Introduction
Osteosarcoma, the most prevalent bone malignancy,
occurred frequently in children and adolescents (1). The survival of osteosarcoma patients
is dependent on the clinical stage of the tumor and whether there
is lung metastasis (2). For
example, osteosarcoma without lung metastasis has an ideal
prognosis, with 5-year overall survival (OS) rate range from 20 to
70% after radical tumor resection, whereas osteosarcoma with lung
metastasis had an ~20% five-year OS rate (3,4).
Despite markedly improved diagnostic and treatment strategies,
recurrence and metastasis, especially lung metastasis, is the main
obstacle for the long-term survival of osteosarcoma (5). Previous studies reported that changes
in gene expression patterns were the main cause of osteosarcoma
metastasis (6), and identified a
number of some critical metastasis-associated genes, such as p53
and Rb (7,8). To date, the mechanism of osteosarcoma
metastasis remains elusive. Thus, it is urgently required to
elucidate the mechanisms underlying metastasis of osteosarcoma
which will help research on novel strategies for better treatment,
biomarkers for prognosis prediction.
Osteosarcoma metastasis is frequent to the lung via
the bloodstream, which is involved in multiple biological changes
of tumor cells, including cell proliferation, migration, invasion,
enabling tumor cells to invade into the vessels and escape from the
vessels to form a metastatic nodule in distant organs (9). Recently, substantial evidence also
indicate epithelial-mesenchymal transition (EMT), a normal biologic
process that enables an epithelial cell change into a mesenchymal
cell phenotype, is involved in the metastasis of osteosarcoma
(10,11). Actin-like protein 6A (ACTL6A), which
is a core 53 kDa subunit of the BRG1/BRM-associated factor complex,
involved in diverse cellular processes, such as vesicular
transport, spindle orientation, nuclear migration and chromatin
remodeling (12,13). ACTL6A is essential for neural
progenitor cell proliferation, differentiation and migration during
gastrulation (14). ACTL6A is a
subunit of the SWI/SNF ATP-dependent chromatin-remodeling complex,
which also functions in osteoblast differentiation (15). It was also reported that ACTL6A
could suppress epidermal differentiation and maintain the
progenitor state (16). The
conditional knockout of ACTL6A led to terminal differentiation of
the epidermis, whereas the ectopic expression of ACTL6A enhanced
and maintained the progenitor state of the epidermis (16), indicating that ACTL6A was involved
in EMT. Intriguingly, ACTL6A has been identified as a
transcriptional regulator and driving pathways that are of specific
benefit to the malignant elements within the tumor (17,18).
These pathways facilitate cell adhesion, proliferation, migration
and invasion (19). The above data
indicate the oncogenic role of ACTL6A in osteosarcoma. However, the
expression and clinical significance of ACTL6A in osteosarcoma it
is still unknown.
In this study, we assessed the ACTL6A expression in
osteosarcoma tissues, and the association of ACTL6A expression with
clinicopathological features and prognosis of osteosarcoma
patients. Then we explored the role of ACTL6A in osteosarcoma by
in vitro and in vivo assays, expecting to provide
insightful information for identifying ACTL6A is a novel biomarker
to predict the survival of osteosarcoma patients and exploring
ACTL6A as a potential target for osteosarcoma.
Materials and methods
Patients and tissue specimens
Three independent cohorts of osteosarcoma samples
totaling 216 osteosarcoma patients from two medical institutions
were enrolled in this study. Firstly, 30 pairs of frozen fresh
primary tumor tissues (PTs), matched non-cancerous bone tissues
(NCBTs) and five biopsy lung metastatic nodule tissues from
osteosarcoma patients after radical surgical resection were
collected from the Department of Orthopedic Surgery, the Second
Xiangya Hospital, Central South University (Hunan, China) between
January 2009 and December 2010. These tissue samples (screening
cohort) were used to screen the expression of ACTL6A mRNA and
protein. Another two independent cohorts of formaldehyde-fixed,
paraffin-embedded osteosarcoma tissue samples from 186 osteosarcoma
patients with radical surgical resection including training cohort
(n=110) and validation cohort (n=76) were randomly collected from
Department of Orthopedics Surgery, the Second Xiangya Hospital and
the Affiliated Cancer Hospital of Xiangya School of Medicine,
Central South University, respectively, between June 2007 and April
2010. All patients were pathologically diagnosed with osteosarcoma
according to the WHO bone tumor diagnosis criteria and staging
system. Data of clinical and pathological variables were also
collected for analysis. Prior informed consent was obtained and the
study protocol was approved by the Ethics Committee of Xiangya
School of Medicine, Central South University.
Patient follow-up
All osteosarcoma patients received regular follow-up
by the experienced medical staff in these two hospitals. The
follow-up period was defined as the interval between the date of
operation and that of the patient's death or the last follow-up.
All patients had a mean follow-up time of 63 months (ranging from 8
to 107 months). Recurrence and metastasis were monitored and
diagnosed by using clinical examination, ALP levels, computed
tomography (CT) or magnetic resonance imaging (MRI) at a
three-month interval. OS was defined as the time interval between
tumor resection and death or the last follow-up. Patients alive at
the end of follow-up or dead from causes without sign of recurrence
were censored. Disease-free survival (DFS) was calculated from
tumor resection to the first radiological evidence of metastasis
or/and recurrence or biopsies with histologically confirmed
metastasis or/and recurrence.
Cell lines and cell culture
The human fetal osteoblastic cell line hFOB 1.19,
and three human osteosarcoma cell lines MG-63, U2-OS and SAOS-2
were obtained from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and cultured in RPMI-1640 medium (Gibco-BRL,
Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum
(FBS; HyClone, Logan, UT, USA) in a humidified incubator with 5%
CO2 at 37°C.
Quantitative real-time polymerase
chain reaction (qRT-PCR)
Total RNA was isolated from fresh osteosarcoma
tissue samples and cell lines by using a TRIzol® reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. After quantification using a
spectrophotometer (Shimadzu, Kyoto, Japan), RNA samples were
reversely transcribed into cDNA using a universal cDNA synthesis
kit (Toyobo, Osaka, Japan). The cDNA was subjected to qRT-PCR using
the SYBR-Green PCR kit (Toyobo) and the assay was performed on an
PRISM 7300 Sequence Detection System (Applied Biosystems, Foster
City, CA, USA). The PCR cycling parameters were as follows: 50
cycles of 95°C for 5 sec and 60°C for 20 sec. The primers for
ACTL6A were used as follows: sense, 5′-CCAGGTCTCTATGGCAGTGTAA-3′
and antisense, 5′-CGTAAGGTGACAAAAGGAAGGTA-3′; the primers for
GAPDH: sense, 5′-CCACCCATGGCAAATTCC-3′ and antisense,
5′-GATGGGATTTCCATTGATGACA-3′. The relative levels of mRNA
expression were calculated using the 2−ΔΔCt method based
on the threshold cycle (Ct) values and then normalized to the
internal control of GAPDH. All the assays were performed in
triplicate.
Western blot analysis
Total cellular or tissue protein was extracted by
RIPA lysis buffer. The protein concentrations of the lysates were
determined according to the bicinchoninic acid (BCA) method using a
protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Cell or tissue lysates containing 100 µg proteins were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred onto PVDF membranes (Millipore,
Billerica, MA, USA). The PVDF membranes were then blocked by a 5%
skim milk solution for 30 min and incubated primary antibody
ACTL6A, E-cadherin and N-cadherin (all from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), Snail (Abcam, Cambridge,
MA, USA), Twist-1 and vimentin (both from Santa Cruz Biotechnology,
Inc.). Membranes were subsequently incubated with an HRP-conjugated
secondary antibody (KPL, Gaithersburg, MD, USA). After this, the
membranes were subjected to an enhanced chemiluminescence reagent
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and exposed to
x-films to detect protein bands. The β-actin antibody
(Sigma-Aldrich, St. Louis, MO, USA) was used as a loading control.
Protein expression was quantified by BandScan software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and defined as the ratio of
target protein relative to β-actin.
Immunohistochemistry
Paraffin-embedded tissues were cut into 4-µm
sections, and then 4-µm sections deparaffinized and rehydrated.
