Introduction
Head and neck cancer is the sixth most common
carcinoma worldwide (1). Head and
neck cancer accounts for approximately 3.6% of new cancer cases in
the United States of America, and an estimated 59,340 new diagnoses
and 12,290 deaths occurred in 2015 (1). More than 90% of head and neck cancers
are squamous cell carcinomas (2).
Current standard treatments for head and neck squamous cell
carcinoma (HNSCC) are multimodal, including surgery, radiotherapy
and chemotherapy. Despite advances in multimodality therapies,
long-term survival rates remain poor, between 40–50%, and further
improvements in therapeutic strategies are needed, particularly in
individuals with advanced-stage cancer (1–5). Thus,
new strategies, such as molecular targeted therapies, are needed.
Moreover, the discovery of molecular targets having synergistic
effects with conventional chemotherapy is necessary because various
oncogenes and underlying signaling pathways may be involved in
cancer progression and treatment resistance of cancer cells.
Inhibition of apoptosis is a crucial mechanism
involved in tumorigenesis and confers cancer cells with
chemoresistance (6). Inhibitor of
apoptosis proteins (IAPs) comprise a group of structurally related
proteins with anti-apoptotic potential (7,8). Livin
is an important member of the human IAP family (9). Several studies have suggested that
overexpression of Livin in neoplasms correlates with more
aggressive behavior such as shorter disease-free survival, shorter
overall survival and chemoresistance (10,11).
Furthermore, Livin has been shown to be highly expressed in various
human cancer tissues, including melanoma, breast, colon, prostate
cancer and hepatoma (10–12). Therefore, Livin has become the focus
of increased research in recent years; however, little is known
about the role of Livin in human HNSCC. In our previous studies, we
reported that Livin is also associated with invasive and oncogenic
phenotypes in human HNSCC (13–15).
The responsiveness of HNSCC to chemotherapy affects
prognosis. Cisplatin-based concurrent chemoradiotherapy (CRT) has
become a popular treatment that enables organ preservation in
locally advanced HNSCC (16). A
regimen of cisplatin, 5-fluorouracil (FU) and docetaxel has been
established as the standard induction chemotherapy regimen for
locally advanced HNSCC on the basis of large randomized phase III
trials (17–19). Failure of chemotherapy resulting
from drug resistance remains a challenging problem for treatment in
patients with HNSCC. The role and mechanisms of Livin in
chemoresistance in HNSCC have not been elucidated. Therefore, in
the present study, we investigated the role of Livin in determining
susceptibility to commonly used chemotherapeutic drugs, such as
docetaxel, cisplatin and 5-FU, in human HNSCC cell lines. Although
several studies have described that Livin contribute to the
resistance of various chemotherapeutic drugs including cisplatin in
various cancers (20–34), the present study is the first to
demonstrate the correlation between Livin and chemoresistance in
human HNSCC.
Materials and methods
Cell culture and transfection
The human HNSCC cell lines SNU1041, PCI1 and PCI50
were kindly provided by M.W. Sung (Seoul National University,
Seoul, South Korea). Cells were cultured in RPMI-1640 (Invitrogen,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
HyClone Laboratories, Inc., Logan, UT, USA), 50 U/ml penicillin and
50 µg/ml streptomycin (Gibco, Grand Island, NY, USA) at 37°C in a
humidified atmosphere containing 5% CO2.
Small-interfering RNA (siRNA) was used to knockdown endogenous
Livin expression in cells. Cells were transfected for 48 h with
Livin-specific siRNA (Bioneer Corp., Daejeon, Korea) or negative
control siRNA (Qiagen Sciences, Inc., Germantown, MD, USA) using
Lipofectamine 2000 (Invitrogen).
RNA isolation and reverse
transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen), reverse transcribed and amplified using
specific primers for Livin and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH). Primer sequences were as follows:
5′-CACACAGGCCATCAGGACAAG-3′ and 5′-ACGGCACAAAGACGATGGAC-3′ (Livin α
and Livin β); and 5′-ACCACAGTCCATGCCATCAC-3′,
5′-TCCACCACCCTGTTGCTGTA-3′ (GAPDH). PCR products were separated by
electrophoresis on a 1% agarose gels containing ethidium bromide.
The signals were quantified by densitometric analysis using
LabWorks Image Acquisition software (UVP, LLC, Upland, CA,
USA).
Protein isolation and western blot
analysis
Cells were lysed in RIPA buffer. Resolved proteins
were electrophoretically transferred to polyvinylidene fluoride
(PVDF) membranes. Specific proteins were sequentially blotted with
primary antibodies against Livin, cleaved caspase-3, cleaved
caspase-7, cleaved poly(ADP-ribose)polymerase (PARP), cleaved PARP,
and the X-linked inhibitor of apoptosis protein (XIAP), purchased
from Cell Signaling Technology (Danvers, MA, USA) and against GAPDH
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight.
The primary antibody against Livin detected Livin α (36 kDa) and
Livin β (34 kDa). Immunoreactive proteins were visualized using an
enhanced chemiluminescence detection system with a horseradish
peroxidase (HRP) substrate (Millipore, Billerica, MA, USA) and were
analyzed using a LAS-4000 luminescent image analyzer (Fujifilm,
Tokyo, Japan).
