Introduction
Among all cell types in the tumor microenvironment,
immune system cells are involved in the strongest interaction with
tumor cells as a result of the primary necessity of cancer cells to
maintain immune cells in a suppressed state to ensure tumor growth
and development (1–3). Thus, tumor cells release paracrine
signals such as pro-tumor cytokines (IL-4 and TGF-β) that induce
the ‘education’ of cells in the tumor microenvironment converting
these initial ‘antitumor fighters’ into ‘protumor slaves’. Among
the immune cell populations present in tumor tissue, ‘the educated’
macrophages, so-called tumor-associated macrophages (TAMs) seem
most important in supporting tumor growth via coordination of
angiogenesis, inflammation, oxidative stress, invasion, and the
metastatic capacity of tumors (3–5). The
protumor education of macrophages is facilitated by high phenotypic
plasticity of this cell type that has multiple intermediary
phenotypes ranging from the antitumor M1 or ‘classically activated’
macrophage type (characterized mainly by high levels of IL-12 and
low levels of IL-10) to the protumor, M2 type (characterized mainly
by high levels of IL-10 and low levels of IL-12) specific for TAMs
(6,7). Thus, TAMs are known to be an important
source of pro-inflammatory molecules (TNFα, IL-1, 6, 8, 10, 12p40,
MCP-1 and PGE2), pro-angiogenic proteins (VEGF, bFGF,
IGF, TNF-α and MMPs) as well as immunosuppressive cytokines (IL-10
and TGF-β) (2,3,5,8).
Moreover, TAM-induced chronic inflammation produces reactive oxygen
species (ROS) with a key role in the activation of redox sensitive
transcription factors (NF-κΒ, AP-1 and Egr-1) that orchestrate all
processes involved in tumor progression such as immunosuppression,
cell proliferation, angiogenesis, and metastasis (9–15).
Nevertheless, the role of TAMs in the development of
colon carcinoma is still controversial as the presence of
infiltrated macrophages in the tumor microenvironment is associated
with good (16–18), as well as poor prognosis (19).
Therefore, the present study aimed to investigate
the main TAM-driven processes that can affect colon carcinoma
development. In this respect, we aimed to ascertain how
TAM-mediated angiogenesis, inflammation, and oxidative stress can
affect the proliferative capacity of C26 murine colon carcinoma
cells, when they are co-cultivated with TAMs in vitro. To
gain insight into the mechanisms by which TAMs influence the
proliferation of C26 cells, key molecules involved in the
coordination of the protumor processes presented above were
screened. Our results demonstrated that TAM-regulated oxidative
stress is the main process that affects C26 colon carcinoma cell
proliferation via the activity of macrophage NADPH oxidase that
maintains the physiological range of the proliferative levels of
reactive oxygen species (ROS) and enhances the production of
angiogenic proteins in the tumor microenvironment.
Materials and methods
Cell line and culture conditions
C26 murine colon carcinoma cells (Cell Lines Service
GmbH, Eppelheim, Germany) were cultured in RPMI-1640 medium (Lonza,
Group AG, Basel, Switzerland), supplemented with 10%
heat-inactivated fetal bovine serum (FBS) (HyClone, GE Healthcare
Life Sciences, Logan, UT, USA), as a monolayer at 37̊C in a
humidified atmosphere containing 5% CO2.
Co-culture of C26 tumor cells and
macrophages
Macrophages were freshly harvested from the
peritoneal cavity of 6- to 8-week BALB/c mice (Cantacuzino
Institute, Bucharest, Romania) that had been intraperitoneally
injected with 1 ml of 3% thioglycollate (Sigma-Aldrich Chemie GmbH,
Munich, Germany). After 3 days, elicited macrophages were isolated
by intraperitoneal lavage as previously reported (20). Co-cultures were prepared by seeding
C26 tumor cell suspensions on macrophage monolayers; the density
ratio between macrophages and tumor cells in co-cultures being 1:4.
Previous studies have proved that this cell density ratio ensures
the optimal cytokine interplay between tumor cells and macrophages
that provides an approximation of physiological conditions for
colon carcinoma development in vivo (21). Experiments were performed according
to national regulations and were approved by the University Animal
Experiments Ethics Committee (registration no.
31375/06.04.2015).
Cell proliferation assay
The proliferative capacity of the C26 colon
carcinoma cells was evaluated for the standard culture of C26 cells
as well as for the co-culture of C26 cells with murine peritoneal
macrophages. Thus, C26 cells (1×103/well) cultured alone
or along with peritoneal macrophages at a density ratio of 1:4,
were seeded into 96-well plates and incubated for 48 h. The
proliferation rate was expressed as number of absorbance
units/hours of incubation (22) and
tested using ELISA BrdU-colorimetric immunoassay (Roche Applied
Science, Penzberg, Germany) according to the manufacturer's
instructions as previously described (23). This method is based on the
incorporation of bromodeoxyuridine (BrdU), a pyridine analogue,
instead of thymidine into the DNA of the proliferating cells. C26
colon carcinoma cells were incubated with BrdU solution for 24 h
and the culture medium was completely removed from each well.
Following this step, the cells were fixed and the DNA was
denatured. The incorporated BrdU in the newly synthesized cellular
DNA was detected by adding a monoclonal antibody anti-BrdU-POD to
each well, conjugated with peroxidase. The antibody was removed
after 1 h of incubation, and the cells were washed 3 times with
phosphate-buffered saline. A peroxidase substrate
(tetramethyl-benzidine) was added to each well, and the immune
complexes were detected by measuring the absorbance of the reaction
product at 450 nm with a reference wavelength of 655 nm.
Preparation of cell culture
lysates
Cells cultures were lysed with lysis buffer
containing 10 mM HEPES (pH 7.0), 200 mM NaCl, 1% Triton X-100, 10
mM MgCl2, 1 mM dithiothreitol and protease inhibitor
cocktail tablets (cOmplete; Roche Diagnostics GmbH, Mannheim,
Germany). The homogenate was incubated for 30 min on ice, and then
centrifuged for 10 min at 12,000 × g, at 4̊C, and the supernatant
was collected and stored at −80̊ for further molecular
measurements. For nuclear extraction, cell cultures were lysed with
extraction buffer containing 20 mM HEPES (pH 7.6), 20% glycerol
(v/v), 10 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1%
Triton X-100 (v/v), 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride
(PMSF), 10 µg/ml leupeptin, 10 µg/ml pepstatin, 100 µg/ml
aprotinin, and phosphatase inhibitor PhosSTOP tablets (Roche
Diagnostics GmbH). Then, cell lysates were centrifuged for 5 min at
2,500 × g, at 4̊C, and the pellet was resuspended in extraction
buffer supplemented with NaCl up to 500 mM (24). After 1 h of incubation on the
rocking platform, at 4̊C, the nuclei were lysed and separated by
centrifugation (10 min at 18,000 × g, at 4̊C). The supernatants
were retained (nuclear fractions) and the protein
concentration was determined by the Bradford assay (Sigma-Aldrich
Chemie GmbH) (25).
