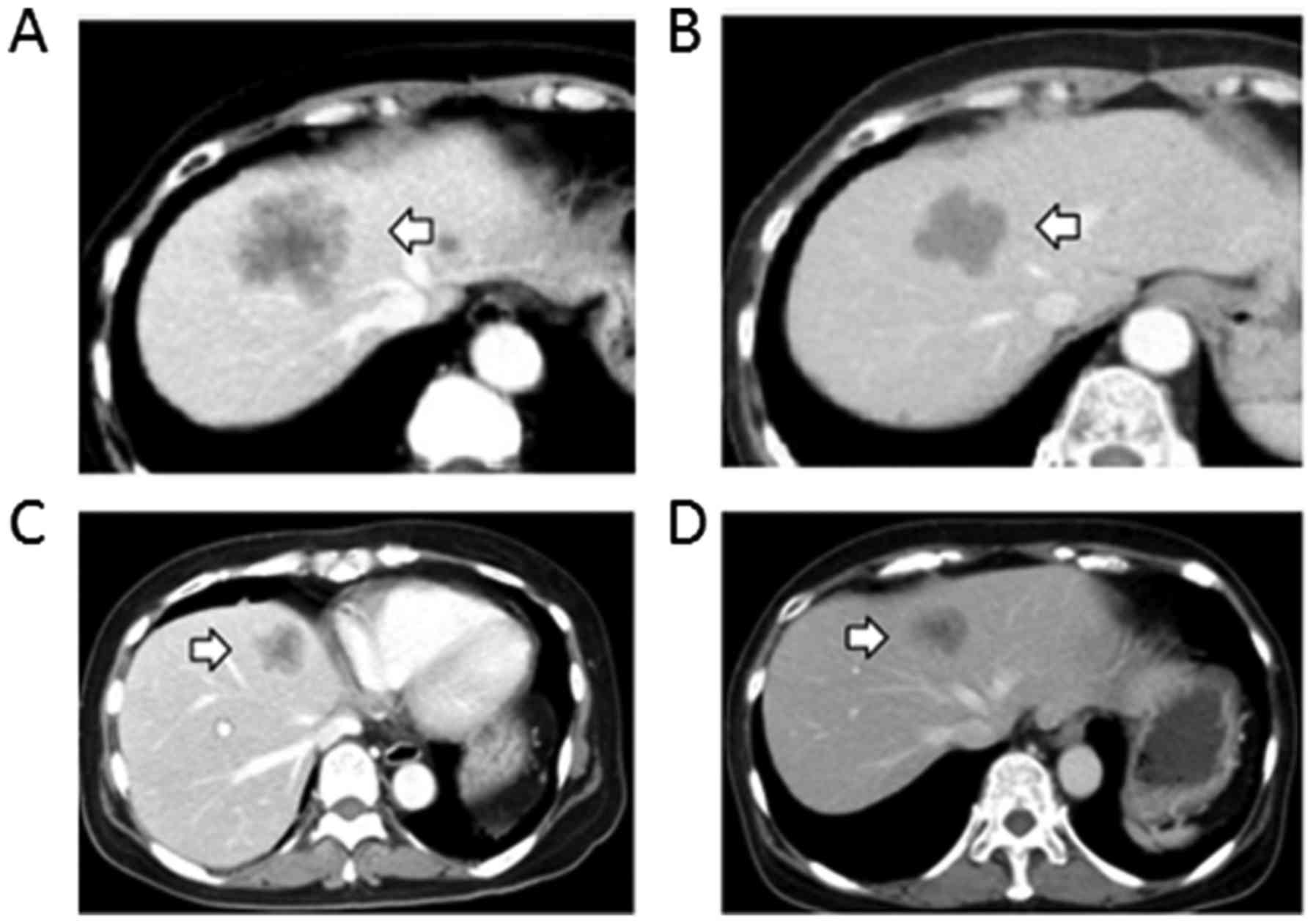
Morphologic changes on enhanced
CT
Four patients showed morphologic changes concerning
incomplete response on CT images (Fig.
