Introduction
Breast Cancer is still the most frequent malignant
disease worldwide, and the most frequent cause of death in women
(1). One out of eight women is
diagnosed with breast cancer during her lifetime. Although
lethality has declined in the last 40 years, still 30% of the
affected patients die from the consequences of breast cancer
(2).
Approximately 10–20% of all diagnosed breast cancers
are characterized by a lack of expression or a only very weak
expression of the hormone receptors, estrogen- and
progesterone-receptor, and of the human epidermal growth factor
receptor 2 (Her2) (3). These quite
aggressive tumours, which are termed ‘triple-negative breast
cancer’ [TNBC (4)], occur
frequently in younger women, many of them show up with BRCA-1
mutations (5). Patients suffering
from TNBC may develop visceral metastases, have a high risk of
recurrence and a reduced overall survival (6), independent of tumour size, staging and
lymph node affection (7).
Furthermore, the possibilities for treatment are
sparse, as endocrine therapy with Tamoxifen or aromatase-inhibitors
as well as anti-Her2 therapy with Trastuzumab are ineffective.
Actually, TNBCs are treated postoperatively with a dose-dense or
metronome chemotherapy using anthracyclins or taxans and radiation
(8). Only in the neoadjuvant
setting TNBC shows a better follow-up as non-TNBC (9). Recent therapeutic concepts introduced
for example inhibitors of poly(ADP-ribose) polymerase (PARP) in
addition to chemotherapy, such as Iniparib or Olaparib, which are
normally responsible for the repair of single- and double-strand
DNA breaks. The aim is, to accumulate severe DNA-damage within the
tumour cells, that the cells stop entering mitosis, thereby
stopping cell division. Thus disease-free survival can be prolonged
(10). The risk of recurrence can
be reduced by the additional application of VEGF inhibitors such as
bevacizumab, which decelerates tumour growth by reduction of
neoangiogenesis (11). However, the
disadvantage of this treatment is, that bevacizumab has rather
strong side effects (12,13).
From all those facts the importance of new treatment
options can be explained. New therapeutic strategies could target
for example inter- and intracellular signal transduction pathways,
regulation of cell adhesion and proliferation. Clinical studies are
carried out using cetuximab, an inhibitor of epithelial growth
factor receptor (EGFR) (14,15)
which seems to improve survival rates without further tumour
progression (16), or the
mTOR-inhibitor everolimus (17).
Another treatment strategy could target the Notch1 pathway. Notch1
plays an important role in normal breast development and cell fate
determination. In breast cancer tissue, especially TNBC, this
pathway is activated in an aberrant manner. Additionally it has
been shown, that an inhibition of Notch signalling by
gamma-secretase inhibitors (GSIs) results in antitumour activity by
cell cycle arrest, apoptosis and disruption of angiogenesis
(18,19).
Notch-pathway members are moreover regulated by
HIF1α, which thereby constitutes another possibility for TNBC
treatment. Overexpression of the transcription factor HIF1α is
associated with a poor prognosis for the affected patients, and in
a mouse model it was shown, that it is important for promoting
carcinoma onset and formation of lung metastasis. The deletion of
HIF1α resulted in reduced primary tumour growth, suppression of
lung metastasis and prolonged survival (20). HIF1α and XBP1 in turn form a
transcriptional complex, which is known to drive tumorigenicity.
XBP1 thereby regulates the expression of HIF1α targets via
recruitment of polymerase II. Hence, XBP1 also plays a role in
tumour progression, especially in TNBC, contributing to a poor
prognosis. In a cell culture model it was shown, that an inhibition
of XBP1 results in a retardation of tumour growth, giving hints to
another therapeutic target (21).
Another transcription factor, which is highly
expressed in tumour cells is FOXP3, a potent repressor of several
oncogenes. Its involves in TNBC susceptibility and prognosis
(22), but the prognosis is
dependent on the cellular localisation of FOXP3: If it is located
in the cytoplasm, it is a marker for poor OAS, but if it remains in
the nucleus, OAS is markedly improved (23). Furthermore the Wnt/β-catenin
signalling pathway could be used for therapeutical intervention, as
this pathway regulates the cell cycle, cell growth and tumour
progression and is responsible for poor clinical outcomes.
