Introduction
Astrocytoma, which originates from star-shaped glial
cells, is the most commonly occurring primary brain tumor in
adults. According to the World Health Organization (WHO) scheme, it
represents a heterogeneous group of diseases with different degrees
of malignancy from relatively indolent diffuse astrocytomas to
highly aggressive glioblastoma multiforme (GBM) (1). Among these tumors, grade II
astrocytomas are characterized by low level proliferative activity,
but often recur, and some of the types tend to progress to higher
grades of malignancy (1,2). The prognosis of patients with GBM, the
most malignant astrocytoma, remains rather dismal. Even with the
best standard of care currently available, these patients only have
a median life expectancy of ~10.6 months after diagnosis (3). The current standard therapy for GBM
patients includes maximal debulking surgery, radiation and adjuvant
chemotherapy. Temozolomide, a current chemotherapeutic agent of
choice for GBM patients, has yielded only very modest improvements
in disease outcomes (4).
Considerable research efforts have been focused on
the identification of genetic alterations in GBMs so as to help
define subgroups of GBM patients with different prognosis. Several
genes, including TP53, PTEN, EGFR and IDH1, are
altered in gliomas. The TP53/IDH1 mutation seems to occur
early during the development of an astrocytoma (5), whereas the loss or mutation of
PTEN and amplification of EGFR are characteristic of
primary glioblastomas (6,7). The discovery of these genetic
alterations helps the diagnosis of GBM and allows us to better
define the prognosis of GBM patients and provides further
characterization and understanding of gliomagenesis.
The ATP2A2 gene encodes a protein called
sarco (endo)plasmic reticulum Ca2+-ATPase isoform 2
(SERCA2), which transports Ca2+ from the cytosol to the
endoplasmic reticulum (ER) lumen. Mutations in ATP2A2 cause
Darier's disease, an autosomal dominant skin disorder characterized
by loss of adhesion between epidermal cells and abnormal
keratinization (8,9). Disturbances of Ca2+
homeostasis have been implicated in the development of many types
of tumor, such as colon cancer, liposarcoma, leukemia and head and
neck squamous cancer (10–15). ATP2A2 heterozygous mutant
(ATP2A2+/−) mice showed increased incidence of
hyperkeratinized tumors in regions of stratified squamous
epithelia, including the oral mucosa, tongue, esophagus, palate,
skin, genitalia, and non-glandular mucosa of the stomach (16). The role of ATP2A2 in the oncogenesis
of astrocytomas has not been elucidated. We hypothesized that,
given the importance of Ca2+ homeostasis in maintaining
the physiological processes, the expression of ATP2A2 could be
altered in astrocytomas and the definition of such alterations may,
as a prognostic marker, help identify subgroups of patients with a
distinct prognosis, or, as a diagnostic marker, help distinguish
astrocytomas of different grades.
Although ATP2A2 has recently been implicated in
certain cancer types (19,20), no information is available on its
expression in human astrocytoma tissues. In this study, we examined
the expression of ATP2A2 in 109 human astrocytoma samples by
immunohistochemistry and further analyzed the correlation between
ATP2A2 expression and clinicopathological characteristics of these
astrocytoma patients. We also investigated the effect of ATP2A2
overexpression on the growth of GBM cells in vitro and in a
mouse xenograft model.
Materials and methods
Tissue specimens
In total, 109 formalin-fixed and paraffin-embedded
surgically resected astrocytic tumor specimens (84 primary ones and
17 secondary GBM and 8 AA developed from DA) were acquired from the
Department of Pathology, Changzheng Hospital, Second Military
Medical University, Shanghai, China. Samples of oligodendrogiomas
with IDH1 mutation (n=20) and pilocytic astrocytomas (PAs) (n=20)
without IDH1 mutation were also acquired to further explore the
relation between ATP2A2 expression and IDH1 mutation. These
specimens were archived between January, 2007 and December, 2014.
All analyzed brain tumors were subjected to consensus review by two
neuropathologists (Wei-Qing Li, and Hui-Min Liu) according to the
WHO Classification (1). Table I lists the types of brain tumors
analyzed, including DA (n=39), AA (n=19) and GBM (n=51). There were
61 (60.0%) males and 48 (40.0%) females with a median age of 45
(range, 5–73) years. No patients had received preoperative chemo-
or radiotherapy. The average period of follow-up was 27.6±20.5
(range, 1–90) months. Tumor size, location, extent of surgical
resection, and postoperative consolidated treatment were recorded
(Table I). In addition, normal
brain tissue samples were obtained from patients undergoing
decompressive craniectomy and patients undergoing selective
temporal lobe resection for intractable epilepsy.
 | Table I.Clinicopathological characteristics
of 109 diffuse astrocytic tumor patients. |
Table I.
Clinicopathological characteristics
of 109 diffuse astrocytic tumor patients.
| Variables | N (%) |
|---|
| Gender |
|
|
Male | 61 (60.0) |
| Age
(years)a |
|
|
≥55 | 67 (61.5) |
| Tumor site |
|
|
Temporal lobe | 16 (14.7) |
|
Parietal lobe | 46 (42.2) |
| Frontal
lobe | 31 (28.4) |
|
Occipital lobe | 16 (14.7) |
| Tumor size |
|
| >4
cm | 61 (56.0) |
| Extent of
resection |
|
|
Total | 91 (83.5) |
|
Subtotal | 18 (16.5) |
| Radiotherapy |
|
|
Yes | 47 (43.1) |
| Chemotherapy |
|
|
Yes | 60 (55.0) |
| WHO
gradeb |
|
| II | 39 (35.8) |
|
III | 19 (17.4) |
| IV | 51 (46.8) |
Acquisition of all tissue specimens was approved by
the local ethics committee at the authors' affiliated institution
and was carried out in accordance with established institutional
and national ethical guidelines regarding use of human tissues for
research.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded, 3-µm tissue
sections were cut with 3 adjacent sections chosen from each sample
for routine immunohistochemical staining. After deparaffinization
in xylol and rehydration in gradient ethanol, sections were
immersed in 1 µM EDTA buffer (pH 8.0) and microwaved for 20 min for
antigen retrieval. Endogenous peroxidase was inactivated by 3%
methanolic hydrogen peroxide solution for 30 min. Non-specific
binding was blocked by incubation with non-immune serum for 30 min
followed by overnight incubation at 4°C with anti-ATP2A2 (Abcam,
San Diego, CA, USA), anti-IDH1 and anti-GFAP antibodies (Maxin
Biotechnology, Fujian, China). After staining with the DAB kit
(Maxin Biotechnology), the slides were counterstained with
hematoxylin.
For evaluating ATP2A2 and IDH1 mutation
immunoreactivity, two experienced pathologists examined
representative visual fields (x200 magnification) independently to
identify positively stained tumor cells. The intensity of positive
staining was scored using a scale from 0 to 3 (0 for no
immunostaining, 1 for light-brown color, 2 for medium-brown color,
and 3 for dark-brown color). The percentage of positive staining
cells was also scored (0, no staining; 1, positive staining in
<25% of the tumor cells; 2, positive staining in 25–75% of the
tumor cells; and 3, positive staining in >75% of the tumor
cells). The percentage of cells showing positive staining with the
antibodies was calculated in 5 high-powered fields. The two scores
were then multiplied, and the results were regarded as the
expression score of the sample. All discrepancies in scoring were
reviewed, and a consensus was reached. Samples were scored totally
as follows: strong (+++, total score = 6), moderate (++, total
score = 4–5), weak (+, total score = 1–3), and null (−, total score
= 0). ATP2A2 was recorded as high expression (++ and +++), and low
or negative expression (+ and -) according to the rate of labeled
tumor cells and cytoplasm staining intensity.