Western blot analysis of NF-κΒ
levels
To determine the link between the levels of the
inflammatory transcription factor NF-κΒ (nuclear factor-κB)
(26) and C26 cell proliferation,
we performed western blot analysis for the expression of the active
form of NF-κΒ [when the p65 subunit of NF-κΒ is phosphorylated
(pNF-κΒ-p65)] as well as expression of the total NF-κΒ-p65 subunit
(pNF-κΒ-p65 and inactive form of NF-κΒ when p65 subunit is
unphosphorylated form of NF-κΒ). To this aim, the levels of
pNF-κΒ-p65 in nuclear extracts were determined and expressed as the
percentage of the total amount of NF-κB in the whole cell lysates.
Thus, 25 µg/well of total protein from each lysate were loaded onto
a 10% polyacrylamide gel. Electrophoresis was performed at 95 mV
and then the protein fractions were electro-transferred onto a
nitrocellulose membrane at 100 mV for 40 min. The membranes were
blocked overnight at 4̊C with 5% skimmed milk powder (Bio-Rad
Laboratories, Hercules, CA, USA) in Tris-buffered saline containing
0.1% Tween-20 (TBS-T), under constant shaking. After that, the
membranes were incubated for 2 h at room temperature with either
monoclonal mouse IgG anti-mouse NF-κΒ p65 primary antibody or
polyclonal rabbit IgG anti-mouse pNF-κΒ p65 primary antibody (both
from Santa Cruz Biotechnology, Dallas, TX, USA) diluted 1:500 in
TBS-T, with 5% skimmed milk powder. As the loading control, β-actin
expression was determined using a polyclonal rabbit IgG anti-mouse
β-actin primary antibody (Santa Cruz Biotechnology) diluted 1:500
with 5% skim milk powder in TBS-T. Membranes were washed with TBS-T
and incubated at room temperature for 1 h with goat IgG anti-mouse
IgG secondary antibody HRP-conjugated (Santa Cruz Biotechnology)
diluted 1:3,000 in TBS-T, for NF-κB p65 detection. To determine
β-actin and pNF-κB-p65 levels, a goat IgG anti-rabbit IgG secondary
antibody HRP-conjugated (Santa Cruz Biotechnology) diluted 1:4,000
in TBS-T was used. Proteins were detected using Clarity™ Western
ECL (Bio-Rad Laboratories) and the membranes were exposed to an
X-ray film (Kodak, Knoxville, TN, USA) for 2 min. The films were
developed and analyzed using TotalLab Quant Software version 12 for
Windows. Each sample was determined in duplicate.
Angiogenic protein array analysis
The expression levels of inflammatory/angiogenic
proteins in cells cultivated under both culture conditions were
investigated by performing a screening for 24 proteins involved in
angiogenesis using RayBio® Mouse Angiogenic Cytokine
Antibody Array kit (RayBiotech, Inc., Norcross, GA, USA) as
previously described (23). One
array membrane containing 24 types of primary antibodies against
specific proteins was incubated with 200 µg of proteins of cell
lysates, for 2 h at room temperature. Then, a mixture of secondary
biotin-conjugated antibodies against the same angiogenic factors as
those for primary antibodies, was added to the membranes and
incubated overnight at 4̊C, followed by incubation with
HRP-conjugated streptavidin for 2 h. Each incubation step was
followed by 5 washing steps. Thereafter, the membranes were
incubated with a mixture of 2 detection buffers for 1 min, exposed
to an X-ray film (Kodak) for 2 min, and then the films were
developed. The protein expression levels were quantified by
measuring the intensity of the color of each spot on the membranes,
in comparison to the positive control spots already bound to the
membranes, using TotalLab Quant Software version 12 for Windows.
The expression of each angiogenic/inflammatory protein in cell
lysates was determined in duplicate.
HPLC assessment of malondialdehyde
levels
To investigate whether TAM-generated oxidative
stress affects C26 cell proliferation, we quantified the amount of
malondialdehyde (MDA) through high-performance liquid
chromatography (HPLC) in lysates obtained from C26 cells as well as
C26 cells and macrophages (27).
Before HPLC quantification of MDA, sample deproteinization was
performed using HClO4 (27). Then, the samples were centrifuged at
4,500 × g for 5 min and 100 µl of each supernatant was analyzed by
HPLC. The column type was RP18 (5 µm) (Supelco, Bellefonte, PA,
USA) and the mobile phase consisted of 30 mM
KH2PO4/methanol in a volume ratio of 65:35.
Flow rate was set at 0.5 ml/min and MDA was measured using a UV
detector set at 254 nm. The retention time of MDA was ~5.4 min.
Data were normalized to the protein concentration from the cell
lysates and expressed as µg MDA/mg protein. Each sample was
determined in duplicate.
Assessment of total antioxidant
nonenzymatic capacity
This assay was based on the method described by Erel
(28) and was used to determine the
ability of nonenzymatic antioxidants (e.g. uric acid, vitamins,
sulfhydryl groups of proteins and glutathione) to decolorize the
blue-green oxidized
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate (ABTS+)
proportionally to cell nonenzymatic antioxidant concentrations and
it can be monitored by measuring the absorbance 660 nm. The results
are expressed as µmoles of nonenzymatic antioxidants/mg of protein.
The samples were measured in duplicate.
Measurement of catalase activity
The catalytic activity of catalase was assessed via
the method described by Aebi (29).
Catalase activity was monitored by measuring the decrease of
hydrogen peroxide absorbance at 240 nm during 1 min. One catalytic
unit of catalase was defined as the amount of enzyme that
decomposed 1 µmol hydrogen peroxide/min at 25°C and pH, 7.0.
Catalase activity is expressed as units of catalytic activity/mg of
protein.
Inhibition of NADPH oxidase
function
To assess indirectly the role of macrophage NADPH
oxidase in the generation of physiological levels of ROS in
co-culture microenvironment, cells were treated with 300 µM of
apocynin, a NADPH oxidase inhibitor that acts by blocking the
assembly of NADPH oxidase subunits (Santa Cruz Biotechnology) for
48 h. Moreover, to determine whether NADPH oxidase-generated
oxidative stress can modulate C26 cell proliferation, tumor
angiogenesis and inflammation, all assays described above were
performed after co-culture incubation with apocynin.
Statistical analysis
Data from different experiments are expressed as
mean ± standard deviation (SD). The differences between different
protumor processes under standard and co-culture conditions were
evaluated using unpaired t-test. The differences between the
production of angiogenic proteins in cells from standard culture
and co-culture were analyzed by two-way ANOVA with Bonferroni
correction for multiple comparisons. Correlations between different
parameters were evaluated using Pearson correlation coefficient, r.
All statistical analyses were performed using GraphPad Prism
version 6 for Windows, GraphPad Software (San Diego, CA, USA). A
P-value <0.05 was considered significant.
