Introduction
Synovial sarcoma (SS) is a malignant tumor that
accounts for 7–10% of soft tissue sarcomas, which arises mostly in
young adults with high risk of metastasis and recurrence (1). SS occurs in a wide variety of organs,
with higher incidence near the joints of the lower extremities
(2). The histological types of SS
include biphasic type synovial sarcoma (BSS), monophasic type
synovial sarcoma (MSS), and poorly differential type synovial
sarcomas (PDSS) (1).
Immunohistochemistry plays an important role in the identification
and diagnosis of SS (3). EMA and
CD99 are the commonly used markers for diagnosis of epithelial and
mesenchymal tumors, recently, they have also been used in the
diagnosis of SS. Approximately 60% of CD99 expression was positive
and may be related to the histological type of SS. Most of the
studies reported that CD99 showed high levels of expression in
single phase synovial sarcoma and poorly differentiated synovial
sarcoma. The positive expression rate of CD99 in the poorly
differentiated SS was >90% (2).
The cellular origin of SS remains unknown, SS is
currently classified as a miscellaneous tumor of uncertain
histological origin, which is considered to arise from
undifferentiated mesenchymal cells (2). Studies have supported the histogenesis
and initiation of tumor may involve cancer stem-like cells (CSLCs)
that may derive from a small population of pluripotent stem cells
(4–6). These stem-like cells initiate and
sustain tumor growth in epithelial tumors including various soft
tissue sarcoma such as synovial sarcoma (7–12).
Specific surface markers such as CD133, CD44 and nestin have been
identified to isolate the cancer stem cells in breast cancer,
pancreatic cancer and gastric cancer (13–15).
However, the relationship between SS and tumor stem cells are still
poorly understood. Studies have indicated that CD133 and other
tumor stem cell-related markers are highly expressed in SS. The
identification of cancer stem cell marker expression level has
contributed to the understanding of the pathogenesis and origin of
SS, and provides a new perspective for the diagnosis and treatment
of SS.
Recent studies showed that >90% of synovial
sarcoma with chromosomal translocation t(X;18) (p11.2;q11.2) leads
to formation of SYT-SSX fusion protein (16). It is generally believed that the
SYT-SSX fusion gene is an important event in the early stage of
synovial sarcoma. SYT-SSX is thought to be responsible for sarcoma
initiation and development. The value of SYT-SSX fusion gene in the
diagnosis of synovial sarcoma has been widely recognized, however,
the mechanism remains elusive (17).
This study explored immunohistochemical expression
level of stem cell-associated markers to determine the possible
histogenesis and pathogenesis of SS. Fusion gene SYT-SSX was
evaluated to assess diagnostic value and the molecular pathological
features.
Materials and methods
Patients and tissue specimens
A total of 20 SS patients were included from the
Department of Pathology, the First Affiliated Hospital, Shihezi
University School of Medicine between 1978 and 2016.
Histopathological diagnosis was evaluated independently by two
certified pathologists from the Department of Pathology, the First
Affiliated Hospital, Shihezi University School of Medicine. The
collected data included age, gender, sites, tumor size,
histological type, tumor stage, molecular pathology, metastases,
and the follow-up surveys (Table
I). The study was approved by the institutional ethics
committee at the First Affiliated Hospital of Shihezi University
School of Medicine. Consent was obtained from the subjects for
participation in the study and the use of their tissue.
 | Table I.Clinical data in 20 cases of synovial
sarcomas. |
Table I.
Clinical data in 20 cases of synovial
sarcomas.
| Patient ID | Gender/Age | Site | Size (cm) | Diagnosis | TNM | Fusion gene | Metastases | Outcome |
|---|
| 1 | M/14 | Left elbow
fossa | 6 | MFSS | IIB | SYT-SSX2 | NM | DOD |
| 2 | F/19 | Left thigh | 7.5 | MFSS | III | SYT-SSX | NM | NA |
| 3 | M/32 | Oral | 3 | MFSS | I | SYT-SSX1 | Lung | DOD |
| 4 | M/37 | Right elbow | 2 | MFSS | III | SYT-SSX2 | NM | DOD |
| 5 | M/40 | Left hip | 22 | MFSS | IV | SYT-SSX2 | Liver | Alive |
| 6 | M/47 | Left leg | 5 | MFSS | IV | SYT-SSX2 | Lung | DOD |
| 7 | F/40 | Left bone | 7.5 | PDSS | III | SYT-SSX | NM | DOD |
| 8 | F/10 | Right elbow | 5 | BSS | IV | SYT-SSX1 | Bone marrow
cavity | DOD |
| 9 | F/15 | Right neck | 4 | BSS | IIA | NA | NM | NA |
| 10 | F/21 | Right thigh | 7.5 | BSS | I | SYT-SSX1 | NM | Alive |
| 11 | F/22 | Left heel | 3 | BSS | IIA | SYT-SSX1 | NM | NA |
| 12 | F/32 | Left groin | NA | BSS | IIA | NA | NM | DOD |
| 13 | M/36 | Left forearm | 11 | BSS | III | SYT-SSX1 | NM | DOD |
| 14 | F/37 | Left hand and
forearm | 6 | BSS | III | SYT-SSX1 | NM | DOD |
| 15 | M/40 | Right femoral | 2.8 | BSS | IV | SYT-SSX1 | NM | DOD |
| 16 | F/43 | Right kidney | 13 | BSS | III | SYT-SSX1 | NM | DOD |
| 17 | M/52 | Left ilium | 5 | BSS | III | SYT-SSX2 | NM | Alive |
| 18 | F/55 | Right foot | 10 | BSS | IV | SYT-SSX1 | Lung | DOD |
| 19 | M/55 | Left thigh | 5.2 | BSS | I | SYT-SSX1 | Lung | DOD |
| 20 | M/64 | Left hip | 11.5 | BSS | IV | SYT-SSX1 | Lung | DOD |
Immunohistochemistry
Sections (4 µm) prepared from formalin-fixed and
paraffin-embedded tissue were obtained for immunohistochemical
analysis. EnVisions two-step immunohistochemical kit (EnVision;
Dako, Glostrup, Denmark) were used to detect specific target
proteins. Briefly, the baked sections were deparaffinized with
xylene and rehydrated in graded ethanol. Then sections were
performed by microwave in citrate buffer (pH 6.0), heated at 100°C
and quenched with 3% hydrogen peroxide. The samples were incubated
with specific target antibodies at 4°C overnight. The information
of stem cell markers (including CD133, CD29, CD44, nestin and
ALDH1) are shown in Table II.
