Introduction
As the most common digestive malignancy, colon
cancer is the most devastating primary tumor (1). Colon cancer is the third most commonly
diagnosed cancers worldwide, and it is the third leading cause of
cancer-related death worldwide (2).
Despite the great progress including surgery and combined radio and
chemotherapy, colon cancer remains a ruinous disease with
invariable manifestation of tumor recurrence, the 5-year survival
rate of colon cancer is still poor (3). Therefore, researchers have intensive
interest in the therapeutic strategies aimed at preventing and
delaying the disease.
Tumorigenesis is often initiated by mutations
activated oncogenes or inhibited by tumor suppressor genes
(4). Clinical data and experimental
models have shown that signal transducers and activators of
transcription (STAT3) played an important role in colon cancer
including cell proliferation and survival, invasion, migration, and
angiogenesis (5). STAT3 is known to
adjust a number of cytokines such as vascular endothelial growth
factor (VEGF) and interleukins (ILs). It has been found that STAT3
can be responsible for cellular transformation, and STAT3 may
prevent apoptosis by being a downstream protein of Src (6). STAT3 has various effects in malignant
transformation, targeting STAT3 can decrease many types of cells
and malignant transformation susceptibility (7). In addition to cellular transformation,
STAT3 also participates in cellular survival, proliferation,
metastasis and angiogenesis.
MicroRNAs (miRNAs) are a group of non-coding RNAs.
miRNAs have been reported to be deregulated in a variety of human
cancers, and miRNAs were proved to be involved in several
physiological and pathological states. Several miRNAs such as
miR-9, miR-31, and miR-182 are known to be deregulated in colon
cancer, but the expression and function of miR-1299 in colon cancer
are unclear. Akao et al found that when SW480 colon cancer
cells were overexpression with miR-143 and miR-145, the cell
viability was decreased (8). When
cells were transfected with hsa-let7a-1 or miR-126 the cell
proliferation was decreased. Studies showed that let-7a-1 can
inhibit c-MYC and RAS activation, and miR-126 can inhibit the
expression of AKT (9,10). In addition, miRNAs have been found
to be related to drug resistance, metastasis and prognosis
(11–14). All these studies indicate that
miRNAs may become therapeutic targets in colon cancer treatment.
miR-1299 has been reported that it could affect PIM1 pathway in
PI003-induced apoptosis in HeLa cells, however there are no studies
on miR-1299 in colon cancer.
In this study, we detected miR-1299 and STAT3 in 60
cases of colon cancer patients with tumor tissues and adjacent
tissues to clarify their relationship. We found that miR-1299 as a
negative control of STAT3 had a negative correlation with the TNM
grade of colon cancer, and that it could affect the growth of colon
cancer cells.
Materials and methods
Patients and tissue samples
Sixty cases of tumor tissues and adjacent tissues
were obtained from patients undergoing resection of their tumor in
Central Hospital Affiliated to Shenyang Medical College. All
patients had a clear histologic diagnosis of colon cancer based on
the AJCC. All cases were diagnosed with colon cancer and treated
between January 2009 and December 2009 at Central Hospital
Affiliated to Shenyang Medical College. All cases were classified
as shown in Table I. Each patient
provided agreement for use of their tissue for research, and the
Institutional Research Medical Ethics Committee of Central Hospital
Affiliated to Shenyang Medical College granted approval for this
study.
 | Table I.The relationship between miR-1299 and
colon cancer. |
Table I.
The relationship between miR-1299 and
colon cancer.
|
|
| miR-1299
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Parameters | No. of patients | Low | High | χ2 | P-value |
|---|
| Sex |
|
|
| 0.028 | 0.866 |
| Male | 29 | 20 | 9 |
|
|
|
Female | 31 | 22 | 9 |
|
|
| Age (years) |
|
|
| 0.082 | 0.775 |
|
<40 | 25 | 18 | 7 |
|
|
| ≥40 | 35 | 24 | 11 |
|
|
| Size (maximal
diameter) |
|
|
| 4.898 | 0.027a |
|
<5 | 18 | 9 | 9 |
|
|
| ≥5 | 42 | 33 | 9 |
|
|
| Family history |
|
|
| 0.003 | 0.955 |
| No | 27 | 19 | 8 |
|
|
|
Yes | 33 | 23 | 10 |
|
|
| TNM grade |
|
|
| 10.051 | 0.018a |
| I | 13 | 11 | 2 |
|
|
| II | 9 | 7 | 2 |
|
|
|
III | 29 | 15 | 14 |
|
|
| IV | 9 | 9 | 0 |
|
|
Cell culture
Two human colon cancer cell lines, HCT116 and SW480,
were obtained from American Type Culture Collection and cultured
according to their recommendations.