Cell culture and lentiviral
infections
Human glioblastoma cell line U251MG was obtained
from the Cell Bank, Type Culture Collection, Chinese Academy of
Sciences (CBTCCCAS, Shanghai, China) and cultured at 37°C in a
humidified atmosphere containing 5% CO2 in Dulbecco's
modified Eagle's medium (DMEM) containing 10% fetal bovine serum
(FBS) supplemented with 100 U⁄ml penicillin and 100 µg/ml
streptomycin.
The lentivirus vector expressing ATP2A2,
pcDNA3.1-SERCA2 ATPase, was constructed using the pcDNA3.1
(−)-3Flag-Myc-His expression system according to the manufacturer's
instructions (Sunbio Medical Biotechnology, Shanghai, China). The
recombinant lentiviruses were produced by co-transfecting human
embryonic kidney (HEK) 293T cells using Lipofectamine 2000
according to the manufacturer's protocol (Invitrogen, Carlsbad, CA,
USA). U251MG cells were infected with lentiviruses overexpressing
ATP2A2 and then selected with 800 µg/ml G418 (Invitrogen) in
complete medium for 20–30 days. Stable U251MG cells were maintained
in α-MEM containing 10% FBS and 800 µg/ml G418. Total cellular RNA
and proteins were extracted at 10 days after transfection for
further analysis.
Quantitative real-time RT-PCR
For quantitative real-time RT-PCR, total cellular
RNA was extracted with TRIzol (Invitrogen). Reverse transcription
was performed using a Reverse Transcriptase kit (Invitrogen)
according to the manufacturer's instructions. The expression of
ATP2A2 mRNA was normalized against β-actin mRNA. The
comparative threshold cycle (ct) method was used, and the fold
difference = 2− (∆ct of target gene − ∆ct of
reference). Quantitative real-time PCR was performed using
the SYBR Premix Ex Taq™ kit (Takara, Kyoto, Japan) on the
Takara TP800 System. The PCR was run at 95°C for 15 sec, 40 cycles
of 95°C for 5 sec and 60°C for 30 sec. The sequences of the PCR
primers used were as follows: ATP2A2,
5′-CTCGGATCCAACACTACAGGTGTTGAATGG-3′ (sense), and
5′-CGGAATTCATGCGCAGTGATAAATTGAC-3′ (antisense); β-actin,
5′-CGTGACATTAAGGAGAAGCTG-3′ (sense), and 5′-CTAGAAGCATTTGCGGTGGAC-3
(antsense).
Western blot assays
Cellular lysates were prepared of U251MG cells using
RIPA lysis buffer. Proteins were quantified using the Bradford
method and samples were resolved on 10% SDS denatured
polyacrylamide gel. Immunoblotting was performed as previously
described (18) and the following
antibodies were used: mouse anti-flag antibody (Sigma, St. Louis,
MO, USA), rabbit anti-SERCA2 ATPase antibody (Abcam), and goat
anti-mouse IgG and goat anti-rabbit IgG (all from Santa Cruz
Biotechnology, Santa Cruz, CA, USA). The LabWorks™ Image
Acquisition and Analysis Software (UVP) was used for densitometric
analysis.
Cell proliferation and clonogenic
assays
For cell proliferation studies, cells were plated
onto 96-well plates at 2×103 cells/well and cultured
overnight to allow cell attachment. The number of viable cells was
determined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) bromide
colorimetric assays at daily intervals (24, 48, 72, 96 and 120 h).
For soft agar clonogenic survival assays, GBM cells were plated
onto the top agar at 2.0×102 cells per well and grew for
14 days at 37°C. Colonies were visualized using the cell staining
Giemsa solution (Chemicon, Millipore, Billerica, Danvers, MA, USA).
An aggregate of ≥50 cells was considered a colony and the number of
colonies was counted under the microscope. Each experiment was
carried out in triplicate and at least three times
independently.
Apoptosis assays
Cells were harvested 8 days after lentiviral
infection and washed once with phosphate-buffered saline (PBS),
trypsinized, and washed again in PBS with 2% FBS and resuspended in
binding buffer containing 10 mM HEPES (pH 7.4); 2.5 mM
CaCl2, and 140 mM NaCl, and stained with Annexin V-R-PE
and 7-AAD according to the manufacturer's protocol (Southern
Biotech, Birmingham, AL, USA). Stained cells were analyzed on a
FACSCalibur flow cytometer (BD Biosciences) using CellQuest
software, and the Mod-Fit program (Verity Software House Inc.,
Topsham, ME, USA) was used to analyze apoptosis. Each experiment
was conducted in triplicate and at least three times
independently.
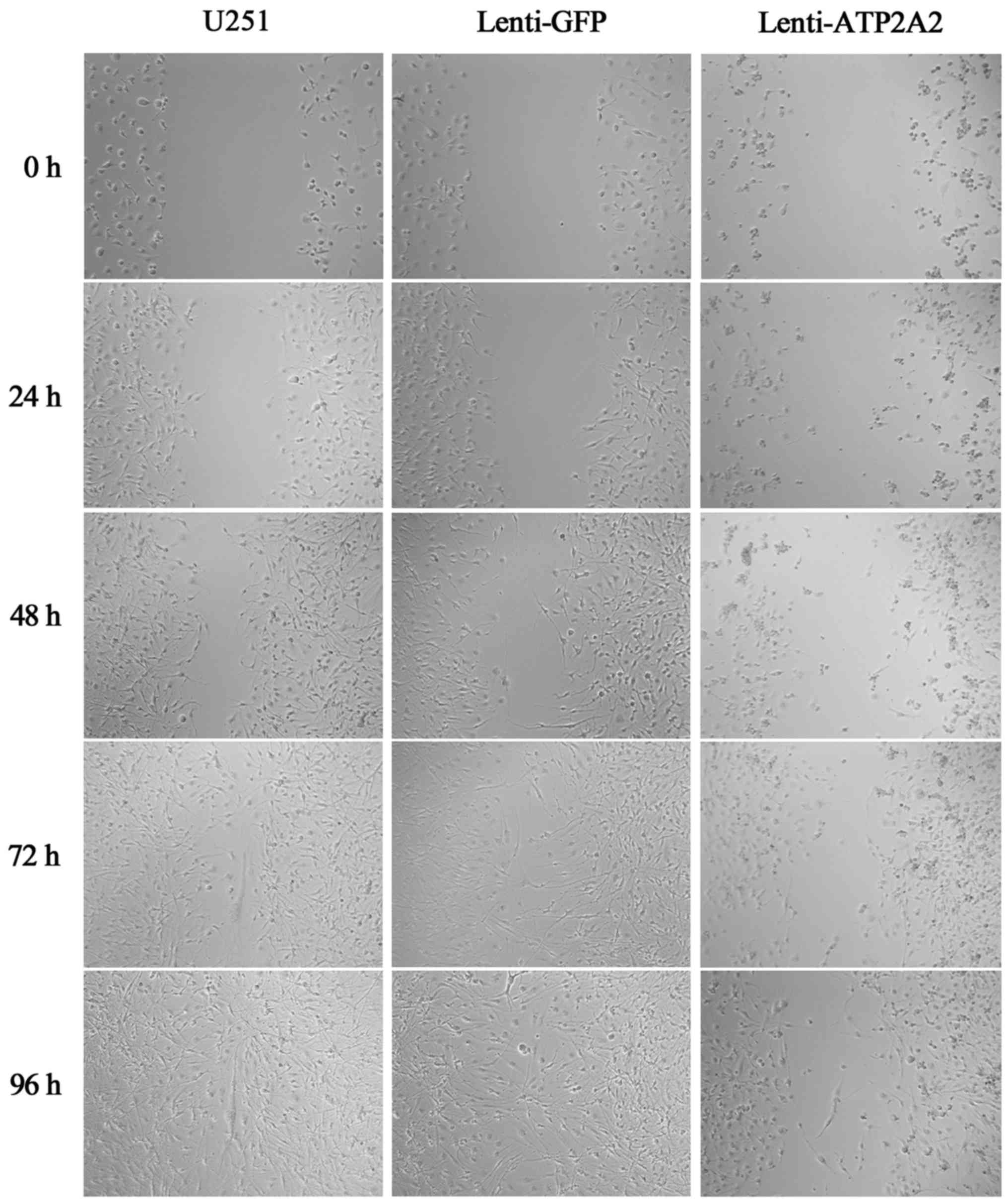
Wound healing assays
The scratch assay was performed to investigate the
effect of ATP2A2 overexpression on the migration of GBM cells.
