Introduction
Lectins can act specifically on malignant cells
thanks to the presence on the surface of transformed cells of the
Thomsen-Friedenreich antigen or T-antigen, a disaccharide,
Galβ1-3GalNAc. This disaccharide, that is hidden in normal cells
(1), allows selective binding of
lectins through serine or threonine of glycoproteins.
Mushroom derived lectins have been considered to
this purpose by taking advantage of their ligand-binding
specificity (2). For instance,
Agaricus bisporus, a lectin contained in edible mushrooms,
has been shown to selectively inhibit the proliferation of human
malignant epithelial cell lines without toxicity for normal cells
(3).
Recently, we obtained a lectin with antineoplastic
properties from the wild mushroom Boletus edulis (4). This protein was named Boletus
edulis lectin (BEL) β-trefoil and it could represent an
interesting tool for therapeutic applications against cancer.
Malignant melanoma is a lethal type of cancer with
an incidence that is increased in the last decades (1). It is a highly metastatic tumor
generally resistant to apoptosis as well as to chemotherapeutic
treatments and local tumor surgery. Radiotherapy and chemotherapy
are the standard treatment used. Since metastatic melanoma has a
poor prognosis, the challenge is to develop new and more effective
therapeutic tools.
Transcription factors have been considered in recent
years crucial targets in strategies against this malignancy, as
well as other cancer types.
Among other transcription factors, RUNX2 regulates
the expression of genes involved in tumor progression, invasion and
metastasis such as osteopontin, bone sialoprotein, and collagenases
(5). RUNX2 play an important role
in tumor progression by regulating proliferation, migration and
invasion pathways. In melanoma, RUNX2 has been considered a
molecular regulator of epithelial-to-mesenchymal transition
(6). In general, we found in
patients with bone metastases an increased expression of RUNX2
gene, suggesting that this transcription factor may be considered a
mesenchymal stem marker for cancer (7).
The aim of this study was to use BEL β-trefoil
against A375 and MeWo melanoma cells, focusing the study on effects
in key mechanisms involved in neoplasia, such as apoptosis and Ki67
expression as well as migration properties.
Materials and methods
BEL β-trefoil purification
Commercial king bolete mushrooms (Boletus
edulis) were homogenized in a blender with an equal volume of
PBS (10 mM NaH2PO4 pH 7.5 and 150 mM NaCl)
and centrifuged at 7,500 × g for 30 min at 4°C. Proteins in the
supernatant were precipitated by adding 50% w/v ammonium sulphate,
separated from the supernatant after centrifugation at 12,000 × g
for 15 min at 4°C, resuspended and dialyzed in buffer A (20 mM
Tris-HCl pH 7.5). The protein solution was loaded onto a
DEAE-cellulose column previously equilibrated with buffer A and
then washed with buffer A containing 30 mM NaCl to eliminate
contaminants. The fraction containing BEL β-trefoil was eluted with
20 mM Tris-HCl pH 7.5, 250 mM NaCl and further purified by size
exclusion chromatography using a Superdex G75 column in 20 mM
Tris-HCl pH 7.5, 150 mM NaCl.
The protein solution, previously equilibrated in
buffer A was loaded onto a stronger anionic exchanger MonoQ to
discriminate among isoforms. A linear sodium chloride gradient was
performed and the most abundant isoform eluted with NaCl 90 mM was
defined as BEL-β trefoil.
Recombinant BEL β-trefoil expression
and purification
BEL β-trefoil coding sequence (4) was inserted into pET22b plasmid using
NdeI and XhoI restriction sites. The construct was
used to transform E. coli BL21 C41 expression strain
following a standard heat shock protocol. When cell culture reach
an OD600 of 0.8 in LB media protein expression was
induced by adding 0.25 mM IPTG and shacking overnight at 20°C.
Cells were harvested by centrifugation at 7500 × g, lysed by
sonication and the soluble fraction was loaded onto a
Nickel-Sepharose column previously equilibrated with buffer B (20
mM Tris-HCl pH 7.5, 500 mM NaCl and 10 mM imidazole). After washing
the column with buffer B BEL β-trefoil was eluted with a linear
imidazole gradient from 10 to 350 mM. Next, thrombin was added to
cleave the histidine tag. Tag-free protein was separated from the
uncut through Nickel-Sepharose column.
BEL β-trefoil was then collected, loaded onto
Superdex G75 column and eluted with 20 mM Tris-HCl pH 7.5, 150 mM
NaCl. The fluorescent BEL β-trefoil was obtained by cloning the
protein coding sequence into pWaldo-GFP plasmid using NdeI
and KpnI restriction enzymes; this protocol allowed the
expression of recombinant BEL β-trefoil fused with a C-terminal
GFP. After the heat shock transformation of E. coli BL21
(DE3) the chimeric protein was expressed and purified following the
same protocol of BEL β-trefoil alone.
