Introduction
Hepatocellular carcinoma (HCC) is the most common
liver cancer, accounting for 90% of primary liver cancers and is
currently the third major cause of cancer-related deaths worldwide
(1). Although, recent progress in
diagnostic and treatment technologies has improved survival, the
long-term survival of HCC patients remains dismal. One of the
reasons for the dismal prognosis is that current treatment cannot
eliminate cancer-initiating cells (CICs) (2,3). A
comprehensive understanding of the molecular basis of CICs of HCC
may contribute to the identification of novel therapeutic targets
to improve patient outcome.
Sorafenib (Nexavar), a multiple kinase inhibitor, is
the first and only drug that is clinically approved for patients
with advanced HCC (4–8). The major target of sorafenib is serine
threonine kinase Raf-1, which is involved in the
Ras/Raf/MEK/mitogen-activated protein kinase signaling cascade
(9,10). In an in vitro kinase assay,
sorafenib efficiently inhibited the activity of Raf-1 at a very low
dose (IC50 of 6 nM) (11,12).
Other receptor tyrosine kinases are also suppressed by sorafenib,
including vascular endothelial growth factor receptors 1, 2 and 3,
platelet-derived growth factor receptor and fibroblast growth
factor receptor (12,13). Although, sorafenib has exhibited
survival benefits in large randomized phase III studies, the
response rate to sorafenib is actually quite low (2–3%) (4,14). In
addition, therapeutic biomarkers that may predict the response to
sorafenib are not currently available. Therefore, to improve the
treatment response in HCC, it is important to identify the
molecular mechanism of sorafenib resistance.
Adenine nucleotide translocator (ANT) which is
abundant in the inner mitochondrial membrane, plays an important
role in cellular energy metabolism by catalyzing the exchange of
mitochondrial adenosine triphosphate for cytosolic adenosine
diphosphate, thus, influencing mitochondrial bioenergetics
(15). In addition, it is involved
in the formation of the mitochondrial permeability transition pore
complex that interacts with the Bcl2 family of proteins,
contributing to mitochondrial-mediated apoptosis (16). Human ANT has 4 isoforms (ANT1, ANT2,
ANT3 and ANT4), and the relative expression of these isoforms is
dependent on the developmental stage, proliferation status and cell
or tissue types. Among these isoforms, ANT2 is specifically
expressed in proliferative and undifferentiated cells (16). It has been reported that ANT2
suppression by shRNA can exert anticancer effects in HCC through
the regulation of different pathways (17–19).
High-intensity focused ultrasound (HIFU) is based on
the unique characteristic of ultrasound beams (0.8–3.5 MHz), which
can be focused at a distance from the radiating transducer
(20). The accumulated energy at
the focal region induces tissue necrosis of the targeted lesion
without causing damage to the surrounding vital structures
(20). The ability of inducing
immediate cell death at a distance from the ultrasound source
without the need for surgery or insertion of ablation instruments
makes HIFU an attractive treatment option for HCC (20).
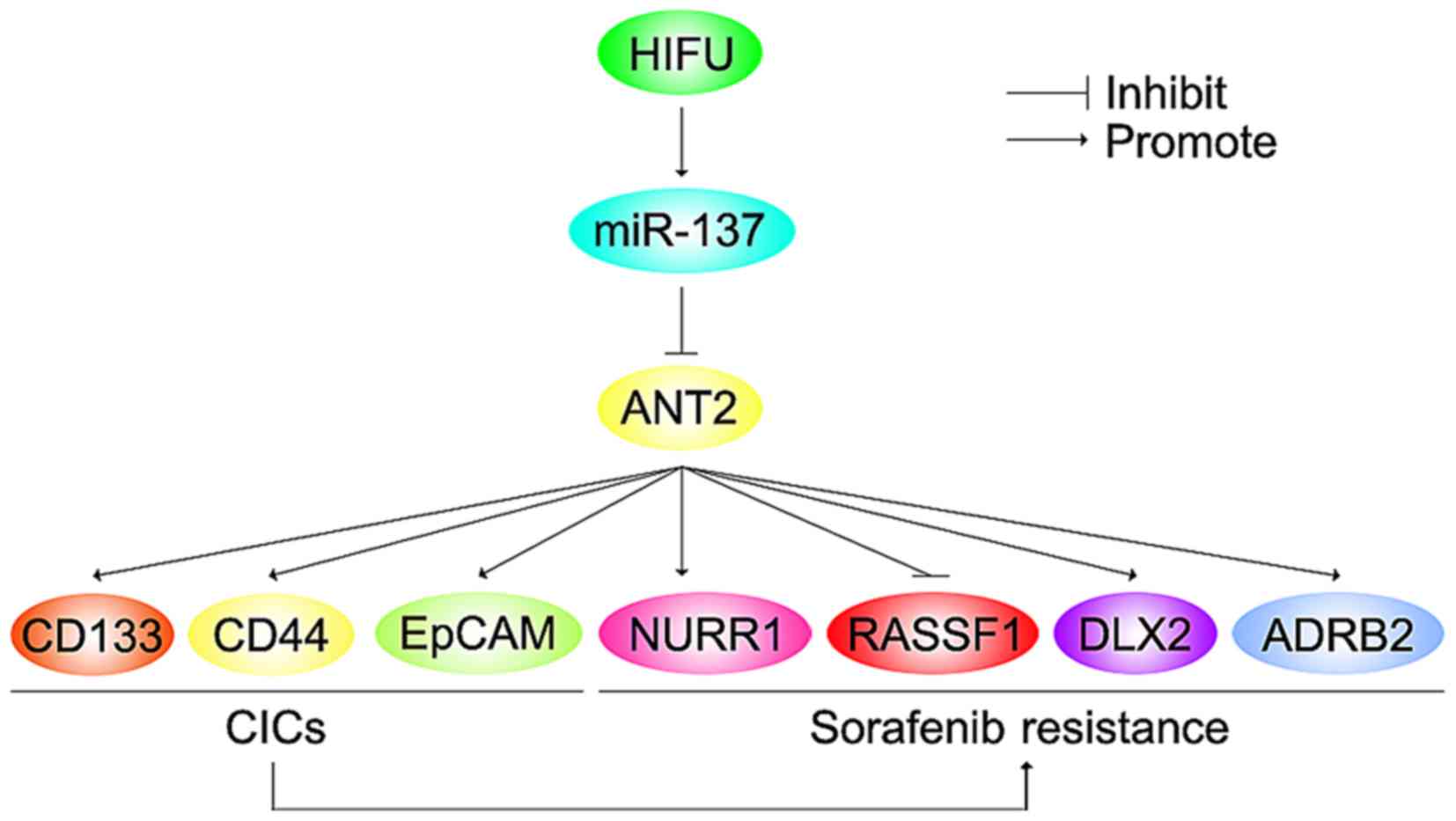
In the present study, we found that ANT2 was
upregulated in sorafenib-resistant HCC Huh7 cells (Huh7-R) and its
overexpression promoted sorafenib resistance. ANT2 induced the
formation of CIC phenotypes and promoted metastasis-associated
traits in the Huh7 cells. Silencing of miR-137 upregulated ANT2
protein expression in the Huh7 cells. miR-137 was downregulated in
the Huh7-R cells, compared with the Huh7 cells and its restoration
reversed sorafenib resistance in the Huh7-R cells. Restoration of
miR-137 inhibited formation of CIC traits and attenuated the
abilities of migration and invasion in the Huh7-R cells. Moreover,
we demonstrated that HIFU in unresectable HCC upregulated serum
miR-137. Combining HIFU and sorafenib may be a wise option for
advanced and unresectable HCC.