Results
TAMs stimulate the proliferation of
C26 colon carcinoma cells
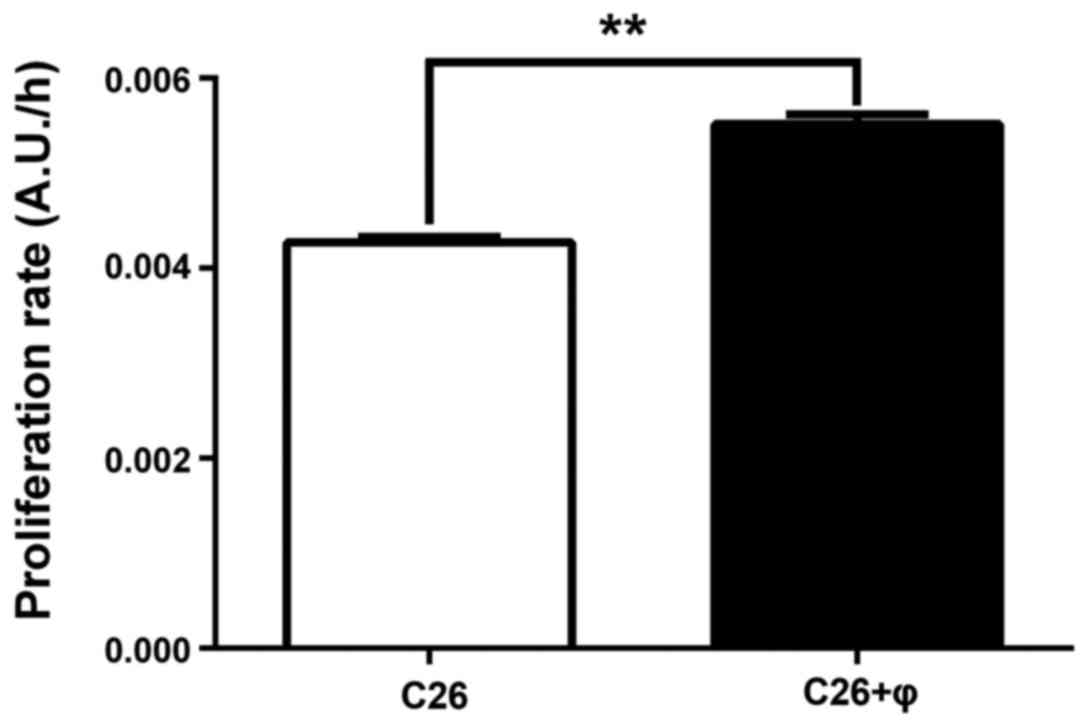
The effect of TAMs on C26 cell proliferation after
48 h of incubation was evaluated by comparing the proliferation
rate of C26 murine carcinoma cells cultivated alone with the
proliferation rate of C26 cells co-cultivated with macrophages
(Fig. 1) at a cell density ratio of
4:1 (C26 cells:macrophages) that ensures an approximation of
physiological conditions for colon carcinoma development in
vivo (21). Our data showed
that C26 cells proliferated more rapidly in the presence of
macrophages (by 28%; P<0.01) than those cultivated alone.
Therefore, the involvement of TAMs in the coordination of main
processes responsible for C26 cell proliferation was further
investigated.
Anti-inflammatory effects of TAMs on
C26 colon carcinoma cells
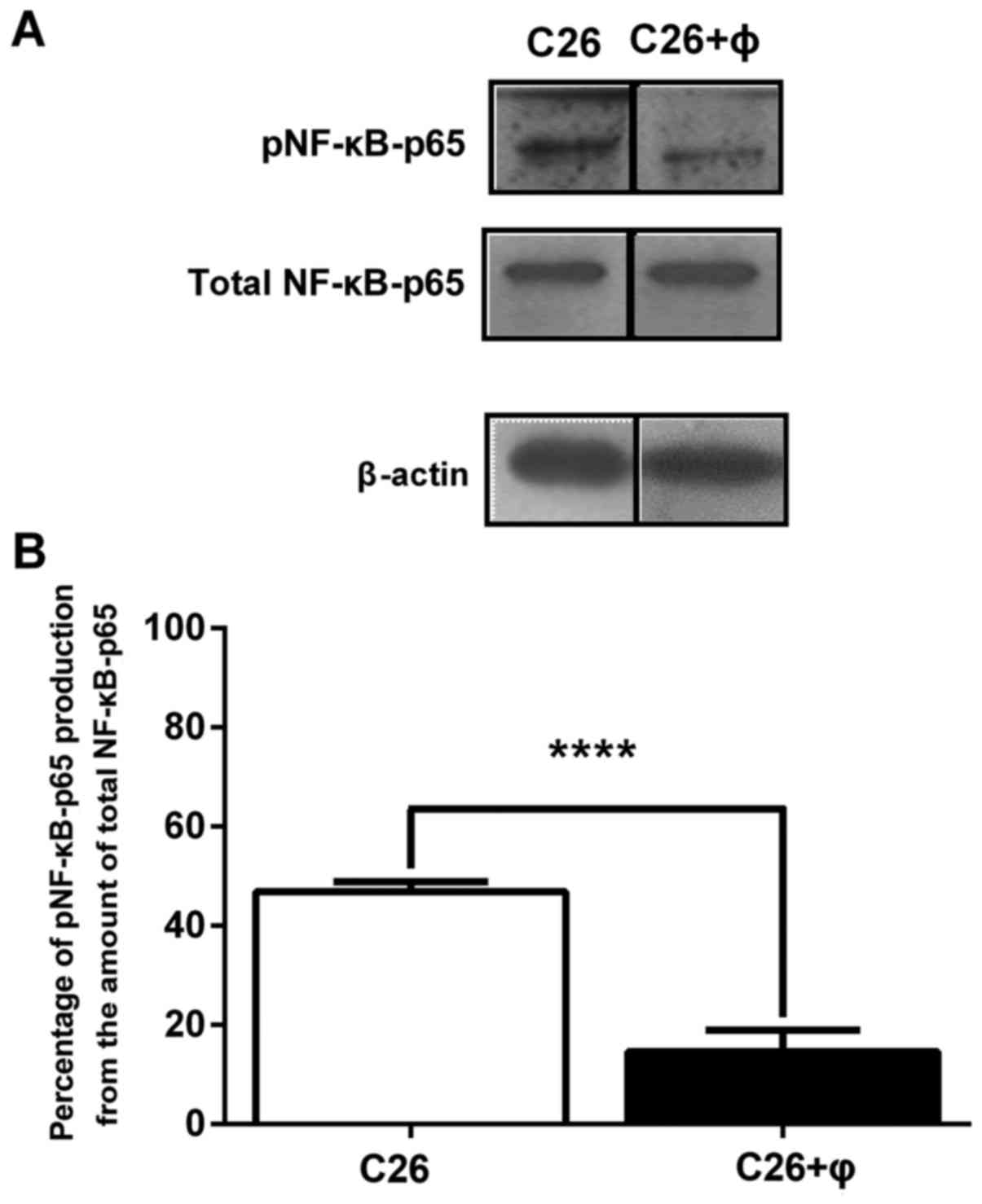
To investigate whether TAMs stimulate the
proliferation of C26 cells through the modulation of the production
of the key inflammatory transcription factor, NF-κB, we analyzed by
western blotting the levels of the active form of this protein
(NF-κB with p65 subunit phosphorylated, pNF-κB-p65) as well as the
production of total NF-κB in C26 cells cultured alone as well as in
C26 cells co-cultured with macrophages. The results are presented
as percentages of pNF-κB-p65 production from the amount of total
NF-κB-p65 in the cell lysates (Fig.
2). Although, the levels of total NF-κB were similar in lysates
obtained from standard culture of C26 cells with those from
co-culture, the co-cultivation of C26 cells with TAMs reduced
drastically the levels of the active form of NF-κB by 70%
(P<0.001) compared to its production in C26 cells cultivated
under standard conditions. These results are in accordance with the
previously described anti-inflammatory role of TAMs in the tumor
microenvironment due to the suppression of NF-κB activation
(8,30).