Sections were then washed with PBS and incubated with secondary
antibodies at 37°C. 3, 3′-Diaminobenzidine (DAB) was used as a
chromogen. Finally, slides were counterstained with hematoxylin,
gradient alcohol and xylene dehydration, and mounted. The
expression of stem cell markers was scored semi-quantitatively
according to the percentage of positive cells and staining
intensity. The samples were scored according to the staining
intensity and the ratio of positive cells as follows: distribution
(0, 0%; 1, ≤10%; 2, 10–50%; 3, ≥50%) and intensity (0, negative; 1,
weak; 2, moderate; and 3, strong). A score of 0 was given for
sections with no staining, and 1 was the lowest positive score. The
product of the two scores determined the final score values; a
score of 0 indicated negative expression (−), whereas a score of
1–3 represented weak positive expression (+). Similarly, a score of
4–12 was considered as strong positive expression (++). All of the
results were confirmed by at least two senior pathologists
independently.
 | Table II.Primary antibodies used in the
immunohistochemistry staining. |
Table II.
Primary antibodies used in the
immunohistochemistry staining.
| Antigen | Antibody
species | Location | Company | Clone number | Dilution |
|---|
| CD133 | Rabbit
Polyclonal | Cytoplasm | ARP, Waltham, MA,
USA | 05-PA1021 | 1:200 |
| CD29 | Rabbit
monoclonal | Cytoplasm | Abcam, Cambridge,
UK | EP1041Y | 1:800 |
| CD44 | Mouse
monoclonal | Cytomembrane | Dako, Glostrup,
Denmark | DF1485 | 1:300 |
| Nestin | Rabbit
monoclonal | Cytoplasm | Abcam, Cambridge,
UK | SP103 | 1:200 |
| ALDH1 | Rabbit
monoclonal | Cytoplasm | Abcam, Cambridge,
UK | EP1933Y | 1:200 |
Reverse transcription polymerase chain
reaction (RT-PCR)
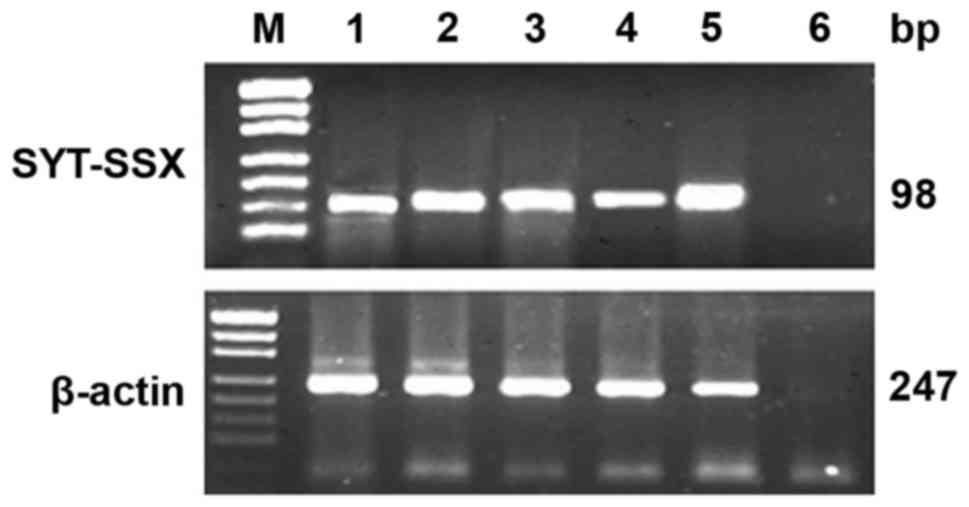
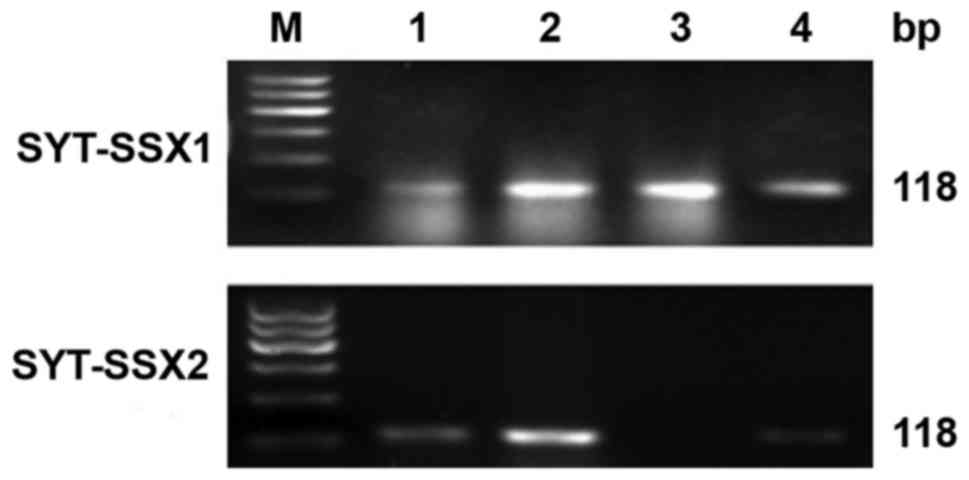
Total RNA was extracted from 18 of 20
paraffin-embedded tissues of SS using TRIzol, the other 2 cases
were from consultations which did not provide paraffin tissue.
Since 2 of 18 paraffin tissues could not be evaluated by RT-PCR
because of the low RNA quality, 16 cases of RNA were successfully
tested. To detect SYT-SSX transcript and positive control β-actin
transcript, the design of the primers is shown in Table III. One-step RT-PCR was conducted
using RNA PCR kit. The PCR products were visualized by
electrophoresis on 2% agarose gels.
 | Table III.The primers of SYT-SSX and
β-actin. |
Table III.
The primers of SYT-SSX and
β-actin.
| Primer | Sequence |
|---|
| SYT |
5′-CCAGCAGAGGCCTTATGGATA-3′ |
| SSX |
5′-TTTGTGGGCCAGATGCTTC-3′ |
|
SSX1 |
5′-GTGCAGTTGTTTCCCATCG-3′ |
|
SSX2 |
5′-GCACAGCTCTTTCCCATCA-3′ |
| β-actin-F |
5′-CAGTTTGGAGCTCCTGGAAG-3′ |
| β-actin-R |
5′-TGCAAATCCAGGGTGCAGTG-3′ |
Statistical analysis
Data were analyzed by SPSS software 17.0, and Fisher
exact test was used to compare the relationship between the stem
cell associated markers expression and clinicopathological data.
