Introduction
As the most frequent tumors affecting humans, glioma
originates in the cerebral glia of brain tissue. Glioblastoma (GBM)
is the most common malignant form of glioma with a high mortality.
The survival rate at present for GBM patients is only one year
(1), and the dilemma has not been
greatly overcome over the last decade. Specifically, the available
treatments including targeted drugs, chemotherapy, radiotherapy and
surgical resection, have displayed slight improvement on survival
of GBM patients (2). No distinct
borderline exists between brain tissue and glioma due to the nature
of aggressive invasion (3).
Uncovering the molecular mechanism of malignant glioma invasion
will be beneficial for the clinical diagnosis and therapy of
malignant gliomas. This will not only prolong the progression-free
survival but also suggest new approaches for the innovation of
potent anti-glioma drugs.
As a crucial step in the initiation of metastatic
status, epithelial-mesenchymal transition (EMT) has drawn worldwide
attention for therapeutic interventions targeting metastasis.
During the process of EMT, cells gradually replace their epithelial
phenotypes with a mesenchymal characteristic, which is accompanied
with augmented migration and invasion. Decrement in epithelial
junction protein (E-cadherin) as well as an increment in
mesenchymal markers (N-cadherin and vimentin) is commonplace in
tumors with metastatic potential (4). An emerging hypothesis is that EMT
endows cancer cells to invade, migrate and subsequently disseminate
to form distant metastases.
It is well known that the complex interactions
between the microenvironment and tumor are critical to orchestrate
the destiny of carcinoma development (5). A tumor microenvironment is composed of
an intricate crosstalk of various cell types and cytokines and
growth factors, which have been proposed to modify the EMT
procession. Among them, the polypeptide cytokine transforming
growth factor beta (TGF-β) is widely involved in cell biological
events such as migration, invasion, differentiation and
proliferation (6,7). TGF-β1 has been demonstrated to
facilitate the expression of extracellular matrix proteins, thus
inducing EMT. Particularly in glioma, high level and secretion of
TGF-β1 has been observed, indicating the important role of TGF-β1
in glioma invasion. Moreover, TGF-β1 is closely associated with the
regulation of epithelial or mesenchymal markers, such as
fibronectin and E-cadherin (8).
Both phosphatidylinositol 3-kinase (PI3K)/AKT and
Smad pathways play important roles in translational regulating
effect of TGF-β during EMT. p-AKT also functions in
differentiation, apoptosis, and proliferation of glioma cells.
Glycogen synthase kinase-3β (GSK-3β), a ubiquitously expressed
serine/threonine kinase, is a downstream target of p-AKT and can
inactivate various substrates, including HIF-1, cyclin D1, glycogen
synthase and β-catenin (9). GSK-3β
is active in resting epithelial cells (10). β-catenin is a crucial structure
adaptor. PI3K/AKT signaling inhibited the GSK3β-mediated
degradation of β-catenin. This fosters the translocation of
cytoplasmic β-catenin into the nucleus where it can interact with
the lymphoid enhancer factor and T-cell factor (LEF/TCF)
transcriptional complexes (11,12).
This interaction will further modulate the expression of various
downstream target genes including Slug and E-cadherin.
Bioactive components emerging from traditional
medicine have exhibited potential anticancer efficacy in recent
years. Also known as heterogeneous polyphenols, flavonoids are rich
in fruits, vegetables, tea and wine. Nobiletin
(3′,4′,5,6,7,8-hexamethoxyflavone) is a polymethoxy flavonoid found
in citrus fruits, which have been widely used in traditional
Chinese medicine with potent anti-inflammatory, antidepressant,
antioxidant, anti-hypertension and antibacterial effects. Previous
studies have demonstrated the growth inhibitory effects of
nobiletin in various cancer cell lines and tumor models such as
glioma cancer, lung cancer, and breast cancer. Anticancer
activities of nobiletin involve repression of proliferation,
migration, invasion and angiogenesis.
Recently, it was reported that nobiletin regulates
the cell cycle via MAPK and AKT pathways, accompanied with the
suppression of glioma cell proliferation (13). Nobiletin inhibits tumor growth and
angiogenesis of ovarian cancers via the AKT pathway (14). Moreover, metastasis was also
attenuated via both ERK and PI3K/AKT pathways in HGF-treated liver
cancer HepG2 cells (15). In summary, these data proved the
essential role of AKT in the anticancer effect of nobiletin, as an
encouraging bioactive compound with distinguishing anticancer
effects. However, the downstream effector and the molecular
mechanism need to be explored. Given that EMT plays a fundamental
role in metastasis of glioma tumors, we therefore examined the
potential of nobiletin to prevent EMT, migration, and invasion. We
demonstrated that nobiletin inhibited EMT of U87 and U251 cells, in
which expression of Slug can be observed, while exerting weak
effects on U343 cells which expresses rather low level of Slug.
Nobiletin inhibited TGF-β-induced EMT in vivo and in
vitro, with AKT/GSK3β/β-catenin signaling pathway greatly
involved. Collectively, our data indicated that nobiletin may have
potential in glioma cancer treatment.
Materials and methods
Reagents
Nobiletin, LY294002, TGF-β1 was from Merck Millipore
(Darmstadt, Germany). AZD1080, SKL2001 and diamidino-phenyl-indole
(DAPI) were purchased from Funakoshi (Tokyo, Japan). Slug plasmid
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) was transfected
into U343 cells according to the manufacturer's protocol.
Antibodies against p38, p-p38, ERK, p-ERK, p-AKT, AKT, β-catenin,
GSK-3β, p-GSK-3β, p-Smad3 were from Cell Signaling Technology
(Danvers, MA, USA). Antibodies against E-cadherin, N-cadherin,
occluding, fibronectin, Slug, Twist1, and Snail were obtained from
Santa Cruz Biotechnology.
Cell culture and viability assay
U87, U251 and U43 cells from the American Type
Culture Collection (ATCC, Manassas, VA, USA) were maintained at
37°C, 5% CO2, in DMEM supplemented with 10% fetal bovine
serum, penicillin (500 U/ml) and streptomycin (100 µg/ml). Cell
proliferation was determined using the CCK-8 (Beyotime Institute of
Biotechnology, Shanghai, China) method. U87 and U251 cells
(3×103 cells/well) were plated in 96-well plates. At 24
h incubation with nobiletin, CCK-8 reagents were added and
incubated for another two hours. The absorbance at 450 nm
represents the number of viable cells with a Multiskan Spectrum
microplate reader.
Migration and invasion
In the migration assays, scratches were made in the
cells and presented by the average of distance differences between
24 and 0 h. To assess invasion potential, Matrigel-coated Transwell
was used. Nobiletin-treated cells were seeded in the upper chamber
of the Transwell insert. The migration capacity over the Matrigel
and the membrane towards the bottom chamber containing 10% FBS was
examined. Cells were fixed in formaldehyde solution, and stained
with crystal violet and counted.
Immunofluorescence staining
Cells were grown on glass coverslips and blocked
with 5% normal fetal bovine serum containing 0.1% Triton X-100 for
2 h at room temperature, thereafter, coverslips were incubated with
anti-E-cadherin antibodies overnight with DAPI (Molecular Probes).
Fluorescence was visualized with a microscope and Image-Pro Plus
4.0 software.
Signaling experiments
Signaling experiments were performed as described
previously with slight modifications (16). Nuclear and cytosolic fractions were
carried out according to manufacturer's instructions (Pierce
Biotechnology, Rockford, IL, USA). In western blot assays, protein
samples were separated on SDS polyacrylamide gel and then blotted
onto nitrocellulose membranes using a Semi-dry electrotransfer mini
Trans-Blot Cell (Bio-Rad, Hercules, CA, USA) and transfer buffer.