Briefly, cells were seeded in 6-well plates at a density of
2×105 cells/well. When the cells were 90–100% confluent,
the monolayer was scratched manually with a plastic pipette tip
across the diameter of each plate, and after two washes with PBS,
the wounded cellular monolayer was allowed to heal for 96 h. Images
of central wound edges were taken at time 0 and at the indicated
time-points using PowerShot G10 camera (Canon, Tokyo, Japan). Cell
migration was observed by microscopy at 24, 48, 72 and 96 h.
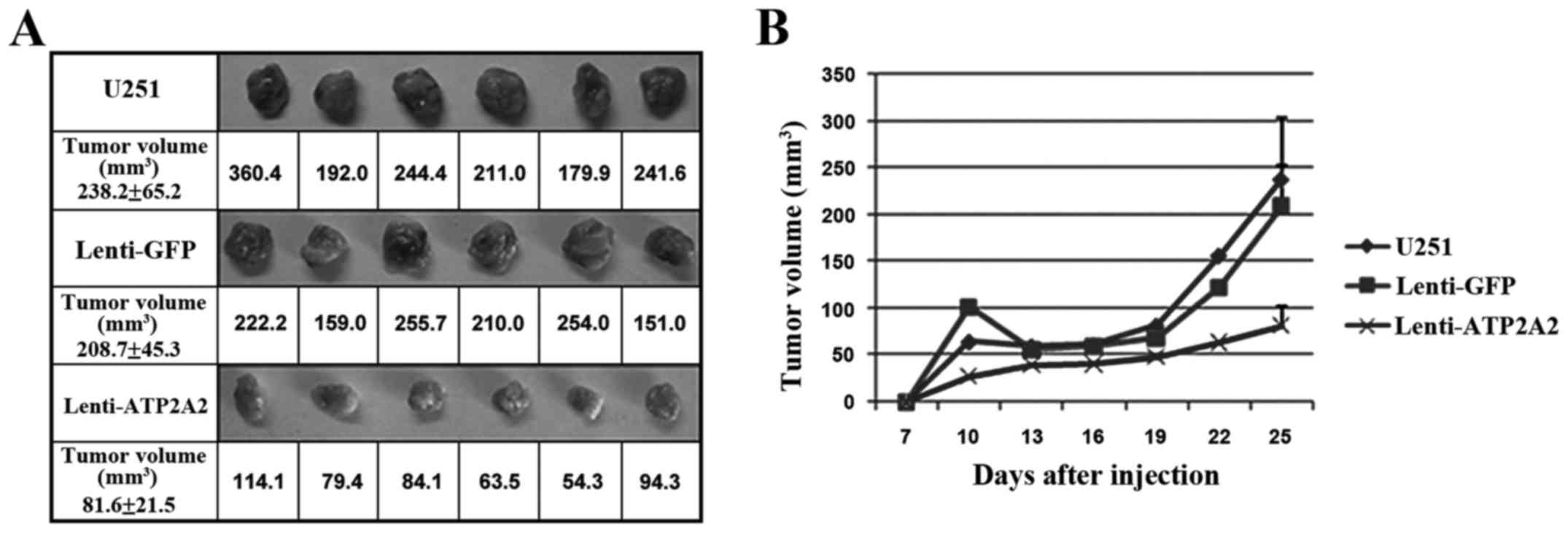
Xenograft studies
Forty-five female BALB⁄c-nu mice, 7–8 weeks of age,
were obtained from the Institute of Zoology, Chinese Academy of
Sciences, Shanghai, China and kept under specific pathogen-free
conditions in accordance with procedures and guidelines set by the
Institutional Animal Care and Use Committee (IACUC) of the Second
Military Medical University, Shanghai, China. The study protocol
was approved by the local institutional review board. Each mouse
was inoculated subcutaneously in the forelimb with 1×107
U251 cells in 0.2 ml of medium. After 5 days, mice were randomized
to receive twice weekly intratumoral injections (30 ml⁄mouse at 3–5
sites) of Lenti-ATP2A2 (pcDNA3.1-SERCA2 ATPase, 5×109
TU/ml), or intratumoral injections of control lentiviruses
(5×109 TU/ml), or vehicle control. Tumor size was
measured every 3 days in two dimensions using a caliper, and tumor
volume (mm3) was calculated using the formula V =
0.5×larger diameter×(smaller diameter)2. Tumor volume
was normalized against the initial volume before intratumoral
injection for plotting the curve of astrocytoma cell growth rate.
Four weeks post injection, 6 mice from each group were randomly
selected and sacrificed for weighing and photographing.
Statistical analysis
All statistical analysis and graphs were performed
with the SPSS 22.0 analysis software (SPSS Inc., Chicago, IL, USA).
The correlation between ATP2A2 expression and clinicopathologic
characteristics was analyzed using the Kruskal-Wallis test and
Chi-square test. The post hoc Dunn's test of multiple comparisons
was used after the Kruskal-Wallis test to examine the sample
contrasts between individual sample pairs. Besides, the correlation
between ATP2A2 expression and IDH1 mutation in secondary
glioblastoma and AA developed from DA was investigated by the
McNemar test. The difference of ATP2A2 expression in
oligodendrogliomas with IDH1 mutation and PAs without IDH1 mutation
was investigated by Chi-square test. Survival was defined from the
date of surgical diagnosis to death from any cause. Correlation of
ATP2A2 expression with survival was determined using the
Kaplan-Meier product-limit method, and differences between survival
curves were tested using the log-rank test. For other experiments,
differences between groups were measured by Student's t-test, and
for comparing means of more than 2 groups, one-way ANOVA was used.
P-values <0.05 were considered as statistically significant.