4); in these 4 patients, there were no morphologic changes
during bevacizumab treatment. All responses during regorafenib
treatment were incomplete; however, the patients with an incomplete
response had a longer PFS than the median PFS of 3.5 months in
patients who experienced treatment discontinuation because of PD.
These changes were detected within 2–3 months from the beginning of
regorafenib treatment.
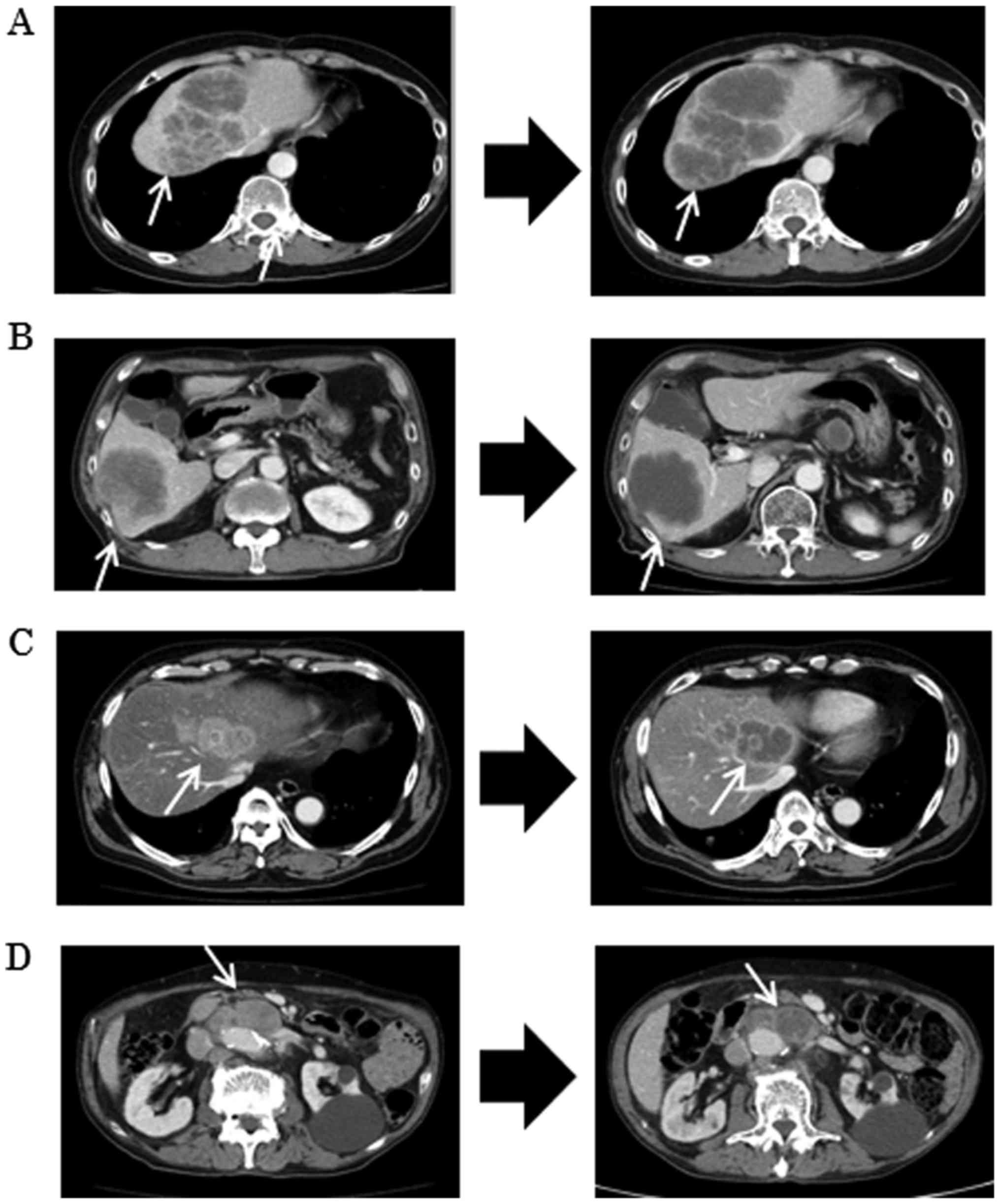
KRAS status in circulating tumor
DNA
The emergence of the KRAS mutation in ctDNA
was observed in 3 patients among 11 patients without the
KRAS mutation in the tumor tissue. Fig. 5 shows the change in KRAS
status in ctDNA along with the sequential treatments in these three
patients. The emergence of the KRAS mutation in ctDNA was
observed during anti-EGFR antibody therapy. The KRAS
mutation in ctDNA was detectable in the blood prior to radiographic
detection of PD. Moreover, the mutation in ctDNA in 2 out of 3
patients declined during regorafenib treatment. These 2 patients
underwent anti-EGFR antibody as re-challenge treatment with the
anti-EGFR antibody; 1 patient exhibited the KRAS mutation in
ctDNA again after treatments with the anti-EGFR antibody.