The membranes were incubated overnight at 4°C with primary
antibodies and then incubated with anti-IgG Antibody. Protein
G-agarose beads (Invitrogen) were used for immunoprecipitations.
Pierce Clean Blot IP Detection reagent (Thermo Fisher Scientific,
Rockford, IL, USA) were applied for immunoprecipitation
experiments. ECL substrates were used for protein band
visualization (Bio-Rad). The bands densitometry was analyzed with
ImageJ software.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was performed as previously described
with slight modification (17).
β-catenin antibody was used, and qPCR was performed with specific
primers to the slug promoter. PCR amplifications were conducted
using Blend Taq DNA polymerase and the PCR products were analyzed
by electrophoresis on agarose gel and then qPCR. Cross-link
released chromatin was saved and reversed by proteinase K digestion
and phenol/chloroform extraction. Immunoprecipitated samples were
compared to the housekeeping gene RPL30.
Antitumor efficiency
Athymic nude mice (4 weeks old) were maintained
under pathogen-free conditions. U87-Luc cells were harvested and
resuspended. Suspended cells (1×107) were subcutaneously
injected into the flank of each nude mouse. U87-Luc-bearing nude
mice were randomly divided into four groups as follows: Vehicle
group (saline daily); Cyclophosphamide positive group (20 mg/kg,
i.p. every 3 days); nobiletin group (15 and 30 mg/kg, oral
administration daily). The tumor growth was examined with a caliper
every 3 days and calculated by the following equation: V =
length×width2/2. Mice were observed by the IVIS in
vivo imaging system after 24 day-treatment.
Immunohistochemical analysis
(IHC)
The primary tumors were immunostained for
E-cadherin, N-cadherin and Slug as previously described (18).
Statistical analysis
Statistical analysis was conducted with GraphPad
Prism software (GraphPad Software Inc., San Diego, CA, USA).
One-way ANOVA was used to compare between multiple groups. All
results are expressed as mean ± SD. p<0.05 and p<0.01 were
considered to indicate statistically significant differences.
Results
Nobiletin inhibits viability,
migration, invasion and EMT of glioma cells
In the CCK-8 assays, nobiletin at 5, 10 and 15 µM
exhibited no significant cytotoxic effect to U87 and U251 cells
(Fig. 1A). Based on our previous
data, these concentrations intervene with neither metabolism nor
function of mitochondria, so they were then used in the following
experiments. Wound healing models were used to assess the effect of
nobiletin on the migratory of glioma cells. It can be seen from
Fig. 1B that the migratory
potential was remarkably inhibited by nobiletin. Twenty-four hours
after the scratch, cells in the vehicle group almost filled the
scratched area, whereas an obvious gap was observed in the wound in
nobiletin-treated cells. The quantification by scratch width showed
that the inhibition rate of nobiletin (10 and 15 µM) was
approximately 25 and 45% in U251 cells and 33 and 50% in U87 cells,
respectively. Next, the regulatory effect of nobiletin on the
invasion of U87 and U251 cells were validated via matrigel
transmembrane assay. The results showed that cells in the control
group invaded through the matrigel faster than nobiletin-treated
cells, and the inhibitory rate of nobiletin (15 µM) reached
approximately 65 and 60% in U87 and U251 cells, respectively
(Fig. 1C).
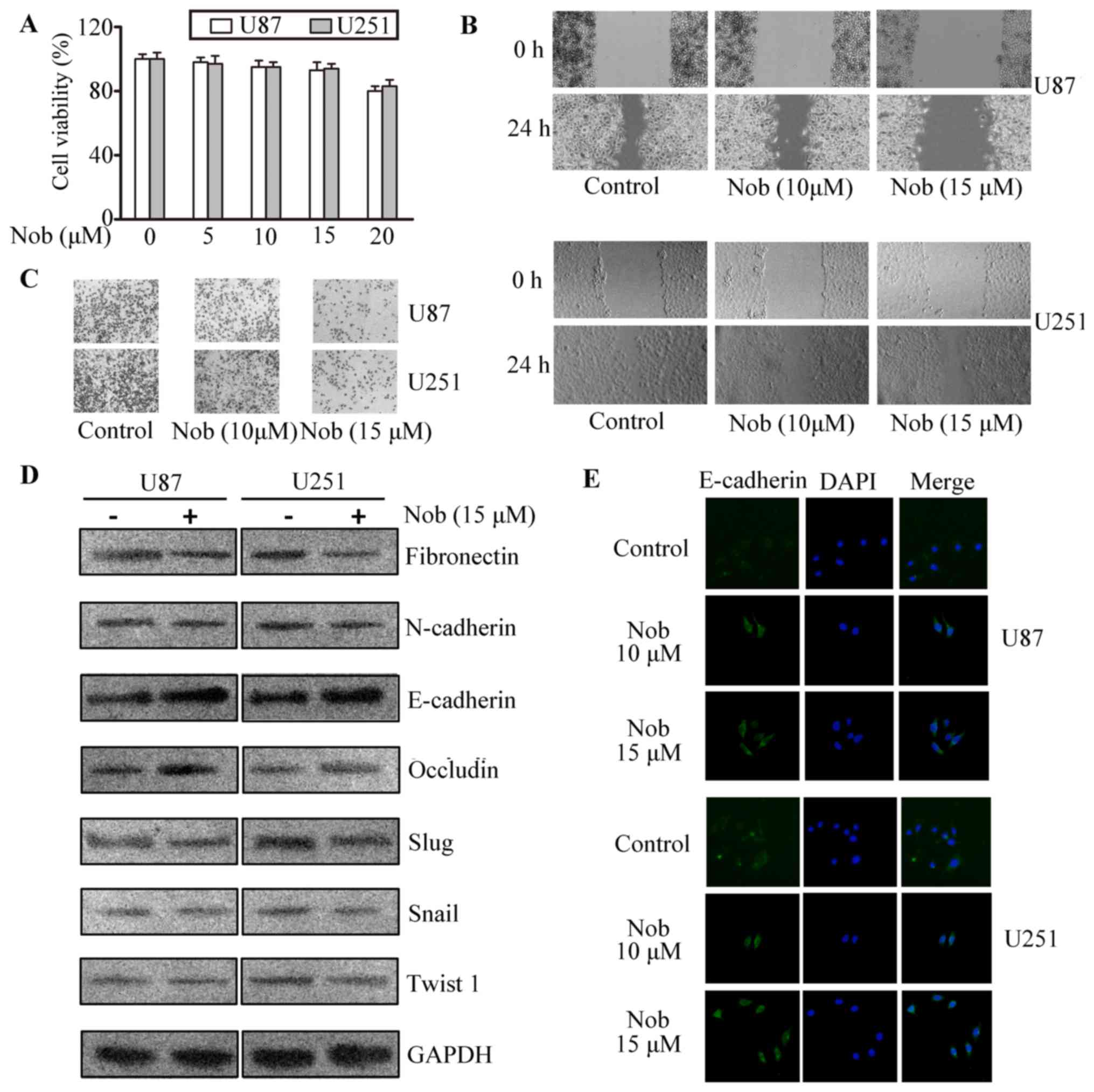 | Figure 1.Nobiletin repressed invasion,
migration and EMT in glioma cancer cells. (A) CCK-8 assays of cell
viability after 48 h of incubation with nobiletin at indicative
concentrations. p<0.05; p<0.01, versus control. (B) Migration
of U87 and U251 cells was assessed with scratch-wound healing
experiments. (C) The invasion of U87 and U251 cells were quantified
by calculating the crystal violet-stained cells invading to the
lower surface of the membrane. (D) U87 and U251 cells were treated
with nobiletin (15 µM) for 24 h. Cell lysates were subjected to
western blot analysis for E-cadherin, occludin, N-cadherin,
fibronectin, Slug, Snail, Twist1 and GAPDH. (E) U87 and U251 cells
treated with nobiletin (15 µM) for 24 h. Then the cells were
incubated with anti-E-cadherin antibodies, respectively, and
assessed by immunofluorescence. The nucleus was stained with DAPI
(blue). |
E-cadherin, occluding, N-cadherin, and fibronectin
play an essential role during the progress of cancer metastasis. In
western blotting assays, the expression of EMT-related proteins in
glioma cells were examined. In Fig.
