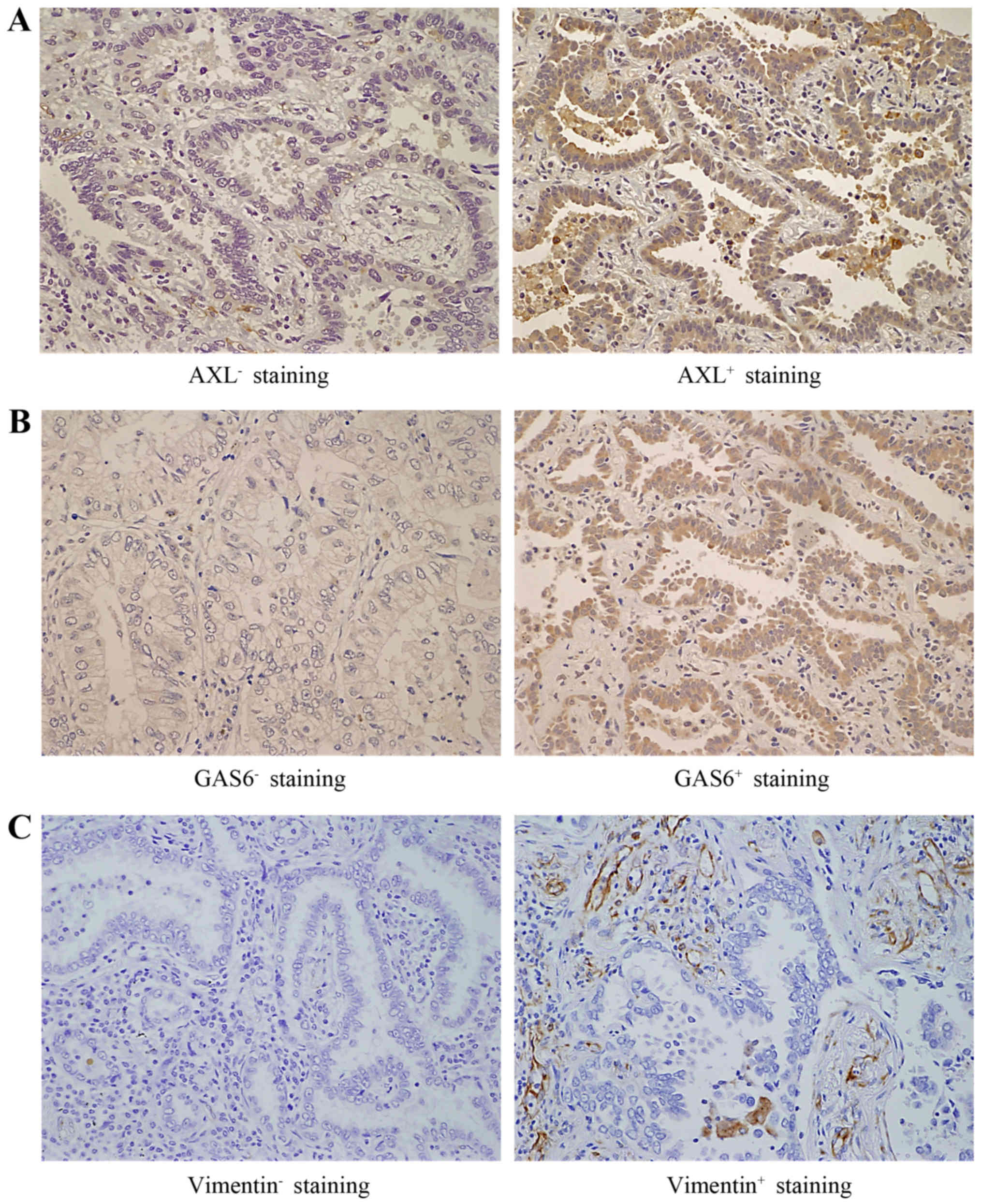
Introduction
Lung cancer is the leading cause of
cancer-associated deaths worldwide, contributing to approximately
1.4 million deaths each year despite major advances in diagnostics
and treatment in the last decade (1). Approximately 80% of lung cancers are
classified histologically as non-small cell lung cancers (NSCLCs),
of which the most common type is adenocarcinoma (AD), accounting
for approximately half of all NSCLCs (2). Therapeutic strategies for lung AD
patients currently focus on inhibiting target molecules or
oncogenic pathways such as receptor tyrosine kinases (3–5).
Unfortunately, despite initial marked responses to tyrosine kinase
inhibitors (TKIs), most AD patients with oncogenic driver mutations
eventually acquire resistance. Therefore, identification of
predictive and prognostic biomarkers and precision medicine using
the biomarkers could have a clinically significant impact on
treatment strategies for lung AD patients.
Signaling by AXL, a receptor tyrosine kinase,
induces the downstream activation of phosphoinositide 3-kinase
(PI3K)/AKT, signal transducer and activator of transcription 3
(STAT3), mitogen-activated protein kinase (MAPK), and nuclear
factor κB (6–8). Growth arrest-specific 6 (GAS6) is a
ligand of AXL and a member of the vitamin K-dependent protein
family. AXL overexpression is associated with cell survival,
proliferation, invasion, migration, cell-to-cell adhesion,
metastasis, and anti-apoptosis in different types of tumors
(9,10). In human cancers, increased
expression of AXL has been observed in glioma and cancer cells of
the breast, stomach and lung, and is associated with invasion and
metastasis (11–14). Furthermore, recent studies revealed
that AXL overexpression led to resistance to EGFR-TKI in NSCLC
cells undergoing epithelial-to-mesenchymal transition (EMT), making
AXL a potential therapeutic target in patients with acquired
resistance to EGFR-TKIs (15,16).
In this study, we examined the correlation of AXL
and GAS6 expression with clinicopathologic parameters and prognoses
in patients with complete lung AD resection. We ultimately found
that the combination of AXL and GAS6 expression was useful in
distinguishing those with a worse prognosis, particularly among
stage I AD patients.