MTT assays
3-(4,5-Dimethylthiazolyl) 2,5 diphenyltetrazolium
bromide (MTT) assay was used to analyzed the cell viability. Cells
(1×103) were seeded to one well of 96-well plates; 12 h
later, the transfection was performed. At different time points, 5
mg/ml MTT was added and incubated at 37°C in a CO2
incubator for 4 h, then medium was replaced by 0.1 ml DMSO and the
absorbance was measured spectrophotometrically at 490 nm using
Microplate Reader (Bio-Rad).
Hochest 33258 staining
After transfected with different miRNAs, cells were
fixed with 4% polyoxymethylene, incubated with 10 µg/ml Hochest
33258. Cells were observed with a fluorescence microscope.
Reverse transcription and quantitative
real-time PCR
Total RNA was extracted by TRIzol (Invitrogen)
through the protocol. The expression of miR-1299 was detected with
Stem-Loop RT-PCR assay as reported (15,16).
Primer sequences were synthesized as follows: miR-1299, F:
5′-ACACTCCAGCTGGGTTCTGGAAUUCTC-3′, R:
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCCCTCAC-3′; U6, F:
5′-CTCGCTTCGGCAGCACA-3′; R: 5′-AACGCTTCACGAATTTGCGT-3′; STAT3, F:
5′-GGAACAAGCCCCAACCGG-3′, R: 5′-CTAAAATCAGGGGTCCCAACTG-3′; CDK4, F:
5′-CAGAGCTCTTAGCCGAGCGT-3′, R: 5′-GGCACCGACACCAATTTCAG-3′; CDK6, F:
5′-AGTCTGATTACCTGCTCCGC-3′, R: 5′-CCTCGAAGCGAAGTCCTCAA-3′; Bcl-2,
F: 5′-GGTGAACTGGGGGAGGATTG-3′, R: 5′-GGCAGGCATGTTGACTTCAC-3′; Bax,
F: 5′-AGCTGAGCGAGTGTCTCAAG-3′, R: 5′-GTCCAATGTCCAGCCCATGA-3′;
GAPDH, F: 5′-CTCTGCTCCTCCTGTTCGAC-3′, R:
5′-GCGCCCAATACGACCAAATC-3′. All the reactions were carried out as
previously described (17).
Western blot analyses
RIPA lysis buffer were used to split cells and
tissues. Sample were subjected to 10% SDS-PAGE and transferred onto
a PVDF membrane. Target proteins were probed with specific
antibodies, STAT3, bcl-2, bax, CDK4, CDK6, MMP2 and Gapdh (Santa
Cruz Biotechnology).
Dual luciferase reporter assay
The STAT3 3′-UTR was PCR amplified and cloned into
the pMIR-REPORT™ vector (Ambion). The primers were: STAT3-WT, F:
5′-GGAACAAGCCCCAACCGG-3′, R: 5′-CTAAAATCAGGGGTCCCAACTG-3′;
STAT3-DEL, F: 5′-CGAATGGGCCTGACAAT-3′, R: 5′-CTAAAATCAGGGGTCCC-3′.
Cells were seeded at a 24-well plate and co-transfected with WT or
DEL reporter vector and 10 ng pMIR-REPORT βgal control plasmid in
miR-1299 mimic and miR-1299 AS. After 36 h, the luciferase activity
was measured using the Dual Luciferase Reporter Assay System
(Promega).
Immunostaining
All the reactions were carried out as previously
described (18). All slices were
independently assessed by two experienced pathologists who were
blinded to the patient clinicopathology and other information.
STAT3 expression level was evaluated via positive staining
proportion and intensity of tumor cells. Slices with inconsistent
results were re-examined by the original two pathologists and a
senior pathologist until a consensus was reached. Sections were
immunostained with STAT3 antibody (1:200).
Annexin V-FITC
By Annexin V-FITC apoptosis detection kit
instructions determination, the specific steps were as follows:
cells were washed twice with cold PBS, then re-suspended with
binding buffer at a concentration of 1×106 cells/ml.
