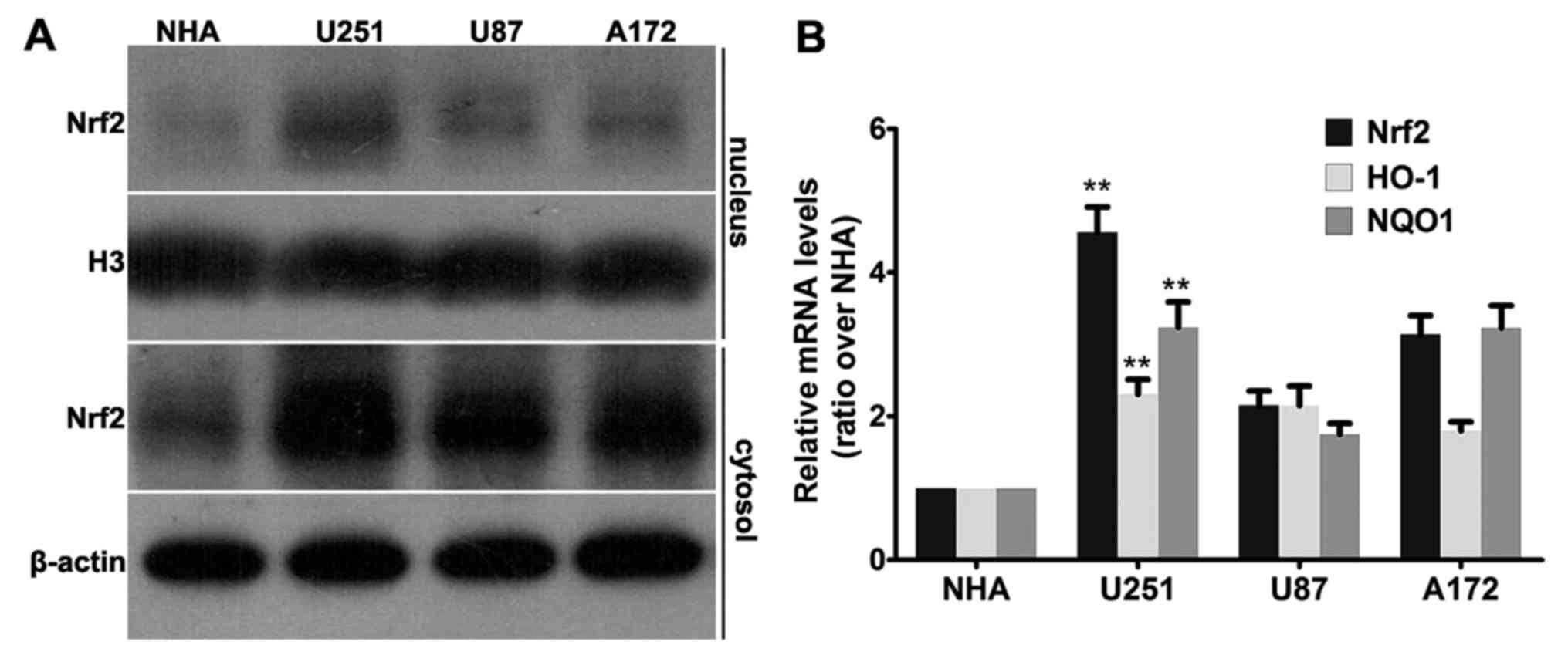
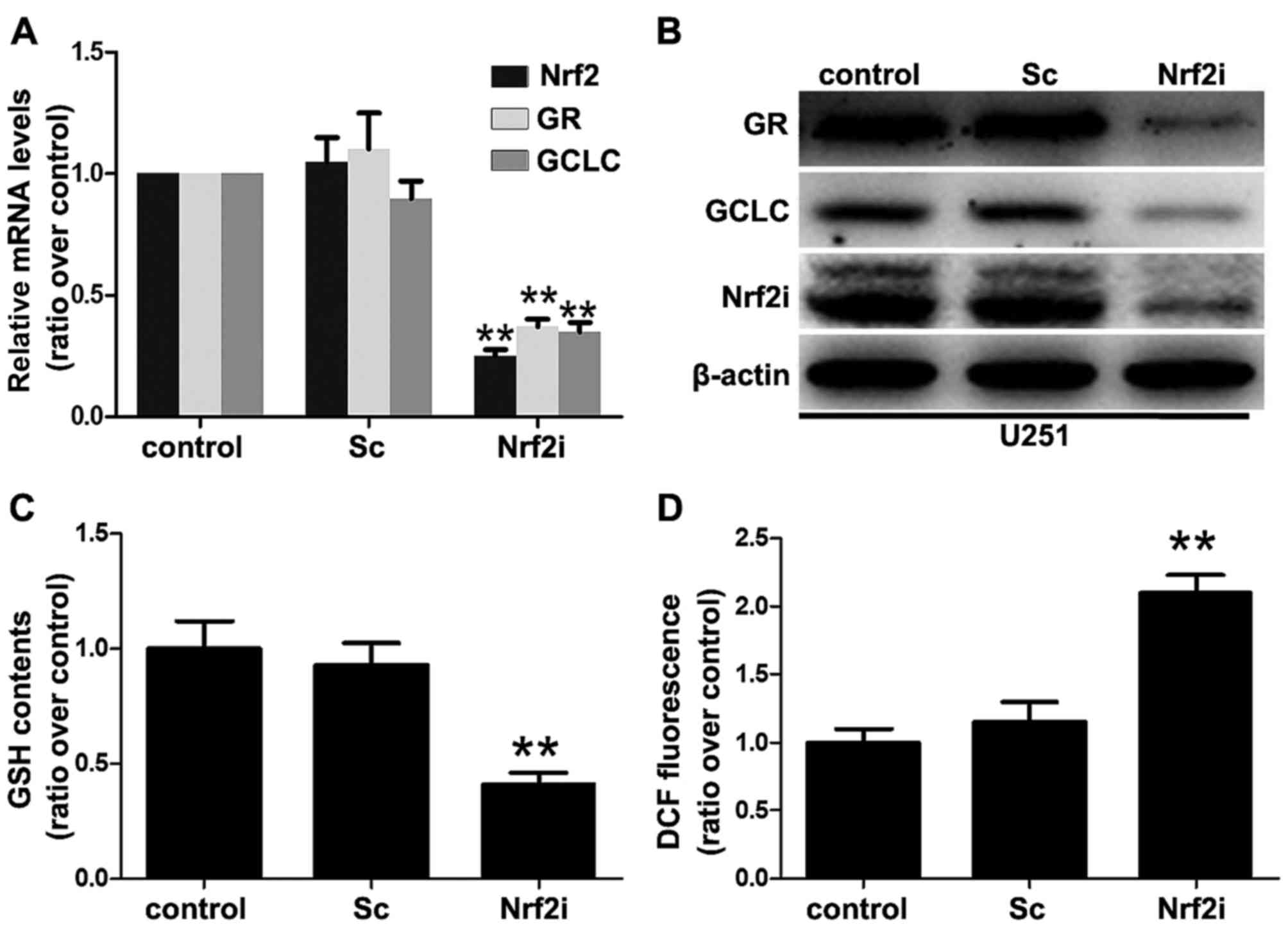
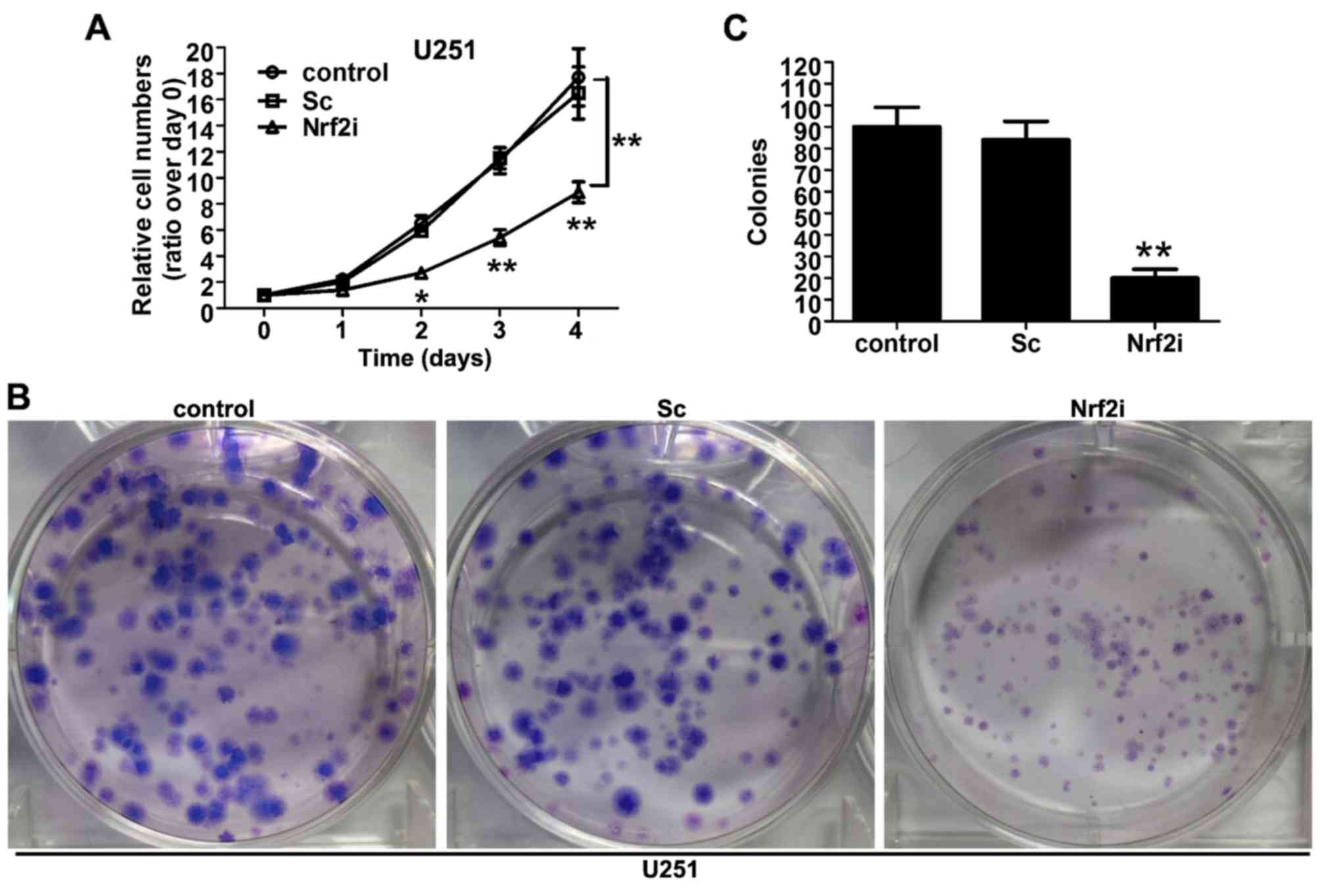
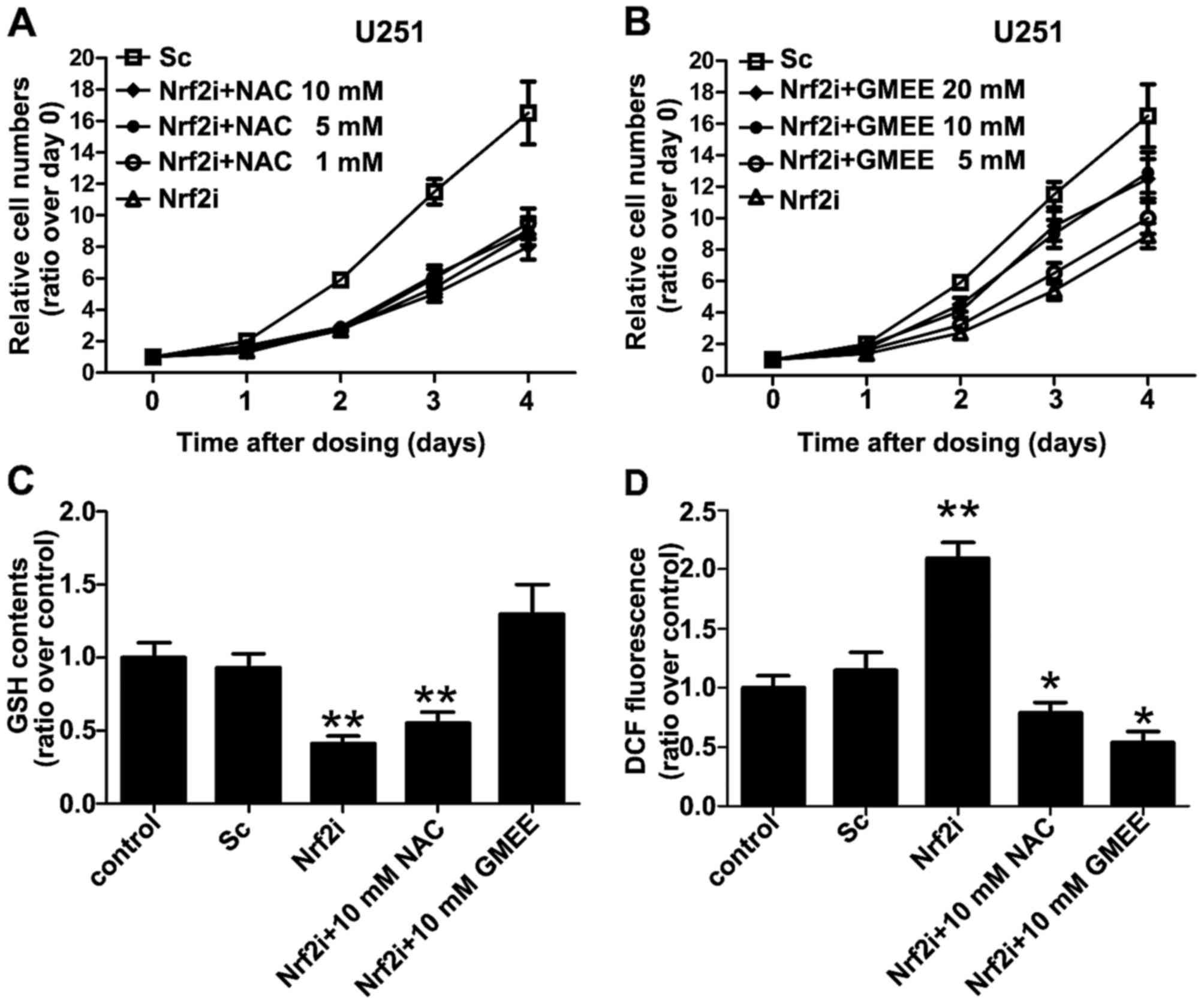
|
1
|
Jolly C and Morimoto RI: Role of the heat
shock response and molecular chaperones in oncogenesis and cell
death. J Natl Cancer Inst. 92:1564–1572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh KK: Mitochondrial dysfunction is a
common phenotype in aging and cancer. Ann NY Acad Sci.
1019:260–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mena S, Ortega A and Estrela JM: Oxidative
stress in environmental-induced carcinogenesis. Mutat Res.