Materials and methods
Patients
Between November 2012 and October 2014, 13 patients
with HCC were enrolled in this clinical study. Four patients were
not included in the present study since they either had >4 HCC
foci (n=2) or hepatic dysfunction (Child-Pugh class C, n=2). The
present study was approved by the Ethics Committee of the First
Peoples Hospital of Jining, and each patient signed an informed
consent form at the time of enrollment.
Human HCC cell line
Huh7 cells were purchased from the Biochemistry and
Cell Biology Institute of Shanghai, Chinese Academy of Sciences,
within 3 months of the experiments. To obtain sorafenib-resistant
Huh7 cells (Huh7-R cells), we treated Huh7 cells with increasing
concentrations of sorafenib from 107 to 105
M. The established Huh7-R cells grew at a similar rate in the
presence or absence of 105 M sorafenib for 3 days (data
not shown). The IC50 (half maximal inhibitory
concentration) of Huh7-R cells increased by 12-fold, compared with
the Huh7 cells (data not shown). They were cultured in Dulbeccos
modified Eagles medium (DMEM) supplemented with 10% fetal bovine
serum and antibiotics (100 mg/ml penicillin, 100 U/ml streptomycin)
in a 5% CO2 incubator at 37̊C.
Western blotting
Whole cell lysates were subjected to
SDS-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes (Bio-Rad, Berkeley, CA, USA).
Then, proteins were probed with primary antibodies against human
ANT2 (1:500; ab195630), RASSF1 (1:500; ab180801), SIRT3 (1:500;
ab217319), NURR1 (1:500; ab60149), DLX2 (1:500; ab30339), ADRB2
(1:500; EP795Y), CD133 (1:500; ab19898), CD44 (1:500; ab157107),
EpCAM (1:500; ab71916) and β-actin (1:500; ab8227; all from Abcam,
Cambridge, MA, USA) overnight at 4̊C. Secondary antibodies
(1:10,000; ab150077; Abcam) were used for 30 min at room
temperature. The specific proteins were visualized by Odyssey™
Infrared Imaging System (Gene Company, Lincoln, NE, USA). β-actin
expression was used as an internal control to show equal loading of
the protein samples.
MTT assay
The proliferation of cells was assessed by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay
(Sigma-Aldrich, St Louis, MO, USA). The MTT analysis was performed
as previously described (21–26).
In brief, the cells were plated in 96-well plates in DMEM
containing 10% fetal bovine serum at a density of 8×103
cells/well at 37 °C in a 5% CO2 incubator for 12 h.
Cells were transfected with ANT2-expressing plasmid or empty
vectors and were then treated with sorafenib or dimethyl sulfoxide
(DMSO) (10−4-102) for 24 h. Alternatively,
cells were transfected with pre-miR-137 or control miR (mock) for
24 h. Then MTT solution (5 mg·ml−1) was added to the
wells (20 µl/well). Subsequently the plates were incubated in a
cell incubator for 4 h, then the supernatant was removed and 150 µl
of DMSO was added to each well. After incubation for 10 min, the
absorbance of each well was assessed using a Synergy™ 4 Hybrid
microplate reader (BioTek Instruments, Winooski, VT, USA) at a
wavelength of 570 nm, with the reference wavelength set at 630 nm.
The absorbance was directly proportional to the number of cells
that survived.
Sphere formation assay
Cells (103/ml) in serum-free RPMI-1640/1
mM Na-pyruvate were seeded on 0.5% agar precoated 6-well plates.
After 10 days, half the medium was replaced with fresh medium every
third day. Single spheres were selected and counted.
Real-time PCR for microRNAs
(miRs)
Total RNA was extracted using the miRNeasy Mini kit
and RNase-free DNase Set (Qiagen, Valencia, CA, USA) following the
protocol provided by the manufacturer. The expression level of
microRNA was analyzed using TaqMan MicroRNA Assay kit (Applied
Biosystems) following the manufacturer's protocol.
Immunofluorescence staining
Immunofluorescence staining was performed as
previously described (27). After
transfection, the cells were fixed with 4% paraformaldehyde for 10
min and permeabilized with phosphate-buffered saline (PBS)
containing 1% Triton X-100 for 10 min at room temperature. Then,
the coverslips were blocked with BSA and incubated with the primary
antibodies against ANT2 (Abcam) overnight. The following day, the
cells were incubated with the secondary antibodies (Abcam).
Bioinformatics method
The analysis of potential microRNA target sites was
performed with the commonly used prediction algorithm, miRanda
(http://www.microrna.org/).
Migration and invasion assays
Migration and invasion assays were performed as
previously described (28).
Wound healing assay
Cells were seeded onto 6-well plates. Monolayers of
cells were wounded with yellow pipette tips (volume range, 200µl).
After washing, the cells were incubated in fresh culture medium.
The wounded areas were photographed at 0 and 36 h using a Nikon
inverted microscope.
Anoikis assays
Anoikis resistance was evaluated by seeding
7×104 cells in ultralow attachment plates (Corning Inc.,
Corning, NY, USA). After 24 h of anchorage-independent culture,
cells were transfected as indicated and resuspended in 0.4% trypan
blue (Sigma-ALdrich) and the viability of the cells was assessed
using the CellTiter-Glo® Luminescent Cell Viability
Assay (Promega, Madison, WI, USA). The cells were harvested with
Triton X-100 lysis buffer at indicated times.
Statistical analysis
Data are presented as the mean ± SEM. A Student's
t-test (two-tailed) was used to compare differences between two
groups (P<0.05 was considered significant).
