Introduction
Microvesicles (MVs) are small vesicular structures
(30–1,000 nm in diameter) that are formed and shed directly from
the surfaces of cells under physiological and pathological
conditions (1). They play vital
roles in intercellular communication. Many studies suggest that
de-regulated biogenesis of MVs is responsible for several diseases,
especially cancers such as hepatocellular carcinoma (HCC) (2).
The formation of MVs involves tightly regulated
processes. There are generally two types of MVs depending on the
membrane vesicles from which they are derived, microparticles
(100–1000 nm) and exosomes (30–100 nm). Microparticles bud from
micro-domains within the plasma membrane (known as lipid rafts),
then are loaded with the specific cargo and are finally released
into the extracellular environment (3). In contrast, the biogenesis of exosomes
uses the multivesicular body (MVB) sorting pathway, in which
proteins and lipids are initially destined for degradation in
lysosomes. The biogenesis of MVs results in dramatic morphological
changes in the cells. RhoA signaling, which is responsible for
actin-cytoskeletal rearrangements and cell morphology, is able to
stimulate the formation of MVs in different cancer cell lines
(4). An increase of the
intracellular Ca2+ has also been shown to promote the
formation of MVs (5,6). Gelsolin (GSN) is a potent
actin-severing protein that removes the capping proteins and
regulates the assembly and disassembly of the actin filaments
(F-actin) (7). The breakdown of
actin filaments by GSN depends on the cytosolic Ca2+
concentration (8). Ca2+
serves as a rapid activator for GSN and assists GSN in
participating in the regulation of cell cytoskeleton and vesicle
secretion. However, thus far, the molecular aspects associated with
the regulation of gene expression of GSN, especially at
post-transcriptional level, have not been clearly elucidated.
MVs can transport several types of cargo including
proteins, lipid, and nucleic acids (mRNA, microRNA, and DNA) to
recipient cells to regulate the functional or behavioral aspects of
the recipient cells. The biological function of MVs appears to be
dependent on the content transported by them. Our previous study
suggested that overexpression of miR-483 in HL7702 cells might lead
to an intercellular transfer of miR-483 between HL7702 and LX-2
cells (9). Chen et al also
confirmed that miR-214 could be transferred by exosomes from
hepatocytes to hepatic stellate cells and be involved in
fibrogenesis (10). However, it is
unclear whether microRNA regulates the secretion of MVs.
Increased MV levels have been reported in patients
with HCC and might serve as a potential biomarker for the diagnosis
and prognosis of HCC (2,11). Kogure et al have shown that
HCC-derived MVs can carry a specific subset of miRNAs that can be
internalized by other cells to regulate transforming growth
factor-β (TGF-β)-activated kinase-1 (TAK1) expression and enhance
transformed cell growth in recipient cells (12). Furthermore, the expression of miRNAs
in serum exosomes could be a potential diagnostic marker for HCC.
Serum exosomal miR-21 expression in patients with HCC has been
reported to be higher than healthy volunteers (13). In the past decade, extensive and
in-depth studies on microRNAs in liver diseases, especially in HCC,
have been conducted. miR-21, miR-122, miR-200, and miR-221 have
been reported to be de-regulated in HCC progression and implicated
in cancer cell proliferation and metastasis (14–17).
Nevertheless, whether de-regulated microRNA could regulate the
secretion of MVs in HCC is unclear.
In this study, we proposed that miR-200a could
inhibit the secretion of MVs derived from liver cancer cell lines
and regulate the proliferation of the adjacent cells. Herein, we
first identified the amount of MVs released from normal liver cell
lines and HCC cells. Second, we explored the effect of miR-200a on
the secretion of MVs. Subsequently, GSN was predicted and
identified as the functional target of miR-200a. Finally, the
upregulation of miR-200a was able to regulate the proliferation of
the adjacent cells. To the best of our knowledge, this is the first
study to propose that microRNA regulates the secretion of MVs. The
results of this study can help in understanding the functions of
microRNA and the secretion of MVs in liver diseases.
Materials and methods
Cell culture
The human HCC cell line HepG2 and Huh7 and human
normal hepatocyte cell line HL7702 were conserved in our
laboratory. HCC cell lines and normal hepatocyte cell line were
cultured in Dulbecco's modified Eagle's medium and RPMI-1640 medium
(Invitrogen, Carlsbad, CA, USA), respectively, supplemented with
10% fetal bovine serum and 1% penicillin/streptomycin in a humid
atmosphere containing 5% CO2 at 37°C.
Transfection and luciferase assay
Cells were seeded in 6-well plates and transfected
with miR-200a or miR-483 mimics, or non-targeting control (NC)
(GenePharma Co., Ltd., Shanghai, China) using Lipofectamine-2000
(Invitrogen). The NC was synthetic scrambled double
oligonucleotide, non-targeting against any mRNA. Protein and mRNA
expression levels were analyzed 48-h post-transfection. For the MV
isolation, we used 10-cm plates to do the transfection. The
luciferase assay was performed in HEK-293T cells. Briefly, we
generated two Luc-M 3′-UTR constructs, including the potential
biding sequence (GSN-WT-UTR) and mutated the potential biding
sequence (GSN-MUT-UTR). Cells were seeded in 24-well plates and
co-transfected with 100 ng Luc-GSN 3′-UTR reporter vector, 40 ng TK
and 30 nM miR-200a mimics and incubated overnight. Luciferase
activity was measured using the Dual-Glo Luciferase assay system
(Promega, Madison, WI, USA).
Isolation of MVs and electron
microscopy
MVs were purified from cell culture medium as
previously described (18). Cell
culture medium was centrifuged at 300 × g for 10 min, at 1200 × g
for 10 min, and then at 10,000 × g for 30 min. The supernatant was
ultracentrifuged at 110,000 × g for 2 h at 4°C. Pelleted MVs were
resuspended in culture medium and sent to the Center for Electron
Microscopy, Harbin Medical University for transmission electron
microscopy (TEM) analysis.