After antigen retrieval by heat-induced epitopes in the 1 mM, pH
8.0 EDTA buffer (Zhongshan Golden Bridge Biotechnology Co.,
Shanghai, China) for 20 min, endogenous peroxidases of tissue were
blocked for 20 min using 0.3% H2O2 (Zhongshan
Golden Bridge Biotechnology Co.) and then incubated with primary
antibody ACTL6A (1:200 dilution; Santa Cruz Biotechnology, Inc.) at
4°C overnight. The next day, the sections were incubated with
horseradish peroxidase-labeled secondary antibody (Zhongshan Golden
Bridge Biotechnology Co.) after washing with phosphate-buffered
saline (PBS). Subsequently, the sections were counterstained with
hematoxylin solution (Zhongshan Golden Bridge Biotechnology Co.)
and mounted with a coverslip. The immunohistochemical score of
ACTL6A was calculated according to the percentage of positive tumor
cells. The percentage of positive staining was defined as follow:
0, <5% positive cells; 1, 5–30% positive cells; 2, 31–50%
positive cells; 3, 51–80% positive cells; and 4, >80% positive
cells (20). Based on the protein
expression, osteosarcoma tissue specimens were divided into the
low-expression group (0–1) and high-expression group (≥2) for data
analysis.
Vector construction and stable
transfection
ACTL6A-knockdown plasmids carrying short hairpin
RNAs (shRNA) for ACTL6A (shACTL6A), ACTL6A-expressing plasmid
carrying ACTL6A expression vector inserted with ACTL6A coding
sequences (CDSs) and its negative control were purchased from
GeneChem (Shanghai, China). The sequences of the three shRNAs used
to knockdown ACTL6A expression were as follows: ACTL6A-shRNA-1,
5′-TCCAAGTATGCGGTTGAAA-3′; ACTL6A-shRNA-2,
5′-GTACTTCAAGTGTCAGATT-3′; ACTL6A-shRNA-3,
5′-GGGATAGTTTCCAAGCTAT-3′.
MG-63 cells used for this study were transfected
with shACTL6A, ACTL6A expressing or negative control plasmids.
Briefly, exponentially growing MG-63 cells were transfected with
plasmids using Lipofectamine 2000 (Invitrogen Life Technologies)
according to manufacturer's instructions. Cells transfected with
negative controls were used as controls. The cells were selected
with 3 µg/ml puromycin (Invitrogen Life Technologies) to obtain the
stable cell population. Then, the ACTL6A-knockdown efficiency of
three shRNAs, ACTL6A overexpression was validated by qRT-PCR and
western blot analysis.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
MTT assays were used to determine the level of cell
proliferation. Cells (5×103) were seeded into each well
of 96-well plates and incubated in a humidified incubator with 5%
CO2 at 37°C. Three wells of each group were detected
every day for up to 7 days. Fresh medium (100 µl) containing 0.5
mg/ml MTT (Sigma-Aldich) was added to each well and incubated at
37°C for 4 h. The culture medium was then replaced with 100 µl of
dimethyl sulfoxide (DMSO) (Sigma-Aldrich) and incubated at room
temperature for 10 min. The optical density of the cells was
measured at 570 nm using a spectrophotometer (BioTek Instruments,
Inc., Winooski, VT, USA). The experiments were repeated at least
three times.
Wound healing assay
Wound healing assays were used to assess the cell
migration capacity (21,22). MG-63 cells with ACTL6A-knockdown,
overexpression or control were grown in RPMI-1640 medium with 10%
FBS to reach ~90% confluence. Cells were incubate with 10 µg/ml
puromycin for 1 h, and then rinsed with ice-cold PBS, and then
further cultured in serum-free medium for 24 h. Then, three
separate parallel wounds were created using a sterile 10 µl pipette
tip and then rinsed with ice-cold PBS. The wound closure was
recorded after 24 h. The experiment was performed in
triplicate.
Transwell assay
Transwell assays were performed to analyze the cell
invasion capacity (23). Briefly,
the cells at a density of 1×105 in RPMI-1640 containing
0.1% bovine serum albumin (BSA) were seeded into the upper chambers
of Transwell insert precoated with Matrigel (BD Biosciences, San
Jose, CA, USA), while the bottom chambers were filled with 200 µl
of RPMI-1640 containing 10% FBS. The cells were cultured at 37°C
for 24 h. The cells remaining in the upper chamber were removed
using a cotton swap. After fixing with 20% methanol and staining
with a solution containing 0.1% crystal violet (Beyotime Institute
of Biotechnology, Beijing, China), the number of cells invading
into the lower membrane of the inserts was counted and imaged using
an inverted fluorescence microscope TE-2000S (Nikon, Tokyo, Japan).
For each experiment group, the assay was performed in triplicate,
and five random fields of each replicate were chosen for
analysis.
Immunofluorescence analysis
Immunofluorescence analysis was used to analyze the
effect of ACTL6A on actin cytoskeleton of osteosarcoma cells. To
allow direct fluorescence of actin cytoskeleton, the cells were
stained with 0.25 mM tetramethylrhodamine isothiocyanate
(TRITC)-conjugated phalloidin (Sigma-Aldrich). Nuclei were
visualized with 4′,6-diamidino-2-phenylindole (DAPI). The slides
were imaged by an inverted fluorescence microscope TE-2000S
(Nikon).
In vivo metastatic assays
The animal study was approved by the Institutional
Animal Care and Use Committee (IACUC) of The Second Xiangya
Hospital, Central South University. The animal experiments were
performed in accordance with standard IACUC procedures. In briefly,
4-week-old BALB/c nude mice were obtained from Medical Animal
Research Institute, Central South University and used to assay the
metastatic potential of osteosarcoma cells. MG-63 cells
(5×106) of each group were suspended in 0.1 ml of saline
and injected into the tail vein of each mouse (n=6). After 30 days,
the animals were sacrificed and autopsied. Lung tissue of each
mouse was harvested and then fixed in 10% buffered formalin,
embedded in paraffin, serially sectioned, and then stained with
hematoxylin and eosin (H&E). The number of lung tumor
metastatic nodules was counted under the microscope (Nikon).
Statistical analysis
All statistical analyses were performed using SPSS
18.0 statistical software (SPSS Inc., Chicago, IL, USA).
Categorical data were analyzed using Fisher's exact test.
Continuous data were summarized as mean ± SD and analyzed by using
an independent t-test when the variance was homogeneous, or a
Mann-Whitney U test if the variance was not homogeneous. OS and DFS
curves were plotted using the Kaplan-Meier method and compared by
the log-rank test. The univariate and multivariate Cox proportional
hazards regression model was used to identify independent
predictors for OS and DFS. All tests were two-tailed and P<0.05
was considered statistically significant.
Results
ACTL6A is overexpressed in human
osteosarcoma tissues and cell lines
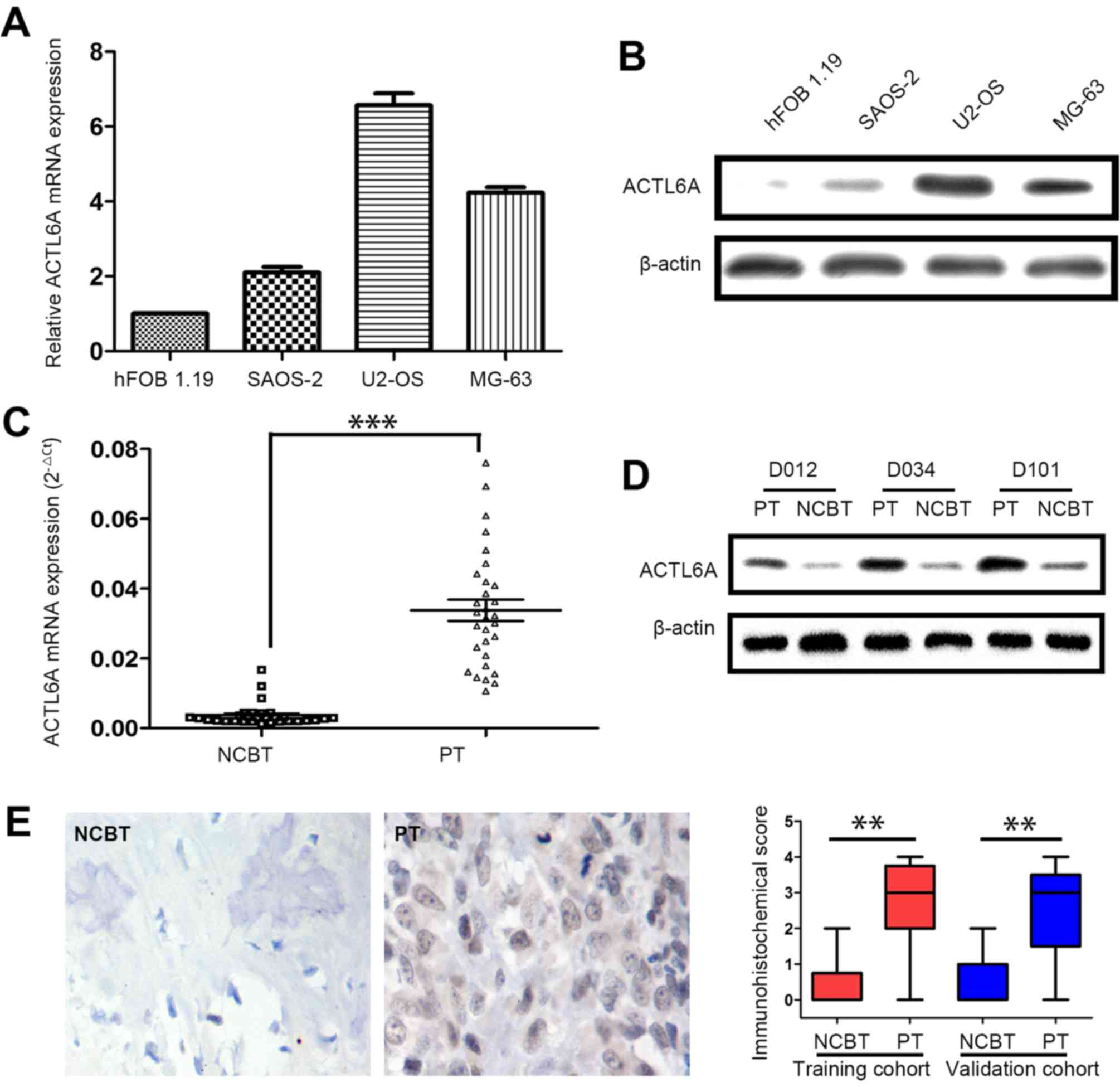
Firstly, the level of ACTL6A expression was assessed
in osteosarcoma cell lines by qRT-PCR and western blot analysis.
