Introduction
Esophageal cancer is the sixth leading cause of
cancer-related death in the world and the fourth leading cause of
cancer-related death in China (1).
There are two types of esophageal cancer, squamous cell carcinoma
(SCC) and adenocarcinoma (AC), and most Chinese esophageal cancers
are ESCCs (2). Although diagnostic
methods and cancer treatments have improved in recent years, the
prognosis is still poor because of widespread lymph node metastasis
and relatively frequent distant metastasis. Therefore,
understanding the mechanism of esophageal carcinogenesis will lay
the foundations for improving clinical management and outcomes.
Mini-chromosome maintenance protein complex (MCM) is
a eukaryotic DNA helicase complex required for initiation of DNA
replication. The MCM proteins include six members, MCM2 to MCM7,
and are considered as molecular markers of proliferation in several
types of cancer (3–6). MCM7 is a critical component of DNA
replication licensing complex, and is overexpressed in multiple
human malignancies including hepatocellular carcinoma, head and
neck squamous cell carcinoma, prostate carcinoma and esophageal
squamous cell carcinoma (7–10). Kim et al found that
upregulation of MCM7 was associated with cisplatin resistance in
bladder cancer (11). In liver
cancer MHCC-97 cells, silence of MCM7 dramatically reduced the cell
proliferation, migration, invasion and increased the apoptotic
cells (12).
Our previous and other studies found that MCM7 was
amplified and overexpressed in ESCC, and overexpression of MCM7 was
significantly linked with poor prognosis (7,13,14),
however the roles and mechanisms of MCM7 amplification and
overexpression in ESCC were largely unclear. In the present study,
we revealed that MCM7 promoted tumor cell proliferation, colony
formation and migration of ESCC cells via inhibiting the AKT1/mTOR
signaling pathway.
Materials and methods
TCGA and GEO datasets
Genomic and expression data of MCM7 are publically
available from the Cancer Genome Atlas and the NCBI Gene Expression
Omnibus. The copy number alterations and mutations of TCGA datasets
were analyzed by Cbioportal (www.cbioportal.org). For GSE datasets of GSE20347,
GSE38129 and GSE29001, the mRNA expression levels are detected by
microarray, and the difference of MCM7 between ESCC tissues and
paracancerous tissues was analyzed using the paired t-test by SPSS
19.
Cell culture
The human esophageal cancer cell lines were supplied
by Peking Union Medical College and Chinese Academy of Medical
Sciences. All cell lines were cultured in RPMI-1640 medium with 10%
fetal bovine serum, 100 U/ml penicillin and 100 g/ml streptomycin
at 37°C in a humidified incubator containing 5% carbon dioxide.
siRNAs and transfection
The synthetic negative control siRNA, MCM7 siRNA-1
and MCM7 siRNA-2 were purchased from Shanghai Gene Pharma Co. Ltd.
The ESCC cell lines were transiently transfected using
Lipofectamine® 2000 Transfection Reagent from Invitrogen
according to the manufacturer's protocol. The sequences of negative
control siRNA, MCM7 siRNA-1 and MCM7 siRNA-2 were as follows:
Negative control siRNA: 5′-UUCUCCGAACGUGUCACGUTT-3′; MCM7 siRNA-1:
5′-ATCGGATTGTGAAGATGAA-3′; MCM7 siRNA-2:
5′-AAGAUGUCCUGGACGUUUACA-3′.
Total RNA extraction and real-time PCR
assay
Total RNA was isolated from cancer cells using the
RNeasy mini kit as described by the manufacturer (Qiagen, Hilden,
Germany) and used for Real-time PCR assay.
Real-time PCR was used to detect the mRNA expression
levels of cyclin D1, cyclin E2 and CDK2. The PCR reactions were
performed in a total volume of 20 µl, including 10 µl of 2X Power
SYBR® Green PCR Master Mix (Applied Biosystems,
Warrington, UK), 2 µl of cDNA (5 ng/µl) and 1 µl of primer mix (10
µM each). PCR amplification and detection were performed in a
LightCycler 480 II (Roche Applied Science) as follows: an initial
denaturation at 95°C for 10 min; 40 cycles at 95°C for 15 sec and
60°C for 1 min. The relative gene expression was calculated using
the comparative CT method. The gene expression of the target gene
were normalized to an endogenous reference (GAPDH), and relative to
the calibrator were given by the formula 2-∆∆Ct. ∆CT was calculated
by subtracting the average GAPDH CT from the average CT of the gene
of interest. The ratio defines the level of relative expression of
the target gene to that of GAPDH. The primers were as follows:
Cyclin D1 forward primer, 5′-ACGCTTACCTCAACCATCCTG-3′; Cyclin D1
reverse primer, 5′-GGCCTCTCGATACACACAACA-3′; Cyclin E2 forward
primer, 5′-GCCCGGCCTATATATTGGGTT-3′; Cyclin E2 reverse primer,
5′-AACGGCTACTTCGTCTTGACA-3′; CDK2 forward primer,
5′-TCTTTGCTGAGATGGTGACTCG-3′; CDK2 reverse primer,
5′-TCTTCATCCAGGGGAGGTACA-3′; GAPDH forward primer,
5′-AAATCCCATCACCATCTTCCAG-3′; GAPDH reverse primer,
5′-GAGTCCTTCCACGATACCAAAGTTG-3′.