1D-E, the epithelial markers occludin and E-cadherin were
increased while mesenchymal markers such as fibronectin and
N-cadherin were reduced in both cell lines. As is known, Snail,
Slug and Twist1, are potent repressors of E-cadherin. Herein,
transcriptional factors involved in EMT were further tested to
prove the efficacy of nobiletin. We found nobiletin considerably
inhibited Slug expression while displaying negligible effect on the
levels of Snail and Twist1.
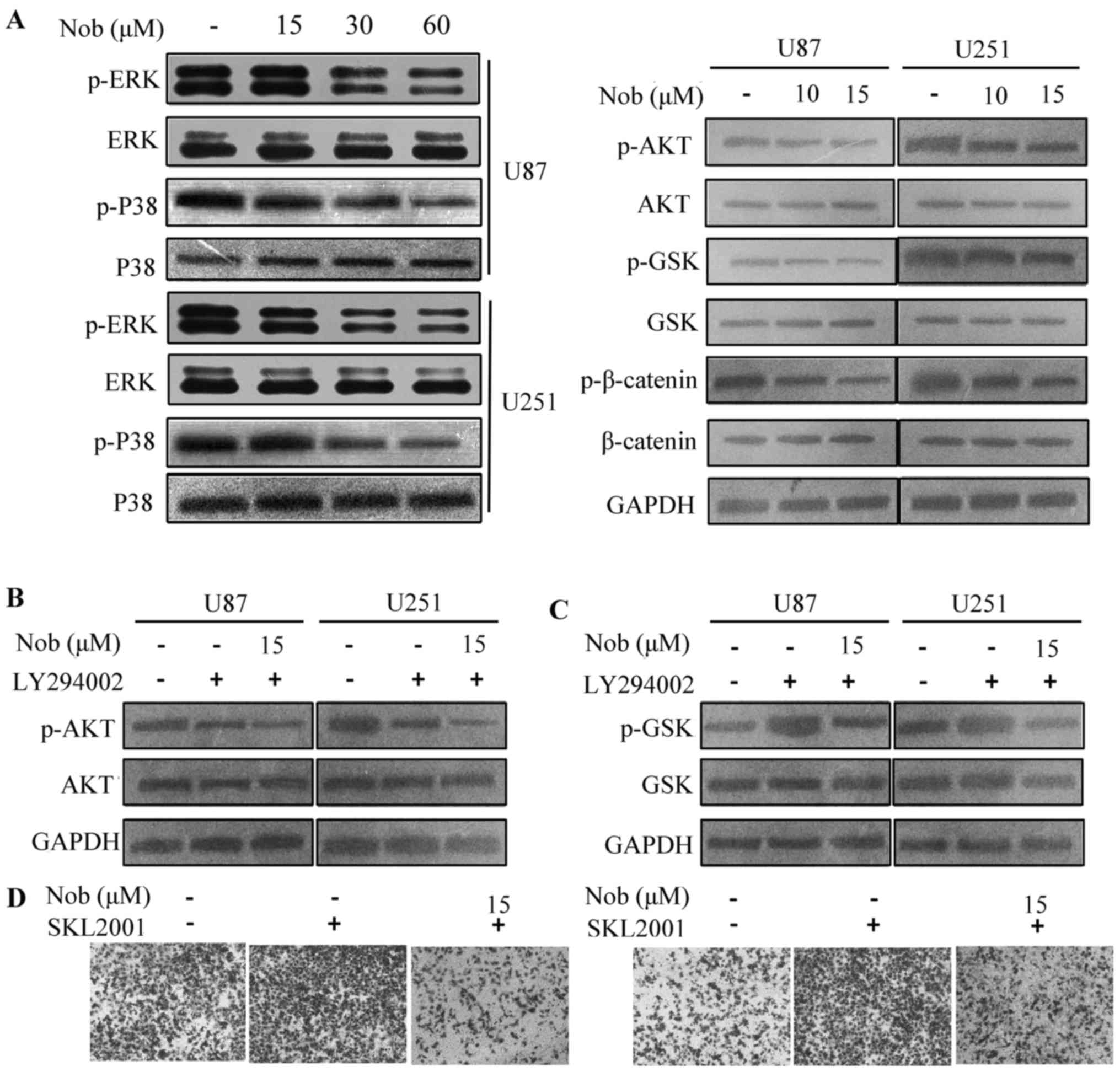
Nobiletin inhibits cell migration by
blunting the AKT/GSK-3β/β-catenin signaling pathway
To uncover the molecular mechanisms of nobiletin,
essential and crucial pathways manipulating cancer invasion are
examined, such as ERK/p38, PI3K/AKT and Wnt/β-catenin axis. The
ERK/p38 signaling was only subtly affected by nobiletin treatment
at 15 µM. At the concentration of 30 and 60 µM, nobiletin displayed
a potent inhibitory effect on ERK and p38 phosphorylation.
Moreover, PI3K/AKT pathway was remarkably suppressed at 15 µM.
Decrement in the phosphorylation of AKT, GSK3β and β-catenin
proteins was observed in nobiletin-treated U87 and U251 cells
(Fig. 2A). Combination of nobiletin
and LY294002 (a specific PI3K inhibitor) synergistically repressed
the activation of AKT pathway in glioma cells (Fig. 2B). As a potent inhibitor of GSK3β,
AZD1080 was also used in the study. Nobiletin reversed
AZD1080-triggered GSK-3β phosphorylation in both U87 and U251
cells. SKL2001, a small molecule inhibitor of β-catenin
degradation, was chosen to promote β-catenin stabilization
(19). SKL2001 (40 µM) enhanced
cell invasion, and the increment was partially abrogated by
nobiletin (Fig. 2D). In summary,
these results imply that the AKT/GSK-3β/β-catenin axis plays a
critical role in the function of nobiletin on glioma cell
migration.
