Introduction
Pancreatic cancer is the fourth leading cause of
cancer-related deaths in the US, with 46,420 estimated new cases
and 39,590 estimated deaths each year (1). Even patients who undergo surgery for
localized disease show a 5-year survival rate of only ~20%
(2). Therefore, it is important to
determine the mechanism of pancreatic cancer progression and
develop new treatments.
Tumor progression depends on microenvironmental
interactions (3,4). Cancer-associated fibroblasts (CAFs)
are the most important host cells in the micro-ecological
environment. They are formed from cells of different origins and
can be derived from the differentiation of quiescent fibroblasts,
epithelial, endothelial and mesenchymal stem cells. These cells
play an important regulatory role in tumor occurrence and
development (5).
Metabolic reprogramming is considered to be a
hallmark of tumor cells (6).
Aerobic glycolysis, known as the Warburg effect, is a
characteristic metabolic feature of cancer cells (7). Recent studies have found that
fibroblasts are forced into glycolysis, a phenomenon known as the
‘anti-Warburg effect’, since the changes in cell metabolism occur
in stromal cells rather than in tumor cells (8). This change in cell metabolism may be
associated with tumor progression (9). According to previous studies (10–12) in
co-cultured cancer cell and fibroblast models, the mitochondrial
mass in tumor cells is significantly increased compared to that in
a separate cultured model. Since co-culture with fibroblasts can
more accurately reflect the tumor microenvironment, the Warburg
effect may not occur in in vitro experiments. Furthermore,
treating tumor cells with lactate also significantly improves the
mitochondrial mass, indicating a parasitic relationship between
tumor cells and fibroblasts, with the tumor cells acting as
‘parasites’. After modification, the stromal cells are forced to
glycolysis, providing aerobic oxidation to the tumor cells. In the
pancreatic cancer microenvironment, it remains unclear whether
there is a metabolic coupling mechanism between the cancer cells
and CAFs. In the present study, we extracted pancreatic CAFs and
evaluated the ability of these cells to promote pancreatic cancer
progression from a metabolic perspective.
Materials and methods
Materials
The glucose detection checkerboard and lactic acid
checkerboard were obtained from the Jiancheng Institute of
Biological Engineering (Nanjing, China). RIPA cracking liquid kits
were purchased from Biyuntian Biological Co., Ltd. (Shanghai,
China). Dimethyl sulfoxide was obtained from Sigma Co., Ltd.
(Beijing, China). Dulbecco's modified Eagles medium (DMEM) and
fetal bovine serum (FBS) were purchased from HyClone (Logan, UT,
USA). Transwell chambers were purchased from Millipore (Billerica,
MA, USA). Matrigel and the One-Step RT-PCR kit were purchased from
BD Biosciences (Franklin Lakes, NJ, USA). LDHA, PKM2,
monocarboxylate transporter 1 (MCT1), SDH, fumarate hydratase (FH),
matrix metalloprotease (MMP)-2 and MMP-9 and β-actin antibodies
were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
The MCT1-specific blocker was from Sigma.
Cell cultures and treatments
Human pancreatic cancer cells [BxPc-3, Panc-1;
obtained from the American Tissue Type Collection (ATCC; Manassas,
VA, USA)] were maintained in DMEM supplemented with penicillin (100
U/ml), streptomycin (100 µg/ml), 0.1 mM nonessential amino acids,
0.2 mM glutamine, 1 mM pyruvate, and 10% heat-inactivated FBS and
incubated in 5% CO2 humidified atmosphere at 37̊C. Cells
were grown to 80% confluence. In the invasion and migration
experiments, the cells were cultured in DMEM without FBS.
CAF cell separation, culture and
purification
Normal fibroblast (NF) and CAF cells were derived
from patients with pancreatic cancer and pancreatic trauma from the
Second Affiliated Hospital of Xi'an Jiaotong University. All
patients were newly diagnosed and had not received any relevant
treatment prior to surgery. Informed consent was obtained from all
patients prior to obtaining the specimens. Fibroblast isolation was
conducted as previously described (13). First, the tissue was trimmed to
1×1×1 mm and washed gently with phosphate-buffered saline (PBS)
three times (5 min each). Next, the tissues were washed once with
the medium and placed in fresh cell cultural medium containing 15%
fetal calf serum, 2 mM L-glutamine and 10% penicillin. The tissue
was cut with a sterile scalpel blade and sections of cells were
gently scraped with a blunt blade. The cells were cultured in an
incubator for 3–5 days at 37̊C and 5% CO2. The medium
was replaced once and every three days thereafter; after 14 days,
the cells fully covered the Petri dish. When the cell density
reached 80–90%, the cells were digested with trypsin and
regenerated at a rate of 1:3. The CAFs and NFs used in the
experiment were the 3rd and 5th generations of cells cultured in
vitro, respectively, and showed no obvious aging phenotype.
Medium preparation of pancreatic CAFs
and NFs
CAFs were added to 6-well plates at a density of
1.5×105/ml and rinsed with PBS after 24 h. The medium
was replaced with serum-free medium and cultured for 48 h, after
which the culture broth was collected and centrifuged to remove the
cells and debris; the supernatant obtained was the CAF conditioned
medium. These samples were stored at 4̊C. The conditioned medium of
NFs was collected in the same manner.
Indirect co-culture model of CAFs and
pancreatic cancer cells
The pancreatic cancer cells BxPc-3 and Panc-1 were
added to Petri dishes at a density of 1.5×105/ml; after
24 h, CAF-CM was added and the cells were cultured for 48 h. Cells
in PBS or serum-free medium were used as controls. An inverted
phase contrast microscope was used to observe the morphology and
growth of pancreatic cancer cells in each Petri dish. Proteins were
extracted from the cells.
Cell migration assay
Cell migration capability was evaluated using a
scratch test. First, 10×105 BxPc-3 and Panc-1 cells were
seeded into 1.5 ml media in each well of a 24-well plate. The cells
were grown to a confluent layer (48 h), and then, a scratch was
made in each well using a pipette tip. Subsequently, the cells were
washed gently with PBS, and then culture broths of CAFs and NFs
were added to the respective wells. An image was captured at time
point 0. The cells were then incubated at 37̊C in 5% CO2
and images were acquired after 24 h. The 24 h time point was chosen
to decrease the potential impact of proliferation on the closing of
the scratch. ImagePro Plus 5.0 from the NIH (Bethesda, MD, USA) was
used to standardize the results.
Cell invasion assay
Cell invasion was examined using Transwell assays.
Following incubation for 48 h, 3×104 cells were
transferred to the top of the Matrigel-coated invasion chambers (BD
Biosciences) in serum-free DMEM. DMEM containing 10% FBS was added
to the lower chamber. After 24 h, the non-invading cells were
removed and the invading cells were fixed using 95% ethanol,
stained with 0.1% crystal violet, and photographed at a
magnification of ×100 under an inverted phase contrast microscope
(Olympus CKX31/41; Olympus, Tokyo, Japan). The experiments were
repeated three times.