The Wnt/β-catenin pathway was shown to be activated
in TNBC, the inhibition of Wnt-receptors by, e.g. salinomycin, is
already known as therapeutic target, as it induces LRP6 degradation
(24). LRP6 in turn, is frequently
produced in response to obesity stimuli and induces cell
proliferation by interaction with Ki67 and reduces efficiency of
treatment. LRP6 is of great use in TNBC-therapy, as it has no
relation with ER, PR, and Her2. Its use as drug target receptor
significantly prolonged survival time in a mouse model (25). Another signalling molecule within
the Wnt-signal transduction pathway, which might be of therapeutic
significance, is MCL1, which modulates mitochondrial physiology,
and is associated with enhanced metastasis formation and decreased
DFS (26).
Materials and methods
Patient samples
Tumour tissue of breast cancer patients, who
underwent breast cancer surgery between 2001 and 2002 were
collected by the Department of Obstetrics and Gynaecology of
Ludwig-Maximilians University of Munich, and subsequently embedded
in paraffin. Ethics approval compliant to the Declaration of
Helsinki for the collection of these samples was available (LMU
048-08 and 148–12). Hormone receptor and Her2 status were
determined pathologically. Thirty-one patients had a
triple-negative receptor state and were studied by
immunhistochemical analysis. At the time of surgery, patients had
an average age of 62 years. Further tumour characteristics are
listed in Table I.
 | Table I.Patient/tumour characteristics. |
Table I.
Patient/tumour characteristics.
| Characteristics | No. of patients |
|---|
| Tumour size |
|
|
pT1 | 19 |
|
pT2 | 8 |
|
pT3 | 1 |
|
pT4 | 2 |
|
pTx | 1 |
| Lymph node
affection |
|
|
pN0 | 17 |
|
pN1 | 11 |
|
pN2 | 2 |
|
pNx | 1 |
| Metastatic
stage |
|
|
pM0 | 20 |
|
pM1 | 1 |
|
pMx | 10 |
| Grading |
|
| G1 | 1 |
| G2 | 7 |
| G3 | 14 |
| Gx | 9 |
| Histology |
|
|
Ductal | 18 |
|
Lobular | 4 |
|
Medullar | 7 |
|
Other | 2 |
Immunohistochemical staining
The paraffin-embedded tissues samples were cut in
thin sections by a sliding microtome and transferred onto specially
covered microscope slides (SuperFrost Plus, Menzel GmbH,
J1800AMNZ/ground 90°). The slides were air-dried overnight at
56–58°C. For the staining procedure paraffin was removed by
incubation of the slides in xylol (Merck, 81500) for 20 min,
followed by washes in different dilutions of ethanol (100, 90,
75%). Slides were then incubated in 3% H2O2
(VWR International, ACRO42600100) to reduce activity of endogenous
peroxidase and thereby to prevent unspecific staining of tissue
samples. Following slides were again washed in ethanol and water
and boiled in Na-Citrate (Merck, 106448) Buffer (pH 6.00) for 5 min
to reconstitute the antigens. After cooling down, samples were
again washed in water and PBS (Biochrom, order no. L1835). To
prevent unspecific binding of the primary antibody, samples are
blocked in 10% normal goat serum (Vector Laboratories, S-1012) for
20 min, then the blocking solution was removed and primary
antibodies were applied in the appropriate concentrations (see
Table II).
 | Table II.Primary antibodies used for
staining. |
Table II.
Primary antibodies used for
staining.
| Antibody | Clonality | Working
dilution | Distributor | Order no. |
|---|
| Anti-HIF1α | Monoclonal
Rabbit-IgG | 1:2000 | Sigma Aldrich | HPA001275 |
| Anti-β-catenin | Polyclonal
Rabbit-IgG | 1:300 | Diagnostic
Biosystems | RP080 |
| Anti-XBP1 | Monoclonal
Rabbit-IgG | 1:400 | Sigma Aldrich | HPA044305 |
| Anti-FOXP3 | Monoclonal Mouse
IgG1 | 1:300 | Abcam | ab20034 |
| Anti-NOTCH1 | Monoclonal
Mouse-IgG | 1:100 | Sigma Aldrich | N5163 |
| Anti-MCL1 | Monoclonal
Rabbit-IgG | 1:1000 | Abcam | ab53709 |
| Anti-LRP6 | Rabbit-IgG | 1:80 | Millipore | 06-017 |
Incubation of primary antibodies was carried out at
4°C for 18 h, following slides were washed twice with PBS and
incubated with the biotinylated secondary antibody for 30 min at
room temperature. When the secondary antibody was removed, the
samples were treated with ABC-reagent (Vector Laboratories, order
no. AK-5200) for 30 min, then DAB-reagent (Dako, K-3468), diluted
in H2O2 was added to the slides for 1 min.