Data showed that, compared to the normal cell line hFOB 1.19,
ACTL6A mRNA was overexpressed in osteosarcoma cell lines by qRT-PCR
(Fig. 1A). Western blot analysis
also showed ACTL6A protein was highly expressed in osteosarcoma
cell lines (Fig. 1B). Next, we
screened the ACTL6A expression in 30 pairs of fresh osteosarcoma
tissues in screening cohort. Notably, the levels of ACTL6A mRNA and
protein were dramatically higher in PTs than in matched
non-cancerous bone tissues (NCBTs) (Fig. 1C and D). Furthermore,
immunohistochemical (IHC) analysis also showed the expression of
ACTL6 protein was significantly higher in PT than in NCBTs from
training and validation cohort (Fig.
1E).
ACTL6A overexpression is associated
with osteosarcoma metastasis
Then, we analyzed ACTL6A expression among 30
different fresh PTs. Interestingly, ACTL6A expression was higher in
PTs of tumor node metastasis (TNM) stage III than that of TNM stage
I/II (Fig. 2A). Additionally,
ACTL6A expression was much higher in PTs from patients who
developed metastases and/or recurrence than those who did not
(Fig. 2B). Importantly, ACTL6A
expression was higher in lung metastatic nodules (LMNs) than in
their corresponding PTs and NCBTs from same patients (n=5) and the
ACTL6A expression exhibited the gradient increase from NCBTs, PTs
to LMN (Fig. 2C and D). Taken
together, these data indicate that ACTL6A may contribute to
osteosarcoma progression and metastasis.
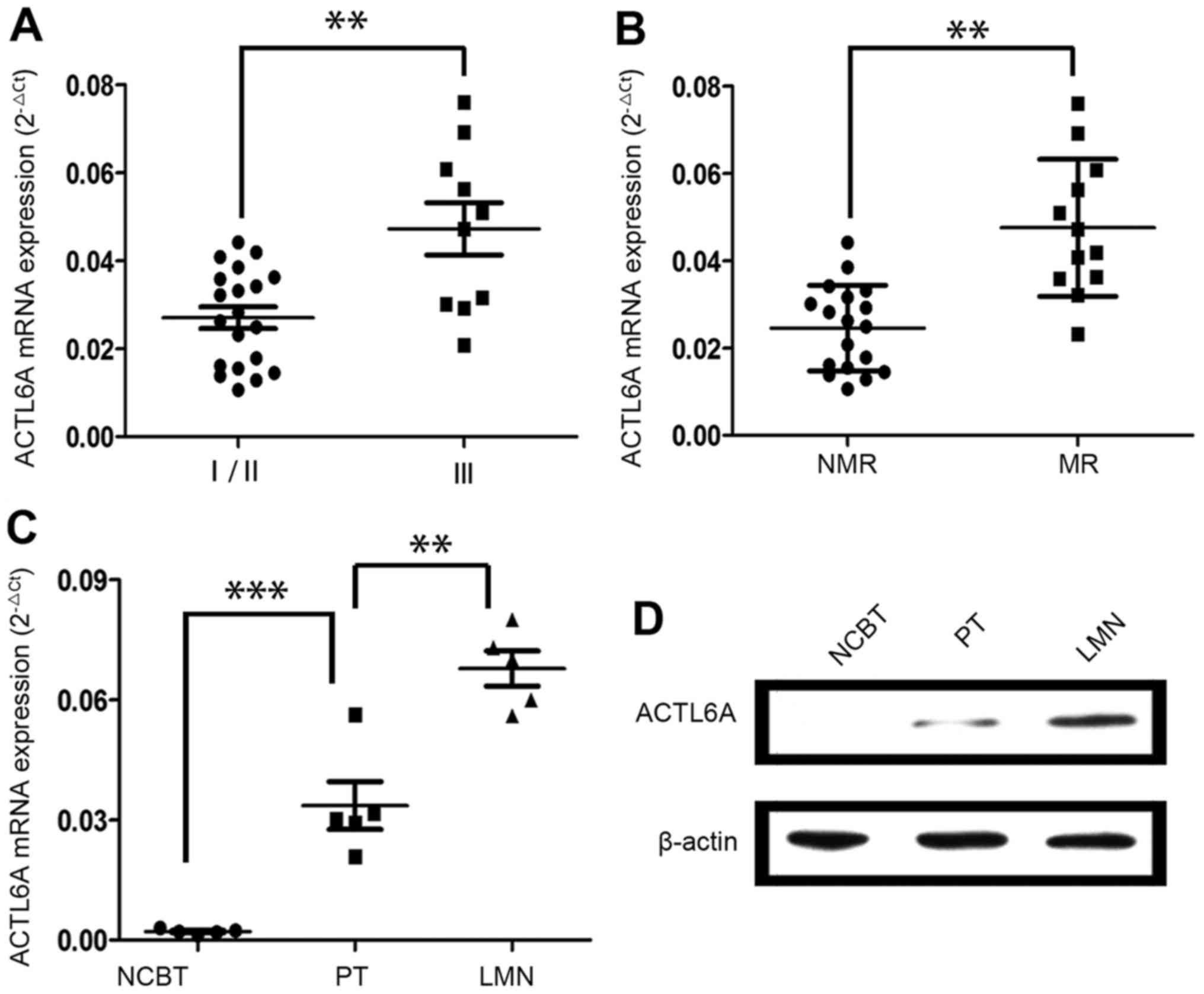 | Figure 2.ACTL6A overexpression is associated
with osteosarcoma metastasis. (A) ACTL6A expression was higher in
PTs at TNM stage III than in those at TNM stage I/II. (B) ACTL6A
expression was much higher in PTs from patients who developed
metastases or recurrence than those who did not. MR, with
metastases or recurrence; NMR, without metastases or recurrence. (C
and D) ACTL6A expression in LMNs, PTs and their corresponding NCBTs
from the same patient (n=5). (C) ACTL6A mRNA expression exhibited
the gradient increase from NCBTs, PTs to LMNs. (D) Representative
images of western blot analysis. Results showed the ACTL6A protein
expression exhibited the gradient increase from NCBTs, PTs to LMNs.
**P<0.01, ***P<0.001. ACTL6A, actin-like protein 6A; TNM,
tumor node metastasis; PTs, primary tumor tissues; LMNs, lung
metastatic nodules; NCBTs, non-canerous bone tissues. |
Association of ACTL6A expression with
clinicopathological characteristics in osteosarcoma patients
Further, IHC was used to detect the ACTL6A
expression in osteosarcoma samples from two cohorts of patients
including training cohort (n=110) and validation cohort (n=76).
Detail demographics and clinicopathological characteristics of two
cohorts of patients are described in the Table I. In training cohort, patients were
divided into two groups, the high ACTL6A expression (Fig. 3A) and the low ACTL6A expression
(Fig. 3B) based on ACTL6A
expression. We found that 60.0% (66/110) of PTs exhibited high
ACTL6A expression, as compared with 10.9% (12/110) in corresponding
NCBTs. By analyzing the relationship between the levels of ACTL6A
expression in PTs and the clinicopathological characteristics,
results showed that high ACTL6A expression was significantly
associated with large tumor size (P<0.001), presence of
pathological facture (P=0.018), high Ennecking grade (P=0.015),
high histologic grade (P<0.006) and advanced TNM stage
(P=0.005). However, it was not associated with patient gender
(P=0.676), age (P=0.275), anatomical localization of tumor
(P=0.843) or ALP level (P=0.525) (Table II).
 | Table I.Patient demographics and
clinicopathological characteristics in the training and validation
cohorts. |
Table I.