Results
ATP2A2 is variably expressed in
astrocytoma tissues and correlated with astrocytoma grade
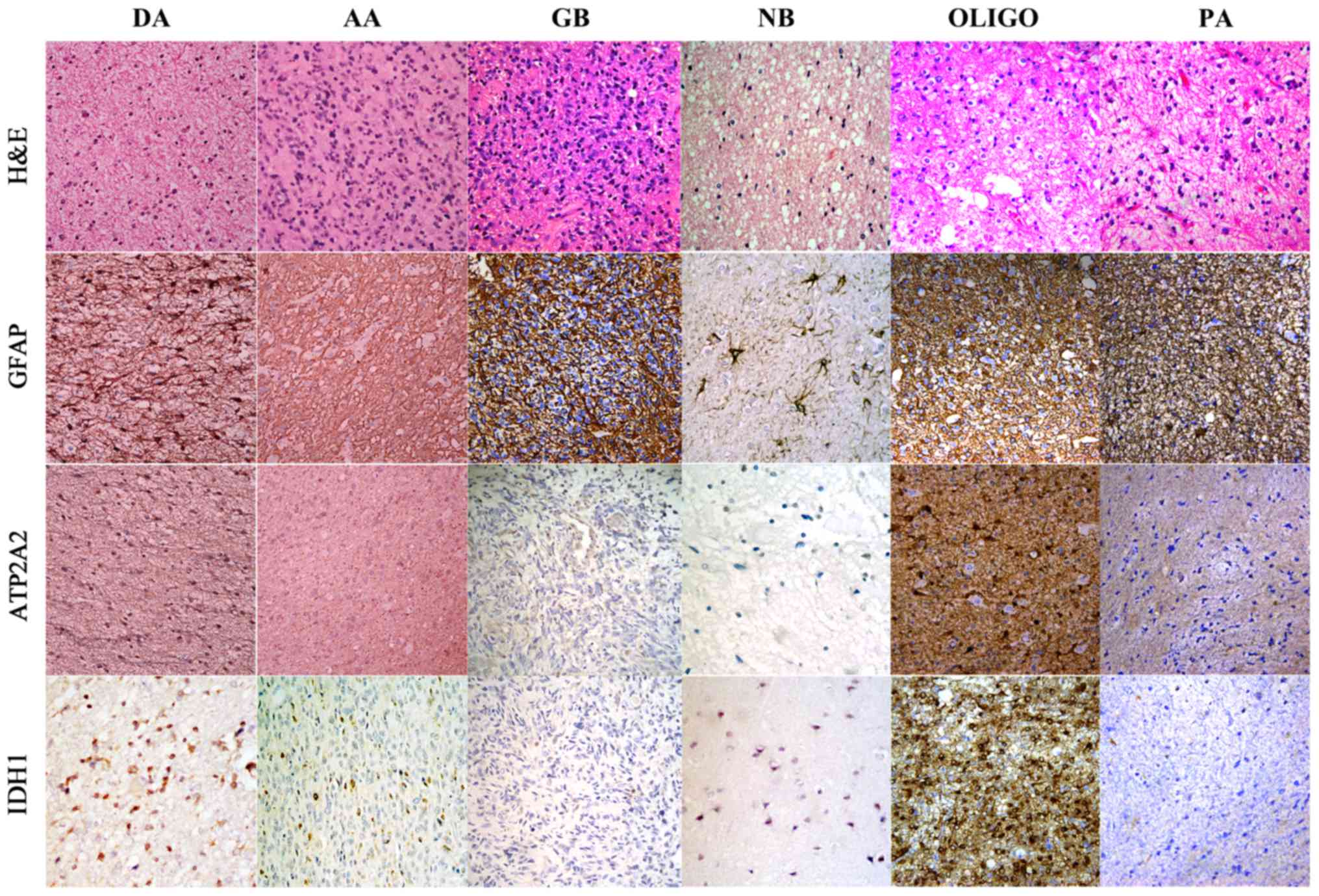
We found weak staining of ATP2A2 in some of the
neurons but not in the astrocytes of normal brains (Fig. 1). ATP2A2 was not expressed in 26.6%
(29/109) of the patients, mildly positive in 25.7% (28/109),
moderately positive in 23.9% (26/109) and strongly positive in
23.9% (26/109) of the patients (Table
II). It was further examined whether ATP2A2 expression was
associated with astrocytoma grades. ATP2A2 was positive in 76.9%
(30/39) of grade II patients, 94.7% (18/19) of grade III patients
and 62.8% (32/51) of grade IV patients (P=0.036, Table II). Moreover, 35.3% (18/51) of
grade IV astrocytoma patients showed high positive staining of
ATP2A2, which was significantly lower than that of grade II (61.5%,
24/39) and grade III (52.6%, 10/19) astrocytoma patients (P=0.042,
Table III). The staining pattern
of ATP2A2 in different grades of astrocytomas is illustrated in
Fig. 1. As shown in Table IV, statistical analysis revealed a
close correlation between ATP2A2 expression and WHO grade of
astrocytomas (P=0.025). Furthermore, the results of the post hoc
Dunn's test used after the Kruskal-Wallis test showed that the
difference of ATP2A2 expression rate in grade IV and grade II was
significant (P=0.015). However, there was no significant difference
in the ATP2A2 expression between grade II glioma and grade III
glioma (P=0.765), and grade III glioma and grade IV glioma
(P=0.105). The above findings indicated that, despite variable
expression of ATP2A2 in astrocytoma tissues, the most aggressive
form of astrocytomas, GBM, exhibits noticeably lower expression of
ATP2A2 compared to astrocytoma of lower grades. In addition, high
ATP2A2 expression level is also seen in the patients with the
secondary glioblastoma and AAs developed from DAs (56.0%, 14/25),
which is similar to the relationship between IDH1 mutation and the
secondary glioblastoma and AAs developed from DAs (44.0%, 11/25)
(P=0.508). To further illustrate the correlation of high ATP2A2
expression and IDH1 mutation, ATP2A2 expression was also examined
in oligodendrogiomas with IDH1 mutation and PAs without IDH1
mutation. The result supported that high ATP2A2 was more frequently
observed in astrocytomas with IDH1 mutation (15/20) than in those
without IDH1 mutation (3/20) (Chi-square test, P<0.001)
(Fig. 1).
 | Table II.ATP2A2 immunoexpression based on
staining intensity scores in diffuse astrocytic tumor according to
tumor grade (n=109). |
Table II.
ATP2A2 immunoexpression based on
staining intensity scores in diffuse astrocytic tumor according to
tumor grade (n=109).
|
| Staining intensity
scores of ATP2A2 |
|---|
|
|
|
|---|
|
| -(n=32) | + (n=30) | ++ (n=32) | +++ (n=30) | Total | P-value |
|---|
| WHO grade |
|
|
|
|
| 0.036 |
| II | 9 | 6 | 10 | 14 | 39 |
|
|
III | 1 | 8 | 7 | 3 | 19 |
|
| IV | 19 | 14 | 9 | 9 | 51 |
|
 | Table III.ATP2A2 immunoexpression in diffuse
astrocytic tumor according to tumor grade (n=109). |
Table III.
ATP2A2 immunoexpression in diffuse
astrocytic tumor according to tumor grade (n=109).
|
| Low ATP2A2
expression | High ATP2A2
expression | Total | P-value |
|---|
| WHO grade |
|
|
| 0.042 |
| II | 15 | 24 | 39 |
|
III | 9 | 10 | 19 |
| IV | 33 | 18 | 51 |
 | Table IV.ATP2A2 immunoexpression in low and
high grade diffuse astrocytic tumor (n=109). |
Table IV.