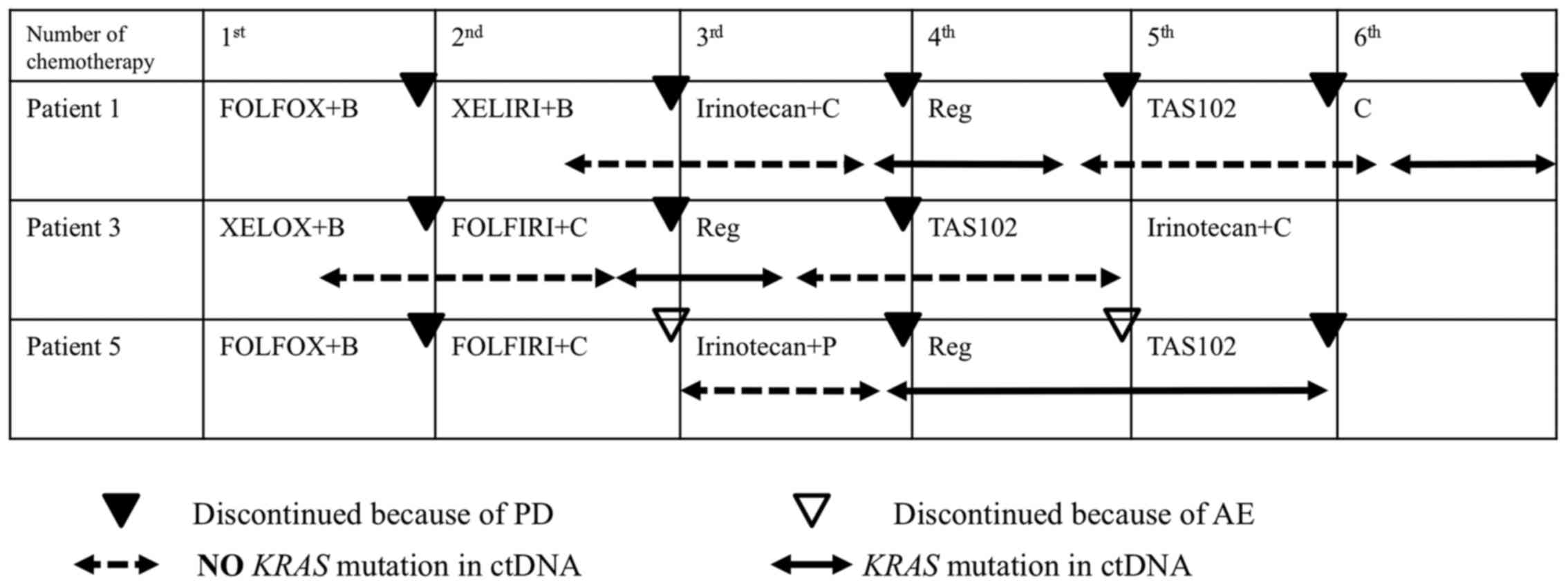 | Figure 5.Emergence of the KRAS mutation
in ctDNA in 3 patients without the KRAS mutation in the
tumor tissue. Patient 1 is a 50-year-old woman with rectal cancer
(the patient shown in Fig. 4A). The
KRAS mutation in ctDNA was detected during the 3rd line
therapy using irinotecan and cetuximab prior to radiological
detection of disease progression (black triangle). Emergence of the
KRAS mutation was shown from the dashed arrow to the solid
arrow. Moreover, the KRAS mutation in ctDNA disappeared
during treatments with regorafenib and TAS102 (from the solid arrow
to the dashed arrow). This patient was, therefore, treated with
cetuximab as re-challenge treatment with the anti-EGFR antibody.