Patient demographics and
clinicopathological characteristics in the training and validation
cohorts.
|
| Training
cohort | Validation
cohort |
|
|---|
|
|
|
|
|
|---|
| Variables | n | % | n | % | P-value |
|---|
| Gender |
|
|
|
| 0.314 |
|
Male | 75 | 68.2 | 57 | 75.0 |
|
|
Female | 35 | 31.8 | 19 | 25.0 |
|
| Age (years) |
|
|
|
| 0.319 |
|
<45 | 57 | 51.8 | 45 | 59.2 |
|
|
>45 | 53 | 48.2 | 31 | 40.8 |
|
| Anatomical
site |
|
|
|
| 0.243 |
|
Femur/tibia | 89 | 80.9 | 56 | 73.7 |
|
|
Elsewhere | 21 | 19.1 | 20 | 26.3 |
|
| Tumor size
(cm) |
|
|
|
| 0.797 |
|
<8 | 50 | 45.5 | 36 | 47.4 |
|
|
>8 | 60 | 54.5 | 40 | 52.6 |
|
| ALP level
(U/l) |
|
|
|
| 0.800 |
|
<150 | 44 | 44.0 | 29 | 38.2 |
|
|
>150 | 66 | 66.0 | 47 | 61.8 |
|
| Pathological
fracture |
|
|
|
| 0.517 |
|
Absent | 91 | 82.7 | 60 | 78.9 |
|
|
Present | 19 | 17.3 | 16 | 21.1 |
|
| Ennecking
grade |
|
|
|
| 0.706 |
| 2a | 61 | 55.5 | 40 | 52.6 |
|
| 2b | 49 | 44.5 | 36 | 47.4 |
|
| Histologic
grade |
|
|
|
| 0.220 |
|
Low | 65 | 59.1 | 38 | 50.0 |
|
|
High | 45 | 40.9 | 38 | 50.0 |
|
| TNM stage |
|
|
|
| 0.882 |
|
I/II | 62 | 56.4 | 42 | 55.3 |
|
|
III | 48 | 43.6 | 34 | 44.7 |
|
 | Table II.Correlations of ACTL6A expression
with clinicopathological variables of osteosarcoma in training and
validation cohort. |
Table II.
Correlations of ACTL6A expression
with clinicopathological variables of osteosarcoma in training and
validation cohort.
|
| Training
cohort | Validation
cohort |
|---|
|
|
|
|
|---|
|
|
| ACTL6A
expression |
|
| ACTL6A
expression |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Variables | N | Low | High | P-value | N | Low | High | P-value |
|---|
| Gender |
|
|
| 0.676 |
|
|
| 0.890 |
|
Male | 75 | 31 | 44 |
| 57 | 20 | 37 |
|
|
Female | 35 | 13 | 22 |
| 19 | 7 | 12 |
|
| Age (years) |
|
|
| 0.275 |
|
|
| 0.621 |
|
<45 | 57 | 20 | 37 |
| 45 | 17 | 28 |
|
|
>45 | 53 | 24 | 29 |
| 31 | 10 | 21 |
|
| Anatomical
site |
|
|
| 0.843 |
|
|
| 0.302 |
|
Femur/tibia | 89 | 36 | 53 |
| 56 | 18 | 38 |
|
|
Elsewhere | 21 | 8 | 13 |
| 20 | 9 | 11 |
|
| Tumor size
(cm) |
|
|
|
<0.001 |
|
|
| 0.003 |
|
<8 | 50 | 30 | 20 |
| 36 | 19 | 17 |
|
|
>8 | 60 | 14 | 46 |
| 40 | 8 | 32 |
|
| ALP level
(U/1) |
|
|
| 0.525 |
|
|
| 0.281 |
|
<150 | 44 | 16 | 28 |
| 29 | 14 | 15 |
|
|
>150 | 66 | 28 | 38 |
| 47 | 23 | 24 |
|
| Pathological
fracture |
|
|
| 0.018 |
|
|
| 0.030 |
|
Absent | 91 | 41 | 50 |
| 60 | 25 | 35 |
|
|
Present | 19 | 3 | 16 |
| 16 | 2 | 14 |
|
| Ennecking
grade |
|
|
| 0.028 |
|
|
| 0.022 |
| 2a | 61 | 30 | 31 |
| 40 | 19 | 21 |
|
| 2b | 49 | 14 | 35 |
| 36 | 8 | 28 |
|
| Histologic
grade |
|
|
| 0.006 |
|
|
| 0.008 |
|
Low | 65 | 33 | 32 |
| 38 | 19 | 19 |
|
|
High | 45 | 11 | 34 |
| 38 | 8 | 30 |
|
| TNM stage |
|
|
| 0.005 |
|
|
| 0.014 |
|
I/II | 62 | 32 | 30 |
| 42 | 20 | 22 |
|
|
III | 48 | 12 | 36 |
| 34 | 7 | 27 |
|
Furthermore, we then confirmed the
results in validation cohort of 76 osteosarcoma patients
Our data showed that ACTL6A protein was
overexpressed in 64.5% (49/76) of PTs, as compared with 13.2%
(10/76) in corresponding NCBTs by IHC. High ACTL6A expression was
significantly associated with large tumor size (P=0.003), presence
of pathological facture (P=0.030), high Ennecking grade (P=0.022),
high histologic grade (P=0.008) and advanced TNM stage (P=0.014),
whereas it was not associated with patient gender (P=0.890), age
(P=0.621), anatomical localization of tumor (P=0.302) or ALP level
(P=0.281) (Table II).
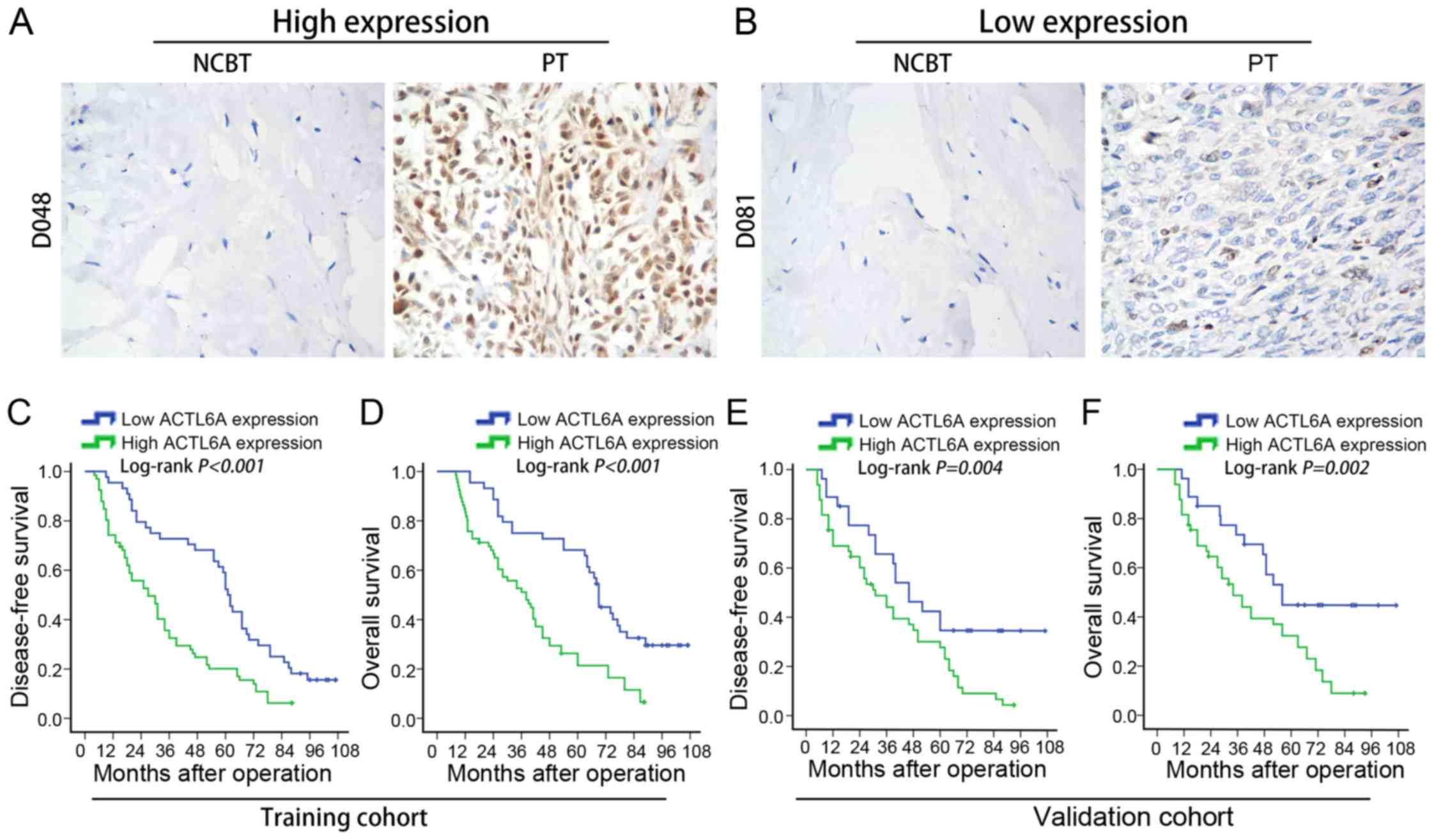
High ACTL6A expression level
correlated with poor prognosis of osteosarcoma patients
To evaluate the prognostic potential of ACTL6A
expression in osteosarcoma tissues, we compared the DFS and OS
between patients with high ACTL6A expression and those with low
ACTL6A expression. In training cohort, we found that patients with
high ACTL6A expression had a shorter DFS (P<0.001; Fig. 3C) and had a shorter OS (P<0.001;
Fig. 3D) than those with low ACTL6A
expression. To determine whether ACTL6A expression was an
independent prognostic factor for osteosarcoma, a univariate
analysis was first performed followed by a subsequent multivariate
Cox proportional hazards analyses. Remarkably, high ACTL6A
expression was found to be a significant and independent predictor
for DFS (HR: 3.409, 95% CI: 2.113–5.500, P=0.004; Table III) and OS (HR: 3.602, 95% CI:
2.021–6.420, P=0.006; Table III)
in osteosarcoma.