ATP2A2 immunoexpression in low and
high grade diffuse astrocytic tumor (n=109).
|
| Low ATP2A2
expression | High ATP2A2
expression | Total | P-value |
|---|
| Glioma grade |
|
|
| 0.025 |
|
Low | 15 | 24 | 39 |
|
|
High | 42 | 28 | 70 |
|
ATP2A2 expression correlates with
survival of astrocytoma patients
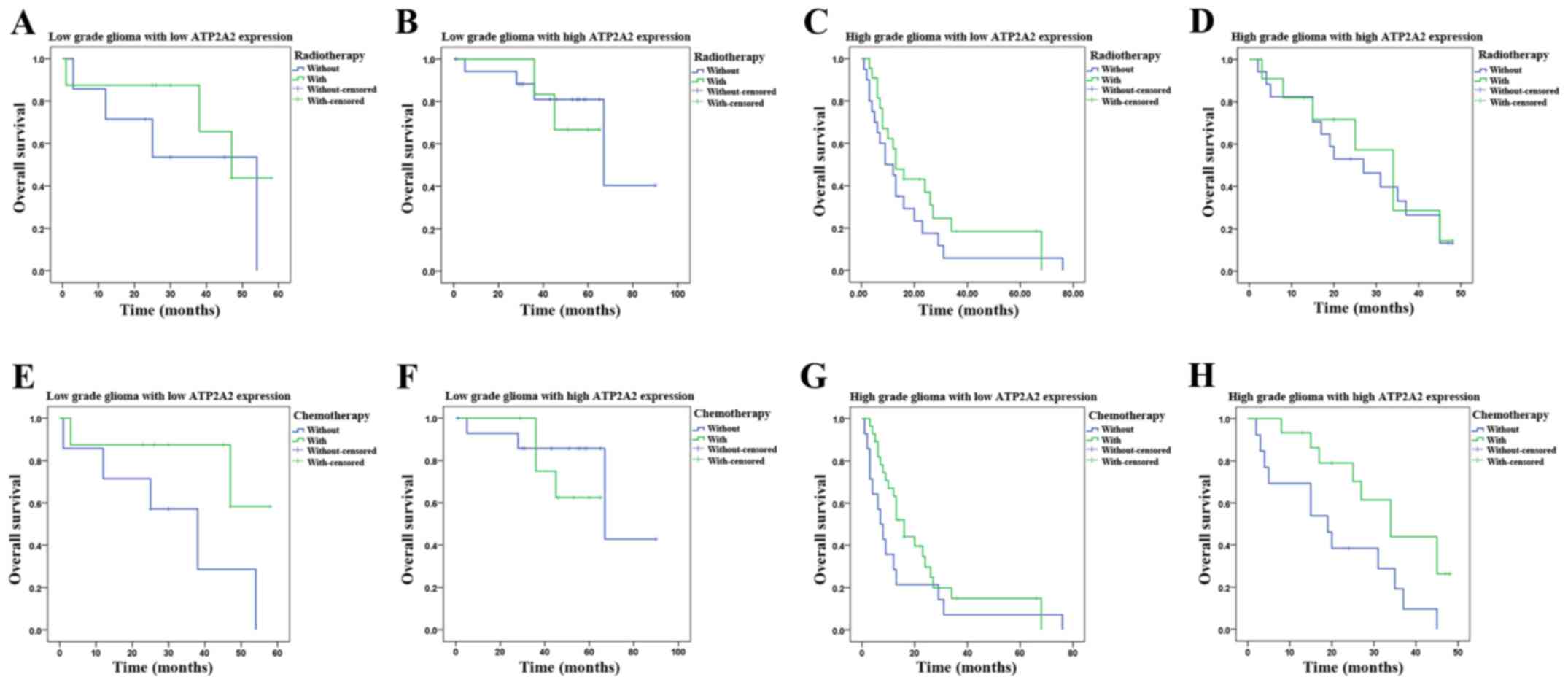
The Kaplan-Meier distribution of survival is shown
in Fig. 2A. The median survival was
45.0±5.3 (95% CI, 34.7–55.3) months in patients with high ATP2A2
expression and 16.0±5.0 (95% CI, 6.3–25.7) months in patients with
low ATP2A2 expression (P<0.0001). There was a significant
correlation between ATP2A2 expression and survival. We further
analyzed the survival of high grade astrocytoma patients stratified
by ATP2A2 expression. High grade astrocytoma patients with high
ATP2A2 expression showed markedly longer survival (median, 31.0±4.9
months; 95% CI, 21.4–40.7) than those with low ATP2A2 expression
(median, 13.0±1.6 months; 95% CI, 9.9–16.1) (P=0.027, Fig. 2B). Low grade astrocytoma patients
with lower ATP2A2 expression showed significantly shorter survival
(47.0±7.2 months, 95% CI, 32.8–61.2) than those with higher ATP2A2
expression (67.0±15.8 months, 95% CI, 36.0–98.0) (P=0.039, Fig. 2C). Besides, we examined whether the
survival time was influenced by postoperative adjuvant therapy
(radiotherapy and chemotherapy) in astrocytoma patients of
different grades with different ATP2A2 expression levels (Fig.3). As shown in Fig. 3H, only in high grade astrocytoma
patients with high ATP2A2 levels, those who received chemotherapy
showed significantly longer survival (34±5.7 months, 95% CI,
22.7–45.3) than those who did not (19±6.7 months, 95% CI, 5.8–32.2)
(P=0.021).
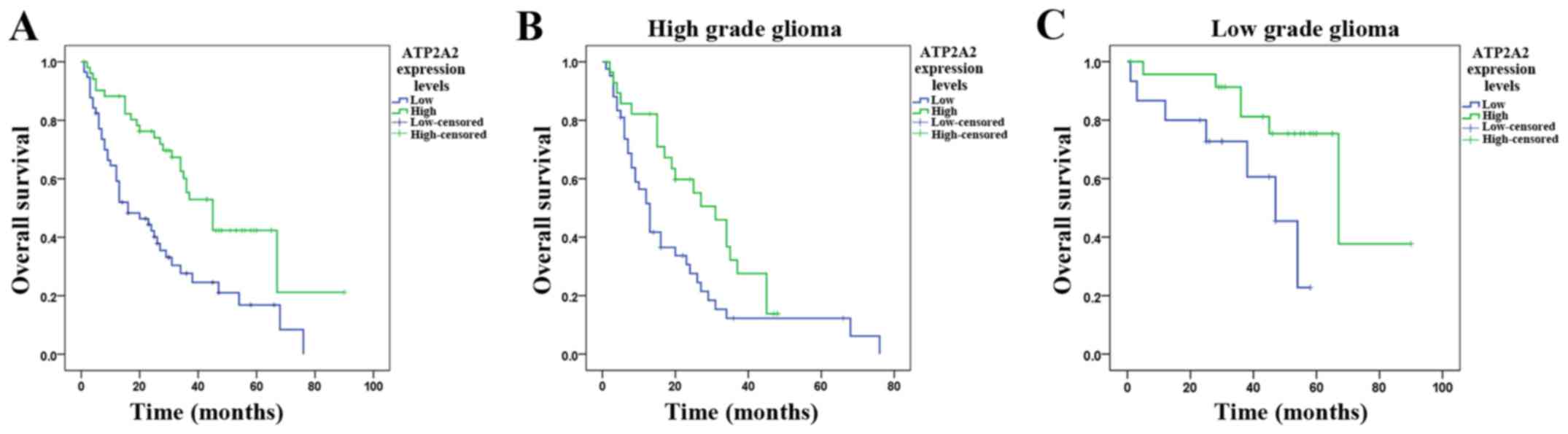 | Figure 2.Kaplan-Meier postoperative survival
curve for astrocytoma patients stratified by ATP2A2 expression. Low
and high ATP2A2 expression is defined in Materials and methods. (A)
The median overall survival of astrocytoma patients with high and
low ATP2A2 expression is 45±5.3 (95% CI, 34.7–55.3) and 16±5.0 (95%
CI, 6.3–25.7), respectively (log-rank test, P<0.0001). (B) High
grade astrocytoma patients with high ATP2A2 expression show
markedly longer survival (31±4.9 months, CI, 21.4–40.7) than those
with low ATP2A2 expression (13±1.6 months, CI, 9.9–16.1) (P=0.027).