Materials and methods
Patients and tumor samples
We carried out a retrospective study of 113 Japanese
patients who had been diagnosed with lung AD and had undergone
complete surgical resection at Nippon Medical School Hospital
between 2001 and 2009. The patients were enrolled consecutively
into the cohort upon undergoing surgery. During a 5-year follow-up,
overall survival was measured from the date of lung cancer surgery
until the date of death, and disease-free survival (DFS) was
measured from the date of surgery until relapse. All tumor samples
were freshly collected during surgery, quickly snap-frozen and
stored at −80°C. TNM staging, including T factor, N factor and
tumor differentiation grade (G), was assessed by the latest TNM
staging system and by following the 7th edition of the American
Joint Committee on Cancer Staging Manual (17–19).
Specimens from lung AD patients were used only for
immunohistochemistry (IHC) analysis. The study protocol was
approved by an ethics committee review board at Nippon Medical
School Hospital. Written informed consent was obtained from all
patients and the specimen of the patients was inspected according
to the Declaration of Helsinki 2008.
Cell culture
Ten lung AD cell lines were used in the present
study: PC-9, HCC827, NCI-H1975, A549, RERF-LC-KJ, RERF-LC-MC,
NCI-H441, PC-14, LC-2/ad and ABC-1. Cell lines were grown in
RPMI-1640 medium (Gibco, Carlsbad, CA, USA), except ABC-1 and
RERF-LCMS, which were grown in minimum essential medium Eagle
(Sigma-Aldrich, St. Louis, MO, USA). All media were supplemented
with 10% fetal bovine serum. The cell line, PC-14, was obtained
from Immuno-Biological Laboratories (Gunma, Japan); HCC827,
NCI-H441, and NCI-H1975 were obtained from the American Type
Culture Collection (ATCC, Manassas, VA, USA); A549, LC-2/ad, PC-9,
and RERF-LCKJ were obtained from the RIKEN BRC Cell Bank (Ibaraki,
Japan); and ABC-1 and RERF-LCMS were obtained from the Japanese
Collection of Research Bioresources Collection (JCRB) Cell Bank
(Osaka, Japan). Of the three cell lines with activating EGFR
mutations, PC-9 and NCI-HCC827 contained EGFR deletions
(delE746-A750) at exon 19, and NCI-H1975 showed double mutations:
L858R at exon 21 and T790M at exon 20. The other cells all had
wild-type EGFR.
Detection of EGFR mutations
The PNA-LNA PCR clamp method was used to identify
EGFR mutations in tissue or cytology specimens by LSI Medience
Corp. (Tokyo, Japan), as previously described (20).
Immunohistochemistry
Immunohistochemistry (IHC) was consecutively
performed on formalin-fixed, paraffin-embedded sections. After
deparaffinization, sections were quenched for endogenous activity
with 0.3% hydrogen peroxide plus absolute methanol for 20 min.
Thereafter, antigen retrieval was carried out in a 10 mmol/l
citrate buffer solution (pH 6.0; LSI Medience Corp.) for 10 min
using an autoclave. After blocking with 2% normal swine serum
(Vector Laboratories Inc., Burlingame, CA, USA), sections were
washed and incubated with goat anti-AXL polyclonal antibody
(#AF154, Rot: DMG0514051) and goat anti-GAS6 polyclonal antibody
(#AB885, Rot: DNH0113121; R&D Systems Inc., Minneapolis, MN,
USA), or anti-vimentin antibody (#3932, Rot: 3; Cell Signaling
Technology Inc., Danvers, MA, USA) overnight at 4°C. After washing,
slides were incubated for 30 min with biotinylated anti-goat
antibody for AXL and GAS6, or anti-rabbit antibody for vimentin
(1:200 dilution; Vector Laboratories), and treated with an
avidin-biotin complex kit (Funakoshi Co., Ltd., Tokyo, Japan).
Finally, slides were exposed to 3, 3′-diaminobenzidine
tetrahydrochloride (Muto Pure Chemicals Co., Ltd., Tokyo, Japan),
followed by counterstaining with Mayer's hematoxylin. Negative
control slides were prepared by omitting the primary antibody in
the above steps.
Evaluation of immunohistochemical
expression of AXL, GAS6, and vimentin
IHC scoring was performed using a Histoscore
(H-score) as previously described (21), where the staining intensity was
graded as follows: 0 (none), 1 (weak), 2 (moderate), and 3
(strong). The percentage of immunoreactive positive tumor cells for
AXL and GAS6 were graded as follows: 0, <10% positive cells; 1,
10–25% positive cells; 2, 25–50% positive cells; 3, 50–75% positive
cells; and 4, ≥75% positive cells. The percentage of
vimentin-positive tumor cells was graded differently as follows: 0,
<5% positive cells; 1, 5–30% positive cells; 2, 30–70% positive
cells; and 3, ≥70% positive cells (22). The final H-score was obtained by
multiplying the intensity grade by the percentage grade. All slides
were reviewed and scored independently by two investigators (C.-H.
Kim and F. Zou) who were blinded to clinical information pertaining
to patients. A tumor was defined as positive for IHC staining if
the AXL H-score ≥1.0, GAS6 H-score, ≥3.0 and vimentin H-score ≥1.0;
in all other cases, a tumor was defined as negative (Fig. 1).
Western blotting
Western blotting was performed as previously
described (23,24). Protein samples (10 µg) were
separated by SDS-PAGE, transferred to nitrocellulose membranes and
incubated in solutions of primary antibodies: anti-AXL (#AF154,
Rot: DMG0514051), anti-GAS6 (#AB885, Rot: DNH0113121),
anti-vimentin (#3932, Rot: 3) and β-actin (#A5316, Rot: 123M4876).
Anti-β-actin was obtained from Sigma-Aldrich. Anti-goat antibody
for AXL and GAS6, anti-rabbit antibody for vimentin or anti-mouse
antibody for β-actin were used as secondary antibodies (1:5000
dilution; Vector Laboratories). Proteins were detected with
Clarity™ ECL Western Blotting Substrate (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and visualized using a chemiluminescence
system (GE Healthcare Japan Corp., Tokyo, Japan).
Statistical analysis
Correlations between IHC staining and
clinicopathological factors were determined using the chi-square
test or the Fisher's exact test. Kaplan-Meier survival curves were
drawn for overall survival and DFS and compared by log-rank test.
The 5-year survival rate was analyzed by the Wilcoxon rank test.
Univariate and multivariate analyses were performed using the Cox
proportional hazard model. All tests were two-sided, and a P-value
of <0.05 was considered statistically significant. Statistical
analyses were performed using IBM SPSS Statistics version 21 (IBM
SPSS, Inc., Armonk, NY, USA).