Adding 5 µl of Annexin V-FITC and 10 µl of PI. Finally, adding 400
µl binding buffer to each tube and the apoptosis rate was measured
by flow cytometry within 1 h.
Statistical analysis
All data were analyzed with SPSS17.0 (SPSS Inc.).
Difference was analyzed by ANOVA test. All experiments were
repeated three times. Group comparison and Chi-square test were
used to analyze the results.
Results
Expression of STAT3 and miR-1299 in
tumor tissues and adjacent tissues
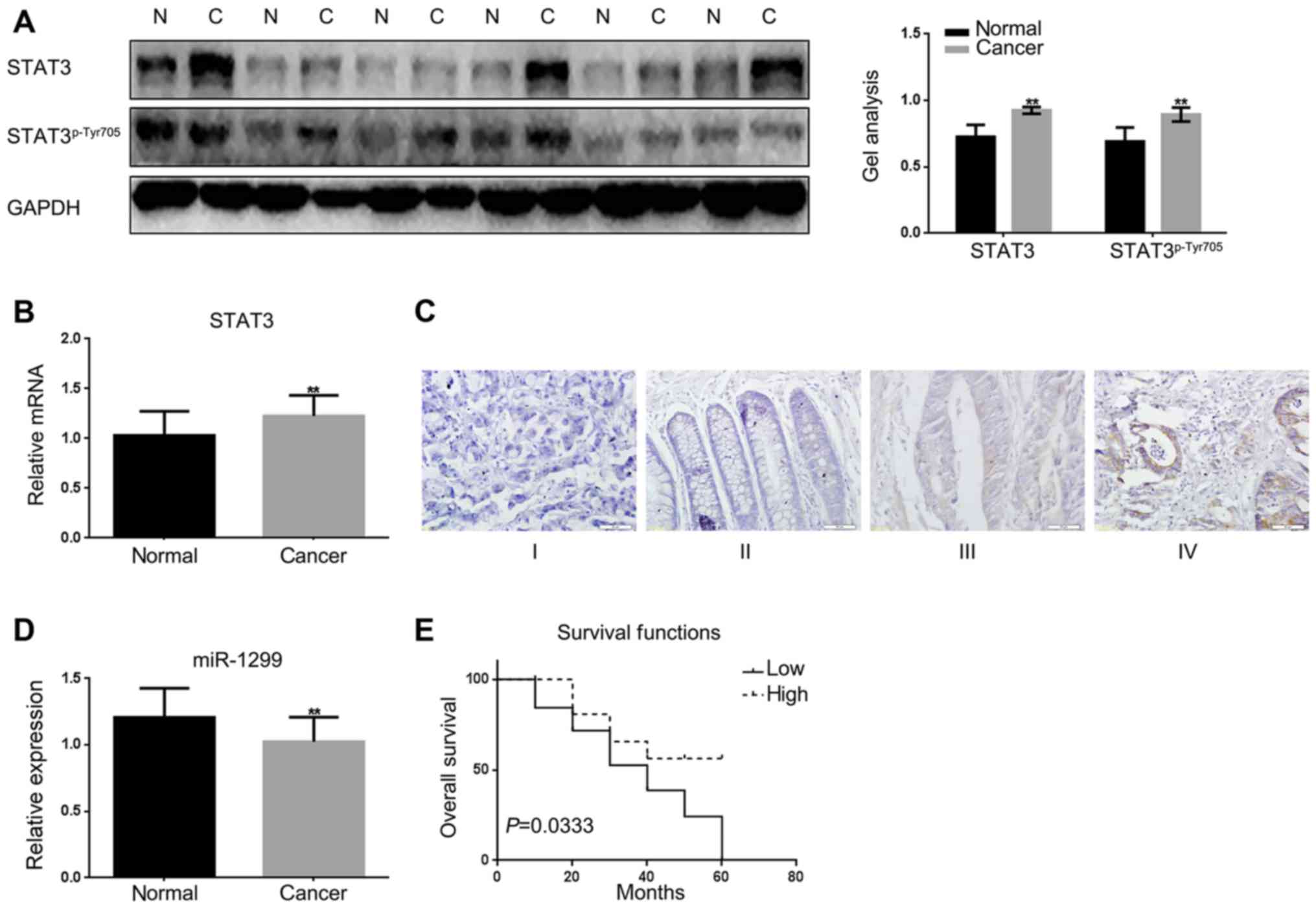
First, the expression levels of STAT3 were detected
in colon cancer tissues and adjacent tissues by real-time PCR,
western blotting (Fig. 1A and B).