Two-tailed P-values at <0.05 were considered statistically
significant.
Results
Clinical findings
The SS patients comprised of 10 males and 10
females, with a median age of 37 years (range: 10–64 years). All
samples were primary tumors located in the limbs (17 cases) and
extra-limb (oral, kidney, and neck). According to the follow-up
survey, 7 patients had a poor prognosis with distant metastases (5
patients with lung metastasis, 1 patient with liver metastasis, and
1 patient with bone metastasis). Three patients still live with
disease after presentation. Three patients dropped out, the other
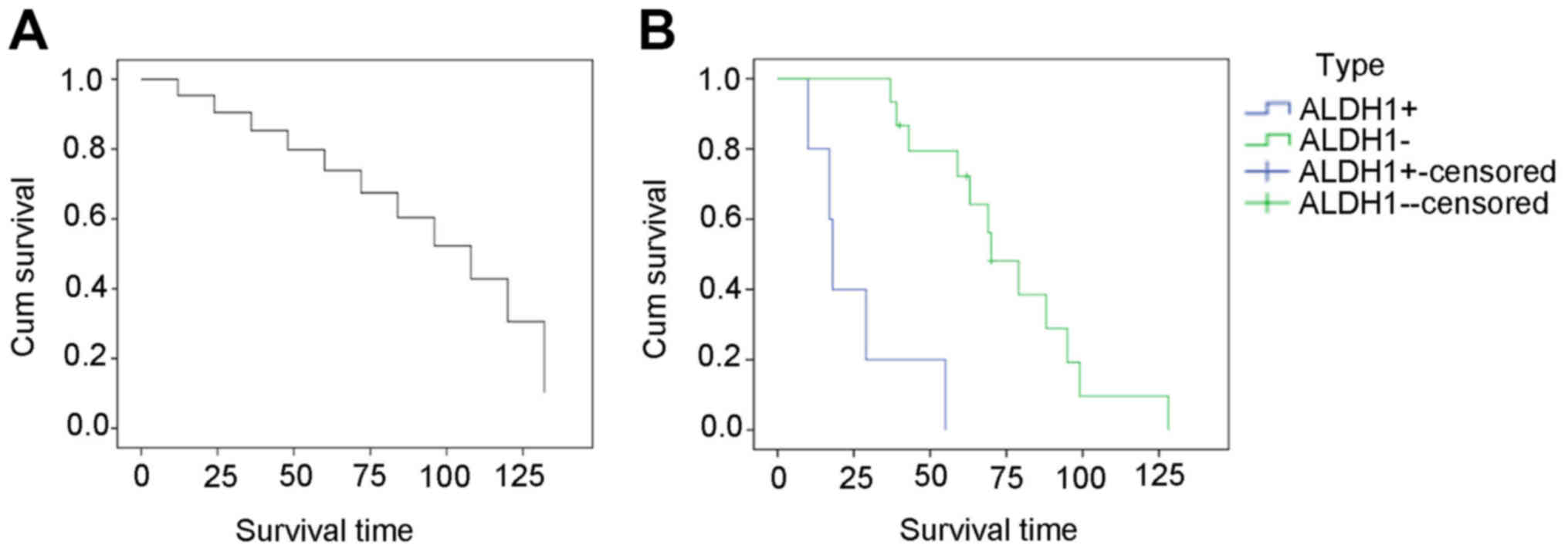
14 patients died of their disease (Table I). ALDH1− SS cases had
good prognosis with higher survival rate than ALDH1+ SS
cases (P<0.05) (Fig. 1, Table IV).
 | Table IV.P-value of the cumulate survival of
ALDH1+ and ALDH1− synovial sarcoma
patients. |
Table IV.
P-value of the cumulate survival of
ALDH1+ and ALDH1− synovial sarcoma
patients.
|
| Overall
comparisons |
|---|
|
|
|
|---|
| Test | Chi-square | df | Sig |
|---|
| Log Rank
(Mantel-Cox) | 18.190 | 1 | <0.001 |
Pathological findings
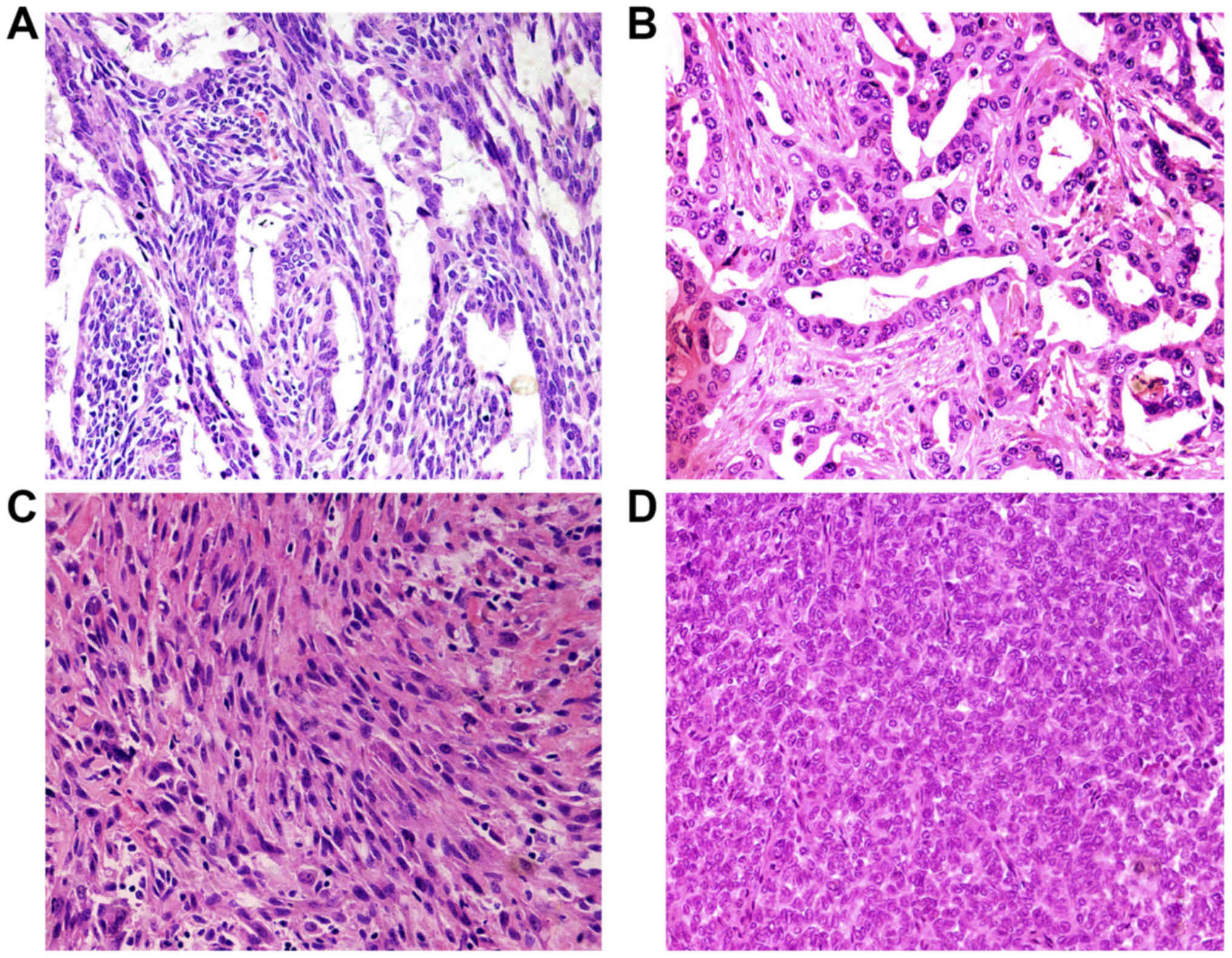
According to the fourth WHO diagnostic criteria SS
can be divided into biphasic synovial sarcoma (BSS), monophasic
fibrous synovial sarcoma (MFSS) and poorly differentiated synovial
sarcoma (PDSS). Histologically, the main characteristic of synovial
sarcoma is epithelial and mesenchymal biphasic differentiation. In
our study, the median tumor size was 7.2 cm, with a range from 2 to
22 cm. The cases were composed of 13 biphasic synovial sarcoma
(BSS), 6 monophasic fibrous synovial sarcoma (MFSS), and 1 poorly