 | Table III.Univariate and multivariate analysis
of DFS and OS in training cohort. |
Table III.
Univariate and multivariate analysis
of DFS and OS in training cohort.
|
|
| DFS | OS |
|---|
|
|
|
|
|
|---|
|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|
|---|
| Variables |
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Gender | Male vs.
female | 1.075
(0.702–1.634) | 0.302 |
| NA | 1.162
(0.603–2.246) | 0.209 |
| NA |
| Age (years) | <45 vs.
>45 | 0.973
(0.672–1.401) | 0.148 |
| NA | 0.802
(0.541–1.192) | 0.131 |
| NA |
| Anatomical
site | Femur/tibia vs.
elsewhere | 0.786
(0.514–1.197) | 0.153 |
| NA | 0.914
(0.635–1.748) | 0.206 |
| NA |
| Tumor size
(cm) | <8 vs.
>8 | 2.043
(1.434–2.915) | 0.004 | 1.824
(1.403–2.371) | 0.035 | 1.908
(1.364–2.652) | 0.009 | 1.565
(1.186–2.064) | 0.028 |
| ALP level
(U/1) | <150 vs.
>150 | 4.252
(2.923–6.181) |
<0.001 | 2.154
(1.731–2.675) | 0.019 | 3.843
(2.324–6.353) |
<0.001 | 2.023
(1.612–2.564) | 0.017 |
| Pathological
fracture | Absent vs.
present | 3.063
(1.833–5.114) | 0.002 | 2.034
(1.562–2.642) | 0.026 | 3.301
(2.343–4.654) | 0.005 | 1.914
(1.502–2.431) | 0.020 |
| Ennecking
grade | 2a vs. 2b | 1.378
(0.834–2.262) | 0.082 | 1.036
(0.693–1.542) | 0.185 | 1.431
(0.931–2.191) | 0.071 | 1.053
(0.902–1.231) | 0.093 |
| Histologic
grade | Low vs. high | 1.643
(1.011–2.665) | 0.045 | 1.084
(0.762–1.552) | 0.064 | 1.176
(0.913–1.512) | 0.105 |
| NA |
| TNM stage | I/II vs. III | 2.972
(2.061–4.283) | 0.003 | 3.213
(2.042–5.051) | 0.007 | 3.082
(2.174–4.369) | 0.002 | 2.401
(1.792–3.217) | 0.011 |
| ACTL6A
expression | Low vs. high | 4.943
(2.925–8.366) |
<0.001 | 3.409
(2.113–5.500) | 0.004 | 4.482
(3.101–6.472) |
<0.001 | 3.602
(2.021–6.420) | 0.006 |
Furthermore, we confirmed the correlation between
ACTL6A expression level and osteosarcoma prognosis in validation
cohort and also found that osteosarcoma patients with high ACTL6A
expression had a worse DFS (P=0.004; Fig. 3E) and a worse OS (P=0.002; Fig. 3F) than those with low ACTL6A
expression. Multivariate Cox regression analysis also showed that
high ACTL6A expression was an independent predictor for DFS (HR:
2.718, 95% CI: 1.514–4.879, P=0.010; Table IV) and OS (HR: 2.834, 95% CI:
2.006–4.003, P=0.013; Table IV).
Above of all, these data suggest that high ACTL6A expression
predicts poor prognosis in osteosarcoma patients and may contribute
to the metastasis of osteosarcoma.
 | Table IV.Univariate and multivariate analysis
of DFS and OS in validation cohort. |
Table IV.
Univariate and multivariate analysis
of DFS and OS in validation cohort.
|
|
| DFS | OS |
|---|
|
|
|
|
|
|---|
|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|
|---|
| Variables |
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Gender | Male vs.
female | 1.244
(0.741–2.064) | 0.401 |
| NA | 1.125
(0.672–1.873) | 0.148 |
| NA |
| Age (years) | <45 vs.
>45 | 0.867
(0.573–1.294) | 0.109 |
| NA | 0.912
(0.673–1.234) | 0.126 |
| NA |
| Anatomical
site | Femur/tibia vs.
elsewhere | 0.782
(0.531–1.143) | 0.130 |
| NA | 0.773
(0.514–1.165) | 0.153 |
| NA |
| Tumor size
(cm) | <8 vs.
>8 | 3.802
(2.071–4.584) |
<0.001 | 2.603
(1.815–3.186) | 0.017 | 2.643
(1.871–3.722) | 0.009 | 1.794
(1.247–2.581) | 0.036 |
| ALP level
(U/1) | <150 vs.
>150 | 2.715
(1.682–4.372) | 0.007 | 2.525
(1.503–4.232) | 0.023 | 3.434
(2.311–5.094) | 0.004 | 2.396
(1.523–3.752) | 0.016 |
| Pathological
fracture | Absent vs.