Cells and BEL β-trefoil treatment
A375 and MeWo melanoma cells were cultured under
humidified atmosphere of 5% CO2 and passaged in growth
medium: DMEM/F12 containing 10% FBS (fetal bovine serum)
supplemented with antibiotics (1% penicillin and streptomicyn) and
1% glutamin. Cells were then harvested using trypsin, washed and
counted on a microscope using a Burker hemocytometer and plated
again in growth medium. Once 80% confluence was reached BEL
β-trefoil at concentration ranging from 0 to 100 µg/ml was
added.
Cell viability
Cell viability was evaluated by a colorimetric assay
based on the reduction of the tetrazolium salt XTT (sodium
3I-[1-phenylamino-carbonyl-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene
sulfonic acid hydrate) by mitochondrial dehydrogenase of viable
cells to a formazan dye (Cell proliferation kit II - XTT
Roche).
Briefly 100 µl XTT labelling mixture was added to
each well and cells were incubated at 37°C in a humidified
atmosphere of 5% CO2 for 4 h. The spectrophotometric
absorbance of the samples was measured every hour using a
microtitre plate (ELISA) reader at a wavelength of 450 nm.
Real-time PCR
PCR was performed in a total volume of 20 µl
containing 1X Premix Ex Taq™ (2X), 1X Rox Reference Dye (50X) and
20 ng of cDNA; probe sets for gene Runx2, (Hs00231692_m1)
actin and GAPDH obtained from Assay-on-Demand Gene Expression
Products (Applied Biosystems). Real-time RT-PCR reactions were
carried out in two-tube system and in multiplex.
The amplification conditions included 30 sec at 95°C
(initial denaturation), followed by 50 cycles at 95°C for 5 sec
(denaturation) and at 60°C for 31 sec (annealing/extension).
Thermocycling and signal detection were performed with ABI PRISM
7000 Sequence Detector (Applied Biosystems). Signals were detected
according to the manufacturer's instructions.
As previously reported, the Ct value correlates to
the starting quantity of target mRNA (8); we selected the ∆Rn in the exponential
phase of amplification plots to determine the Ct values. ∆∆Ct
values were then calculated with respect to control.
To normalize mRNA expression for sample-to-sample in
RNA input, quality and reverse transcriptase efficiency, we
amplified the β-actin and β-2 microglobulin housekeeping genes.
β-actin and β-2 microglobulin endogenous/internal control genes
were abundant and remained constant proportionally to total RNA
among the samples.
Ct data
Ct values for each reaction were determined using
TaqMan SDS analysis software. For each amount of RNA tested,
triplicate Ct values were averaged. Because Ct values vary linearly
with the logarithm of the amount of RNA, this average represents a
geometric mean.
Cell proliferation test
Proliferating cell nuclei were identified by
Ki67-positive immunofluorescence on cells cultured in slide glass
chambers, acetone fixed and stored at −20°C. Briefly, cells were
grown in slides with different BEL β-trefoil concentrations for 48
h under humidified atmosphere of 5% CO2 at 37°C; then,
cultured cells were fixed with cold acetone and stored at −20°C.
For immunofluorescence, cells were firstly rinsed twice in PBS and
permeabilized with washing solution (Triton X-100 0.1% in PBS).
After incubating the cells with anti-Ki67 primary antibody (clone
Ki67, Dako), the secondary antibody (FITC-conjugated rabbit
anti-mouse IgG, Dako) was applied. Finally, the nuclei were
counterstained with DAPI (Sigma) and observed at 40X objective
using Nikon epifluorescence microscope. Semiquantitative analysis
of protein expression was achieved by counting fluorescent cells in
three fields of each of six slides at magnification of ×40. The
estimate was calculated as the percentage of positive cells with
respect to the total DAPI-stained nuclei.
Annexin staining
The exposure of phospholipid phosphatidylserine on
the plasma membrane of apoptotic cells was detected using the
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
detection kit (Bender Med System, Vienna, Austria), as previously
reported (7). Briefly, cells were
treated with lectin for 48 h. After incubation, cells were
trypsinized and 250×103 cells were centrifuged and
re-suspended in 500 µl of 1X binding buffer. Next, cells were
stained with Annexin V-FITC for 10 min. Propidium iodide (PI) was
added just before the analysis with the FACSCanto™ flow cytometer
(BD Biosciences, San Jose, CA, USA). At least 10,000 events were
acquired and samples were analyzed by FlowJo software (TreeStar,
Ashland, OR, USA). Viable cells were defined as Annexin V-negative
and PI-negative; early apoptotic cells were defined as Annexin
V-positive and PI-negative; late apoptotic/necrotic cells were
defined as Annexin V-positive and PI-positive.