Western blot assay
Cells were lysed in the lysis buffer (20 mM Tris, 2
mM EDTA, 50 mM 2-mecaptoethanol, 10% glycerol, pH 7.4). The
homogenates were placed on ice for 30 min and centrifuged at 12,000
× g for 15 min at 4°C. Afterward, the protein concentrations of the
lysates were determined using a Protein Assay kit (Bio-Rad,
Richmond, CA, USA). Equal amounts of total proteins were loaded
onto a 10% gradient polyacrylamide gel, electrophoretically
transferred to polyvinylidene difluoride membrane, and then blocked
with 10% non-fat milk for 2 h at room temperature. The membranes
were incubated with specific primary antibodies overnight at 4°C
and probed with corresponding secondary antibodies for 1 h at room
temperature. The protein bands were visualized using ECL Blotting
Detection Reagents (Applygen, Beijing, China). The primary
antibodies were as follows: MCM7 (1:1000; Santa Cruz Biotechnology,
Santa Cruz, CA, USA), p-AKT1 (1:1000; Santa Cruz Biotechnology),
AKT1 (1:1000; Santa Cruz Biotechnology), p-mTOR (1:1000; Santa Cruz
Biotechnology), mTOR (1:1000; Santa Cruz Biotechnology) and β-actin
(1:5000; Santa Cruz Biotechnology).
Cell proliferation assay
The Cell Counting Kit-8 (Dojindo Laboratories,
Kumamoto, Japan) was performed to quantify the proliferation of
KYSE510 and EC9706 cells. Cells were cultured at 1000/well in
96-well plates. After incubated for 24, 48, 72, 84, 96, or 120 h,
10 µl of CCK-8 was added to each well and incubated for 1 h. The
absorbance of each well was read at 450 nm. Three independent
experiments were performed.
Colony formation assay
For each group, 5000 cells were plated in 6-well
plate. After cultured for 10 days, the cells were washed with PBS,
fixed with methanol and 0.1% crystal violet. Then, the colonies
were counted and photographed. Three independent experiments were
performed.
Transwell assay
The migration assay was performed on Transwell
plates. For cell migration assay, 1×105 cells were
seeded on a polycarbonate membrane insert in a Transwell apparatus
(Costar, Cambridge, MA, USA) and cultured in RPMI-1640 without
serum. RPMI-1640 containing 20% fetal bovine serum was added to the
lower chamber. After incubation for 24 h at 37°C in a
CO2 incubator, the insert was washed with PBS, and cells