The adjacent tissues were used as a control. The results showed
that there was a higher expression of STAT3 in colon cancer tissues
than the adjacent tissues. Then STAT3 in different stages of colon
cancer tissues were detected by immunohistochemical staining. The
results showed that STAT3 increased with the increasing of the
stage of colon cancer (Fig. 1C).
There are few reports on the expression of miR-1299 in cancer; we
wondered whether there was a different expression of miR-1299 in
colon cancer tissues and adjacent tissues. Real-time PCR was used
to test the expression of miR-1299 in colon cancer tissues and
adjacent tissues (Fig. 1D). We
found that the expression of miR-1299 was significantly
downregulated in colon cancer tissues. We analyzed the relationship
between miR-1299 and colon cancer in 60 pairs of colon cancer
tissues. We found there was little correlation between miR-1299
with the sex, age and family history in the tissues we detected;
however there was correlation between the miR-1299 and the tumor
size and TNM stage of colon cancer (Table I).
miR-1299 inhibits the expression of
STAT3 in HCT-116 cells
We found there was a variety of proteins could be
the target genes of miR-1299 through miRDB tool (Fig. 2A). We evaluated whether there is a
correlation between STAT3 and miR-1299 in colon cancer. The miRDB
tool showed that miR-1299 could be combined with 3′-UTR of STAT3,
which confirmed our conjecture (Fig.
2B). For further confirmation the luciferase reporter assay was
carried out to determine whether STAT3 is a potential target gene
of miR-1299 (Fig. 2C). The results
indicated that miR-1299 inhibited the expression of STAT3 at the
gene level. On the other hand, we found that the expression of