Migration assay
Transwell plates with 8-µm pore size membranes
(Corning, NY, USA) were used to analyze cell migration, according
to the mmanufacturer's instructions. A375 and MeWo cells in RPMI
with or without BEL β-trefoil were added to the top of the
Transwell inserts and cultured for 48 h in humidified environment
with 5% CO2 at 37°C. Migrated cells (passed through
polyethylene membrane) were fixed with methanol and stained with a
0.1% crystal violet solution and counted in six random fields.
Statistical analysis
Results are expressed as mean ± SE. The Wilcoxon
test was used for non-parametric data. For analysis of treatment
responses, multiple measurement ANOVA followed by Bonferroni as
post hoc analysis was performed. A probability value of <0.05
was considered statistically significant. Spearman correlation
coefficient and regression curve estimations were performed when
indicated. Analyses were applied to experiments carried out at
least three times. Statistical analyses were performed using SPSS
for Windows, version 16.0 (SPSS Inc, Chicago, IL, USA).
Results
Cells viability, apoptosis and
proliferation
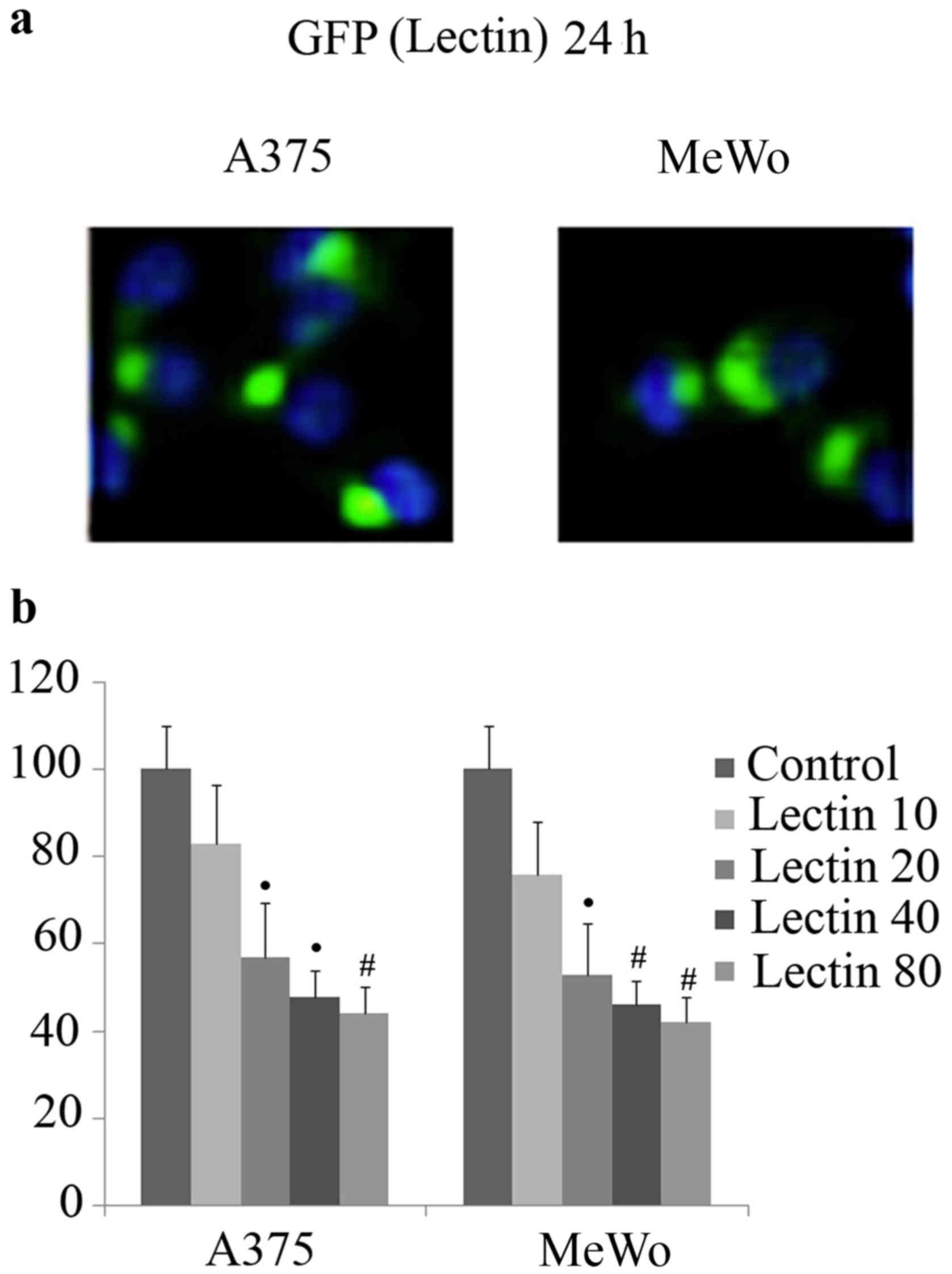
Recombinant-fluorescent BEL β-trefoil entered the
cells after 24 h at concentration starting from 20 to 80 µg/ml
(Fig. 1a). In order to study the
effect of the treatment, we analyzed cell viability by XTT test in
cells incubated at 37°C and exposed to BEL β-trefoil up to 80
µg/ml. After 48 h we observed a dose-dependent reduction of cell
viability. The BEL β-trefoil decreased cell viability in a manner
of statistical significance versus non-treated cells when it was
added at 20 µg/ml (p<0.05), 40 and 80 µg/ml (p<0.01)
(Fig. 1b).