The KRAS mutation in ctDNA was then detected again during
the treatment, resulting in discontinuation of treatment because of
disease progression. Patient 3 (the patient shown in Fig. 4C) is a 65-year-old man with rectal
cancer. KRAS mutation in ctDNA was detected during the 2nd
line therapy using FOLFIRI (5-fluorouracil + irinotecan) and
cetuximab prior to radiological detection of disease progression.
The KRAS mutation in ctDNA disappeared during treatments
with regorafenib and TAS102. This patient was being treated with
cetuximab as re-challenge treatment with the anti-EGFR antibody.
Patient 5 (not shown before) is a 53-year-old woman with colon
cancer. The KRAS mutation in ctDNA was detected during the
3rd line therapy using irinotecan and panitumumab prior to
radiological detection of disease progression. The KRAS
mutation in ctDNA did not disappear during treatments with
regorafenib and TAS102, resulting in discontinuation of treatment
because of disease progression. The dashed arrow represents the
period without KRAS mutation in ctDNA, and the solid arrow
represents the period with the KRAS mutation in ctDNA. The
black triangle indicates the point at which radiological detection
of disease progression was recognized. The white arrow shows the
point at which treatment was discontinued because of adverse
events. B, bevacizumab; P, panitumumab; C, cetuximab; Reg,
regorafenib; FOLFOX, 5-fluorouracil and oxaliplatin; XELOX,
capecitabine and oxaliplatin; FOLFIRI, 5-fluorouracil and
irinotecan; XELIRI, capecitabine and irinotecan. |
Discussion
The present study revealed that patients with poor
PS or multiple bilateral liver metastases are more likely to show
hardly manageable severe AEs resulting in the discontinuation of
regorafenib monotherapy. These patients displayed poor prognosis
with an extremely short PFS of 2.1 months. In contrast, patients
whose tumors exhibited morphological change had a median PFS that
was as long as or much longer than 3.5 months. These patients
probably benefited from regorafenib; thus, they should receive
adequate management and treatment should not be discontinued
because of AEs. Furthermore, KRAS monitoring in ctDNA could
be useful for the assessment of treatment response and decision
making regarding treatment strategy.
Comparison of the profile of AEs in the present
study with that in the CORRECT trial revealed that a similar
profile in AEs was seen in both. The most common grade 3 or higher
AEs were fatigue, skin reaction and hyperbilirubinemia. These AEs
were manageable by modification of the dose and/or treatment
periods for most of the patients. AEs such as the hand-foot skin
reaction, rash and fatigue are most likely to occur during the
first or second treatment cycles. Some reports have explored the
initiation of regorafenib at a reduced dose as a means of avoiding
early toxic effects (1,2,12). In
the privious study, successful modification during the early period
of treatment was achieved in patients with treatment
discontinuation caused by PD. They did not show severe AEs, which
resulted in a longer PFS time than that in patients with treatment
discontinuation caused by AEs (Figs.
1 and 2). However, severe
fatigue and hyperbilirubinemia in patients with a poor PS and
multiple bilateral liver metastases, respectively, were hardly
manageable and resulted in discontinuation of regorafenib in a
short period; these patients were unlikely candidates for
regorafenib monotherapy.