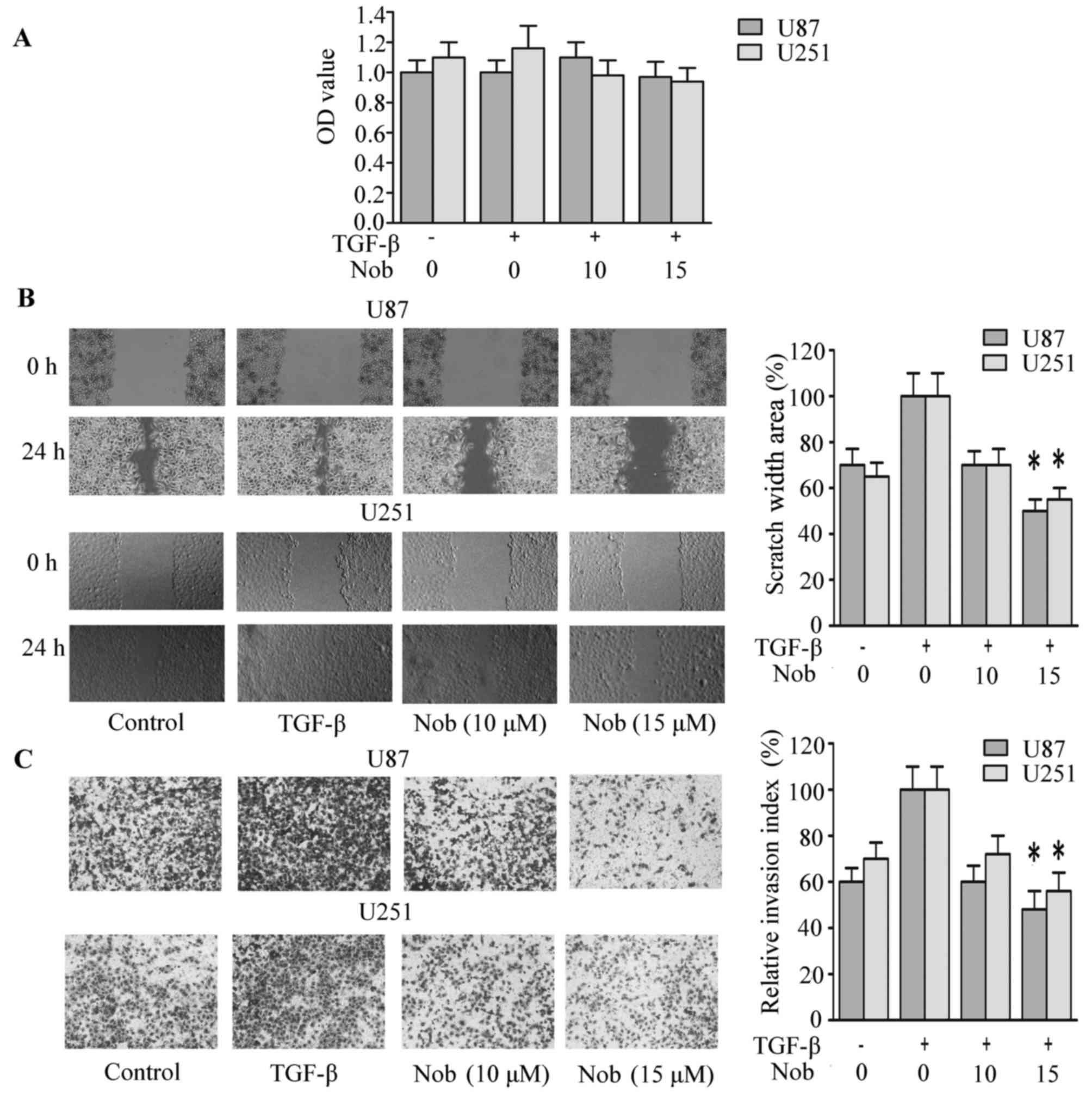
Nobiletin inhibits TGF-induced cell
invasion and EMT in glioma cells
Amplified secretion of TGF-β by carcinoma cells, and
augmented TGF receptor expression, leading to autocrine TGF-β loop,
are regarded to drive or to be requisite for EMT progression in
tumor cells. Matrigel invasion and wound-healing assay was
performed to assess whether nobiletin inhibits invasion stimulated
by TGF-β. In the CCK-8 analysis, medium containing TGF-β (10 ng/ml)
and nobiletin (10, 15 µM) exerted no distinguished cytotoxicity on
cell viability (Fig. 3A).
Therefore, TGF-β (10 ng/ml) and nobiletin (10, 15 µM) were used for
following assays. After exposure to TGF with or without nobiletin
for 24 h, U87 cells were harvested and reseeded into a
Transwell-coated with matrigel. After 24 h, cells that penetrated
through the inserts was stained with crystal violent and assessed
under microscopy. TGF-β enhanced both invasion and migration, but
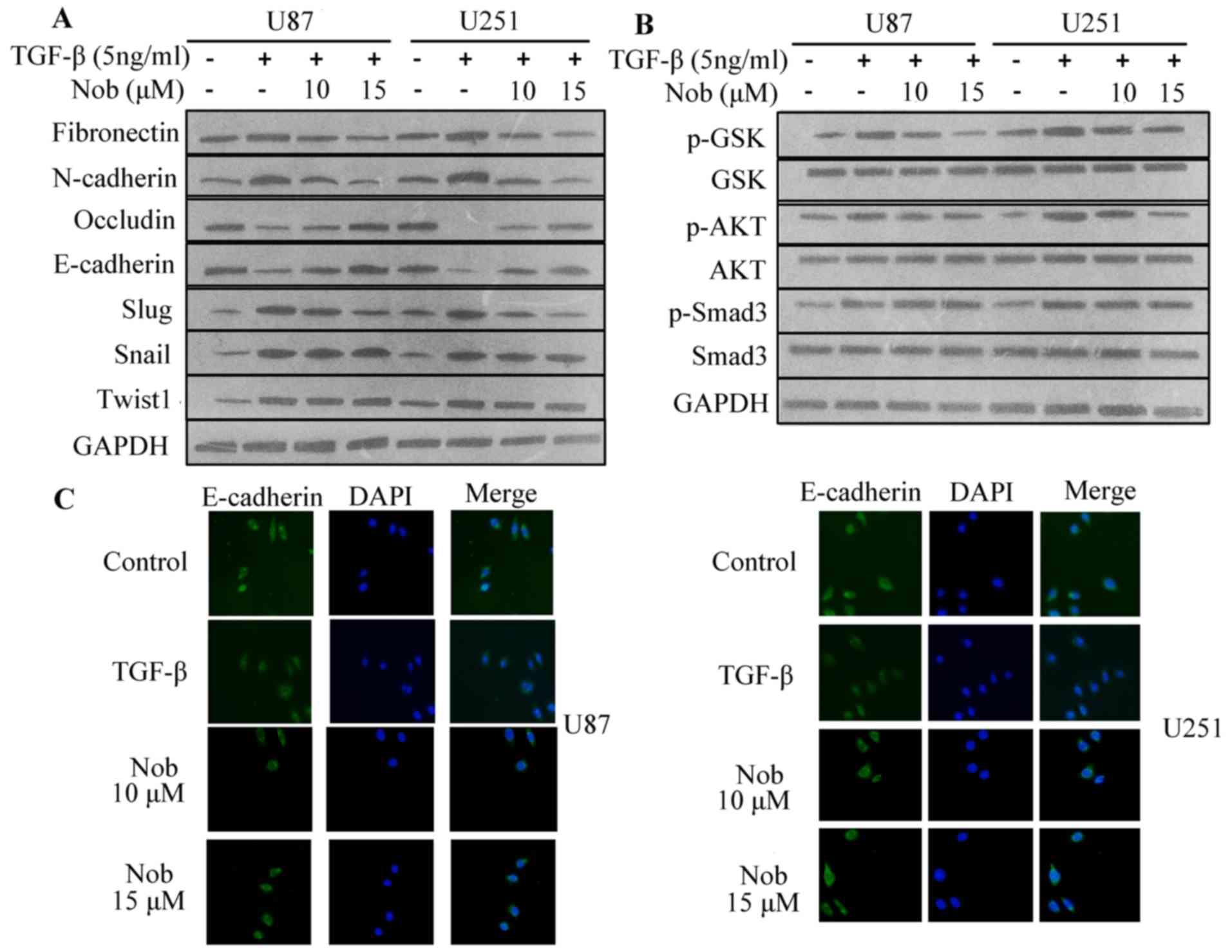
the tendency was reversed by nobiletin (Fig. 3B and C). Cells that had undergone
EMT in response to TGF-β accompanied with fibronectin and
N-cadherin increment and E-cadherin and occludin decrement. In line
with inhibitory effect on basal level of mesenchymal markers,
nobiletin reversed TGF-β-induced epithelial-mesenchymal transition
(Fig. 4A).