Chemotherapeutic drug treatment
Cell were treated with different concentrations of
cisplatin (Dong-A ST, Co., Ltd., Seoul, Korea), 5-FU (JW
Pharmaceutical, Seoul, Korea), or docetaxel (Boryung
Pharmaceutical, Co., Ltd., Seoul, Korea) for 24 h at 37°C.
Cell viability assay
Cells were seeded in 96-well plates
(5×103 cells/well) and were then transfected the next
day with Livin siRNA or negative control siRNA. After incubation
for 48 h, cell proliferation and viability were measured using an
EZ-Cytox (tetrazolium salts, WST-1) cell viability assay kit (Daeil
Lab Inc., Seoul, Korea). Following addition of WST-1 reagent for
1–2 h at 37°C, absorbance at 460 nm was determined using a
microplate reader (Infinite M200; Tecan, Austria GmbH, Grödig,
Austria) with Magellan V6 data analysis software (Tecan).
Pretreated cells served as the indicator of 100% cell
viability.
Cell apoptosis assay
Apoptosis was determined using Annexin V-FITC
assays. Cells were washed in phosphate-buffered saline (PBS) twice
and resuspended in binding buffer (BD Biosciences, San Diego, CA,
USA). Annexin V-FITC and 7-amino-actinomycin D (7-AAD; BD
Biosciences) were added to the cells, and the cells were then
incubated in the dark for 15 min and resuspended in 400 µl of
binding buffer. Cells were analyzed using a FACSCalibur flow
cytometer (Becton-Dickinson, San Jose, CA, USA). Data analysis was
performed using standard CellQuest software (Becton-Dickinson).
Statistical analysis
All experiments were performed independently at
least three times. Experimental differences between the Livin
knockdown group and control group were tested using Students
t-tests. Statistical Package for the Social Sciences (SPSS) version
20.0 (Microcal Software Inc., Chicago, IL, USA) was used for all