on the top surface of the insert were removed by wiping with a
cotton swab. Cells that migrated to the bottom surface of the
insert were fixed with methanol, stained with 0.4% crystal violet,
and counted in five random fields at ×200.
Statistical analysis
The data were analyzed by Student's t-test and
one-way analysis of variance using the SPSS and Graphpad Prism 5.0.
P<0.05 was considered to indicate a statistical significant
difference.
Results
MCM7 is amplified and overexpressed in
many types of cancer including ESCC
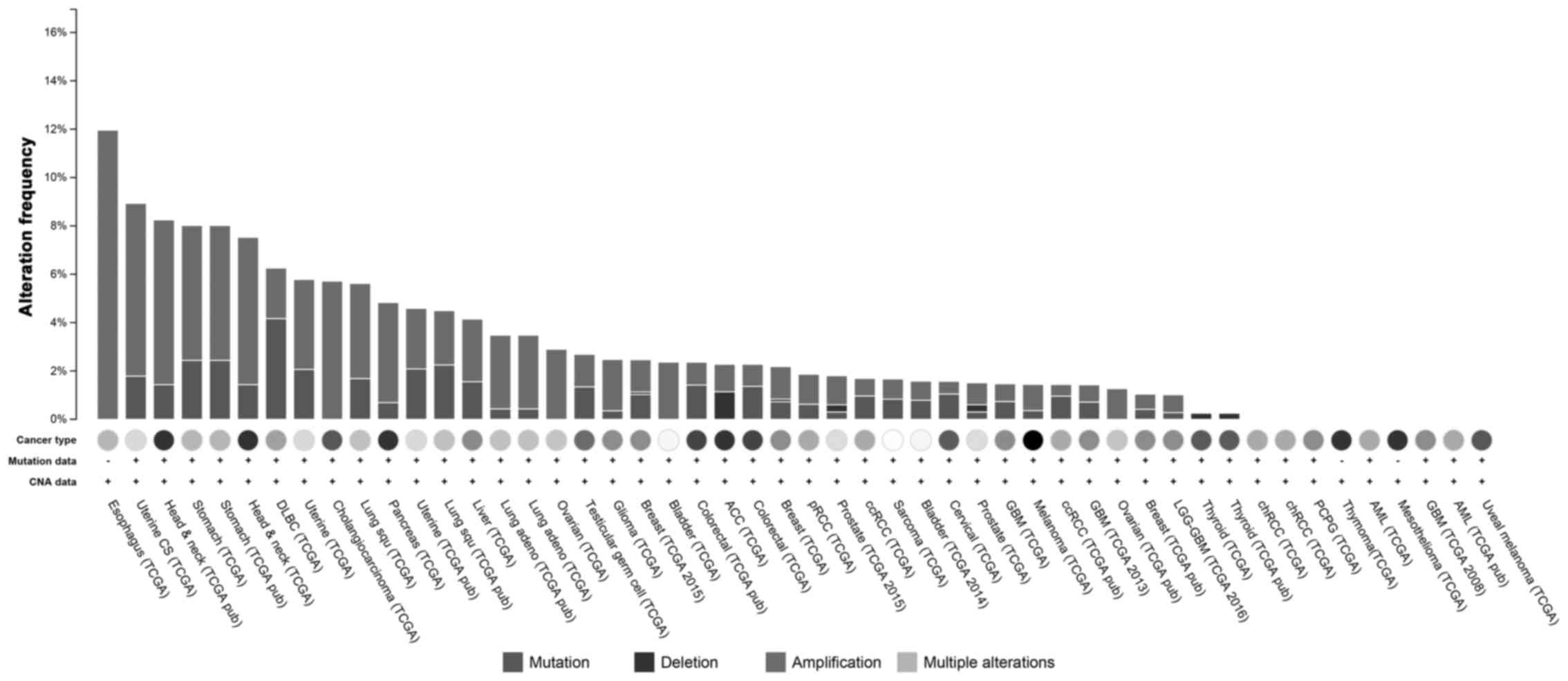
The datasets of The Cancer Genome Atlas (TCGA)
showed that MCM7 was amplified in approximately 12% of ESCCs, and
in >4% of head and neck squamous cell carcinomas and stomach
carcinomas, and the amplification was the dominant form of changes
in DNA level; however in diffuse large B-cell lymphoma (DLBC),
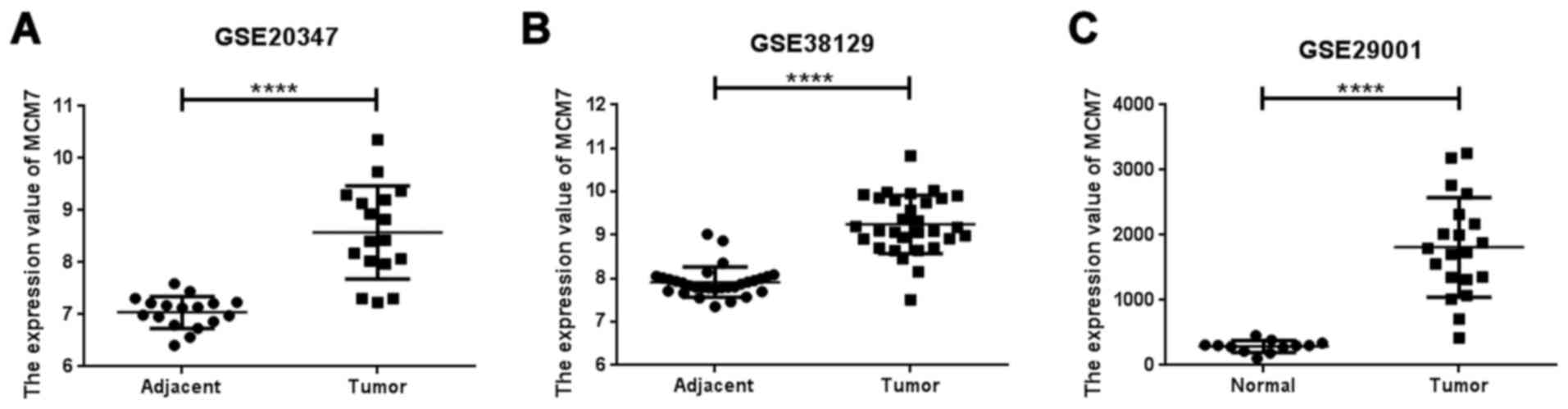
mutation was more frequent than amplification (Fig. 1). MCM7 was overexpressed in ESCC
tissues in the datasets of GSE20347, GSE38129 and GSE29001
(Fig. 2).
Knockdown of MCM7 suppresses the