Glucose uptake assay
According to Fischer et al (14) the glucose uptake rate is reflected
by the amount of [3H]-2DG taken up by the cells. After
24 h in serum-free culture, the medium was changed to low-sugar
DMEM, 37 kBq/ml [3H]-2DG was added, and the cells were
cultured for another 24 h. After digestion, the small fraction of
cells remaining was counted and other cells were lysed in 0.5 M
NaOH for 15 min; the same volume of 0.5 M hydrochloric acid was
added for neutralization. The dpm value in the cell lysate solution
was examined using a microplate reader. [3H]-2DG intake
by the cells was calculated as follows: Total cellular
radioactivity - non-specific binding radioactivity)/24 h.
Lactic acid detection in the cell
culture medium
Cells in the 12-well plate were washed once with
PBS, the medium was replaced with phenol-free red medium, and the
cells were cultured for 20 h. The supernatant was collected
according to the instructions of the lactic acid detection kit.
Lactic acid content was examined using a DRY-CHEM FDC3500 analyzer
(Fuji, Tokyo, Japan); additionally, digested cells were counted.
The result reflected the amount of lactic acid
generated/106 cells.
Mitochondrial activity detection
After culturing the NF and CAF cells for 24 h, the
solution was used to culture pancreatic cancer cells for 24 h.
Cells grown in PBS or serum-free medium were used as blank
controls. Fresh DMEM containing mitochondrial fluorescent probes
(1:200) was added at 500 µl/well and incubated for an additional 30
min. The cells were stored and produced in a darkroom and
immediately observed using an inverted fluorescence microscope.
RT-PCR
Total RNA was extracted from the cells using TRIzol
reagent. In total, 2 µg RNA was reversed-transcribed into
first-strand cDNA using the RevertAid First Strand cDNA Synthesis
kit (Thermo Scientific, Waltham, MA, USA). PCR primer sequences
were as follows: MMP-2 forward primer, 5′-TGGTCCTGGTGCTCCTGGTG-3′
and reverse primer, 5′-GCTGCCTGTCGGTGAGATTGG-3′; MMP-9 forward
primer, 5′-TGGTCCTGGTGCTCCTGGTG-3′ and reverse primer,
5′-GCTGCCTGTCGGTGAGATTGG-3′; LDHA forward primer,
5′-CCAACATGGCAGCCTTTTCC-3′ and reverse primer,
5′-TCACGTTACGCTGGACCAAA-3′; PKM2 forward primer,
5′-ATTATTTGAGGAACTCCGCCGCCT-3′ and reverse primer,
5′-ATTCCGGGTCACAGCAATGATGG-3′; MCT1 forward primer,
5′-TCGGTATCTTTGGATGGAGAGG-3′ and reverse primer,
5′-TGGTAACTTCATTTGGCTTCCC-3′; SDH forward primer,
5′-CGGAAGAGTGTATGGACCA-3′ and reverse primer,
5′CGACGTAGTCCTTGTTGAC-3′; FH forward primer,
5′-GCGGATCCGGACATTGAGT-3′ and reverse primer,
5′-CGAATTCTCACTTTGGACCCA-3′; β-actin forward primer,
5′-ATCGTGCGTGACATTAAGGAGAAG-3′ and reverse primer,
5′-AGGAAGGAAGGCTGGAAGAGTG-3′. The PCR conditions were as follows:
an initial reaction at 42̊C for 1 h was used for cDNA synthesis,
followed by denaturation at 94̊C for 5 min and 22 cycles of the
following reactions: 94̊C for 30 sec, 55̊C for 30 sec and 72̊C for
30 sec. After the final cycle, the reaction was amplified at 72̊C
for 10 min. The housekeeping gene β-actin was used as an internal
reference.
Western blotting
In total, 5×105 cells in the logarithmic
growth phase were added to 0.5 ml of pre-chilled cell lysis buffer
and incubated on ice for 30 min. After centrifugation, the
supernatant was collected and the protein contents were measured.
The proteins were separated by 10% SDS-PAGE and blotted onto a
nitrocellulose membrane by semi-dry transfer. Next, the membrane
was immersed in Tris-buffered saline with Tween-20 containing 5%
skim milk for blocking followed by overnight incubation with the
primary antibody at 4̊C. On the following day, the membrane was
incubated with the secondary antibody conjugated to horseradish
peroxidase at 1:2,000 dilution (Santa Cruz Biotechnology) at room
temperature for 2 h, and then an enhanced chemiluminescence kit
(Amersham Pharmacia Biotech, Amersham, UK) was used for staining.
The membrane was photographed and the results were analyzed.
Statistical analysis
Each experiment was repeated at least three times.
The data are expressed as mean ±SD and analyzed using the Student's
t-test. P<0.05 indicates a statistically significant
difference.