differentiated synovial sarcoma (PDSS). Biphasic SS contained
epithelial cells and spindle cells. The variable biphasic
differentiation presented glands with a tubular or papillary
architecture or solid nests with a population of oval or rounded
cells. Epithelioid cells formed glands. Glands are irregular and
covered by columnar or cuboidal cells. Tumor cells had eosinophilic
cytoplasm, nucleus was round or oval and hyperchromatic (Fig. 2A and B). The spindle cells resemble
histologically the monophasic fibrous synovial sarcoma and were
ovoid to uniform spindle-shaped with elongated nuclear features
forming dense cellular sheets and fascicles. The cell atypia was
inconspicuous with pale-stained or eosinophilic cytoplasm. Nucleus
showed short spindle shape, granular chromatin, mesenchymal
vascular slightly enriched, slit-shaped thin wall (Fig. 2C). Poorly differentiated type
synovial sarcoma was composed of round hyperchromatic atypical
tumor cells with diffuse distribution. The cell nucleus was large,
obviously atypical and eosinophilic (Fig. 2D).
Immunohistochemical findings of stem
cell marker expression in SS
In 20 specimens, 17 were stained positive for CD133,
11 of 20 were positive for CD29 and CD44, 6 of 20 were positive for
nestin (Tables V and VI). CD133 was detected in epithelial
cells (Figs. 3A and B, and 4A and B). CD29 were also detected in the
vascular endothelial cells (Figs. 3C
and D, and 4C and D). CD44 and
nestin displayed focal immunoreactivity in spindle cells (Figs. 3E-H; 4E-H). Of the 20 specimens, 5 were
ALDH1-positive in scattered tumor cells (Figs. 3J and 4J) and negative in 15 cases (Figs. 3I and 4I). There was no statistically significant