TGF/Smads regulates the activities of EMT
transcription factors such as Slug (Snai2), which suppresses
E-cadherin transcription. TGF-β also activates non-Smad pathway,
such as AKT and GSK-3β. The role of above-mentioned pathways in
regulating TGF-β-stimulated EMT was assessed. Consistently,
nobiletin blunted the acitivity of AKT/GSK-3β axis induced by
TGF-β, however, showing little effect on TGF-β-activated Smad3
phosphorylation (Fig. 4B).
Collectively, these data indicated that nobiletin did not retard
EMT via directly targeting Smad3 signaling.
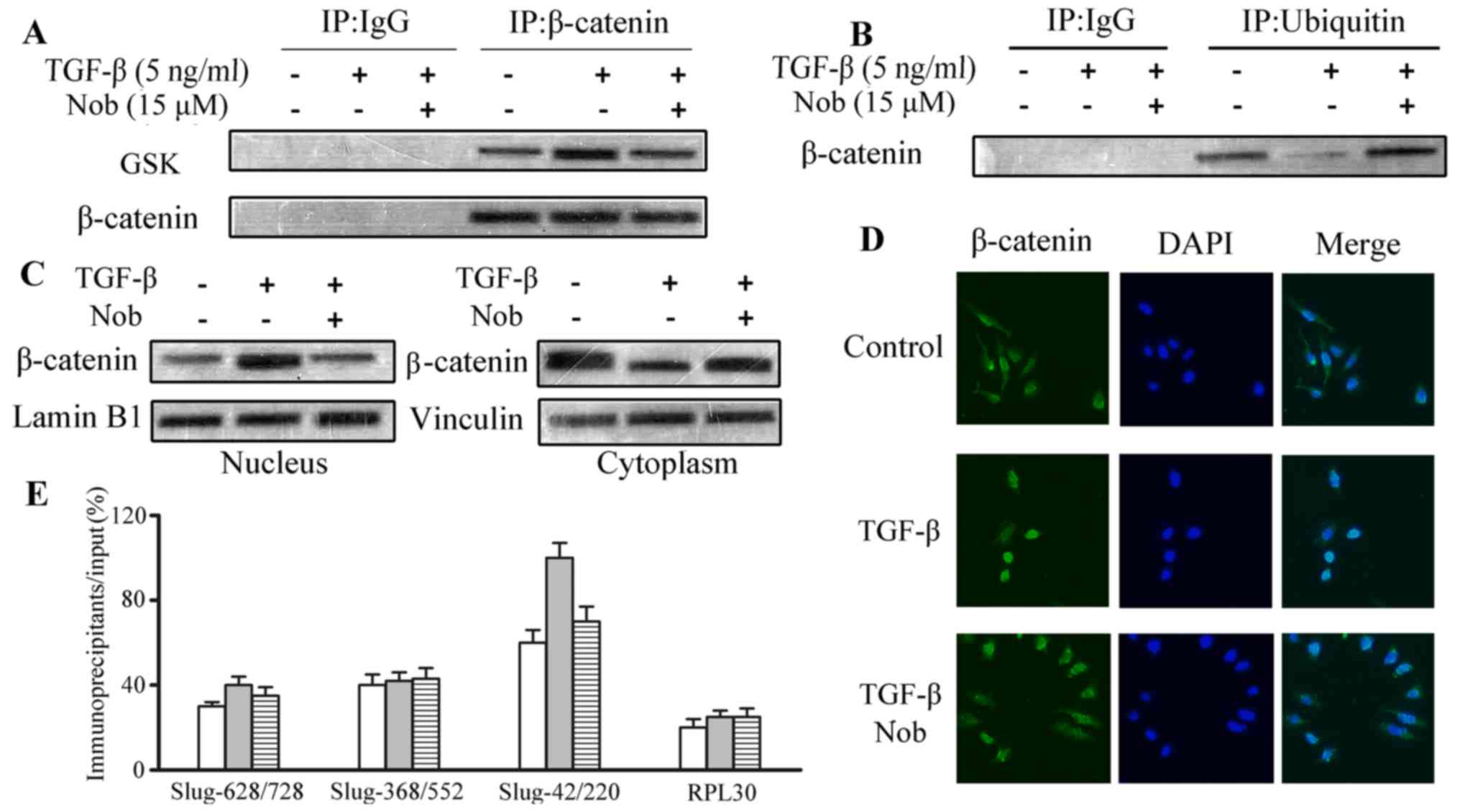
Nobiletin intervenes with
TGF-β-induced β-catenin nuclear translocation, and abolishes
interactions between β-catenin and Slug
By interacting with E-cadherin, β-catenin is
important in EMT progression. On the other hand, active GSK-3β will
form a complex with β-catenin to hinder the translocation of
β-catenin into nucleus. Phosphorylated GSK-3β is an inactive form
of GSK-3β, which would abolish the interaction with β-catenin,
subsequently promoting nuclear translocation and inhibiting
β-catenin degradation. As revealed in the immunoprecipation assay,
an increase was observed in the β-catenin/GSK-3β complex in TGF-β
treated U87 cells (Fig. 5A).
Western blot assays for β-catenin ubiquitination displayed that
TGF-β considerably reduced β-catenin proteasome degradation, and
the decrement was greatly alleviated by nobiletin treatment
(Fig. 5B). Furthermore, nucleus
translocation of β-catenin was also upregulated due to TGF stimuli.
Nevertheless, the function was abolished after nobiletin treatment
(Fig. 5C), as demonstrated in the
immunofluorescence staining. Fractionation followed by western blot
also showed similar results (Fig.
5D). ChIP tests were carried out to explore the binding of
β-catenin to the promoter of Slug. Noteworthy, nobiletin
successfully prevented the binding of β-catenin to the Slug
promoter, induced by TGF (Fig. 5E).
It can be concluded that nobiletin might influence EMT via
regulating GSK-3β and the downstream effector β-catenin.