(C) Low grade astrocytoma patients with lower ATP2A2 expression
show significantly shorter survival (47.0±7.2 months, 95% CI,
32.8–61.2) than those with higher ATP2A2 expression (67.0±15.8
months, 95% CI, 36.0–98.0) (P=0.039). |
ATP2A2 overexpression suppresses the
clonogenic growth and migration of GBM cells
The above findings suggest that ATP2A2 may play a
hitherto undiscovered role in the oncogenesis and development of
astrocytomas. To decipher the role of ATP2A2 in astrocytoma
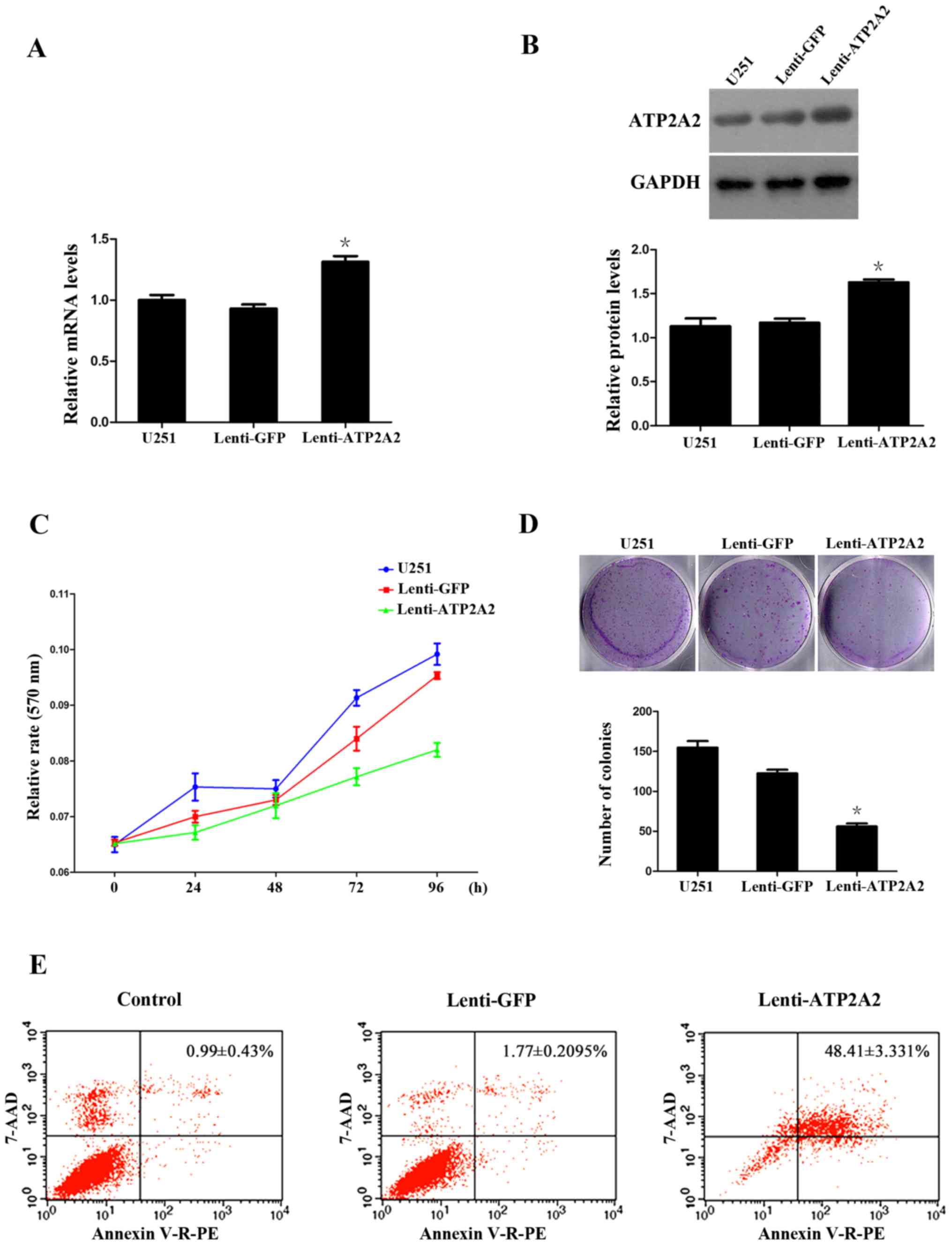
genesis, we infected GBM U251MG cells with lentiviral vectors
overexpressing ATP2A2 (Lenti-ATP2A2), which resulted in a 132%
increase in the mRNA transcript levels and 163% increase in the
protein levels of ATP2A2 (Fig. 4A and
B). The MTT assays showed that ATP2A2 overexpression
significantly suppressed the growth of U251MG cells 72 and 96 h
post transfection (P<0.05 or <0.001), with a 15.2±0.7%
reduction in the growth of U251MG cells at 96 h (Fig. 4C). Additionally, the clonogenic
assays showed that ATP2A2 overexpression caused a 54.2±1.9%
decrease in the number of colonies (Fig. 4D) [P=0.0004 vs. Lenti-green
fluorescent protein (Lenti-GFP)]. We further investigated whether
the growth-inhibitory effect of ATP2A2 was associated with
induction of apoptosis of GBM cells. Our flow cytometric analysis
revealed that ATP2A2 overexpression was associated with a
significant increase in the percentage of apoptotic U251MG cells
(Lenti-ATP2A2, 48.4±3.3% vs. Lenti-GFP, 1.7±0.2%, P=0.0008)
(Fig. 4E).
Moreover, we sought to investigate whether ATP2A2
impacted on the migration of GBM cells. Our wound healing assays
revealed that ATP2A2 overexpression markedly suppressed the
migration of U251MG cells (Fig.
5).
ATP2A2 overexpression inhibits GBM
xenograft growth in mice
It was examined whether ATP2A2 overexpression
suppressed the growth of GBM xenografts in mice. We found that,
compared with control xenografts expressing GFP, tumors stably
overexpressing ATP2A2 were markedly smaller in size 3 weeks after
xenograft implantation (Fig. 6).
The mean tumor volume was 81.6±21.5 mm3 in xenografts
overexpressing ATP2A2 and 238.2±65.2 cm3 in the control
xenografts respectively at week 4 post inoculation (P<0.05).