relationship between the expression of stem cell-associated markers
(CD133, CD29, CD44, nestin, and ALDH1) and clinical data (age,
gender, sites, tumor size, histological type, tumor stage, and
metastases) (P>0.05), the expression of ALDH1 was significantly
related to the metastases of SS (P<0.05) (Tables VII and VIII).
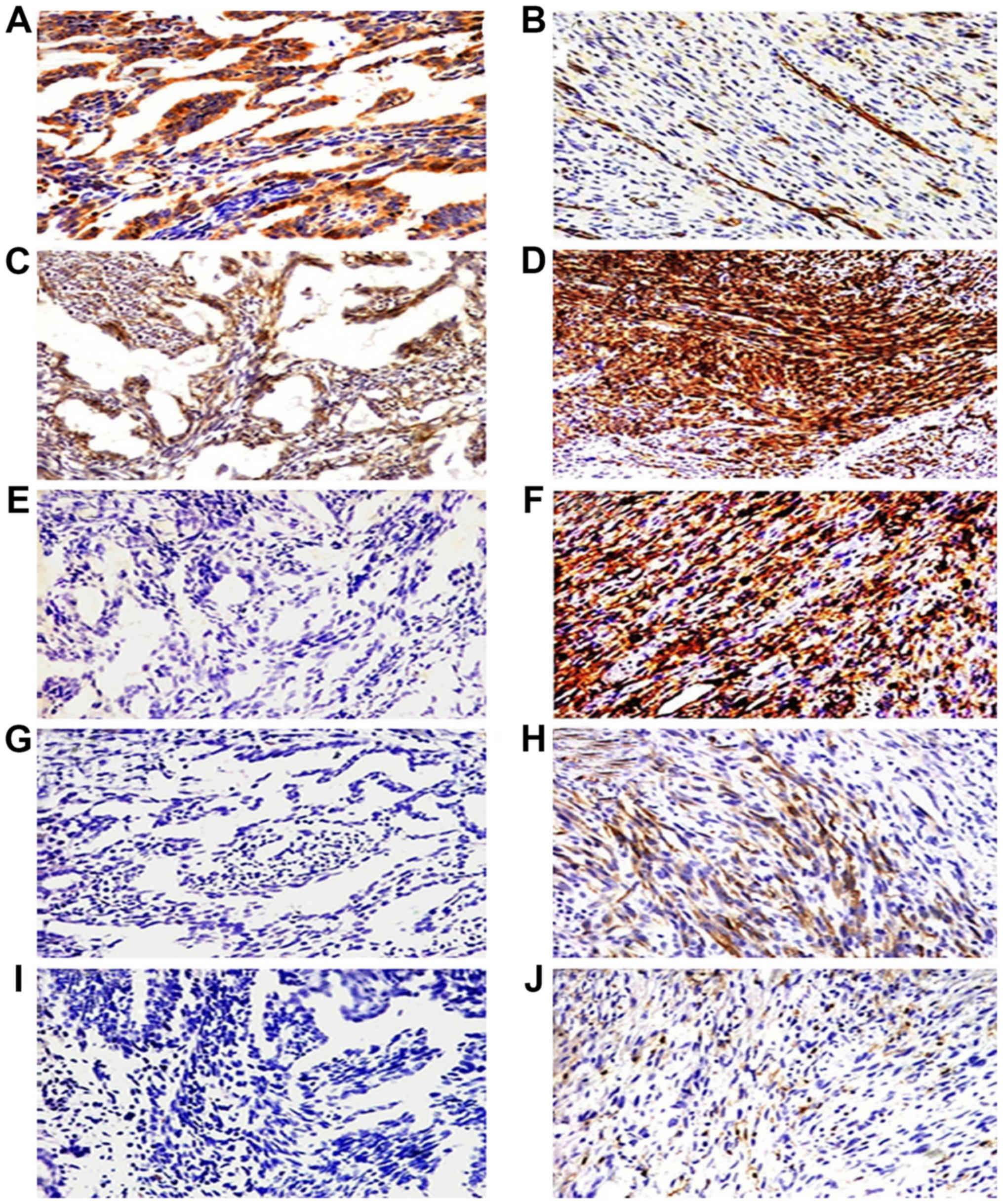 | Figure 3.Immunohistochemical staining of the
five cancer stem marker expression including CD133, CD29, CD44,
nestin and ALDH1 in epithelial cells and spindle cells of biphasic
type synovial sarcoma (BSS). Epithelial cells of BSS positive
expression for CD133 and CD29 (A and C, ×200) and negative
expression for CD44, nestin and ALDH1 (E, G and I, ×200). Spindle
cells of BSS overexpressed CD29 and CD44 (D and F, ×400), scattered
expression of CD133, nestin and ALDH1 (B, H and J, ×400). |
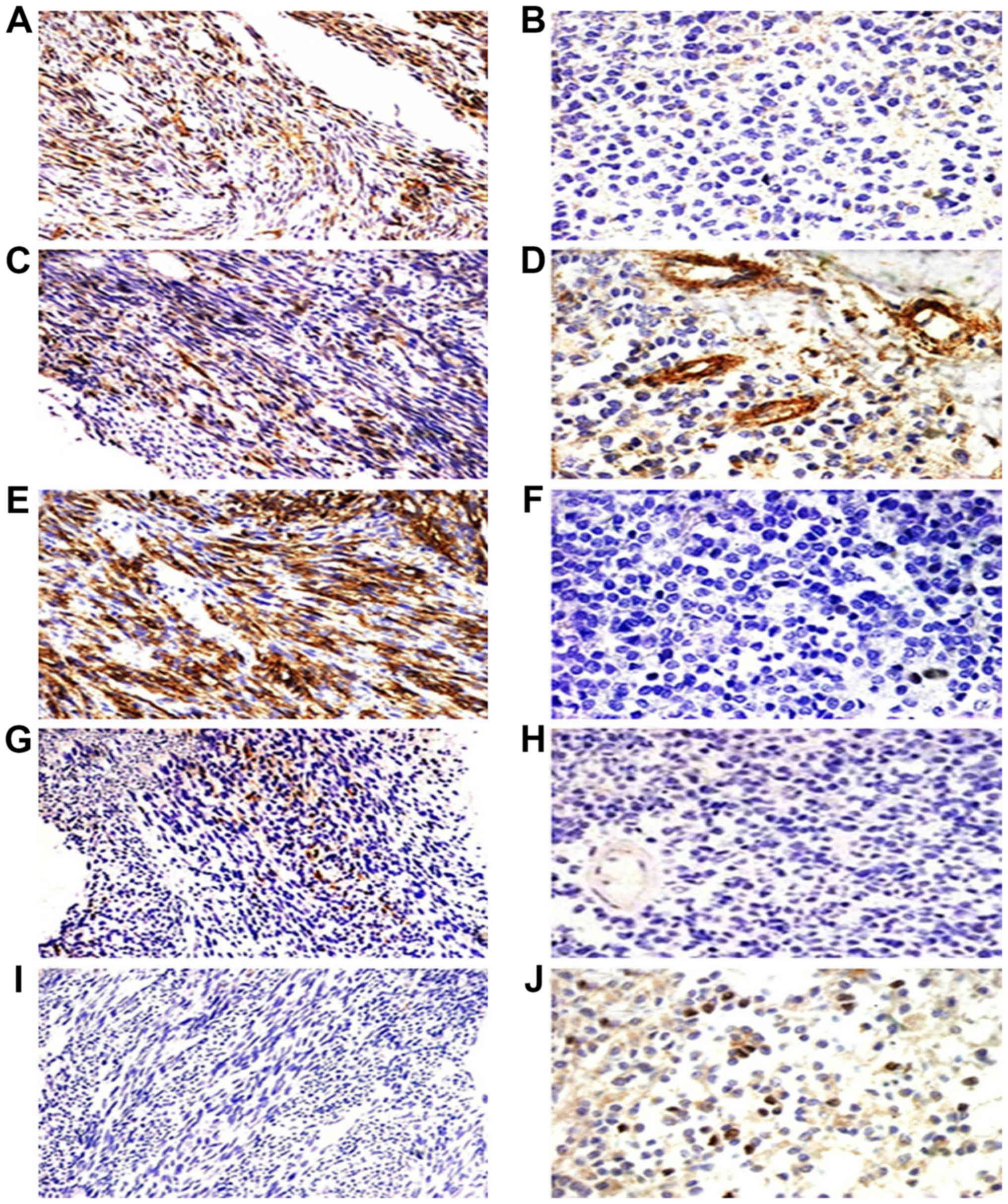 | Figure 4.Immunohistochemical staining of the
five cancer stem marker expression including CD133, CD29, CD44,
nestin and ALDH1 in monophasic fibrous synovial sarcoma (MFSS) and
poorly differential synovial sarcoma (PDSS). MFSS positive
expression of CD133, CD29 and CD44 (A, C and E, ×200) and scattered
expression of ALDH1 (J, ×200), scattered expressed of nestin (G,
×200), negative expression of CD133, CD44 and nestin (B, F, and H,
×400). PDSS scattered expression of CD29 and ALDH1 (D and J, ×400),
and with positive expression of CD29 in vascular (D, ×400),
negative expression of ALDH1 (I, ×200). |
 | Table V.Immunohistochemical expression for 5
stem cell markers in synovial sarcoma. |
Table V.