statistical analyses. Differences with P<0.05 were considered
statistically significant.
Ethical considerations
Local research ethics committee approval was
obtained from the Chonnam National University Hwasun Hospital
Institutional Review Board.
Results
Response of HNSCC cell lines to
chemotherapeutic drugs
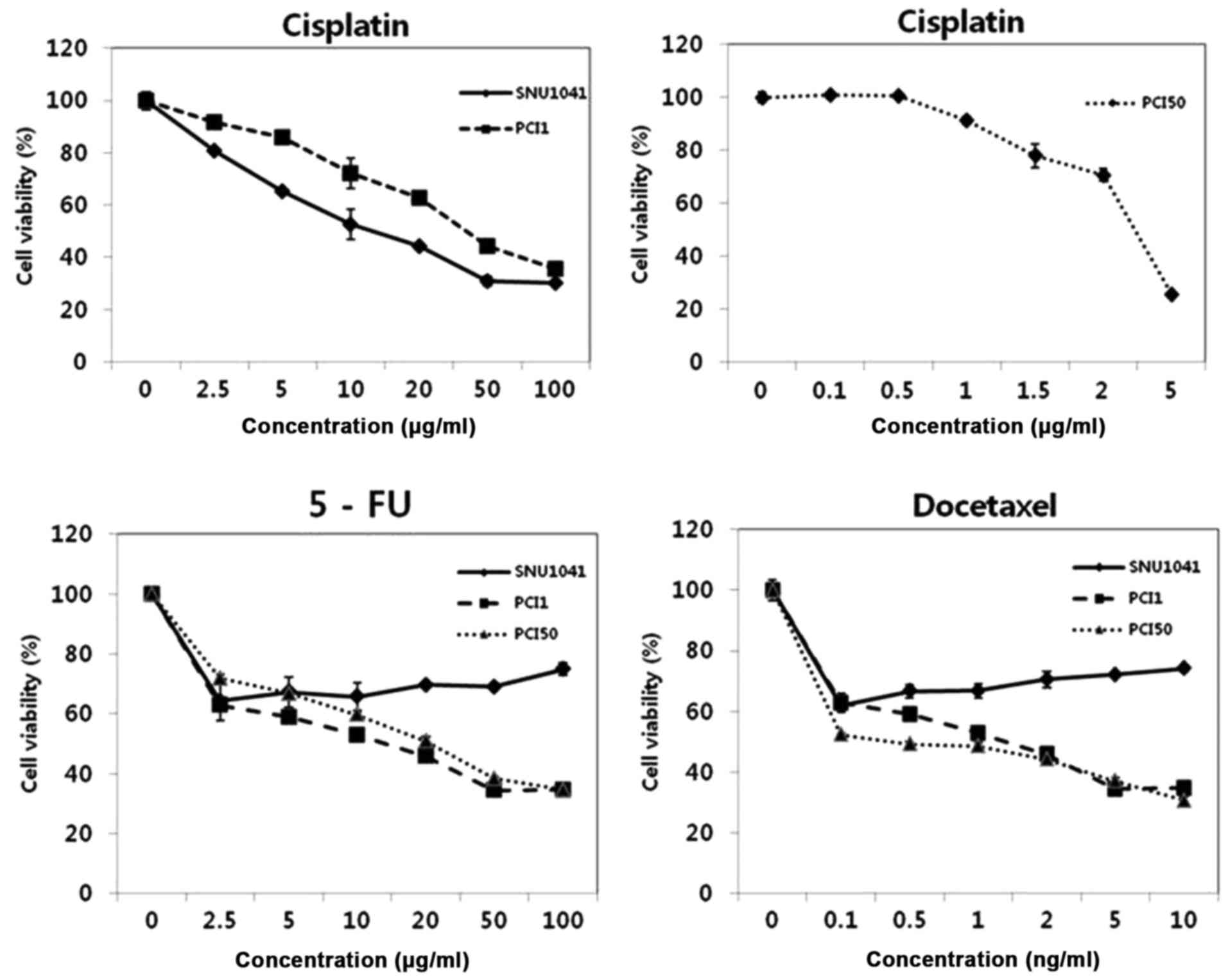
To test the effects of cisplatin, 5-FU and
docetaxel, we treated three HNSCC cell lines, SNU1041, PCI1 and
PCI50, with different concentrations of cisplatin (0.1–100 µg/ml),
5-FU (2.5–100 µg/ml), or docetaxel (0.1–10 ng/ml) for 24 h. Viable
cells were determined by measurement of absorbance, and pretreated
cells served as an indicator of 100% cell viability. Cisplatin
treatment of SNU1041, PCI1 and PCI50 cells resulted in a
significant reduction in cell viability in a
concentration-dependent manner. PCI50 cells were more sensitive to
cisplatin than SNU1041 and PCI1 cells (Fig. 1). 5-FU and docetaxel treatment of
PCI1 and PCI50 cells also resulted in significantly reduced cell
viability in a concentration-dependent manner. However, SNU1041
cells maintained >60% cell viability at high concentrations of
5-FU or docetaxel. Thus, our results showed that SNU1041 cells were
resistant to 5-FU or docetaxel treatment (Fig. 1).
Livin knockdown enhances the
chemosensitivity of human HNSCC cells to cisplatin, 5-FU and
docetaxel
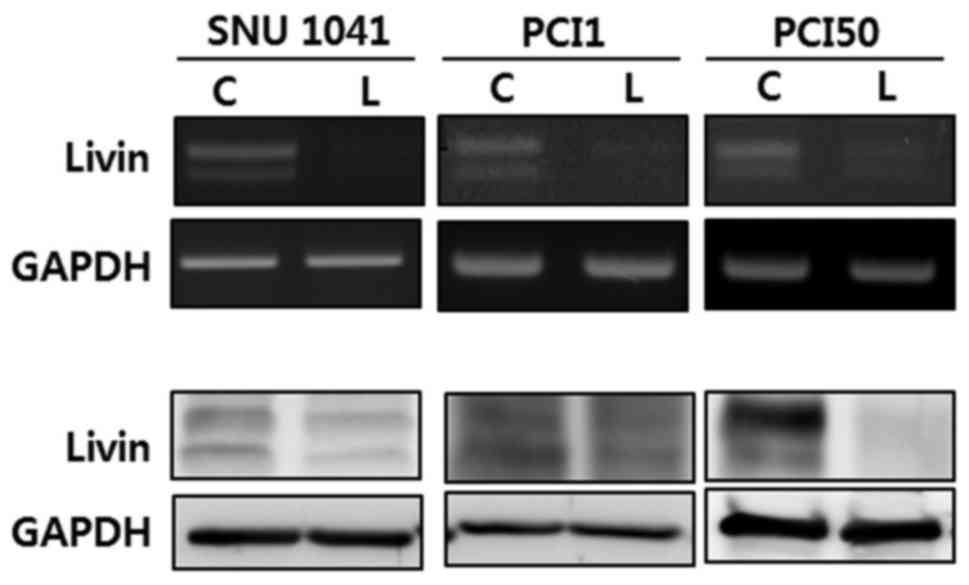
To explore the role of Livin in the chemotherapy
response in HNSCC cells, we used siRNA to inhibit endogenous Livin
expression in human HNSCC cell lines, including SNU1041, PCI1 and
PCI50 cells. Expression of Livin mRNA and protein was reduced by
Livin siRNA in SNU1041, PCI1 and PCI50 cells as compared with that
in cells transfected with negative control siRNA (Fig. 2).