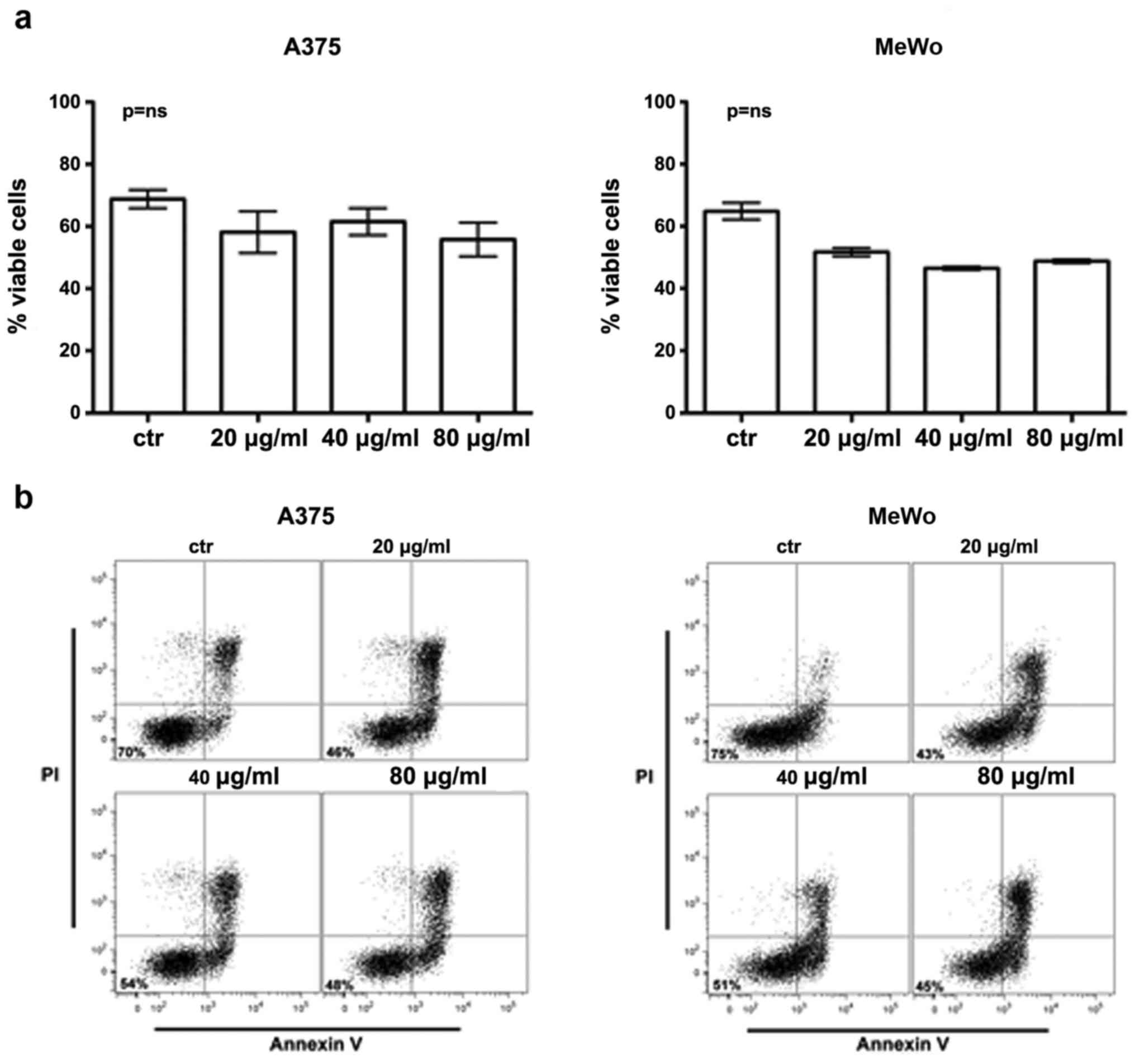
To clarify whether BEL β-trefoil effect was
pro-apoptotic or/and it inhibited proliferation, we evaluated the
exposure of the phospholipid phosphatidylserine on the plasmatic
membrane (apoptotic feature) by annexin staining and the
proliferation index by in situ expression of Ki67 protein
(proliferative marker).
