Introduction
Globally, the incidence of gastric cancer (GC) ranks
fourth in men and fifth in women, and affects more than one million
individuals per year. The death rate for GC is next to lung cancer,
and the number of deaths caused by GC ranks third in men and fourth
in women among total cancer-related deaths (1,2).
Accurate figures vary in regards to different populations and world
regions (3,4). Despite the declining morbidity and
improved standardized treatment, GC carries a poor prognosis with
the mortality-to-incidence ratio ranging from 0.35 to 0.8 (5). This situation probably results from
the fact that patients with GC are often diagnosed at the advanced
stage, along with a high incidence of metastasis and
recurrence.
Carcinomas arising from epithelial tissues progress
to higher pathological grades of malignancy, as reflected in local
invasion and distant metastasis. Along with this process, the
associated cancer cells develop alterations in their shape as well
as in their attachment to other cells and to the extracellular
matrix (ECM). Loss of epithelial-cadherin (E-cadherin) by carcinoma
cells, a key cell-to-cell adhesion molecule, is well characterized
in this alteration. Inactivation of E-cadherin induces expression
of transcriptional repressors such as Snail and ZEB family numbers,
subsequently inducing epithelial-mesenchymal transition (EMT) which
is a hallmark of tumor progression (6,7).
Rudolf Virchow described leukocyte infiltrates
within tumors in the 19th century (8), and now it is clear that the
infiltrates can exert both tumor-suppressive and tumor-promoting
effects. Chemokines and chemokine receptors, which were initially
researched for their role in the regulation of leukocyte
trafficking to inflammatory sites, were found to be involved in
enhancing the immunity of tumor-associated antigens, regulating new
blood vessel formation, promoting cancer cell proliferation and
directing cancer cell metastasis (9–11).
CXCR6 was reported to be positively correlated with
Gr-1+ neutrophil infiltration and microvessel growth, to
lead to a protumor inflammatory microenvironment, and to predict
poor prognosis in hepatocellular carcinoma (HCC) (12). GC is inflammation-related and
Helicobacter pylori infection increases the risk of GC 3- to
6-fold (13). However, a recent
study showed that blockade of CXCR6 reduced the migration and
invasion of GC cells (14). Thus,
CXCR6 is possibly involved in gastric tumorigenesis and metastasis.
We explored CXCR6 expression in a cohort of 352 GC cases, and
analyzed the association of CXCR6 expression with
clinicopathological parameters and survival in GC.
Materials and methods
Patients and specimens
The present study was approved by the Ethics
Committee of Fudan University Shanghai Cancer Center (Shanghai,
China). Written informed consent was obtained from all of the
patients enrolled in the present study. A cohort of 352 surgically
resected GC patients recruited between 2010 and 2011 at Fudan
University Shanghai Cancer Center were enrolled in the study.
Patients did not have signs of distant metastasis nor had they
received anticancer therapy before surgery. Tumor stage was
determined according to the 2010 American Joint Committee on Cancer
(AJCC)/International Union Against Cancer (UICC)
tumor-node-metastasis (TNM) classification system. Conventional
clinicopathologic variables, including age, gender,
carcinoembryonic antigen (CEA), tumor size, degree of
differentiation, vascular invasion, tumor stage, therapy and
status, were recorded and are documented in Table I.
 | Table I.Clinicopathological features of the GC
patients. |
Table I.
Clinicopathological features of the GC
patients.
| Variables | Results |
|---|
| Age (years): median
(range) | 62 (21–84) |
| Gender:
male/female | 275/77 |
| CEA (preoperative)
(ng/ml): median (range) | 2.07 (0–539.26) |
| Tumor size (cm):
median (range) | 5 (0.9–15) |
| Vascular invasion:
absence/presence | 161/191 |
| Tumor
differentiation: well or moderate/poor | 171/181 |
| AJCC/UICC TNM stage:
I/II/III/IV | 20/65/204/63 |
| Adjuvant therapy:
none/chemotherapy | 26/326 |
| Alive with recurrence
(without recurrence)/death due to tumor (non-tumor) | 26 (222)/66 (38) |
Eight pairs of fresh frozen human GC tumor and
matched peritumoral tissues were obtained for western blot
analysis. Ethical approval was obtained from Fudan University
Cancer Center Research Ethics Committee and written informed
consent was obtained from each patient.
Follow-up and postoperative
treatment
After surgery, patients with stage II or III were
treated with chemotherapy. The adjuvant treatment lasted for 1 year
which included oxaliplatin with fluoropyrimidine or oxaliplatin
with capecitabine for 6–8 courses, and oral fluoropyrimidine or
capecitabine for the rest of the year. Certain patients interrupted
the treatment due to toxic side-effects. Follow-up was conducted
following our standard protocol (every 3 months for at least 2
years, every 6 months for the next 3 years, and after 5 years every
12 months for the duration of life) (15). Patient monitoring included physical
examination, tumor marker assessment, ultrasound, chest
radiography, computed tomographic scan and endoscopic examination.
A diagnosis of recurrence was based on typical imaging appearance
in computed tomography and/or endoscopic examination. The treatment
modality after relapse varied among the individuals. OS was defined
as the interval between surgery and death, or between surgery and
the last observation for surviving patients. Data were censored at
the last follow-up for patients without relapse, or death.