Results
AXL and GAS6 expression in lung
AD
Among 113 patients with IHC staining for AXL and
GAS6 proteins, 43 (38.1%) were positive for AXL (AXL+),
38 (33.6%) were positive for GAS6 (GAS6+), and 20
(17.7%) were positive for both AXL and GAS6
(AXL+/GAS6+; Fig.
1; Table I). Associations
between patient clinicopathological parameters and AXL+
or GAS6+ status are shown in Table II. There were no significant
associations between AXL+ or GAS6+ and
parameters such as age, sex, smoking, T factor, N factor, tumor
grade, post-operative recurrence, and EGFR status. The 5-year
survival rates for patients who were AXL+ or
GAS6+ were significantly lower than those for
AXL− or GAS6− patients (51% vs. 75%; P=0.028;
53% vs. 72%; P=0.040).
 | Table I.Association between AXL and GAS6
expressions and patient characteristics. |
Table I.
Association between AXL and GAS6
expressions and patient characteristics.
| Variables | N |
AXL+ |
AXL− | P-value |
GAS6+ |
GAS6− | P-value |
|---|
| Total, n=113 |
| 43 (38.1) | 70 (61.9) |
| 38 (33.6) | 75 (66.4) |
|
| Age (years) |
|
<65 | 41 (36.3) | 16 (37.2) | 25 (35.7) |
| 12 (31.6) | 29 (38.7) |
|
|
≥65 | 72 (63.7) | 27 (62.8) | 45 (64.3) | 0.87 | 26 (68.4) | 46 (61.3) | 0.46 |
| Sex |
|
Male | 57 (50.4) | 24 (55.8) | 33 (47.1) |
| 17 (44.7) | 40 (53.3) |
|
|
Female | 56 (49.6) | 19 (44.2) | 37 (52.9) | 0.37 | 21 (55.3) | 35 (46.7) | 0.39 |
|
Smokinga |
| Current
and former smoker | 68 (60.7) | 29 (69.0) | 39 (55.7) |
| 22 (57.9) | 46 (62.2) |
|
|
Non-smoker | 44 (39.3) | 13 (31.0) | 31 (44.3) | 0.16 | 16 (42.1) | 28 (37.8) | 0.66 |
| T factor |
| T1 | 34 (30.1) | 15 (34.9) | 19 (27.1) |
| 13 (34.2) | 21 (28.0) |
|
|
T2-4 | 79 (69.9) | 28 (66.1) | 51 (72.9) | 0.38 | 25 (65.8) | 54 (72.0) | 0.50 |
| N factor |
| N0 | 78 (69.0) | 27 (62.8) | 51 (72.9) |
| 25 (65.8) | 53 (70.7) |
|
|
N1-2 | 35 (31.0) | 16 (37.2) | 19 (27.1) | 0.26 | 13 (34.2) | 22 (29.3) | 0.60 |
| Stage |
| I | 64 (56.6) | 24 (55.8) | 40 (57.1) |
| 20 (52.6) | 44 (58.7) |
|
|
II–III | 49 (43.4) | 19 (44.2) | 30 (42.9) | 0.89 | 18 (47.4) | 31 (41.3) | 0.54 |
| Grade |
| G1 | 34 (30.1) | 14 (32.6) | 20 (28.6) |
| 14 (36.8) | 20 (26.7) |
|
|
G2-3 | 79 (69.1) | 29 (67.4) | 50 (71.4) | 0.65 | 24 (63.2) | 55 (73.3) | 0.27 |
| EGFR |
|
Wild-type | 91 (80.5) | 34 (79.1) | 57 (81.4) |
| 34 (89.5) | 57 (76.0) |
|
|
Mutant | 22 (19.5) | 9
(20.9) | 13 (18.6) | 0.76 | 4
(10.5) | 18 (24.0) | 0.09 |
| Post-operative
recurrence |
| No | 60 (53.1) | 21 (48.8) | 39 (55.7) |
| 19 (50.0) | 41 (54.7) |
|
|
Yes | 53 (46.9) | 22 (51.2) | 31 (44.3) | 0.40 | 19 (50.0) | 34 (45.3) | 0.64 |
| Vimentin
IHCb |
|
Negative | 66 (78.6) | 23 (67.6) | 43 (86.0) |
| 21 (65.6) | 45 (86.5) |
|
|
Positive | 18 (21.4) | 11 (32.4) | 7
(14.0) | 0.044 | 11 (34.4) | 7
(13.5) | 0.023 |
| 5-year survival
rate, % |
| 51 | 75 | 0.028c | 53 | 72 | 0.040c |
 | Table II.Univariate and multivariate Cox
proportional hazards models of factors associated with death in
stages I–III and stage I patients. |
Table II.
Univariate and multivariate Cox
proportional hazards models of factors associated with death in
stages I–III and stage I patients.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | Comparison
Reference vs. risk group | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Stages I–III cases
(n=113) |
| Age
(years) | <65 vs. ≥65 | 0.75 | 0.38, 1.49 | 0.41 |
|
|
|
|
Sex | Male vs.
female | 0.90 | 0.46, 1.79 | 0.77 |
|
|
|
|
Smoking | Non vs. smoker | 0.71 | 0.36, 1.41 | 0.33 |
|
|
|
| T
factor | T1 vs. T2-4 | 0.94 | 0.46, 1.94 | 0.87 |
|
|
|
| N
factor | N0 vs. N1-2 | 2.94 | 1.47, 5.90 | 0.002 | 1.57 | 0.50, 4.94 | 0.44 |
|
p-stage | I vs. II–III | 2.61 | 1.30, 5.24 | 0.007 | 1.87 | 0.61, 5.78 | 0.28 |
|
Grade | G1 vs. G2+3 | 0.92 | 0.44, 1.89 | 0.81 |
|
|
|
|
EGFR | Wild-type vs.
mutant | 2.05 | 0.99, 4.24 | 0.05 |
|
|
|
|
Vimentin IHC | Negative vs.
positive | 0.44 | 0.13, 1.46 | 0.18 |
|
|
|
|
AXL/GAS6 classification | The
othersa vs.