Angiogenic effects of TAMs on C26
colon carcinoma cells
To assess the link between proliferative effects of
TAMs on C26 cells and TAM-driven angiogenesis, 24 proteins involved
in this process were screened by protein array (RayBio Mouse
Angiogenic Cytokine Antibody Array kit; RayBiotech, Inc.) in C26
cell lysates, and also in lysates obtained after C26 cell
cultivation with TAMs for 48 h. The results presented in Table I show an overall enhancement of the
production of the angiogenic proteins with 112% in the cell lysates
obtained under co-culture conditions compared to their production
in C26 cells cultivated alone. More specifically, after 48 h of
incubation of C26 cells with macrophages, the levels of
insulin-like growth factor-II (IGF-II), interleukin-9 (IL-9),
interleukin-12 subunit p40 (IL-12p40), Fas ligand (FasL), vascular
endothelial growth factor (VEGF) and thrombopoietin (TPO), were
stimulated very strongly (by 200–260%), and the levels of
macrophage-colony stimulating factor (M-CSF), interleukin-1α
(IL-1α), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α),
monocyte chemoattractant protein-1 (MCP-1), leptin and platelet
factor-4 (PF-4), were strongly enhanced (by 100–200%), while
interleukin-6 (IL-6), eotaxin, basic fibroblast growth factor
(bFGF), tissue inhibitor of metalloproteinase-2 (TIMP-2) and
interferon-γ (IFN-γ) were moderately stimulated (by 30–100%) in
comparison with the production of the same proteins in standard C26
cell culture. Only the expression of granulocyte-colony stimulating
factor (G-CSF) and interleukin-13 (IL-13), was inhibited slightly
and statistically significantly (by 25–35%) in the co-culture model
compared to their levels in the C26 cell lysates.
 | Table I.Effects of TAMs on the production of
angiogenic proteins in the co-culture model. |
Table I.
Effects of TAMs on the production of
angiogenic proteins in the co-culture model.
| Angiogenic
proteins | Percentage of
inhibition (−) and stimulation (+) of angiogenic proteins in
co-culture of C26 cells and macrophages compared to standard
culture of C26 cells |
|---|
| Granulocyte
CSF |
−34.65±3.70b |
|
Granulocyte-macrophage CSF |
−26.65±1.34ns |
| Macrophage-CSF |
191.45±4.47d |
| Insulin-like growth
factor II |
204.54±0.15d |
| IL-1α |
190.56±7.36d |
| IL-1β |
178.60±0.23d |
| IL-6 |
45.30±4.99c |
| IL-9 |
202.54±4.50d |
| IL-12p40 |
257.75±0.30d |
| IL-13 |
−27.91±1.51a |
| Tumor necrosis
factor-α |
169.63±17.53d |
| Monocyte
chemoattractant protein-1 |
186.40±3.06d |
| Eotaxin |
91.58±3.85d |
| Fas ligand |
221.19±11.76d |
| Basic fibroblast
growth factor |
31.53±4.89a |
| Vascular
endothelial growth factor |
208.69±17.80d |
| Leptin |
156.92±21.90d |
| Thrombopoietin |
207.97±1.53d |
| TIMP-1 |
−22.52±3.62ns |
| TIMP-2 |
39.54±5.53b |
| Platelet factor
4 |
122.50±0.62d |
| IL-12p70 |
15.10±13.81ns |
| Interferon-γ |
51.94±3.38d |
| Monokine induced by
interferon-γ |
17.38±9.19ns |
Pro-oxidant effects of TAMs on C26
colon carcinoma cells
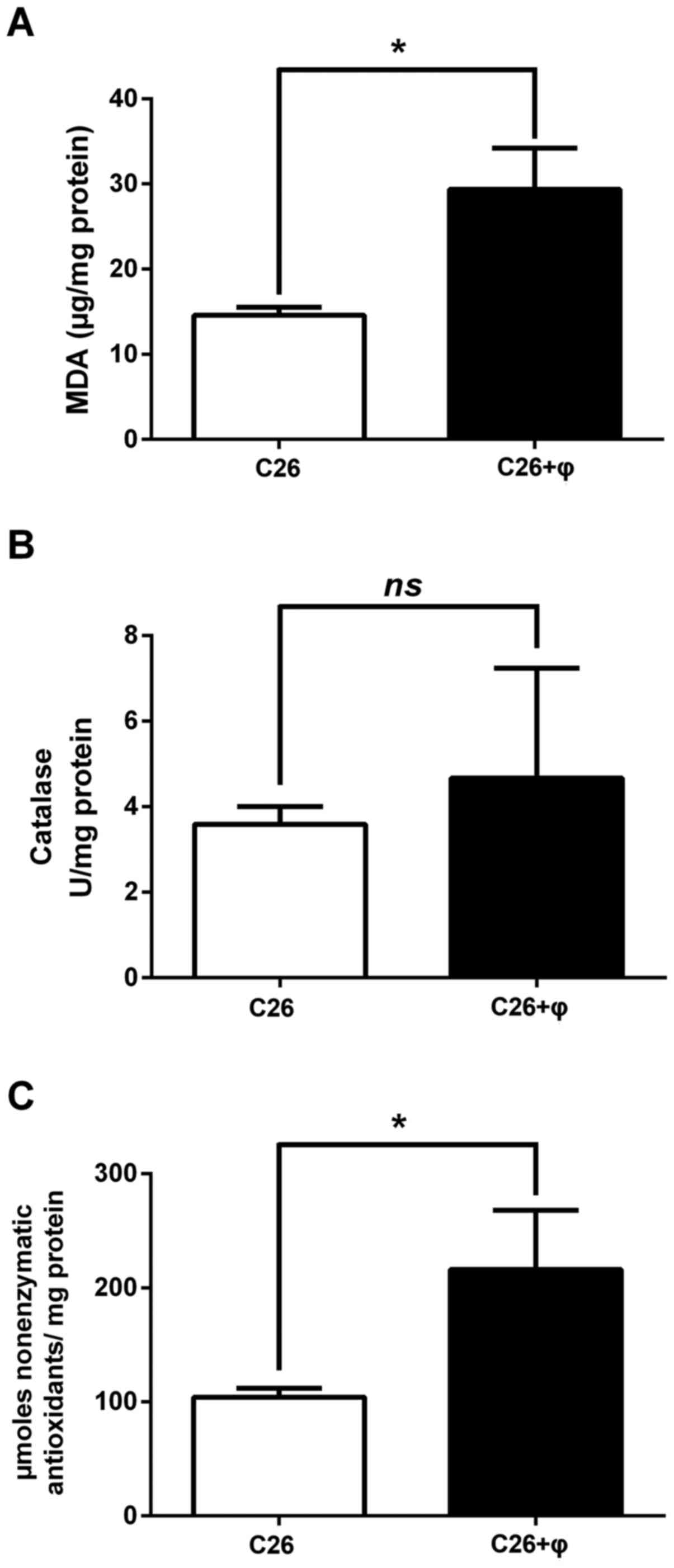
To determine whether the proliferative activity of