proliferation, colony formation and migration of ESCC cells
In order to explore the tumorigenic roles and
mechanisms of MCM7 in esophageal carcinogenesis, we selected cell
lines KYSE510 and EC9706 with higher MCM7 expression for further
study.
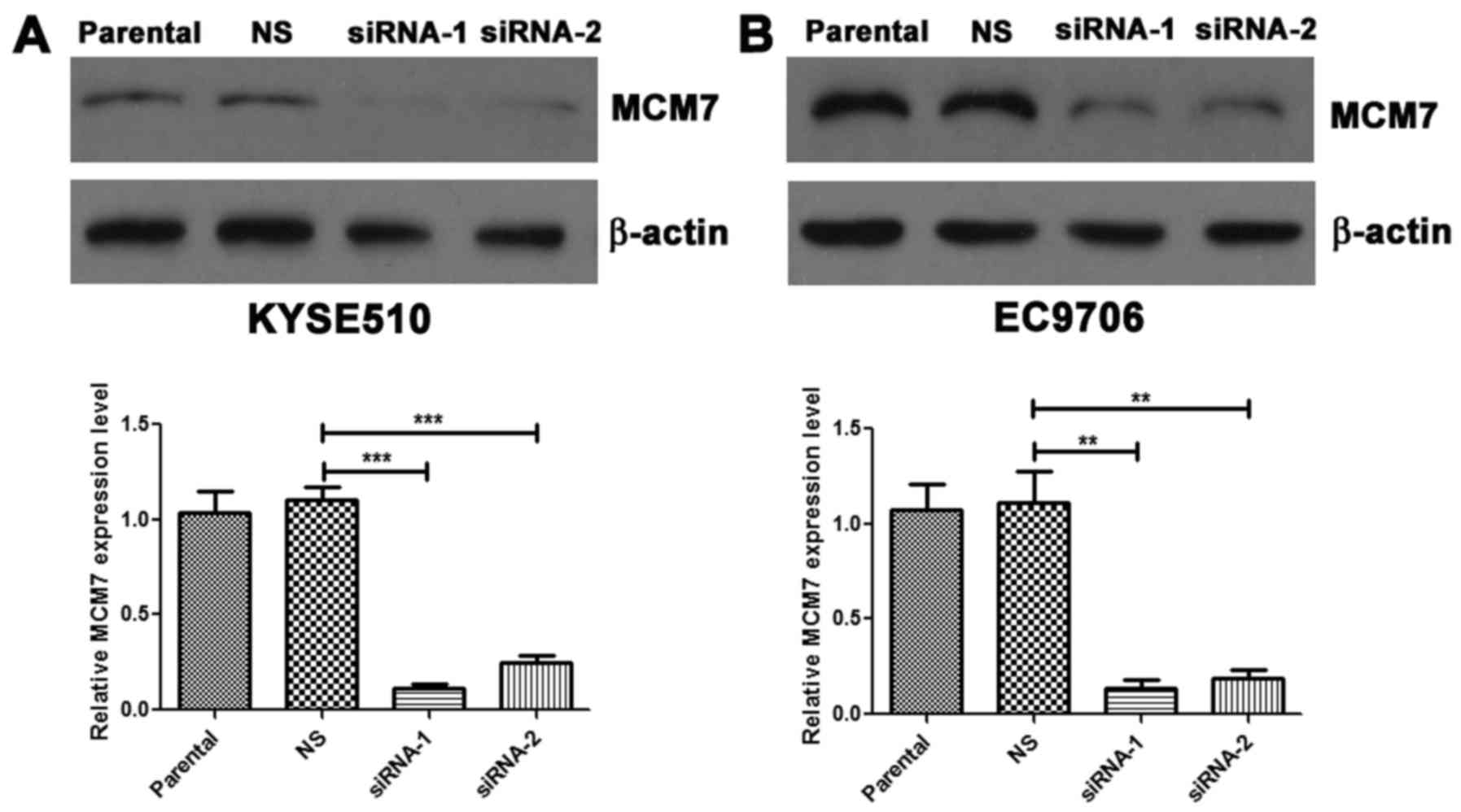
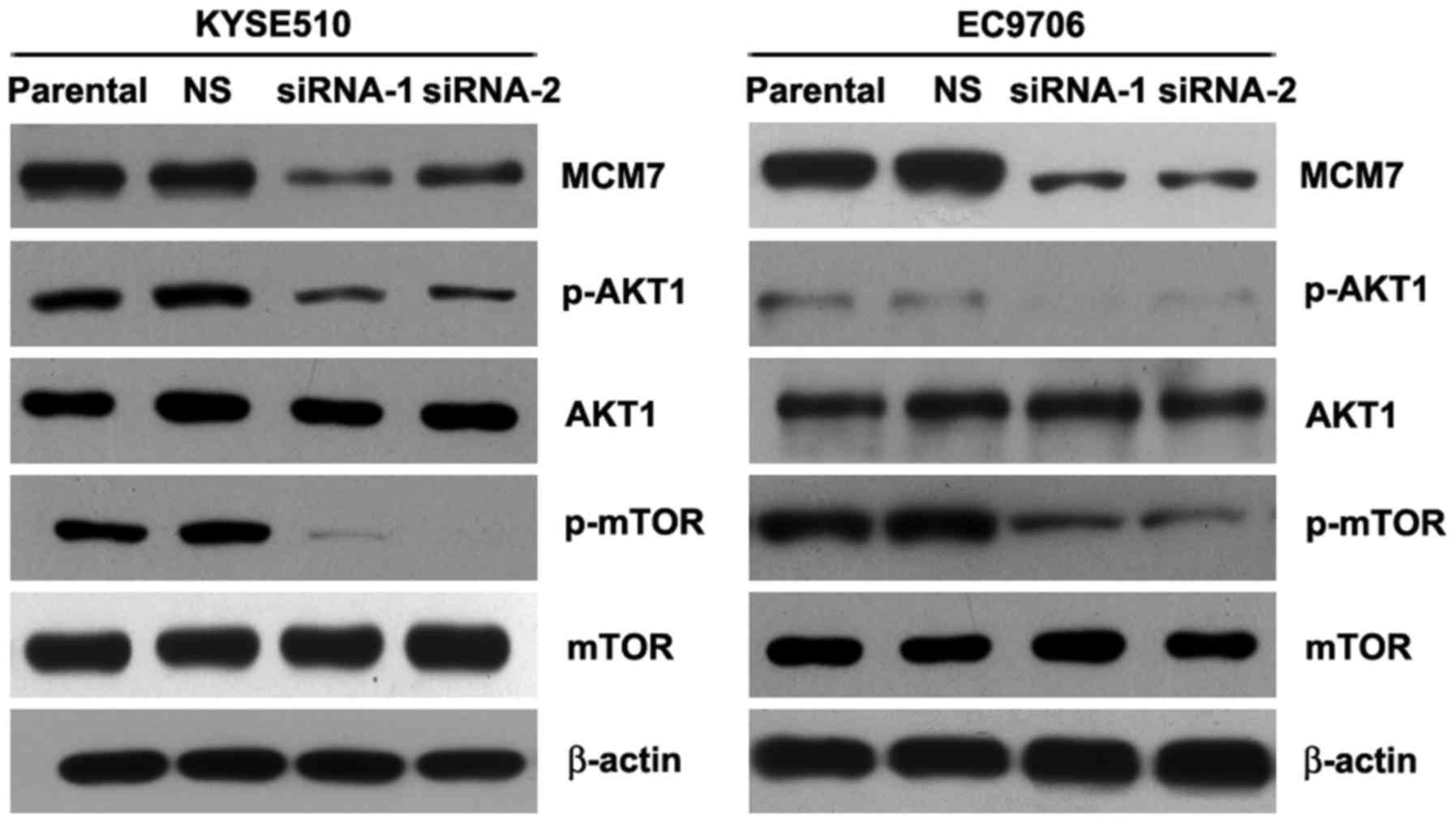
siRNAs were used to knock down the MCM7 expression
in KYSE510 and EC9706 cells, and the RNAi efficiency was determined
by western blot assay (Fig. 3A and
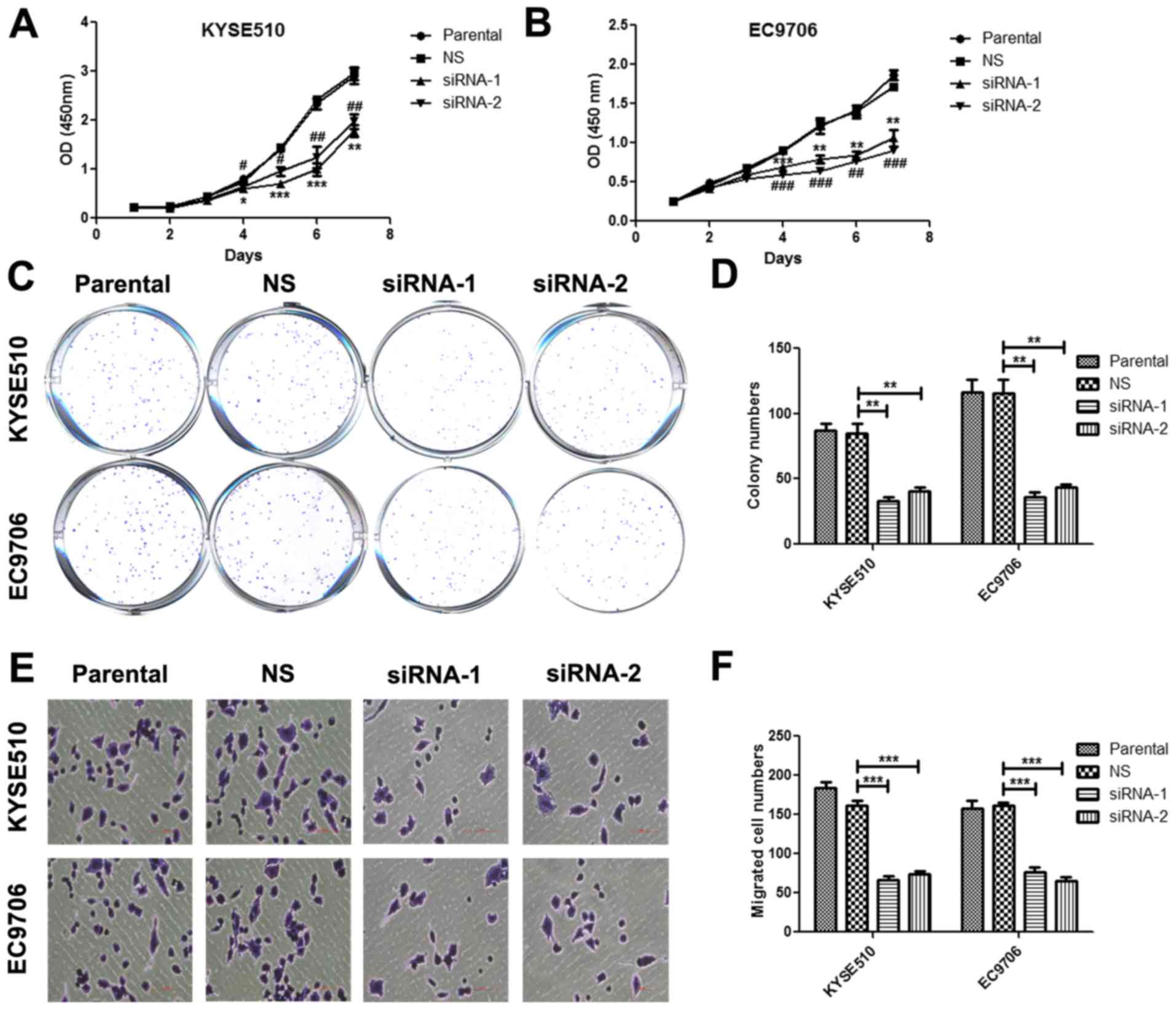
B). Using CCK-8 proliferation assay, we found that silence of
MCM7 significantly inhibited cell proliferation compared with
non-silencing group, and there was significant suppression at days
4, 5, 6 and 7 both in KYSE510 and EC9706 cells (Fig. 4A and B). In colony formation assay,
we observed that colony numbers were significantly lower in MCM7
siRNA-1 and siRNA-2 transfected cells than in the control group
both in KYSE510 and EC9706 cells (Fig.
4C and D). By Transwell assay, we revealed that silence of MCM7
significantly inhibited the migration of KYSE510 and EC9706 cells
(Fig. 4E and F).
