Introduction
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a type II transmembrane protein belonging to the
tumor necrosis factor (TNF) superfamily. It is expressed on the
surface of certain immune cells including T-lymphocytes and natural
killer (NK) cells. TRAIL plays a central role in immune
surveillance. Owing to the fact that it induces selective apoptosis
in tumor cells, but not normal cells, TRAIL has been considered as
a promising therapeutic anticancer agent (1,2). The
apoptotic signaling pathway regulated by TRAIL is initiated by its
binding to agonist death receptors, namely DR4/TRAIL-R1 and
DR5/TRAIL-R2. Oligomerization of these receptors allow the
recruitment of the adaptor protein Fas-associated death domain
(FADD), which subsequently stimulates the formation of
death-inducing signaling complex (DISC) through recruitment of the
pro-caspase-8 (3,4). Activation of pro-caspase-8 within the
DISC, by proximity, activates either a cascade of caspase-cleavage
leading to activation of caspase-3, −6, and −7 (extrinsic pathway)
or stimulates the mitochondrial pathway (intrinsic pathway) via
cleavage of the BH3-interacting domain death agonist (Bid) to
truncated Bid (tBid)/caspase-3, inducing an irreversible cell death
(5). Despite potent anticancer
activity, resistance to TRAIL-induced apoptosis has been reported
in several malignant cells (6–12). The
mechanism by which the tumor cell gains resistance to TRAIL mainly
include i) a deficiency of DR expression, ii) overexpression of
anti-apoptotic proteins, or iii) competition of the decoy receptors
(DcR1/TRAIL-R3 and DcR2/TRAIL-R4) for TRAIL binding (13–15).
However, conventional or non-conventional chemotherapy can restore
TRAIL sensitivity (16–18).
Marine environment encompasses a great variety of
microorganisms living in extreme conditions such as high salinity,
pressure and temperature. These organisms were reported to produce
unique and structurally novel secondary metabolites allowing their
survival in such conditions (19).
Marine actinomycetes are considered as an unexplored source of
biologically active secondary metabolites including anti-bacterial,
anti-fungal, anti-malarial, anti-inflammatory, and antitumor
(20–25). Several natural products derived from
terrestrial and marine actinomycetes were investigated for their
TRAIL-resistance overcoming activities and the molecular mechanisms
that trigger the induction of apoptosis were determined (25–30).
In this study, we investigated TRAIL-resistance overcoming activity
of several crude extracts of marine actinomycetes isolated from the
Red Sea. In TRAIL-resistant MDA-MB-231 breast cancer cells, four
crude extracts obtained from Streptomyces sp. (EGY1, EGY3,
EGY24, and EGY34) showed selective TRAIL synergistic activity, but
had no effect on the normal mouse embryonic fibroblast (MEF).
Analyzing the signaling pathways triggered by the co-treatment
indicate that these crude extracts possibly act through two
distinct mechanisms; either through i) activation of caspase-8 and
−10 pathways or ii) induction of ER-stress via stimulation of
ER-stress sensors, BiP. Our results are expected to provide new
insight into the development of lead marine-derived structures
against cancer.
Materials and methods
Collection of samples
Thirty samples of sediments, sea water and sands
were collected from different parts of Sharm El-Sheikh, South
Sinai, Egypt. The collected samples were kept in 50 ml sterile
Falcon tubes and preserved in refrigerator for further study.
Isolation of actinomycetes
Wet sediment/sand (1 g) was dispersed in 9 ml of
sterilized water. The samples were vortexed for 2 min and subjected
to heat treatment in a water bath at 60°C for 10 min to eliminate
non-sporulating bacteria. Following serial dilution
(10−1, 10−2 and 10−3) of the
suspension with sterile water, a 100 µl aliquot was spread on humic
acid-vitamin agar (31): humic acid
(1.0 g/l), Na2HPO4 (0.5 g/l), KCl (1.71 g/l),
MgSO4.7H2O (0.05 g/l),
FeSO4.7H2O (0.01 g/l), CaCO3 (0.02
g/l), vitamin mixture (1.0 ml) and agar (18.0 g/l); and
starch-casein agar (32): soluble
starch (10.0 g/l), casein (0.3 g/l), K2HPO4
(2.0 g/l), KNO3 (2.0 g/l), NaCl (2.0 g/l),
MgSO4.7H2O (0.05 g/l), CaCO3 (0.02
g/l), FeSO4.7H2O (0.01 g/l), agar (15 g/l).
All media were prepared in 50% filtered Red Sea water and
supplemented with nalidixic acid (75 µg ml−1) and
cycloheximide (50 µg ml−1) as antibacterial and
antifungal agents, respectively. The plates were incubated at 30°C
for 7–30 days until the colonies appeared. Forty-seven actinomycete
strains were picked up, subsequently spread on Waksman agar plates
and labeled with the code numbers EGY1 to EGY47. Characterizations
of the isolated strains were carried by morphological methods
(33) and on the basis of 16S rRNA
partial gene sequences (34).
Fermentation and preparation of crude
extracts
To prepare the cultures, chunks of well grown agar
plate of each strain were used to inoculate 2×100 cm3
Erlenmeyer flasks each containing 100 ml of Waksman medium with 50%
sea water. The Waksman liquid medium contains glucose (2.0 g/100
ml), meat extract (0.5 g/100 ml), peptone (0.5 g/100 ml), dried
yeast (0.3 g/100 ml), NaCl (0.5 g/100 ml) and CaCO3 (0.3
g/100 ml). The cultures were grown at 28°C for 3–4 days with
reciprocal shaking at 200 r.p.m. A library of crude extracts was
constructed through a consecutive extraction of the culture broths
of different strains with ethyl acetate. The cell pellets were
extracted two times with methanol. The solvents from culture broth
and mycelia were evaporated under vacuum, collected together in a
small glass vial, and then stored at −20°C for further use. Each
vial took a serial number identical to the number of its own
bacterial strain. Each crude extract was dissolved in dimethyl
sulfoxide (DMSO) for further investigation.
Cell culture
The human TRAIL-resistant MDA-MB-231 breast cancer
cell line was a kind gift of Dr Patrick Legembre (Rennes, France).
Normal mouse embryonic fibroblast cell line (MEF) was obtained from
the (ATCC-CF-1 SCRC 1040). Monolayer cell cultures were grown in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 4.5 g/l
of glucose, 4 mmol/l of L-glutamine, and 10% heat-inactivated fetal
calf serum (FCS).
Cell viability assay
MDA-MB-231 cells were seeded in 96-well plates
(4×104 cells per well) in 100 µl of DMEM medium. Cells
were incubated at 37°C in a 5% CO2 incubator for 24 h.
Crude extracts at different doses (0.04, 0.1, 0.2, 0.3, 0.6 and 1.2
mg/ml) were added to each well. After 24 h incubation, the culture
media was removed and the cells were washed with 100 µl PBS. Cells
that remained attached were fixed by adding 100 µl of 70% ethanol.