TAMs on C26 colon carcinoma cells is related to their modulatory
effects on oxidative stress, the levels of a general oxidative
stress marker-MDA as well as catalytic activity of catalase and
production of nonenzymatic antioxidant systems were assessed and
are shown in Fig. 3A-C. Moreover,
the involvement of the main pro-oxidant enzyme in macrophages,
NADPH oxidase (31) in the
maintenance of the proliferative levels of oxidative stress in the
tumor microenvironment were addressed (Fig. 4A-D). Our results confirmed that TAMs
are important in the generation of tumor oxidative stress since MDA
levels were significantly enhanced (2-fold higher; P<0.05) in
the lysates from co-culture of C26 cells and macrophages compared
to their levels in C26 cell lysates (Fig. 3A). These data were also supported by
the results regarding the higher amount of nonenzymatic antioxidant
systems in co-culture lysates compared to C26 cell lysates (2-fold
higher production in co-culture lysates compared to their
production in C26 cell lysates, P=0.04) (Fig. 3C). As catalase activity was only
slightly increased and not statistically significant in the
presence of macrophages (Fig. 3B;
P=0.61), it seems that the main protective mechanism against high
levels of ROS in the co-culture model is mediated rather by
nonenzymatic antioxidant systems than by catalase. To evaluate
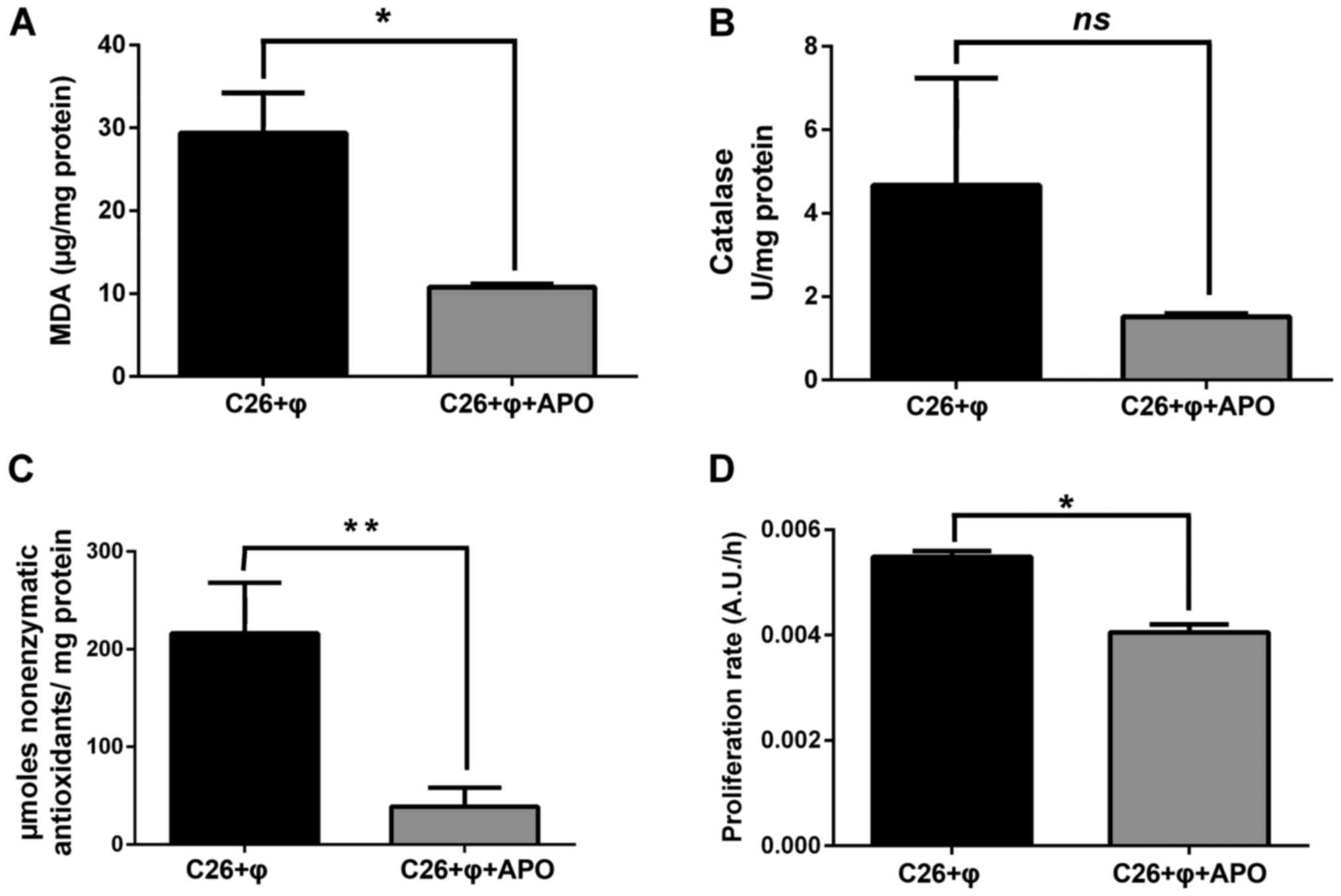
further the involvement of the TAM-expressed NADPH-oxidase in
maintaining the C26 carcinoma oxidative stress, TAMs co-cultivated
with C26 cells were incubated for 48 h with an inhibitor of this
enzyme (300 µM apocynin) and the oxidative stress parameters
presented above were evaluated (Fig.
4A-C). As a negative control, a standard culture of C26 cells
treated with apocynin was used. No effects on the levels of MDA and
nonenzymatic antioxidants and catalase activity were noted after
incubation of C26 cells with apocynin (data not shown). Our data
clearly confirmed the principal role of NADPH oxidase in supporting
the C26 carcinoma oxidative stress as the MDA levels in cell
lysates obtained from co-culture treated with apocynin were reduced
to its levels in C26 cells cultivated alone (Fig. 4A compared to Fig. 3A; P=0.08). Neutralization of
oxidative stress in the co-culture microenvironment via NADPH
oxidase suppression was also accompanied by the reduction in the
production of nonenzymatic antioxidant systems (5-fold lower amount
after co-culture treatment with apocynin than their amount in cell
co-culture with active NADPH oxidase, P=0.0027) (Fig. 4C). Moreover, to link the role of
NADPH oxidase to stimulatory effects of TAMs on C26 cells, the
proliferation of these cancer cells under co-culture conditions in
the presence of apocynin was tested. Our data demonstrated that the
stimulatory effects of TAMs on C26 cell proliferation were
abrogated after NADPH oxidase inhibition in macrophages. Thus,
after cell co-culture treatment with apocynin, the C26 cell
proliferation was similar to that of the C26 cells cultivated alone
(Fig. 4D compared to Fig. 1; P=0.06).