Livin knockdown enhances the
cytotoxicity of cisplatin, 5-FU and docetaxel in human HNSCC
cells
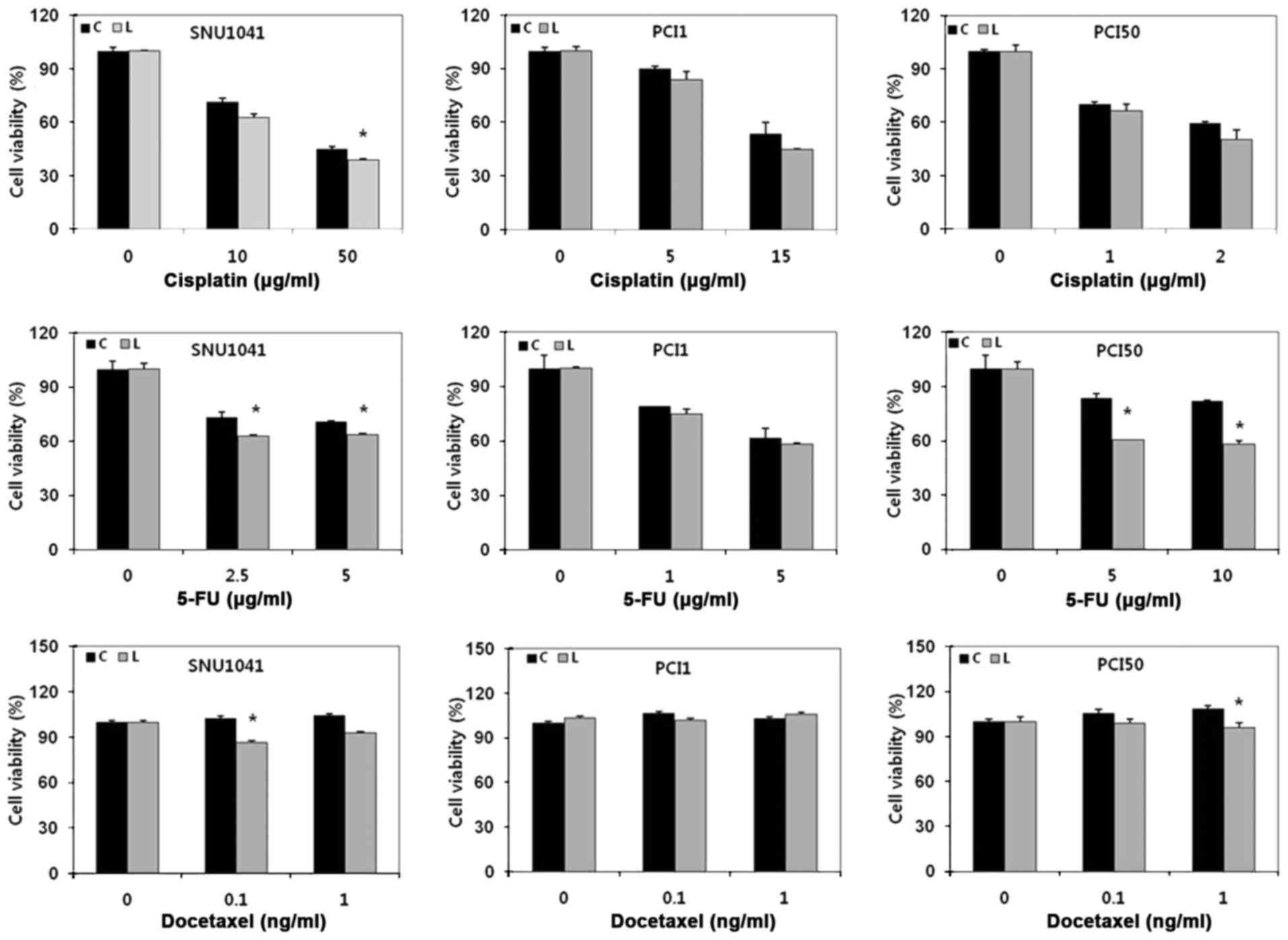
To determine whether Livin knockdown affected the
cytotoxicity of cisplatin, 5-FU and docetaxel in HNSCC cells, cells
were transfected with Livin siRNA or negative control siRNA and
then treated with cisplatin, 5-FU, or docetaxel for 24 h. Because
each HNSCC cell line had different sensitivity to chemotherapeutic
drugs, SNU1041, PCI1 and PCI50 cells were treated with different
concentrations of drugs. Viable cells were determined by
measurement of absorbance, and pretreated cells were used as a
control, indicating 100% cell viability. Cells with Livin knockdown
showed reduced cell survival in response to cisplatin, 5-FU and
docetaxel treatment as compared with that in negative control
cells. Additionally, the viability of Livin-knockdown cells was
significantly lower than that of negative control cells in response
to cisplatin, 5-FU and docetaxel treatment in SNU1041 cells and
5-FU or docetaxel treatment in PCI50 cells (P<0.05; Fig. 3). These results showed that Livin
knockdown enhanced the cytotoxicity of cisplatin, 5-FU and
docetaxel in human HNSCC cells.