Results
ANT2 is upregulated in
sorafenib-resistant Huh7 cells (Huh7-R cells) and its
overexpression promotes sorafenib resistance in sorafenib-sensitive
Huh7 cells
In order to detect whether sorafenib resistance is
associated with ANT2 protein expression, we analyzed the protein
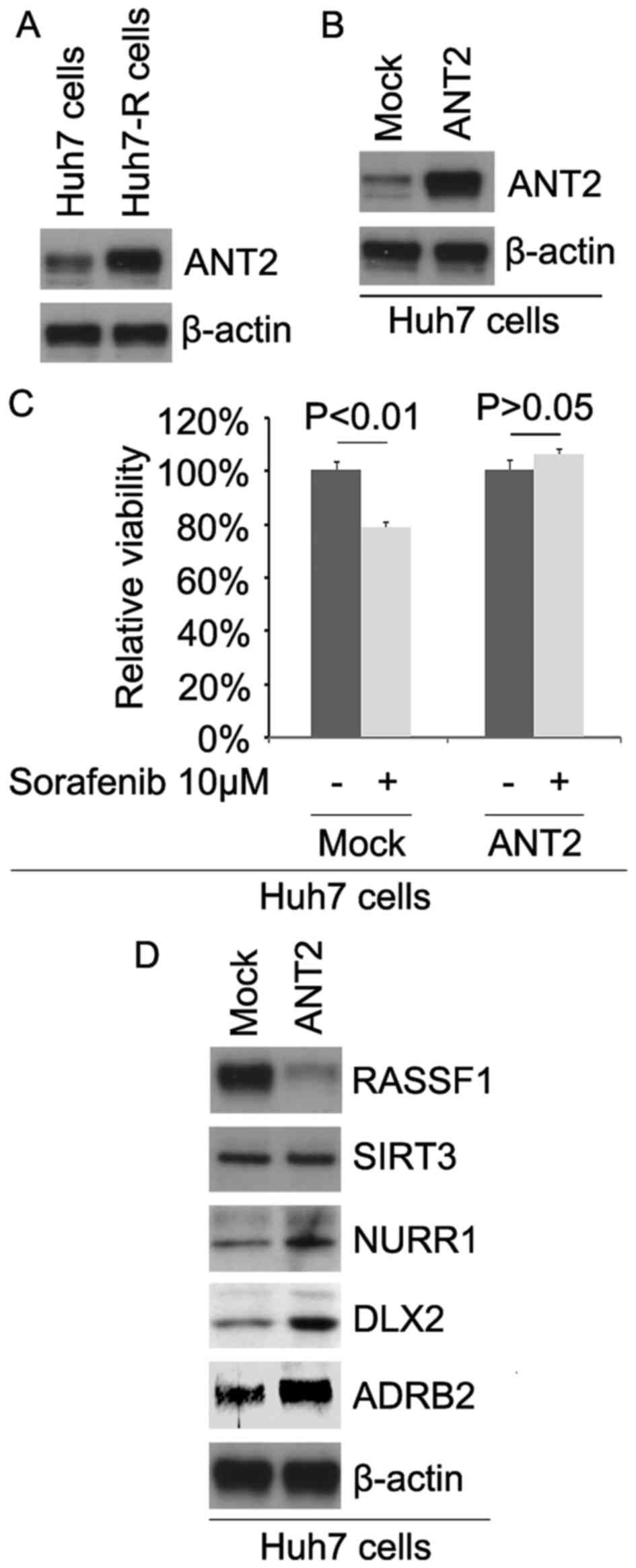
expression of ANT2 in Huh7 and Huh7-R cells. The results revealed
that the ANT2 protein was upregulated in the Huh7-R cells (Fig. 1A). To identify the role of ANT2, we
ascertained whether ANT2-expressing plasmids could cause stable
expression of the ANT2 protein in the Huh7 cells. The results
showed that the ANT2 protein was significantly increased by
ANT2-expressing plasmids in the cells (Fig. 1B). To further determine whether ANT2
affects sorafenib efficacy in HCC cells, we transfected Huh7 cells
with the ANT2-expressing plasmids. Then, we performed an MTT assay
in the cells transfected with the ANT2-expressing plasmids. The
results revealed that ANT2 transformed Huh7 to Huh7-R cells
(Fig. 1C), suggesting that its
overexpression promotes sorafenib resistance.
To identify whether ANT2 affects RASSF1, SIRT3,
NURR1, DLX2 and ADRB2, we performed western blotting to detect
their expression in Huh7 cells. Our results showed that RASSF1 was
downregulated and NURR1, DLX2 and ADRB2 were upregulated in the
Huh7 cells transfected with ANT2 (Fig.
1D).
ANT2 promotes formation of CIC
phenotypes in Huh7 cells
In order to identify whether ANT2 affects CIC traits
in Huh7 cells, we performed a sphere-forming assay to assess the
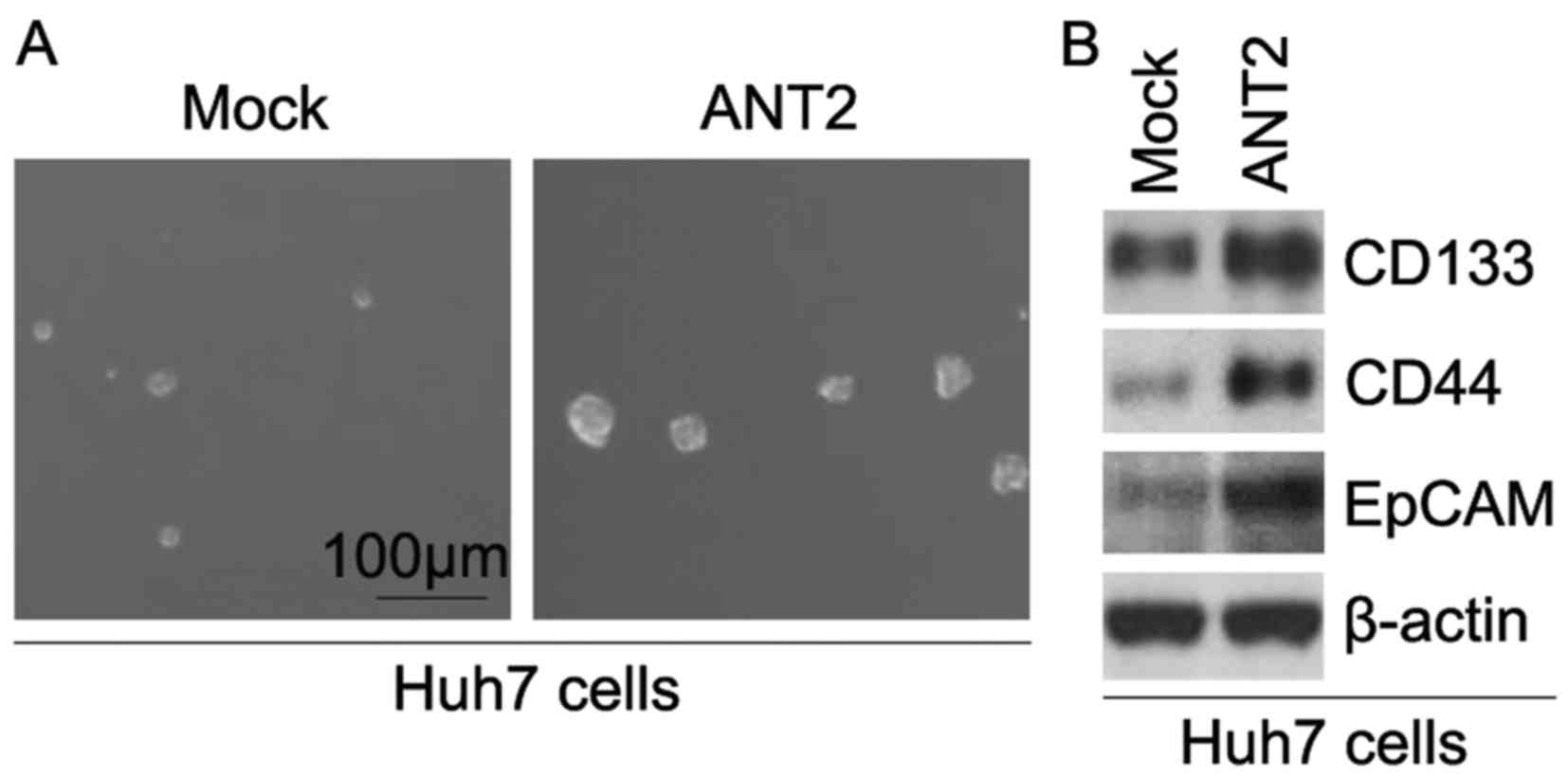
ability of CIC or CIC-like cell-self renewal in Huh7 cells. The
sphere-forming assay revealed that ANT2-overexpressing cells formed
much bigger spheres after 14 days of culture when compared to the
control cells, indicating markedly increased CIC traits by ANT2
(Fig. 2A). CD133, CD44 and EpCAM
are positively associated with CIC-like characteristics in HCC
(29–31). To determine whether ANT2 regulates
CD133, CD44 and EpCAM protein expression, we performed western
blotting in Huh7 cells transfected with the ANT2-expressing
plasmids and empty vectors. The results showed that the protein
expression of CD133, CD44 and EpCAM was upregulated in the Huh7
cells transfected with the ANT2-expressing plasmids (Fig. 2B).