The plate was then incubated at room temperature for 1 h to remove
ethanol and 100 µl of methylene blue dye was added. The plates were
incubated at room temperature for 15 min. To remove the excess of
dye, the plate was washed three times with tap water and then dried
for 2 h at 37°C. Dye was eluted from the attached cells by adding
100 µl of 0.1 M HCl in each well and then incubated for 5 min at
room temperature. The developed blue color was measured using an
ELISA reader at 630 nm.
To investigate whether the crude extracts have a
synergistic effect with TRAIL, MDA-MB-231 breast cancer cells were
treated with the crude extracts at various concentrations (0.04,
0.1, 0.2, 0.3, 0.6 and 1.2 mg/ml) for 24 h, followed by stimulation
with TRAIL at final concentration of 250 ng/ml for an additional 24
h. Controls referred to wells containing only cells and medium with
and without 10% DMSO.
Detection of apoptosis
The MDA-MB-231 cells (1×105 cells per
well) were seeded in a 12-well plate, treated with different doses
of crude extracts (EGY1, EGY3, EGY24, EGY34) and TRAIL (250 ng/ml)
then incubated for 16 h at 37°C. The cells were harvested by
centrifugation and stained with Annexin V and propidium iodide (PI)
according to the manufacturer's instructions (AbCys SA).
Quantification of apoptosis-induced by the crude extracts with and
without TRAIL was analyzed by flow cytometry using a FACScanto II
flow cytometer (BD Becton-Dickinson).
Western blot analysis
Approximately 1×106 of the cells were
seeded in 6-well plates, treated with the crude extracts, and then
incubated for 24 h. The cells were stimulated with TRAIL (250
ng/ml) and incubated for 24 h. Afterwards, the cells were collected
and lysed in lysis buffer (1% NP-40, Tris-HCl, 3 M NaCl, 5%
glycerol). The concentration of protein was determined by Bradford
reagent (Bio-Rad, Marnes-la-Coquette, France). Proteins were
resolved by electrophoresis on 12% sodium dodecyl
sulfate-polyacrylamide gel and transferred by electro-blotting to
polyvinylidene difluoride (PVDF) membranes (Bio-Rad). The membranes
were incubated overnight with the following primary antibodies.
Anti-TRAIL-R1 (cat# AB16955) and TRAIL-R2 (cat# AB16942) antibodies