MVs labeling and flow cytometry
MVs were labeled with PE anti-human CD9 (#312105,
BioLegend, London, UK) for 30 min at room temperature in the dark
according to the manufacturer's instructions. CD9 is a specific
molecular marker for exosomes (19). After incubation with CD9, the
samples were immediately analyzed using flow cytometry. The
procedure was carried out as previously described (20). To establish a MV gate, we used
size-calibrated fluorescent beads with a size of 1 µm (#L2778,
Sigma, St. Louis, MO, USA). The upper limit of the MV gate was
established using the 1 µm beads, while the lower limit was set by
the medium (Fig. 1B). We defined
MVs as particles within the MV gate, which had positive staining
for CD9. The formula, number of events of defined MVs/number of
cells used for the MVs isolation, was used for MV
quantification.
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen). Reverse-transcribed complementary DNA was synthesized
with random primers or microRNA specific stem-loop primers (Applied
Biosystems, Foster City, CA, USA). Subsequently, the cDNA was
subjected to real-time PCR on a 7500 real-time PCR system (Applied
Biosystems). The 2−∆∆Ct method was used to calculate the
relative expression levels of the genes of interest. GAPDH and U6
were used as internal controls. The primer sequences used were as
follows: GSN sense: 5′-CAGACAGCCCCTGCCAGCACCC-3′, antisense:
5′-GAGTTCAGTGCACCAGCCTTAGGC-3′; GAPDH sense:
5′-AGCCTCCCGCTTCGCTCTCT-3′, antisense:
5′-GCGCCCAATACGACCAAATCCGT-3′; miR-200a sense:
5′-GGCGTAACACTGTCTGGTAA-3′, antisense: 5′-CGTATCCAGTGCGTGTCGTG-3′;
U6 sense: 5′-GCTTCGGCAGCACATATACTAAAAT-3′, antisense:
5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Cell indirect co-culture and MTT
assay
For the indirect co-culture assay, polycarbonate
membrane inserts in multidishes (Nunc, Beijing, China) were used.
The HepG2 or Huh7 cells transfected with carboxyfluorescent labeled
miR-200a mimics or NC were seeded in the upper transwell inserts,
and the HL7702 cells were seeded in the lower 3.5-cm plates. HepG2
or Huh7 cells expressing green fluorescence was visualized directly
by fluorescence microscopy and at least twenty fields were recorded
per experiment. The formula - % transfection efficiency = (number
of cells stained with fluorescent positive-green/total number of
cells per field) was used to calculate the transfection efficiency.
The HepG2 or Huh7 cells with transfection efficiency exceeding 70%
was used in the indirect co-culture experiment. After transfection,
we changed the culture medium with medium containing CD9 in the
upper transwell inserts. The CD9+ HL7702 cells were
observed by fluorescence microscopy and we calculated the
percentage of CD9+ HL7702 cells by choosing ten fields
for each experiment. Cell viability was assessed using the MTT
assay. To each well of cells 100 µl of 5 mg/ml solution of