ANT2 promotes metastasis-associated
traits in Huh7 cells
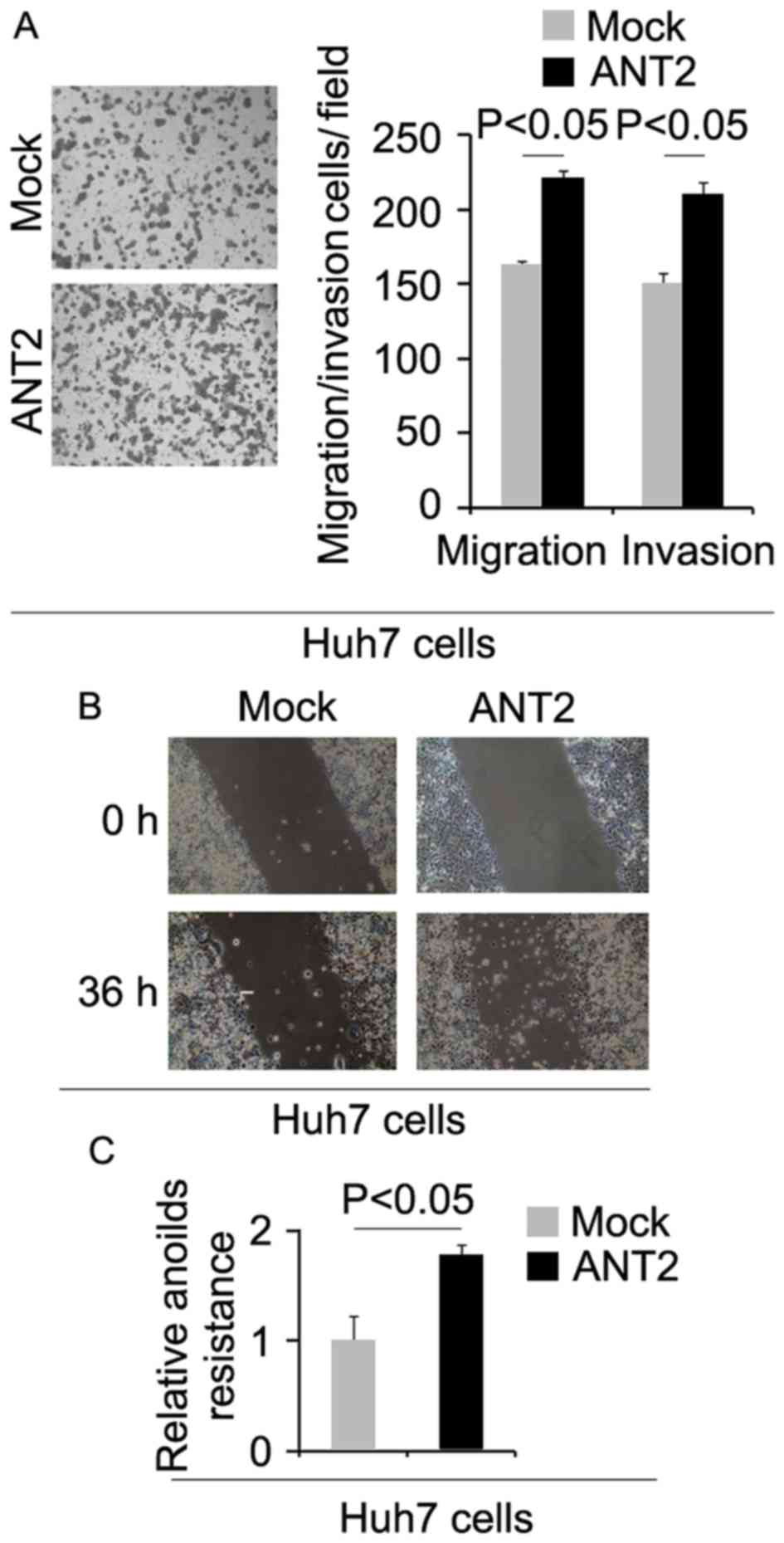
To determine whether cells with increased CIC
characteristics have increased metastatic ability, we performed
migration, invasion, wound healing and anoikis assays. We found
that migration, invasion and anoikis resistance were increased by
ANT2 (Fig. 3A-C).
Silencing of miR-137 upregulates ANT2
protein expression in Huh7 cells
Having demonstrated that ANT2 was upregulated in
Huh7-R cells and that its overexpression promoted sorafenib
resistance in Huh7 cells and promoted formation of CIC phenotypes,
we next studied the mechanisms regulating ANT2 expression in Huh7
cells. miRs are a class of small non-coding RNAs (~22 nucleotides),
that negatively regulate protein-coding gene expression by
targeting mRNA degradation or translation inhibition (32,33).
To further confirm whether ANT2 could be regulated
by microRNAs, we employed the commonly used prediction algorithm,
miRanda (http://www.microrna.org/microrna/home.do) to analyze
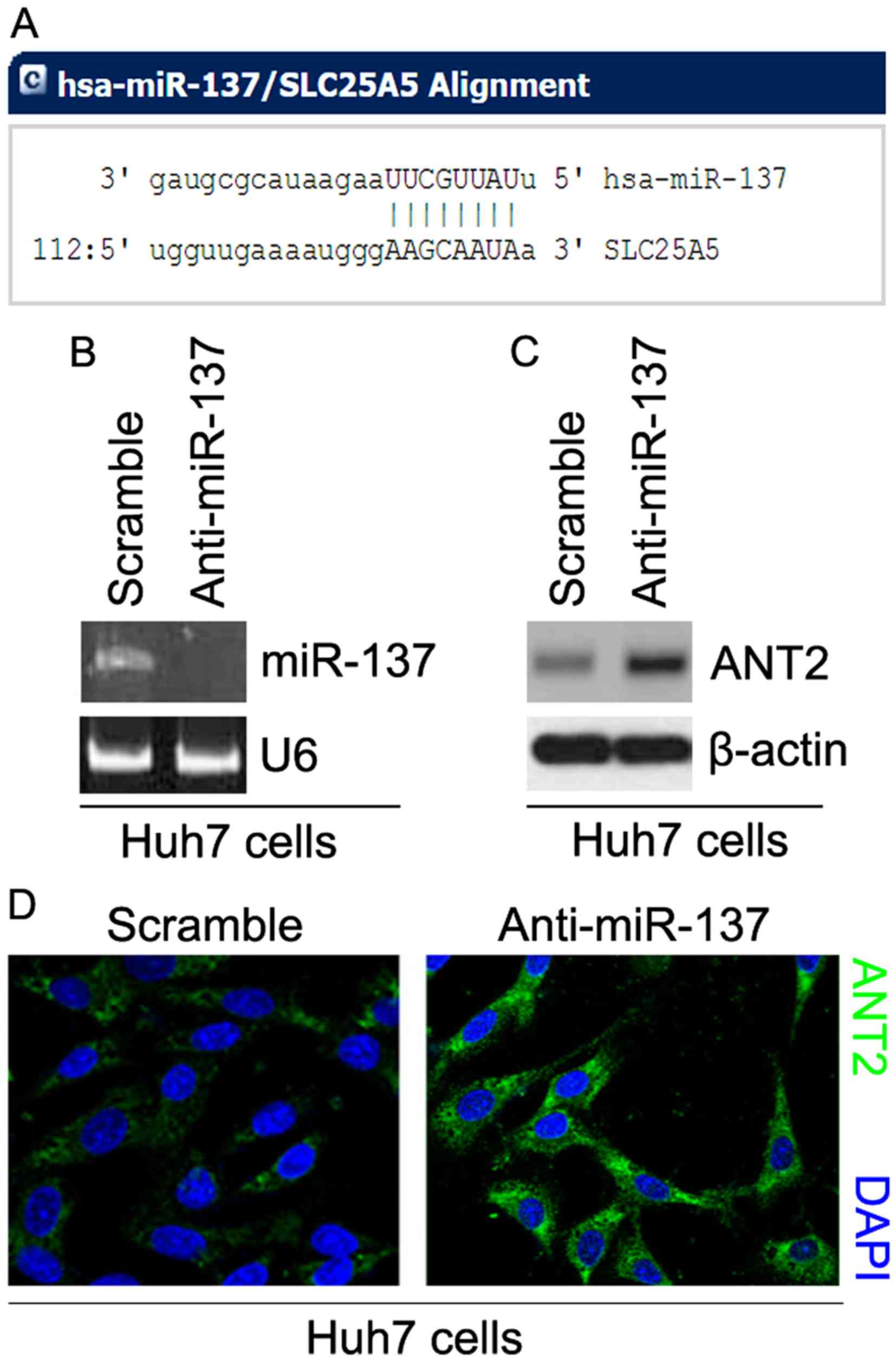
the 3 untranslated region (3′UTR) of ANT2. Twelve miRs were
identified by the algorithm. However, our interests concerned
miR-137, since it has been reported that miR-137 is significantly
downregulated in HCC. Its decreased expression is associated with
vascular invasion, incomplete involucrum and distant metastasis
(28). Target sites on the 3′UTR of
ANT2 are shown in Fig. 4A. We
reasoned that miR-137 downregulated ANT2 expression by targeting
its 3′UTR in HCC cells. Sliencing of miR-137 contributes to the
upregulation of ANT2 and sorafenib resistance in Huh7 cells.