AXL+/GAS6+ | 2.56 | 1.24, 5.29 | 0.011 | 2.45 | 1.16, 5.17 | 0.018 |
| Stage I cases
(n=64) |
| Age
(years) | <65 vs. ≥65 | 0.80 | 0.28, 2.30 | 0.68 |
|
|
|
|
Sex | Male vs.
female | 1.15 | 0.40, 3.28 | 0.80 |
|
|
|
|
Smoking | Non vs. smoker | 0.60 | 0.21, 1.72 | 0.34 |
|
|
|
| T
factor | T1 (IA) vs. T2
(IB) | 0.91 | 0.32, 2.62 | 0.86 |
|
|
|
|
Grade | G1 vs. G2+3 | 2.56 | 0.57,
11.46 | 0.22 |
|
|
|
|
EGFR | Wild-type vs.
mutant | 6.22 | 2.14,
18.13 | 0.001 | 9.30 | 3.00, 28.88 | 0.0001 |
|
Vimentin IHC | Negative vs.
positive | 0.42 | 0.09, 1.91 | 0.26 |
|
|
|
|
AXL/GAS6 classification | The
othersa vs.
AXL+/GAS6+ | 3.89 | 1.35,
11.24 | 0.012 | 5.90 | 1.88, 18.53 | 0.0024 |
Association between AXL or GAS6 and
vimentin expression
The overexpression of AXL and GAS6 is associated
with an EMT phenotype (15,16). Therefore, we evaluated vimentin
expression as a mesenchymal marker. Of 113 patients, 84 were
assessed by IHC assessment for vimentin. Eighteen cases (21.4%)
were vimentin positive (vimentin+; Fig. 1; Table
I). A vimentin+ status significantly correlated with
AXL+, GAS6+, and
AXL6+/GAS6+ status (P=0.044, P=0.023 and
P=0.004, respectively; Fig. 2A-C).
The frequency of vimentin+ increased with the extent of
AXL+ or GAS6+, which was highest for the
AXL+/GAS6+ group (44.4%), followed by the
single-positive group (AXL+/GAS6− plus
AXL−/GAS6+; 33.3%) and then the
AXL−/GAS6− group (22.2%; P=0.006,
linear-by-linear association; Fig.
2D). With regard to EGFR status, none of the 18 patients who
were vimentin+ had a mutant EGFR. On the other hand, 16
(24.2%) of 66 patients who were vimentin− showed EGFR
mutations (P=0.018; Fig. 2E).
Similarly, two (10%) of 20 patients with
AXL+/GAS6+ had a mutant EGFR, while 20
(21.5%) of 93 patients in the other groups
(AXL+/GAS6− plus
AXL−/GAS6+ plus
AXL−/GAS6−) showed the presence of EGFR
mutations, although the difference was not statistically
significant (P=0.354; Fig. 2F).
This suggested that high expression levels of AXL and GAS6 were
associated with vimentin overexpression and a wild-type EGFR
status.
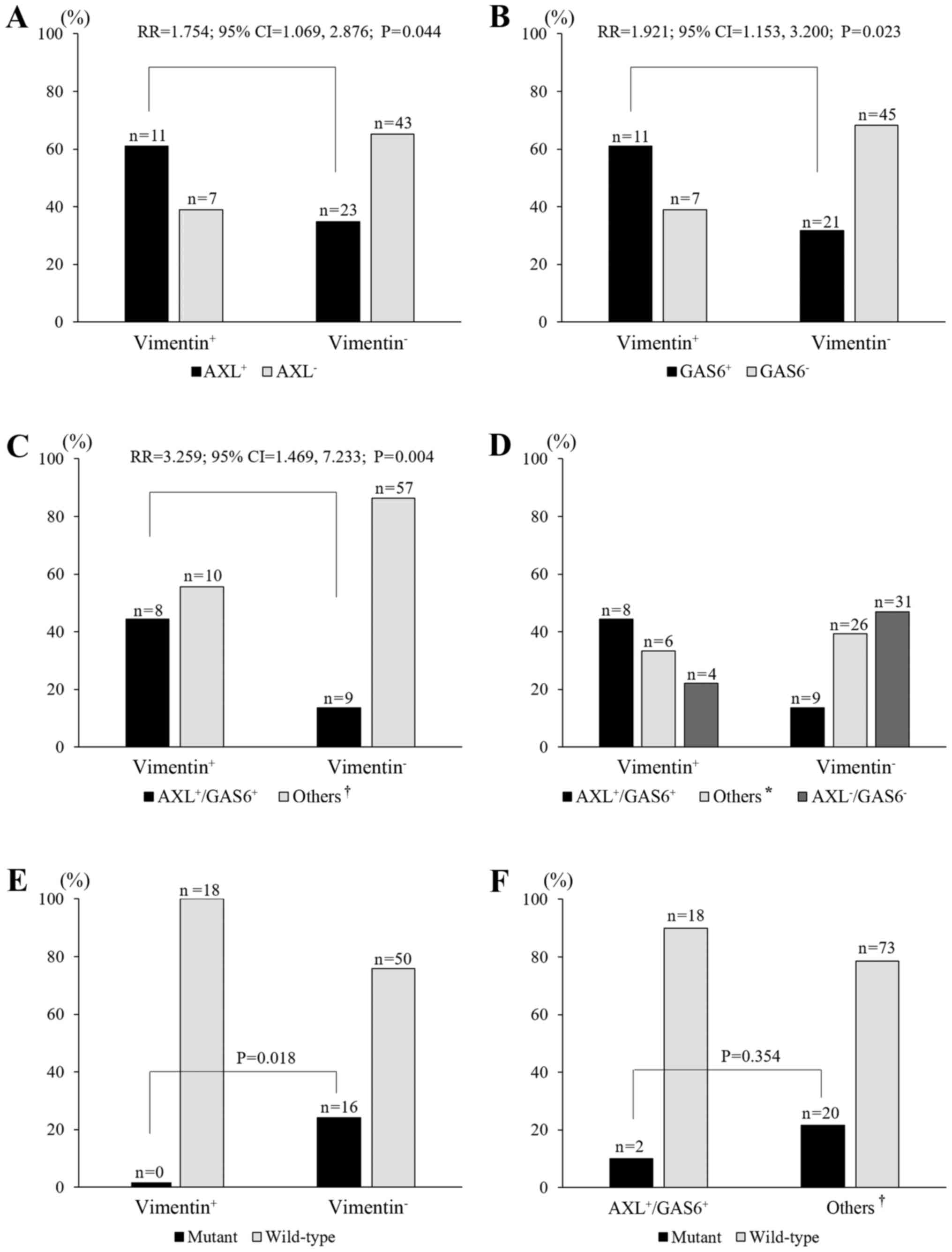 | Figure 2.Relationships between AXL and/or GAS6
and vimentin expression, and of AXL/GAS6 and vimentin expression
with EGFR mutation status. (A-C) The frequency of AXL+
[relative risk (RR)=1.754, P=0.044], GAS6+ (RR=1.921,
P=0.023), and AXL+/GAS6+ (RR=3.259, P=0.004)
correlated with vimentin+ expression. (D) The frequency