Knockdown of MCM7 suppressed the
AKT1/mTOR signaling pathway in ESCC
Many studies showed that activated AKT1/mTOR
signaling pathway promoted cancer cell proliferation,
epithelial-mesenchymal transition (EMT), tumor metastasis and
invasion (15–20). Noteworthy, our study revealed that
silencing MCM7 significantly inhibited the phosphorylation of AKT1
and mTOR (Fig. 5). In addition,
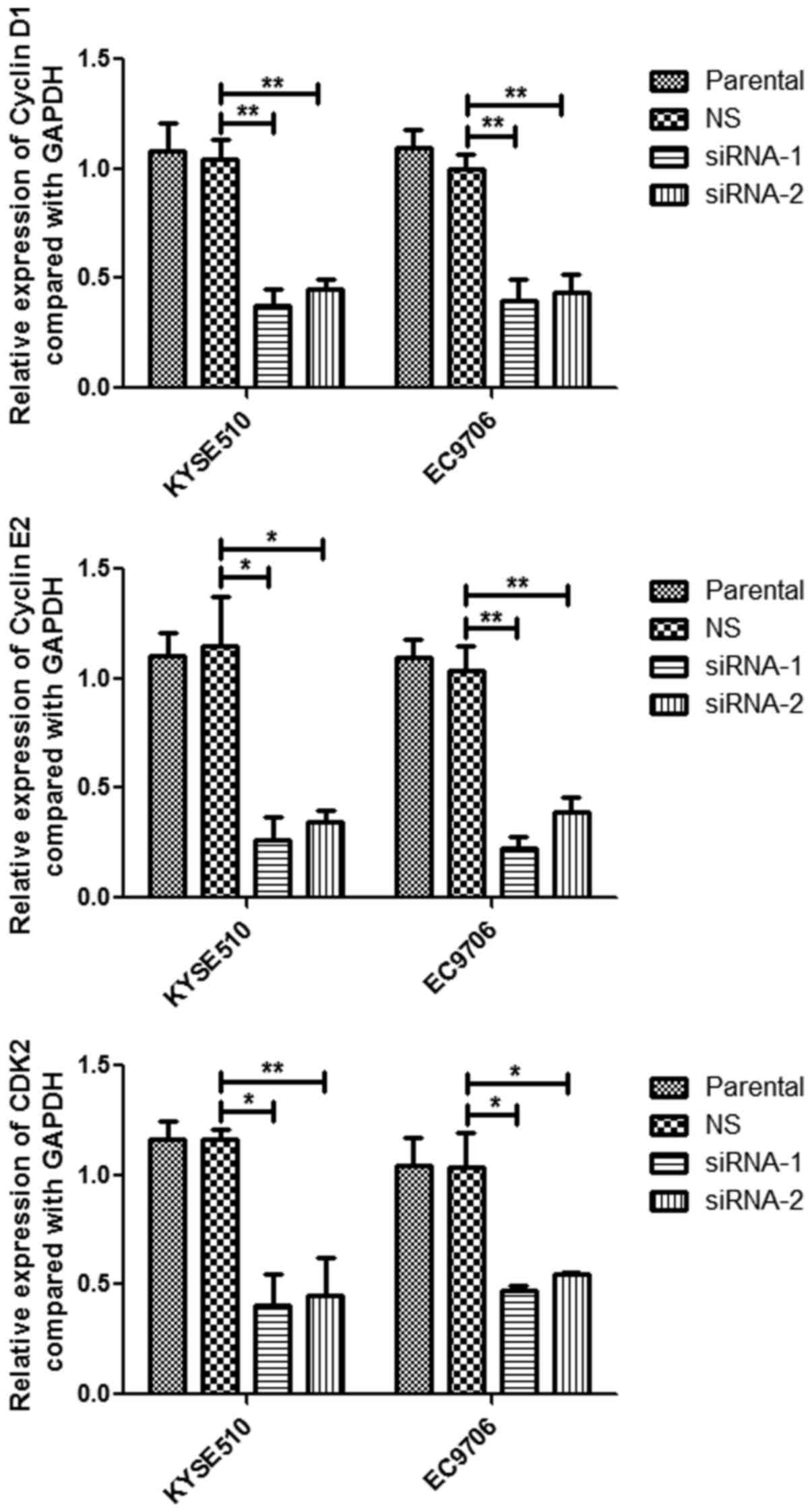
knockdown of MCM7 reduced the cell cycle regulatory genes cyclin
D1, cyclin E2 and CDK2 in mRNA expression levels (Fig. 6). These results indicated that MCM7
amplification and overexpression promoted cell proliferation,
colony formation and migration via activating the AKT1/mTOR
signaling pathway.
Discussion
Genomic aberrations can contribute to carcinogenesis
and tumor progression. In the past decades, the understanding of
molecular pathogenesis of ESCC has developed, but is still limited.
Thus, elucidation of the mechanism of esophageal carcinogenesis was
very important for tumor diagnosis and therapy. Recently, it was
shown that MCM proteins were overexpressed in several types of
tumors, and high expression levels of MCM proteins including MCM2,
MCM3 and MCM7 were positively correlated with Ki-67 positive
staining in Laryngeal squamous cell carcinoma (LSCC) (5).
MCM7 is a critical DNA replication licensing factor
in both yeast and xenopus oocytes, and serves as a
co-transcriptional and co-translational enhancing factor of
androgen receptor, which regulates cell growth and proliferation
(21). Increased MCM7 expression is
common in various human cancers including esophageal squamous cell
carcinoma, prostate cancer and pancreatic cancer (7,10,22,23),
and MCM7 has been considered as a tumorigenesis-related gene
(24). In pituitary adenoma,
diffuse-type primary gastric adenocarcinoma and colorectal cancer,
the patients with MCM7 overexpression had a shorter
recurrence/progression-free survival respectively (25,26).
MCM7 promotes tumor cell proliferation and invasion in papillary
urothelial neoplasia and liver cancer (27), and depletion of MCM7 inhibits
glioblastoma multiforme tumor growth in vivo (28). In ESCC, G9a and MCM7 overexpression
levels were correlated with poor prognosis (7), however, the roles and mechanisms of
MCM7 amplification and overexpression in ESCC were largely unknown.
Our results showed that MCM7 promoted tumor cell proliferation,
colony formation and migration of ESCC cells.