Results
Preliminary identification of pancreas
CAFs and NFs
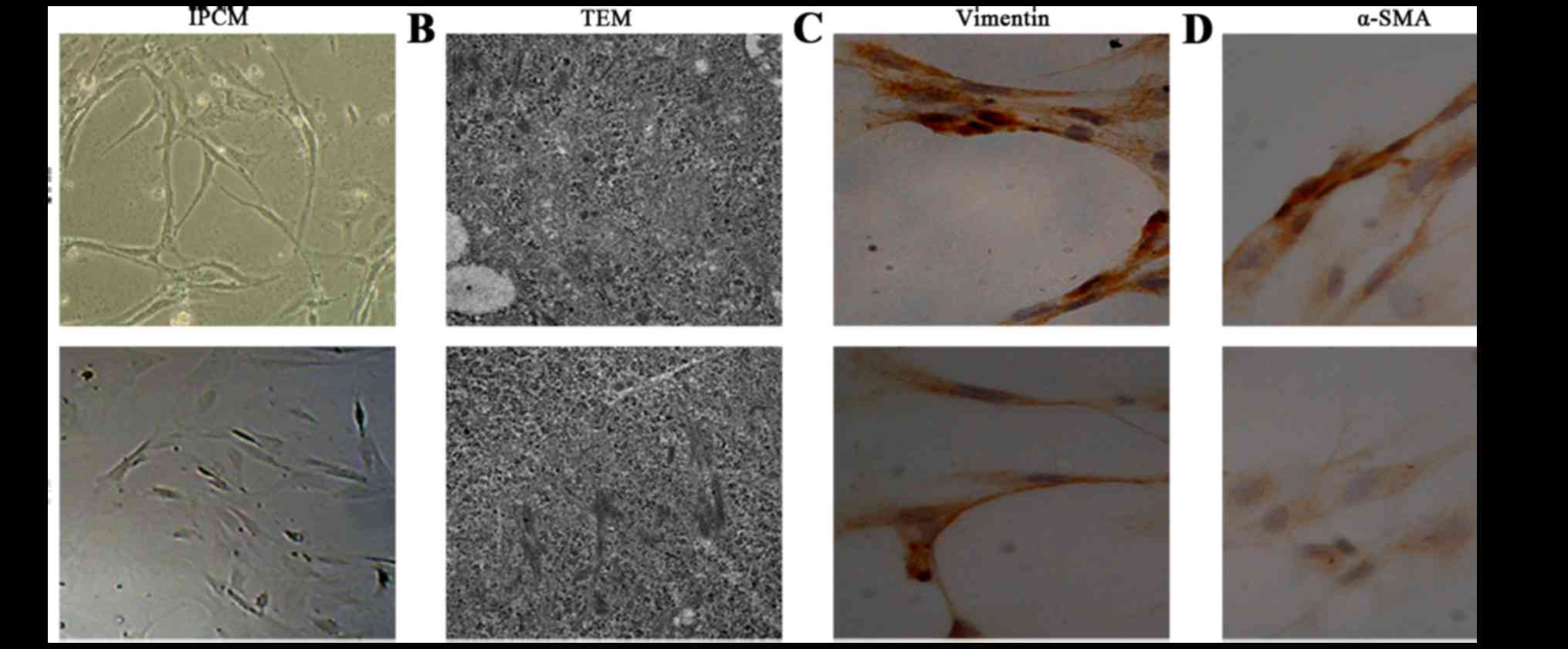
After 10 days, cell morphology was observed using an
inverted phase contrast microscope, and NFs and CAFs both showed a
spindle shape (Fig. 1). The CAFs
were spindle- or fusiform-shaped, had inconsistent sizes, showed
dense growth and exhibited a disordered arrangement. For NFs, the
cells showed multiple flat stellate shapes with similar cell sizes,
were arranged in the same direction, and had a radially outward
appearance (Fig. 1A). Transmission
electron microcopy observations showed that CAFs had large cell
nuclei, were evenly colored, and contained one or two nucleoli. In
the cytoplasm, a large number of rough endoplasmic reticulum,
mitochondria, and bundles of parallel subcapsular filaments were
observed. For NFs, the cells had an irregular cell nucleus, were
rich in rough endoplasmic reticulum in the cytoplasm, and had no
filaments (Fig. 1B).
Immunohistochemical results of CAFs showed that vimentin and
α-smooth muscle actin expression was positive. In NFs, vimentin
showed positive expression, whereas α-smooth muscle actin
expression was negative (Fig. 1C and
D).
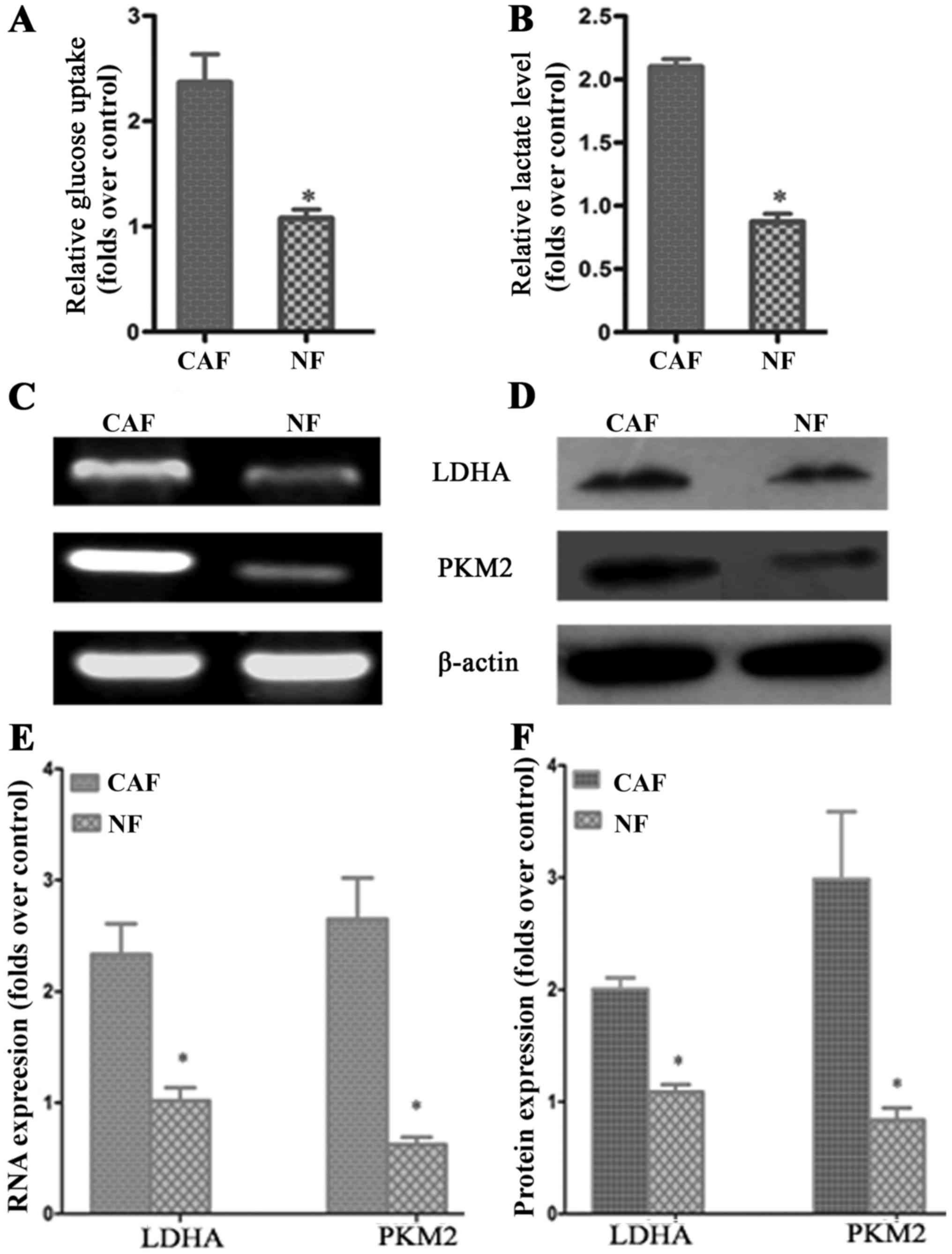
CAF glycolytic metabolism
According to previous studies, CAF cells exhibit the
‘Warburg effect’, similarly to tumor cells; lactate is produced,
secreted and absorbed by tumor cells to synthesize anabolic biomass
to facilitate the rapid proliferation of tumor cells. In order to
determine whether glycolysis occurs in CAFs, we examined the
lactate levels of glucose uptake in the culture medium. The results
demonstrated that glucose uptake and lactate production in CAFs
were significantly increased compared to that in NFs (Fig. 2A and B). To further verify the
change in glycolysis in CAFs, RT-PCR was conducted to detect the
mRNA expression of the CAF glycolytic enzymes lactate dehydrogenase
(LDHA) and pyruvate kinase m2 (PKM2). The results showed that LDHA
and PKM2 were highly expressed in CAFs (Fig. 2C and E). The western blotting and
PCR test results were consistent: LDHA and PKM2 protein expression
in CAFs was higher than that in the control group (Fig. 2D and F). These results further
confirmed that in vitro, glycolysis occurs in CAFs.