miR-1299 was negatively correlated with the expression of STAT3 in
colon cancer tissue by real-time PCR, which was further evidence
that miR-1299 could regulate STAT3 (Fig. 2D). In order to confirm our
hypothesis, we overexpressed or inhibited miR-1299 in HCT-116
cells, and then detected the expression of STAT3 by real-time PCR
and western blotting. The results show that miR-1299 can negatively
regulate the expression of STAT3 in the protein and RNA levels
(Fig. 2E-H).
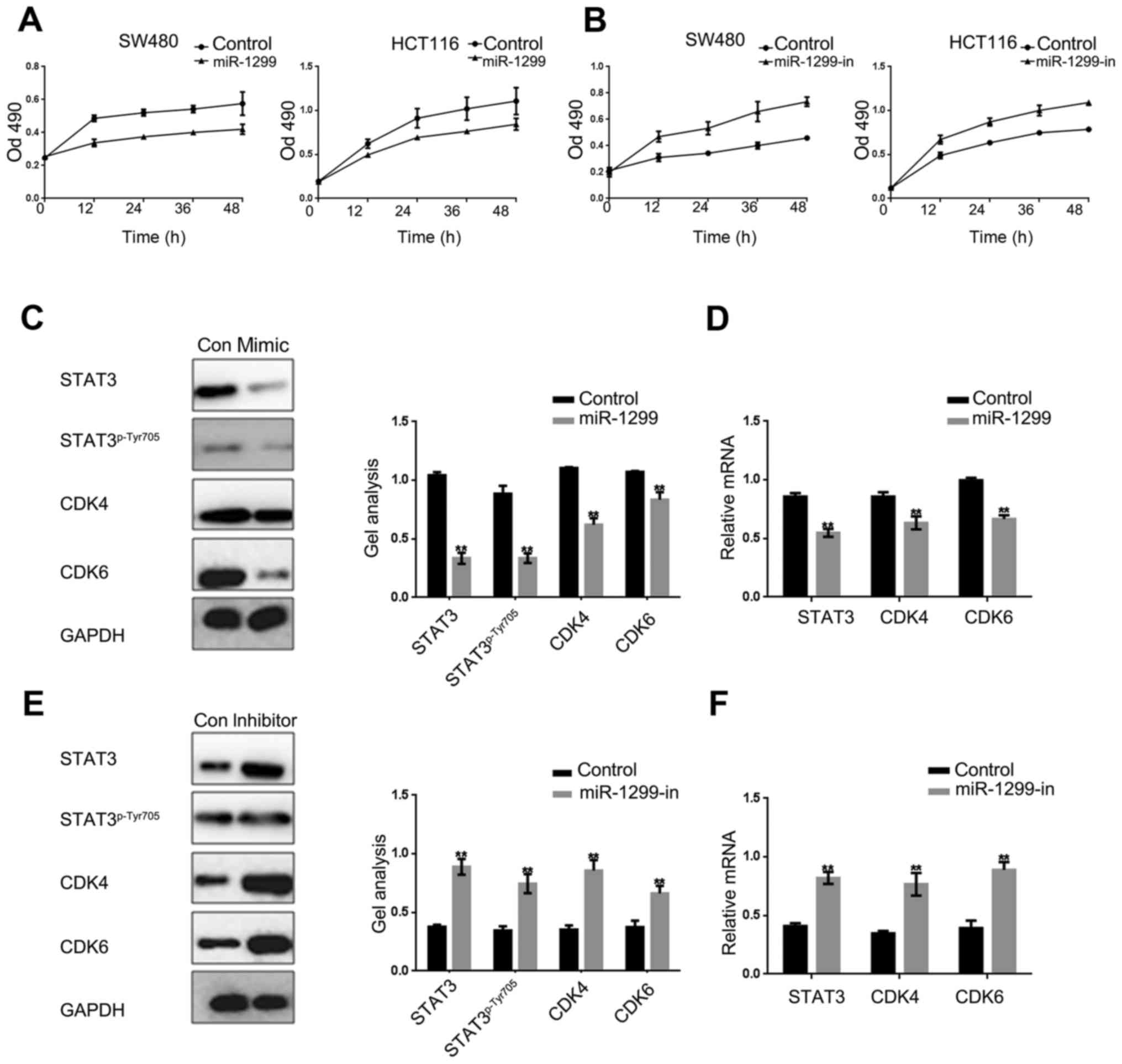
miR-1299 inhibits the proliferation of
colon cancer cells
It is well known that STAT3 can regulate cell growth
by promoting cell proliferation and inhibiting cell apoptosis. We
used MTT assay to examine the effect of miR-1299 to HCT-116 and
SW480 cells. With the overexpression or inhibition of miR-1299, we
found that miR-1299 can suppress the proliferation of HCT-116 cells
and SW480 cells (Fig. 3A and B).
Further, we examined the CDK4 and CDK6 downstream proteins of the
STAT3 pathway in HCT-116 cells. Results showed that the expression
of STAT3, STAT3p-Tyr705, CDK4 and CDK6 were negatively
correlated with the expression of miR-1299 (Fig. 3C-F). These results were sufficient
to explain that miR-1299 can inhibit cells proliferation by
negatively regulating phosphorylation of STAT3, CDK4 and CDK6.
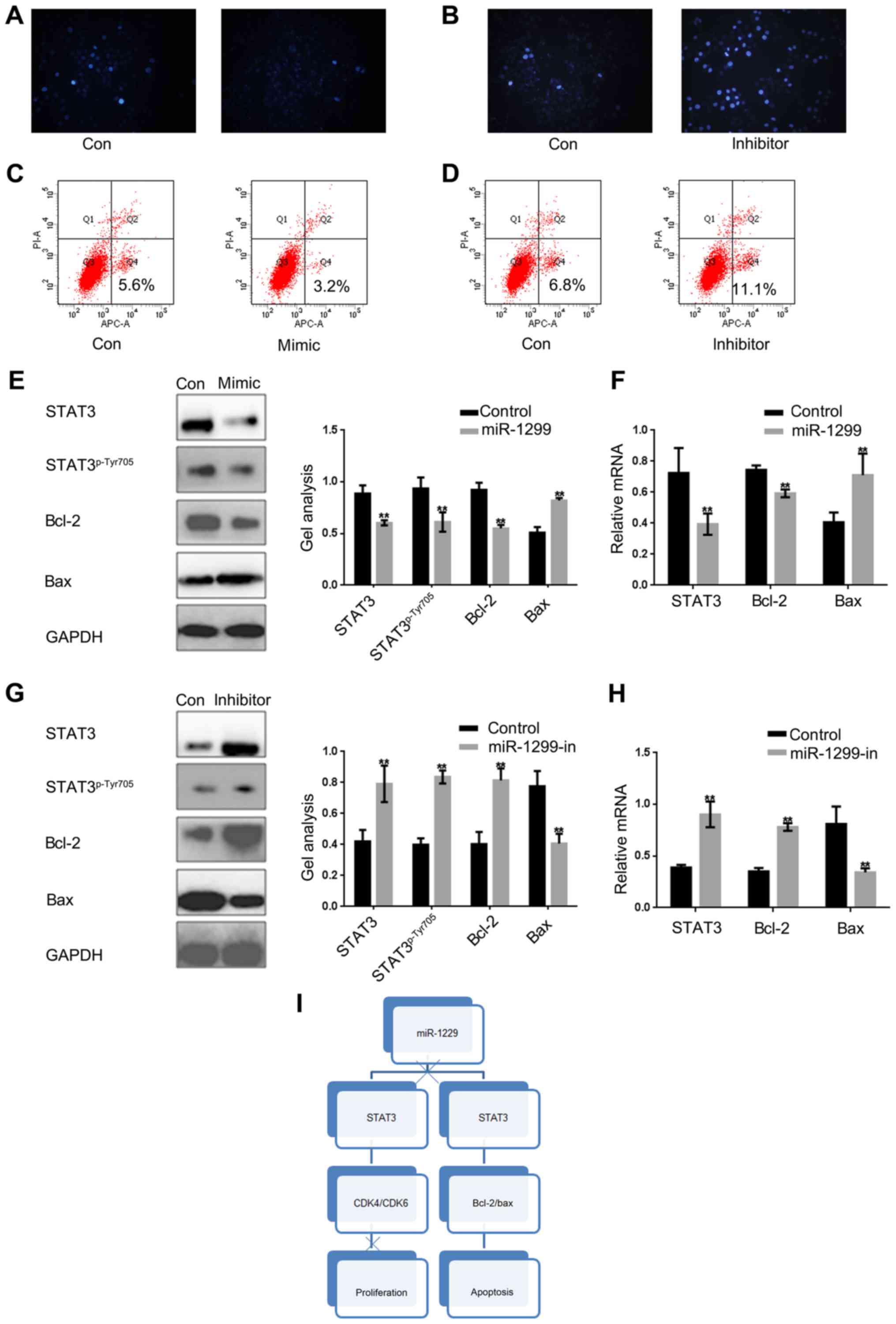
miR-1299 promotes apoptosis of colon
cancer cells by suppressing the STAT3 pathway
STAT3 has been proved to be able to promote the
growth of tumor cells by inhibiting apoptosis. Since miR-1299 can
inhibit cell proliferation through negative regulation of STAT3, we
investigated whether miR-1299 can affect cell apoptosis by
regulating the expression of STAT3. Hochest 33258 staining was used
to detect whether mi-1299 can affect cell apoptosis by regulating