In an attempt to identify the role of miR-137 in the
regulation of ANT2 expression in Huh7 cells, we transfected Huh7
cells with anti-miR-137 and scramble. After transfection, miR-137
expression was detected by real-time PCR and the results revealed
that miR-137 was significantly decreased by anti-miR-137 in the
cells (Fig. 4B). To ascertain the
reason, we performed western blotting to detect ANT2 protein
expression in Huh7 cells transfected with anti-miR-137 and
scramble. The results revealed that the protein expression of ANT2
was significantly upregulated by anti-miR-137 (Fig. 4C). We next performed
immunofluorescence analyses in Huh7 cells transfected with
anti-miR-137 and scramble. The results showed that the protein
expression of ANT2 was clearly increased in the cells transfected
with anti-miR-137 (Fig. 4D).
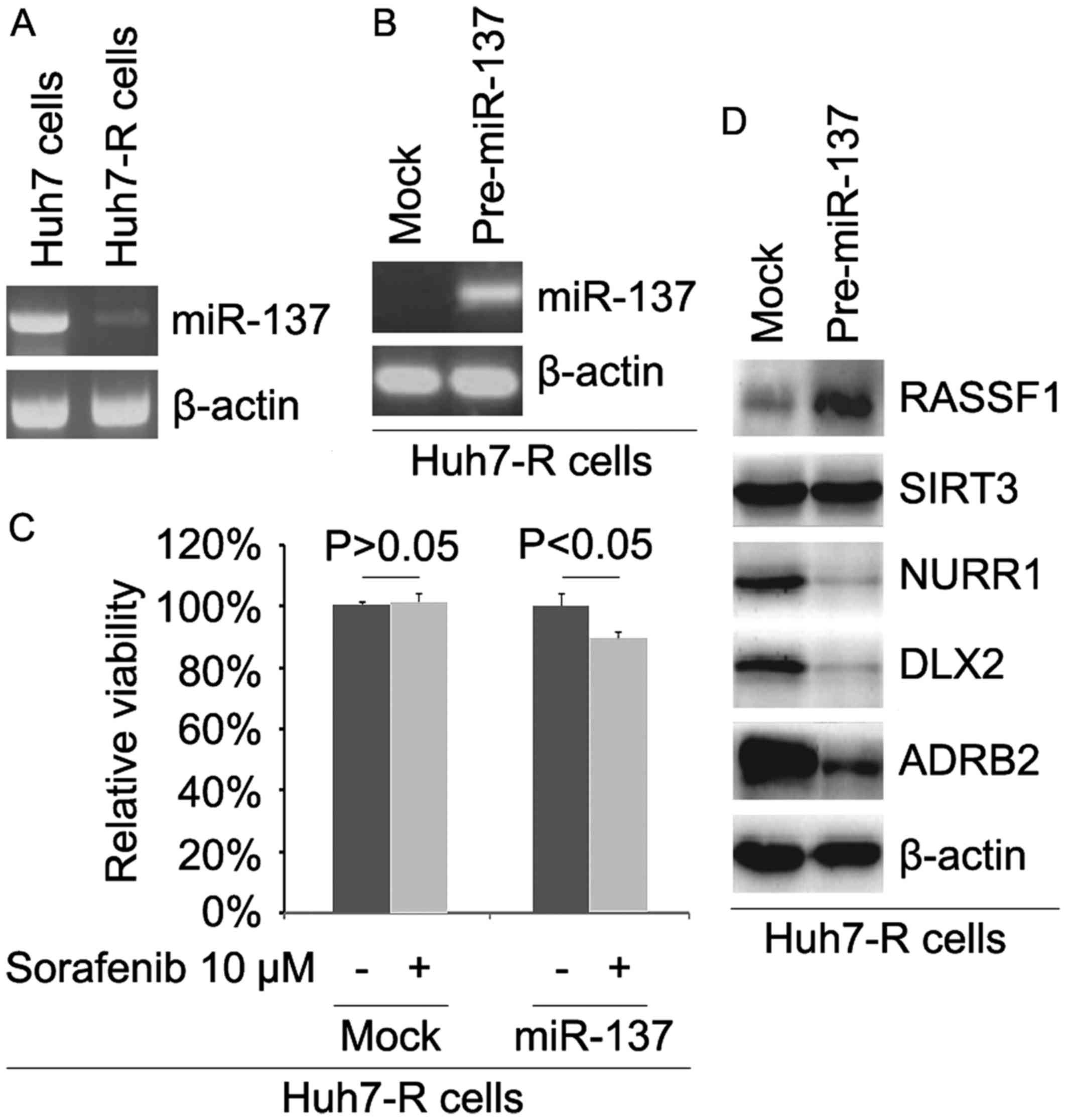
miR-137 is downregulated and its
restoration reverses sorafenib resistance in Huh7-R cells
In order to detect whether sorafenib resistance is
associated with miR-137 expression, we analyzed miR-137 expression
in Huh7 and Huh7-R cells. The results revealed that miR-137
expression was downregulated in the Huh7-R cells (Fig. 5A). To identify the role of miR-137,
we determined whether pre-miR-137 could cause stable expression of
miR-137 in Huh7-R cells. The results showed that the expression of
miR-137 was significantly increased by pre-miR-137 in the cells
(Fig. 5B). To further ascertain
whether miR-137 affects sorafenib efficacy in HCC cells, we
transfected Huh7-R cells with pre-miR-137. Then, we performed an
MTT assay in Huh7-R cells transfected with pre-miR-137. The results
revealed that miR-137 transformed Huh7-R to Huh7 cells (Fig. 5C), suggesting that its
overexpression reversed sorafenib resistance. To identify whether
miR-137 affects RASSF1, SIRT3, NURR1, DLX2 and ADRB2, we performed
western blotting to detect their expression in Huh7-R cells. Our
results showed that RASSF1 was upregulated and NURR1, DLX2 and
ADRB2 were downregulated in the Huh7-R cells transfected with
pre-miR-137 (Fig. 5D).