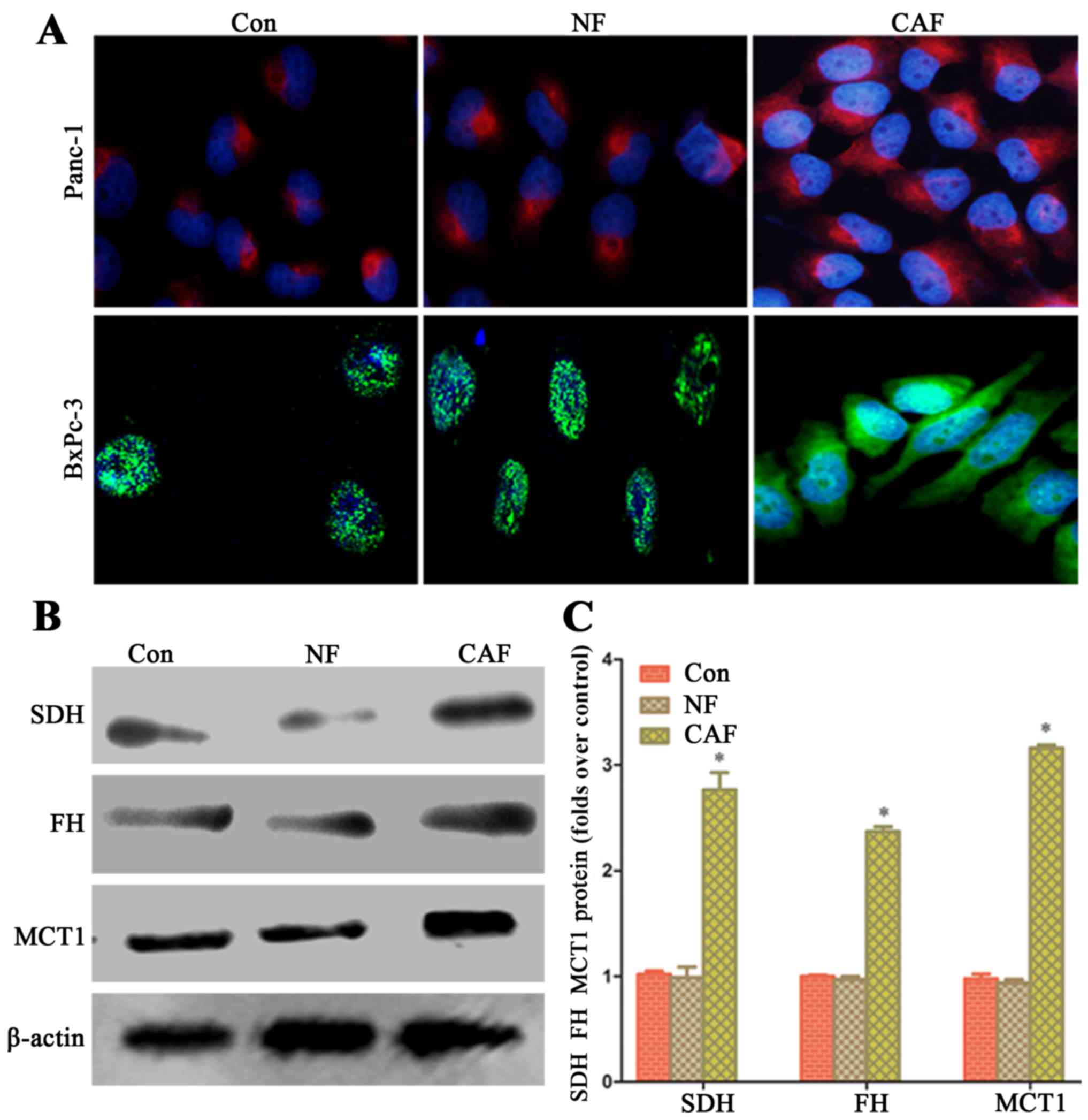
Enhanced oxidative phosphorylation
activity in pancreatic cancer cells after co-culture
In order to explore whether the capacity of aerobic
oxidation is strengthened in cancer cells after co-culture, we
observed the fluorescence intensity in the mitochondria of cancer
cells by fluorescence microscopy. After co-culture, pancreatic
cancer cell mitochondrial activity in the CAF group was higher than
that in the NF group and cells cultured alone (Fig. 3A). The western blot results showed
that in cancer cells, SDH, FH and MCT1 protein expression in the
CAF group was higher than that in the NF group and for cells
cultured alone (Fig. 3B and C).
This indicates that a CAF (glycolysis)-cancer cell (aerobic
oxidation) metabolic coupling mode exists.