Follow-up was completed on July 30, 2014. The median follow-up was
40 months (range, 1–59 months).
Tissue microarray and
immunohistochemistry
All GC cases were histologically reviewed by
hematoxylin and eosin (H&E) staining and representative areas
were pre-marked in the paraffin blocks, away from necrotic and
hemorrhagic materials. A duplicate of 1.5-mm diameter cylinders was
included in each case to ensure reproducibility of the slides.
Thus, 8 different tissue microarray blocks were constructed.
Sections of 4-µm thickness were placed on
3-aminopropyltriethoxysilane-coated slides. CXCL16 (1:100) and
CXCR6 (1:100) polyclonal antibodies were both purchased from Abcam
(Cambridge, UK).
Immunohistochemistry of tissue microarrays was
carried out using a two-step protocol. Briefly, paraffin sections
were deparaffinized, hydrated and washed in Tris-buffered saline
containing Tween-20 (TBST). After microwave antigen retrieval,
endogenous peroxidase activity was blocked as required by
incubating the slides in 0.3% H2O2 and
non-specific binding sites were blocked with Protein Block
(Novocastra Laboratories, Newcastle upon Tyne, UK). Then, the
tissues were incubated with primary antibodies for 12 h at 4°C, and
then washed off. The components of the EnVision Plus detection
system were applied (EnVision+/HRP/Mo; Dako,
Carpinteria, CA, USA), and the sections were developed in
3,3-diaminobenzidine solution under microscopic observation and
counterstained with hematoxylin. Negative controls identically
treated, but with the primary antibodies omitted were included in
all assays.
All slides were independently evaluated by two
experienced pathologists. Immunoreactivity scores of CXCR6 and
CXCL16 staining were determined by a semi-quantitative method
multiplying the proportion and intensity of positively stained
tumor cells. The percentage of positive cells was scored as 0 (no
positive cells), 1 (<25%), 2 (26–50%), 3 (51–75%), and 4
(76–100%). Staining intensity was scored as 0 (negative), 1 (weak),
2 (moderate), and 3 (strong). The staining intensity score
multiplied by the percentage of positive staining was used to
define the expression levels of CXCR6. Median value of all scores
was used as a cut-off point for classification of protein
expression. The GC patients were divided into two groups: a low
expression (scores, 0–6) and a high expression group (scores,
6–12), for the CXCR6 protein.
Cell lines and transfection
Gastric carcinoma cell lines HGC-27 and SGC-7901
were obtained from Shanghai Cell Bank, Chinese Academy of Sciences
and maintained in RPMI-1640 medium (HyClone, Logan, UT, USA)
containing 10% fetal bovine serum (FBS) (Biological Industries,
Beit-Haemek, Israel), 100 U/ml penicillin G and 100 µg/ml
streptomycin. Lentiviral expression plasmid pCDH-cmV-EF1-copGFP
(purchased from System Biosciences, Mountain View, CA, USA) was
used to generate CXCR6 (NM_006564) expression plasmid by Genesent
Technologies (Shanghai, China). To silence the expression of CXCR6,
a short hairpin RNA (shRNA) sequence targeting the CXCR6 gene was
purchased from Genesent Technologies. The lentivirus was harvested
48 h after co-transfection of the targeted plasmids, with psPAX2
and pMD2.G or the corresponding empty vector into HEK-293T cells
using Lipofectamine 2000 transfection reagent (Invitrogen,
Carlsbad, CA, USA). Target cells were infected with the filtered
lentivirus plus 6 µg/ml Polybrene (Sigma-Aldrich, St. Louis, MO,
USA) for 24 h.
Western blot analysis
Expression levels of CXCR6 in tumor and peritumoral
tissues were evaluated via western blot analysis. Total protein was
extracted in lysis buffer for 30 min on ice. Equal amounts of
protein were separated by sodium dodecylsulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto polyvinylidene
fluoride membranes. The primary antibody against CXCR6 (1:1,000;
Abcam) was used. A monoclonal antibody against GAPDH (1:1,000) was
used as an internal control. Each experiment was repeated at least
3 times.
Transwell assays
The migration ability of the tested cells was
evaluated in 24-well Corning chambers (8-µm pore size) (Corning,
NY, USA). A total of 5×104 cells in serum-free medium
was added to the upper chamber. Invasion assay was conducted using
the Transwell inserts coated with Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA). Cells (2×105) were seeded into
the upper chambers in serum-free medium. After 48 h of incubation
at 37°C in 5% CO2, the cells that had invaded were fixed
and stained in dye solution (Beyotime, NKG, China). The cells that
had migrated/invaded were counted in 5 random fields at a
magnification of ×100, and imaged using an IX71 inverted microscope
(Olympus Corp., Tokyo, Japan). Each experiment was carried out in
triplicate.
Cell proliferation assay
Cell proliferation was determined using Cell
Counting Kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan). Tested
cells were seeded into 96-well plates (Corning) at a density of
1,000 cells/well. Cells were allowed to grow for 1, 2, 3, 4, 5, 6
and 7 days, and then 10 µl of CCK-8 solution was added to each well
and incubated at 37°C for 4 h. Cell viability was detected by
measurement of absorbance at 490 nm using a microplate reader
(ELx800NB; BioTek Instruments, Winooski, VT, USA).