of vimentin+ increased with the co-expression of
AXL+/GAS6+, followed by the ‘others’ category
and AXL−/GAS6− (P=0.006, linear-by-linear
association). (E) Mutant EGFR was lacking in vimentin+
cases (P=0.018, versus the vimentin− group). (F) Mutant
EGFR was observed in two cases (10%) of the
AXL+/GAS6+ group and in 10 cases (22%) of the
‘others’ group (P=0.354). *, ‘others’ includes the single-positive
groups (AXL+/GAS6− plus
AXL−/GAS6+); †, ‘others’ includes the
single-positive and double-negative groups
(AXL+/GAS6− plus
AXL−/GAS6+ plus
AXL−/GAS6−). |
Association between AXL and/or GAS6
expression and clinical outcome
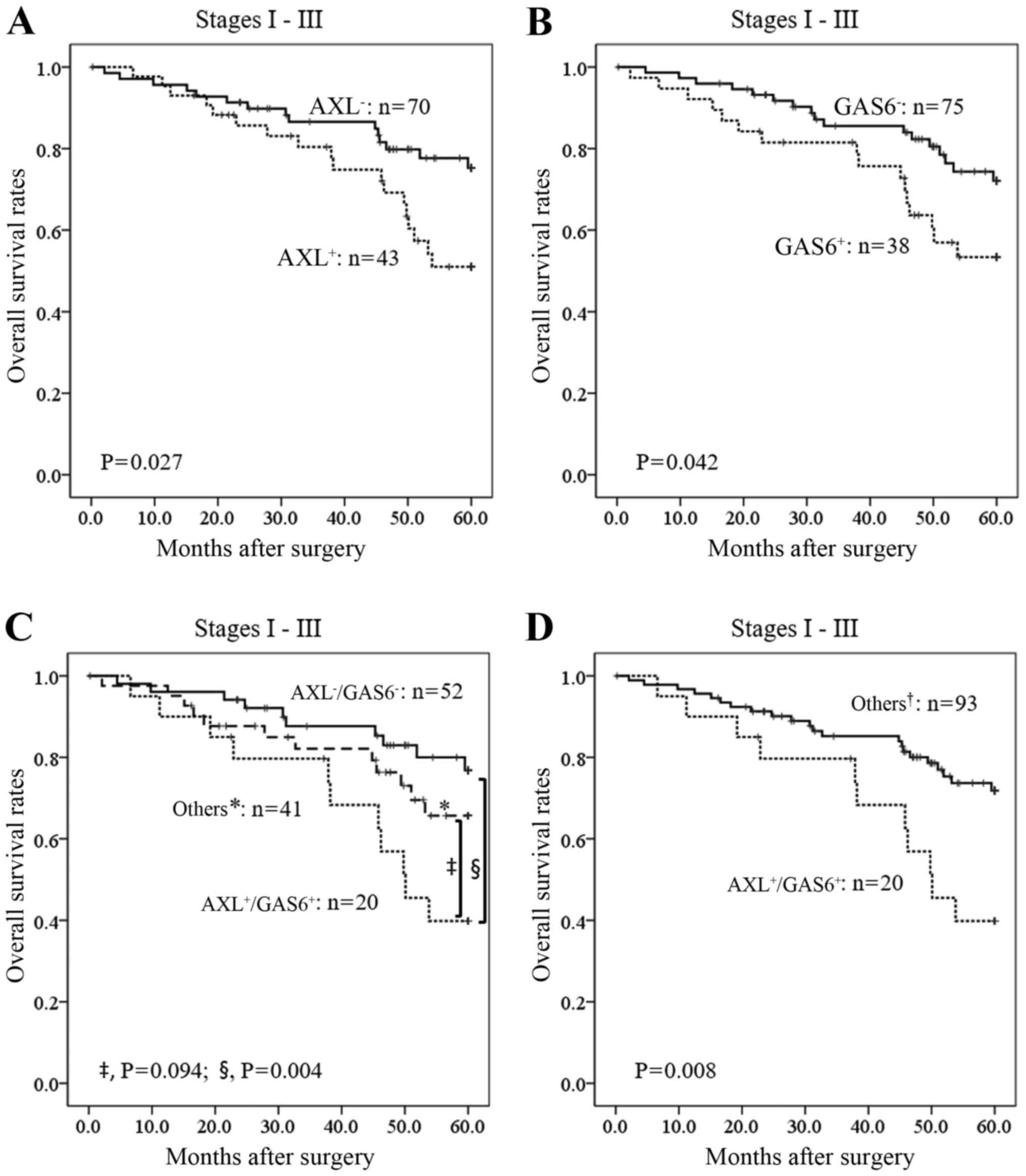
We next evaluated correlations between AXL, GAS6,
vimentin expression, and patient prognosis. Overall survival was
not significantly different between vimentin+ and
vimentin− for stages I–III lung AD (P=0.167; data not
shown). In contrast, AXL+ or GAS6+ status was
significantly associated with poor overall survival compared to
AXL− or GAS6− among patients with stages
I–III lung AD (P=0.027 and P=0.042, respectively; Fig. 3A and B). Furthermore, patients
showing AXL+/GAS6+ were also significantly
associated with poor overall survival compared to
AXL−/GAS6− or other groups
(AXL+/GAS6− plus
AXL−/GAS6+ plus
AXL−/GAS6−; P=0.004 and P=0.008,
respectively; Fig. 3C and D);
however, there was no significant association between
AXL+/GAS6+ and
AXL+/GAS6− plus
AXL−/GAS6+ (P=0.094; Fig. 3C). These results suggested that AXL
and GAS6 co-expression (AXL+/GAS6+) was
associated with a poor prognosis for lung AD. Next, we investigated
whether a patient's prognosis was affected by AXL+ or
GAS6+ expression among patients stratified according to
stage and EGFR status. For stage I cases, overall survival and DFS
rates for AXL+/GAS6+ cases were significantly
shorter than those for other cases (P=0.007 and P=0.006,
respectively; Fig. 3E and F). In
stage I patients with wild-type EGFR, the overall survival and DFS
rates for AXL+/GAS6+ patients were also
significantly shorter than those for the other patients (P=0.0001
and P=0.0004, respectively; Fig. 3G and
H). In contrast, AXL+/GAS6+ as a negative
prognostic factor was not observed in stage I patients with mutant
EGFR (data not shown). Thus, AXL and GAS6 expression in combination
significantly correlated with a poor outcome for stage I lung AD
and an EGFR wild-type status.