AKT, a serine/threonine kinase, has a wide tissue
distribution and regulates many processes including cell
metabolism, proliferation, survival and tumor growth (29). Notably, AKT has been considered as a
critical oncogene, and it can activate downstream signaling
pathways through phosphorylation of a plethora of AKT substrates
(30). In recent studies, the
phosphorylation of AKT was shown correlated with poor prognosis in
ESCC (31,32). AKT was responsible for the cisplatin
resistance, and could promote metastasis of ESCC by targeting
epithelial-mesenchymal transition (33–35).
mTOR activation was able to also prcomote the cell proliferation
and tumor progression of ESCC (36,37).
However, the direct relationship between AKT/mTOR and MCM7 has not
been reported. Our findings further revealed that knockdown of MCM7
significantly inhibited the phosphorylation of AKT and mTOR.
In summary, our data revealed that MCM7 promoted
tumor cell proliferation, colony formation and migration via
activating the AKT1/mTOR signaling pathway. Future studies should
focus on how MCM7 regulates the AKT1/mTOR signaling pathway, and
explore the antitumor activity of MCM7 silencing in an animal
model.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81460425) and the Yunnan
Provincial Research Foundation for Basic Research, China (no.
2013FD012) and Foundation for the Talents of Kunming University of
Science and Technology (no. KKSY201226099).
References
|
1
|
Chen W, Zheng R, Zuo T, Zeng H, Zhang S
and He J: National cancer incidence and mortality in China, 2012.
Chin J Cancer Res. 28:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He YT, Hou J, Chen ZF, Qiao CY, Song GH,
Meng FS, Jin HX and Chen C: Decrease in the esophageal cancer
incidence rate in mountainous but not level parts of Cixian County,
China, over 29 years. Asian Pac J Cancer Prev. 6:510–514.
2005.PubMed/NCBI
|
|
3
|
Chang CC, Huang CC, Yang SH, Chien CC, Lee
CL and Huang CJ: Data on clinical significance of GAS2 in
colorectal cancer cells. Data Brief. 8:82–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Juríková M, Danihel Ľ, Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowinska K, Chmielewska M, Piotrowska A,
Pula B, Pastuszewski W, Krecicki T, Podhorska-Okołow M, Zabel M and
Dziegiel P: Correlation between levels of expression of
minichromosome maintenance proteins, Ki-67 proliferation antigen
and metallothionein I/II in laryngeal squamous cell cancer. Int J
Oncol. 48:635–645. 2016.PubMed/NCBI
|
|
6
|
Gann PH, Deaton RJ, Rueter EE, van Breemen
RB, Nonn L, Macias V, Han M and Ananthanarayanan V: A phase II
randomized trial of lycopene-rich tomato extract among men with
high-grade prostatic intraepithelial neoplasia. Nutr Cancer.
67:1104–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong X, Chen X, Guan X, Zhang H, Ma Y,
Zhang S, Wang E, Zhang L and Han Y: Overexpression of G9a and MCM7
in oesophageal squamous cell carcinoma is associated with poor
prognosis. Histopathology. 66:192–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou YM, Zhang XF, Cao L, Li B, Sui CJ, Li
YM and Yin ZF: MCM7 expression predicts post-operative prognosis
for hepatocellular carcinoma. Liver Int. 32:1505–1509. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honeycutt KA, Chen Z, Koster MI, Miers M,
Nuchtern J, Hicks J, Roop DR and Shohet JM: Deregulated
minichromosomal maintenance protein MCM7 contributes to oncogene
driven tumorigenesis. Oncogene. 25:4027–4032. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren B, Yu G, Tseng GC, Cieply K, Gavel T,
Nelson J, Michalopoulos G, Yu YP and Luo JH: MCM7 amplification and
overexpression are associated with prostate cancer progression.
Oncogene. 25:1090–1098. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SH, Ho JN, Jin H, Lee SC, Lee SE, Hong
SK, Lee JW, Lee ES and Byun SS: Upregulated expression of BCL2,
MCM7, and CCNE1 indicate cisplatin-resistance in the set of two
human bladder cancer cell lines: T24 cisplatin sensitive and T24R2
cisplatin resistant bladder cancer cell lines. Investig Clin Urol.
57:63–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Tian L, Chen BA and Xia JR:
Biological effects of lentivirus-mediated silencing of
minichromosome maintenance protein 7 with shRNA on the liver cancer
MHCC-97H cells. Int J Clin Exp Med. 8:8433–8441. 2015.PubMed/NCBI
|
|
13
|
Kan T, Sato F, Ito T, Matsumura N, David
S, Cheng Y, Agarwal R, Paun BC, Jin Z, Olaru AV, et al: The
miR-106b-25 polycistron, activated by genomic amplification,
functions as an oncogene by suppressing p21 and Bim.
Gastroenterology. 136:1689–1700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi ZZ, Shang L, Jiang YY, Hao JJ, Zhang
Y, Zhang TT, Lin DC, Liu SG, Wang BS, Gong T, et al: Consistent and
differential genetic aberrations between esophageal dysplasia and
squamous cell carcinoma detected by array comparative genomic
hybridization. Clin Cancer Res. 19:5867–5878. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leu WJ, Swain Sh P, Chan SH, Hsu JL, Liu
SP, Chan ML, Yu CC, Hsu LC, Chou YL, Chang WL, et al:
Non-immunosuppressive triazole-based small molecule induces
anticancer activity against human hormone-refractory prostate
cancers: The role in inhibition of PI3K/AKT/mTOR and c-Myc
signaling pathways. Oncotarget. 7:76995–77009. 2016.PubMed/NCBI
|
|
16
|
Liu L, Pan Y, Song Y, Su X, Ke R, Yang L,
Gao L and Li M: Activation of AMPK α2 inhibits airway smooth muscle
cells proliferation. Eur J Pharmacol. 791:235–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moravčík R, Stebelová K, Boháč A and Zeman
M: Inhibition of VEGF mediated post receptor signalling pathways by
recently developed tyrosine kinase inhibitor in comparison with
sunitinib. Gen Physiol Biophys. 35:511–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Divine LM, Nguyen MR, Meller E, Desai RA,
Arif B, Rankin EB, Bligard KH, Meyerson C, Hagemann IS, Massad M,
et al: AXL modulates extracellular matrix protein expression and is
essential for invasion and metastasis in endometrial cancer.