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
was added and incubated at 37°C for 4 h and then lysed in 700 µl of
dimethyl sulfoxide (DMSO) at room temperature for 15 min. The
absorbance of each well at a wavelength of 492 nm was read on a
spectrophotometer. Three independent experiments were performed in
quadruplicate.
Western blotting
Total proteins from cells were extracted using RIPA
buffer. Cellular protein extracts were separated in a 10%
SDS-polyacrylamide gel and electrophoretically transferred onto a
PDVF membrane (Millipore, Bedford, MA, USA). Membranes were blocked
with 5% non-fat dried milk and incubated with antibodies against
GSN (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and GAPDH (Cell
Signaling Technology, Danvers, MA, USA) overnight at 4°C. After
washing with PBST, the membranes were incubated with horseradish
peroxidase-linked secondary antibody. The proteins were visualized
using ECL chemiluminescence and detected by X-ray film. Bands were
quantified with ImageJ (National Institutes of Health, Bethesda,
MD, USA).
F-actin staining
The F-actins in fixed cells were stained by
CytoPainter F-actin Staining kit (Abcam) according to the
manufacturer instructions. The cells were rinsed with PBS and fixed
with 4% paraformaldehyde for 30 min at room temperature followed by
permeabilizing with 0.1% Triton X-100 in PBS for 5 min. The cells
were rinsed in PBS and 1X red fluorescent phalloidin conjugate
working solution was added at room temperature for 45 min.
Statistical analysis
All experiments were repeated at least three times
before statistical analysis. All experimental data are shown as the
mean ± SEM. Differences between samples were analyzed using the
two-tailed Student's t-test. Statistical significance was accepted
at P<0.05.
Results
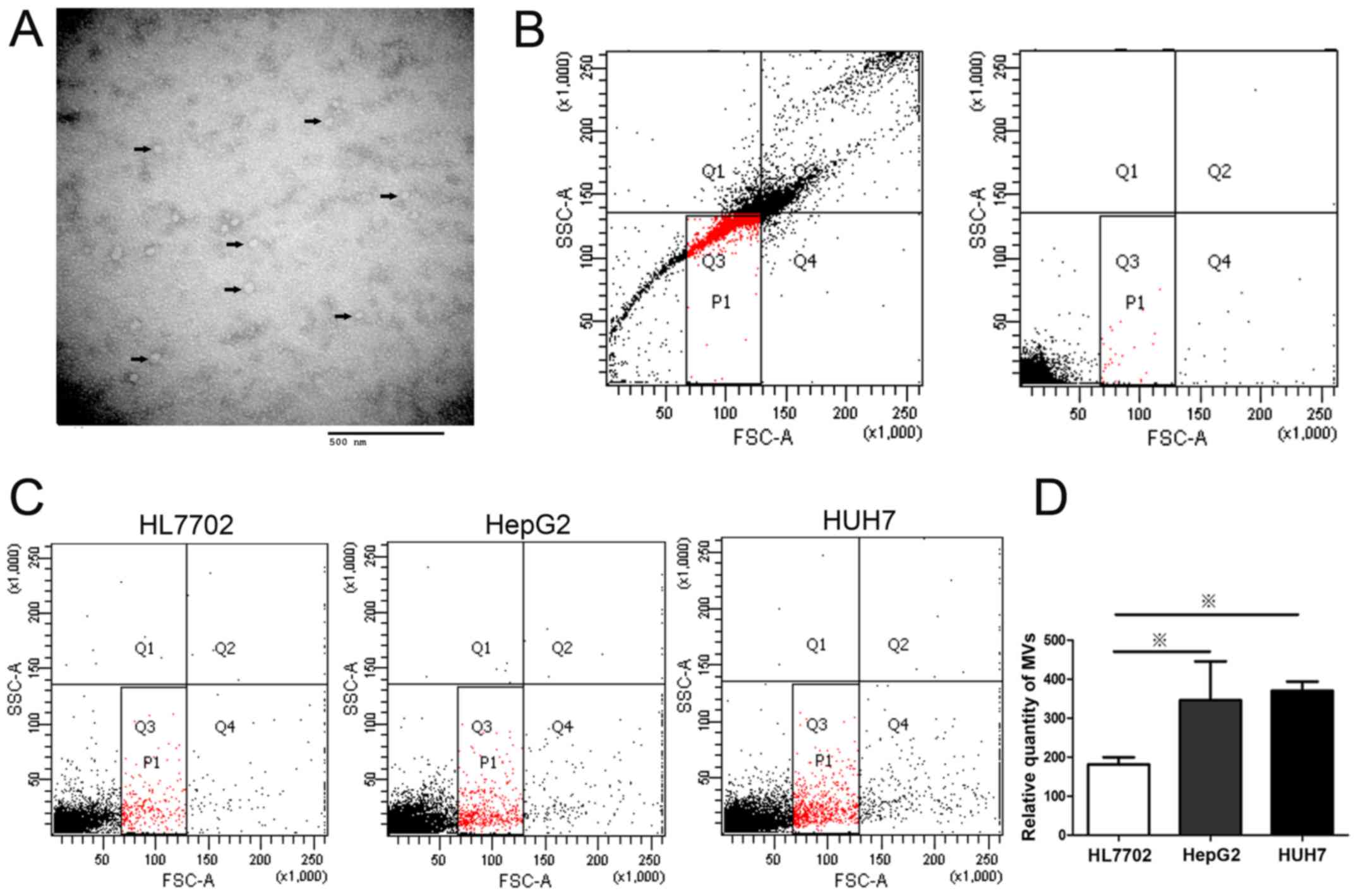
Hepatocellular carcinoma cells release
more microvesicles than normal hepatocytes
MVs are one of the key vectors of intercellular
communication and transport regulatory molecules from donor cells
to recipient cells. Our previous study suggested that hepatocytes
might secrete MVs containing miR-483 and transferred the MVs to
hepatic stellate cells (9).
However, the amount of MVs released by normal hepatocytes and
hepatocellular carcinoma cells remains unclear. The presence of
exosomes was confirmed by several criteria including i) appearance
as 50–90 nm bi-membrane vesicles assessed by electron microscopy
ii) complementary biochemical (immunoblotting), mass spectrometry,
and imaging techniques to identify exosomal proteins including
tetraspanins (CD9, CD63), Alix, TSG101 and ESCRT (12,13).
In our study, we chose CD9 for exosomal
identification because CD9 is a well-characterized marker of
exosomes and is highly expressed on the surface of exsomes. MVs
extracted from the media of HCC cells, HepG2, were visualized by