were purchased from Chemicon (Millipore, Molsheim, France),
antibodies against caspase-3 (clone 8G10), caspase-8 (clone 5F7)
and caspase-10 (clone 4C1) were all from Medical & Biological
Laboratories (Clinisciences, Montrouge, France). Anti-BIP (cat#
3177) and CHOP (cat# 2895) were obtained from Cell Signaling
Technology (Ozyme, St. Quentin Yvelines, France). Anti-FLIP
antibody (clone Dave-2) was from Adipogen (Coger, Paris, France),
anti-cleaved lamin A/C Asp230 (cat# 3596-1) from Epitomics (Abcam,
Paris, France). Anti-GAPDH (clone 0411) antibody was from Santa
Cruz Biotechnology (Clinisciences, Nantere, France). Anti-actin
antibody (cat# 4970) was from Cell Signaling Technology.
HRP-conjugated anti-rabbit or mouse secondary antibodies were from
Jackson Immuno Research (Interchim, Montluçon, France),
HRP-conjugated anti-mouse IgG1-, Ig2a- and Ig2b-specific antibodies
were from Southern Biotech (Clinisciences). After 3 washes in
PBS-Tween 0.5% for 10 min, membranes were incubated for 1 h with
the corresponding secondary antibody and washed as above. Blots
were developed using the Advansta WesternBright chemiluminescence
substrate according to the manufacturer's protocol (Diagomics,
Blagnac, France) followed by image processing using the Bio-Rad
imaging system.
Results
Cytotoxic effect of crude extracts and
their sensitization to TRAIL in breast cancer cells
Forty-seven actinomycete strains were isolated from
different areas of the Red Sea shore of Sharm El-Sheikh, South
Sinai, Egypt. Microscopic observations revealed that both aerial
and vegetative hyphae were abundant and well developed. The color
of aerial and substrate mycelium of 21 strains varied from yellow
to red. As we are interested in pigmented secondary metabolites,
the cytotoxic effect of 21 crude extracts at concentration of 0.1
mg/ml was screened sequentially with and without TRAIL (250 ng/ml)
in MDA-MB-231 breast cancer cells (Table I). The crude extracts that produced
approximately >20% inhibition along with TRAIL than the agent
alone were selected for further investigation. Four extracts
corresponding to actinomycetes EGY1, EGY3, EGY24 and EGY34 showed
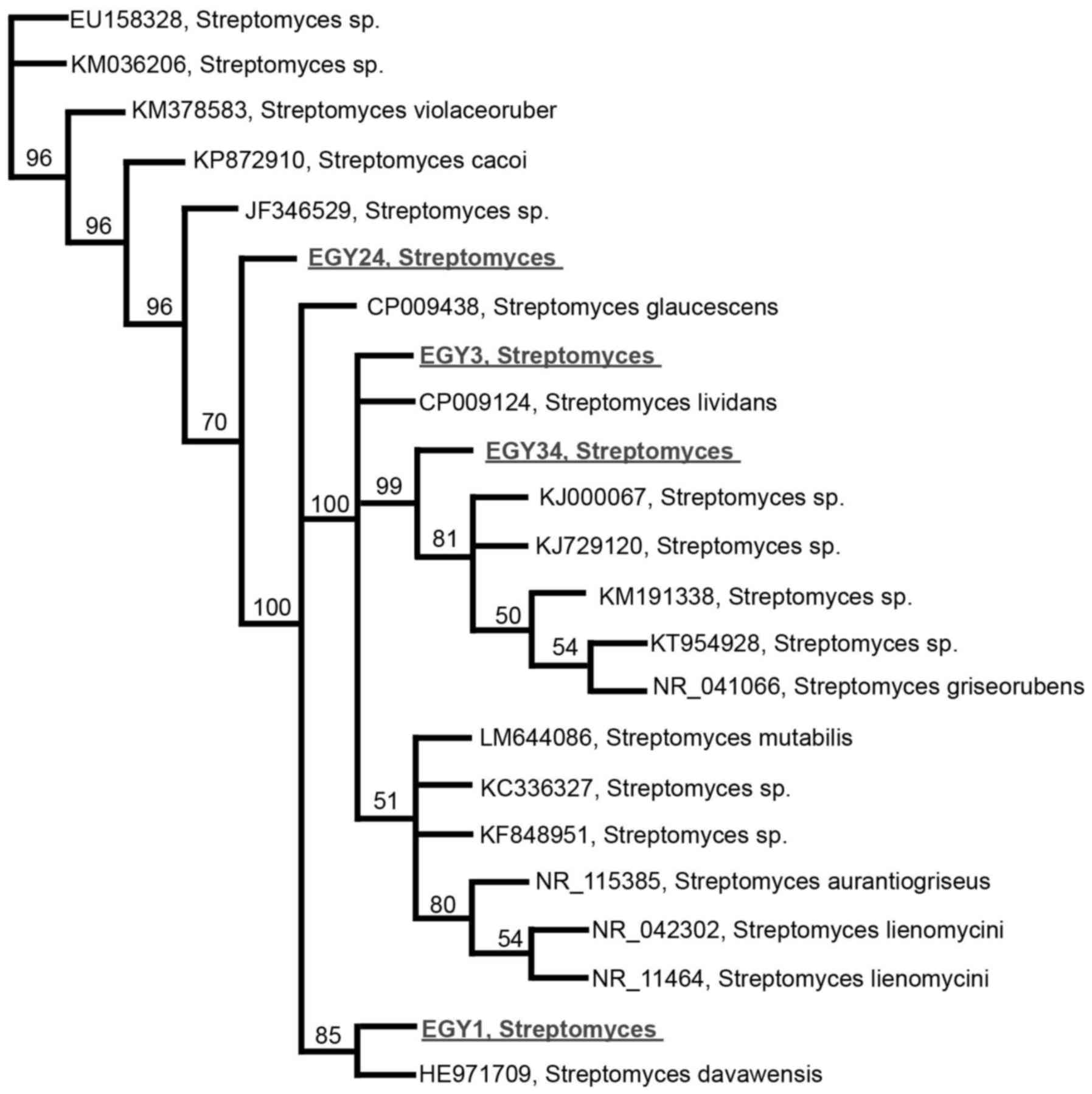