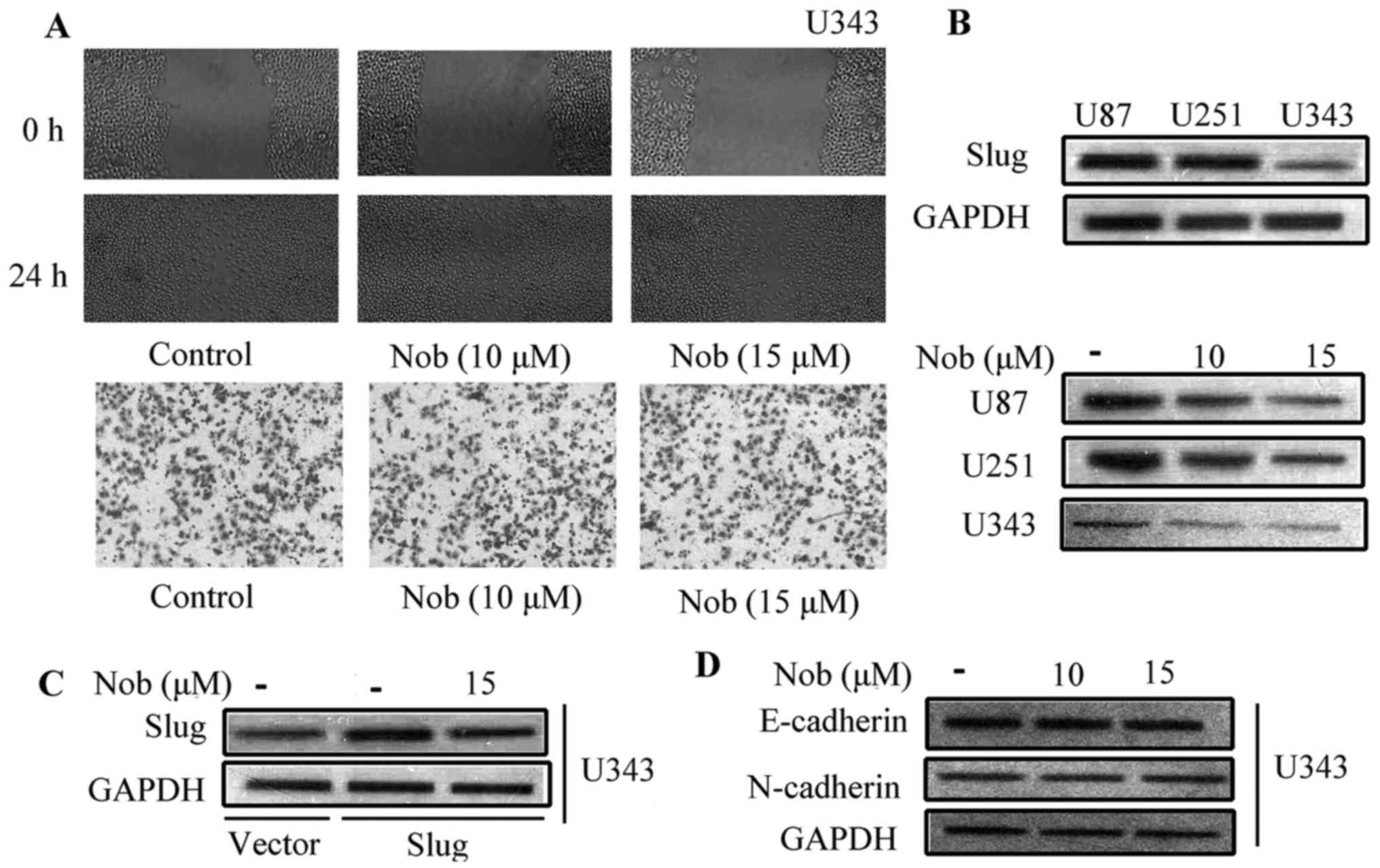
Nobiletin fails to inhibit the
migration and invasion of glioma U343 cells, which do not express
Slug
To confirm the essential role of Slug in the
anti-metastasis effect of nobiletin, a non-Slug-expressing glioma
cell line was applied to compare the effect of nobiletin on
migration and EMT. According to preliminary research, these three
cell lines have divergent Slug protein levels. The protein
expression of Slug was rather low, almost undetectable in U343
cells, and high level of Slug was observed in both U87 and U251
cells (Fig. 6A). Nobiletin had
little effect on the invasion of U343 cells at 10 and 15 µM.
However, at the same concentration, the inhibitory rate reached 45
and 60% in U87 and U251 cell, respectively (Fig. 6B). Moreover, nobiletin failed to
change Slug protein expression in U343 cells. Notably, nobiletin
treatment brought down Slug protein expression in Slug
plasmid-transfected U343 cells (Fig.
6C). The phenomenon that nobiletin enlarged E-cadherin
expression and inhibited the level of N-cadherin in both U87 and
U251 cells was not observed in U343 cells (Fig. 6D). EMT-related proteins remained
unaffected in U343 cells. These data implied that Slug, either at
transcriptional or post-translational level, is greatly involved in
the regulatory effect of nobiletin.
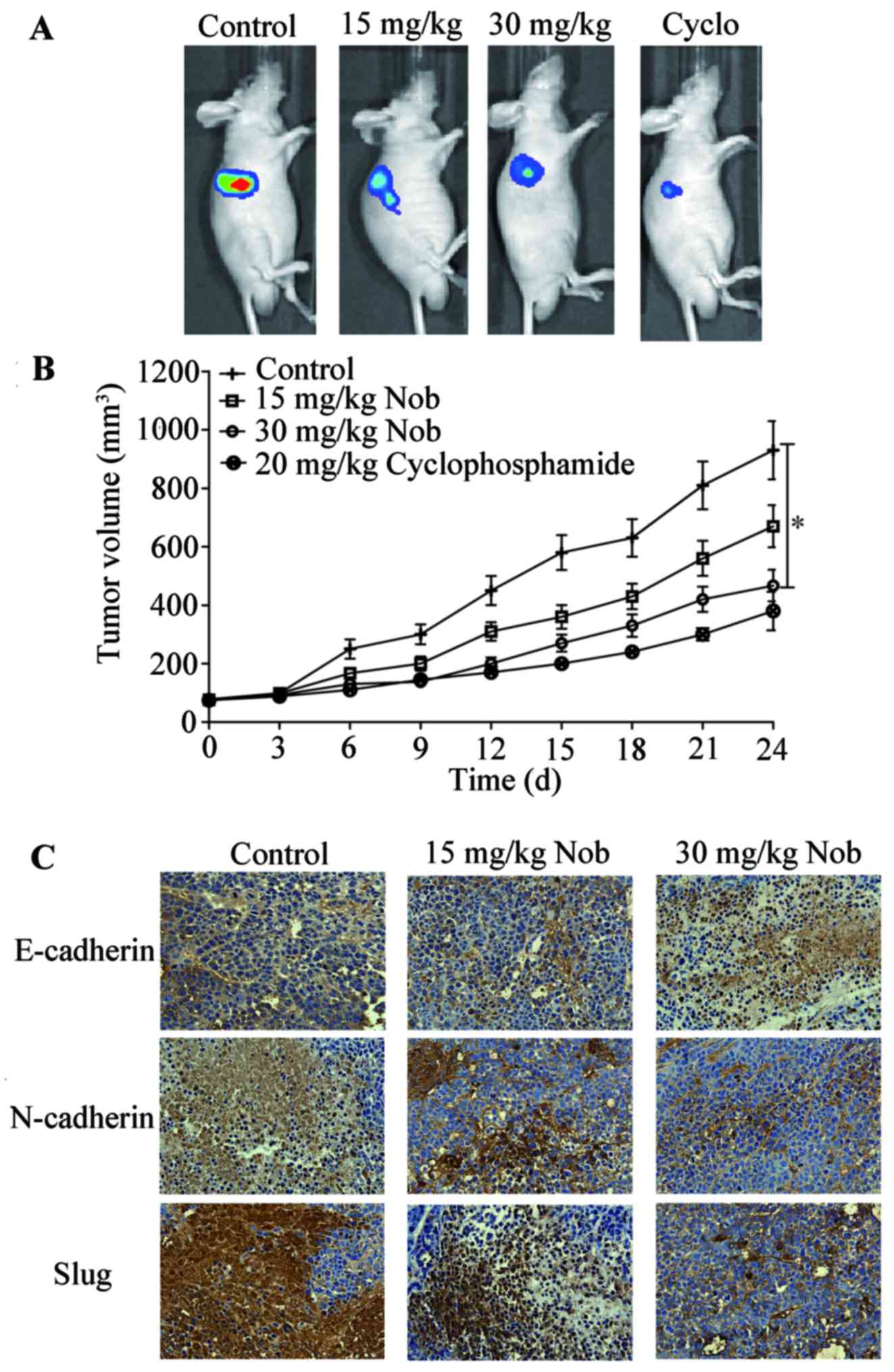
Nobiletin suppresses tumor growth in
vivo
To further assess the antitumor efficiency of
nobiletin in vivo, athymic nude mouse model bearing U87-Luc
implanted xenografts were used. After 24-day treatment, the
bioluminescence imaging results demonstrated impressive repression
of tumor volume in nobiletin-treated nude mice (p<0.01, versus
the vehicle group). Tumor sections were then subjected to IHC
analysis for detection of E-cadherin, N-cadherin and Slug.