Statistical analysis
Statistical analyses were conducted using SPSS
version 19.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism
version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Statistically significant differences were analyzed by the
Chi-square test for categorical variables and the Student's t-test
for continuous variables. Cumulative survival rates were calculated
by the Kaplan-Meier method, and differences between the survival
curves were analyzed by the log-rank test. Univariate and
multivariate analyses were performed using the Cox
proportional-hazards model. Statistically significant differences
were determined at a P-value of <0.05.
Results
CXCR6 is correlated with lymph node
and distant metastasis, and advanced clinical stage in patients
with GC
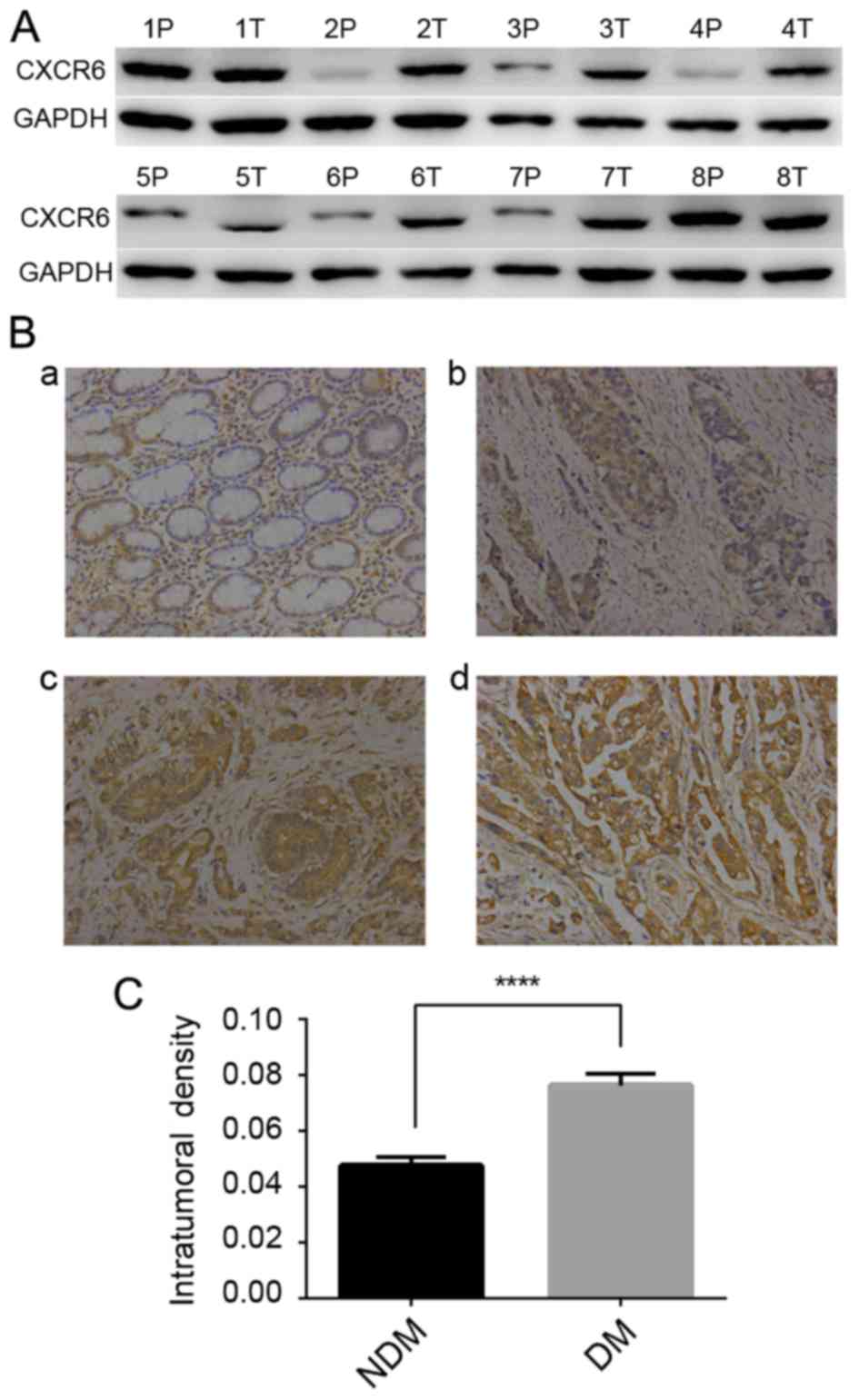
To investigate the role of CXCR6 in GC, we first
examined CXCR6 protein in 8 pairs of frozen GC tumor and
peritumoral tissues by western blotting, and found that CXCR6
expression was elevated in the tumor tissues (Fig. 1A). Further study was conducted in
352 GC specimens with immunohistochemical staining for CXCR6. The
results showed that in GC tissues, CXCR6 staining was strong in 58,
moderate in 152, weak in 100 and negative in 42 cases, located
diffusely in the cytoplasm and cell membrane, while in paired
peritumoral tissues, CXCR6 expression was moderate or weak
(Fig. 1B). Moreover, in patients
with distant metastasis (DM) (n=63), CXCR6 staining densities were
significantly higher than that in patients without DM (n=289;
Mann-Whitney test; P<0.0001; Fig.