Livin knockdown enhances
chemotherapy-induced apoptosis in response to cisplatin, 5-FU and
docetaxel treatment in human HNSCC cells
We addressed whether Livin knockdown enhanced
chemosensitivity by measuring induction of apoptosis in SNU1041,
PCI1 and PCI50 cells. After transfection with Livin siRNA or
negative control siRNA, cells were treated with cisplatin, 5-FU or
docetaxel for 24 h. The combination treatment with Livin siRNA and
chemotherapeutic drugs resulted in a marked increase in apoptosis
as compared with drug treatment alone (Fig. 4). The proportions of early and late
apoptotic cells induced by Livin siRNA transfection plus cisplatin
were greater than those induced by negative control siRNA
transfection plus cisplatin (32.2 vs. 49.9, 53.5 vs. 84.6 and 45.7
vs. 58.1%, respectively) in SNU1041, PCI1 and PCI50 cells (Fig. 4A). The proportions of early and late
apoptotic cells induced by Livin siRNA transfection plus 5-FU were
greater than that induced by negative control siRNA transfection
plus 5-FU (32.4 vs. 55.5, 65.0 vs. 74.4 and 58.1 vs. 78.0%,
respectively) in SNU1041, PCI1 and PCI50 cells (Fig. 4A). The proportions of early and late
apoptotic cells induced by Livin siRNA and docetaxel were greater
than that induced by negative control siRNA transfection plus
docetaxel (40.8 vs. 75.8, 33.3 vs. 58.2 and 44.7 vs. 62.7%,
respectively) in SNU1041, PCI1 and PCI50 cells (Fig. 4A). Consistent with this, Livin
knockdown cells showed greater expression of cleaved caspase-3 and
−7 and PARP compared with that in control cells after cisplatin,
5-FU, or docetaxel treatment (Fig.
4B). These findings suggested that combination therapy with
Livin knockdown plus chemotherapeutic drugs may have synergistic
apoptosis-inducing effects in human HNSCC cells.
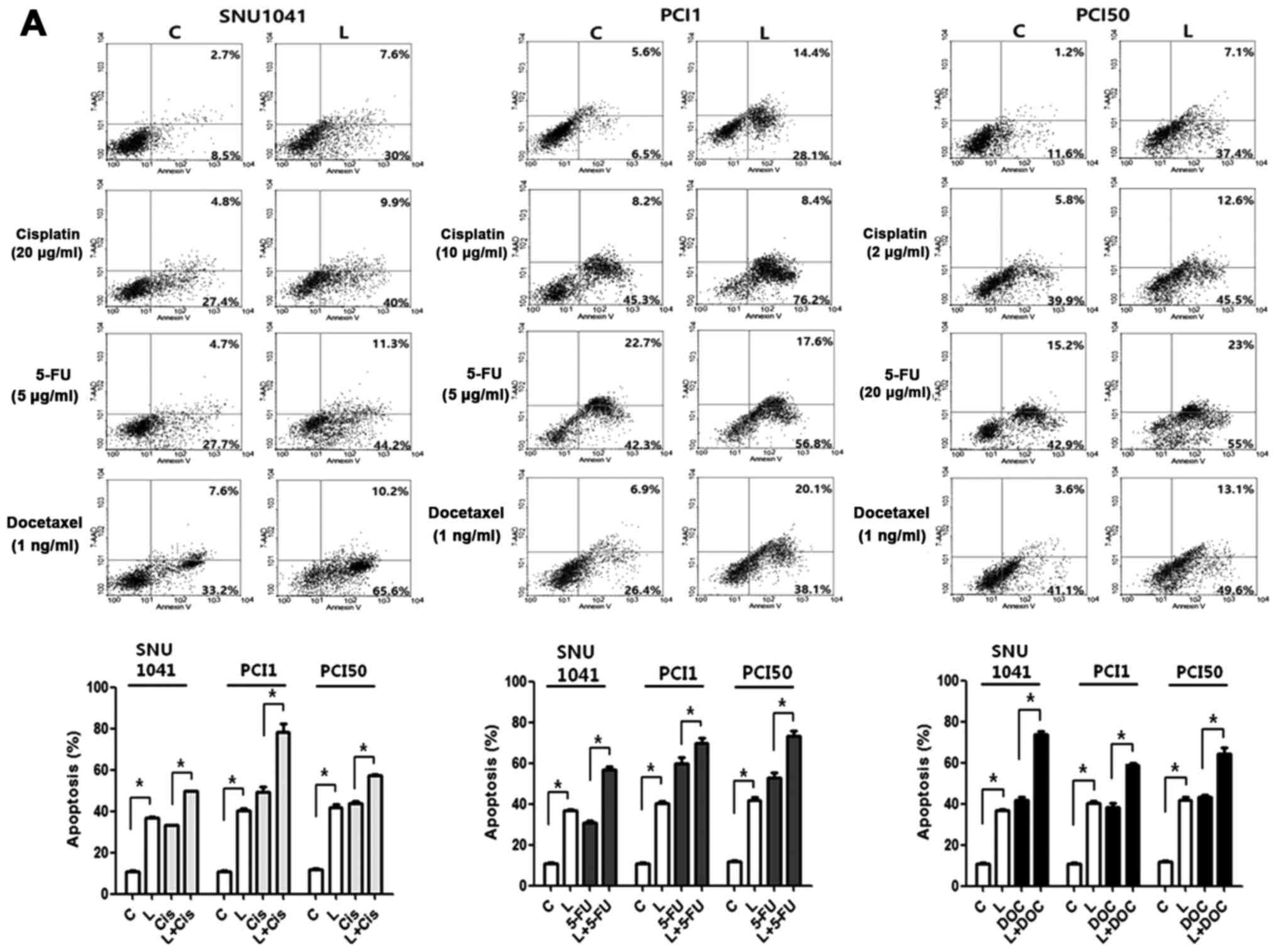 | Figure 4.Effects of Livin knockdown on
chemosensitivity in human head and neck squamous cell carcinoma
cells. (A) Combination treatment with Livin siRNA and cisplatin,
5-FU, or docetaxel resulted in significantly increased apoptosis in
SNU1041, PCI1 and PCI50 cells compared with that in control cells
treated with cisplatin, 5-FU, or docetaxel alone (*P<0.05). (B)
Livin-knockdown cells showed greater expression of cleaved
caspase-3 and −7 and cleaved poly(ADP-ribose)polymerase (PARP) than
did control cells after cisplatin, 5-FU, or docetaxel treatment
(*P<0.05). C, cells transfected with negative control siRNA; L,
cells transfected with Livin-specific siRNA; Cis, cisplatin
treatment; FU, 5-FU treatment; DC, docetaxel treatment. |
Discussion
HNSCC is potentially curable at an early stage using
single modality therapy of either surgery or radiotherapy. However,
most patients with HNSCC present with locally or locoregionally
advanced disease. Surgery followed by combined chemoradiotherapy or
primary concurrent chemoradiotherapy with/without induction
chemotherapy is the treatment of choice in locally advanced HNSCC
(35). Chemotherapy benefits
patients by improving locoregional control and reducing distant
metastasis (36). Thus, overcoming
chemoresistance is necessary to improve prognosis in patients with
advanced HNSCC.