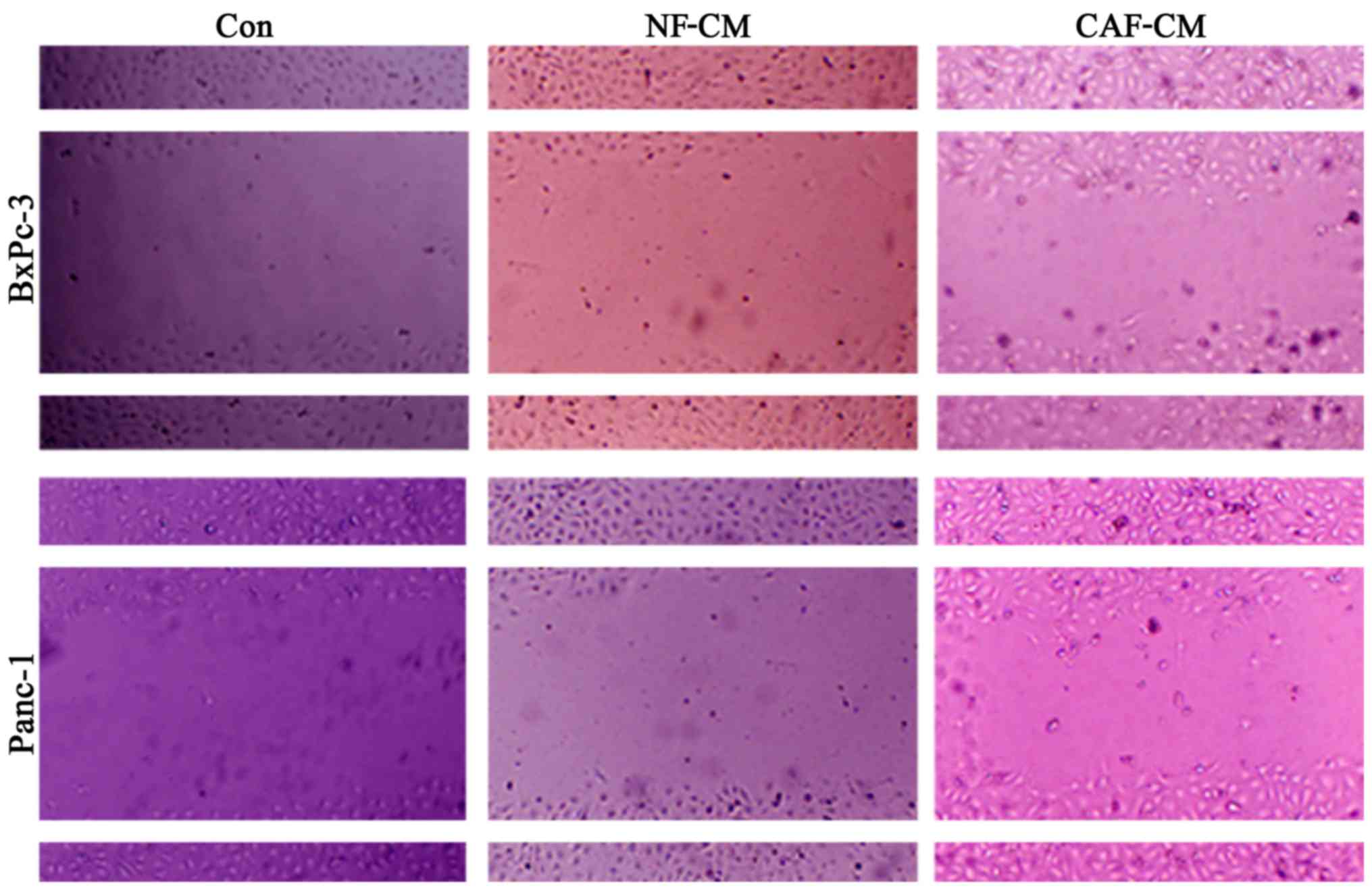
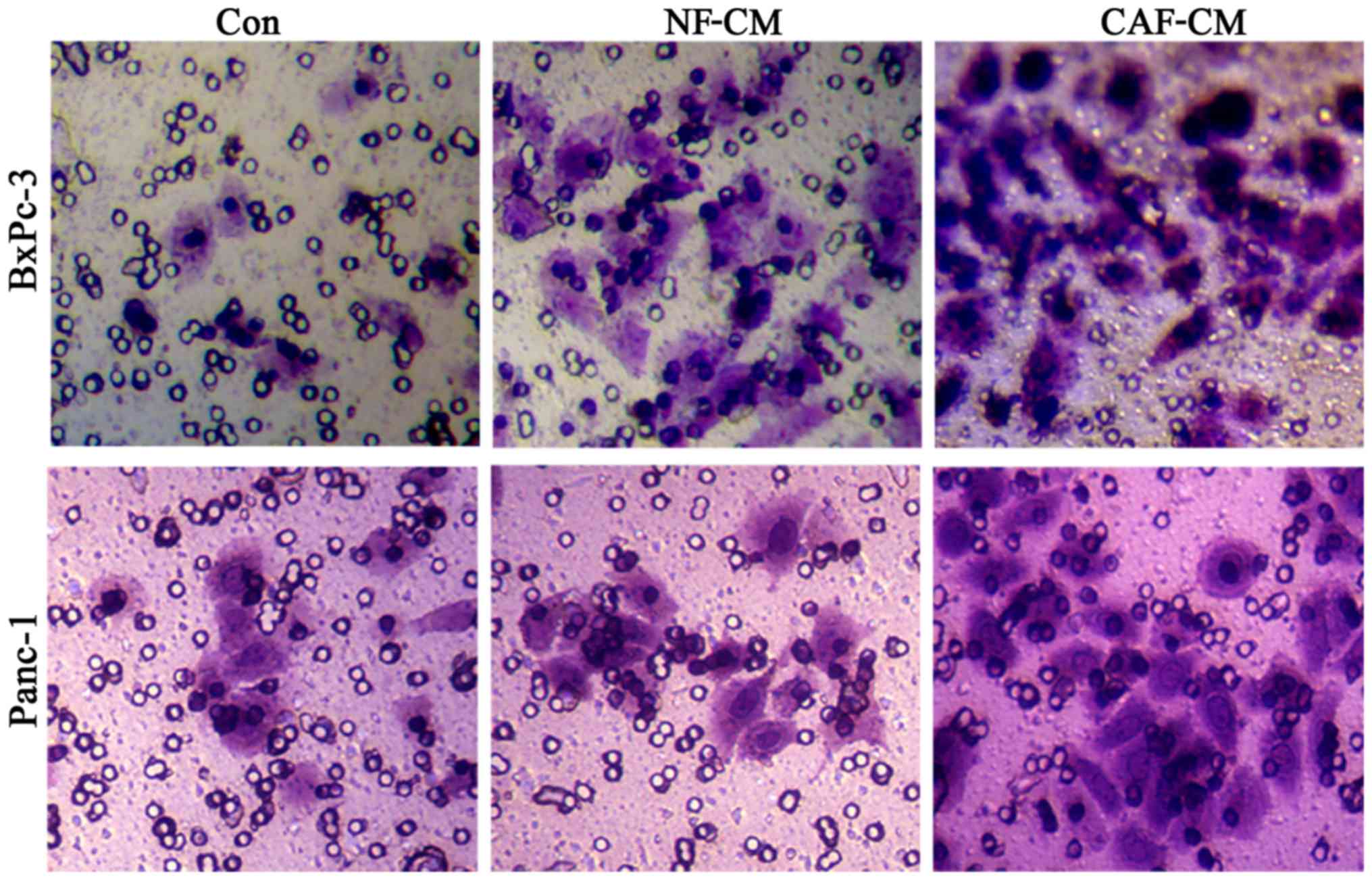
CAFs promote the migration of
pancreatic cancer cells in vitro
In order to determine whether CAFs promote the
migration of pancreatic cancer cells, an indirect co-culture model
was developed for 48 h, and the influence of CAFs on cancer cell
migration was examined in a scratch test. The results showed that
compared with that in the CAF-CM group, PBS alone or NF-CM induced
the migration abilities in the BxPc-3 and Panc-1 cells (Fig. 4). This indicates that CAFs enhance
the migration of pancreatic cancer cells in vitro.
CAF promotes the invasion of
pancreatic cancer cells in vitro
In order to detect the influence of CAFs on
pancreatic cancer cell invasion, we first adopted an indirect
co-culture model and processed the cells for 48 h, after which a
Transwell assay was used to examine cell invasion capability. The
results showed that the number of cells penetrating the CAF-CM
group was significantly increased compared to that in the PBS or
NF-CM groups (Fig. 5), indicating
that CAF enhances the invasive ability of pancreatic cancer
cells.