transmission electron microscopy (TEM) (Fig. 1A). The results showed that most MVs
from supernatants were membrane particles with a diameter of
approximately 50 nm. The size and ultrastructural morphology
suggested that the extracted MVs were predominantly exosomes
(21). To quantify the MVs released
from HCC cells, the number of MVs from different liver cell lines
was determined by flow cytometry after labeling with PE-conjugated
CD9 antibody (Fig. 1C). The MV
levels of HepG2 and Huh7 cells were higher than that of normal
liver cell line, HL7702 (Fig. 1D).
Considering that MVs derived from cancer cells are capable of
transferring oncogenic cargo to recipient cancer cells (3), this result suggested that high level
of cellular MV secretion might be associated with the abnormal cell
behavior growing out of control in liver cells. Further studies
were conducted to determine the mechanism of the de-regulation of
MV levels.
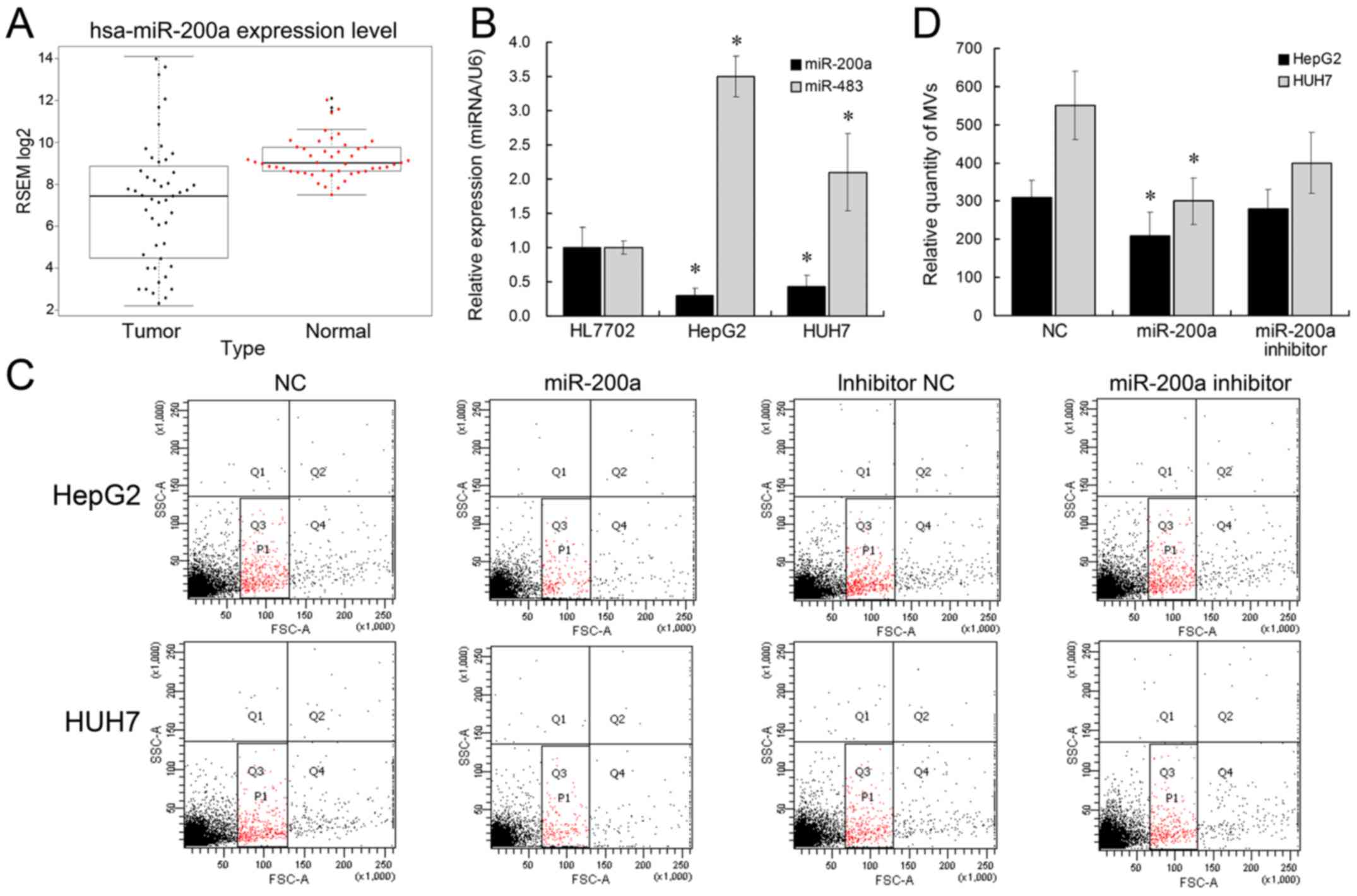
Overexpression of miR-200a suppresses
the secretion of HCC cell-derived microvesicles
We have previously showed that miR-483 might be
transported by MVs to influence adjacent cells (9). Other microRNAs with significantly
differential expression in HCC, such as miR-200a, are downregulated
in HCC (22). Herein, we aimed to
determine whether miR-483 or miR-200a could be involved in the MV
biogenesis. We initially analyzed miR-200a expression pattern in
TCGA data and found that miR-200a expression was significantly low
(Fig. 2A). We subsequently
identified the expression levels of miR-483 and miR-200a by
qRT-PCR. miR-483 expression was >3-fold higher, and miR-200a
expression was 2-fold lower in HepG2 and Huh7 cells, compared with
HL7702 (Fig. 2B), which is also in
accordance with previous studies (22,23).
Further, we tested the effect of ectopic miR-483 or miR-200a
expression on liver cell MV secretion. The results showed that
miR-200a overexpression was able to inhibit the release of MVs from
liver cancer cell lines (Fig. 2C and
D). miR-200a inhibitor treatment did not have a significant
effect on MV release, which might be caused by the low expression
level of endogenous miR-200a in HCC cells. Nevertheless, the number
of MVs derived from HL7702 showed no differences between the
miR-200 overexpression group and negative control group. This might
be due to the de-regulated expression of miR-200a in liver cancer
cells. However, miR-483 did not affect the MV release in liver
cells. Collectively, these results suggested that miR-200a
overexpression could suppress the release of MVs in liver cancer
cells.
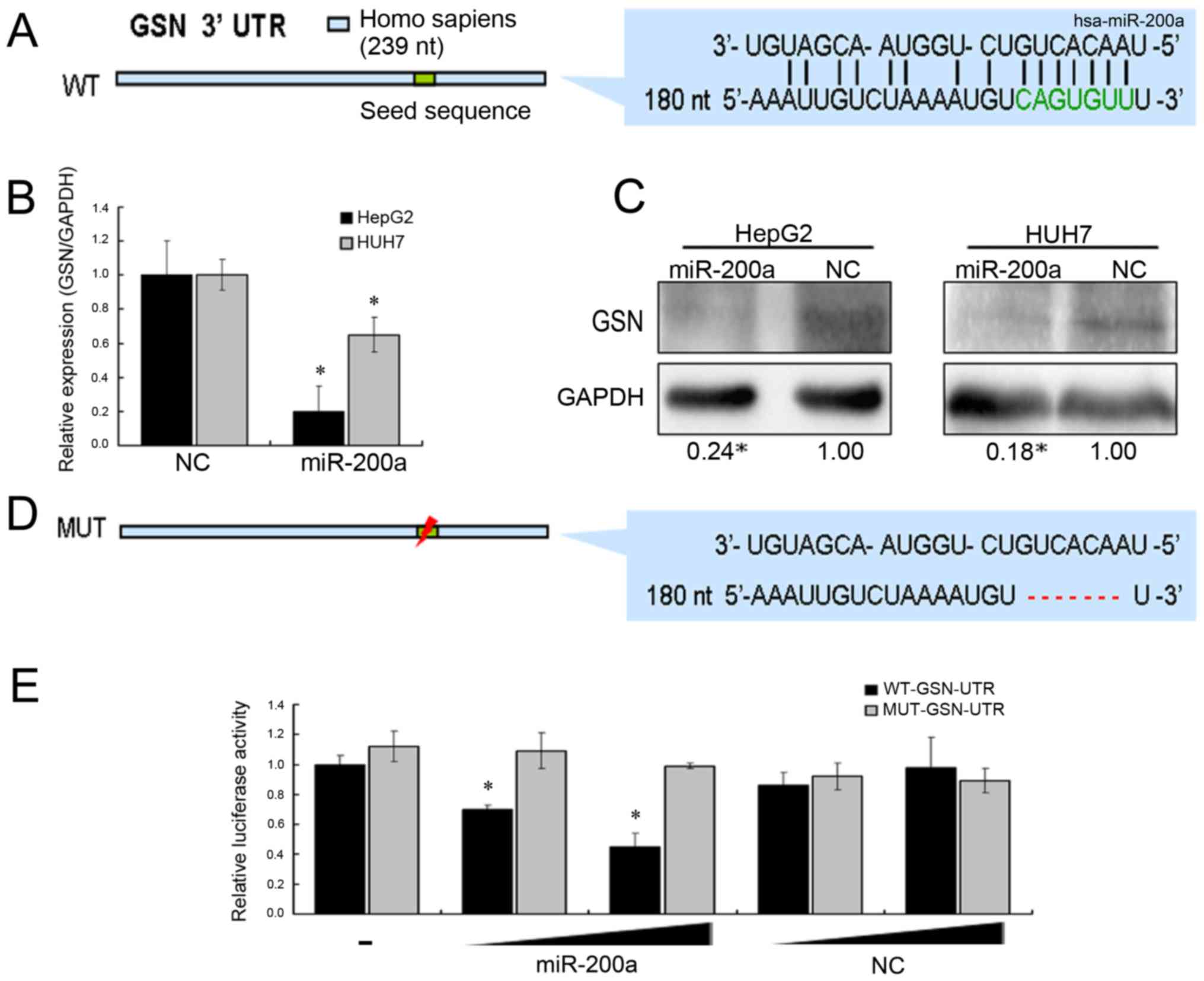
miR-200a inhibits the release of MVs
of HCC cells by functionally regulating GSN
Many studies have shown that miR-200a could regulate
the proliferation, migration, and epithelial-mesenchymal transition
(EMT) of cancer cells (16,24–26).