Chemoresistance results from a variety of
complicated factors, including mutations in specific drug targets,
impaired drug transporters, DNA repair activation, increased drug
efflux and evasion of apoptosis by cancer cells (37,38).
Among these mechanisms, evasion of apoptosis is considered a major
cause of drug resistance since many chemotherapeutic agents act
through the induction of apoptosis (6). Thus, the IAP family has become the
focus of increased research related to chemoresistance in various
human malignancies.
IAP family members include NAIP, c-IAP1, c-IAP2,
XIAP, survivin, Apollon, ILP-2 and Livin (7,9). These
proteins contain one or more baculovirus IAP repeat (BIR) domains,
which are generally required for the suppression of apoptosis, and
harbor a COOH-terminal RING finger domain (7,8,39,40).
Livin, a recently discovered IAP, is composed of a single BIR
domain and a RING motif (9). Livin
has been implicated in chemoresistance in various cancers (20–34).
It has been reported to play a role in resistance to cisplatin in
bladder cancer, renal carcinoma, gastic cancer, hepatocellular
carcinoma, lung adenocarcinoma/non-small cell carcinoma,
osteosarcoma, colon cancer and ovarian carcinoma (20–29,31).
Furthermore, a few studies described that silencing Livin enhances
the cytotoxic effects of one or more anticancer drugs. Wang et
al (29) reported that
shRNA-mediated silencing of Livin induces chemosensitivity to
cisplatin and 5-FU in gastric cancer. Wang et al (30) and Oh et al (32) showed that Livin contributes to the
resistance to 5-FU/vincristine/etoposide or
5-FU/leucovorin/oxaliplatin in colon cancer. Livin knockdown also
increased chemosensitivity to adriamycin and cisplatin in non-small
cell lung cancer (31). As these
studies suggest, the effectiveness of Livin silencing in increasing
sensitivity to multiple anticancer drugs is an important advantage,
because different types of cancer have different chemotherapeutic
regimens, and combined treatment of multiple anticancer drugs is
popular in many cancers. However, the effects of Livin on
chemoresistance in human HNSCC have not been studied yet. Different
types of cancer have different expression of and sensitivity to
specific molecular target such as Livin. Thus, we studied whether
Livin is a specific molecular target to overcome the resistance of
chemotherapeutic drugs commonly used in head and neck cancer.
In the present study, we showed that siRNA-mediated
Livin knockdown enhanced the cytotoxicity of cisplatin, 5-FU and
docetaxel in human HNSCC cells. Additionally, we found that Livin
knockdown increased chemotherapy-induced apoptosis in response to
cisplatin, 5-FU and docetaxel. These findings were further
supported by significantly elevated levels of cleaved caspase-3 and
−7 and PARP, which are key enzymes involved in apoptosis, in Livin
knockdown HNSCC cells after chemotherapy. This study provides
highly valuable information because our findings were consistently
observed in all three HNSCC cell lines examined and we evaluated
three popular chemotherapeutic agents, which is the standard
induction chemotherapy regimen in HNSCC. Our findings suggested
that Livin knockdown may promote tumor cell regression, having
synergistic effects when applied with cisplatin, 5-FU and docetaxel
chemotherapy in human HNSCC.
There are several studies on upstream and downstream
regulation of Livin involving chemoresistance in cancers. Zhu et
al (22) demonstrated that
miRNA-20a induces cisplatin resistance via targeting
cylindromatosis (CYLD), leading to activation of nuclear factor
(NF)-κB and downstream target Livin in gastric cancer. Activation
of NF-κB pathway and downstream target Livin by SHANK-associated RH
domain interacting protein (SHARPIN) contributed to docetaxel
resistance in prostate cancer (34). In addition, Livin silencing
increased cisplatin chemosensitivity involving Bcl-2 and Akt
pathway in renal cell carcinoma (21). Further studies are needed to support
the regulation of Livin in HNSCC.