We observed an apoptotic induction in treated
compared to untreated cells. In fact Annexin V staining confirmed
cell reduction showed by XTT assay in both cell lines even if the
difference was not statistically significant (Fig. 2).
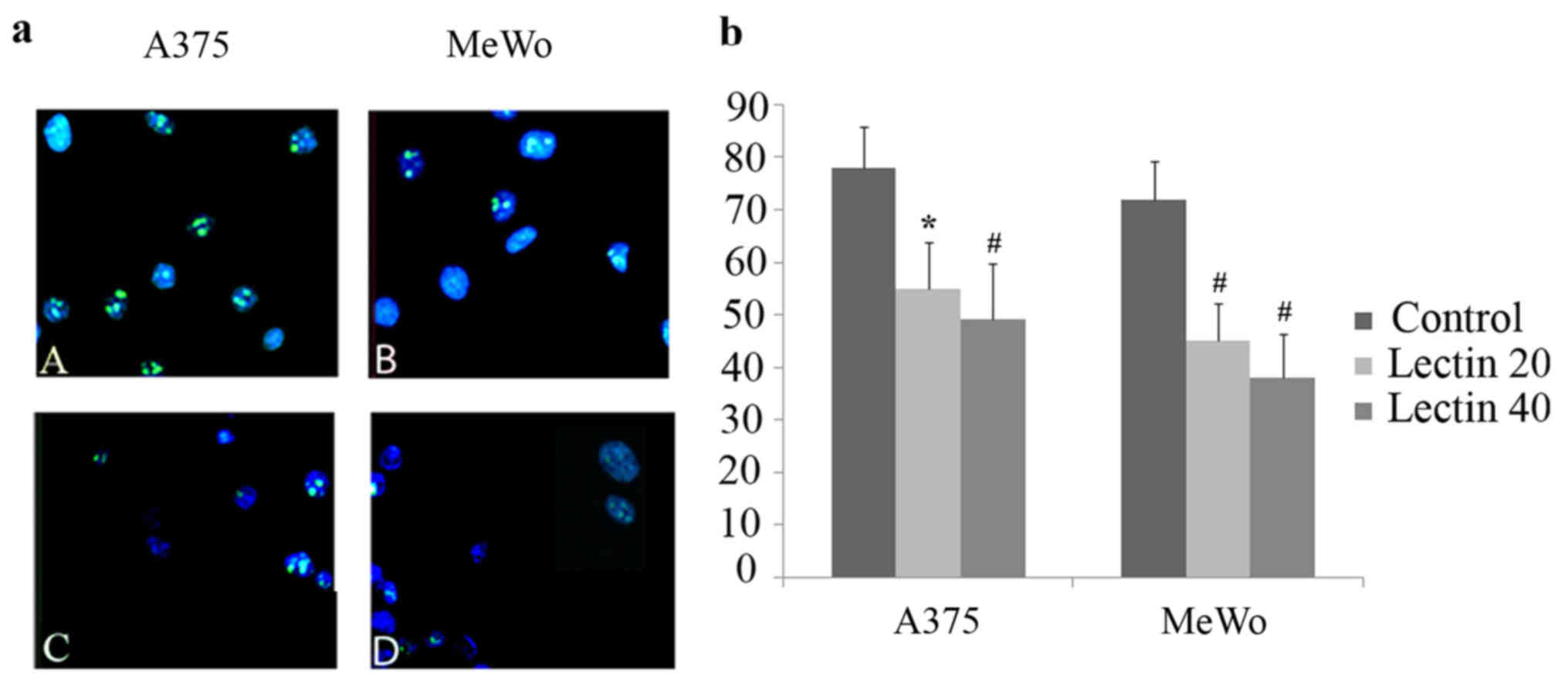
When proliferating cells were evaluated by Ki67
immunofluorescence, positively stained nuclei were reduced in
treated A375 and MeWo cells as compared to non-treated cells. This
proliferative reduction was statistical significant in both cell
lines treated with 20 and 40 µg/ml of BEL β-trefoil (Fig. 3)
Migration ability and RUNX2 gene
expression
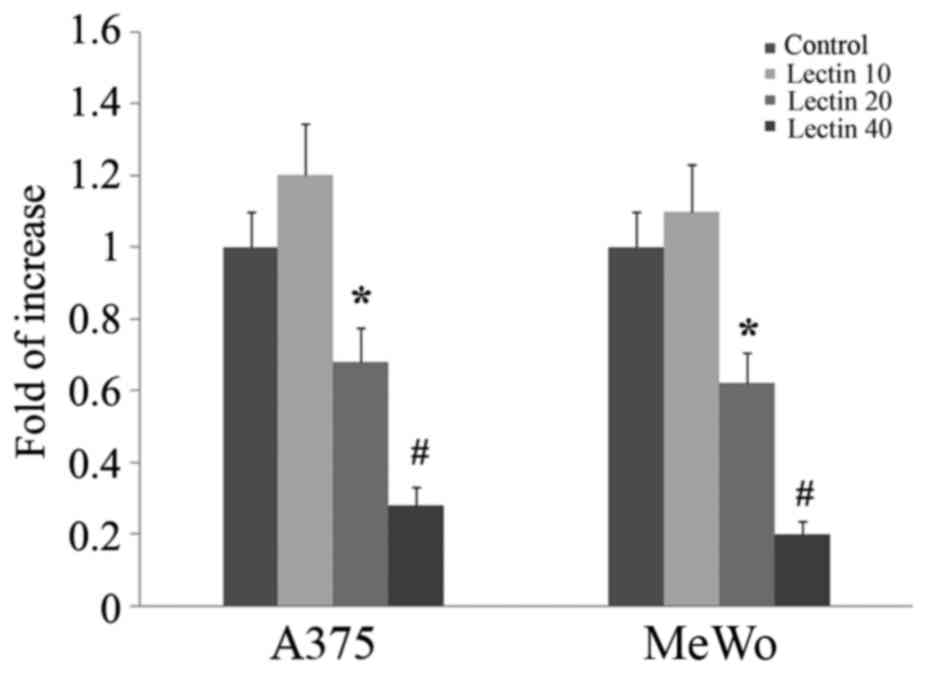
RUNX2 expression has been shown to be involved in
promoting migration of melanoma cells. To evaluate if BEL β-trefoil
treatment would modulate RUNX2 expression, we treated both MeWo and
A375 lines with increasing doses up to 80 µg/ml. After 24 h of
treatment Runx2 gene expression was significantly reduced in
treated cells already from 40 µg/ml of BEL β-trefoil (p<0.05)
(Fig. 4).
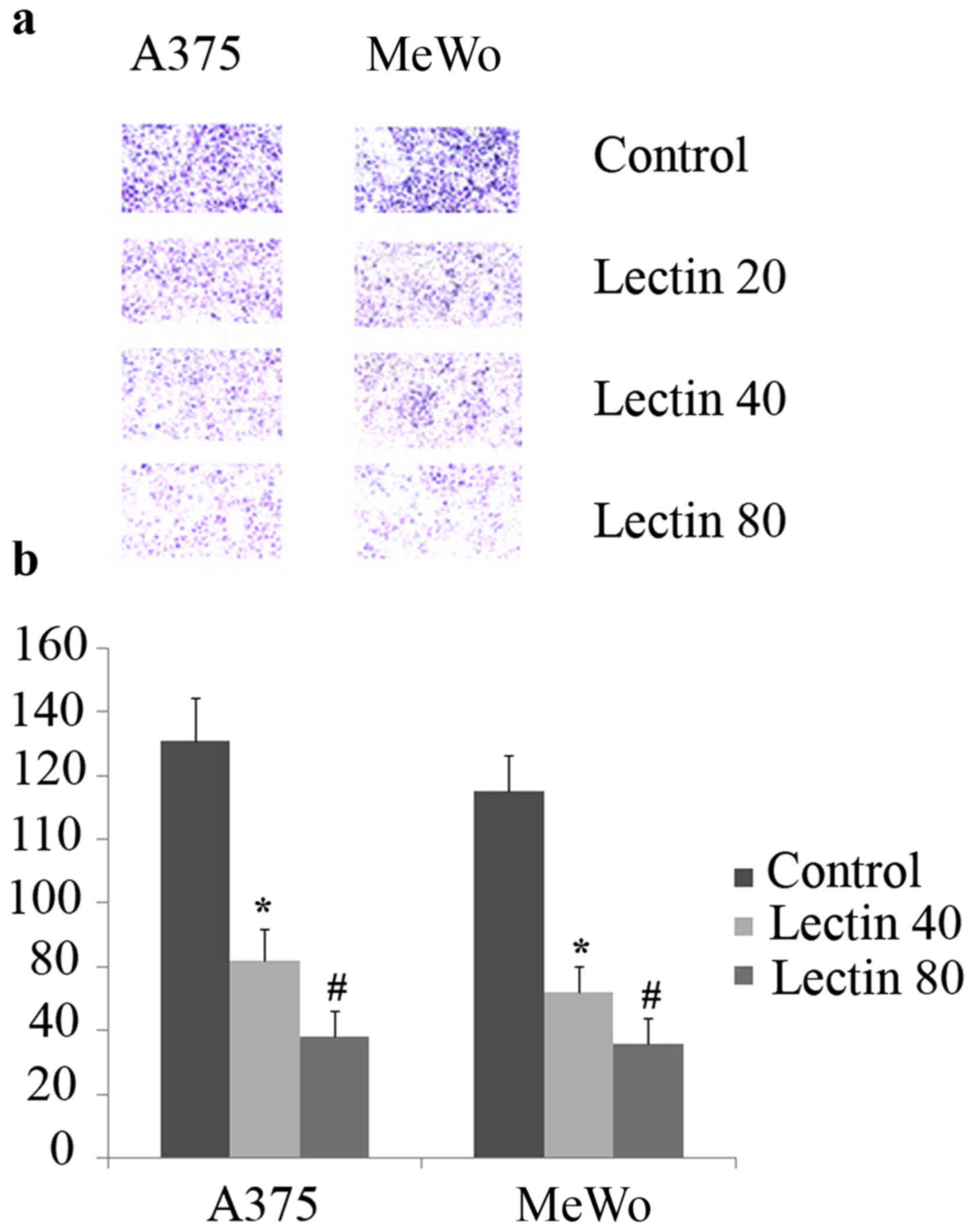
To evaluate the impact of exposing cells to BEL