synergistic effects with TRAIL. Partial 16S rRNA sequence analysis
revealed that the effective strains are new member of the genus
Streptomyces sp., indicating <98.2% sequence similarities
(Fig. 1). The 16S rRNA gene
sequence of EGY1, EGY3, EGY24, and EGY34 was submitted to GeneBank
with the accession numbers, KX218228, KP895876, KP792816, and
KP792818, respectively.
 | Table I.Cytotoxic effects of 21 marine
actinomycete crude extracts with and without TRAIL (250 ng/ml) on
TRAIL-resistant MDA-MB-231 breast cancer cell line. |
Table I.
Cytotoxic effects of 21 marine
actinomycete crude extracts with and without TRAIL (250 ng/ml) on
TRAIL-resistant MDA-MB-231 breast cancer cell line.
|
| Cell viability
(%) |
|---|
|
|
|
|---|
| Crude extract (0.1
mg/ml) | −/− TRAIL (0
ng/ml) | +/+ TRAIL (250
ng/ml) | Difference (%) |
|---|
| EGY1 | 75.7 | 43.2 | 32.5 |
| EGY2 | 97.5 | 89.3 |
8.2 |
| EGY3 | 75.8 | 48.2 | 27.6 |
| EGY4 | 98.1 | 92.8 |
5.3 |
| EGY7 | 93.6 | 86.5 |
7.1 |
| EGY8 | 95.6 | 84.3 | 11.3 |
| EGY12 | 94.7 | 82.5 | 12.2 |
| EGY16 | 92.6 | 83.8 |
8.8 |
| EGY20 | 89.3 | 75.5 | 13.8 |
| EGY22 | 91.4 | 81.2 | 10.2 |
| EGY23 | 90.5 | 82.0 |
8.5 |
| EGY24 | 76.3 | 43.3 | 33.0 |
| EGY26 | 93.7 | 88.3 |
5.4 |
| EGY27 | 85.2 | 78.4 |
6.8 |
| EGY30 | 87.8 | 77.5 | 10.3 |
| EGY31 | 85.6 | 78.2 |
7.4 |
| EGY34 | 76.2 | 43.1 | 33.1 |
| EGY39 | 95.8 | 88.6 |
7.2 |
| EGY40 | 86.4 | 72.2 | 14.2 |
| EGY41 | 87.4 | 75.6 | 11.8 |
| EGY47 | 92.4 | 77.3 | 15.1 |
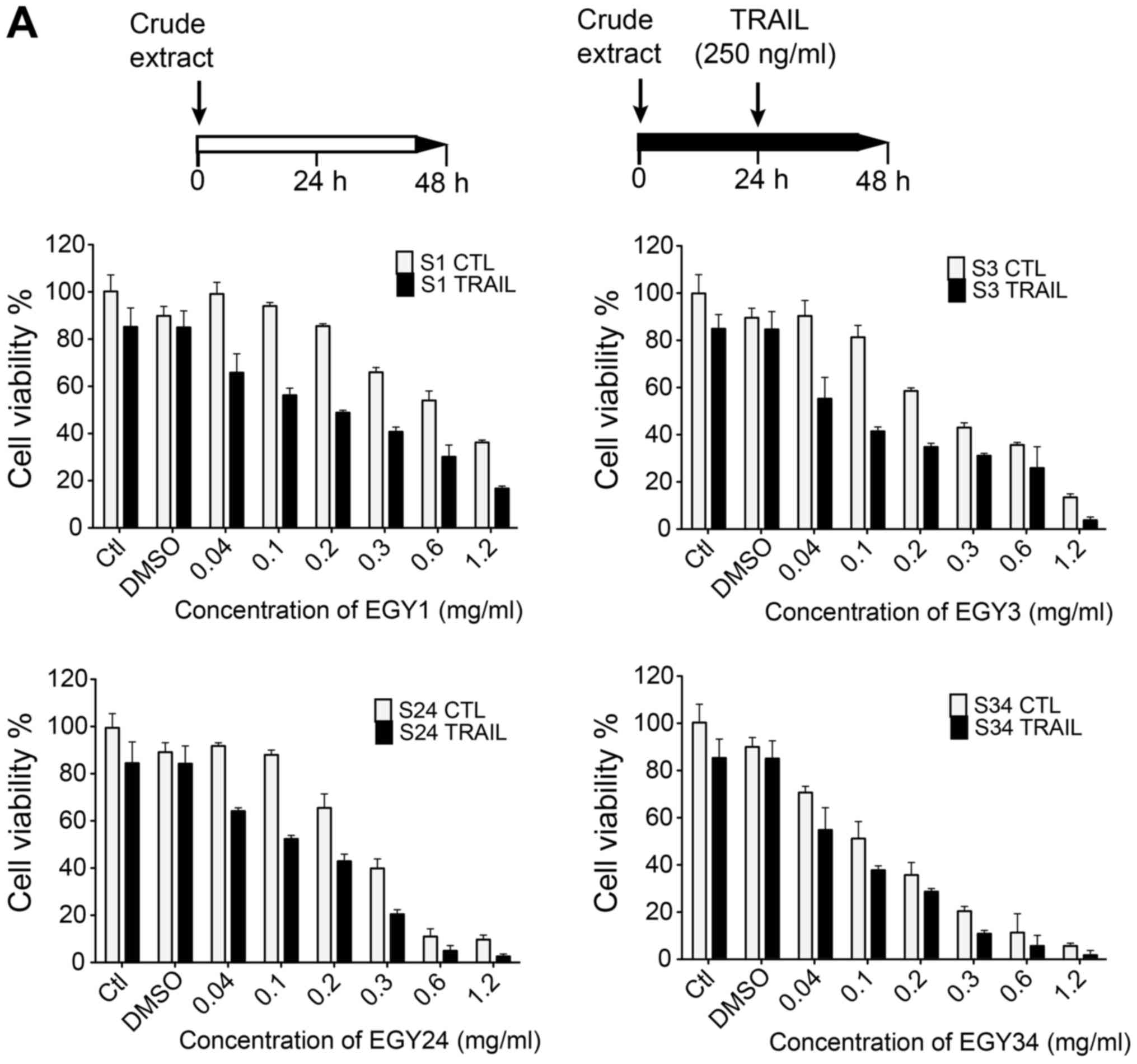
The sensitizing effect of the crude extracts of
EGY1, EGY3, EGY24 and EGY34 to TRAIL-induced apoptosis in
MDA-MB-231 breast cancer cell line in both sequential and combined
treatment with TRAIL was tested. Cells were incubated with various
concentrations of the crude extracts (0.04, 0.1, 0.2, 0.3, 0.6 and
1.2 mg/ml) for 24 h. A significant concentration-dependent decrease
of the cell viability of MDA-MB-231 cancer cell line was observed
(Fig. 2A). When TRAIL (250 ng/ml)
was sequentially added for an additional 24 h, cell viability was
significantly reduced in cells pre-stimulated with these crude
extracts as compared to TRAIL alone. Extracts EGY1, EGY3, EGY24 and