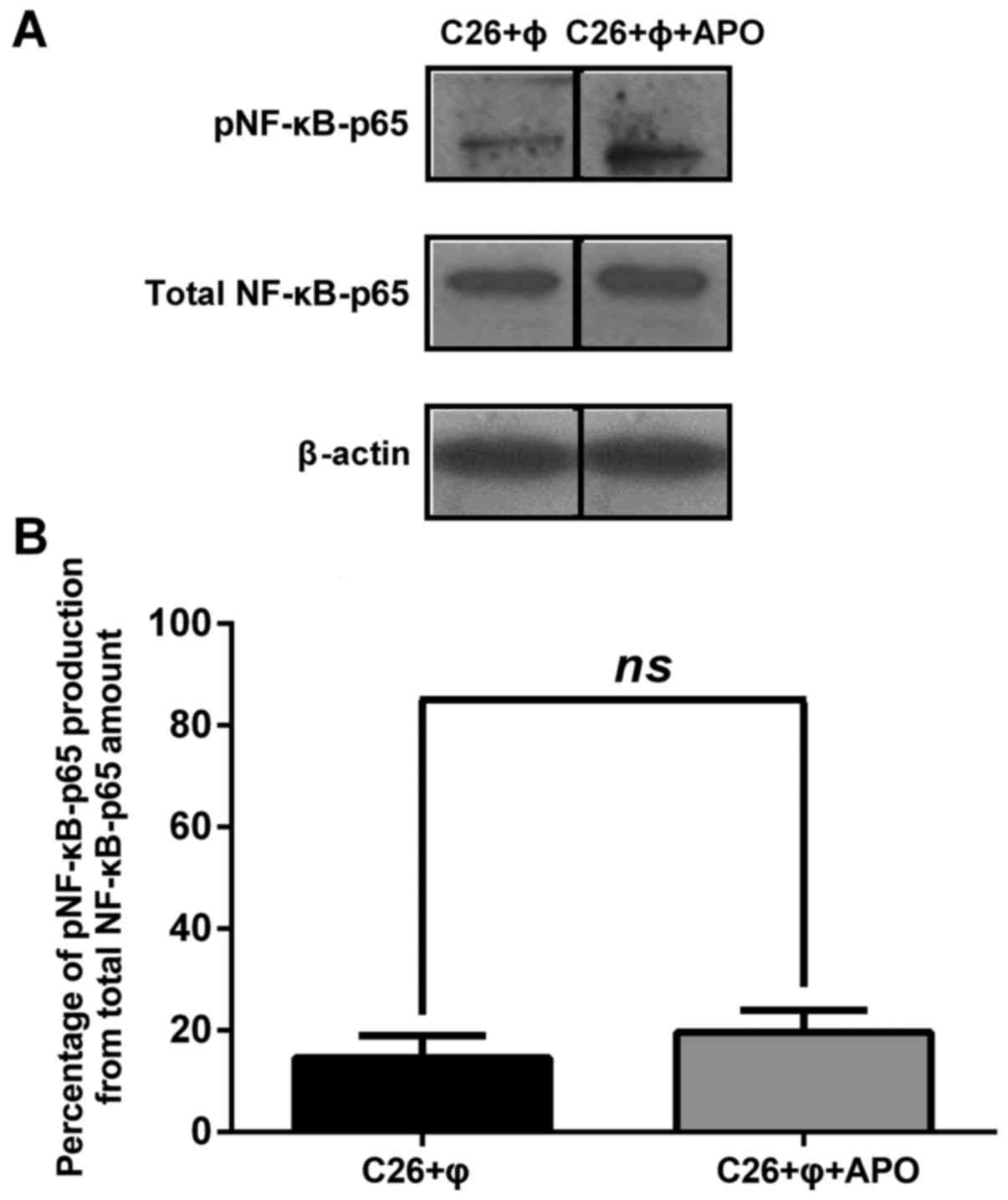
Role of NADPH oxidase in the
modulation of the protumor actions of TAMs on C26 cells
To evaluate whether the pro-oxidant effects of TAMs
generated via activity of NADPH oxidase can be linked to potential
stimulatory actions of TAMs on C26 cell proliferation, C26 murine
carcinoma cells were cultured with macrophages in the presence of
apocynin for 48 h and then the production of NF-κB (Fig. 5A and B) as well as of different
angiogenic proteins (Table II)
were assessed. Our results showed that inhibition of NADPH oxidase
in macrophages did not affect the levels of the active form of
NF-κB as well as of total NF-κB in cell co-culture lysates (P=0.35)
(Fig. 5A and B). When C26 cells
co-cultured with macrophages were treated with apocynin, the levels
of all angiogenic proteins tested were statistically significantly
reduced (P<0.0001) compared to their production in untreated
cell co-culture lysates. On average, NADPH oxidase inhibition
reduced the production of these proteins by 63% in the
apocynin-incubated cells compared to their production in the
untreated cell co-culture. Except for the TNF-α level that was
slightly reduced (by 35%), all angiogenic and inflammatory protein
levels were suppressed moderately (by 35–75%) to strongly (>75%)
after apocynin treatment (Table
II).
 | Table II.Involvement of TAM-induced
NADPH-oxidase in the production of angiogenic proteins in the
co-culture model. |
Table II.
Involvement of TAM-induced
NADPH-oxidase in the production of angiogenic proteins in the
co-culture model.
| Angiogenic
proteins | Percentage of
inhibition (−) of angiogenic proteins in the co-culture of C26
cells and macrophages compared to apocynin-treated co-culture of
C26 cells and macrophages |
|---|
| Granulocyte
CSF |
−49.54±1.05a |
|
Granulocyte-macrophage CSF |
−55.20±1.93a |
| Macrophage-CSF |
−74.51±1.87a |
| Insulin-like growth
factor II |
−75.18±5.44a |
| IL-1α |
−67.89±1.74a |
| IL-1β |
−71.64±2.02a |
| IL-6 |
−43.87±2.12a |
| IL-9 |
−79.25±0.50a |
| IL-12p40 |
−84.57±14.15a |
| IL-13 |
−48.17±1.92a |
| Tumor necrosis
factor-α |
−32.23±4.19a |
| Monocyte
chemoattractant protein-1 |
−77.30±0.40a |
| Eotaxin |
−45.41±15.04a |
| Fas ligand |
−56.75±2.55a |
| Basic fibroblast
growth factor |
−60.08±1.74a |
| Vascular
endothelial growth factor |
−65.72±0.81a |
| Leptin |
−85.54±4.01a |
| Thrombopoietin |
−69.97±0.41a |
| TIMP-1 |
−54.77±4.51a |
| TIMP-2 |
−53.56±0.14a |
| Platelet factor
4 |
−65.65±1.09a |
| IL-12p70 |
−28.79±6.82a |
| Interferon-γ |
−74.38±0.56a |
| Monokine induced by
interferon-γ |
−55.09±4.12a |
Discussion
Although, a large body of data suggest that the
infiltration of TAMs into tumors to a great extent are related with
poor prognosis in most types of cancer (breast, bladder,
urogenital, head and neck cancer) (32–34),
the role of this microenvironmental cell type in colon carcinoma
development is still controversial (16–19,34–38).
Thus, the present study aimed to investigate the role of TAMs in
colon carcinoma cell development. Therefore, the link between
TAM-driven protumor processes and C26 murine colon carcinoma cell
proliferation was evaluated in an in vitro co-culture model
of murine macrophages and C26 tumor cells at a cell density ratio
that approximates physiological conditions for colon carcinoma
development in vivo (20).
Our results demonstrated that TAMs enhanced proliferation of C26
colon carcinoma cells when they were co-cultivated (Fig. 1).
To gain insight into the molecular mechanisms of the
proliferative actions of TAMs on C26 cells, TAM-mediated protumor
processes such as inflammation, angiogenesis and oxidative stress
were investigated. Firstly, the expression of the inflammatory
transcription factor, NF-κB was assessed as the active form as well
as total protein (as the active and inactive form). It is known
that NF-κB is a pleiotropic transcriptional regulator
constitutively expressed and activated in most colon carcinoma
cells including C26 cells (39).
This protein controls the expression of numerous genes encoding for
growth factors (VEGF and FGF), cytokines and chemokines (TNF-α and
IL-8), and metalloproteinases, which are involved in tumor
inflammation, angiogenesis, cell proliferation, tumor survival and
chemoresistance (40,41). Moreover, NF-κB ensures crosstalk
between colon carcinoma and stromal cells in the tumor
microenvironment which is essential for tumor progression (42,43).