β-trefoil on migration ability, we cultured the cells in Transwell
plates. As shown in Fig. 5, the
presence of lectin significantly reduced cell migration in both
cell lines either at 40 (p<0.05) or at 80 (p<0.01) compared
to untreated cells after 24 h of treatment.
Discussion
BEL β-trefoil in vitro treatment affected
viability of both melanoma cell lines: A375 and MeWo. In order to
clarify the underlying mechanism, we assayed the treated and
untreated cells for Ki67 expression as well as for Annexin V-FITC
and PI staining.
The Ki67 protein is a marker of proliferative
activity and its overexpression has been correlated with a poor
prognosis in various tumors, including cutaneous melanoma where
Ki67 immunohistochemical staining is considered a specific and
sensitive method to diagnose and to predict prognosis (9). Our results showed Ki67 expression
significantly decreased in both treated cell lines, respect to the
untreated controls. On the contrary, Annexin V-FITC and PI staining
performed to assess cell apoptosis showed reduced ability of BEL
β-trefoil in inducing cellular death, in both melanoma cells. Taken
together, these results demonstrated that BEL β-trefoil treatment
reduces cell viability mainly by affecting proliferative activity
rather than by inducing apoptosis. On the other hand, a
pro-apoptotic effect should not be completely ruled out since
melanomas are thought to redundantly prevent apoptosis by
upregulating multiple signaling pathways. Constitutive signaling
mediated by Ras, MAPKs (10,11)
and involving PI3K could be acting on Fas cascade (12) minimizing pro-apoptotic signaling
mediated by the lectin. Yet, the inhibitory effect on cell
proliferation was more striking in both, A375 and MeWo cells.
Recently, the involvement of the transcription
factor RUNX2 in melanomagenesis was demonstrated at multiple
levels, including cell proliferation (13). RUNX2 was overexpressed by melanoma
cell lines and its down modulation by siRNA, inhibited migration
ability.
Interestingly, the treatment with lectin decreased
RUNX2 expression in both cell lines and the effect was
dose-dependent. The role of RUNX2 in the different steps of
malignancies has been largely demonstrated for inducing gene
expression of molecular targets associated to tumor progression,
invasion, metastasis as well as migration and invasion.