Immunohistochemical expression for 5
stem cell markers in synovial sarcoma.
|
| Stem cell
markers |
|---|
|
|
|
|---|
| Patient ID | CD133 | CD29 | CD44 | Nestin | ALDH1 |
|---|
| 1 | ++ | − | ++ | − | − |
| 2 | + | − | + | − | − |
| 3 | + | − | ++ | − | − |
| 4 | + | + | + | + | − |
| 5 | ++ | + | + | − | − |
| 6 | + | − | − | − | + |
| 7 | − | − | − | − | + |
| 8 | ++ | − | − | − | + |
| 9 | ++ | ++ | − | − | − |
| 10 | ++ | ++ | + | − | − |
| 11 | + | + | − | − | − |
| 12 | + | ++ | − | − | − |
| 13 | − | ++ | ++ | + | − |
| 14 | ++ | + | ++ | − | − |
| 15 | + | − | − | − | − |
| 16 | − | ++ | ++ | − | − |
| 17 | ++ | ++ | ++ | + | + |
| 18 | ++ | − | − | + | − |
| 19 | ++ | − | − | ++ | − |
| 20 | + | ++ | ++ | ++ | + |
 | Table VI.Five stem cell marker expression in
synovial sarcoma. |
Table VI.
Five stem cell marker expression in
synovial sarcoma.
|
|
| Positive |
|
|---|
|
|
|
|
|
|---|
| Markers | Negative (−) | (+) | (++) | Positive rate
(%)a (n) |
|---|
| CD133 | 3 | 9 | 8 | 85 (17) |
| CD29 | 9 | 4 | 7 | 55 (11) |
| CD44 | 9 | 4 | 7 | 55 (11) |
| Nestin | 14 | 4 | 2 | 30 (6) |
| ALDH1 | 15 | 4 | 1 | 25 (5) |
 | Table VII.Association between expression of 5
stem cell markers, fusion gene and clinicopathological
features. |
Table VII.
Association between expression of 5
stem cell markers, fusion gene and clinicopathological
features.
|
| CD133 |
|
| CD29 |
|
| CD44 |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Factors | Negative | Positive | χ2
value | P-value | Negative | Positive | χ2
value | P-value | Negative | Positive | χ2
value | P-value |
|---|
| Age |
|
| 0.131 | >0.05 |
|
| 0.067 | >0.05 |
|
| 0.067 | >0.05 |
|
<50 | 2 | 13 |
|
| 7 | 8 |
|
| 7 | 8 |
|
|
|
≥50 | 1 | 4 |
|
| 2 | 3 |
|
| 2 | 3 |
|
|
| Gender |
|
| 0.392 | >0.05 |
|
| 1.181 | >0.05 |
|
| 0.202 | >0.05 |
| F | 2 | 8 |
|
| 3 | 7 |
|
| 5 | 5 |
|
|
| M | 1 | 9 |
|
| 6 | 4 |
|
| 4 | 6 |
|
|
| Size (cm) |
|
| 1.644 | >0.05 |
|
| 0.024 | >0.05 |
|
| 2.170 | >0.05 |
|
<5 | 0 | 6 |
|
| 3 | 3 |
|
| 4 | 2 |
|
|
| ≥5 | 3 | 10 |
|
| 6 | 7 |
|
| 4 | 9 |
|
|
| TNM |
|
| 0.669 | >0.05 |
|
| 0.900 | >0.05 |
|
| 3.104 | >0.05 |
| I,
II | 1 | 10 |
|
| 3 | 6 |
|
| 3 | 8 |
|
|
| III,
IV | 2 | 7 |
|
| 6 | 5 |
|
| 6 | 3 |
|
|
| Metastases |
|
| 1.900 | >0.05 |
|
| 0.642 | >0.05 |
|
| 0.020 | >0.05 |
| + | 0 | 7 |
|
| 4 | 3 |
|
| 3 | 4 |
|
|
| − | 3 | 10 |
|
| 5 | 8 |
|
| 6 | 7 |
|
|
| Fusion gene |
|
| 1.039 | >0.05 |
|
| 0.042 | >0.05 |
|
| 0.95 | >0.05 |
|
SYT-SSX1 | 2 | 9 |
|
| 5 | 6 |
|
| 5 | 6 |
|
|
|
SYT-SSX2 | 0 | 5 |
|
| 2 | 3 |
|
| 1 | 4 |
|
|
| Histology |
|
| 1.644 | >0.05 |
|
| 1.310 | >0.05 |
|
| 2.328 | >0.05 |
|
MFSS | 0 | 6 |
|
| 4 | 2 |
|
| 1 | 5 |
|
|
|
BSS | 3 | 10 |
|
| 5 | 8 |
|
| 7 | 6 |
|
|
 | Table VIII.Association between expression of 5
stem cell markers and clinicopathological features. |
Table VIII.
Association between expression of 5
stem cell markers and clinicopathological features.