These reports revealed the functional targets of miR-200a, such as
ZEB1/ZEB2, DEK, SPAG9, and EPHA2. To identify the target of
miR-200a in the secretion of MVs, we used bioinformatics tools
(miRanda and PicTar) to predict the potential target of miR-200a,
which might be involved in the stability of cell membrane (Fig. 3A). Among these potential targets,
GSN has been reported to play an important role in regulating the
assembly and disassembly of actin filament (F-actin).
Actin-cytoskeletal rearrangements have been implicated in the
release of MVs (4,27,28).
Additionally, we found that GSN expression was upregulated in HCC
by analyzing TCGA data (Fig. 4A).
mRNA and protein levels of GSN were investigated after transfection
with miR-200a mimics or negative control sequences by qRT-PCR and
western blotting. The results suggested that miR-200a could inhibit
the expression of GSN gene (Fig. 3B and
C). Next, we proved that miR-200a directly targeted the 3′-UTR
of GSN using the dual luciferase reporter assay (Fig. 3D). Further, the lack of GSN induced
by miR-200a overexpression resulted in marked stabilization of the
polymerized actin networks, as shown by F-actin staining in HCC
cells (Fig. 4B). In summary, we
assumed that a miR-200a-GSN-F-actin pathway might promote the
release of liver cancer cell MVs.
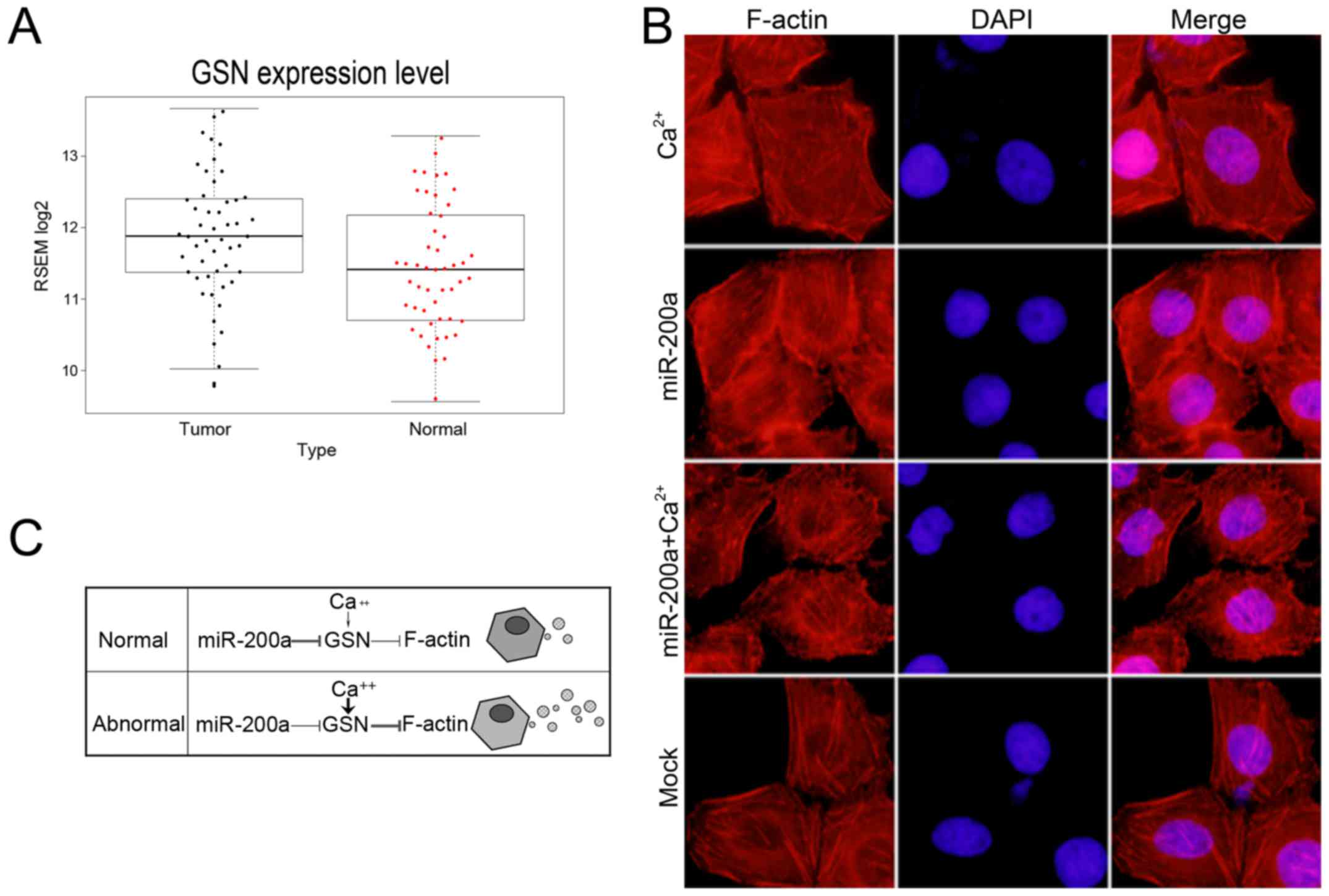
Function of GSN is dually controlled
by miR-200a and calcium
In the presence of micromolar Ca2+
concentrations, GSN is activated to bind and sever assembled actin
filaments (29). To determine
whether GSN could be regulated by both miR-200a and
Ca2+, we treated the cells with 1 mM CaCl2
for 30 min to increase the intracellular Ca2+
concentration. We observed more short actin filaments in the
presence of Ca2+ (Fig.