present | 4.013
(2.782–5.786) |
<0.001 | 3.046
(2.017–4.593) |
<0.001 | 3.644
(2.383–5.562) |
<0.001 | 2.632
(1.947–3.572) | 0.005 |
| Ennecking
grade | 2a vs. 2b | 1.205
(1.051–1.372) | 0.048 | 1.044
(0.902–1.212) | 0.097 | 1.067
(0.834–1.352) | 0.182 |
| NA |
| Histologic
grade | Low vs. high | 1.108
(0.914–1.332) | 0.104 |
| NA | 1.038
(0.724–1.473) | 0.207 |
| NA |
| TNM stage | I/II vs. III | 2.601
(1.534–4.410) | 0.008 | 2.149
(1.373–3.344) | 0.039 | 2.847
(1.681–4.850) | 0.006 | 2.437
(1.421–4.297) | 0.025 |
| ACTL6A
expression | Low vs. high | 3.046
(2.293–4.046) | 0.004 | 2.718
(1.514–4.879) | 0.010 | 3.801
(2.412–5.990) | 0.002 | 2.834
(2.006–4.003) | 0.013 |
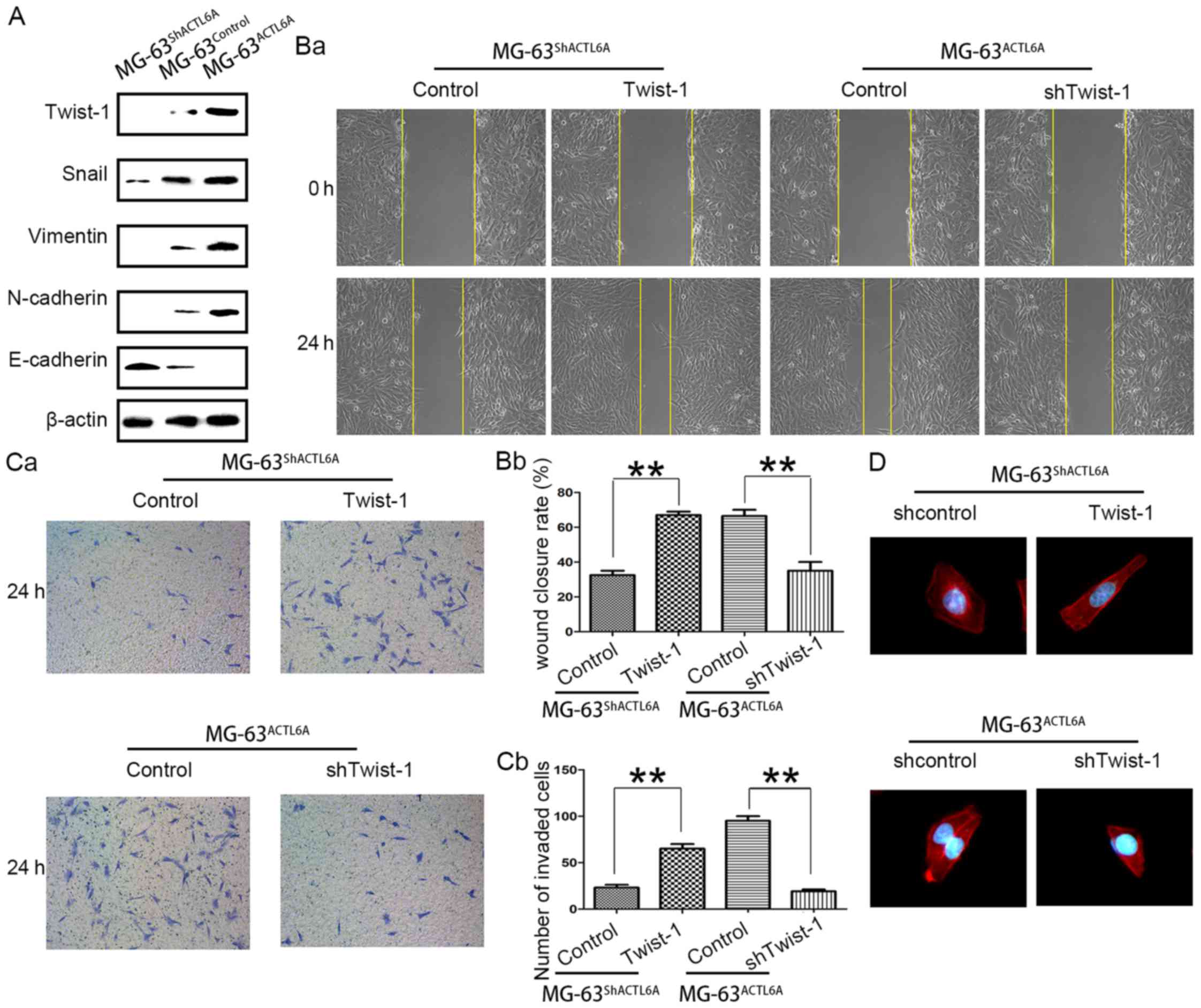
ACTL6A enhances invasion and
metastasis of osteosarcoma cells in vitro and in vivo
To determine the roles of ACTL6A in osteosarcoma
invasion and metastasis, we established ACTL6A overexpression cell
line MG-63ACTL6A, ACTL6A-knockdown cell line
MG-63shACTL6A and its control cell line
MG-63Control (Fig.
4A-a-b) according to the expression level of ACTL6A in
osteosarcoma cell lines and biological characteristics of
osteosarcoma cell lines. By wound healing assays, we evidenced that
overexpression of ACTL6A enhanced MG-63 cell migration, whereas
knockdown of ACTL6A dramatically inhibited migration (Fig. 4B-a-b). Transwell invasion assays
showed that, compared with MG-63Control, a significant
increase in the number of invaded cells was observed in
MG-63ACTL6A group, but an obvious decrease in the number
of invaded cells was observed in MG-63shACTL6A group
(Fig. 4C-a-b). Immunofluorescence
was used to analyze the cell cytoskeleton by F-actin staining. The
results showed that compared to MG-63Control and
MG-63shACTL6A cells showed the stress fiber-like
structures disappeared and the morphology regressed to cobble-stone
shape, but MG-63ACTL6A cells showed obvious
reorganization of actin cytoskeleton and the cell appearance
changed into more spindle-like, fibroblastic morphology (Fig. 4D). Also, we observed overexpression
of ACTL6A significantly accelerated MG-63 cell proliferation, while
knockdown of ACTL6A dramatically inhibited MG-63 cells
proliferation by MTT assays (Fig.
4E). These data support a metastasis-promoting role of ACTL6A
in osteosarcoma.
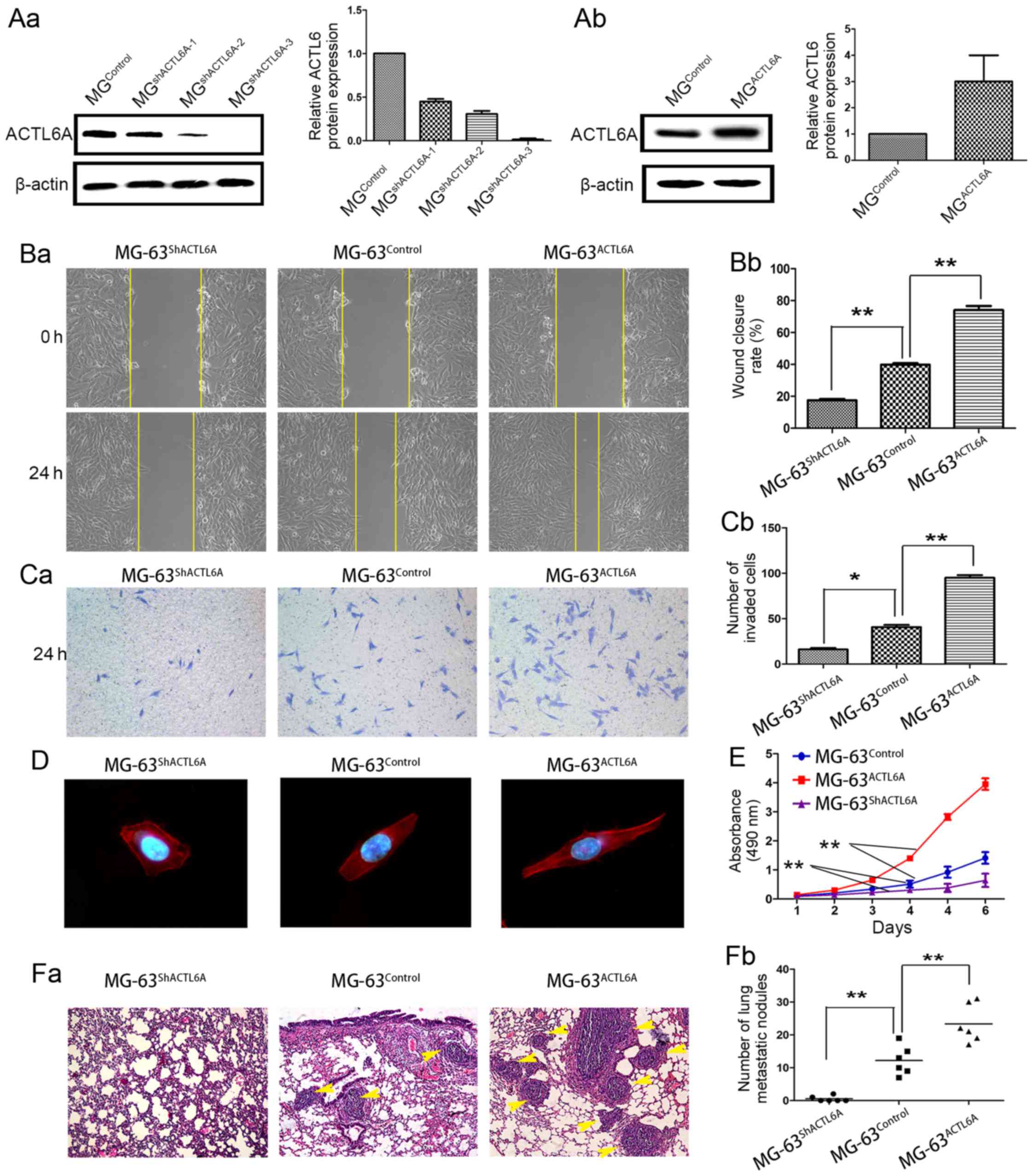 | Figure 4.ACTL6A promotes invasion and
metastasis of osteosarcoma cells in vitro and in
vivo. (A) ACTL6A overexpression and knockdown cells were stably
established. (A-a) MG-63 osteosarcoma cells were stably transfected
with shRNA plasmids and (A-b) ACTL6A-expressing plasmids, and then
subjected to analysis of ACTL6A expression by western blot
analysis. (B-a-b) Wound healing assays showed ACTL6A promoted
migration of osteosarcoma cells. (B-a) Representative micrographs
of wound healing assays in each group. (B-b) The rate of wound
healing in each group was calculated and compared. Results showed
ACTL6A-overexpression in osteosarcoma cells MG-63ACTL6A
exhibited a faster wound healing capacity than control cells
MG-63Control, whereas ACTL6A-knockdown osteosarcoma
cells MG-63shACTL6A showed lower closure than control
cells MG-63Control. (C-a-b) Transwell invasion assays
showed that ACTL6A enhanced invasion of osteosarcoma cells. (C-a)
Representative micrographs of Transwell assays in each group. (C-b)
The number of invaded cells in each group was calculated and
compared. Results showed that ACTL6A-overexpression MG-63 cells
MG-63ACTL6A exhibited increase in invasion compared with
control cells MG-63Control. In contrast,
ACTL6A-knockdown MG-63 cells MG-63shACTL6A showed
decrease in invasion compared with control cells
MG-63Control. (D) ACTL6A affected cell morphological
changes of osteosarcoma cells. Immunofluorescence was used to
analyze the morphological changes of MG-63 cells infected with
plasmids expressing ACTL6A shRNA, ACTL6A and its negative control.