1C). For further analysis, patients were classified into a
CXCR6-low (negative and weak; n=142) or -high (moderate and strong;
n=210) group. Clinicopathologic analysis revealed that expression
of CXCR6 was positively correlated with larger tumor size (≥5 cm;
P=0.004), poor differentiation status (P=0.001), LN metastasis
(P=0.001), DM (P=0.011) and advanced TNM stages (stage III and IV;
P=0.006) (Table II). Taken
together, CXCR6 was significantly correlated with LN metastasis, DM
and advanced TNM stage in patients with GC.
 | Table II.Correlation between tumor CXCR6
expression and clinicopathological features of the gastric cancer
cases. |
Table II.
Correlation between tumor CXCR6
expression and clinicopathological features of the gastric cancer
cases.
|
| CXCR6 expression |
|
|---|
|
|
|
|
|---|
| Characteristics | High (n=210) n
(%) | Low (n=142) n
(%) | P-value |
|---|
| Age, years |
|
| 0.155 |
|
<60 | 82 (56.2) | 64 (43.8) |
|
| ≥60 | 128 (62.1) | 78 (27.9) |
|
| Gender |
|
| 0.115 |
| Male | 159 (57.8) | 116 (42.2) |
|
|
Female | 51 (58.6) | 26 (41.4) |
|
| Tumor size
(cm) |
|
| 0.004 |
|
<5 | 99 (52.9) | 88 (47.1) |
|
| ≥5 | 111 (67.3) | 54 (32.7) |
|
| Differentiation
status |
|
| 0.001 |
|
Well/moderately | 123 (69.5) | 54 (30.5) |
|
|
Poor | 87 (49.7) | 88 (50.3) |
|
| Vascular
invasion |
|
| 0.069 |
|
Absent | 103 (64.3) | 57 (35.7) |
|
|
Present | 107 (56) | 84 (44) |
|
| Infiltration
depth |
|
| 0.193 |
| T1,
T2 | 20 (69) | 9 (31) |
|
| T3,
T4 | 190 (58.8) | 133 (41.2) |
|
| Lymph node
metastasis |
|
| 0.001 |
|
Absent | 61 (77.2) | 18 (21.8) |
|
|
Present | 149 (54.6) | 124 (45.4) |
|
| Distant
metastasis |
|
| 0.011 |
|
Absent | 164 (56.7) | 125 (43.3) |
|
|
Present | 46 (73) | 17 (27) |
|
| TNM stage |
|
| 0.006 |
| I,
II | 61 (71.8) | 24 (28.2) |
|
| III,
IV | 149 (53.8) | 118 (46.2) |
|
CXCR6 is an independent prognostic
factor for OS and DFS in GC
Survival analysis was conducted to determine
prognostic factors for overall survival (OS) and disease-free
survival (DFS) in GC patients. Univariate analysis showed that
larger tumor size (P=0.010), elevated preoperative serum CEA level
(P=0.012), advanced TNM stage (P<0.001), and high CXCR6
expression (P=0.030) indicated both worse OS and DFS in GC.
Nevertheless vascular invasion predicted shorter OS time, but had
no impact on DFS in GC (Table
III). As shown in Fig. 2,
patients in the CXCR6-high group had significantly shorter OS and
DFS time than those in the CXCR6-low group. Results remained
significant in cases with stage III and IV (median DFS time; 29 vs.
40 months; P<0.001). On the basis of these results, multivariate
Cox regression analysis was conducted, TNM stage and CXCR6
expression were verified to be independent prognostic factors for
both OS and DFS in GC, and preoperative serum CEA level was an
independent prognostic factor for DFS in GC (Table IV). Hence, conclusions were drawn
that CXCR6 expression was an independent prognostic factor for both
OS and DFS in GC.
 | Table III.Univariate analysis of prognostic
variables for gastric cancer. |
Table III.
Univariate analysis of prognostic
variables for gastric cancer.
|
| OS | DFS |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) (<60
vs. ≥60) | 0.95
(0.65–1.41) | 0.806 | 0.94
(0.64–1.38) | 0.742 |
| Gender (male vs.
female) | 1.04
(0.65–1.67) | 0.867 | 1.03
(0.65–1.66) | 0.867 |
| Tumor size (cm)
(<5 vs. ≥5) | 1.66
(1.23–2.45) | 0.010 | 1.62
(1.09–2.39) | 0.015 |
| Differentiation
status (well vs. poor) | 0.83
(0.57–1.22) | 0.352 | 0.82
(0.56–1.20) | 0.352 |
| Vascular invasion
(absent vs. present) | 1.57
(1.05–2.35) | 0.030 | 1.47
(0.98–2.18) | 0.057 |
| CEA (ng/ml)
(<5.2 vs. ≥5.2) | 1.69
(1.12–2.55) | 0.012 | 1.78
(1.18–2.68) | 0.012 |
| TNM stage (II vs.
III/IV) | 0.13
(0.05–0.32) | 0 | 0.13
(0.05–0.32) | 0 |
| CXCR6 expression
(low vs. high) | 2.32
(1.49–3.64) | 0.001 | 2.39
(1.53–3.37) | 0.001 |
 | Table IV.Multivariate analysis of prognostic
variables. |
Table IV.