Oncotarget. 7:77291–77305. 2016.PubMed/NCBI
|
|
19
|
Yang Y, Gao Z, Ma Y, Teng H, Liu Z, Wei H,
Lu Y, Cheng X, Hou L and Zou X: Fucoidan inhibits lymphangiogenesis
by downregulating the expression of VEGFR3 and PROX1 in human
lymphatic endothelial cells. Oncotarget. 7:38025–38035.
2016.PubMed/NCBI
|
|
20
|
Wei L, Li K, Pang X, Guo B, Su M, Huang Y,
Wang N, Ji F, Zhong C, Yang J, et al: Leptin promotes
epithelial-mesenchymal transition of breast cancer via the
upregulation of pyruvate kinase M2. J Exp Clin Cancer Res.
35:1662016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi YK, Yu YP, Zhu ZH, Han YC, Ren B,
Nelson JB and Luo JH: MCM7 interacts with androgen receptor. Am J
Pathol. 173:1758–1767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lau KM, Chan QK, Pang JC, Li KK, Yeung WW,
Chung NY, Lui PC, Tam YS, Li HM, Zhou L, et al: Minichromosome
maintenance proteins 2, 3 and 7 in medulloblastoma: Overexpression
and involvement in regulation of cell migration and invasion.
Oncogene. 29:5475–5489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang JW, Shi ZZ, Shen TY, Che X, Wang Z,
Shi SS, Xu X, Cai Y, Zhao P, Wang CF, et al: Identification of
genomic alterations in pancreatic cancer using array-based
comparative genomic hybridization. PLoS One. 9:e1146162014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huber AR, Tan D, Sun J, Dean D, Wu T and
Zhou Z: High expression of carbonic anhydrase IX is significantly
associated with glandular lesions in gastroesophageal junction and
with tumorigenesis markers BMI1, MCM4 and MCM7. BMC Gastroenterol.
15:802015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coli A, Asa SL, Fadda G, Scannone D,
Chiloiro S, De Marinis L, Lauretti L, Ranelletti FO and Lauriola L:
Minichromosome maintenance protein 7 as prognostic marker of tumor
aggressiveness in pituitary adenoma patients. Eur J Endocrinol.
174:307–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishibashi Y, Kinugasa T, Akagi Y, Ohchi T,
Gotanda Y, Tanaka N, Fujino S, Yuge K, Kibe S, Yoshida N, et al:
Minichromosome maintenance protein 7 is a risk factor for
recurrence in patients with Dukes C colorectal cancer. Anticancer
Res. 34:4569–4575. 2014.PubMed/NCBI
|
|
27
|
Guan B, Wang X, Yang J, Zhou C and Meng Y:
Minichromosome maintenance complex component 7 has an important
role in the invasion of papillary urothelial neoplasia. Oncol Lett.
10:946–950. 2015.PubMed/NCBI
|
|
28
|
Erkan EP, Ströbel T, Lewandrowski G,
Tannous B, Madlener S, Czech T, Saydam N and Saydam O: Depletion of
minichromosome maintenance protein 7 inhibits glioblastoma
multiforme tumor growth in vivo. Oncogene. 33:4778–4785. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Z, Yu W, Fu X, Sun M, Wei Q, Li D,
Chen H, Xiang J, Li H, Zhang Y, et al: Phosphorylated AKT1 is
associated with poor prognosis in esophageal squamous cell
carcinoma. J Exp Clin Cancer Res. 34:952015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kircher DA, Arave RA, Cho JH and Holmen
SL: Melanoma metastases caught in the AKT. Mol Cell Oncol.
3:e11285162016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shan B, Man H, Liu J, Wang L, Zhu T, Ma M,
Xv Z, Chen X, Yang X and Li P: TIM-3 promotes the metastasis of
esophageal squamous cell carcinoma by targeting
epithelial-mesenchymal transition via the Akt/GSK-3β/Snail
signaling pathway. Oncol Rep. 36:1551–1561. 2016.PubMed/NCBI
|
|
34
|
Liu T, Li R, Zhao H, Deng J, Long Y, Shuai
MT, Li Q, Gu H, Chen YQ and Leng AM: eIF4E promotes tumorigenesis
and modulates chemosensitivity to cisplatin in esophageal squamous
cell carcinoma. Oncotarget. 7:66851–66864. 2016.PubMed/NCBI
|
|
35
|
Li DJ, Shi M and Wang Z: RUNX3 reverses
cisplatin resistance in esophageal squamous cell carcinoma via
suppression of the protein kinase B pathway. Thorac Cancer.
7:570–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji YM, Zhou XF, Zhang J, Zheng X, Li SB,
Wei ZQ, Liu T, Cheng DL, Liu P, Song K, et al: DEPTOR suppresses
the progression of esophageal squamous cell carcinoma and predicts
poor prognosis. Oncotarget. 7:14188–14198. 2016.PubMed/NCBI
|
|
37
|
Zhang W, Lei C, Fan J and Wang J: miR-18a
promotes cell proliferation of esophageal squamous cell carcinoma
cells by increasing cylin D1 via regulating PTEN-PI3K-AKT-mTOR
signaling axis. Biochem Biophys Res Commun. 477:144–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|