Additionally, RUNX2 regulates transcription factors such as SOX9,
SNAI2 and SMAD3 involved in epithelial to mesenchymal transition
(EMT) process (14).
In accordance with the role of RUNX2 on cell
migration demonstrated in other systems, we observed that treating
melanoma cells with BEL β-trefoil simultaneously and
dose-dependently affected RUNX2 expression and migration ability in
both melanoma cell lines.
Even if in recent years melanoma treatment has
improved by the development of immunological approaches, a certain
percentage of melanoma affected patients are unresponsive to
imunotherapy (15).
In conclusion, this study showed that BEL β-trefoil
inhibited cell viability through cytostatic effects and reduced
migration ability in melanoma cells.
Further studies are required to elucidate BEL
β-trefoil molecular mechanism, nonetheless it could represent a
valid alternative therapeutic approach to treat melanoma based on
its inhibitory effects on proliferation and migration.
Acknowledgements
We acknowledge Mr. Andy Robinson for revising the
English language.
References
|
1
|
Bovi M, Carrizo ME, Capaldi S, Perduca M,
Chiarelli LR, Galliano M and Monaco HL: Structure of a lectin with
antitumoral properties in king bolete (Boletus edulis)
mushrooms. Glycobiology. 21:1000–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh RS, Bhari R and Kaur HP: Mushroom
lectins: Current status and future perspectives. Crit Rev
Biotechnol. 30:99–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu L, Fernig DG, Smith JA, Milton JD and
Rhodes JM: Reversible inhibition of proliferation of epithelial
cell lines by Agaricus bisporus (edible mushroom) lectin.
Cancer Res. 53:4627–4632. 1993.PubMed/NCBI
|
|
4
|
Bovi M, Cenci L, Perduca M, Capaldi S,
Carrizo ME, Civiero L, Chiarelli LR, Galliano M and Monaco HL: BEL
β-trefoil: A novel lectin with antineoplastic properties in king
bolete (Boletus edulis) mushrooms. Glycobiology. 23:578–592.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blyth K, Vaillant F, Jenkins A, McDonald
L, Pringle MA, Huser C, Stein T, Neil J and Cameron ER: Runx2 in
normal tissues and cancer cells: A developing story. Blood Cells
Mol Dis. 45:117–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riminucci M, Corsi A, Peris K, Fisher LW,
Chimenti S and Bianco P: Coexpression of bone sialoprotein (BSP)
and the pivotal transcriptional regulator of osteogenesis,
Cbfa1/Runx2, in malignant melanoma. Calcif Tissue Int. 73:281–289.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valenti MT, Zanatta M, Donatelli L,
Viviano G, Cavallini C, Scupoli MT and Carbonare L Dalle: Ascorbic
acid induces either differentiation or apoptosis in MG-63
osteosarcoma lineage. Anticancer Res. 34:1617–1627. 2014.PubMed/NCBI
|
|
8
|
Heid CA, Stevens J, Livak KJ and Williams
PM: Real time quantitative PCR. Genome Res. 6:986–994. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim DK, Kim DW, Kim SW, Kim DY, Lee CH and
Rhee CS: Ki67 antigen as a predictive factor for prognosis of
sinonasal mucosal melanoma. Clin Exp Otorhinolaryngol. 1:206–210.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ivanov VN, Bhoumik A and Ronai Z: Death
receptors and melanoma resistance to apoptosis. Oncogene.
22:3152–3161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herr I and Debatin KM: Cellular stress
response and apoptosis in cancer therapy. Blood. 98:2603–2614.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu B, Wang L, Stehlik C, Medan D, Huang C,
Hu S, Chen F, Shi X and Rojanasakul Y: Phosphatidylinositol
3-kinase/Akt positively regulates Fas (CD95)-mediated apoptosis in
epidermal Cl41 cells. J Immunol. 176:6785–6793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boregowda RK, Olabisi OO, Abushahba W,
Jeong BS, Haenssen KK, Chen W, Chekmareva M, Lasfar A, Foran DJ,
Goydos JS, et al: RUNX2 is overexpressed in melanoma cells and
mediates their migration and invasion. Cancer Lett. 348:61–70.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen-Solal KA, Boregowda RK and Lasfar A:
RUNX2 and the PI3K/AKT axis reciprocal activation as a driving
force for tumor progression. Mol Cancer. 14:1372015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ott PA, Hodi FS and Buchbinder EI:
Inhibition of immune checkpoints and vascular endothelial growth
factor as combination therapy for metastatic melanoma: An overview
of rationale, preclinical evidence, and initial clinical data.
Front Oncol. 5:2022015. View Article : Google Scholar : PubMed/NCBI
|