Enzyme reaction was stopped by washing the slides in water. Nuclei
were then counterstained by Hemalaun (Applichem, A0884) for 5 min
before slides are again dehydrated with ethanol and xylol and
embedded in Eukitt (Sigma, 03989).
To be sure of the function of the primary antibody,
and also to determine its optimal working dilution, a tissue
sample, which was confirmed to express the complimentary antigen
was stained (tissues used for the antibodies and used
concentrations are also given in Table
II). Furthermore an isotype control was carried out, staining
the same tissue as for the positive control, replacing the primary
antibody by a control serum. Thereby the unspecific background of
each antibody could be determined.
Microscopy
Staining of the samples was observed and evaluated
by two independent persons by a Leitz Diaplan light microscope
(Ernst Leitz GmbH, Wetzlar, Germany), equipped with four objectives
for different magnifications (x6.3, ×10, ×25, ×40). Evaluation was
carried out following the immunoreactive score [IRS (27)]. In brief, staining intensity is
rated in groups from 0 (no staining) to 3 (strong colour reaction),
and number of stained cells is also classified in groups from 0 (no
stained cells) to 4 (81–100% of cells stained). A multiplication of
both values results in the IRS score, ranging from 0 to 12. The IRS
is then set into reference with different tumour
characteristics.
Statistical evaluation
Statistical analysis was performed by SPSS (SPSS
Inc., Chicago, IL, USA) version 22.0. Correlations were calculated
by the non-parametric Kruskal-Wallis test. A p-value of ≤0.05 was
regarded to be statistically significant.
Results
Correlation of staining with different
tumour characteristics
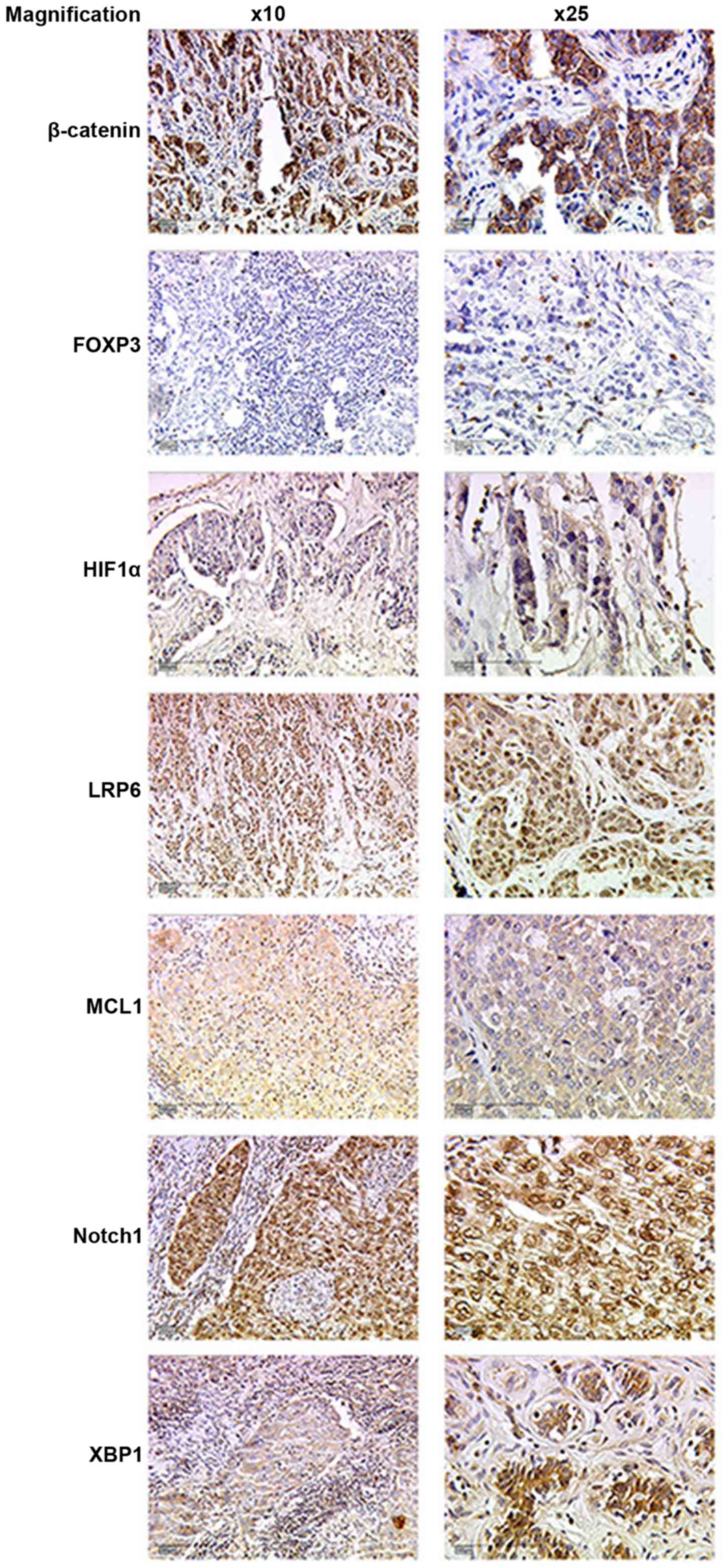
The TNBC-tissue sections were prepared and stained
immunohistochemically (Fig. 1) as
described above and an IR-Score was calculated. The IRS was then
set into correlation with different tumour characteristics using
SPSS software for calculation (Table
III). The non-parametric Kruskal-Wallis test revealed a