The impact of AXL/GAS6 expression on
lung AD patient survival
We finally evaluated whether the prognostic ability
of AXL+/GAS6+ was affected by underlying
clinicopathological covariates using univariate and multivariate
Cox regression analyses. Among stages I–III patients, N factor
[hazard ratio (HR)=2.94, P=0.002], p-stage (HR=2.61, P=0.007) and
AXL/GAS6 classification (HR=2.56, P=0.011) were significant
predictors of survival in univariate analysis (Table II). Multivariate analysis, adjusted
for N factor, p-stage, and AXL/GAS6 classification, showed that
only AXL+/GAS6+ (HR=2.45, P=0.018) was a
statistically significant predictor of survival (Table II). In stage I cases, univariate
analysis showed that the EGFR mutation status (HR=6.22, P=0.001)
and AXL/GAS6 classification (HR=3.89, P=0.012) were significantly
associated with death. Finally, the EGFR mutation status (HR=9.30,
P=0.0001) and AXL/GAS6 classification (HR=5.90, P=0.0024) were
found to be independent predictors of death in multivariate
analysis (Table II). Thus,
co-expression of AXL and GAS6 significantly correlated with death
for stages I–III and I lung AD patients.
AXL, GAS6, and vimentin expression in
lung AD cell lines
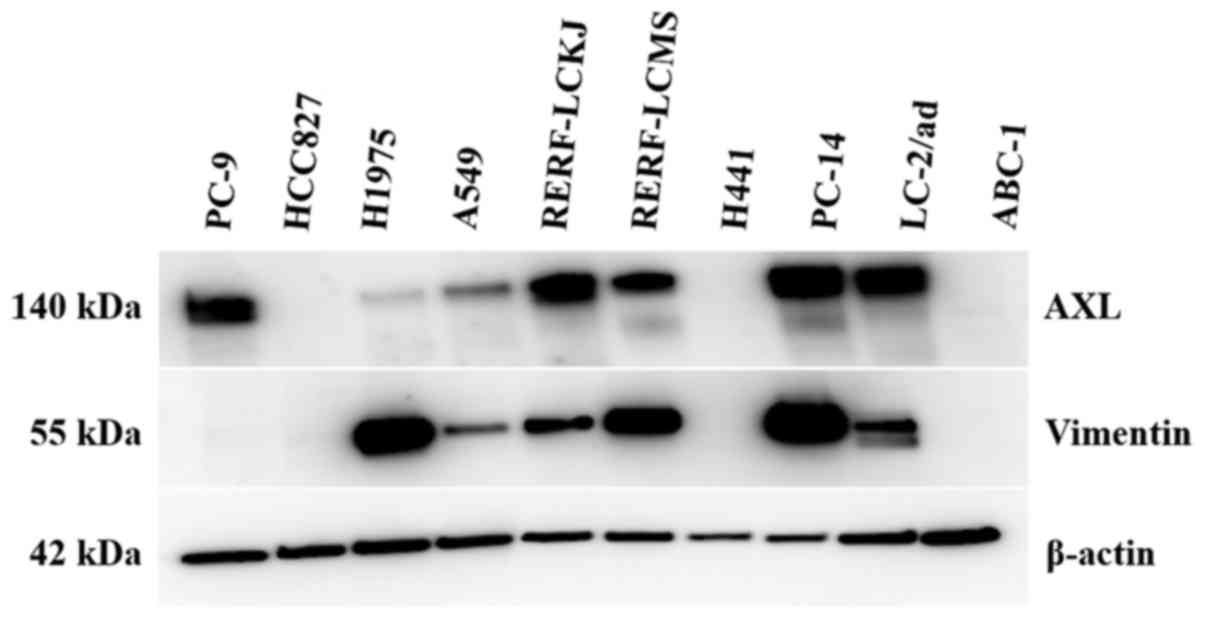
We also evaluated protein expression levels of AXL,
GAS6, and vimentin in 10 lung AD cell lines (Fig. 4). Unfortunately, GAS6 proteins were
not detected in cells, probably because this is a secreted protein.
Among seven AD cell lines with wild-type EGFR, five cell lines
(A549, RERF-LC-KJ, RERF-LC-MS, PC-14, and LC-2/ad) strongly
expressed AXL and vimentin protein. Of three mutant EGFR cell
lines, PC-9 and H1975 showed strong AXL and vimentin expression,
respectively.
Discussion
In this study, we found that the positive expression
of AXL and GAS6 in combination could be used as a marker of a poor
prognosis in lung AD patients. AXL protein expression has, in the
past, correlated with lymph node metastasis and clinical stage
(13,25), while high expression levels of AXL
and GAS6 have been associated with poor survival in lung AD
patients with stage I–III (25).
Consistent with these findings, we observed that AXL and GAS6
expression levels significantly correlated with poor survival in
lung AD cases. Furthermore, we showed the negative impact that the
high expression of both AXL and GAS6 had on the survival of
patients with stage I lung AD. Of note, AXL+ expression
significantly correlated with vimentin+ expression, as
reported in an earlier in vitro study (26). The co-expression of AXL and GAS6 was
mostly associated with vimentin positive expression in this study.
Thus, rather than the individual expression of either protein, the
expression of both in combination may be more closely associated
with the biological features of vimentin, suggesting that
AXL+/GAS6+ tumor cells may represent abundant
vimentin. Vimentin may actually induce AXL expression (27). However, vimentin expression was not
a prognostic factor in this study. Besides vimentin, AXL expression
could be also regulated by other factors, including TGF-β1
(26). Therefore, AXL and GAS6
co-expression, but not vimentin expression, may be critical for
patient survival, as well as in the carcinogenesis of lung AD.