EGY34 induced a marked decrease in cell viability in the presence
of TRAIL (250 ng/ml), compared to TRAIL alone (Fig. 2A). Combined treatments were also
assessed and cells were treated with various concentrations of the
crude extracts and TRAIL (250 ng/ml) for 16 h (Fig. 2B). Of note, TRAIL sensitizing
activity of these crude extracts, even at lower concentrations, was
greatly enhanced. This gives a slight indication that the
synergistic effect-induced by both sequential and combined
treatment may be due to early apoptotic events including DISC
assembly and subsequent activation of initiator caspases.
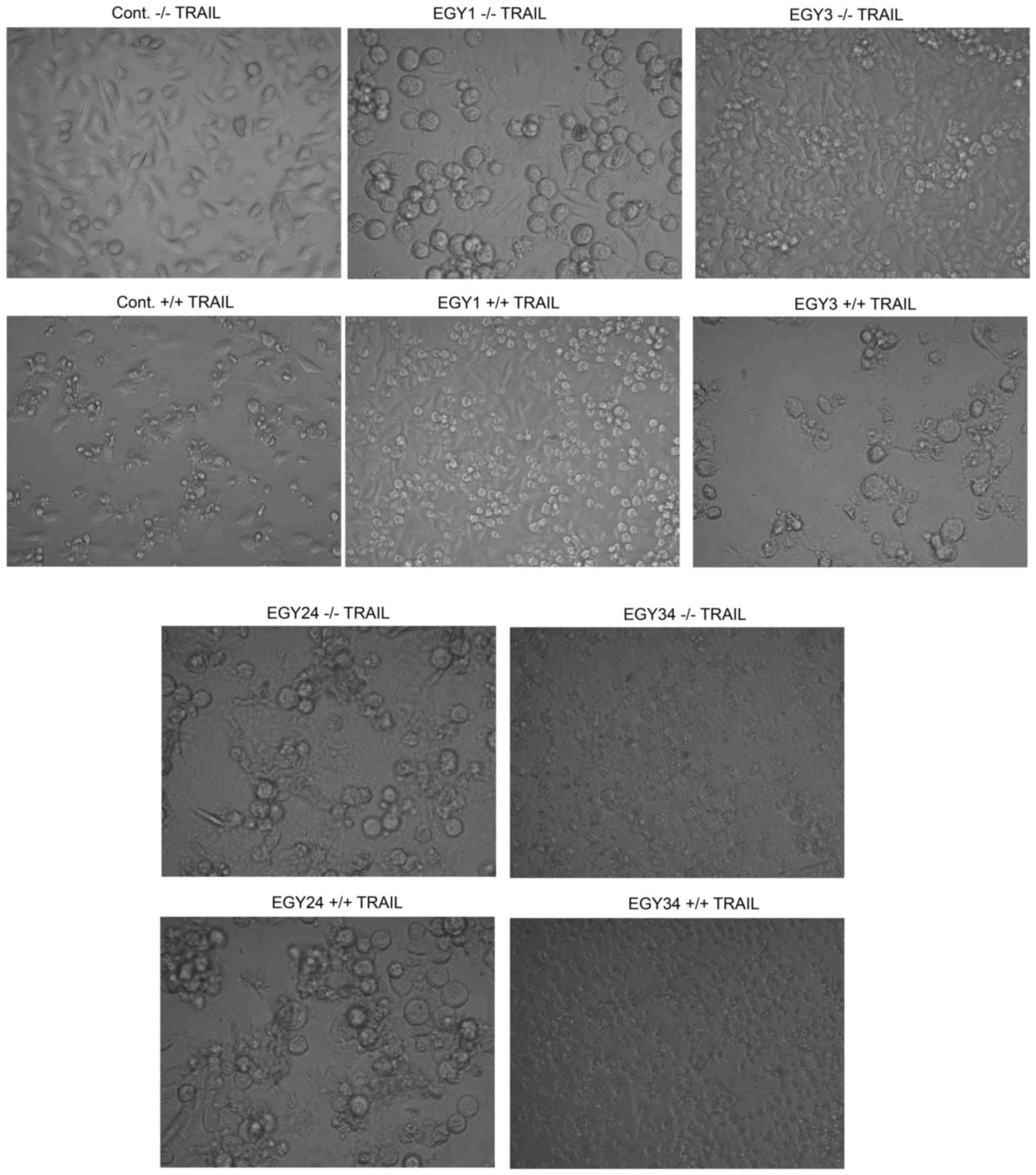
Phenotypic changes of cells pretreated with the
crude extracts and after sequential stimulation with TRAIL were
analyzed by microscopic investigation (Fig. 3). Pretreatment of MDA-MB-231 cells
with the identified crude extracts induced an early apoptosis,
observed by the appearance of granulated cellular lumen, indicative
of apoptotic bodies. On the other hand, cells stimulated with both
crude extracts and TRAIL showed marked apoptosis in which the
condensation of the genetic material in the cell center was clearly
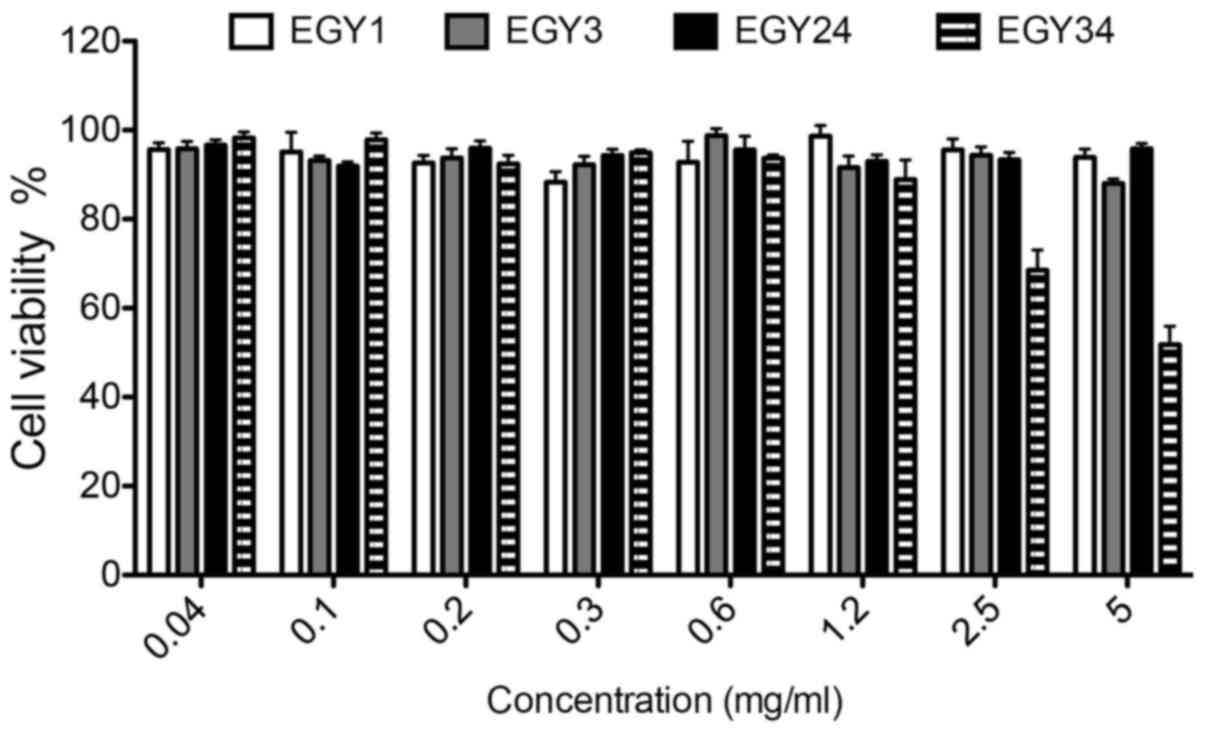
detected. In terms of selectivity, the cytotoxic effect of the
crude extracts on normal mouse embryonic fibroblast (MEF) cell line
was tested (Fig. 4). The crude
extracts showed no cytotoxicity toward the MEF cell line. Only
crude extract EGY34 induced a significant cytotoxicity at the
higher concentrations (2.5 and 5.0 mg/ml) compared to the
non-treated cells.
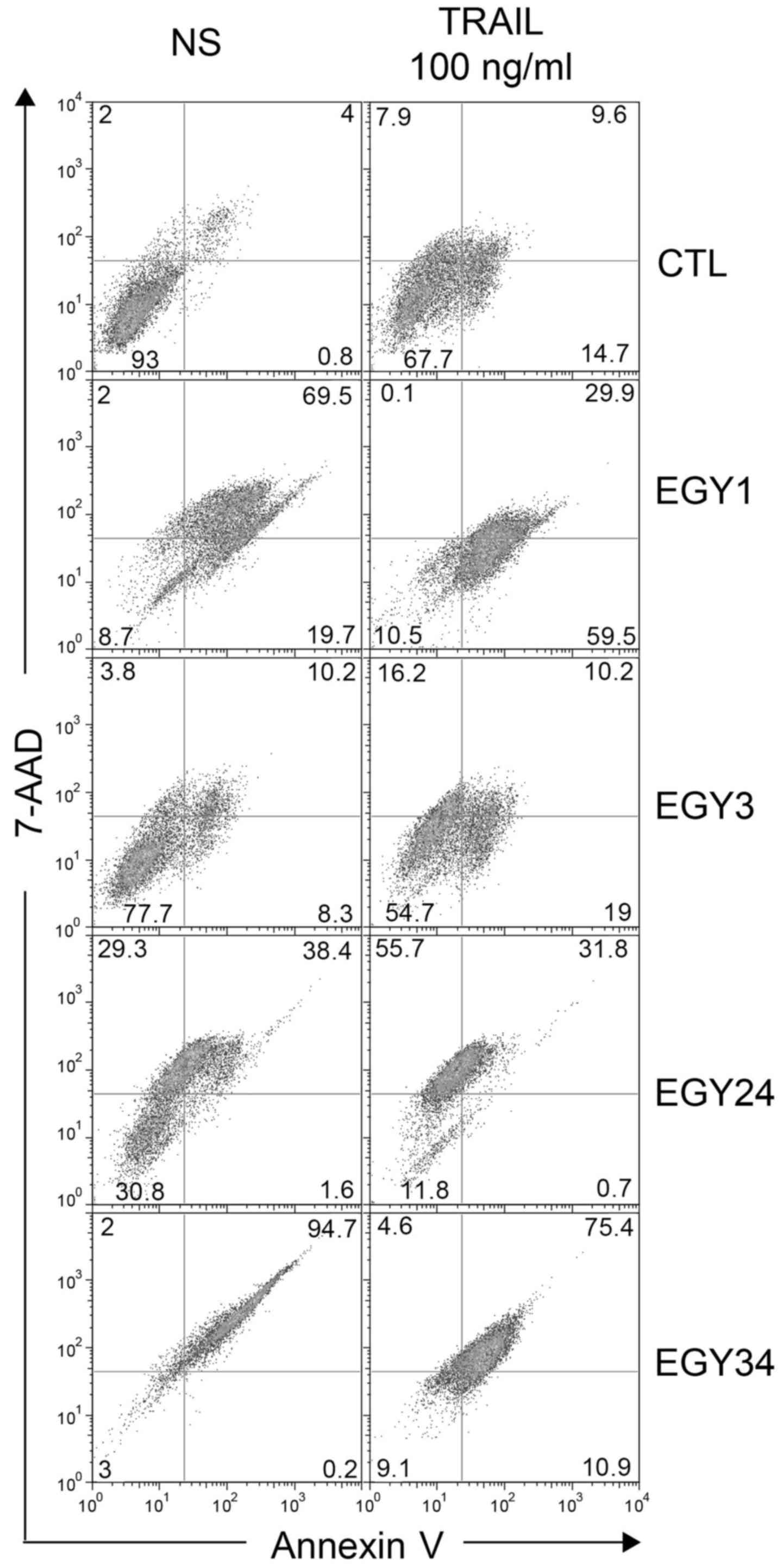
Flow cytometric analysis of
apoptosis
Annexin V and PI staining were used to measure
apoptosis induced by the crude extracts alone and in combination
with TRAIL (Fig. 5). Cells were
treated with the crude extracts at the lowest effective
concentrations and TRAIL (250 ng/ml) for 16 h. The results
correlated with the increase in the percentage of apoptosis
compared to the non-stimulated cells. Flow cytometric analysis
detected a slight increase in the percentage of the apoptotic cells
in the control with and without TRAIL from 6.2 to 18.7% confirming
the resistance of the MDA-MB-231 cells to TRAIL-induced apoptosis.