statistically significant correlation of cytoplasmic HIF1α-staining
and tumour grading (p=0.030). A borderline significance was seen
for the cytoplasmic staining of β-catenin and menopausal state
(p=0.068). Furthermore, an association of Notch1 staining,
cytoplasmic and nuclear, and lymph node affect on tumours were
detected (p=0.049 and p=0.063, respectively). For the other tumour
parameters such as tumour size, metastatic affection and
histological classification no significant correlations could be
found. In addition, the other signal molecules investigated in our
study, such as nuclear HIF1α, and nuclear β-catenin, XBP1, MCL1,
LRP6 and FOXP3 did not seem to have an influence on tumour
characteristics.
 | Table III.Statistical correlation of stainings
with tumour characteristics (Kruskal-Wallis test). |
Table III.
Statistical correlation of stainings
with tumour characteristics (Kruskal-Wallis test).
|
| HIF1α | β-catenin |
| Notch1 | Mcl1 |
| FoxP3 |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Tumour trait | Cybottomlasm | Nucleus | Cybottomlasm | Nucleus | XBPI | Cybottomlasm | Nucleus | LRP6 | Cybottomlasm | Nucleus | Cybottomlasm | Nucleus |
|---|
| Grading | 0.030 | 0.269 | 0.980 | 0.516 | 0.225 | 0.530 | 0.537 | 0.302 | 0.279 | 0.443 | 0.711 | 0.445 |
| Size | 0.849 | 0.331 | 0.384 | 0.701 | 0.154 | 0.112 | 0.369 | 0.672 | 0.705 | 0.355 | 0.282 | 0.369 |
| Lymph node
affection | 0.208 | 0.751 | 0.377 | 0.656 | 0.615 | 0.049 | 0.063 | 0.605 | 0.233 | 0.154 | 0.189 | 0.707 |
| Metastases | 0.704 | 0.982 | 0.189 | 0.659 | 0.921 | 0.294 | 0.706 | 0.769 | 0.938 | 0.458 | 0.667 | 0.627 |
| Histology | 0.594 | 0.317 | 0.192 | 0.426 | 0.984 | 0.350 | 0.315 | 0.426 | 0.556 | 0.315 | 0.755 | 0.392 |
| Menopausal
state | 0.750 | 0.591 | 0.068 | 0.609 | 0.767 | 0.476 | 0.298 | 0.557 | 0.151 | 0.802 | 0.663 | 0.542 |
Correlation of staining with
recurrence
In the following we investigated, if the signal
transduction molecules could be correlated to different types of
recurrence, such as local recurrence, lymph node recurrence or
metastatic recurrence (Table IV).