Furthermore, high vimentin expression correlated with an EGFR
wild-type status. AXL and vimentin-positive expression were also
found for most AD cell lines showing wild-type EGFR. Therefore, AXL
and GAS6 may play a critical role in tumor progression and patient
survival in lung AD patients with wild-type EGFR. As for the
significance of AXL/GAS6/vimentin for the EGFR mutant-type, small
numbers of stage I–III AD patients as well as AD cells with the
EGFR mutation have been analyzed. Further studies are planned to
perform using large-scale samples with an EGFR mutation, including
stage IV, to evaluate the correlation between AXL/GAS6/vimentin
expression and EGFR status as a prognostic factor.
Recently, AXL upregulation and activation by GAS6
has been implicated in the EMT of breast cancer and hepatocellular
carcinoma (28,29). Likewise, the expression of both AXL
and GAS6 is deemed to be closely related to full-blown EMT in a
subset of lung AD. AXL-related EMT resulting in drug resistance has
been reported in patients with prior EGFR-TKI therapy, as well as
in in vitro studies using NSCLC cell lines (15,16,30).
Aberrant AXL signaling and the development of the EMT phenotype
were also associated with ALK inhibitor resistance in ALK-driven
neuroblastoma cells (31). Our
clinical data support the concept that EMT under AXL or GAS6 high
expression apparently exists in patients with prior surgical
resection for lung AD, which consequently leads to de novo
resistance to EGFR-TKI (15,30).
Unfortunately, approximately 20–30% of early stage
NSCLC patients undergo a relapse, even after complete surgical
treatment (32). Sensitive
biomarkers can help identify patients with early-stage or locally
advanced NSCLC who have a high risk of relapse and a poor
prognosis. High expression levels of excision repair
cross-complementation group 1 (ERCC1), ribonucleotide reductase
subunit M2 (RRM2), and thymidylate synthase (TS) were suggested as
negative prognostic factors for patients with resected NSCLC
(33,34). In addition, cyclooxygenase-2 and
amplification of the actin-4 (ACTN4) gene were considered markers
for a poor prognosis in stage I disease (35,36).
However, a conceivable prognostic biomarker for patients with stage
I AD has not yet been established. In the present study, we
demonstrated that the co-expression of AXL and GAS6 had a greater
effect on survival in patients with stage I lung AD, especially in
those with wild-type EGFR. AXL has been recognized as a potential
therapeutic target for overcoming EGFR-TKI resistance (30). The BATTLE study using AXL inhibitor
and EGFR-TKI demonstrated synergistic effects in some patients with
wild-type EGFR (15). Our findings
suggest that the combination of AXL and GAS6 was significantly
associated with poor overall survival and DFS in the AD subgroup of
stage I disease with wild-type EGFR. Therefore, AXL and GAS6 may be
promising predictive biomarkers of a drug response and crucial
therapeutic targets in lung AD with wild-type EGFR. The prognostic
significance of the co-expression of AXL and GAS6 needs to be
further validated in large-scale studies of AD samples. Further
investigation is also needed to determine whether the
overexpression of AXL and/or GAS6 modulate different internal
signaling pathways, depending on the EGFR status and EMT
signature.
Our study demonstrated that the co-expression of AXL
and GAS6 in a tumor was a significant independent predictor of a
poor outcome in patients with stage I lung AD, as well as stages
I–III lung AD. An AXL and GAS6 expression status may be useful for
the identification of lung AD patients at high risk of
post-operative death and who will benefit from adjuvant
chemotherapy.
Acknowledgements
This study was supported in part by a grant-in-aid
from the Ministry of Education, Culture, Sports, Science, and
Technology of Japan (grant no. 25461172 to A.G.), and the Clinical
Rebiopy Bank Project for Comprehensive Cancer Therapy Development
in Nippon Medical School.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alberg AJ, Brock MV, Ford JG, Samet JM and
Spivack SD: Epidemiology of lung cancer: Diagnosis and management
of lung cancer, 3rd ed: American College of Chest Physicians
evidence-based clinical practice guidelines. Chest. 143 Suppl
5:e1–e29. 2013. View Article : Google Scholar
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: North-East Japan Study Group: Gefitinib or chemotherapy for
non-small-cell lung cancer with mutated EGFR. N Engl J Med.
362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: West Japan Oncology Group: Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): An
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hasanbasic I, Cuerquis J, Varnum B and
Blostein MD: Intracellular signaling pathways involved in
Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart
Circ Physiol. 287:H1207–H1213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee WP, Wen Y, Varnum B and Hung MC: Akt
is required for Axl-Gas6 signaling to protect cells from
E1A-mediated apoptosis. Oncogene. 21:329–336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vajkoczy P, Knyazev P, Kunkel A, Capelle
HH, Behrndt S, von Tengg-Kobligk H, Kiessling F, Eichelsbacher U,
Essig M, Read TA, et al: Dominant-negative inhibition of the Axl
receptor tyrosine kinase suppresses brain tumor cell growth and
invasion and prolongs survival. Proc Natl Acad Sci USA. 103:pp.
5799–5804. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lay JD, Hong CC, Huang JS, Yang YY, Pao
CY, Liu CH, Lai YP, Lai GM, Cheng AL, Su IJ, et al: Sulfasalazine
suppresses drug resistance and invasiveness of lung adenocarcinoma
cells expressing AXL. Cancer Res. 67:3878–3887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Linger RM, Keating AK, Earp HS and Graham
DK: Taking aim at Mer and Axl receptor tyrosine kinases as novel
therapeutic targets in solid tumors. Expert Opin Ther Targets.