E-cadherin expression was enhanced while N-cadherin and Slug was
inhibited in nobiletin-treated group (Fig. 7). All these date indicated that
nobiletin prevented EMT and growth of glioma cancer cells in
vivo.
Discussion
Amplified TGF-β expression secreted by tumors is a
critical hallmark in cancer progression, and it makes contributions
to tumor growth and metastasis via autocrine and paracrine
signaling (20). Cells motivated
with TGF-β will be equipped with a greater invasive capacity than
that of the control cells. An essential role of TGF-β in cancer
progression is attributed to the ability of inducing EMT, along
with cell phenotype changes and enhanced cell invasion and
migration (21). Using human glioma
cells models such as U87 and U251 that undergo EMT triggered by
TGF-β, we found that enhanced cell invasion in response to TGF-β
stimuli go along with an increment in protein content of
EMT-related markers. Cells treated with TGF-β and nobiletin showed
weaken invasive ability than cells triggered with TGF-β only. A
number of studies indicate a critical role for PI3K in TGF-β
signaling (16–18). Besides, TGF-β activates
AKT/GSK3β/β-catenin pathway and that this signaling is involved in
the anti-metastasis effect of nobiletin. In addition to modulating
EMT through above-mentioned signaling, nobiletin remarkably reduced
TGF-β-induced β-catenin nuclear translocation and the binding to
the Slug promoter. Finally, we demonstrated that nobiletin
repressed the tumor growth and EMT progression in nude mice bearing
glioma cell xenografts. Our findings suggested that nobiletin might
have great potential for treating glioma.
Glioblastoma multiform (GBMs) represents the most
lethal and most aggressive central nervous system tumor (22). GBM is characteristic of invasion all
through the brain, distinguished heterogeneity in appearance and
gene expression. Surgical resection followed by traditional
radio-chemotherapy and newly-developed targeted approaches remains
the standard therapy, but tumor recurrence is unavoidable and
almost always causes mortality owning to their infiltrative nature.
EMT is a multiple process which is regarded a crucial event in
tumor progression and is responsible for introducing pathological
variation in organisms. EMT reprogramming is characteristic of loss
of cell-cell adhesions and apical polarity which ultimately leads
to the formation of spindeloid morphology with cytoskeleton
reorganization and increment in migratory capacity. A hallmark of
EMT is the loss of E-cadherin, which is an important constituent of
adhesion junctions that plays a vital role in maintaining the
epithelial integrity (23).
A group of specific transcription factors, including
Slug, Snail, Twist and ZEB, have been implicated in the
manipulating of EMT, acting as transcriptional repressors of the
E-cadherin (24). Snail family
proteins regulate transcription of molecules for cell-cell adhesion
during EMT. As a mediator of EMT and metastasis, Slug/Snail2
expression is strengthened in glioblastoma and can be stimulated by
TGF-β and HGF (25). TGF-β-induced
EMT, which is fundamentally studied as a model, incorporates
classic Smad and non-Smad signaling, and transmits signaling via
downstream PI3K/AKT and ERK/MAPK pathways (26). Invasion and EMT of TGF-induced cells
as well as normal cells were all reversed by nobiletin in glioma
cells. It is obvious that the protein levels of epithelial markers
E-cadherin and occludin were upregulated after incubated with
nobiletin for 24 h, along with reduced mesenchymal markers such as
fibronectin and N-cadherin.
The oncogenic kinase AKT encourages EMT as well as
the stabilization of β-catenin via phosphorylating GSK-3β.
β-catenin was first discovered as a component of a mammalian cell
adhesion complex (27).
Phosphorylation of β-catenin by AKT promotes the dissociation of
β-catenin from cell junctions and the gathering in nucleus
(28). Additionally, β-catenin,
which initially conglomerates with the cytoplasmic tail of
E-cadherin at the C-terminus, exhibits nuclear translocation to
promote expression of EMT-related genes and molecules associates
with PI3K/AKT signaling (29).
To uncover the antitumor mechanisms of nobiletin,
the effects of nobiletin during normal, repressed and motivated AKT
phosphorylation protein levels was examined in glioma cells. The
data showed that nobiletin can hinder AKT phosphorylation in all of
these conditions. Combined treatment of nobiletin and LY294002 (a
specific PI3K inhibitor) synergistically blocked AKT activation in
glioma cells. GSK-3β phosphorylation induced by AKT obstructs
GSK-3β activity. Accordingly, nobiletin downregulated the level of
p-GSK-3β and p-β-catenin. We further used AZD1080, an inhibitor of
GSK-3β, and found that nobiletin reversed AZD1080-triggered GSK-3β
phosphorylation in glioma cells. Herein, it can be concluded that
nobiletin might influence EMT via regulating GSK-3β and the
downstream effector β-catenin. Our study indicated that nobiletin
can abrogate the aberrant glioma cell invasion via blunting the
activity of the AKT/GSK-3β/β-catenin signaling.
As is known, β-catenin mediated activation of Slug
(30). AKT inhibition impeded Slug
accumulation and blocked EMT and stemness of human thyroid cancer
cells (31). TGF-β1 also regulates
the degradation of the Slug protein, probably through GSK-3β
inactivation (32). Overexpression
of Slug will, in turn, repress E-cadherin. Mutation of GSK-3β
phosphorylation sites (S92/96A or S100/104A) promoted the
Slug-mediated EMT properties of E-cadherin suppression and vimentin
stimulation, compared with wild-type Slug.
In clinical samples, both Slug and nuclear β-catenin
scores were considerably higher in the sarcomatous components than
carcinomatous elements (30). In
the ChIP tests, nobiletin reversed TGF-induced binding of β-catenin
to the Slug promoter. Nobiletin considerably inhibited Slug
expression, however, without significant effect on Snail and Twist1
expressions. Interactions between β-catenin and Slug were also
greatly abolished by nobiletin treatment. To further explore the
role of Slug, U343 cell line was applied, which do not express
Slug. However, high level of Slug was observed in both U87 and U251
cells. Of note, nobiletin failed to inhibit the migration and
invasion of U343 cells. However, Slug protein expression was
brought down by nobiletin treatment in Slug plasmid-transfected
U343 cells. These data demonstrated the essential role of Slug in
the progression of EMT and invasion of glioma cells, and suggested
that nobiletin inhibits invasion probably via inhibiting
AKT/GSK3β/β-catenin signaling pathway in Slug-expressing glioma
cells.
Acknowledgements
This work was supported the Natural Science
Foundation of Hebei Province (no. H2016201238), and Science
Foundation of Affiliated Hospital of Hebei University (no.
2015Z003).
References
|
1
|
Wirsching HG and Weller M: The role of
molecular diagnostics in the management of patients with gliomas.