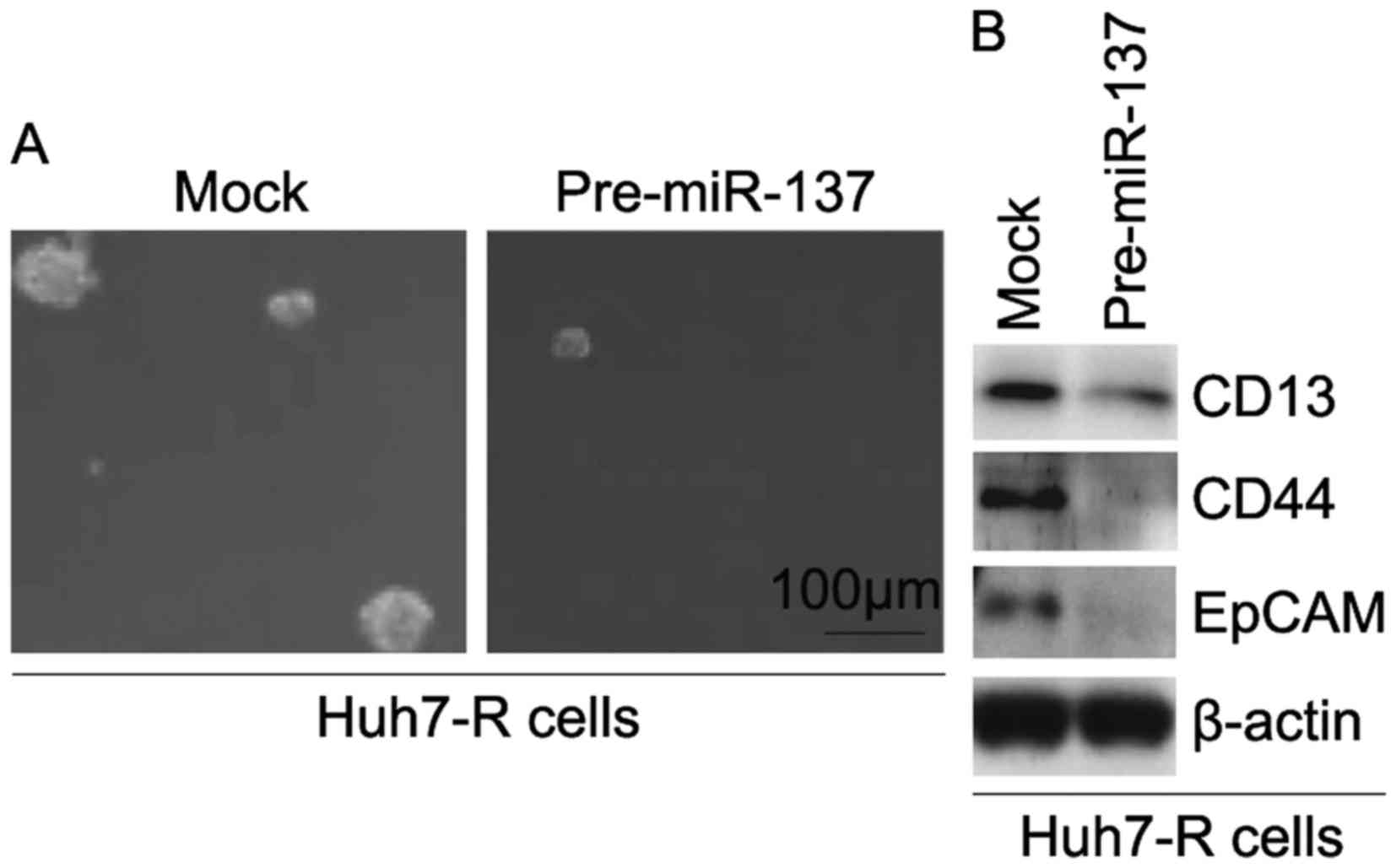
miR-137 inhibits formation of CIC
phenotypes in Huh7-R cells
In order to identify whether miR-137 affects CIC
traits in Huh7-R cells, we performed a sphere-forming assay to
assess the ability of CIC or CIC-like cell self-renewal in Huh7-R
cells. The sphere-forming assay revealed that
miR-137-overexpressing cells formed much smaller spheres after 14
days of culture as compared with the control cells, indicating
markedly increased CIC traits by miR-137 (Fig. 6A). To determine whether miR-137
regulates the protein expression of CD133, CD44 and EpCAM, we
performed western blotting in Huh7-R cells transfected with
pre-miR-137 and control miR. The results revealed that the protein
expression of CD133, CD44 and EpCAM was downregulated in the Huh7-R
cells transfected with pre-miR-137 (Fig. 6B).
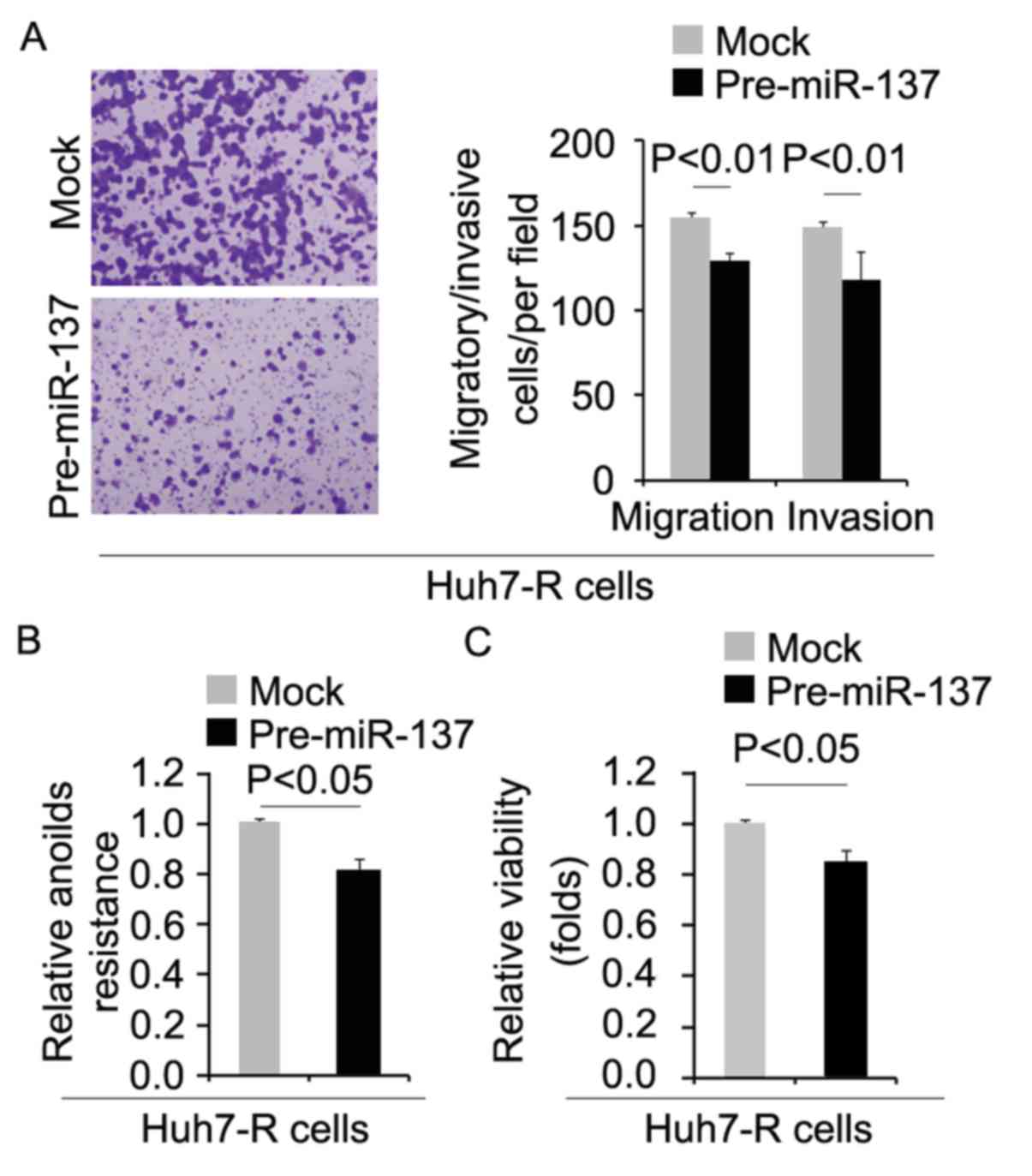
miR-137 inhibits metastasis-associated
traits in Huh7-R cells
To determine whether cells with decreased CIC
characteristics had attenuated metastatic ability, we performed
migration, invasion and anoikis assays. We found that migration,
invasion and anoikis resistance were inhibited by pre-miR-137 in
the Huh7-R cells (Fig. 7A and B).