Our results showed that the total production of NF-κB was not
influenced by the co-cultivation of C26 cells with TAMs albeit the
activation of NF-κB was drastically affected (by 70%) in the cell
co-culture lysates compared to transcription factor activation in
C26 cell lysates (Fig. 2A and B).
Several studies are consistent with our data showing the
inactivation of NF-κB in TAMs as a result of the primary necessity
of cancer cells to maintain these immune cells in a suppressed
state vital for tumor cell proliferation and invasion (44,45).
Moreover, the antitumor role of NF-κB activation in TAMs is also
supported by previous findings regarding its antimetastatic
activity in a mammary tumor lung metastasis model (46). In addition to these data, several
studies proved the dual role of NF-κB in tumorigenesis in a manner
dependent on cell type (47). Thus,
NF-κB activation in tumor cells is responsible for the expression
of anti-apoptotic, proliferative, and pro-angiogenic molecules
while its activation in endothelial cells is linked to inhibition
of tumor angiogenesis (47).
Although our studies involved macrophages instead of endothelial
cells, we noted that the levels of the active form of NF-κB
correlated negatively with the proliferation of C26 cells (Pearson
correlation coefficient r=−0.991; P<0.0001) and also with the
levels of pro-angiogenic molecules (Pearson correlation coefficient
r=−0.793; P=0.0007) in cell lysates. Collectively, our data suggest
that the proliferative effects of TAMs on C26 cells may be related
to the anti-inflammatory role of these cells in the tumor
microenvironment via reduction of NF-κB activation (Fig. 2A and B) as well as the stimulatory
activity of TAMs on the production of most of the angiogenic
proteins tested in the cell co-culture (Table I).
Furthermore, since pro-angiogenic protein production
and NF-κB activation are modulated by ROS levels in the tumor
microenvironment (48–52), we evaluated oxidative stress in C26
cells as well as in the co-culture of C26 cells and TAMs. Thus we
assessed the levels of the oxidative stress marker, MDA and total
nonenzymatic antioxidant systems, and catalytic activity of
catalase under both culture conditions. Our results suggested that
TAMs are important contributors in producing the proliferative
levels of ROS in the C26 carcinoma microenvironment (4,52)
since MDA levels doubled when C26 cells were co-cultivated with
macrophages (Fig. 3A). Moreover,
TAMs may help C26 cells to protect themselves from
microenvironmental oxidative stress mainly via enhancement of the
production of nonenzymatic antioxidant defense systems under
co-culture conditions (Fig. 3A-C).
Furthermore, our data proved that NADPH oxidase has the main role
in supporting the C26 carcinoma cell oxidative stress as the levels
of the oxidative stress marker (MDA) as well as antioxidant defense
mechanisms in the tumor microenvironment (particularly via
nonenzymatic antioxidant systems) were reduced significantly after
quenching NADPH oxidase activity under co-culture conditions
(Fig. 4A-C). The activity of NADPH
oxidase from the plasma membrane of macrophages was also
responsible for the stimulatory effects of TAMs on the
proliferation of C26 cells (Fig.
4D) mainly via enhancement of the pro-angiogenic protein
production in the co-culture microenvironment (Table II). Thus, abolishment of NADPH
oxidase activity after apocynin administration reduced strongly the
levels of all angiogenic proteins tested in the cell co-culture
lysates. Nevertheless, the apocynin effect on the co-culture of C26
cells with TAMs did not affect the anti-inflammatory effects of
these cells on the activation of NF-κB in the C26 carcinoma milieu
(Fig. 5A and B) and did not totally
counteract the pro-angiogenic actions of TAMs on C26 cells [average
enhancing effect of TAMs on angiogenic protein production in cell
co-culture (~112%) (Table I) was
higher than reducing effect of the same protein production after
apocynin administration (by 63%) (Table II)]. These findings suggest that
TAMs also support C26 carcinoma cell proliferation via additional
effects to the main mechanism based on NADPH oxidase-generated
tumor oxidative stress, probably via ROS-signaling-independent
regulatory effects on different transcription factors involved in
tumor-associated inflammation and angiogenesis (21).
In conclusion, our data support the stimulatory role
of TAMs on C26 murine colon carcinoma cell proliferation via
anti-inflammatory, pro-angiogenic and pro-oxidant effects on the
co-culture microenvironment. The main mechanism of the protumor
function of TAMs in C26 tumor development is based on the
maintenance of the physiological range of the oxidative stress and
angiogenic capacity of the C26 colon carcinoma milieu via NADPH
oxidase activity. Moreover, in addition to this principal mechanism
of action, the anti-inflammatory and pro-angiogenic effects of TAMs
on these cancer cells create a favorable microenvironment for C26
colon carcinoma development and progression. These findings provide
new insights into tumor-targeted therapies based on ‘re-education’
strategies of TAMs.
Acknowledgements
The present study was supported by the UEFISCDI
(Romanian Ministry of Education, Research and Innovation) (project
PN-II-PT-PCCA-2011-3-2-1060, contract no. 95/2012 and project
PN-II-RU-TE-2014-4-1191, contract no. 235/01.10.2015), and the
Sectorial Operational Programme for Human Resources Development
2007–2013, co-financed by the European Social Fund (project
POSDRU/187/1.5/S/155383-‘Quality, Excellence, Transnational
Mobility in Doctoral Research’).
References
|
1
|
Albini A, Tosetti F, Benelli R and Noonan
DM: Tumor inflammatory angiogenesis and its chemoprevention. Cancer
Res. 65:10637–10641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allavena P, Sica A, Garlanda C and
Mantovani A: The Yin-Yang of tumor-associated macrophages in
neoplastic progression and immune surveillance. Immunol Rev.
222:155–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alupei MC, Licarete E, Patras L and Banciu
M: Liposomal simvastatin inhibits tumor growth via targeting
tumor-associated macrophages-mediated oxidative stress. Cancer
Lett. 356:946–952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Banciu M, Metselaar JM, Schiffelers RM and
Storm G: Antitumor activity of liposomal prednisolone phosphate
depends on the presence of functional tumor-associated macrophages
in tumor tissue. Neoplasia. 10:108–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumor-associated macrophages (TAM) as major players of
the cancer-related inflammation. J Leukoc Biol. 86:1065–1073. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown NS and Bicknell R: Hypoxia and
oxidative stress in breast cancer. Oxidative stress: Its effects on
the growth, metastatic potential and response to therapy of breast
cancer. Breast Cancer Res. 3:323–327. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown NS, Jones A, Fujiyama C, Harris AL
and Bicknell R: Thymidine phosphorylase induces carcinoma cell
oxidative stress and promotes secretion of angiogenic factors.