|
| Nestin |
|
| ALDH1 |
|
| Fusion gene |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Factors | Negative | Positive | χ2
value | P-value | Negative | Positive | χ2
value | P-value | SYT-SSX1 | SYT-SSX2 | χ2
value | P-value |
|---|
| Age |
|
| 2.260 | >0.05 |
|
| 0.800 | >0.05 |
|
| 0.097 | >0.05 |
|
<50 | 13 | 4 |
|
| 12 | 3 |
|
| 8 | 4 |
|
|
|
≥50 | 1 | 2 |
|
| 3 | 2 |
|
| 3 | 1 |
|
|
| Gender |
|
| 0.952 | >0.05 |
|
| 0.267 | >0.05 |
|
| 4.364 | >0.05 |
| F | 8 | 2 |
|
| 7 | 3 |
|
| 6 | 0 |
|
|
| M | 6 | 4 |
|
| 8 | 2 |
|
| 5 | 5 |
|
|
| Size (cm) |
|
| 0.012 | >0.05 |
|
| 3.132 | >0.05 |
|
| 0.097 | >0.05 |
|
<5 | 4 | 2 |
|
| 6 | 0 |
|
| 3 | 1 |
|
|
| ≥5 | 9 | 4 |
|
| 8 | 5 |
|
| 8 | 4 |
|
|
| TNM |
|
| 0.087 | >0.05 |
|
| 0 | >0.05 |
|
| 0.428 | >0.05 |
| I,
II | 8 | 3 |
|
| 9 | 3 |
|
| 4 | 1 |
|
|
| III,
IV | 6 | 3 |
|
| 6 | 2 |
|
| 7 | 4 |
|
|
| Metastases |
|
| 0.848 | >0.05 |
|
| 12.381 | 0.001 |
|
| 0.042 | >0.05 |
| + | 4 | 3 |
|
| 13 | 0 |
|
| 5 | 2 |
|
|
| − | 10 | 3 |
|
| 2 | 5 |
|
| 6 | 3 |
|
|
| Fusion gene |
|
| 0.019 | >0.05 |
|
| 0.873 | >0.05 |
|
| − | − |
|
SYT-SSX1 | 7 | 4 |
|
| 9 | 2 |
|
| − | − |
|
|
|
SYT-SSX2 | 3 | 2 |
|
| 3 | 2 |
|
|
|
|
|
|
| Histology |
|
| 0.903 | >0.05 |
|
| 3.041 | >0.05 |
|
| 8.045 | 0.013 |
|
MFSS | 5 | 1 |
|
| 9 | 4 |
|
| 1 | 4 |
|
|
|
BSS | 8 | 5 |
|
| 8 | 0 |
|
| 10 | 1 |
|
|
RT-PCR
Total RNA was extracted and evaluated successfully
from 18 cases of SS. The β-actin was tested in 247 bp. The results
of RT-PCR showed that fusion gene SYT-SSX (98 bp) was available in
all the cases, SYT-SSX1 and SYT-SSX2 fusion gene were tested in 118
bp. SYT-SSX1 and SYT-SSX2 transcript were detected in 11 cases and
5 cases respectively. Among 11 cases of SYT-SSX1, 10 cases were BSS
and 1 case was MFSS, the 5 cases of SYT-SSX2 included 4 cases of
MFSS and 1 case of BSS. Two cases showed no explicit typing
(Table I; Figs. 5 and 6).
Discussion
Synovial sarcoma (SS) is classified as uncertain
differentiation tumor in the 4th WHO Classification of soft tissue
tumors that occur in a widely variety of organs, especially near
the joints of the limbs. SS occurs more frequently in young adults,
in males more common than females. SS is a highly malignant soft
tissue tumor with poor prognosis, the recurrence rate was
approximately 50% within two years after operation and metastasis
occurred in 40% of patients. The 5-year survival rate was <30%,
lung and lymph node metastasis may be negative prognostic factors
of long-term survival. Thus, confirming the tumor cellular and
tissue origin is of great significance in the process of making a
correct diagnosis and differential diagnosis of SS (1,16,17).
In the present study, distant metastasis occurred in
7 patients, in addition to 3 patients lost to follow-up and 3
patients still alive, the other 14 patients all died of a tumor.
Recent studies supported that cancer is initiated by a population
of tumor cells with stem cell characteristics (18–20).
The cancer stem cell hypothesis suggests that cancer stem cells may
contribute to the initiation, progression and recurrence of cancer
(21,22). Expression of cancer stem cell makers
have been reported in leukemia, breast cancer, colon cancer and
sarcoma (7,8,10,11,23,24).
CD133 was first described as a surface antigen specific to human
hematopoietic stem and progenitor cells, but has also recently been
recognized as a common stem cell marker (14,25–28).
CD133 may be responsible for tumorigenesis and represents a pool of
tumor progenitor cells or tumor stem cells (12). Terry et al isolated a small
amount of CD133+ cells from SS cell line for the first
time, this suggested the relationship between SS and cancer
stem-like cells (25).
In our immunohistochemical study, CD133 was
positively expressed in 85% SS cases. It implies SS may be derived
from stem cells and progenitor cells. ALDH1 has been considered to
be cancer stem cell marker in lung cancer, liver cancer, colon
cancer, prostate cancer, and breast cancer. Recently, some evidence
proved that ALDH1 staining was observed as stem cell marker in soft
tissue tumors, such as solitary fibrous tumor (29,30).
Bouvier et al (29) reported
that ALDH1 expression may play an important role in distinguishing
SS from soft tissue tumors such as solitary fibrous tumors. Many
studies have found that the expression of ALDH1 protein is related
to the histologic subtypes, recurrence, metastasis and invasion of
the tumor. Increasing evidence has demonstrated ALDH1 as
therapeutic target and a malignant tumor stem cell marker that
indicates poor prognosis for tumors, decreased ALDH1 may suggest a
good prognosis (31,32).
In our study, only 5 cases were positive for ALDH1,
while ALDH1-positive cases are characteristic of poorly
differentiated, tumors ≥5 cm, and TNM III/IV, especially in
metastases. ALDH1− SS cases had better survival rate
than ALDH1+ SS cases. This suggested that the SS may
originate from cancer stem like cells. The high expression of ALDH1
may suggest an immature undifferentiated state and high malignancy,
which leads to poor prognosis. The high expression of ALDH1 may be
responsible for the failure of traditional chemotherapy of some
malignancies including colon cancer (33), prostate cancer (34) and breast cancer (35).
More studies need to be done to explore the role of
ALDH1 in the development of SS. SS occur in multiple sites of the
body, the cellular origin may be cells with multiple
differentiation ability, such as stem cells. Studies show high
expression of MSC surface markers in SS. CD29, nestin, and CD44 are
surface proteins associated with mesenchymal stem cells (MSCs)
(14,15,36).
MSC markers CD29 and CD44 displayed high expression (55%). The
mesenchymal stem/progenitor cells markers CD29 or CD44 could be
identified and separated from SS, this suggested that SS may
originate from mesenchymal stem/progenitor cells (37). Recently, nestin was considered as a
stem cell or progenitor cell surface marker. Nestin was detected in
6 SS cases in this investigation, it was positive in MFSS or
spindle cells of BSS in our study. This suggested spindle cells of
SS may originate from MSCs. However, the relationship between stem
cells and SS is poorly understood, a larger number of SS patients
needs to be explored for the histogenesis of SS.
SS is characterized by a chromosomal translocation
t(X;18) (p11.2;q11.2), and this molecular genetic feature does not
appear in other tumors (38–40).