In summary, our results demonstrated that Livin
knockdown increased apoptosis and enhanced the chemosensitivity of
three HNSCC cells to cisplatin, 5-FU and docetaxel. Although
further studies are needed to confirm these findings, our results
suggested that the novel therapeutic strategies with combined use
of siRNA targeting Livin and chemotherapeutic agents may have
applications in the treatment of advanced HNSCC.
Acknowledgements
The present study was supported by a grant (HCRI
14002-1) from the Chonnam National University Hwasun Hospital
Institute of Biomedical Science. We thank Dr M.W. Sung (Seoul
National University) for the SNU1041, PCI1 and PCI50 cell
lines.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grégoire V, Lefebvre JL, Licitra L and
Felip E; EHNS-ESMO-ESTRO Guidelines Working Group, : Squamous cell
carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 21
Suppl 5:v184–v186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choong N and Vokes E: Expanding role of
the medical oncologist in the management of head and neck cancer.
CA Cancer J Clin. 58:32–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parfenov M, Pedamallu CS, Gehlenborg N,
Freeman SS, Danilova L, Bristow CA, Lee S, Hadjipanayis AG, Ivanova
EV, Wilkerson MD, et al: Cancer Genome Atlas Network:
Characterization of HPV and host genome interactions in primary
head and neck cancers. Proc Natl Acad Sci USA. 111:pp. 15544–15549.
2014; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matta A and Ralhan R: Overview of current
and future biologically based targeted therapies in head and neck
squamous cell carcinoma. Head Neck Oncol. 1:62009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deveraux QL, Stennicke HR, Salvesen GS and
Reed JC: Endogenous inhibitors of caspases. J Clin Immunol.
19:388–398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ashhab Y, Alian A, Polliack A, Panet A and
Ben Yehuda D: Two splicing variants of a new inhibitor of apoptosis
gene with different biological properties and tissue distribution
pattern. FEBS Lett. 495:56–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu B, Han M, Wen JK and Wang L:
Livin/ML-IAP as a new target for cancer treatment. Cancer Lett.
250:168–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Zhang Q, Liu B, Han M and Shan B:
Challenge and promise: Roles for Livin in progression and therapy
of cancer. Mol Cancer Ther. 7:3661–3669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vucic D, Stennicke HR, Pisabarro MT,
Salvesen GS and Dixit VM: ML-IAP, a novel inhibitor of apoptosis
that is preferentially expressed in human melanomas. Curr Biol.
10:1359–1366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SA, Yoon TM, Lee DH, Lee JK, Park YL,
Chung IJ, Joo YE and Lim SC: Livin enhances tumorigenesis by
regulating the mitogen-activated protein kinase signaling pathway
in human hypopharyngeal squamous cell carcinoma. Mol Med Rep.
14:515–520. 2016.PubMed/NCBI
|
|
14
|
Lee DH, Yoon TM, Kim SA, Park YL, Lee KH,
Lim SC, Lee JK and Joο YE: Relationship between expression of Livin
and the biological behavior of human oral squamous cell carcinoma.
Oncol Rep. 32:2453–2460. 2014.PubMed/NCBI
|
|
15
|
Yoon TM, Kim SA, Lee DH, Lee JK, Park YL,
Lee KH, Chung IJ, Joo YE and Lim SC: Expression of Livin and the
inhibition of tumor progression by Livin silencing in
laryngohypopharyngeal cancer. In Vivo. 28:751–759. 2014.PubMed/NCBI
|
|
16
|
Pignon JP, Bourhis J, Domenge C and
Designé L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: Three meta-analyses of updated
individual data. MACH-NC Collaborative Group. Meta-Analysis of
Chemotherapy on Head and Neck Cancer. Lancet. 355:949–955. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hitt R, López-Pousa A, Martínez-Trufero J,
Escrig V, Carles J, Rizo A, Isla D, Vega ME, Martí JL, Lobo F, et
al: Phase III study comparing cisplatin plus fluorouracil to
paclitaxel, cisplatin, and fluorouracil induction chemotherapy
followed by chemoradiotherapy in locally advanced head and neck
cancer. J Clin Oncol. 23:8636–8645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: TAX 324 Study Group: Cisplatin and
fluorouracil alone or with docetaxel in head and neck cancer. N
Engl J Med. 357:1705–1715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: EORTC 24971/TAX 323 Study Group: Cisplatin,
fluorouracil, and docetaxel in unresectable head and neck cancer. N
Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin L, Liu S, Li C, Ding S, Bi D, Niu Z,
Han L, Li W, Gao D, Liu Z, et al: CYLD downregulates Livin and
synergistically improves gemcitabine chemosensitivity and decreases
migratory/invasive potential in bladder cancer: The effect is
autophagy-associated. Tumour Biol. 37:12731–12742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Liu S, Ding K, Ding S, Li C, Lu J,
Gao D, Zhang T and Bi D: Silencing Livin induces apoptotic and
autophagic cell death, increasing chemotherapeutic sensitivity to
cisplatin of renal carcinoma cells. Tumour Biol. 37:15133–15143.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu M, Zhou X, Du Y, Huang Z, Zhu J, Xu J,
Cheng G, Shu Y, Liu P, Zhu W, et al: miR-20a induces cisplatin
resistance of a human gastric cancer cell line via targeting CYLD.