The highest apoptotic effect was measured for the combined
treatment of crude extracts EGY3 and EGY24 with TRAIL. These
combinations increased apoptosis in MDA-MB-231 cells by
approximately 5- and 2-fold, respectively. Some extracts, including
EGY1, EGY24 and EGY34 induced, alone, a large amount of cell death
as evidenced by Annexin V and PI staining (Fig. 5). Stimulation in the presence of
TRAIL, however, and consistent with cell viability assays (Fig. 2), further increased cell death in
resistant MDA-MB-231 cells, as evidenced with EGY3 and EGY24
extracts (Fig. 5).
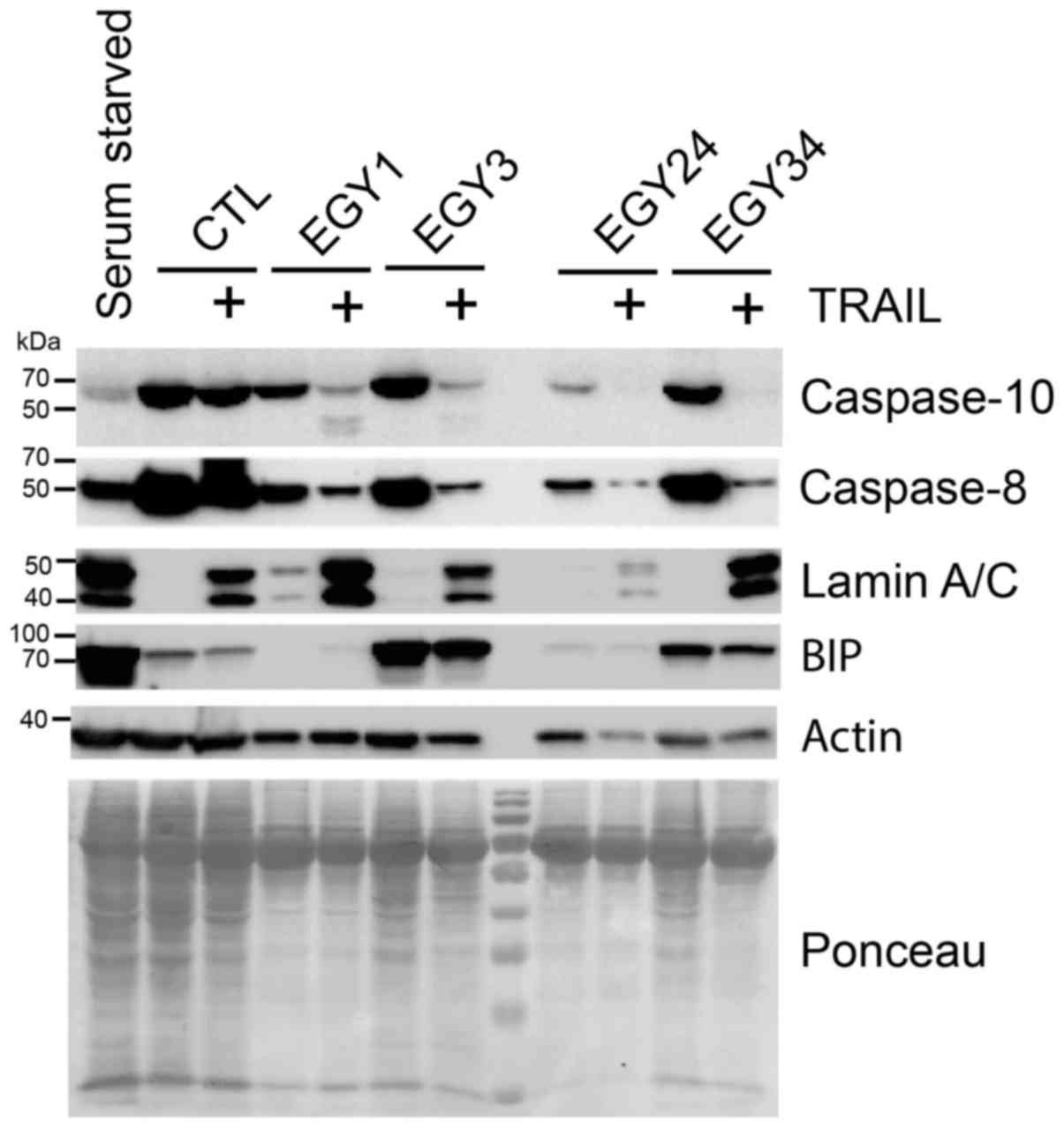
Analysis of the signaling pathways
triggered by the combined treatment
The molecular mechanisms by which the identified
crude extracts trigger the sensitization of the breast cancer cells
to TRAIL-induced cell death were explored by western blot analysis
(Fig. 6). All crude extracts
facilitated activation of the initiator caspase-8/-10 as a result
of combined treatment with TRAIL. Consistent with the increase in
Annexin V staining (Fig. 5), an
increase in cleaved lamin A/C was detected in cells stimulated with
TRAIL and EGY1 or EGY34, but less so with EGY3 and EGY24.
Noteworthy, some crude extracts were also able to induce an ER
stress response. Likewise, as compared to serum starvation, EGY3
and EGY34, but not EGY1 and EGY24 induced by the appearance of the
ER-stress sensor, binding immunoglobulin protein (BiP). These
results therefore suggest that the actinomycete crude extracts
identified here exhibit TRAIL-sensitizing activity, and are likely
to act at least at the level of the TRAIL DISC.