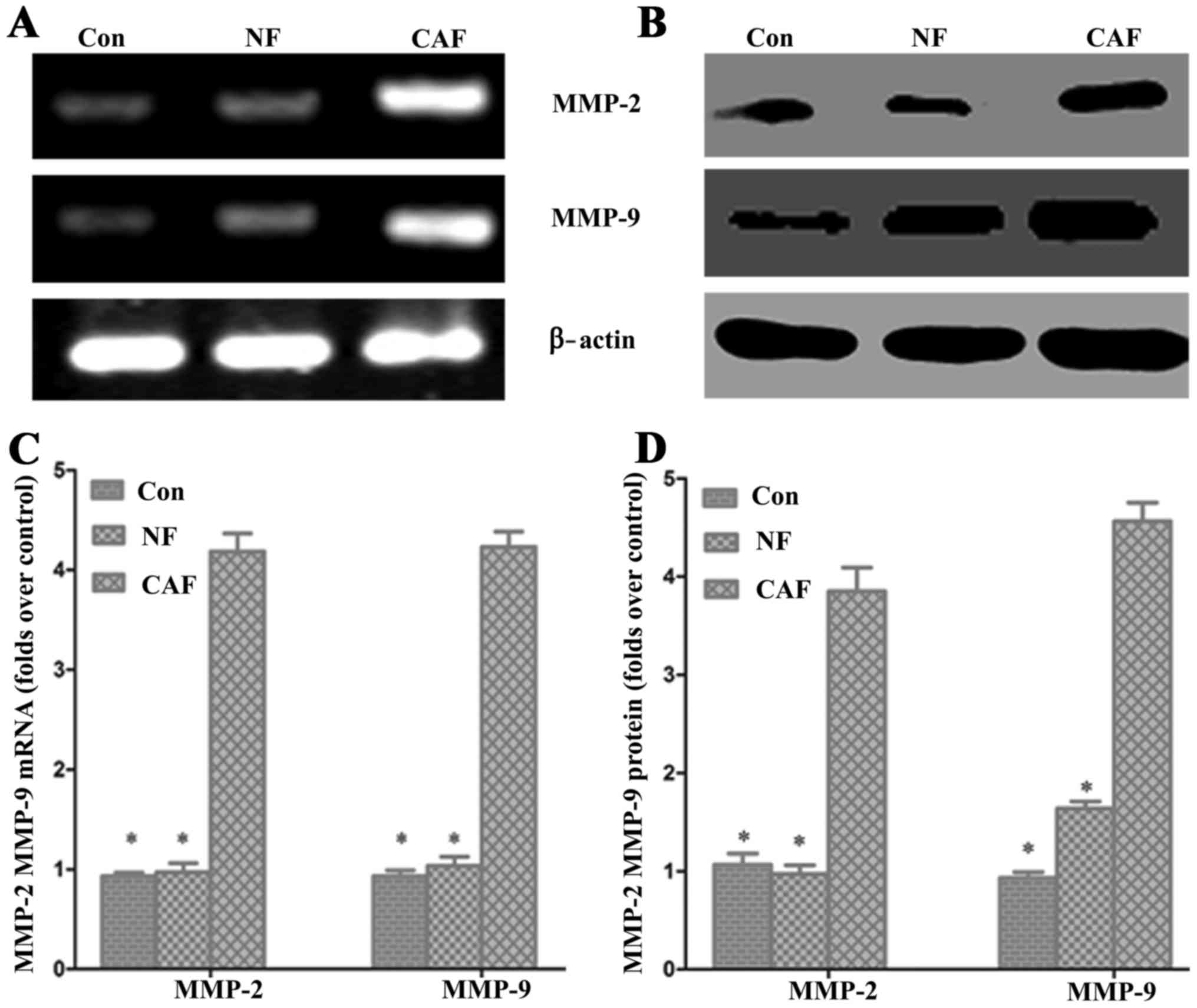
Effect of CAF on pancreatic cancer
MMP-2 and MMP-9 mRNA and protein levels
To further verify the impact of CAFs on pancreatic
cancer invasion and metastasis, we used RT-PCR and western blotting
to detect the expression of matrix MMP-2 and MMP-9 mRNA and
protein, respectively (Fig. 6). The
PCR results showed that MMP-2 and MMP-9 mRNA levels were
significantly higher in the CAF-CM group than levels in the control
group. Western blot results showed that the MMP-2 and MMP-9 protein
expression levels were significantly increased in the CAF-CM group
compared with levels in the control group (Fig. 6). These results indicate that CAF
enhances cell invasion and migration abilities.
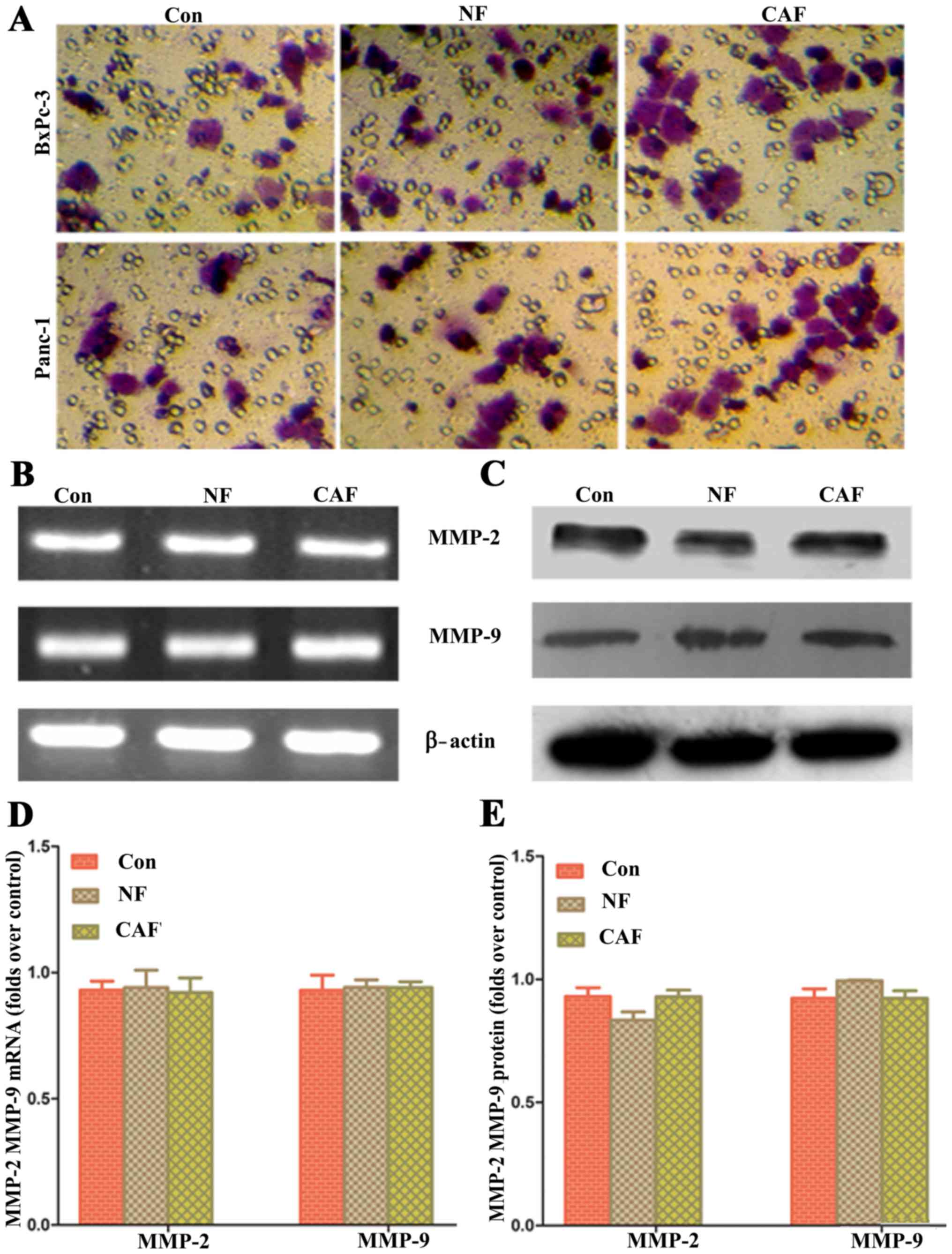
Impact of removing ‘CAF-cancer cell’
metabolic coupling upon pancreatic cancer progression
Based on the above results, CAFs are generally
glycolytic and release lactic acid as nutrients through MCT4/1
transport; these nutrients can be used by pancreatic cancer cells
to maintain their progression. In order to verify whether removing
metabolic coupling affects the progression of pancreatic cancer, an
MCT1-specific blocker was used to pre-treat pancreatic cancer cells
and for co-culture with CAFs. The results showed that after removal
of ‘cancer cell-CAF’ metabolic coupling, there was no significant
difference in the cell invasion ability among the three groups
(Fig. 7). These data indicate that
the ‘CAF-cancer cell’ metabolic coupling ring is an important
mechanism for enhancing the progression of pancreatic cancer
cells.
Discussion
CAFs are known to be involved in tumor progression
(15). Nonetheless, the positive
regulatory mechanism in tumors has not been fully elucidated.
Certain growth factors are believed to be involved. Recently,
several studies (16–19) examining the cell cycle described
possible mechanisms from an energy metabolism perspective.
Oxidative phosphorylation is considered to be partially abandoned
in activated CAFs; thus, these cells use the glycolytic metabolic
pathway instead. Through this passive metabolic pattern, released
nutritional products such as lactate, can be absorbed by tumor
cells to maintain growth (8,17).
This process is known as the reverse Warburg effect. However,
whether this mechanism exists in pancreatic cancer cells was
unclear. The present study revealed that pancreatic CAFs exhibit a
similar reverse Warburg effect. Lactic acid secretion was
significantly increased; in addition, cancer cell oxidation and
metastasis were enhanced after co-culture. After blocking this
coupling phenomenon, the pancreatic cancer cell migration and
invasion abilities were decreased. This is consistent with results
of previous studies (11,20–22)
indicating that changes in mesenchymal cell metabolism are
important for tumor progression. Furthermore, a recently published
study (23) confirmed that MCT1 and
MCT4 regulated the migration and invasion of pancreatic cancer,
indicating that interaction of metabolic pattern changes in the