F-actin filaments were visualized in cells using
rhodamine-phalloidin. (E) MTT was used to assay the proliferation
ability of MG-63ACTL6A, MG-63shACTL6A and
MG-63Control cells. (F-a-b) ACTL6A enhanced osteosarcoma
cell metastasis in vivo. (F-a) Representative images of lung
metastasis, (F-b) the number of lung metastatic nodules was
calculated and compared. Magnification, ×100. *P<0.05,
**P<0.01. Error bar, SD. ACTL6A, actin-like protein 6A; SD,
standard deviation. |
To verify the in vitro results, we further
evaluated the role of ACTL6A by using an in vivo mouse
metastasis model. We observed that nude mice injected with
MG-63ACTL6A cells had a markedly larger size and number
of lung metastasis tumor nodules than those injected with
MG-63Control cells (Fig.
4F-a-b). However, nude mice injected with
MG-63shACTL6A cells had a smaller size and number of
lung metastatic tumors than those injected with
MG-63Control cells (Fig.
4F-a-b). By in vitro and in vivo assays, we
demonstrated ACTL6A have the potential of enhancing invasion and
metastasis of osteosarcoma cells.
ACTL6A enhances metastasis of
osteosarcoma by promoting EMT
ACTL6A suppress epidermal differentiation, and
maintain the progenitor state (16). Such a phenomenon was associated with
EMT in carcinoma cells, an important mechanism on metastasis.
Interestingly, knockdown of ACTL6A resulted in the cell appearance
with more cobble-like, epithelial morphology and the cell
appearance became more spindle-like, fibroblastic morphology when
ACTL6A overexpressed in MG-63 cells (Fig. 4D), these suggested that ACTL6A may
be associated with EMT. Western blot analysis showed the expression
of mesenchymal marker such as Twist-1, Snail, vimentin, and
N-cadherin was significantly lower in MG-63shACTL6A
cells than in MG-63Control cells, while the expression
of epithelial marker E-cadherin was much higher in
MG-63shACTL6A than in MG-63Control cells.
Conversely, relative to MG-63Control cells, the
expression of mesenchymal marker such as Snail, Twist-1, vimentin
and N-cadherin was significantly increased in
MG-63ACTL6A cells and the expression of epithelial
marker E-cadherin was highly reduced in MG-63ACTL6A
cells (Fig. 5A). These indicate
that ACTL6A may enhance metastasis of osteosarcoma by facilitating
EMT.
Next, to confirm whether ACTL6A promotes invasion
and metastasis of hepatocellular carcinoma (HCC) by facilitating
EMT, we reintroduced Twist-1 into MG-63shACTL6A and
knocked down Twist-1 in MG-63ACTL6A (data not shown), as
Twist-1, a key EMT driver, has been implicated in regulating the
osteogenic cell lineage differentiation (24,25).
The wound healing assays showed that cells migrated faster after
Twist-1 was reintroduced into MG-63shACTL6A cells, when
downregulation of Twist-1 expression in MG-63ACTL6A
restrain the ACTL6A-promoted migration (Fig. 5B-a-b). Similarly, the Transwell
invasion assays also showed that the numbers of
MG-63shACTL6A cells passed through the Matrigel was
significantly increased after Twist-1 was reintroduced, while the
number of MG-63ACTL6A passed through the Matrigel was
significantly decreased after Twist-1 knockdown (Fig. 5C-a-b). Further,
MG-63shACTL6A showed the reorganization of actin
cytoskeleton and changed the cell appearance to more spindle-like,
fibroblastic morphology after Twist-1 was reintroduced, but the
stress fiber-like structures disappeared and the morphology
regressed to cobble-stone shape in MG-63ACTL6A cells
after Twist-1 knockdown (Fig. 5D).
In all, these studies suggested that ACTL6A could promote
osteosarcoma cell metastasis by enhancing EMT.
Discussion
Metastasis, mainly present as pulmonary metastasis,
significantly affects the prognosis of osteosarcoma patients
(26). The mechanism of
osteosarcoma metastasis remains to be determined. ACTL6A, a nuclear
actin-related protein (Arp), is a component of a number of
chromatin-modifying complexes SWI/SNF and is indispensable for
SWI/SNF complex regulation of chromatin structure (27). SWI/SNF, in its role as a
chromatin-remodeling complex and transcription, is a convergent
point for signaling from the various hormones, growth factors, and
kinase cascades that influence lineage choice in stem cells,
especially in an increasing tendency of bone marrow stromal cells
(BMSCs) to differentiate into adipocytes at the expense of
osteoblasts (15). These biological
functions are also involved in oncogenic phenomena, suggesting the
possible oncogenic role of ACTL6A in osteosarcoma.
In the present study, we demonstrated that both
ACTL6A mRNA and protein levels elevated significantly in
osteosarcoma tissues and cell lines. High ACTL6A expression was
found to be positively correlated with the metastatic potential of
osteosarcoma cells. These suggest ACTL6A may play a role in
osteosarcoma metastasis. Further analysis of the association of
ACTL6A expression with the clinicopathologic characteristics in
osteosarcoma patients of training cohort reveals that ACTL6A
overexpression was significantly correlated with malignant
clinicopathological variables, including large tumor size, presence
of pathological facture, high Ennecking grade and high histologic
grade. These clinicopathological variables are associated with poor
prognosis of the patients with osteosarcoma (28), suggesting ACTL6A expression may be
important for the acquirement of malignant potential in
osteosarcoma. Moreover, compared with early stage osteosarcoma,
ACTL6A expression was also significantly upregulated in tumors in
advanced osteosarcoma, suggesting ACTL6A plays an important role in
the progression of osteosarcoma. The Kaplan-Meier analysis shows
that the osteosarcoma patients with high ACTL6A expression in
general had worse prognosis than those with low expression, and
multivariable Cox regression analysis indicates that high ACTL6A
expression is an independent risk factor for the prognosis of
osteosarcoma patients. The validity of ACTL6A to predict
osteosarcoma prognosis was validated in validation cohort,
suggesting that ACTL6A may be a useful biomarker to predict the
survival of osteosarcoma patients as well as the necessity for
further study of ACTL6A as a novel target for future control of
osteosarcoma. These data also manifest that ACTL6A shows the
biological function of metastasis in osteosarcoma.
In vitro experiments were performed to
confirm that overexpression of ACTL6A in osteosarcoma cells
significantly enhanced cell migration, invasion and proliferation,
while knockdown of ACTL6A expression in osteosarcoma cells markedly
inhibited cell migration, invasion and proliferation. In
vivo nude mouse model data of lung metastasis further confirmed
that ACTL6A promoted metastasis of osteosarcoma cells. Our data
mirrored the biological function of ACTL6A in cell proliferation
and migration in developmental biology (14), suggesting that ACTL6A may have a
potential role in promotion of tumor metastasis. Our data are novel
in osteosarcoma, although also consistent with a recent study
showing ACTL6A expression upregulated in HCC tissues and ACTL6A
promoted HCC cell proliferation, invasion and metastasis (29).
EMT, enabling the tumor cells to gain invasive and
metastatic properties, plays a vital role in the initiation of
cancer metastasis (24,30). ACTL6A suppresses epidermal
differentiation, and maintains the progenitor state (16), which is associated with EMT.
Therefore, it is of interest to investigate whether ACTL6A promotes
invasion and metastasis of osteosarcoma cells by facilitating EMT.
Our findings indicate that ACTL6A could modulate the reorganization
of F-actin and the formation of stress fiber-like structures.