Discussion
The hallmark features for initiation and progression
of cancer is mainly assigned to the defect in the apoptotic
signaling pathways. Despite the potent anticancer effect of TRAIL,
several tumor cell lines including lung and breast cancer cells
were found to possess resistance to TRAIL-induced apoptosis
(35). Overcoming TRAIL-resistance
by combined treatment using a chemotherapeutic agent is considered
as a promising drug target in the treatment of cancer. Marine
microorganisms, particularly actinomycetes still remain a rich
source for the discovery of marine natural products. They are a
group of bacteria, reported to produce great variety of bioactive
secondary metabolites and represent a focal point in the search for
novel antimicrobials and anticancer agents (36,37).
The cytotoxic effect of several plant and marine sponge extracts
against various tumor cell lines was reported (38–43).
However, the anticancer effect of crude extracts isolated from
marine microorganisms has not been studied yet.
In this study, 21 crude extracts of marine
actinomycetes isolated from the Red Sea were found to produce
yellow to red pigments based on the morphological properties.
Actinomycetes are characterized by the production of various types
of colored compounds (i.e. anthraquinones and phenazines) with
TRAIL-resistance overcoming activity in different cancer cell lines
(25,26,28,29).
Therefore, the TRAIL-resistance overcoming activities of the 21
crude extracts were investigated. Four crude extracts (EGY1, EGY3,
EGY24, and EGY34) revealed a significant sensitizing effect to
TRAIL-induced cell death. The extracts exhibited significant
cytotoxic and synergistic effect by combined treatment rather than
by sequential treatment with TRAIL. The significant impact of the
crude extracts on the sensitization of the breast cancer cells to
TRAIL-induced cell death was also confirmed by flow cytometry. It
was shown that the combined treatment of the crude extracts
potentially enhanced the effect of TRAIL by increasing the
reduction of the cell viability. We are still in the
characterization phase of the identified crude extracts represented
in the purification and structural elucidation of the bioactive
components as potent therapeutic agents against breast cancer.
Analyzing the molecular mechanisms for the
restoration of apoptotic cell death by western blotting initially
revealed two possible signaling pathways; i) stimulation of
extrinsic pathway and DISC formation via activation of the
initiator caspase-8/-10 and ii) stimulation of ER-stress-induced
cell death. The combined treatment of MDA-MB-231 cells with the
identified crude extracts and TRAIL was assumed to sensitize the
cells to TRAIL-induced apoptosis by the activation of the initiator
caspase-8/-10, the main components required for the assembly of
DISC (44). Lamins are intermediate
filament proteins lining the inner surface of the inner nuclear
membrane and help maintain the structural integrity of the nucleus.
Caspase-mediated proteolytic cleavage of lamins facilitates the
nuclear disintegration on the apoptotic signaling pathway (45). We found that the combined treatment
of MDA-MB-231 cells with EGY1 and TRAIL induces the activation of
cleaved lamin A/C. Conversely, the single treatment of MDA-MB-231
with EGY3, EGY34 alone indicated an apoptotic signaling
pathway-induced by ER-stress and subsequent activation of the
ER-stress sensor BiP. This protein was reported to play a central
role in the upregulation of DR5 thus inducing apoptosis (46).
The present study revealed the TRAIL-resistant
overcoming activity of some crude extracts isolated from pigmented
marine actinomycetes. The combinatorial treatment of the identified
crude extracts (EGY1, EGY3, EGY24 and EGY34) with TRAIL showed a
great enhancement in cell cytotoxicity at lower concentrations.
Analyzing the molecular mechanism involved in the sensitization of
the breast cancer cells to TRAIL-induced cell death by western
blotting revealed two possible apoptotic signaling pathways; i)
stimulation of the extrinsic pathway via activation of both
caspase-8/-10 and ii) induction of apoptosis mediated by the
activation of the ER-stress sensor BiP as a result of the
combinatorial treatment of MDA-MB-231 cells with EGY3, EGY34, and
TRAIL. Our results are expected to provide new insight into the
development of lead compounds against breast cancer and other types
of cancer.
Acknowledgements
This work was supported by the Science and
Technology Development Fund (STDF), Egypt (grant no. 4930).
References
|
1
|
Almasan A and Ashkenazi A: Apo2L/TRAIL:
Apoptosis signaling, biology, and potential for cancer therapy.
Cytokine Growth Factor Rev. 14:337–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Micheau O, Shirley S and Dufour F: Death
receptors as targets in cancer. Br J Pharmacol. 169:1723–1744.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peter ME and Krammer PH: The
CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelley SK and Ashkenazi A: Targeting death
receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol.
4:333–339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falschlehner C, Emmerich CH, Gerlach B and
Walczak H: TRAIL signalling: Decisions between life and death. Int
J Biochem Cell Biol. 39:1462–1475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griffith TS and Lynch DH: TRAIL: A
molecule with multiple receptors and control mechanisms. Curr Opin
Immunol. 10:559–563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu H, Zhang L, Huang X, Davis JJ, Jacob
DA, Teraishi F, Chiao P and Fang B: Overcoming acquired resistance
to TRAIL by chemotherapeutic agents and calpain inhibitor I through
distinct mechanisms. Mol Ther. 9:666–673. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Greco FA, Bonomi P, Crawford J, Kelly K,
Oh Y, Halpern W, Lo L, Gallant G and Klein J: Phase 2 study of
mapatumumab, a fully human agonistic monoclonal antibody which
targets and activates the TRAIL receptor-1, in patients with
advanced non-small cell lung cancer. Lung Cancer. 61:82–90. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Henson ES, Johnston JB and Gibson SB: The
role of TRAIL death receptors in the treatment of hematological
malignancies. Leuk Lymphoma. 49:27–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Humphreys RC and Halpern W: Trail
receptors: Targets for cancer therapy. Adv Exp Med Biol.
615:127–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ndozangue-Touriguine O, Sebbagh M, Mérino
D, Micheau O, Bertoglio J and Bréard J: A mitochondrial block and
expression of XIAP lead to resistance to TRAIL-induced apoptosis
during progression to metastasis of a colon carcinoma. Oncogene.
27:6012–6022. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thorburn A, Behbakht K and Ford H: TRAIL
receptor-targeted therapeutics: Resistance mechanisms and
strategies to avoid them. Drug Resist Updat. 11:17–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mahalingam D, Szegezdi E, Keane M, de Jong
S and Samali A: TRAIL receptor signalling and modulation: Are we on
the right TRAIL? Cancer Treat Rev. 35:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: Roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Srivastava RK: TRAIL/Apo-2L: Mechanisms
and clinical applications in cancer. Neoplasia. 3:535–546. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morizot A, Mérino D, Lalaoui N, Jacquemin
G, Granci V, Iessi E, Lanneau D, Bouyer F, Solary E, Chauffert B,
et al: Chemotherapy overcomes TRAIL-R4-mediated TRAIL resistance at
the DISC level. Cell Death Differ. 18:700–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacquemin G, Granci V, Gallouet AS,
Lalaoui N, Morlé A, Iessi E, Morizot A, Garrido C, Guillaudeux T
and Micheau O: Quercetin-mediated Mcl-1 and survivin downregulation
restores TRAIL-induced apoptosis in non-Hodgkin's lymphoma B cells.