Two statistically strong correlations were seen for β-catenin: the
cytoplasmic staining correlated with the occurrence of remote
metastases (p=0.007), whereas the nuclear staining was associated
with a lymph node recurrence (p=0.018). The nuclear β-catenin
staining was also correlated in a borderline manner to the
appearance of remote metastasis (p=0.100). Significance of the
three more borderline values were found: XBP-1 could be linked to a
lymph node recurrence (p=0.059), nuclear Notch1-staining was
connected to remote metastasis formation (p=0.082) and nuclear
FOXP3 seemed to be related to the appearance of local recurrence
(p=0.083). No statistically significant correlations could be found
for HIF1α, cytoplasmic Notch1 and FOXP3, Mcl1 and LRP6.
 | Table IV.Statistical correlation of stainings
with tumour recurrence (Kruskal-Wallis test). |
Table IV.
Statistical correlation of stainings
with tumour recurrence (Kruskal-Wallis test).
|
| HIF1α | β-catenin |
| Notch1 | Mcl1 |
| FoxP3 |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Type of
recurrence | Cybottomlasm | Nucleus | Cybottomlasm | Nucleus | XBPI | Cybottomlasm | Nucleus | Cybottomlasm | Nucleus | LRP6 | Cybottomlasm | Nucleus |
|---|
| Local | 0.188 | 0.787 | 0.946 | 0.209 | 0.909 | 0.188 | 0.163 | 0.621 | 0.112 | 0.270 | 0.448 | 0.083 |
| Lymph node | 0.312 | 0.953 | 0.239 | 0.018 | 0.059 | 0.312 | 0.418 | 0.638 | 0.241 | 0.470 | 0.508 | 0.906 |
| Metastatic | 0.896 | 0.965 | 0.007 | 0.100 | 0.225 | 0.370 | 0.082 | 0.387 | 0.560 | 0.426 | 0.661 | 0.761 |
Discussion
The results clearly indicate that some of the
analysed signal transduction molecules have a correlation to the
incidence of patient and tumour traits, and are furthermore
correlated to different recurrences. The novelty of this study lies
in the combination of marker molecules of breast cancer tissue
samples and their correlation to other tumour and patient
characteristics, especially of the rather aggressive and hard to
treat triple-negative breast cancer subtype.
A drawback of the presented study is of course the
small number of patient samples analysed, so that the results have
to be considered as preliminary. However, the data already show up
a certain trend, which should be clarified by the analysis of a
larger patient collective. A higher number of samples analysed
would also improve the statistical significance.
Nevertheless, some of these findings are in
accordance to recently published data, either in breast cancer or
in other tumour entities. The correlation of HIF1α to the
histological grading was already described for ovarian cancer
(28), where this factor might
become important for prognostic evaluation and clinical treatment.
Moreover, the linkage between β-catenin and menopausal stage might
be of clinical relevance, as Wang et al found, that the
Wnt-pathway influenced the pathogenesis of postmenopausal
osteoporosis (29). The
Wnt/β-catenin pathway also contributes to radioresistence of TNBC
cells. Niclosamide, a potent inhibitor of the Wnt/β-catenin pathway
might be able to abolish the radioresistence of these cells,
improving the TNBC-therapy (30).
Wnt/β-catenin and Notch1 are normally responsible
for mammary gland morphogenesis during embryonal development and
are frequently found to be upregulated during tumorigenesis,
creating another treatment option (31). The role of Notch1 in lymph node
affected infiltrating ductal carcinomas was already described in
the literature, claiming the involvement of Notch1 in
epithelial-mesenchymal transition (32) which is a rather important process in
metastasis formation. Furthermore patients with high expression of
Notch1 had a worse OAS and DFS (33). Recently, the microRNA, miR9, was
identified to regulate Notch1 in a manner to suppress its
tumorigenic capacity (34).
Furthermore, the ATPase, a2V-ATPase, was identified, which is
necessary for the processing of Notch1-receptor. A deficiency of
this ATPase was shown to disrupt Notch signalling and mammary gland
development, which might also represent a new way in breast cancer
treatment (35).
However, we did not find correlations of tumour
characteristics with XBP1, MCL1, LRP6 and FOXP3, and no relation
could be shown between tumour size, remote metastasis formation and
tumour histology and one of the investigated signalling
molecules.