ACTL6A depletion markedly attenuated the expression of mesenchymal
markers such as Twist-1, Snail, vimentin and N-cadherin, while
increased the expression of epithelial marker E-cadherin.
Conversely, ACTL6A overexpression significantly upregulated the
expression of mesenchymal markers such as Twist-1, vimentin and
N-cadherin, while downregulated the expression of epithelial marker
E-cadherin. Twist-1 is a key EMT driver that directly binds to CDH1
promoter and inhibits E-cadherin transcription (31). Analysis of EMT transcription factors
Twist-1 in ACTL6A-mediated EMT program found that reintroduced
Twist-1 into MG-63shACTL6A recover the function of
ACTL6A deletion including promoting cell migration, invasion and
morphologic changes in cytoskeleton organization. On the contrary,
knockdown of Twist-1 in MG-63ACTL6A compromised the
function induced by ACTL6A overexpression. Our results also provide
a link between ACTL6A and Twist-1 in EMT and Twist-1 was the
potential target of ACTL6A in osteosarcoma. There was a study
proposed that ACTL6A played a pivotal role in supporting the
pro-oncogenic functions of Notch1 in EMT (29). Twist-1 also is promoted by the
activated Notch1 signaling (32).
Available data indicated that ACTL6A may transactivate Twist-1
expression via Notch1 signaling. However, further studies are
needed to explore the possible role of ACTL6A in EMT-related signal
transduction pathway in osteosarcoma.
In conclusion, the present study showed that ACTL6A
overexpression was significantly associated with malignant behavior
and poor prognosis of osteosarcoma. Furthermore, we have
demonstrated that ACTL6A could promote the metastasis of
osteosarcoma via facilitating EMT. Collectively, our data indicate
that ACTL6A functions as a potential oncogene and a biomarker to
predict prognosis in osteosarcoma, supporting the pursuit of ACTL6A
as a prospective therapeutic target for osteosarcoma in clinical
practice.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21:(Suppl 7). vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klein MJ and Siegal GP: Osteosarcoma:
anatomic and histologic variants. Am J Clin Pathol. 125:555–581.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren HY, Sun LL, Li HY and Ye ZM:
Prognostic significance of serum alkaline phosphatase level in
osteosarcoma: a meta-analysis of published data. BioMed Res Int.
2015:1608352015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Zhang L, Zhang G, Li S, Duan J,
Cheng J, Ding G, Zhou C, Zhang J, Luo P, et al: Osteosarcoma
metastasis: prospective role of ezrin. Tumour Biol. 35:5055–5059.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scott MC, Sarver AL, Tomiyasu H, Cornax I,
Van Etten J, Varshney J, O'Sullivan MG, Subramanian S and Modiano
JF: Aberrant retinoblastoma (RB)-E2F transcriptional regulation
defines molecular phenotypes of osteosarcoma. J Biol Chem.
290:28070–28083. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Del Mare S, Husanie H, Iancu O, Abu-Odeh
M, Evangelou K, Lovat F, Volinia S, Gordon J, Amir G, Stein J, et
al: WWOX and p53 dysregulation synergize to drive the development
of osteosarcoma. Cancer Res. 76:6107–6117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aguirre-Ghiso JA: Models, mechanisms and
clinical evidence for cancer dormancy. Nat Rev Cancer. 7:834–846.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang G, Yuan J and Li K: EMT transcription
factors: implication in osteosarcoma. Med Oncol. 30:6972013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou CH, Lin FL, Hou SM and Liu JF: Cyr61
promotes epithelial-mesenchymal transition and tumor metastasis of
osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 signaling pathway. Mol
Cancer. 13:2362014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao K, Wang W, Rando OJ, Xue Y, Swiderek
K, Kuo A and Crabtree GR: Rapid and phosphoinositol-dependent
binding of the SWI/SNF-like BAF complex to chromatin after T
lymphocyte receptor signaling. Cell. 95:625–636. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krasteva V, Buscarlet M, Diaz-Tellez A,
Bernard MA, Crabtree GR and Lessard JA: The BAF53a subunit of
SWI/SNF-like BAF complexes is essential for hemopoietic stem cell
function. Blood. 120:4720–4732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lessard J, Wu JI, Ranish JA, Wan M,
Winslow MM, Staahl BT, Wu H, Aebersold R, Graef IA and Crabtree GR:
An essential switch in subunit composition of a chromatin
remodeling complex during neural development. Neuron. 55:201–215.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen KH, Xu F, Flowers S, Williams EA,
Fritton JC and Moran E: SWI/SNF-mediated lineage determination in
mesenchymal stem cells confers resistance to osteoporosis. Stem
Cells. 33:3028–3038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bao X, Tang J, Lopez-Pajares V, Tao S, Qu
K, Crabtree GR and Khavari PA: ACTL6a enforces the epidermal
progenitor state by suppressing SWI/SNF-dependent induction of
KLF4. Cell Stem Cell. 12:193–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu W, Fang L, Ouyang B, Zhang X, Zhan S,
Feng X, Bai Y, Han X, Kim H, He Q, et al: Actl6a protects embryonic
stem cells from differentiating into primitive endoderm. Stem
Cells. 33:1782–1793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park J, Wood MA and Cole MD: BAF53 forms
distinct nuclear complexes and functions as a critical
c-Myc-interacting nuclear cofactor for oncogenic transformation.
Mol Cell Biol. 22:1307–1316. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao D, Pan C, Sun J, Gilbert C,
Drews-Elger K, Azzam DJ, Picon-Ruiz M, Kim M, Ullmer W, El-Ashry D,
et al: VEGF drives cancer-initiating stem cells through
VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene.
34:3107–3119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yong KJ, Gao C, Lim JS, Yan B, Yang H,
Dimitrov T, Kawasaki A, Ong CW, Wong KF, Lee S, et al: Oncofetal
gene SALL4 in aggressive hepatocellular carcinoma. N Engl J
Med. 368:2266–2276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pullar CE, Chen J and Isseroff RR: PP2A
activation by beta2-adrenergic receptor agonists: novel regulatory
mechanism of keratinocyte migration. J Biol Chem. 278:22555–22562.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang F, Chang RM, Yu L, Lei X, Xiao S,
Yang H and Yang LY: MicroRNA-188-5p suppresses tumor cell
proliferation and metastasis by directly targeting FGF5 in
hepatocellular carcinoma. J Hepatol. 63:874–885. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su S, Liu Q, Chen J, Chen J, Chen F, He C,
Huang D, Wu W, Lin L, Huang W, et al: A positive feedback loop
between mesenchymal-like cancer cells and macrophages is essential
to breast cancer metastasis. Cancer Cell. 25:605–620. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiang L and He YY: Autophagy deficiency
stabilizes TWIST1 to promote epithelial-mesenchymal transition.
Autophagy. 10:1864–1865. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horvai AE, Roy R, Borys D and O'Donnell
RJ: Regulators of skeletal development: a cluster analysis of 206
bone tumors reveals diagnostically useful markers. Mod Pathol.
25:1452–1461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kempf-Bielack B, Bielack SS, Jürgens H,
Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G,
Kabisch H, et al: Osteosarcoma relapse after combined modality
therapy: an analysis of unselected patients in the Cooperative
Osteosarcoma Study Group (COSS). J Clin Oncol. 23:559–568. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee K, Shim JH, Kang MJ, Kim JH, Ahn JS,
Yoo SJ, Kwon Kim Y and Kwon H: Association of BAF53 with mitotic
chromosomes. Mol Cells. 24:288–293. 2007.PubMed/NCBI
|
|
28
|
Ferrari S, Bertoni F, Mercuri M, Picci P,
Giacomini S, Longhi A and Bacci G: Predictive factors of
disease-free survival for non-metastatic osteosarcoma of the
extremity: an analysis of 300 patients treated at the Rizzoli
Institute. Ann Oncol. 12:1145–1150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao S, Chang RM, Yang MY, Lei X, Liu X,
Gao WB, Xiao JL and Yang LY: Actin-like 6A predicts poor prognosis
of hepatocellular carcinoma and promotes metastasis and
epithelial-mesenchymal transition. Hepatology. 63:1256–1271. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial- mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang W, Chen Z, Shang X, Tian D, Wang D,
Wu K, Fan D and Xia L: Sox12, a direct target of FoxQ1, promotes
hepatocellular carcinoma metastasis through up-regulating Twist1
and FGFBP1. Hepatology. 61:1920–1933. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu KW, Hsieh RH, Huang KH, Li Fen-Yau A,
Chi CW, Wang TY, Tseng MJ, Wu KJ and Yeh TS: Activation of the
Notch1/STAT3/Twist signaling axis promotes gastric cancer
progression. Carcinogenesis. 33:1459–1467. 2012. View Article : Google Scholar : PubMed/NCBI
|