Haematologica. 97:38–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morlé A, Garrido C and Micheau O:
Hyperthermia restores apoptosis induced by death receptors through
aggregation-induced c-FLIP cytosolic depletion. Cell Death Dis.
6:e16332015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blunt JW, Copp BR, Munro MH, Northcote PT
and Prinsep MR: Marine natural products. Nat Prod Rep. 23:26–78.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayer AM, Rodríguez AD, Berlinck RG and
Hamann MT: Marine pharmacology in 2003-4: Marine compounds with
anthelmintic antibacterial, anticoagulant, antifungal,
anti-inflammatory, antimalarial, antiplatelet, antiprotozoal,
antituberculosis, and antiviral activities; affecting the
cardiovascular, immune and nervous systems, and other miscellaneous
mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol.
145:553–581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williams PG: Panning for chemical gold:
Marine bacteria as a source of new therapeutics. Trends Biotechnol.
27:45–52. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blunt JW, Copp BR, Hu WP, Munro MH,
Northcote PT and Prinsep MR: Marine natural products. Nat Prod Rep.
26:170–244. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fenical W, Sethna KM and Lloyd GK: Marine
microorganisms as a developing resource for drug discovery. Pharm
News. 9:489–494. 2002.
|
|
24
|
Abdelfattah MS: A new bioactive
aminophenoxazinone alkaloid from a marine-derived actinomycete. Nat
Prod Res. 27:2126–2131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abdelfattah MS, Ishikawa N, Karmakar UK,
Yamaku K and Ishibashi M: New phenazine analogues from Streptomyces
sp. IFM 11694 with TRAIL resistance-overcoming activities. J
Antibiot (Tokyo). 69:446–450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abdelfattah MS, Toume K and Ishibashi M:
Yoropyrazone, a new naphthopyridazone alkaloid isolated from
Streptomyces sp. IFM 11307 and evaluation of its TRAIL
resistance-overcoming activity. J Antibiot (Tokyo). 65:245–248.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdelfattah MS, Ishikawa N, Karmakar UK
and Ishibashi M: Sulfotanone, a new alkyl sulfonic acid derivative
from Streptomyces sp. IFM 11694 with TRAIL resistance-overcoming
activity. J Nat Med. 70:266–270. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdelfattah MS, Kazufumi T and Ishibashi
M: New pyranonaphthoquinones and a phenazine alkaloid isolated from
Streptomyces sp. IFM 11307 with TRAIL resistance-overcoming
activity. J Antibiot (Tokyo). 64:729–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abdelfattah MS, Kazufumi T and Ishibashi
M: Izumiphenazines A-C: Isolation and structure elucidation of
phenazine derivatives from Streptomyces sp. IFM 11204. J Nat Prod.
73:1999–2002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Elmallah MIY and Micheau O: Marine drugs
regulating apoptosis induced by tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL). Mar Drugs. 13:6884–6909. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hayakawa M and Nonomura H: Humic
acid-vitamin agar, a new medium for the selective isolation of soil
actinomycetes. J Ferment Technol. 65:501–509. 1987. View Article : Google Scholar
|
|
32
|
Kuester E and Williams ST: Selection of
media for the isolation of streptomycetes. Nature. 202:928–929.
1964. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kieser T, Bibb MJ, Buttner MJ, Chater KF
and Hopwood DA: Practical Streptomyces Genetics. 2nd. The John
Innes Center Foundation; Norwich: 2000
|
|
34
|
Abdelfattah MS, Elmallah MIY, Hawas UW,
El-Kassema LT Abou and Eid MAG: Isolation and characterization of
marine-derived actinomycetes with cytotoxic activity from the Red
Sea coast. Asian Pac J Trop Biomed. 6:651–657. 2016. View Article : Google Scholar
|
|
35
|
LeBlanc HN and Ashkenazi A: Apo2L/TRAIL
and its death and decoy receptors. Cell Death Differ. 10:66–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takahashi Y and Omura S: Isolation of new
actinomycete strains for the screening of new bioactive compounds.
J Gen Appl Microbiol. 49:141–154. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blunt JW, Copp BR, Hu WP, Munro MH,
Northcote PT and Prinsep MR: Marine natural products. Nat Prod Rep.
24:31–86. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Slambrouck S, Daniels AL, Hooten CJ,
Brock SL, Jenkins AR, Ogasawara MA, Baker JM, Adkins G, Elias EM,
Agustin VJ, et al: Effects of crude aqueous medicinal plant
extracts on growth and invasion of breast cancer cells. Oncol Rep.
17:1487–1492. 2007.PubMed/NCBI
|
|
39
|
Ferretti C, Marengo B, De Ciucis C, Nitti
M, Pronzato MA, Marinari UM, Pronzato R, Manconi R and Domenicotti
C: Effects of Agelas oroides and Petrosia ficiformis crude extracts
on human neuroblastoma cell survival. Int J Oncol. 30:161–169.
2007.PubMed/NCBI
|
|
40
|
Oliveras-Ferraros C, Fernández-Arroyo S,
Vazquez-Martin A, Lozano-Sánchez J, Cufí S, Joven J, Micol V,
Fernández-Gutiérrez A, Segura-Carretero A and Menendez JA: Crude
phenolic extracts from extra virgin olive oil circumvent de novo
breast cancer resistance to HER1/HER2-targeting drugs by inducing
GADD45-sensed cellular stress, G2/M arrest and hyperacetylation of
Histone H3. Int J Oncol. 38:1533–1547. 2011.PubMed/NCBI
|
|
41
|
Szliszka E, Zydowicz G, Janoszka B, Dobosz
C, Kowalczyk-Ziomek G and Krol W: Ethanolic extract of Brazilian
green propolis sensitizes prostate cancer cells to TRAIL-induced
apoptosis. Int J Oncol. 38:941–953. 2011.PubMed/NCBI
|
|
42
|
Abdelhamed S, Yokoyama S, Hafiyani L,
Kalauni SK, Hayakawa Y, Awale S and Saiki I: Identification of
plant extracts sensitizing breast cancer cells to TRAIL. Oncol Rep.
29:1991–1998. 2013.PubMed/NCBI
|
|
43
|
Lee YS, Lee DG, Lee JY, Kim TR, Hong SS,
Kwon SW and Kim YS: A formulated red ginseng extract upregulates
CHOP and increases TRAIL-mediated cytotoxicity in human
hepatocellular carcinoma cells. Int J Oncol. 43:591–599.
2013.PubMed/NCBI
|
|
44
|
Thorburn A: Death receptor-induced cell
killing. Cell Signal. 16:139–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rao L, Perez D and White E: Lamin
proteolysis facilitates nuclear events during apoptosis. J Cell
Biol. 135:1441–1455. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|