Multivariate analysis of prognostic
variables.
|
| OS | DFS |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Tumor size (cm)
(<5 vs. ≥5) | 1.28
(0.86–1.89) | 0.214 | 1.24
(0.84–1.83) | 0.283 |
| Vascular invasion
(yes vs. no) | 1.10
(0.73–1.65) | 0.623 |
|
|
| CEA (ng/ml)
(<5.2 vs. ≥5.2) | 1.45
(0.96–2.20) | 0.730 | 1.54
(1.02–2.32) | 0.040 |
| TNM stage (I/II vs.
III/IV) | 0.12
(0.05–0.30) | 0.000 | 0.12
(0.05–0.29) | 0.000 |
| CXCR6 expression
(low vs. high) | 2.78
(1.77–4.37) | 0.003 | 2.85
(1.81–4.47) | 0.010 |
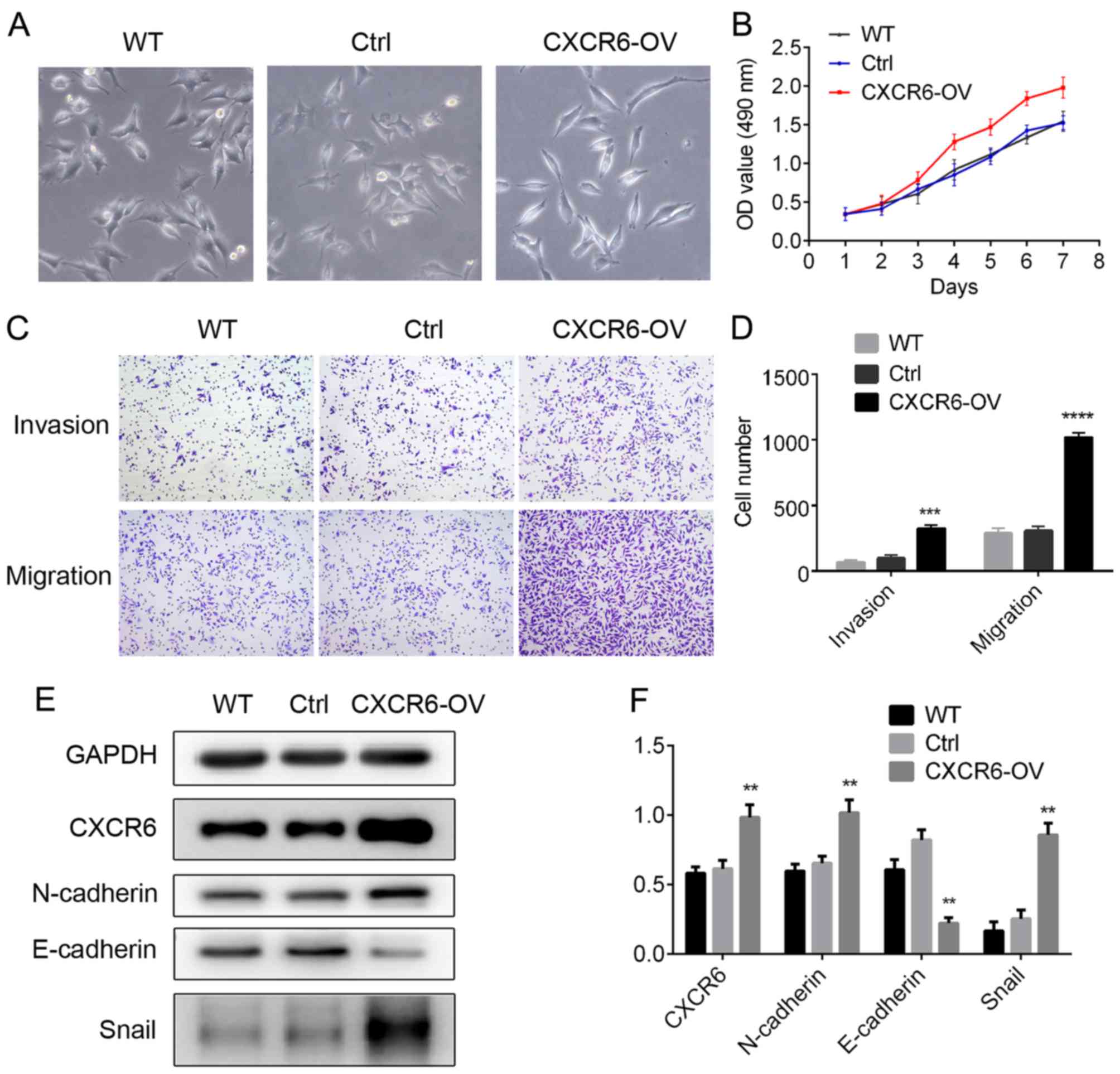
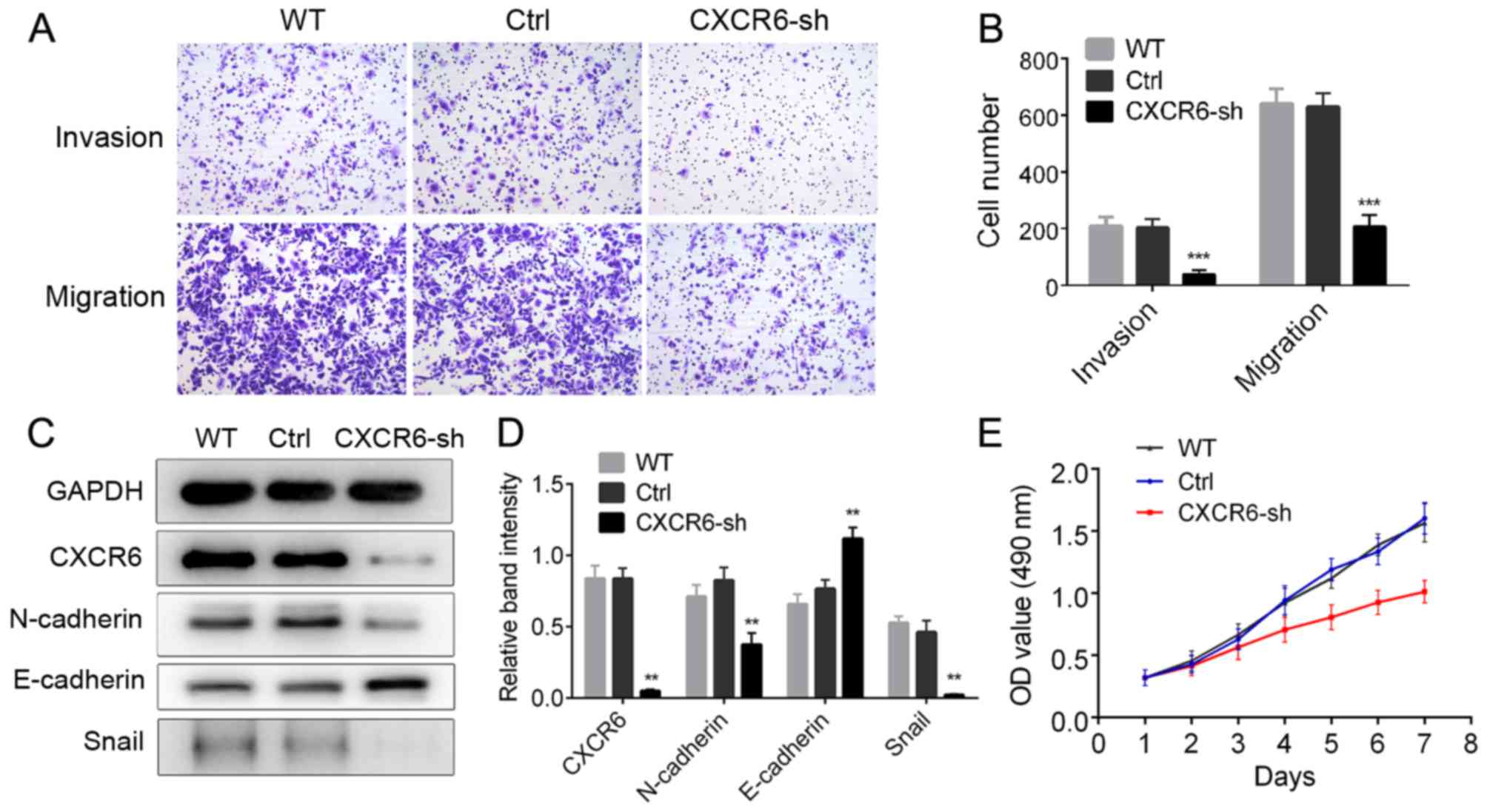
CXCR6 promotes proliferation, invasion
and migration in GC cells
Considering the clinical significance of CXCR6 in GC
patients, we examined the biological effects of CXCR6 in GC cell
lines. CXCR6-shRNA and CXCR6 expression vector, and the
corresponding negative control vectors were transfected into the GC
cell lines, SGC-7901 and HGC-27, respectively. Western blotting
showed that CXCR6 was upregulated in the HGC-27-CXCR6-OV cells
(Fig. 3E and F), and was reduced in
the SGC-7901-CXCR6-sh cells (Fig. 4C
and D). CCK-8 assay was used to examine cell proliferation.
Results showed that CXCR6 overexpression significantly increased GC
cell proliferation (Fig. 3B), while
CXCR6 reduction inhibited GC cell proliferation (Fig. 4E). Transwell assays were used to
assess the migration and invasion abilities of the GC cells.
Results showed that HGC27-CXCR6-OV cells exhibited higher migration
and invasion abilities compared with the control cells (P<0.001
and P<0.0001; Fig. 3C and D),
while SGC7901-CXCR6-sh cells displayed decreased migration and
invasion abilities compared with the control cells (P<0.001;
Fig. 4A and B). In conclusion,
CXCR6 increased proliferation, and promoted the migration and
invasion of GC cells.