In the present study, we attempted to evaluate the
morphologic change in tumors on CT images and identify patients
likely to benefit from regorafenib. Patients with morphologic
changes in their tumors displayed better prognosis with a longer
PFS of >3 months. This morphologic change was most likely to
occur within 2–3 months of regorafenib treatment; therefore,
initial evaluation using CT imaging could provide important
information such as treatment outcome in patients likely to benefit
from regorafenib. The criteria based on morphologic changes had a
significant association with pathologic response and prognosis in
mCRC patients with liver metastasis, who underwent chemotherapy
including bevacizumab (13). It has
been reported that a change in tumor morphology, as determined
using CT imaging, presented as vascular reconstruction induced by
bevacizumab (14). Tumor
morphologic response correctly predicted the pathological changes
produced by the antitumor effect of VEGF signaling inhibitor; this
indicated that it had predictive value in the prognosis of the
patients treated with regorafenib as well as bevacizumab (15). We believed that patients with a
better response in the first or second line treatment with
bevacizumab would also show better response to treatment with
regorafenib, but no association regarding the response was seen.
This may have been the result of the difference between the
inhibition of VEGF-A in bevacizumab and the multi-kinase inhibitor
effect of regorafenib.
The dynamic change in the genomic profiles including
KRAS status was monitored in the present study using liquid
biopsy. We and other groups (7,12,15,16)
have reported that colorectal cancer patients with KRAS
wild-type in the tumor display the KRAS mutation in ctDNA
during several treatments, including anti-VEGF and EGFR antibody.
As we expected, the emergence of the KRAS mutation in ctDNA
was observed in three patients during treatment with anti-EGFR
antibody. The KRAS mutation in ctDNA in 3 patients was
detectable in the blood prior to radiographic detection of PD,
suggesting that KRAS monitoring could provide significant
information concerning drug resistance before routine check-up on
CT (9,10,17).
Furthermore, we showed for the first time the disappearance of the
KRAS mutation in ctDNA in 2 of 3 patients with the
KRAS mutation in ctDNA during regorafenib treatment; this
indicated the recovery of drug sensitivity to anti-EGFR antibody.
These patients underwent re-challenge treatment with the anti-EGFR
antibody. For this challenge, regorafenib is the important key drug
harboring the power to alter KRAS status with a long period
of response. Notably, 1 patient showed KRAS mutation in
ctDNA again after treatment with the anti-EGFR antibody. These
changes in KRAS status in ctDNA represent the alteration in
genomic profile during treatment, suggesting that KRAS
monitoring in the blood could be a biomarker not only for treatment
response but also in decision making regarding treatment
strategy.
In conclusion, our data could provide insights into
the clinical value of selecting patients likely to benefit from
regorafenib monotherapy. It is important, however, to interpret our
results within the context of the study limitations and further
studies will be required to draw definitive conclusions.
Acknowledgements
The present study was supported in part by a
grant-in-aid of post graduate students from the Jichi Medical
University, a grant-in-aid from the Ministry of Education, Culture,
Sports, Science and Technology, and the JKA Foundation through its
promotional funds from Keirin Race.