4B). However, overexpression of miR-200a resulted in
considerably more polymerized and elongated actin filaments. To
further confirm whether high level of cellular Ca2+
concentrations could recover the function of GSN in miR-200a
mimic-transfected cells. We added 1 mM CaCl2 for 30 min
after transfection of miR-200a mimics for 48 h and observed the
cytoskeletal change. We observed the stabilization of the
polymerized actin networks and more elongated actin filaments,
following the ectopic expression of miR-200a and CaCl2
treatment. Collectively, we assumed that in normal cells miR-200a
is the predominant regulator of GSN function and governs the
secretion of MVs, while in abnormal cells the expression of
miR-200a is inhibited and high level cellular Ca2+
activates GSN and leads to the release of more MVs (Fig. 4C). Take together, the results
suggested that the function of GSN was controlled by miR-200a and
calcium.
Overexpression of miR-200a inhibits
the proliferation of adjacent cells
It has been reported that MVs derived from cancer
cells can promote the proliferation of other cancer cells and
enable normal fibroblasts and epithelial cells to have the
transformed characteristics of cancer cells (30). To further explore the role of
hypothetic miR-200a-GSN-F-actin pathway in normal liver cells, we
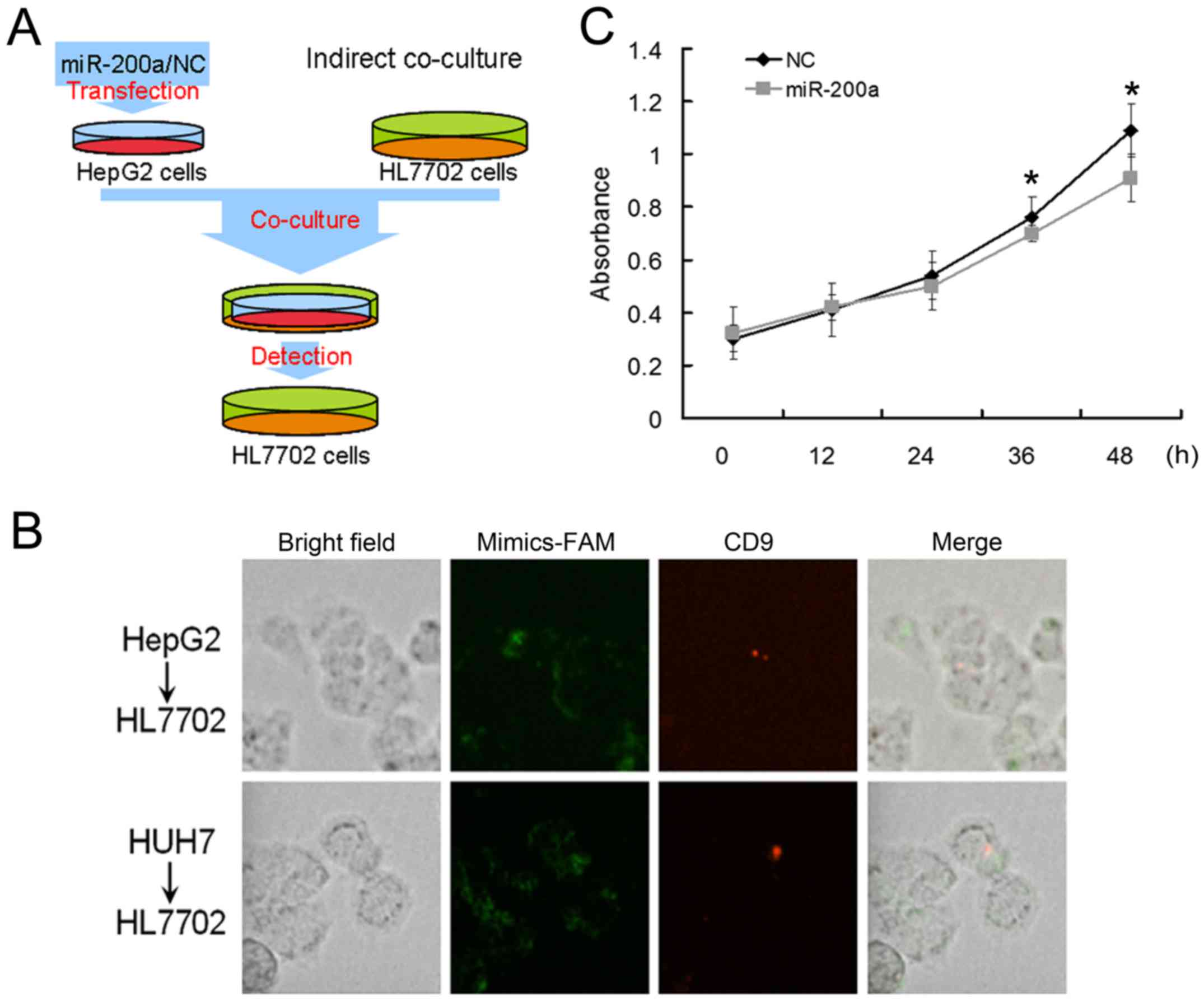
performed indirect co-culture experiments in HL7702 cells (Fig. 5A). The HepG2 or Huh7 cells
transfected with carboxyfluorescent labeled miR-200a mimics or NC
were seeded in the upper transwell inserts, and the HL7702 cells
were seeded in the lower 3.5-cm plates. After transfection, we
changed the culture medium with medium containing CD-9 in the upper
transwell inserts. We observed that CD-9 labeled MVs could be found
in the HL7702 cells, which suggested that MVs could be transported
from HepG2 or Huh7 cells to HL7702 cells in the lower 3.5-cm plates
of indirect co-culture experiments.
We also observed the existence of fluorescently
labelled mir-200a in the HL7702 cells, which suggested that
miR-200a may serve as the cargo of MVs and be transported into the
HL7702 cells (Fig. 5B). Further, we
calculated the percentages of CD9+ HL7702 cells in the
lower plates and found that approximately 9% of HL7702 cells were
CD9-positive. Later, the cell viability of HL7702 cells in the
lower 3.5-cm plates were measured by MTT assay and the
proliferation of HL7702 cells cocultured with miR-200a
mimic-transfected Huh7 cells was inhibited (Fig. 5C). We assumed that miR-200a might
inhibit the release of HCC cell MVs and effect the proliferation of
adjacent cells. In summary, MVs could be an important vector for
intercellular communication among cancer cells.