the STAT3 in HCT-116 cells (Fig. 4A and
B). Then by Annexin V-FITC apoptosis detection we obtained
similar results (Fig. 4C and D). We
found that apoptosis of HCT-116 cells was stimulated when miR-1299
was overexpressed. On the contrary, apoptosis of HCT-116 was
obviously decreased when the expression of miR-1299 was inhibited.
Moreover, we found that regardless of the protein level or the RNA
level, miR-1299 can promote apoptosis of HCT-116 cells by
inhibiting the phosphorylation of STAT3 (Fig. 4E-H).
These above results confirmed that miR-1299 could
inhibit the growth of colon cancer cells by regulating the
expression of STAT3, the phosphorylation level of STAT3 and the
expression of its downstream protein.
Discussion
Signal transducers and activators of transcription
(STAT) family proteins were found in 1994, they are latent
cytoplasmic transcription factors (19). STAT3 as a very important protein in
the STAT family is activated by phosphorylation of a tyrosine
residue located at position 705. There is convincing evidence to
show that STAT3 activation plays a critical role in the occurrence
and development of tumors. Cancer cell growth, survival,
metastasis, and angiogenesis are closely related to the activation
of STAT3. Researchers have shown that STAT3 could be responsible
for cellular transformation in NIH3T3 cells and in mouse skin
epithelial cells (20). In a number
of cell types when STAT3 was inhibited the malignant transformation
was decreased. There is also research showing that STAT3 can
participate in cellular growth. Sufficient evidence shows that the
upregulation of cyclin D1, CDK4, CDK6 and cMyc are associated with
the STAT3 pathway (21). STAT3 has
also been shown to upregulate the expression of PIM1 (22).