Moreover, in order to detect whether miR-137 affects proliferation,
we performed an MTT assay in the Huh7-R cells. We found that its
overexpression inhibited proliferation in the Huh7-R cells
(Fig. 7C).
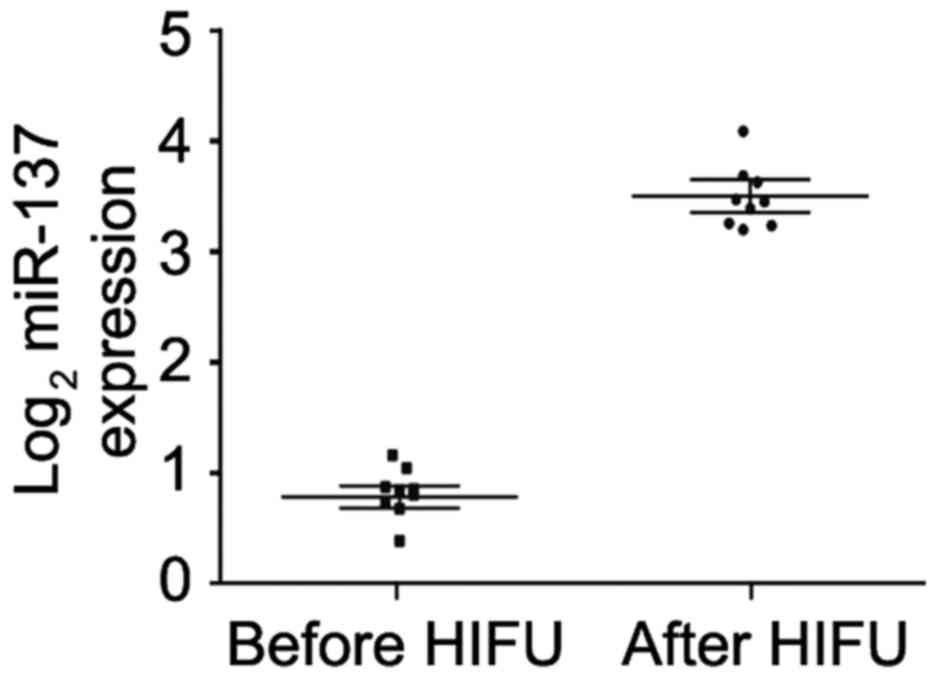
HIFU promotes serum miR-137 expression
in unresectable HCC patients
In order to determine whether HIFU affects serum
miR-137 expression in unresectable HCC patients, we recruited 9
unresectable HCC patients who received HIFU treatment. Real-time
PCR was performed to compare the difference in serum miR-137 before
and after treatment. We found that receiving HIFU significantly
promoted miR-137 expression in the serum of these patients
(Fig. 8).
Discussion
Sorafenib, a multikinase inhibitor, is the only
standard clinical drug used against patients with advanced
hepatocellular carcinoma (HCC). However, development of sorafenib
resistance in HCC often prevents its long-term efficacy. Therefore,
novel targets and strategies are urgently needed to improve the
antitumor effect of sorafenib.
miR-137 expression is significantly downregulated in
HCC. Its decreased expression is associated with vascular invasion,
incomplete involucrum and distant metastasis (34). Decreased miR-137 expression is an
independent indicator for poor survival (34). Overexpression of miR-137 suppresses
cell proliferation, migration and invasion in vitro
(34). Consistent with the previous
studies, we demonstrated that decreased miR-137 expression is
associated with sorafenib resistance in HCC Huh7 cells. Current
treatment cannot eliminate cancer-initiating cells (CICs) (2,3). This
is a cause for anticancer drug resistance and recurrence. We found
that miR-137 inhibited formation of CIC traits as well as migration
and invasion in Huh7-R cells. RASSF1 is a MAPK signaling factor and
knockdown of RASSF1 increased sorafenib resistance (35). Knockdown of NURR1 significantly
increased cell sensitivity to sorafenib and inhibited the cell
growth, migration and invasion of HCC cells, both in vitro
and in vivo (36). DLX2
facilitates sorafenib resistance by promoting the expression of
markers of epithelial-mesenchymal transition and by activating the
extracellular signal-regulated protein kinase pathway (37). ADRB2 signaling promotes HCC
progression and sorafenib resistance by inhibiting autophagic
degradation of HIF1α (38). We
demonstrated that RASSF1 expression can be induced and NURR1, DLX2
and ADRB2 can be inhibited by miR-137. The results indicate that
miR-137 may be a therapeutic target and a reliable biomarker that
can be used to predict sorafenib resistance and ensure more
effective clinical management.
ANT2 suppression by shRNA exerts anticancer effects
in HCC by regulating different pathways (17–19).
However, its regulatory mechanism has yet to be reported. We found
that silencing of miR-137 significantly upregulated ANT2 protein
expression in Huh7 cells. Moreover, we demonstrated that ANT2 was
upregulated in sorafenib-resistant HCC Huh7 cells (Huh7-R) and its
overexpression promoted sorafenib resistance. ANT2 induced the
formation of CIC phenotypes and promoted metastasis-associated
traits in the Huh7 cells. We demonstrated that ANT2 overexpression
inhibited RASSF1 expression and promoted NURR1, DLX2 and ADRB2
expression in Huh7 cells. In the present study, we discovered the
role for ANT2 in HCC, its association with sorafenib resistance,
and the antitumor effects of a newly identified microRNA, miR-137,
that targets ANT2. These findings have potential therapeutic
significance as testing for the absence of ANT2 at diagnosis may be
helpful for identifying patients who are likely to have a response
to sorafenib.
HIFU is the latest developed local ablation
technique for unresectable HCC (20). We found that after receiving HIFU,
miR-137 expression was upregulated in the serum of patients with
unresectable HCC (Fig. 9). Thus,
combining HIFU and sorafenib may be a wise option for advanced and
unresectable HCC (Fig. 9).
References
|
1
|
Cervello M, McCubrey JA, Cusimano A,
Lampiasi N, Azzolina A and Montalto G: Targeted therapy for
hepatocellular carcinoma: Novel agents on the horizon. Oncotarget.