Additionally we investigated the coherence between
the different signalling molecules and the formation of recurrence
such as lymph node recurrence, local recurrence or remote
metastasis formation. We found a correlation between the expression
of β-catenin and the incidence of remote metastasis. It was
published earlier, that β-catenin plays a role in metastasis
formation by interactions with ECM (extracellular matrix)1 protein,
what increased the progress on EMT and CSC (cancer stem cell)
phenotype maintenance in the cancer cells (36). Furthermore, β-catenin is known to
support the action of matrix-metalloproteinases (MMPs) and uPA
(urokinase plasminogen activator), its inhibition thereby inhibits
EMT (37). The inhibition of
β-catenin seems to correlate with the inhibition of metastasis
formation (38,39). However, β-catenin also plays a role
in lymph node recurrence formation (40), as we also demonstrated with our
experiments. An association of Notch1 and remote metastasis
formation, which we found in our analysis, was also already
described (41) and seems to work
via angiogenesis.
Rather new are the coherence of XBP1 and lymph node
recurrences and FOXP3 and local recurrences, showing ultimately,
that all these signal transduction pathways are rather important in
tumorigenicity and recurrence formation.
As a conclusion of the experiments, the correlation
of staining of different signal transduction molecules to tumour
traits, indicated that signal transduction pathways influence
tumour progression and recurrence or metastasis formation. As the
number of samples used in the study is rather small, the results
have to be considered as preliminary and further research has to be
done to verify these data. Carrying out such experiments could help
to refine prognosis and find ways to inhibit tumour progression and
metastasis formation and thereby might help to find new therapeutic
strategies.
Glossary
Abbreviations
Abbreviations:
|
CSCs
|
cancer stem cells
|
|
DFS
|
disease-free survival
|
|
ECM
|
extracellular matrix
|
|
EGFR
|
endothelial growth factor receptor
|
|
EMT
|
epithelial to mesenchymal
transition
|
|
ER
|
estrogen receptor
|
|
FOXP3
|
forkhead box protein 3
|
|
Her2
|
human epidermal growth factor receptor
2
|
|
HIF1α
|
hypoxia inducible factor 1α
|
|
IRS
|
immunreactive score
|
|
LRP6
|
low-density lipoprotein
receptor-related protein 6
|
|
MCL1
|
myeloid leukemia cell differentiation
protein 1
|
|
MMP13
|
matrix metalloprotease 13
|
|
OAS
|
overall survival
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
PR
|
progesterone receptor
|
|
TNBC
|
triple-negative breast cancer
|
|
VEGF
|
vascular endothelial growth factor
|
|
XBP1
|
X-box binding protein 1
|
References
|
1
|
Robert Koch Institut: Krebs in Deutschland
2011/2012. 10th. Berlin: 2015, (In German). http://www.gekid.de/Doc/krebs_in_deutschland_2015.pdf
|
|
2
|
National Cancer Institute. simplewww.cancer.govNIHAccessed. Nov;2015.
|
|
3
|
Badve S, Dabbs DJ, Schnitt SJ, Baehner FL,
Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR,
et al: Basal-like and triple-negative breast cancers: A critical
review with an emphasis on the implications for pathologists and
oncologists. Mod Pathol. 24:157–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brenton JD, Carey LA, Ahmed AA and Caldas
C: Molecular classification and molecular forecasting of breast
cancer: Ready for clinical application? J Clin Oncol. 23:7350–7360.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dawood S: Triple-negative breast cancer:
Epidemiology and management options. Drugs. 70:2247–2258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hugh J, Hanson J, Cheang MC, Nielsen TO,
Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M, et
al: Breast cancer subtypes and response to docetaxel in
node-positive breast cancer: Use of an immunohistochemical
definition in the BCIRG 001 trial. J Clin Oncol. 27:1168–1176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehta RS: Dose-dense and/or metronomic
schedules of specific chemotherapies consolidate the
chemosensitivity of triple-negative breast cancer: A step toward
reversing triple-negative paradox. J Clin Oncol. 26:3286–3288.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Shaughnessy J, Osborne C, Pippen JE,
Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM and Bradley C:
Iniparib plus chemotherapy in metastatic triple-negative breast
cancer. N Engl J Med. 364:205–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Linderholm BK, Hellborg H, Johansson U,
Elmberger G, Skoog L, Lehtiö J and Lewensohn R: Significantly
higher levels of vascular endothelial growth factor (VEGF) and
shorter survival times for patients with primary operable
triple-negative breast cancer. Ann Oncol. 20:1639–1646. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schutz FA, Jardim DL, Je Y and Choueiri
TK: Haematologic toxicities associated with the addition of
bevacizumab in cancer patients. Eur J Cancer. 47:1161–1174. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
von Minckwitz G, Eidtmann H, Rezai M,
Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C,
Kreienberg R, et al: German Breast Group; Arbeitsgemeinschaft
Gynäkologische Onkologie-Breast Study Groups: Neoadjuvant
chemotherapy and bevacizumab for HER2-negative breast cancer. N
Engl J Med. 366:299–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grob TJ, Heilenkötter U, Geist S,
Paluchowski P, Wilke C, Jaenicke F, Quaas A, Wilczak W, Choschzick
M, Sauter G, et al: Rare oncogenic mutations of predictive markers
for targeted therapy in triple-negative breast cancer. Breast
Cancer Res Treat. 134:561–567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Viale G, Rotmensz N, Maisonneuve P,
Bottiglieri L, Montagna E, Luini A, Veronesi P, Intra M, Torrisi R,
Cardillo A, et al: Invasive ductal carcinoma of the breast with the
‘triple-negative’ phenotype: Prognostic implications of EGFR
immunoreactivity. Breast Cancer Res Treat. 116:317–328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baselga J, Stemmer S, Pego A, Chan A,
Goeminne JC, Graas MP, Kennedy J, Gil Ciruelos EM, Zubel A, et al:
Abstract PD01-01: Cetuximab + cisplatin in estrogen
receptor-negative, progesterone receptor-negative, Her2-negative
(triple-negative) metastatic breast cancer: Results of the
randomized phase II BALI-trial. Cancer Res (Thirty-Third Annual
CTRC-AACR San Antonio Breast Cancer Symposium). 70:PD01–01.
2010.
|
|
17
|
Yunokawa M, Koizumi F, Kitamura Y,
Katanasaka Y, Okamoto N, Kodaira M, Yonemori K, Shimizu C, Ando M,
Masutomi K, et al: Efficacy of everolimus, a novel mTOR inhibitor,
against basal-like triple-negative breast cancer cells. Cancer Sci.
103:1665–1671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu M, Peng Q, Jiang I, Carroll C, Han G,
Rymer I, Lippincott J, Zachwieja J, Gajiwala K, Kraynov E, et al:
Specific inhibition of Notch1 signaling enhances the antitumor
efficacy of chemotherapy in triple negative breast cancer through
reduction of cancer stem cells. Cancer Lett. 328:261–270. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu H, Bhaijee F, Ishaq N, Pepper DJ,
Backus K, Brown AS, Zhou X and Miele L: Correlation of Notch1, pAKT
and nuclear NF-κB expression in triple negative breast cancer. Am J
Cancer Res. 3:230–239. 2013.PubMed/NCBI
|
|
20
|
Schwab LP, Peacock DL, Majumdar D, Ingels
JF, Jensen LC, Smith KD, Cushing RC and Seagroves TN:
Hypoxia-inducible factor 1α promotes primary tumor growth and
tumor-initiating cell activity in breast cancer. Breast Cancer Res.
14:R62012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Iliopoulos D, Zhang Q, Tang Q,
Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, et
al: XBP1 promotes triple-negative breast cancer by controlling the
HIF1α pathway. Nature. 508:103–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lopes LF, Guembarovski RL, Guembarovski
AL, Kishima MO, Campos CZ, Oda JM, Ariza CB, de Oliveira KB,
Borelli SD and Watanabe MA: FOXP3 transcription factor: A candidate
marker for susceptibility and prognosis in triple negative breast
cancer. Biomed Res Int. 2014:3416542014.PubMed/NCBI
|
|
23
|
Takenaka M, Seki N, Toh U, Hattori S,
Kawahara A, Yamaguchi T, Koura K, Takahashi R, Otsuka H, Takahashi
H, et al: FOXP3 expression in tumor cells and tumor-infiltrating
lymphocytes is associated with breast cancer prognosis. Mol Clin
Oncol. 1:625–632. 2013.PubMed/NCBI
|
|
24
|
Kim W, Kim SY, Kim T, Kim M, Bae DJ, Choi
HI, Kim IS and Jho E: ADP-ribosylation factors 1 and 6 regulate
Wnt/β-catenin signaling via control of LRP6 phosphorylation.