Mol Med Rep. 14:1742–1750. 2016.PubMed/NCBI
|
|
23
|
Liu F, Chang H, Xu W and Zhai Y: The
effects of Livin shRNA on the response to cisplatin in HepG2 cells.
Oncol Lett. 10:2957–2961. 2015.PubMed/NCBI
|
|
24
|
Zhuang L, Shen LD, Li K, Yang RX, Zhang
QY, Chen Y, Gao CL, Dong C, Bi Q, Tao JN, et al: Inhibition of
livin expression suppresses cell proliferation and enhances
chemosensitivity to cisplatin in human lung adenocarcinoma cells.
Mol Med Rep. 12:547–552. 2015.PubMed/NCBI
|
|
25
|
Zou AM, Wang HF, Zhu WF, Wang FX and Shen
JJ: Effect of RNAi-mediated silencing of Livin gene on biological
properties of colon cancer cell line LoVo. Genet Mol Res.
13:3832–3841. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding ZY, Liu GH, Olsson B and Sun XF:
Upregulation of the antiapoptotic factor Livin contributes to
cisplatin resistance in colon cancer cells. Tumour Biol.
34:683–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Fan S, Li L, Wang L, Fan G, Zhao Q
and Li Y: RNA interference-mediated knockdown of Livin suppresses
cell proliferation and invasion and enhances the chemosensitivity
to cisplatin in human osteosarcoma cells. Int J Oncol. 43:159–168.
2013.PubMed/NCBI
|
|
28
|
Liu X, Wang A, Gao H, Yuan Z and Jiao Y:
Expression and role of the inhibitor of apoptosis protein livin in
chemotherapy sensitivity of ovarian carcinoma. Int J Oncol.
41:1021–1028. 2012.PubMed/NCBI
|
|
29
|
Wang TS, Ding QQ, Guo RH, Shen H, Sun J,
Lu KH, You SH, Ge HM, Shu YQ and Liu P: Expression of livin in
gastric cancer and induction of apoptosis in SGC-7901 cells by
shRNA-mediated silencing of livin gene. Biomed Pharmacother.
64:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Xu J, Ju S, Ni H, Zhu J and Wang
H: Livin gene plays a role in drug resistance of colon cancer
cells. Clin Biochem. 43:655–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan D, Liu L, Xu H and Gu D: The effects
on cell growth and chemosensitivity by livin RNAi in non-small cell
lung cancer. Mol Cell Biochem. 320:133–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oh BY, Kim KH, Chung SS and Lee RA:
Silencing the livin gene enhances the cytotoxic effects of
anticancer drugs on colon cancer cells. Ann Surg Treat Res.
91:273–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang SR, Hu GR, Fang LJ, Huang SJ, Li JS,
Zhao MY and Meng MJ: CpG oligodeoxynucleotides enhance
chemosensitivity of 5-fluorouracil in HepG2 human hepatoma cells
via downregulation of the antiapoptotic factors survivin and livin.
Cancer Cell Int. 13:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Huang H, Zhou H, Du T, Zeng L,
Cao Y, Chen J, Lai Y, Li J, Wang G, et al: Activation of nuclear
factor κB pathway and downstream targets survivin and livin by
SHARPIN contributes to the progression and metastasis of prostate
cancer. Cancer. 120:3208–3218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Forastiere AA: Head and neck cancer:
Overview of recent developments and future directions. Semin Oncol.
27 Suppl 8:1–4. 2000.PubMed/NCBI
|
|
36
|
Ma J, Liu Y, Huang XL, Zhang ZY, Myers JN,
Neskey DM and Zhong LP: Induction chemotherapy decreases the rate
of distant metastasis in patients with head and neck squamous cell
carcinoma but does not improve survival or locoregional control: A
meta-analysis. Oral Oncol. 48:1076–1084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Endo T, Abe S, Seidlar HB, Nagaoka S,
Takemura T, Utsuyama M, Kitagawa M and Hirokawa K: Expression of
IAP family proteins in colon cancers from patients with different
age groups. Cancer Immunol Immunother. 53:770–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim DK, Alvarado CS, Abramowsky CR, Gu L,
Zhou M, Soe MM, Sullivan K, George B, Schemankewitz E and Findley
HW: Expression of inhibitor-of-apoptosis protein (IAP) livin by
neuroblastoma cells: Correlation with prognostic factors and
outcome. Pediatr Dev Pathol. 8:621–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deveraux QL, Roy N, Stennicke HR, van
Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS and Reed
JC: IAPs block apoptotic events induced by caspase-8 and cytochrome
c by direct inhibition of distinct caspases. EMBO J. 17:2215–2223.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deveraux QL, Takahashi R, Salvesen GS and
Reed JC: X-linked IAP is a direct inhibitor of cell-death
proteases. Nature. 388:300–304. 1997. View
Article : Google Scholar : PubMed/NCBI
|