3:236–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Q, Shi S, Yen Y, Brown J, Ta JQ and
Le AD: A subpopulation of CD133+ cancer stem-like cells
characterized in human oral squamous cell carcinoma confer
resistance to chemotherapy. Cancer Lett. 289:151–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Maio M, Daniele B and Perrone F:
Targeted therapies: Role of sorafenib in HCC patients with
compromised liver function. Nat Rev Clin Oncol. 6:505–506. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bioulac-Sage P, Laumonier H, Couchy G, Le
Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G,
Trillaud H, et al: Hepatocellular adenoma management and phenotypic
classification: The Bordeaux experience. Hepatology. 50:481–489.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scanga A and Kowdley K: Sorafenib: A
glimmer of hope for unresectable hepatocellular carcinoma?
Hepatology. 49:332–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43–9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panka DJ, Wang W, Atkins MB and Mier JW:
The Raf inhibitor BAY 43–9006 (Sorafenib) induces
caspase-independent apoptosis in melanoma cells. Cancer Res.
66:1611–1619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adnane L, Trail PA, Taylor I and Wilhelm
SM: Sorafenib (BAY 43–9006, Nexavar), a dual-action inhibitor that
targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases
VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 407:597–612.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilhelm S, Carter C, Lynch M, Lowinger T,
Dumas J, Smith RA, Schwartz B, Simantov R and Kelley S: Discovery
and development of sorafenib: A multikinase inhibitor for treating
cancer. Nat Rev Drug Discov. 5:835–844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Konopleva M, Shi YX, McQueen T,
Harris D, Ling X, Estrov Z, Quintás-Cardama A, Small D, Cortes J,
et al: Mutant FLT3: A direct target of sorafenib in acute
myelogenous leukemia. J Natl Cancer Inst. 100:184–198. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schönfeld P, Schild L and Bohnensack R:
Expression of the ADP/ATP carrier and expansion of the
mitochondrial (ATP + ADP) pool contribute to postnatal maturation
of the rat heart. Eur J Biochem. 241:895–900. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chevrollier A, Loiseau D, Reynier P and
Stepien G: Adenine nucleotide translocase 2 is a key mitochondrial
protein in cancer metabolism. Biochim Biophys Acta. 1807:562–567.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jang JY, Jeon YK, Lee CE and Kim CW: ANT2
suppression by shRNA may be able to exert anticancer effects in HCC
further by restoring SOCS1 expression. Int J Oncol. 42:574–582.
2013.PubMed/NCBI
|
|
18
|
Baik SH, Lee J, Lee YS, Jang JY and Kim
CW: ANT2 shRNA downregulates miR-19a and miR-96 through the
PI3K/Akt pathway and suppresses tumor growth in hepatocellular
carcinoma cells. Exp Mol Med. 48:e2222016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jang JY, Lee YS, Jeon YK, Lee K, Jang JJ
and Kim CW: ANT2 suppression by shRNA restores miR-636 expression,
thereby downregulating Ras and inhibiting tumorigenesis of
hepatocellular carcinoma. Exp Mol Med. 45:e32013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ng KK, Poon RT, Chan SC, Chok KS, Cheung
TT, Tung H, Chu F, Tso WK, Yu WC, Lo CM, et al: High-intensity
focused ultrasound for hepatocellular carcinoma: A single-center
experience. Ann Surg. 253:981–987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liao XH, Li YQ, Wang N, Zheng L, Xing WJ,
Zhao DW, Yan TB, Wang Y, Liu LY, Sun XG, et al: Re-expression and
epigenetic modification of maspin induced apoptosis in MCF-7 cells
mediated by myocardin. Cell Signal. 26:1335–1346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao XH, Wang Y, Wang N, Yan TB, Xing WJ,
Zheng L, Zhao DW, Li YQ, Liu LY, Sun XG, et al: Human chorionic
gonadotropin decreases human breast cancer cell proliferation and
promotes differentiation. IUBMB Life. 66:352–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao XH, Xiang Y, Yu CX, Li JP, Li H, Nie
Q, Hu P, Zhou J and Zhang TC: STAT3 is required for
MiR-17-5p-mediated sensitization to chemotherapy-induced apoptosis
in breast cancer cells. Oncotarget. Feb 2–2017.(Epub ahead of
print).
|
|
24
|
Liao XH, Li JY, Dong XM, Wang X, Xiang Y,
Li H, Yu CX, Li JP, Yuan BY, Zhou J, et al: ERα inhibited
myocardin-induced differentiation in uterine fibroids. Exp Cell
Res. 350:73–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li
YQ, Yan TB, Sun XG, Hu P and Zhang TC: Estrogen receptor α mediates
proliferation of breast cancer MCF-7 cells via a
p21/PCNA/E2F1-dependent pathway. FEBS J. 281:927–942. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiang Y, Lu DL, Li JP, Yu CX, Zheng DL,
Huang X, Wang ZY, Hu P, Liao XH and Zhang TC: Myocardin inhibits
estrogen receptor alpha-mediated proliferation of human breast
cancer MCF-7 cells via regulating MicroRNA expression. IUBMB Life.
68:477–487. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren ZG, Dong SX, Han P and Qi J: miR-203
promotes proliferation, migration and invasion by degrading SIK1 in
pancreatic cancer. Oncol Rep. 35:1365–1374. 2016.PubMed/NCBI
|
|
28
|
Lu Y, Chopp M, Zheng X, Katakowski M,
Buller B and Jiang F: MiR-145 reduces ADAM17 expression and
inhibits in vitro migration and invasion of glioma cells.
Oncol Rep. 29:67–72. 2013.PubMed/NCBI
|
|
29
|
Suetsugu A, Nagaki M, Aoki H, Motohashi T,
Kunisada T and Moriwaki H: Characterization of CD133+
hepatocellular carcinoma cells as cancer stem/progenitor cells.
Biochem Biophys Res Commun. 351:820–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J and
Li J: Cancer stem/progenitor cells are highly enriched in
CD133+CD44+ population in hepatocellular
carcinoma. Int J Cancer. 126:2067–2078. 2010.PubMed/NCBI
|
|
31
|
Yamashita T, Ji J, Budhu A, Forgues M,
Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al:
EpCAM-positive hepatocellular carcinoma cells are tumor-initiating
cells with stem/progenitor cell features. Gastroenterology.
136:1012–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu LL, Lu SX, Li M, Li LZ, Fu J, Hu W,
Yang YZ, Luo RZ, Zhang CZ and Yun JP: FoxD3-regulated microRNA-137
suppresses tumour growth and metastasis in human hepatocellular
carcinoma by targeting AKT2. Oncotarget. 5:5113–5124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Azumi J, Tsubota T, Sakabe T and Shiota G:
miR-181a induces sorafenib resistance of hepatocellular carcinoma
cells through downregulation of RASSF1 expression. Cancer Sci.
107:1256–1262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Emma MR, Iovanna JL, Bachvarov D, Puleio
R, Loria GR, Augello G, Candido S, Libra M, Gulino A, Cancila V, et
al: NUPR1, a new target in liver cancer: Implication in controlling
cell growth, migration, invasion and sorafenib resistance. Cell
Death Dis. 7:e22692016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu J, Cui X, Qu L, Hua L, Wu M, Shen Z,
Lu C and Ni R: Overexpression of DLX2 is associated with poor
prognosis and sorafenib resistance in hepatocellular carcinoma. Exp
Mol Pathol. 101:58–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu FQ, Fang T, Yu LX, Lv GS, Lv HW, Liang
D, Li T, Wang CZ, Tan YX, Ding J, et al: ADRB2 signaling promotes
HCC progression and sorafenib resistance by inhibiting autophagic
degradation of HIF1α. J Hepatol. 65:314–324. 2016. View Article : Google Scholar : PubMed/NCBI
|