In addition to promoting proliferation, STAT3 can
also achieve the function of promoting cell growth by inhibiting
apoptosis. The effect of STAT3 on cell apoptosis is through
regulating the expression of bcl-2, bax, p53, surviving and clAP2
(23). Evidence has been provided
that STAT3 plays a crucial role in cancer metastasis. Several lines
of evidence strongly implicate that STAT3 can regulate the MMPs to
play a crucial role in cell metastasis (24–27).
There are many studies showing that STAT3 can regulate angiogenesis
of tumors by adjusting the expression of VEGF and HIF-1α (28–30).
MicroRNAs are proved to play crucial roles in cancer
development. There are two main categories of microRNAs that are
involved in cancer progression, one can enhance cell growth,
survival, and metastasis, and the other suppressed these
activities. Several RNA therapeutics, with diverse modes of action,
are being evaluated in large late-stage clinical trials, and many
more are in early clinical development (31). For example, studies identified that
miR-143 and miR-145 as the tumor suppressors were downregulated in
colon cancer. miR-1299 was reported downregulated in many cancers
and reported to negatively adjust the expression of PIM1 (32). Studies showed that miR-1299 may be
involved in the cellular proliferation and apoptosis. However,
there are no previous research on the miR-1299 in colon cancer. We
were the first to find that miR-1299 can target STAT3 and suppress
the expression of STAT3 then inhibiting the STAT3 pathway.
In our study, we found that miR-1299 is closely
related to the occurrence and development of colon cancer. It is
negatively correlated with the TNM staging of colon cancer, and it
is closely related to the prognosis of colon cancer. Furthermore,
the expression of miR-1299 and STAT3 in colon carcinoma was
negatively correlated. We hypothesized that miR-1299 could affect
the occurrence and development of colon cancer by regulating the
expression of STAT3. After a series of studies we found that
miR-1299 can regulate STAT3. miR-1299 inhibited the proliferation
of colon cancer cells by inhibiting the expression of STAT3, and
promoted apoptosis of colon cancer cells.
Our findings suggest that overexpression of miR-1299
may be considered as a promising strategy for targeted therapies in
colon cancer.
Acknowledgements
This work was supported by grants from the
Technological innovation fund of Shenyang Technology Division (no.
F15-139-9-07), National Natural Science Foundation of China (no.
81502333) and China Postdoctoral Science Foundation (no.
2016M591437).
References
|
1
|
Sun KX, Xia HW and Xia RL: Anticancer
effect of salidroside on colon cancer through inhibiting JAK2/STAT3
signaling pathway. Int J Clin Exp Pathol. 8:615–621.
2015.PubMed/NCBI
|
|
2
|
Cekaite L, Rantala JK, Bruun J, Guriby M,
Agesen TH, Danielsen SA, Lind GE, Nesbakken A, Kallioniemi O, Lothe
RA, et al: MiR-9, −31, and −182 deregulation promote proliferation
and tumor cell survival in colon cancer. Neoplasia. 14:868–879.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology. 138:2101–2114
e5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nguyen AV, Wu YY, Liu Q, Wang D, Nguyen S,
Loh R, Pang J, Friedman K, Orlofsky A, Augenlicht L, et al: STAT3
in epithelial cells regulates inflammation and tumor progression to
malignant state in colon. Neoplasia. 15:998–1008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamran MZ, Patil P and Gude RP: Role of
STAT3 in cancer metastasis and translational advances. Biomed Res
Int. 2013:4218212013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turkson J, Bowman T, Garcia R, Caldenhoven
E, De Groot RP and Jove R: Stat3 activation by Src induces specific
gene regulation and is required for cell transformation. Mol Cell
Biol. 18:2545–2552. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frank DA: STAT3 as a central mediator of
neoplastic cellular transformation. Cancer Lett. 251:199–210. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akao Y, Nakagawa Y and Naoe T: MicroRNAs
143 and 145 are possible common onco-microRNAs in human cancers.