References
|
1
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: CORRECT Study Group: Regorafenib monotherapy for previously
treated metastatic colorectal cancer (CORRECT): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:303–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu
J, Bai Y, Chi Y, Wang L, et al: CONCUR Investigators: Regorafenib
plus best supportive care versus placebo plus best supportive care
in Asian patients with previously treated metastatic colorectal
cancer (CONCUR): A randomised, double-blind, placebo-controlled,
phase 3 trial. Lancet Oncol. 16:619–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demetri GD, Reichardt P, Kang YK, Blay JY,
Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M,
Joensuu H, et al: GRID study investigators: Efficacy and safety of
regorafenib for advanced gastrointestinal stromal tumours after
failure of imatinib and sunitinib (GRID): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:295–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Funakoshi T, Latif A and Galsky MD: Safety
and efficacy of addition of VEGFR and EGFR-family oral
small-molecule tyrosine kinase inhibitors to cytotoxic chemotherapy
in solid cancers: A systematic review and meta-analysis of
randomized controlled trials. Cancer Treat Rev. 40:636–647. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krishnamoorthy SK, Relias V, Sebastian S,
Jayaraman V and Saif MW: Management of regorafenib-related
toxicities: A review. Therap Adv Gastroenterol. 8:285–297. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McLellan B, Ciardiello F, Lacouture ME,
Segaert S and Van Cutsem E: Regorafenib-associated hand-foot skin
reaction: practical advice on diagnosis, prevention, and
management. Ann Oncol. 26:2017–2026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chun YS, Vauthey JN, Boonsirikamchai P,
Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H,
Charnsangavej C, et al: Association of computed tomography
morphologic criteria with pathologic response and survival in
patients treated with bevacizumab for colorectal liver metastases.
JAMA. 302:2338–2344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maheswaran S, Sequist LV, Nagrath S, Ulkus
L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ,
Bell DW, et al: Detection of mutations in EGFR in circulating
lung-cancer cells. N Engl J Med. 359:366–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diaz LA Jr, Williams RT, Wu J, Kinde I,
Hecht JR, Berlin J, Allen B, Bozic I, Reiter JG, Nowak MA, et al:
The molecular evolution of acquired resistance to targeted EGFR
blockade in colorectal cancers. Nature. 486:537–540.
2012.PubMed/NCBI
|
|
10
|
Misale S, Yaeger R, Hobor S, Scala E,
Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M,
Siravegna G, et al: Emergence of KRAS mutations and acquired
resistance to anti-EGFR therapy in colorectal cancer. Nature.
486:532–536. 2012.PubMed/NCBI
|
|
11
|
Siravegna G, Mussolin B, Buscarino M,
Corti G, Cassingena A, Crisafulli G, Ponzetti A, Cremolini C, Amatu
A, Lauricella C, et al: Clonal evolution and resistance to EGFR
blockade in the blood of colorectal cancer patients. Nat Med.
21:795–801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tabchi S and Ghosn M: Regorafenib: Start
low and go slow. Target Oncol. 10:445–447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klinger M, Tamandl D, Eipeldauer S, Hacker
S, Herberger B, Kaczirek K, Dorfmeister M and Gruenberger BT:
Bevacizumab improves pathological response of colorectal cancer
liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol.
17:2059–2065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
OConnor JP, Carano RA, Clamp AR, Ross J,
Ho CC, Jackson A, Parker GJ, Rose CJ, Peale FV, Friesenhahn M, et
al: Quantifying antivascular effects of monoclonal antibodies to
vascular endothelial growth factor: insights from imaging. Clin
Cancer Res. 15:6674–6682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki K, Muto Y, Ichida K, Fukui T,
Takayama Y, Kakizawa N, Kato T, Hasegawa F, Watanabe F, Kaneda Y,
et al: Morphologic response contributes to patient selection for
rescue liver resection of chemotherapy patients with initially
unresectable colorectal liver metastasis. Oncol Lett. (In
press).
|
|
16
|
Mross K, Frost A, Steinbild S, Hedbom S,
Buchert M, Fasol U, Unger C, Kratzschmar J, Heinig R, Boix O, et
al: A phase I dose-escalation study of regorafenib (BAY 73–4506),
an inhibitor of oncogenic, angiogenic, and stromal kinases, in
patients with advanced solid tumors. Clin Cancer Res. 18:2658–2667.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nielsen DL, Palshof JA, Larsen FO, Jensen
BV and Pfeiffer P: A systematic review of salvage therapy to
patients with metastatic colorectal cancer previously treated with
fluorouracil, oxaliplatin and irinotecan +/− targeted therapy.
Cancer Treat Rev. 40:701–715. 2014. View Article : Google Scholar : PubMed/NCBI
|