674:36–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gottlieb E and Tomlinson IP: Mitochondrial
tumour suppressors: A genetic and biochemical update. Nat Rev
Cancer. 5:857–866. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motohashi H and Yamamoto M: Nrf2-Keap1
defines a physiologically important stress response mechanism.
Trends Mol Med. 10:549–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gañán-Gómez I, Wei Y, Yang H,
Boyano-Adánez MC and García-Manero G: Oncogenic functions of the
transcription factor Nrf2. Free Radic Biol Med. 65:750–764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malhotra D, Portales-Casamar E, Singh A,
Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler
TW, Wasserman WW, et al: Global mapping of binding sites for Nrf2
identifies novel targets in cell survival response through ChIP-Seq
profiling and network analysis. Nucleic Acids Res. 38:5718–5734.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wild AC, Moinova HR and Mulcahy RT:
Regulation of gamma-glutamylcysteine synthetase subunit gene
expression by the transcription factor Nrf2. J Biol Chem.
274:33627–33636. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki H, Sato H, Kuriyama-Matsumura K,
Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M and Bannai
S: Electrophile response element-mediated induction of the
cystine/glutamate exchange transporter gene expression. J Biol
Chem. 277:44765–44771. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishii T, Itoh K, Takahashi S, Sato H,
Yanagawa T, Katoh Y, Bannai S and Yamamoto M: Transcription factor
Nrf2 coordinately regulates a group of oxidative stress-inducible
genes in macrophages. J Biol Chem. 275:16023–16029. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aoki Y, Hashimoto AH, Amanuma K, Matsumoto
M, Hiyoshi K, Takano H, Masumura K, Itoh K, Nohmi T and Yamamoto M:
Enhanced spontaneous and benzo(a)pyrene-induced mutations in
the lung of Nrf2-deficient gpt delta mice. Cancer Res.