Discussion
It is widely recognized that almost all human cancer
cells and normal cells are able to generate MVs. However, the
amounts of MVs generated by the cells are different. The higher
grade and more aggressive cancer cells generate higher number of
MVs compared with the lower grade and less aggressive cancer cells
(31). However, the cellular
mechanism of the increased MV release in cancer cells is not
clearly understood.
In this study, we demonstrated that HCCs secreted
more MVs than normal hepatocytes by using flow cytometry. We
further confirmed that miR-200a was downregulated in HCC cells,
which were reported to be correlated with tumor progression,
metastasis, and poor prognosis. Furthermore, we found that miR-200a
overexpression in HepG2 and Huh7 cells could inhibit the MV
secretion possibly by targeting GSN.
Previous studies have demonstrated that peripheral
blood MV levels are elevated in HCC patients and tumor-derived
exosomes promote tumor progression and tumor cell migration
(2,32). Wei et al reported that
exosomes derived from HCC cells promoted growth, migration, and
invasion of parental cells (33).
Our results showed that liver cancer cells could release more MVs
and regulate the proliferation of adjacent cells. Considering the
fundamental role of MVs in cancer, more researchers have begun to
focus on the biogenesis of MVs. Vps4A and Annexin A2 were reported
to function in affecting migration and invasion of HCC cells by
regulating the secretion of cancer-derived MVs (33,34).
Given the importance of microRNA in HCC progression,
we assumed that microRNA might be associated with increased MVs
release in cancer cells. We found that miR-200a was able to inhibit
the release of MVs in liver cancer cells. To the best of our
knowledge, this is the first study to demonstrate that the microRNA
miR-200a, can regulate the production of liver cancer-derived MVs.
miR-200a overexpression could lead to actin remodeling by
suppressing GSN expression and cause dramatic morphological
changes, which might be a potential pathway to affect the MV
secretion. GSN was first discovered as a Ca2+-dependent
actin filament severing and capping protein (8). GSN also plays crucial roles in many
different physiological and pathological processes, such as
apoptosis, familial amyloidosis of the Finnish type, inflammation,
Alzheimer's disease, and even cancer-high expression level of GSN
was observed in a subset of non-small cell lung cancers, and a
gradual increase of GSN occurred with an increase in the tumor
grade (35). It is of great
significance to explore the mediators of GSN expression. Besides
the activity and promoter regulation of GSN (36), the regulation of GSN expression at
post-transcriptional level has not been reported. We first revealed
that 3′-UTR of GSN can be targeted by microRNA, miR-200a, and
resulted in the decreased expression of GSN and the changes to cell
cytoskeleton.
With the formation of MVs, several cargoes are
loaded into the MVs, which are released when they arrive at the
recipient cells. The function of MVs relies on the cargoes they
carry. However, in our study, we did not address whether miR-200a
could affect the loaded cargo of cancer cell-derived MVs, which is
vital to the understanding of MV function and needs to be further
investigated. In addition, we suggested that miR-200a could serve
as cargo of MVs and be transferred to recipient cells. Considering
the selective packaging of cargo into MVs, whether the packaging of
miR-200a into MVs and the release of miR-200a targeting to
recipient cells are of specificity needs further investigation.
Moreover, it would be of significance to know whether miR-200a-GSN
pathway serves as a general mechanism for MV release. Gelsolin
overexpression and silencing would provide direct link between
gelsolin and MV secretion. In future, further studies will be
conducted to determine the mechanism of MV biogenesis, selective
cargo loading and MV traffic.
In summary, our results suggested that regulating
the secretion of MVs by microRNA-target pathway is feasible for
further tumor treatment. Second, we improved the understanding of
how GSN expression is regulated. Increased cytosolic
Ca2+ could rapidly activate the activity of GSN, while
miR-200a can act as a post-transcriptional regulator for GSN.
Third, GSN serving as a novel target of miR-200a may provide an
additional mechanism explaining the role of miR-200a in EMT. These
findings may contribute to a better understanding of MV biogenesis
and the identification of new therapeutic targets for cancer.
Acknowledgements
This study was supported by Yu Weihan Academician
Fund for distinguished young Scholars of Harbin Medical University,
the Postdoctoral Fund of Heilongjiang Province (LBH-Q15108), the
National Natural Science Foundation of China (81270511, 81570534,
81611130072) and the Application Technology Research and
Development Plan Major Project of Heilongjiang (GA16C105).
References
|
1
|
Antonyak MA, Wilson KF and Cerione RA:
R(h)oads to microvesicles. Small GTPases. 3:219–224. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang W, Li H, Zhou Y and Jie S: Peripheral
blood microvesicles are potential biomarkers for hepatocellular
carcinoma. Cancer Biomark. 13:351–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antonyak MA and Cerione RA: Microvesicles
as mediators of intercellular communication in cancer. Methods Mol
Biol. 1165:147–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li B, Antonyak MA, Zhang J and Cerione RA:
RhoA triggers a specific signaling pathway that generates
transforming microvesicles in cancer cells. Oncogene. 31:4740–4749.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagner-Britz L, Wang J, Kaestner L and
Bernhardt I: Protein kinase Cα and P-type Ca channel CaV2.1 in red
blood cell calcium signalling. Cell Physiol Biochem. 31:883–891.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nguyen DB, Ly TB, Wesseling MC, Hittinger
M, Torge A, Devitt A, Perrie Y and Bernhardt I: Characterization of
microvesicles released from human red blood cells. Cell Physiol
Biochem. 38:1085–1099. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun HQ, Yamamoto M, Mejillano M and Yin
HL: Gelsolin, a multifunctional actin regulatory protein. J Biol
Chem. 274:33179–33182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin HL and Stossel TP: Control of
cytoplasmic actin gel-sol transformation by gelsolin, a
calcium-dependent regulatory protein. Nature. 281:583–586. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li F, Ma N, Zhao R, Wu G, Zhang Y, Qiao Y,
Han D, Xu Y, Xiang Y, Yan B, et al: Overexpression of miR-483-5p/3p
cooperate to inhibit mouse liver fibrosis by suppressing the TGF-β
stimulated HSCs in transgenic mice. J Cell Mol Med. 18:966–974.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Charrier A, Zhou Y, Chen R, Yu B,
Agarwal K, Tsukamoto H, Lee LJ, Paulaitis ME and Brigstock DR:
Epigenetic regulation of connective tissue growth factor by
microRNA-214 delivery in exosomes from mouse or human hepatic
stellate cells. Hepatology. 59:1118–1129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu Z, Jiang C, Wu J and Ding Y: Exosomes
as potent regulators of HCC malignancy and potential bio-tools in
clinical application. Int J Clin Exp Med. 8:17088–17095.