microenvironment is an important mechanism of tumor
progression.
Recently, the Warburg effect has gained attention;
however, previous studies have only verified that tumor cells
undergo glycolysis and produce lactate (24,25).
However, the role of the mitochondrial respiratory chain was not
determined, and the citric acid cycle and oxidative phosphorylation
were thought to be reduced. Recent studies (11,20–22)
have revised the Warburg effect for the tumor metabolic pattern
(26,27). Numerous recent studies cultured only
independent tumor cells in vitro, which differs from the
actual in vivo tumor microenvironment, whereas the impact of
the interstitial microenvironment has not been evaluated. Second,
numerous studies showed that mitochondrial oxidative
phosphorylation plays a positive role in tumor growth and
progression. For example, in 70% of glioma patients, the mutation
and inactivation of the isocitrate dehydrogenase gene was found to
be associated with better prognosis and survival (28), suggesting that decreased
tricarboxylic cycle activity blocks tumor progression.
In tumor cell and fibroblast co-culture models, the
mitochondrial mass in the tumor cells was significantly enhanced
compared with that in individual culture models (10–12).
Since co-culture with CAFs more accurately reflects the
microenvironment of tumorigenesis, the Warburg effects in in
vitro tumor cells may not reflect the actual situation.
Mitochondria mass was also significantly increased after treating
tumor cells with lactate (29),
indicating a parasitic relationship between tumor cells and CAFs,
with the tumor cells acting as ‘parasites’. After transformation,
mesenchymal cells are forced to conduct glycolysis, assisting the
tumor cells in conducting oxidative phosphorylation. Similarly, in
co-cultured tumor cells and a CAF model, cancerous fibroblasts were
induced and the expression of glycolytic metabolic enzymes was
upregulated, resulting in a large number of lactate metabolites;
additionally, the expression of the lactic acid transporters MCT1
and MCT4 increased accordingly in fibroblasts (30) Thus, a metabolic coupling mechanism
exists between CAFs and cancer cells. We also observed ‘metabolic
co-existence’ in pancreatic cancer studies, and the progression of
pancreatic cancer was significantly affected after removing this
phenomenon, again indicating a reverse Warburg effect.
In conclusion, pancreatic CAFs remodeled the
metabolic transition to reflect cancer cell progression, which may
be an important mechanism for promoting tumor progression in a
non-vascular manner in the tumor microenvironment. Further studies
may focus on the mechanism by which CAF reshapes the metabolic
transition.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China, NSFC (no. 81402583), the
Natural Science Foundation of Shaanxi Province (no. 2014JQ4165),
the Xi'an Jiaotong University Education Foundation, XJTUEF (no.
xjj2014077), and the Hospital Fund of the Second Affiliated
Hospital of the Health Science Center, Xi'an Jiaotong University
[no. RC(XM)201402].