14:1073–1090. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hutterer M, Knyazev P, Abate A, Reschke M,
Maier H, Stefanova N, Knyazeva T, Barbieri V, Reindl M, Muigg A, et
al: Axl and growth arrest-specific gene 6 are frequently
overexpressed in human gliomas and predict poor prognosis in
patients with glioblastoma multiforme. Clin Cancer Res. 14:130–138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meric F, Lee WP, Sahin A, Zhang H, Kung HJ
and Hung MC: Expression profile of tyrosine kinases in breast
cancer. Clin Cancer Res. 8:361–367. 2002.PubMed/NCBI
|
|
13
|
Shieh YS, Lai CY, Kao YR, Shiah SG, Chu
YW, Lee HS and Wu CW: Expression of axl in lung adenocarcinoma and
correlation with tumor progression. Neoplasia. 7:1058–1064. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu CW, Li AF, Chi CW, Lai CH, Huang CL, Lo
SS, Lui WY and Lin WC: Clinical significance of AXL kinase family
in gastric cancer. Anticancer Res. 22:1071–1078. 2002.PubMed/NCBI
|
|
15
|
Byers LA, Diao L, Wang J, Saintigny P,
Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK, et al: An
epithelial-mesenchymal transition gene signature predicts
resistance to EGFR and PI3K inhibitors and identifies Axl as a
therapeutic target for overcoming EGFR inhibitor resistance. Clin
Cancer Res. 19:279–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Lee JC, Lin L, Olivas V, Au V,
LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, et al:
Activation of the AXL kinase causes resistance to EGFR-targeted
therapy in lung cancer. Nat Genet. 44:852–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual. 7th. Springer;
New York, NY: 2010
|
|
20
|
Nagai Y, Miyazawa H, Huqun, Tanaka T,
Udagawa K, Kato M, Fukuyama S, Yokote A, Kobayashi K, Kanazawa M,
et al: Genetic heterogeneity of the epidermal growth factor
receptor in non-small cell lung cancer cell lines revealed by a
rapid and sensitive detection system, the peptide nucleic
acid-locked nucleic acid PCR clamp. Cancer Res. 65:7276–7282. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCarty KS Jr, Miller LS, Cox EB, Konrath
J and McCarty KS Sr: Estrogen receptor analyses. Correlation of
biochemical and immunohistochemical methods using monoclonal
antireceptor antibodies. Arch Pathol Lab Med. 109:716–721.
1985.PubMed/NCBI
|
|
22
|
Hirano H, Maeda H, Takeuchi Y, Susaki Y,
Kobayashi R, Hayashi A, Ose N, Yamaguchi T, Yokota S and Mori M:
Lymphatic invasion of micropapillary cancer cells is associated
with a poor prognosis of pathological stage IA lung
adenocarcinomas. Oncol Lett. 8:1107–1111. 2014.PubMed/NCBI
|
|
23
|
Shimokawa T, Seike M, Soeno C, Uesaka H,
Miyanaga A, Mizutani H, Kitamura K, Minegishi Y, Noro R, Okano T,
et al: Enzastaurin has anti-tumour effects in lung cancers with
overexpressed JAK pathway molecules. Br J Cancer. 106:867–875.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seike M, Goto A, Okano T, Bowman ED,
Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, et
al: MiR-21 is an EGFR-regulated anti-apoptotic factor in lung
cancer in never-smokers. Proc Natl Acad Sci USA. 106:pp.
12085–12090. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishikawa M, Sonobe M, Nakayama E,
Kobayashi M, Kikuchi R, Kitamura J, Imamura N and Date H: Higher
expression of receptor tyrosine kinase Axl, and differential
expression of its ligand, Gas6, predict poor survival in lung
adenocarcinoma patients. Ann Surg Oncol. 20 Suppl 3:S467–S476.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilson C, Ye X, Pham T, Lin E, Chan S,
McNamara E, Neve RM, Belmont L, Koeppen H, Yauch RL, et al: AXL
inhibition sensitizes mesenchymal cancer cells to antimitotic
drugs. Cancer Res. 74:5878–5890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vuoriluoto K, Haugen H, Kiviluoto S,
Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB and Ivaska J:
Vimentin regulates EMT induction by Slug and oncogenic H-Ras and
migration by governing Axl expression in breast cancer. Oncogene.
30:1436–1448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gjerdrum C, Tiron C, Høiby T, Stefansson
I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT,
et al: Axl is an essential epithelial-to-mesenchymal
transition-induced regulator of breast cancer metastasis and
patient survival. Proc Natl Acad Sci USA. 107:pp. 1124–1129. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reichl P, Dengler M, van Zijl F, Huber H,
Führlinger G, Reichel C, Sieghart W, Peck-Radosavljevic M,
Grubinger M and Mikulits W: Axl activates autocrine transforming
growth factor-β signaling in hepatocellular carcinoma. Hepatology.
61:930–941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu F, Li J, Jang C, Wang J and Xiong J:
The role of Axl in drug resistance and epithelial-to-mesenchymal
transition of non-small cell lung carcinoma. Int J Clin Exp Pathol.
7:6653–6661. 2014.PubMed/NCBI
|
|
31
|
Debruyne DN, Bhatnagar N, Sharma B, Luther
W, Moore NF, Cheung NK, Gray NS and George RE: ALK inhibitor
resistance in ALK-driven neuroblastoma is associated with AXL
activation and induction of EMT. Oncogene. 35:3681–3691. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Asamura H, Goya T, Koshiishi Y, Sohara Y,
Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et
al: Japanese Joint Committee of Lung Cancer Registry: A Japanese
Lung Cancer Registry study: Prognosis of 13,010 resected lung
cancers. J Thorac Oncol. 3:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grossi F, Dal Bello MG, Salvi S, Puzone R,
Pfeffer U, Fontana V, Alama A, Rijavec E, Barletta G, Genova C, et
al: Expression of ribonucleotide reductase subunit-2 and
thymidylate synthase correlates with poor prognosis in patients
with resected stages I–III non-small cell lung cancer. Dis Markers.
2015:3026492015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng Z, Chen T, Li X, Haura E, Sharma A
and Bepler G: DNA synthesis and repair genes RRM1 and ERCC1 in lung
cancer. N Engl J Med. 356:800–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mascaux C, Martin B, Paesmans M, Berghmans
T, Dusart M, Haller A, Lothaire P, Meert AP, Lafitte JJ and Sculier
JP: Has Cox-2 a prognostic role in non-small-cell lung cancer? A
systematic review of the literature with meta-analysis of the
survival results. Br J Cancer. 95:139–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Noro R, Honda K, Tsuta K, Ishii G,
Maeshima AM, Miura N, Furuta K, Shibata T, Tsuda H, Ochiai A, et
al: Distinct outcome of stage I lung adenocarcinoma with ACTN4 cell
motility gene amplification. Ann Oncol. 24:2594–2600. 2013.
View Article : Google Scholar : PubMed/NCBI
|