Discussion
Comprehensive elucidation of genetic alterations in
astrocytomas may provide novel therapeutic targets, and diagnostic
as well as prognostic markers for the disease. Furthermore,
detailed delineation of prognostic markers for astrocytomas,
especially GBMs, may lead to the identification of subsets of
astrocytoma patients who may have better prognosis in response to
adjuvant or neoadjuvant chemotherapies or targeted therapies. Here,
we characterized the expression of ATP2A2 in astrocytoma tissues
and documented for the first time that ATP2A2 showed altered
expression in astrocytoma tissues. We found that ATP2A2 was
expressed in the majority (73.4%, 80/109) of the patients and more
than half (62.8%, 32/51) of grade IV astrocytoma patients expressed
ATP2A2. Two thirds (61.5%, 24/39) of grade II astrocytoma patients
exhibited high ATP2A2 expression while only approximately one third
(35.3%, 18/51) of grade IV astrocytoma patients showed high ATP2A2
expression. Our statistical analysis further revealed a close
correlation between ATP2A2 expression and WHO grade of
astrocytomas. These findings implicate ATP2A2 participates in the
oncogenesis in astrocytomas.
ATP2A2 acts as a critical component of several
signaling pathways that have been demonstrated to be aberrant in
astrocytoma. Genetic alterations occur frequently in the
RTK/RAS/PI-3K signaling pathway, the p53 signaling pathway and the
RB signaling pathway (17). RAS
signaling and calcium signaling converge at Raf1 (18) while calcium plays a pivotal role in
p53 signaling (19) and
Ca2+ and CaM-dependent signaling is required for RB
phosphorylation (20,21). ATP2A2 expression is downregulated in
cancer of the lung and head and neck squamous cell cancer, but
upregulated in the colorectal cancer, liposarcoma and prostate
cancer (15,22–25).
Our demonstration of aberrant expression of ATP2A2 in astrocytoma
tissues further adds ATP2A2 in a growing list of malignant cancers
in humans. Besides, high ATP2A2 expression was associated with
better overall survival of astrocytoma patients. Our study also
evaluated whether ATP2A2 could be used as a prognostic marker for
identifying a subset of astrocytoma patients responding
preferentially to particular therapies. The result indicates that
high grade astrocytoma patients with high ATP2A2 expression level
are more sensitive to the postoperative chemotherapy. Another
substantial result of our study is that high ATP2A2 expression
shared a similar distribution pattern with IDH1 mutation in
secondary GBM, AA developed from DA, oligodendroglioma and PA,
suggesting that ATP2A2 may possess a similar prognostic value as
IDH1 in astrocytoma (26). We
hypothesized that the alteration of energy metabolism due to IDH1
mutation (27) may affect the
expression and function of ATP2A2. The aforementioned outcomes are
consistent with findings from previous studies on the role of
ATP2A2 in other tumor types (22,23),
implying that ATP2A2 expression may be a potential molecular marker
for predicting the prognosis of astrocytoma patients.
In this study, we provide further evidence that
increased ATP2A2 expression inhibited the clonogenic growth of GBM
cells in vitro, probably via induction of apoptosis.
Additionally, we observed that ATP2A2 overexpression depressed the
migration of GBM cells in an in vitro wound healing assay.
Moreover, tumor xenograft growth was noticeably suppressed by
intratumoral injection of lentiviral vectors overexpressing ATP2A2.
This may partially explain the survival benefit of high ATP2A2
expression in astrocytoma patients. However, it still needs to be
further validated by additional studies.
Sarco (endo)plasmic reticulum (SER) Ca2+
ATPases are a highly conserved family of Ca2+ pumps.
SERCA2 is implicated in certain cancers. Another isoform, SERCA3,
encoded by the ATP2A3 gene, has been shown to be
downregulated in colon cancer, lung adenocarcinoma and head and
neck squamous cell cancer (11,28,29).
Both proteins are involved in maintaining Ca2+
homeostasis, which is critical to the normal functioning of a
plethora of cellular processes ranging from cellular proliferation
to apoptosis by modulating cellular signaling pathways (30), and the aberration of some of these
Ca2+-mediated signaling pathways is implicated in
tumorigenesis and tumor progression, such as metastasis, invasion
and angiogenesis (31). ER
Ca2+ levels remain undefined in astrocytoma cells.
Astrocytoma cells are known to suffer from low grade of ER stress
(32). Agents affecting ER
Ca2+ homeostasis such as flavonoids could activate ER
stress and induce cell death in glioma cells (33). Kovacs et al (34) found that compared with normal
astrocytes, GBM cells exhibit higher resting cytosolic
Ca2+ levels. It remains to be elucidated how ATP2A2
induces apoptosis of GBM cells in terms of ER stress. We speculate
that in astrocytoma, alteration in ATP2A2 expression may contribute
to tumorigenesis by interfering with the balance between cytosolic
and ER Ca2+ level, thereby affecting certain
Ca2+ signaling pathways, especially the RTK/RAS/PI-3K
signaling, p53 signaling and RB signaling pathways (17). However, the exact mechanisms whereby
ATP2A2 is involved in oncogenesis and development of astrocytomas
require further investigation. In some other studies, the
intervention of ATP2A2 expression, either upregulating or
inhibiting, could exert influence on the progression of various
malignancies (14,35), indicating that ATP2A2 may also
function as a therapeutic target for astrocytoma treatment.
In conclusion, we present here direct clinical
evidence that ATP2A2 is variably expressed in astrocytoma tissues
and its expression correlates with tumor grade, and secondary
glioblastoma and AA developed from DA has a propensity to
overexpress ATP2A2. Importantly, patients with high ATP2A2
expression show better overall survival, thus identifying a subset
of astrocytoma patients with differing prognosis. Furthermore,
ATP2A2 overexpression suppresses growth of astrocytoma cells.
Although the precise correlation of the altered ATP2A2 expression
with astrocytoma grade, molecular subtypes or clinical parameters
and the underlying mechanism requires further investigation, our
findings implicate ATP2A2 in gliomagenesis and suggest that ATP2A2
may serve as a prognostic marker identifying astrocytoma patients
with differing prognoses.
Acknowledgements
This study was supported by grants from the Shanghai
Municipal Science and Technology Commission (no. 10ZR1439000 to
Yi-Ming Li), the National Natural Sciences Fund Project of China
(NSFC no. 81101656/H1609 to Yi-Ming Li, NSFC no. 81201987/H1618 to
Wei-Qing Li and NSFC no. 30930094 to Yi-Cheng Lu).
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parisot S, Darlix A, Baumann C, Zouaoui S,
Yordanova Y, Blonski M, Rigau V, Chemouny S, Taillandier L, Bauchet
L, et al: A probabilistic atlas of diffuse WHO grade II glioma
locations in the brain. PLoS One. 11:e01442002016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dubrow R, Darefsky AS, Jacobs DI, Park LS,
Rose MG, Laurans MS and King JT Jr: Time trends in glioblastoma
multiforme survival: The role of temozolomide. Neuro-oncol.
15:1750–1761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mesti T and Ocvirk J: Malignant gliomas:
Old and new systemic treatment approaches. Radiol Oncol.