Oncol Rep. 16:845–850. 2006.PubMed/NCBI
|
|
9
|
Akao Y, Nakagawa Y and Naoe T: let-7
microRNA functions as a potential growth suppressor in human colon
cancer cells. Biol Pharm Bull. 29:903–906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo C, Sah JF, Beard L, Willson JK,
Markowitz SD and Guda K: The noncoding RNA, miR-126, suppresses the
growth of neoplastic cells by targeting phosphatidylinositol
3-kinase signaling and is frequently lost in colon cancers. Genes
Chromosomes Cancer. 47:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song B, Wang Y, Xi Y, Kudo K, Bruheim S,
Botchkina GI, Gavin E, Wan Y, Formentini A, Kornmann M, et al:
Mechanism of chemoresistance mediated by miR-140 in human
osteosarcoma and colon cancer cells. Oncogene. 28:4065–4074. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burk U, Schubert J, Wellner U, Schmalhofer
O, Vincan E, Spaderna S and Brabletz T: A reciprocal repression
between ZEB1 and members of the miR-200 family promotes EMT and
invasion in cancer cells. EMBO Rep. 9:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schimanski CC, Frerichs K, Rahman F,
Berger M, Lang H, Galle PR, Moehler M and Gockel I: High miR-196a
levels promote the oncogenic phenotype of colorectal cancer cells.
World J Gastroenterol. 15:2089–2096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng J, Wang K, Liu X, Chen S and Chen J:
The quantification of tomato microRNAs response to viral infection
by stem-loop real-time RT-PCR. Gene. 437:14–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patel D and Chaudhary J: Increased
expression of bHLH transcription factor E2A (TCF3) in prostate
cancer promotes proliferation and confers resistance to doxorubicin
induced apoptosis. Biochem Biophys Res Commun. 422:146–151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akira S, Nishio Y, Inoue M, Wang XJ, Wei
S, Matsusaka T, Yoshida K, Sudo T, Naruto M and Kishimoto T:
Molecular cloning of APRF, a novel IFN-stimulated gene factor 3
p91-related transcription factor involved in the gp130-mediated
signaling pathway. Cell. 77:63–71. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu CY, Wang L, Khaletskiy A, Farrar WL,
Larner A, Colburn NH and Li JJ: STAT3 activation is required for
interleukin-6 induced transformation in tumor-promotion sensitive
mouse skin epithelial cells. Oncogene. 21:3949–3960. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dang CV: c-Myc target genes involved in
cell growth, apoptosis, and metabolism. Mol Cell Biol. 19:1–11.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shirogane T, Fukada T, Muller JM, Shima
DT, Hibi M and Hirano T: Synergistic roles for Pim-1 and c-Myc in
STAT3-mediated cell cycle progression and antiapoptosis. Immunity.
11:709–719. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhattacharya S, Ray RM and Johnson LR:
STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents
apoptosis in polyamine-depleted cells. Biochem J. 392:335–344.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Huang C, Huang K, Wu W, Jiang T, Cao
J, Feng Z and Qiu Z: STAT3 knockdown reduces pancreatic cancer cell
invasiveness and matrix metalloproteinase-7 expression in nude
mice. PLoS One. 6:e259412011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F,
Sawaya R and Huang S: Stat3 activation regulates the expression of
matrix metalloproteinase-2 and tumor invasion and metastasis.
Oncogene. 23:3550–3560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dechow TN, Pedranzini L, Leitch A, Leslie
K, Gerald WL, Linkov I and Bromberg JF: Requirement of matrix
metalloproteinase-9 for the transformation of human mammary
epithelial cells by Stat3-C. Proc Natl Acad Sci USA. 101:pp.
10602–10607. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Itoh M, Murata T, Suzuki T, Shindoh M,
Nakajima K, Imai K and Yoshida K: Requirement of STAT3 activation
for maximal collagenase-1 (MMP-1) induction by epidermal growth
factor and malignant characteristics in T24 bladder cancer cells.
Oncogene. 25:1195–1204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Z and Han ZC: STAT3: A critical
transcription activator in angiogenesis. Med Res Rev. 28:185–200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Q, Briggs J, Park S, Niu G, Kortylewski
M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, et al:
Targeting Stat3 blocks both HIF-1 and VEGF expression induced by
multiple oncogenic growth signaling pathways. Oncogene.
24:5552–5560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh MK, Park HJ, Kim NH, Park SJ, Park IY
and Kim IS: Hypoxia-inducible factor-1alpha enhances haptoglobin
gene expression by improving binding of STAT3 to the promoter. J
Biol Chem. 286:8857–8865. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sullenger BA and Nair S: From the RNA
world to the clinic. Science. 352:1417–1420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Z, He W, Gao J, Luo J, Huang X and Gao
C: Computational prediction and experimental validation of a novel
synthesized pan-PIM inhibitor PI003 and its apoptosis-inducing
mechanisms in cervical cancer. Oncotarget. 6:8019–8035. 2015.
View Article : Google Scholar : PubMed/NCBI
|