67:5643–5648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K,
Yamamoto M, Talalay P and Kensler TW: Sensitivity to carcinogenesis
is increased and chemoprotective efficacy of enzyme inducers is
lost in nrf2 transcription factor-deficient mice. Proc Natl
Acad Sci USA. 98:3410–3415. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lau A, Villeneuve NF, Sun Z, Wong PK and
Zhang DD: Dual roles of Nrf2 in cancer. Pharmacol Res. 58:262–270.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayes JD and McMahon M: NRF2 and
KEAP1 mutations: Permanent activation of an adaptive
response in cancer. Trends Biochem Sci. 34:176–188. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayes JD and McMahon M: The double-edged
sword of Nrf2: Subversion of redox homeostasis during the evolution
of cancer. Mol Cell. 21:732–734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kensler TW and Wakabayashi N: Nrf2: Friend
or foe for chemoprevention? Carcinogenesis. 31:90–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Homma S, Ishii Y, Morishima Y, Yamadori T,
Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N,
et al: Nrf2 enhances cell proliferation and resistance to
anticancer drugs in human lung cancer. Clin Cancer Res.
15:3423–3432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamadori T, Ishii Y, Homma S, Morishima Y,
Kurishima K, Itoh K, Yamamoto M, Minami Y, Noguchi M and Hizawa N:
Molecular mechanisms for the regulation of Nrf2-mediated cell
proliferation in non-small-cell lung cancers. Oncogene.
31:4768–4777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lister A, Nedjadi T, Kitteringham NR,
Campbell F, Costello E, Lloyd B, Copple IM, Williams S, Owen A,
Neoptolemos JP, et al: Nrf2 is overexpressed in pancreatic cancer:
Implications for cell proliferation and therapy. Mol Cancer.
10:372011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji XJ, Chen SH, Zhu L, Pan H, Zhou Y, Li
W, You WC, Gao CC, Zhu JH, Jiang K, et al: Knockdown of
NF-E2-related factor 2 inhibits the proliferation and growth of
U251MG human glioma cells in a mouse xenograft model. Oncol Rep.
30:157–164. 2013.PubMed/NCBI
|
|
22
|
Pan H, Wang H, Zhu L, Mao L, Qiao L and Su
X: The role of Nrf2 in migration and invasion of human glioma cell
U251. World Neurosurg. 80:363–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji X, Wang H, Zhu J, Zhu L, Pan H, Li W,
Zhou Y, Cong Z, Yan F and Chen S: Knockdown of Nrf2 suppresses
glioblastoma angiogenesis by inhibiting hypoxia-induced activation
of HIF-1α. Int J Cancer. 135:574–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thimmulappa RK, Lee H, Rangasamy T, Reddy
SP, Yamamoto M, Kensler TW and Biswal S: Nrf2 is a critical
regulator of the innate immune response and survival during
experimental sepsis. J Clin Invest. 116:984–995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rangasamy T, Guo J, Mitzner WA, Roman J,
Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, et
al: Disruption of Nrf2 enhances susceptibility to severe
airway inflammation and asthma in mice. J Exp Med. 202:47–59. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hutter DE, Till BG and Greene JJ: Redox
state changes in density-dependent regulation of proliferation. Exp
Cell Res. 232:435–438. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pani G, Colavitti R, Bedogni B, Anzevino
R, Borrello S and Galeotti T: A redox signaling mechanism for
density-dependent inhibition of cell growth. J Biol Chem.
275:38891–38899. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Menon SG, Sarsour EH, Spitz DR,
Higashikubo R, Sturm M, Zhang H and Goswami PC: Redox regulation of
the G1 to S phase transition in the mouse embryo
fibroblast cell cycle. Cancer Res. 63:2109–2117. 2003.PubMed/NCBI
|
|
29
|
Dalle-Donne I, Carini M, Vistoli G,
Gamberoni L, Giustarini D, Colombo R, Facino Maffei R, Rossi R,
Milzani A and Aldini G: Actin Cys374 as a nucleophilic target of
α,β-unsaturated aldehydes. Free Radic Biol Med. 42:583–598. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reddy NM, Kleeberger SR, Bream JH, Fallon
PG, Kensler TW, Yamamoto M and Reddy SP: Genetic disruption of the
Nrf2 compromises cell-cycle progression by impairing GSH-induced
redox signaling. Oncogene. 27:5821–5832. 2008. View Article : Google Scholar : PubMed/NCBI
|