50:129–138. 2016.PubMed/NCBI
|
|
5
|
Watanabe T, Nobusawa S, Kleihues P and
Ohgaki H: IDH1 mutations are early events in the development of
astrocytomas and oligodendrogliomas. Am J Pathol. 174:1149–1153.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chow LM, Endersby R, Zhu X, Rankin S, Qu
C, Zhang J, Broniscer A, Ellison DW and Baker SJ: Cooperativity
within and among Pten, p53, and Rb pathways induces high-grade
astrocytoma in adult brain. Cancer Cell. 19:305–316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hofer S, Rushing E, Preusser M and Marosi
C: Molecular biology of high-grade gliomas: What should the
clinician know? Chin J Cancer. 33:4–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bchetnia M, Benmously R, Ben Brick AS,
Charfeddine C, Ben Ameur Y, Fajraoui M, Debbiche A, Ben Ayed M,
Mokni M, Fenniche S, et al: New mutations of Darier disease in
Tunisian patients. Arch Dermatol Res. 301:615–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi BJ, Feng J, Ma CC, Yan XN, Li WB, Wei
YP, Hu G and Wang XL: Novel mutations of the ATP2A2 gene in two
families with Darier's disease. Arch Dermatol Res. 301:27–30. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Papp B, Brouland JP, Gélébart P, Kovàcs T
and Chomienne C: Endoplasmic reticulum calcium transport ATPase
expression during differentiation of colon cancer and leukaemia
cells. Biochem Biophys Res Commun. 322:1223–1236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Y, Palad AJ, Wasilenko WJ, Blackmore
PF, Pincus WA, Schechter GL, Spoonster JR, Kohn EC and Somers KD:
Inhibition of head and neck squamous cell carcinoma growth and
invasion by the calcium influx inhibitor carboxyamido-triazole.
Clin Cancer Res. 3:1915–1921. 1997.PubMed/NCBI
|
|
12
|
Baron S, Vangheluwe P, Sepúlveda MR,
Wuytack F, Raeymaekers L and Vanoevelen J: The secretory pathway Ca
(2+)-ATPase 1 is associated with cholesterol-rich microdomains of
human colon adenocarcinoma cells. Biochim Biophys Acta.
1798:1512–1521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsui K, Makino T, Nakano H, Furuichi M,
Sawamura D and Shimizu T: Squamous cell carcinoma arising from
Darier's disease. Clin Exp Dermatol. 34:e1015–e1016. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bleeker NP, Cornea RL, Thomas DD and Xing
C: A novel SERCA inhibitor demonstrates synergy with classic SERCA
inhibitors and targets multidrug-resistant AML. Mol Pharm.
10:4358–4366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Li W, Yang Y, Hu Y, Gu Y, Shu Y,
Sun Y, Wu X, Shen Y and Xu Q: High expression of
sarcoplasmic/endoplasmic reticulum Ca (2+)-ATPase 2b blocks cell
differentiation in human liposarcoma cells. Life Sci. 99:37–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prasad V, Boivin GP, Miller ML, Liu LH,
Erwin CR, Warner BW and Shull GE: Haploinsufficiency of Atp2a2,
encoding the sarco (endo)plasmic reticulum Ca2+-ATPase
isoform 2 Ca2+ pump, predisposes mice to squamous cell
tumors via a novel mode of cancer susceptibility. Cancer Res.
65:8655–8661. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cancer Genome Atlas Research Network:
Comprehensive genomic characterization defines human glioblastoma
genes and core pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshiki S, Matsunaga-Udagawa R, Aoki K,
Kamioka Y, Kiyokawa E and Matsuda M: Ras and calcium signaling
pathways converge at Raf1 via the Shoc2 scaffold protein. Mol Biol
Cell. 21:1088–1096. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sorrentino G, Comel A and Del Sal G: p53
orchestrates calcium signaling in vivo. Cell Cycle. 14:1343–1344.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takuwa N, Zhou W, Kumada M and Takuwa Y:
Ca (2+)-dependent stimulation of retinoblastoma gene product
phosphorylation and p34cdc2 kinase activation in serum-stimulated
human fibroblasts. J Biol Chem. 268:138–145. 1993.PubMed/NCBI
|
|
21
|
Rasmussen CD and Means AR: Calmodulin is
required for cell-cycle progression during G1 and mitosis. EMBO J.
8:73–82. 1989.PubMed/NCBI
|
|
22
|
Korosec B, Glavac D, Rott T and
Ravnik-Glavac M: Alterations in the ATP2A2 gene in correlation with
colon and lung cancer. Cancer Genet Cytogenet. 171:105–111. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Korosec B, Glavac D, Volavsek M and
Ravnik-Glavac M: Alterations in genes encoding
sarcoplasmic-endoplasmic reticulum Ca (2+) pumps in association
with head and neck squamous cell carcinoma. Cancer Genet Cytogenet.
181:112–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang MY, Wang HM, Tok TS, Chang HJ, Chang
MS, Cheng TL, Wang JY and Lin SR: EVI2B, ATP2A2, S100B, TM4SF3, and
OLFM4 as potential prognostic markers for postoperative Taiwanese
colorectal cancer patients. DNA Cell Biol. 31:625–635. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Legrand G, Humez S, Slomianny C, Dewailly
E, Vanden Abeele F, Mariot P, Wuytack F and Prevarskaya N:
Ca2+ pools and cell growth. Evidence for
sarcoendoplasmic Ca2+-ATPases 2B involvement in human
prostate cancer cell growth control. J Biol Chem. 276:47608–47614.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karsy M, Neil JA, Guan J, Mahan MA, Colman
H and Jensen RL: A practical review of prognostic correlations of
molecular biomarkers in glioblastoma. Neurosurg Focus. 38:E42015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reitman ZJ, Duncan CG, Poteet E, Winters
A, Yan LJ, Gooden DM, Spasojevic I, Boros LG, Yang SH and Yan H:
Cancer-associated isocitrate dehydrogenase 1 (IDH1) R132H mutation
and d-2-hydroxyglutarate stimulate glutamine metabolism under
hypoxia. J Biol Chem. 289:23318–23328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flores-Peredo L, Rodríguez G and
Zarain-Herzberg A: Induction of cell differentiation activates
transcription of the Sarco/Endoplasmic Reticulum calcium-ATPase 3
gene (ATP2A3) in gastric and colon cancer cells. Mol Carcinog.
56:735–750. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arbabian A, Brouland JP, Apáti Á, Pászty
K, Hegedűs L, Enyedi Á, Chomienne C and Papp B: Modulation of
endoplasmic reticulum calcium pump expression during lung cancer
cell differentiation. FEBS J. 280:5408–5418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berridge MJ, Bootman MD and Roderick HL:
Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev
Mol Cell Biol. 4:517–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monteith GR, McAndrew D, Faddy HM and
Roberts-Thomson SJ: Calcium and cancer: Targeting Ca2+
transport. Nat Rev Cancer. 7:519–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Johnson GG, White MC and Grimaldi M:
Stressed to death: Targeting endoplasmic reticulum stress response
induced apoptosis in gliomas. Curr Pharm Des. 17:284–292. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Das A, Banik NL and Ray SK: Flavonoids
activated caspases for apoptosis in human glioblastoma T98G and
U87MG cells but not in human normal astrocytes. Cancer.
116:164–176. 2010.PubMed/NCBI
|
|
34
|
Kovacs GG, Zsembery A, Anderson SJ,
Komlosi P, Gillespie GY, Bell PD, Benos DJ and Fuller CM: Changes
in intracellular Ca2+ and pH in response to thapsigargin
in human glioblastoma cells and normal astrocytes. Am J Physiol
Cell Physiol. 289:C361–C371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seo JA, Kim B, Dhanasekaran DN, Tsang BK
and Song YS: Curcumin induces apoptosis by inhibiting
sarco/endoplasmic reticulum Ca2+ ATPase activity in
ovarian cancer cells. Cancer Lett. 371:30–37. 2016. View Article : Google Scholar : PubMed/NCBI
|