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Winter JM, Cameron JL, Campbell KA, Arnold
MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS,
et al: 1423 pancreaticoduodenectomies for pancreatic cancer: A
single-institution experience. J Gastrointest Surg. 10:1199–1211.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koontongkaew S: The tumor microenvironment
contribution to development, growth, invasion and metastasis of
head and neck squamous cell carcinomas. J Cancer. 4:66–83. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshida GJ: Metabolic reprogramming: The
emerging concept and associated therapeutic strategies. J Exp Clin
Cancer Res. 34:1112015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pavlides S, Whitaker-Menezes D,
Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro
MC, Wang C, Fortina P, Addya S, et al: The reverse Warburg effect:
Aerobic glycolysis in cancer associated fibroblasts and the tumor
stroma. Cell Cycle. 8:3984–4001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gonzalez CD, Alvarez S, Ropolo A,
Rosenzvit C, Bagnes MF and Vaccaro MI: Autophagy, Warburg, and
Warburg reverse effects in human cancer. BioMed Res Int.
2014:9267292014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ko YH, Lin Z, Flomenberg N, Pestell RG,
Howell A, Sotgia F, Lisanti MP and Martinez-Outschoorn UE:
Glutamine fuels a vicious cycle of autophagy in the tumor stroma
and oxidative mitochondrial metabolism in epithelial cancer cells:
Implications for preventing chemotherapy resistance. Cancer Biol
Ther. 12:1085–1097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martinez-Outschoorn UE, Pavlides S, Howell
A, Pestell RG, Tanowitz HB, Sotgia F and Lisanti MP:
Stromal-epithelial metabolic coupling in cancer: Integrating
autophagy and metabolism in the tumor microenvironment. Int J
Biochem Cell Biol. 43:1045–1051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Migneco G, Whitaker-Menezes D, Chiavarina
B, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Flomenberg
N, Tsirigos A, Howell A, et al: Glycolytic cancer associated
fibroblasts promote breast cancer tumor growth, without a
measurable increase in angiogenesis: Evidence for
stromal-epithelial metabolic coupling. Cell Cycle. 9:2412–2422.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walter K, Omura N, Hong SM, Griffith M and
Goggins M: Pancreatic cancer associated fibroblasts display normal
allelotypes. Cancer Biol Ther. 7:882–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fischer Y, Thomas J, Sevilla L, Muñoz P,
Becker C, Holman G, Kozka IJ, Palacín M, Testar X, Kammermeier H,
et al: Insulin-induced recruitment of glucose transporter 4 (GLUT4)
and GLUT1 in isolated rat cardiac myocytes. Evidence of the
existence of different intracellular GLUT4 vesicle populations. J
Biol Chem. 272:7085–7092. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franco OE, Shaw AK, Strand DW and Hayward
SW: Cancer associated fibroblasts in cancer pathogenesis. Semin
Cell Dev Biol. 21:33–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balliet RM, Capparelli C, Guido C, Pestell
TG, Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Chiavarina
B, Pestell RG, Howell A, et al: Mitochondrial oxidative stress in
cancer-associated fibroblasts drives lactate production, promoting
breast cancer tumor growth: Understanding the aging and cancer
connection. Cell Cycle. 10:4065–4073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonuccelli G, Whitaker-Menezes D,
Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Witkiewicz AK,
Heiden MG Vander, Migneco G, Chiavarina B, et al: The reverse
Warburg effect: Glycolysis inhibitors prevent the tumor promoting
effects of caveolin-1 deficient cancer associated fibroblasts. Cell
Cycle. 9:1960–1971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martinez-Outschoorn UE, Balliet RM, Lin Z,
Whitaker-Menezes D, Howell A, Sotgia F and Lisanti MP: Hereditary
ovarian cancer and two-compartment tumor metabolism: Epithelial
loss of BRCA1 induces hydrogen peroxide production, driving
oxidative stress and NFκB activation in the tumor stroma. Cell
Cycle. 11:4152–4166. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pavlides S, Tsirigos A, Vera I, Flomenberg
N, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG,
et al: Loss of stromal caveolin-1 leads to oxidative stress, mimics
hypoxia and drives inflammation in the tumor microenvironment,
conferring the ‘reverse Warburg effect’: A transcriptional
informatics analysis with validation. Cell Cycle. 9:2201–2219.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Witkiewicz AK, Kline J, Queenan M, Brody
JR, Tsirigos A, Bilal E, Pavlides S, Ertel A, Sotgia F and Lisanti
MP: Molecular profiling of a lethal tumor microenvironment, as
defined by stromal caveolin-1 status in breast cancers. Cell Cycle.
10:1794–1809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sotgia F, Martinez-Outschoorn UE, Pavlides
S, Howell A, Pestell RG and Lisanti MP: Understanding the Warburg
effect and the prognostic value of stromal caveolin-1 as a marker
of a lethal tumor microenvironment. Breast Cancer Res. 13:2132011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pavlides S1, Vera I, Gandara R, Sneddon S,
Pestell RG, Mercier I, Martinez-Outschoorn UE, Whitaker-Menezes D,
Howell A, Sotgia F, et al: Warburg meets autophagy:
Cancer-associated fibroblasts accelerate tumor growth and
metastasis via oxidative stress, mitophagy, and aerobic glycolysis.
Antioxid Redox Signal. 16:1264–1284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong SC, Nøhr-Nielsen A, Zeeberg K,
Reshkin SJ, Hoffmann EK, Novak I and Pedersen SF: Monocarboxylate
transporters MCT1 and MCT4 regulate migration and invasion of
pancreatic ductal adenocarcinoma cells. Pancreas. 45:1036–1047.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg's contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heiden MG Vander, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodríguez-Enríquez S, Gallardo-Pérez JC,
Avilés-Salas A, Marín-Hernández A, Carreño-Fuentes L,
Maldonado-Lagunas V and Moreno-Sánchez R: Energy metabolism
transition in multi-cellular human tumor spheroids. J Cell Physiol.
216:189–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shestov AA, Mancuso A, Lee SC, Guo L,
Nelson DS, Roman JC, Henry PG, Leeper DB, Blair IA and Glickson JD:
Bonded cumomer analysis of human melanoma metabolism monitored by
13C NMR spectroscopy of perfused tumor cells. J Biol Chem.
291:5157–5171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen JR, Yao Y, Xu HZ and Qin ZY:
Isocitrate dehydrogenase (IDH)1/2 mutations as prognostic markers
in patients with glioblastomas. Medicine. 95:e25832016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Hée VF, Pérez-Escuredo J, Cacace A,
Copetti T and Sonveaux P: Lactate does not activate NF-κB in
oxidative tumor cells. Front Pharmacol. 6:2282015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Curry JM, Tuluc M, Whitaker-Menezes D,
Ames JA, Anantharaman A, Butera A, Leiby B, Cognetti DM, Sotgia F,
Lisanti MP, et al: Cancer metabolism, stemness and tumor
recurrence: MCT1 and MCT4 are functional biomarkers of metabolic
symbiosis in head and neck cancer. Cell Cycle. 12:1371–1384. 2013.
View Article : Google Scholar : PubMed/NCBI
|