CXCR6 promotes EMT in GC cells
We found that HGC27-CXCR6-OV cells displayed a loose
cell-cell contact, and spindle-shaped morphology representative of
EMT (Fig. 3A). Previous studies
have proposed that EMT is associated with cancer cell migration,
tumor metastasis and progression. Therefore, we explored levels of
EMT markers in the CXCR6 overexpressing/silenced cells. Western
blotting showed that in the HGC27-CXCR6-OV cells, expression of
mesenchymal markers, N-cadherin and Snail, was significantly
upregulated, while expression of epithelial marker, E-cadherin, was
suppressed (P<0.01; Fig. 3E and
F); while in SGC7901-CXCR6-sh cells, N-cadherin and Snail
expression was decreased, while E-cadherin was upregulated
(P<0.01; Fig. 4C and D).
Considering the changes in representative cell morphology and EMT
marker expression, we conclude that CXCR6 promotes EMT in GC
cells.
Discussion
We studied the association between CXCR6 expression
and gastric cancer (GC) patients after follow-up for at least 3
years. The 3-year OS rate for patients with high CXCR6 expression
was 63%, while the 3-year OS rate for patients with low CXCR6
expression was 81.6% (log-rank test: HR, 2.334; 95% CI,
1.469–3.188; P<0.001). Notably, multivariate analysis showed for
the first time that CXCR6 is an independent predictor for both OS
and DFS in GC. Patients with stage III/IV and high CXCR6 expression
exhibited worse OS than patients with low CXCR6 expression
(log-rank test: HR, 2.803; 95% CI, 1.771–3.895, P<0.001).
However, CXCR6 had no effect on OS of patients with stage I/II
GC.
In the present study, CXCR6 was also found to be
correlated with lymph node and distant metastases of GC. This
correlation does not exist in GC alone. In breast cancer (BC),
CXCR6 expression was found to be higher in BC nest tissues and
metastatic lymph node, and may be responsible for invasion and
metastasis (16). Another study
showed that CXCR6 promoted HCC invasion and a protumor inflammatory
microenvironment, which promoted metastasis and poor patient
outcome in HCC (12). In lung
cancer, CXCR6 was reported to support metastasis via modulation of
metalloproteinase (17). However,
in prostate cancer, ovarian cancer and schwannomas, the correlation
of CXCR6 with metastasis was not reported (18–20).
Mechanisms underlying the metastasis-promoting effects in different
cancer types were not uniform. The
CXCR6/ERK1/2/RhoA/cofilin/F-actin pathway was identified in BC,
while in HCC, Gr-1+ neutrophil infiltration and
neoangiogenesis were involved.
EMT occurs in carcinoma development. During EMT,
epithelial cells lose their characteristic cell-cell adhesion
structures, change their polarity, modulate the organization of the
cytoskeletal systems, and become isolated, motile and resistant to
anoikis (21–24). These alterations facilitate the
malignant behaviors of cancer cells. It has been shown that EMT can
be induced by the signaling of several growth factor receptors and
chemokine receptors (25–27). In the present study, protein markers
for EMT were also detected. E-cadherin was decreased in the
CXCR6-overexpressing cells. Decreased expression of E-cadherin is
well established as a promotor of invasion and metastasis, while
induction of its expression is known to antagonize these
phenotypes. Furthermore, E-cadherin-inactivating mutations have
been detected in diffuse GC, including both germline and somatic
mutations (28–30). Meanwhile, N-cadherin was upregulated
in the GC cells with CXCR6 overexpression in the present study. The
result is also supported by the fact that N-cadherin is upregulated
in many invasive carcinoma cell lines, including BC, pancreatic and
prostate cancer. N-cadherin is associated with enhanced migration
and invasion, leading to increased metastasis and poor prognosis in
these carcinomas (31). Snail, a
suppressor of E-cadherin and inducer of EMT, was upregulated in the
GC cells with CXCR6 overexpression. Briefly, upregulation of CXCR6
in GC cells induced expression of Snail and N-cadherin, and
simultaneously suppressed E-cadherin formation. Upregulation of
CXCR6 promoted EMT in GC cells.
In conclusion, CXCR6 promoted tumor progression in
GC via modulation of EMT. CXCR6 was found to be an independent
prognostic factor for GC and may be a potential target for novel
therapy.
Acknowledgements
The present study was supported by the General
Program of the National Natural Science Foundation of China (no.
81272726).
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schlesinger-Raab A, Mihaljevic AL, Egert
S, Emeny R, Jauch KW, Kleeff J, Novotny A, Nüssler NC, Rottmann M,
Schepp W, et al: Outcome of gastric cancer in the elderly: A
population-based evaluation of the Munich Cancer Registry. Gastric
Cancer. 19:713–722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen L, Shan YS, Hu HM, Price TJ, Sirohi
B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, et al: Management of
gastric cancer in Asia: Resource-stratified guidelines. Lancet
Oncol. 14:e535–e547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berx G and van Roy F: Involvement of
members of the cadherin superfamily in cancer. Cold Spring Harb
Perspect Biol. 1:a0031292009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Virchow R: An address on the value of
pathological experiments. BMJ. 2:198–203. 1881. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luster AD: Chemokines - chemotactic
cytokines that mediate inflammation. N Engl J Med. 338:436–445.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verbeke H, Struyf S, Laureys G and Van
Damme J: The expression and role of CXC chemokines in colorectal
cancer. Cytokine Growth Factor Rev. 22:345–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng L, Chen N, Li Y, Zheng H and Lei Q:
CXCR6/CXCL16 functions as a regulator in metastasis and progression
of cancer. Biochim Biophys Acta. 1806:42–49. 2010.PubMed/NCBI
|
|
12
|
Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH,
Sun J, Yi Y, Shi JY, Shi GM, Ding ZB, et al: CXCR6 upregulation
contributes to a proinflammatory tumor microenvironment that drives
metastasis and poor patient outcomes in hepatocellular carcinoma.