Oncogene. 32:3390–3396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mahamodhossen YA, Liu W and Rong-Rong Z:
Triple-negative breast cancer: New perspectives for novel
therapies. Med Oncol. 30:6532013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang L, Perez AA, Fujie S, Warden C, Li J,
Wang Y, Yung B, Chen YR, Liu X, Zhang H, et al: Wnt modulates MCL1
to control cell survival in triple negative breast cancer. BMC
Cancer. 14:1242014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.PubMed/NCBI
|
|
28
|
Jin Y, Wang H, Liang X, Ma J and Wang Y:
Pathological and prognostic significance of hypoxia-inducible
factor 1α expression in epithelial ovarian cancer: A meta-analysis.
Tumour Biol. 35:8149–8159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Liu Y, Ma JX, Li BX and Li YK:
Effect of the Wnt/LRP5/β-catenin signaling pathway on the
pathogenesis of postmenopausal osteoporosis. Zhonghua Fu Chan Ke Za
Zhi. 46:769–772. 2011.(In Chinese). PubMed/NCBI
|
|
30
|
Yin L, Gao Y, Zhang X, Wang J, Ding D,
Zhang Y, Zhang J and Chen H: Niclosamide sensitizes triple-negative
breast cancer cells to ionizing radiation in association with the
inhibition of Wnt/β-catenin signaling. Oncotarget. 7:42126–42138.
2016.PubMed/NCBI
|
|
31
|
Rangel MC, Bertolette D, Castro NP,
Klauzinska M, Cuttitta F and Salomon DS: Developmental signaling
pathways regulating mammary stem cells and contributing to the
etiology of triple-negative breast cancer. Breast Cancer Res Treat.
156:211–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cao YW, Wan GX, Sun JP, Cui XB, Hu JM,
Liang WH, Zheng YQ, Li WQ and Li F: Implications of the
Notch1-Snail/Slug-epithelial to mesenchymal transition axis for
lymph node metastasis in infiltrating ductal carcinoma. Kaohsiung J
Med Sci. 31:70–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao YW, Li WQ, Wan GX, Li YX, Du XM, Li YC
and Li F: Correlation and prognostic value of SIRT1 and Notch1
signaling in breast cancer. J Exp Clin Cancer Res. 33:972014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mohammadi-Yeganeh S, Mansouri A and Paryan
M: Targeting of miR9/NOTCH1 interaction reduces metastatic behavior
in triple-negative breast cancer. Chem Biol Drug Des. 86:1185–1191.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pamarthy S, Mao L, Katara GK, Fleetwood S,
Kulshreshta A, Gilman-Sachs A and Beaman KD: The V-ATPase a2
isoform controls mammary gland development through Notch and TGF-β
signaling. Cell Death Dis. 7:e24432016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee KM, Nam K, Oh S, Lim J, Kim RK, Shim
D, Choi JH, Lee SJ, Yu JH, Lee JW, et al: ECM1 regulates tumor
metastasis and CSC-like property through stabilization of
β-catenin. Oncogene. 34:6055–6065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar KJ, Vani MG, Chueh PJ, Mau JL and
Wang SY: Antrodin C inhibits epithelial-to-mesenchymal transition
and metastasis of breast cancer cells via suppression of Smad2/3
and β-catenin signaling pathways. PLoS One. 10:e01171112015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Liang W, Liu J, Lin C, Wu S, Song L
and Yuan Z: Transducin (β)-like 1 X-linked receptor 1 promotes
proliferation and tumorigenicity in human breast cancer via
activation of beta-catenin signaling. Breast Cancer Res.
16:4652014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Bu F, Royer C, Serres S, Larkin
JR, Soto MS, Sibson NR, Salter V, Fritzsche F, Turnquist C, et al:
ASPP2 controls epithelial plasticity and inhibits metastasis
through β-catenin-dependent regulation of ZEB1. Nat Cell Biol.
16:1092–1104. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sehgal P, Kumar N, Kumar Praveen VR, Patil
S, Bhattacharya A, Vijaya Kumar M, Mukherjee G and Kondaiah P:
Regulation of protumorigenic pathways by insulin like growth factor
binding protein2 and its association along with β-catenin in breast
cancer lymph node metastasis. Mol Cancer. 12:632013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dong Y, Zhang T, Li J, Deng H, Song Y,
Zhai D, Peng Y, Lu X, Liu M, Zhao Y, et al: Oridonin inhibits tumor
growth and metastasis through anti-angiogenesis by blocking the
Notch signaling. PLoS One. 9:e1138302014. View Article : Google Scholar : PubMed/NCBI
|