2015.PubMed/NCBI
|
|
12
|
Kogure T, Lin WL, Yan IK, Braconi C and
Patel T: Intercellular nanovesicle-mediated microRNA transfer: A
mechanism of environmental modulation of hepatocellular cancer cell
growth. Hepatology. 54:1237–1248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Hou L, Li A, Duan Y, Gao H and
Song X: Expression of serum exosomal microRNA-21 in human
hepatocellular carcinoma. BioMed Res Int.
2014:8648942014.PubMed/NCBI
|
|
14
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin CJ, Gong HY, Tseng HC, Wang WL and Wu
JL: miR-122 targets an anti-apoptotic gene, Bcl-w, in human
hepatocellular carcinoma cell lines. Biochem Biophys Res Commun.
375:315–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fornari F, Gramantieri L, Ferracin M,
Veronese A, Sabbioni S, Calin GA, Grazi GL, Giovannini C, Croce CM,
Bolondi L, et al: MiR-221 controls CDKN1C/p57 and CDKN1B/p27
expression in human hepatocellular carcinoma. Oncogene.
27:5651–5661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Liu D, Chen X, Li J, Li L, Bian
Z, Sun F, Lu J, Yin Y, Cai X, et al: Secreted monocytic miR-150
enhances targeted endothelial cell migration. Mol Cell. 39:133–144.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guescini M, Genedani S, Stocchi V and
Agnati LF: Astrocytes and glioblastoma cells release exosomes
carrying mtDNA. J Neural Transm Vienna. 117:1–4. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nielsen TB, Nielsen MH and Handberg A: In
vitro incubation of platelets with oxLDL does not induce
microvesicle release when measured by sensitive flow cytometry.
Front Cardiovasc Med. 2:372015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Théry C, Boussac M, Véron P,
Ricciardi-Castagnoli P, Raposo G, Garin J and Amigorena S:
Proteomic analysis of dendritic cell-derived exosomes: A secreted
subcellular compartment distinct from apoptotic vesicles. J
Immunol. 166:7309–7318. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murakami Y, Yasuda T, Saigo K, Urashima T,
Toyoda H, Okanoue T and Shimotohno K: Comprehensive analysis of
microRNA expression patterns in hepatocellular carcinoma and
non-tumorous tissues. Oncogene. 25:2537–2545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma N, Li F, Li D, Hui Y, Wang X, Qiao Y,
Zhang Y, Xiang Y, Zhou J, Zhou L, et al: Igf2-derived intronic
miR-483 promotes mouse hepatocellular carcinoma cell proliferation.
Mol Cell Biochem. 361:337–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu X, Wu G, Wu Z, Yao X and Li G: MiR-200a
suppresses the proliferation and metastasis in pancreatic ductal
adenocarcinoma through downregulation of DEK Gene. Transl Oncol.
9:25–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Jiang F, Song H, Li X, Xian J and
Gu X: MicroRNA-200a-3p suppresses tumor proliferation and induces
apoptosis by targeting SPAG9 in renal cell carcinoma. Biochem
Biophys Res Commun. 470:620–626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsouko E, Wang J, Frigo DE, Aydoğdu E and
Williams C: miR-200a inhibits migration of triple-negative breast
cancer cells through direct repression of the EPHA2 oncogene.
Carcinogenesis. 36:1051–1060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kholia S, Jorfi S, Thompson PR, Causey CP,
Nicholas AP, Inal JM and Lange S: A novel role for peptidylarginine
deiminases in microvesicle release reveals therapeutic potential of
PAD inhibition in sensitizing prostate cancer cells to
chemotherapy. J Extracell Vesicles. 4:261922015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piccin A, Murphy WG and Smith OP:
Circulating microparticles: Pathophysiology and clinical
implications. Blood Rev. 21:157–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin HL, Hartwig JH, Maruyama K and Stossel
TP: Ca2+ control of actin filament length. Effects of
macrophage gelsolin on actin polymerization. J Biol Chem.
256:9693–9697. 1981.PubMed/NCBI
|
|
30
|
Du T, Ju G, Wu S, Cheng Z, Cheng J, Zou X,
Zhang G, Miao S, Liu G and Zhu Y: Microvesicles derived from human
Wharton's jelly mesenchymal stem cells promote human renal cancer
cell growth and aggressiveness through induction of hepatocyte
growth factor. PLoS One. 9:e968362014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Nedawi K, Meehan B, Micallef J, Lhotak
V, May L, Guha A and Rak J: Intercellular transfer of the oncogenic
receptor EGFRvIII by microvesicles derived from tumour cells. Nat
Cell Biol. 10:619–624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luga V, Zhang L, Viloria-Petit AM,
Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and
Wrana JL: Exosomes mediate stromal mobilization of autocrine
Wnt-PCP signaling in breast cancer cell migration. Cell.
151:1542–1556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin
HM, Zhou R, Shang CZ, Cao J, He H, et al: Vps4A functions as a
tumor suppressor by regulating the secretion and uptake of exosomal
microRNAs in human hepatoma cells. Hepatology. 61:1284–1294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang W, Zhao P, Xu XL, Cai L, Song ZS,
Cao DY, Tao KS, Zhou WP, Chen ZN and Dou KF: Annexin A2 promotes
the migration and invasion of human hepatocellular carcinoma cells
in vitro by regulating the shedding of CD147-harboring
microvesicles from tumor cells. PLoS One. 8:e672682013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li GH, Arora PD, Chen Y, McCulloch CA and
Liu P: Multifunctional roles of gelsolin in health and diseases.
Med Res Rev. 32:999–1025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eun DW, Ahn SH, You JS, Park JW, Lee EK,
Lee HN, Kang GM, Lee JC, Choi WS, Seo DW, et al: PKCepsilon is
essential for gelsolin expression by histone deacetylase inhibitor
apicidin in human cervix cancer cells. Biochem Biophys Res Commun.
354:769–775. 2007. View Article : Google Scholar : PubMed/NCBI
|