Cancer Res. 72:3546–3556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SS, Ruiz VE, Carroll JD and Moss SF:
Helicobacter pylori in the pathogenesis of gastric cancer and
gastric lymphoma. Cancer Lett. 305:228–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Fu LX, Zhu WL, Shi H, Chen LJ and Ye
B: Blockade of CXCR6 reduces invasive potential of gastric cancer
cells through inhibition of AKT signaling. Int J Immunopathol
Pharmacol. 28:194–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Long Z, Cai H, Huang H, Shi Y and
Wang Y: Analysis of lymph node metastasis correlation with
prognosis in patients with T2 gastric cancer. PLoS One.
9:e1051122014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao G, Wang X and Wang J, Zu L, Cheng G,
Hao M, Sun X, Xue Y, Lu J and Wang J: CXCL16/CXCR6 chemokine
signaling mediates breast cancer progression by pERK1/2-dependent
mechanisms. Oncotarget. 6:14165–14178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mir H, Singh R, Kloecker GH, Lillard JW Jr
and Singh S: CXCR6 expression in non-small cell lung carcinoma
supports metastatic process via modulating metalloproteinases.
Oncotarget. 6:9985–9998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Lu Y, Wang J, Koch AE, Zhang J and
Taichman RS: CXCR6 induces prostate cancer progression by the
AKT/mammalian target of rapamycin signaling pathway. Cancer Res.
68:10367–10376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gooden MJ, Wiersma VR, Boerma A, Leffers
N, Boezen HM, ten Hoor KA, Hollema H, Walenkamp AM, Daemen T,
Nijman HW, et al: Elevated serum CXCL16 is an independent predictor
of poor survival in ovarian cancer and may reflect pro-metastatic
ADAM protease activity. Br J Cancer. 110:1535–1544. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Held-Feindt J, Rehmke B, Mentlein R,
Hattermann K, Knerlich F, Hugo HH, Ludwig A and Mehdorn HM:
Overexpression of CXCL16 and its receptor CXCR6/Bonzo promotes
growth of human schwannomas. Glia. 56:764–774. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stockinger A, Eger A, Wolf J, Beug H and
Foisner R: E-cadherin regulates cell growth by modulating
proliferation-dependent beta-catenin transcriptional activity. J
Cell Biol. 154:1185–1196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valdés F, Alvarez AM, Locascio A, Vega S,
Herrera B, Fernández M, Benito M, Nieto MA and Fabregat I: The
epithelial mesenchymal transition confers resistance to the
apoptotic effects of transforming growth factor Beta in fetal rat
hepatocytes. Mol Cancer Res. 1:68–78. 2002.PubMed/NCBI
|
|
23
|
Klymkowsky MW and Savagner P:
Epithelial-mesenchymal transition: A cancer researcher's conceptual
friend and foe. Am J Pathol. 174:1588–1593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu M, Marsters S, Ye X, Luis E, Gonzalez L
and Ashkenazi A: E-cadherin couples death receptors to the
cytoskeleton to regulate apoptosis. Mol Cell. 54:987–998. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uttamsingh S, Bao X, Nguyen KT, Bhanot M,
Gong J, Chan JL, Liu F, Chu TT and Wang LH: Synergistic effect
between EGF and TGF-beta1 in inducing oncogenic properties of
intestinal epithelial cells. Oncogene. 27:2626–2634. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morali OG, Delmas V, Moore R, Jeanney C,
Thiery JP and Larue L: IGF-II induces rapid beta-catenin relocation
to the nucleus during epithelium to mesenchyme transition.
Oncogene. 20:4942–4950. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka T, Bai Z, Srinoulprasert Y, Yang
BG, Hayasaka H and Miyasaka M: Chemokines in tumor progression and
metastasis. Cancer Sci. 96:317–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Corso G, Marrelli D and Roviello F:
Familial gastric cancer and germline mutations of E-cadherin. Ann
Ital Chir. 83:177–182. 2012.PubMed/NCBI
|
|
29
|
Becker KF, Atkinson MJ, Reich U, Becker I,
Nekarda H, Siewert JR and Höfler H: E-cadherin gene mutations
provide clues to diffuse type gastric carcinomas. Cancer Res.
54:3845–3852. 1994.PubMed/NCBI
|
|
30
|
Corso G, Carvalho J, Marrelli D, Vindigni
C, Carvalho B, Seruca R, Roviello F and Oliveira C: Somatic
mutations and deletions of the E-cadherin gene predict poor
survival of patients with gastric cancer. J Clin Oncol. 31:868–875.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hazan RB, Qiao R, Keren R, Badano I and
Suyama K: Cadherin switch in tumor progression. Ann NY Acad Sci.
1014:155–163. 2004. View Article : Google Scholar : PubMed/NCBI
|