Cancer Res. 60:6298–6302. 2000.PubMed/NCBI
|
|
11
|
Fruehauf JP, Meyskens FL Jr, et al:
Reactive oxygen species: A breath of life or death? Clin Cancer
Res. 13:789–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo C, Buranych A, Sarkar D, Fisher PB and
Wang XY: The role of tumor-associated macrophages in tumor
vascularization. Vasc Cell. 5:202013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sundaresan M, Yu ZX, Ferrans VJ, Sulciner
DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ and Finkel T:
Regulation of reactive-oxygen-species generation in fibroblasts by
Rac1. Biochem J. 318:379–382. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tammali R, Reddy AB, Srivastava SK and
Ramana KV: Inhibition of aldose reductase prevents angiogenesis in
vitro and in vivo. Angiogenesis. 14:209–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Choksi S, Chen K, Pobezinskaya Y,
Linnoila I and Liu ZG: ROS play a critical role in the
differentiation of alternatively activated macrophages and the
occurrence of tumor-associated macrophages. Cell Res. 23:898–914.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forssell J, Oberg A, Henriksson ML,
Stenling R, Jung A and Palmqvist R: High macrophage infiltration
along the tumor front correlates with improved survival in colon
cancer. Clin Cancer Res. 13:1472–1479. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sickert D, Aust DE, Langer S, Haupt I,
Baretton GB and Dieter P: Characterization of macrophage
subpopulations in colon cancer using tissue microarrays.
Histopathology. 46:515–521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van der Bij GJ, Bögels M, Oosterling SJ,
Kroon J, Schuckmann DT, de Vries HE, Meijer S, Beelen RH and van
Egmond M: Tumor infiltrating macrophages reduce development of
peritoneal colorectal carcinoma metastases. Cancer Lett. 262:77–86.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zins K, Abraham D, Sioud M and Aharinejad
S: Colon cancer cell-derived tumor necrosis factor-alpha mediates
the tumor growth-promoting response in macrophages by up-regulating
the colony-stimulating factor-1 pathway. Cancer Res. 67:1038–1045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calorini L, Bianchini F, Mannini A, Mugnai
G and Ruggieri S: Enhancement of nitric oxide release in mouse
inflammatory macrophages co-cultivated with tumor cells of a
different origin. Clin Exp Metastasis. 22:413–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herbeuval JP, Lelievre E, Lambert C, Dy M
and Genin C: Recruitment of STAT3 for production of IL-10 by colon
carcinoma cells induced by macrophage-derived IL-6. J Immunol.
172:4630–4636. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
James AD, Patel W, Butt Z, Adiamah M,
Dakhel R, Latif A, Uggenti C, Swanton E, Imamura H, Siriwardena AK,
et al: The plasma membrane calcium pump in pancreatic cancer cells
exhibiting the Warburg effect relies on glycolytic ATP. J Biol
Chem. 290:24760–24771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banciu M, Schiffelers RM, Fens MH,
Metselaar JM and Storm G: Anti-angiogenic effects of liposomal
prednisolone phosphate on B16 melanoma in mice. J Control Release.
113:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siu FKY, Lee LTO and Chow BKC:
Southwestern blotting in investigating transcriptional regulation.
Nat Protoc. 3:51–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karatas F, Karatepe M and Baysar A:
Determination of free malondialdehyde in human serum by
high-performance liquid chromatography. Anal Biochem. 311:76–79.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Erel O: A novel automated direct
measurement method for total antioxidant capacity using a new
generation, more stable ABTS radical cation. Clin Biochem.
37:277–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sica A and Bronte V: Altered macrophage
differentiation and immune dysfunction in tumor development. J Clin
Invest. 117:1155–1166. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pick E: Role of the Rho GTPase Rac in the
activation of the phagocyte NADPH oxidase: Outsourcing a key task.
Small GTPases. 5:e279522014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH,
Wang XZ, Zhao YW and Wei YQ: Prognostic significance of
tumor-associated macrophages in solid tumor: A meta-analysis of the
literature. PLoS One. 7:e509462012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Biswas SK, Allavena P and Mantovani A:
Tumor-associated macrophages: Functional diversity, clinical
significance, and open questions. Semin Immunopathol. 35:585–600.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siveen KS and Kuttan G: Role of
macrophages in tumour progression. Immunol Lett. 123:97–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van der Bij GJ, Oosterling SJ, Meijer S,
Beelen RH and van Egmond M: The role of macrophages in tumor
development. Cell Oncol. 27:203–213. 2005.PubMed/NCBI
|
|
36
|
Coffelt SB, Hughes R and Lewis CE:
Tumor-associated macrophages: Effectors of angiogenesis and tumor
progression. Biochim Biophys Acta. 1796:11–18. 2009.PubMed/NCBI
|
|
37
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barbera-Guillem E, Nyhus JK, Wolford CC,
Friece CR and Sampsel JW: Vascular endothelial growth factor
secretion by tumor-infiltrating macrophages essentially supports
tumor angiogenesis, and IgG immune complexes potentiate the
process. Cancer Res. 62:7042–7049. 2002.PubMed/NCBI
|
|
39
|
Patras L, Sesarman A, Licarete E, Luca L,
Alupei MC, Rakosy-Tican E and Banciu M: Dual role of macrophages in
the response of C26 colon carcinoma cells to 5-fluorouracil
administration. Oncol Lett. 12:1183–1191. 2016.PubMed/NCBI
|
|
40
|
Yang Z, Li C, Wang X, Zhai C, Yi Z, Wang
L, Liu B, Du B, Wu H, Guo X, et al: Dauricine induces apoptosis,
inhibits proliferation and invasion through inhibiting NF-kappaB
signaling pathway in colon cancer cells. J Cell Physiol.
225:266–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ryan AE, Colleran A, O'Gorman A, O'Flynn
L, Pindjacova J, Lohan P, O'Malley G, Nosov M, Mureau C and Egan
LJ: Targeting colon cancer cell NF-κB promotes an anti-tumour
M1-like macrophage phenotype and inhibits peritoneal metastasis.
Oncogene. 34:1563–1574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Saccani A, Schioppa T, Porta C, Biswas SK,
Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A and Sica A:
p50 nuclear factor-kappaB overexpression in tumor-associated
macrophages inhibits M1 inflammatory responses and antitumor
resistance. Cancer Res. 66:11432–11440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kühnemuth B and Michl P: The role of CUX1
in antagonizing NF-κB signaling in TAMs. OncoImmunology.
3:e282702014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Connelly L, Barham W, Onishko HM, Chen L,
Sherrill TP, Zabuawala T, Ostrowski MC, Blackwell TS and Yull FE:
NF-kappaB activation within macrophages leads to an anti-tumor
phenotype in a mammary tumor lung metastasis model. Breast Cancer
Res. 13:R832011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tabruyn SP and Griffioen AW: A new role
for NF-kappaB in angiogenesis inhibition. Cell Death Differ.
14:1393–1397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ushio-Fukai M and Alexander RW: Reactive
oxygen species as mediators of angiogenesis signaling: Role of
NAD(P)H oxidase. Mol Cell Biochem. 264:85–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Morgan MJ and Liu ZG: Crosstalk of
reactive oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kabe Y, Ando K, Hirao S, Yoshida M and
Handa H: Redox regulation of NF-kappaB activation: Distinct redox
regulation between the cytoplasm and the nucleus. Antioxid Redox
Signal. 7:395–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu M, Bian Q, Liu Y, Fernandes AF, Taylor
A, Pereira P and Shang F: Sustained oxidative stress inhibits
NF-kappaB activation partially via inactivating the proteasome.
Free Radic Biol Med. 46:62–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Alupei MC, Licarete E, Cristian FB and
Banciu M: Cytotoxicity of lipophilic statins depends on their
combined actions on HIF-1α expression and redox status in B16.F10
melanoma cells. Anticancer Drugs. 25:393–405. 2014. View Article : Google Scholar : PubMed/NCBI
|