Curr Treat Options Oncol. 17:512016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huse JT: Establishing a robust molecular
taxonomy for diffuse gliomas of adulthood. Surg Pathol Clin.
9:379–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zou M, Zhu W, Wang L, Shi L, Gao R, Ou Y,
Chen X, Wang Z, Jiang A, Liu K, et al: AEG-1/MTDH-activated
autophagy enhances human malignant glioma susceptibility to
TGF-β1-triggered epithelial-mesenchymal transition. Oncotarget.
7:13122–13138. 2016.PubMed/NCBI
|
|
4
|
Khan Z and Marshall JF: The role of
integrins in TGFβ activation in the tumour stroma. Cell Tissue Res.
365:657–673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bussard KM, Mutkus L, Stumpf K,
Gomez-Manzano C and Marini FC: Tumor-associated stromal cells as
key contributors to the tumor microenvironment. Breast Cancer Res.
18:842016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:52016. View Article : Google Scholar :
|
|
7
|
Guo L, Zhang Y, Zhang L, Huang F, Li J and
Wang S: MicroRNAs, TGF-β signaling, and the inflammatory
microenvironment in cancer. Tumour Biol. 37:115–125. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cervantes-Arias A, Pang LY and Argyle DJ:
Epithelial-mesenchymal transition as a fundamental mechanism
underlying the cancer phenotype. Vet Comp Oncol. 11:169–184. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adhes Migr. 9:317–324. 2015. View Article : Google Scholar
|
|
10
|
Papkoff J and Aikawa M: WNT-1 and HGF
regulate GSK3 beta activity and beta-catenin signaling in mammary
epithelial cells. Biochem Biophys Res Commun. 247:851–858. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Angers S and Moon RT: Proximal events in
Wnt signal transduction. Nat Rev Mol Cell Biol. 10:468–477.
2009.PubMed/NCBI
|
|
13
|
Lien LM, Wang MJ, Chen RJ, Chiu HC, Wu JL,
Shen MY, Chou DS, Sheu JR, Lin KH and Lu WJ: Nobiletin, a
polymethoxylated flavone, inhibits glioma cell growth and migration
via arresting cell cycle and suppressing MAPK and Akt pathways.
Phytother Res. 30:214–221. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Chen AY, Huang H, Ye X, Rollyson
WD, Perry HE, Brown KC, Rojanasakul Y, Rankin GO, Dasgupta P, et
al: The flavonoid nobiletin inhibits tumor growth and angiogenesis
of ovarian cancers via the Akt pathway. Int J Oncol. 46:2629–2638.
2015.PubMed/NCBI
|
|
15
|
Shi MD, Liao YC, Shih YW and Tsai LY:
Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways
in HGF-treated liver cancer HepG2 cells. Phytomedicine.
20:743–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma HW, Xie M, Sun M, Chen TY, Jin RR, Ma
TS, Chen QN, Zhang EB, He XZ, De W, et al: The pseudogene derived
long noncoding RNA DUXAP8 promotes gastric cancer cell
proliferation and migration via epigenetically silencing PLEKHO1
expression. Oncotarget. Aug 5–2016.(Epub ahead of print).
|
|
17
|
McCormick SM, Gowda N, Fang JX and Heller
NM: Suppressor of cytokine signaling (SOCS)1 regulates
interleukin-4 (IL-4)-activated insulin receptor substrate (IRS)-2
tyrosine phosphorylation in monocytes and macrophages via the
proteasome. J Biol Chem. 291:20574–20587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Navarro-Villarán E, Tinoco J, Jiménez G,
Pereira S, Wang J, Aliseda S, Rodríguez-Hernández MA, González R,
Marín-Gómez LM, Gómez-Bravo MA, et al: Differential antitumoral
properties and renal-associated tissue damage induced by tacrolimus
and mammalian target of rapamycin inhibitors in hepatocarcinoma: In
vitro and in vivo studies. PLoS One. 11:e01609792016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gwak J, Hwang SG, Park HS, Choi SR, Park
SH, Kim H, Ha NC, Bae SJ, Han JK, Kim DE, et al: Small
molecule-based disruption of the Axin/β-catenin protein complex
regulates mesenchymal stem cell differentiation. Cell Res.
22:237–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kucuksayan H, Ozes ON and Akca H:
Downregulation of SATB2 is critical for induction of
epithelial-to-mesenchymal transition and invasion of NSCLC cells.
Lung Cancer. 98:122–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Larsen JE, Nathan V, Osborne JK, Farrow
RK, Deb D, Sullivan JP, Dospoy PD, Augustyn A, Hight SK, Sato M, et
al: ZEB1 drives epithelial-to-mesenchymal transition in lung
cancer. J Clin Invest. 126:3219–3235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao F, Cui Y, Jiang H, Sui D, Wang Y,
Jiang Z, Zhao J and Lin S: Circulating tumor cell is a common
property of brain glioma and promotes the monitoring system.
Oncotarget. 7:71330–71340. 2016.PubMed/NCBI
|
|
23
|
Lau MT, So WK and Leung PC: Fibroblast
growth factor 2 induces E-cadherin down-regulation via
PI3K/Akt/mTOR and MAPK/ERK signaling in ovarian cancer cells. PLoS
One. 8:e590832013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shirai K, Hagiwara N, Horigome T, Hirose
Y, Kadono N and Hirai Y: Extracellularly extruded syntaxin-4 binds
to laminin and syndecan-1 to regulate mammary epithelial
morphogenesis. J Cell Biochem. Jul 27–2016.(Epub ahead of
print).
|
|
25
|
Nagaishi M, Paulus W, Brokinkel B, Vital
A, Tanaka Y, Nakazato Y, Giangaspero F and Ohgaki H:
Transcriptional factors for epithelial-mesenchymal transition are
associated with mesenchymal differentiation in gliosarcoma. Brain
Pathol. 22:670–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang G, Liang Y, Zheng T, Song R, Wang J,
Shi H, Sun B, Xie C, Li Y, Han J, et al: FCN2 inhibits
epithelial-mesenchymal transition-induced metastasis of
hepatocellular carcinoma via TGF-β/Smad signaling. Cancer Lett.
378:80–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCrea PD, Turck CW and Gumbiner B: A
homolog of the armadillo protein in Drosophila (plakoglobin)
associated with E-cadherin. Science. 254:1359–1361. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang D, Hawke D, Zheng Y, Xia Y,
Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T and Lu Z:
Phosphorylation of beta-catenin by AKT promotes beta-catenin
transcriptional activity. J Biol Chem. 282:11221–11229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spano D and Zollo M: Tumor
microenvironment: A main actor in the metastasis process. Clin Exp
Metastasis. 29:381–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inoue H, Takahashi H, Hashimura M, Eshima
K, Akiya M, Matsumoto T and Saegusa M: Cooperation of Sox4 with
β-catenin/p300 complex in transcriptional regulation of the Slug
gene during divergent sarcomatous differentiation in uterine
carcinosarcoma. BMC Cancer. 16:532016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Visciano C, Liotti F, Prevete N, Cali' G,
Franco R, Collina F, de Paulis A, Marone G, Santoro M and Melillo
RM: Mast cells induce epithelial-to-mesenchymal transition and stem
cell features in human thyroid cancer cells through an
IL-8-Akt-Slug pathway. Oncogene. 34:5175–5186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JY, Kim YM, Yang CH, Cho SK, Lee JW
and Cho M: Functional regulation of Slug/Snail2 is dependent on
GSK-3β-mediated phosphorylation. FEBS J. 279:2929–2939. 2012.
View Article : Google Scholar : PubMed/NCBI
|