SYT-SSX was tested positive in 93% cases of SS. The function of
SYT-SSX fusion protein is not clear, but Naka et al reported
that SS may be a stem cell malignancy because of the dysregulation
of self-renewal and differentiation driven by SYT-SSX fusion
protein (37). Garcia et al
(41) reported that mesenchymal
stem cells may be reprogrammed by SS-associated protein SYT-SSX,
the aberrant differentiation of human mesenchymal stem cells were
caused by SYT-SSX2. However, more studies need to be done to
explore the relationship between stem cells and SYT-SSX fusion
gene. Saito (42) reported that the
tumor phenotype may be determined by the SYT-SSX fusion gene. Our
study showed that 77% of BSS had the SYT-SSX1 fusion gene (10/13)
and 67% MFSS the SYT-SSX2 fusion gene (4/6). SYT-SSX can be used as
an excellent diagnostic marker for SS. In our study, SYT-SSX was
detected in 18 cases, of these, 7 had distant metastasis (1 case
metastasis to bone, 1 case metastasis to liver, 5 cases metastasis
to lung), 14 patients died of the disease (3 patients were not
available, 3 patients are still alive). SYT-SSX1 may be related to
the high proliferation rate of tumor cells, tumor distant
metastasis and poor prognosis (38–40).
SYT-SSX1 fusion gene was detected in 71.4% (5/7) of cases of SS
with distant metastasis. Gene therapy for SYT-SSX will be of great
importance for the diagnostics, treatment and prognosis of SS.
In conclusion, we detected stem cell marker (CD133,
CD29, CD44, nestin, and ALDH1) expression to explore the
histogenesis and cellular origin of SS. The immunohistochemical
findings for the stem cell markers indicated that SS may originate
from MSCs. The RT-PCR results showed fusion gene SYT-SSX may play
an important role in the tumor initiation and progression of SS.
Due to the characteristic expression of stem cell markers and
SYT-SSX for SS, these may provide new efficient drug design and
therapy strategy in future. Our results need to be confirmed by
further exploration to study the function of cancer stem cell
markers and fusion gene SYT-SSX for histogenesis and pathogenesis
of SS.
Acknowledgements
This work was supported by National Natural Science
Foundation of China (no. 81560053), the Corps Doctor Foundation
(no. 2014BB018), Shihezi University Outstanding Youth Science and
Technology Talent Cultivation Plan (2013ZRKXJQ05, 2015ZRKXJQ07),
the Pairing Program of Shihezi University with Eminent Scholar in
Elite University (SDJDZ201508), Research Project of High Level
Talents of Shihezi University (RCZX201549).
References
|
1
|
Weiss SW and Goldblum JR: Enzinger and
Weiss's Soft Tissue Tumors. 30. 5th. Mosby Elsevier; Philadelphia,
PA: 2008
|
|
2
|
Fletcher CDM, Bridge JA, Hogendoorn P and
Mertens F: WHO Classification of Tumours of Soft Tissue and Bone.
4th. IARC Press; 2013
|
|
3
|
Haldar M, Hancock JD, Coffin CM, Lessnick
SL and Capecchi MR: A conditional mouse model of synovial sarcoma:
Insights into a myogenic origin. Cancer Cell. 11:375–388. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hemmati HD, Nakano I, Lazareff JA,
Masterman-Smith M, Geschwind DH, Bronner-Fraser M and Kornblum HI:
Cancerous stem cells can arise from pediatric brain tumors. Proc
Natl Acad Sci USA. 100:pp. 15178–15183. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 100:pp.
3983–3988. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chu P, Clanton DJ, Snipas TS, Lee J,
Mitchell E, Nguyen ML, Hare E and Peach RJ: Characterization of a
subpopulation of colon cancer cells with stem cell-like properties.
Int J Cancer. 124:1312–1321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeimet AG, Reimer D, Sopper S, Boesch M,
Martowicz A, Roessler J, Wiedemair AM, Rumpold H, Untergasser G,
Concin N, et al: Ovarian cancer stem cells. Neoplasma. 59:747–755.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walter D, Satheesha S, Albrecht P,
Bornhauser BC, D'Alessandro V, Oesch SM, Rehrauer H, Leuschner I,
Koscielniak E, Gengler C, et al: CWS Study Group: CD133 positive
embryonal rhabdomyosarcoma stem-like cell population is enriched in
rhabdospheres. PLoS One. 6:e195062011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mutsaers AJ and Walkley CR: Cells of
origin in osteosarcoma: Mesenchymal stem cells or osteoblast
committed cells? Bone. 62:56–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu A, Feng B, Gu W, Cheng X, Tong T,
Zhang H and Hu Y: The CD133+ subpopulation of the SW982
human synovial sarcoma cell line exhibits cancer stem-like
characteristics. Int J Oncol. 42:1399–1407. 2013.PubMed/NCBI
|
|
13
|
Kekarainen T, Mannelin S, Laine J and
Jaatinen T: Optimization of immunomagnetic separation for cord
blood-derived hematopoietic stem cells. BMC Cell Biol. 7:302006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schieker M, Pautke C, Haasters F, Schieker
J, Docheva D, Böcker W, Guelkan H, Neth P, Jochum M and Mutschler
W: Human mesenchymal stem cells at the single-cell level:
Simultaneous seven-colour immunofluorescence. J Anat. 210:592–599.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Méndez-Ferrer S, Michurina TV, Ferraro F,
Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A,
Enikolopov GN and Frenette PS: Mesenchymal and haematopoietic stem
cells form a unique bone marrow niche. Nature. 466:829–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clark J, Rocques PJ, Crew AJ, Gill S,
Shipley J, Chan AM, Gusterson BA and Cooper CS: Identification of
novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2)
translocation found in human synovial sarcoma. Nat Genet.
7:502–508. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ladanyi M: Fusions of the SYT and SSX
genes in synovial sarcoma. Oncogene. 20:5755–5762. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boman BM and Wicha MS: Cancer stem cells:
A step toward the cure. J Clin Oncol. 26:2795–2799. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jung Y, Bauer G and Nolta JA: Concise
review: Induced pluripotent stem cell-derived mesenchymal stem
cells: progress toward safe clinical products. Stem Cells.
30:42–47. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sadikovic B, Graham C, Ho M, Zielenska M
and Somers GR: Immunohistochemical expression and cluster analysis
of mesenchymal and neural stem cell-associated proteins in
pediatric soft tissue sarcomas. Pediatr Dev Pathol. 14:259–272.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Santagata S, Ligon KL and Hornick JL:
Embryonic stem cell transcription factor signatures in the
diagnosis of primary and metastatic germ cell tumors. Am J Surg
Pathol. 31:836–845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Passegué E: Hematopoietic stem cells,
leukemic stem cells and chronic myelogenous leukemia. Cell Cycle.
4:266–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakanishi M, Niidome T, Matsuda S, Akaike
A, Kihara T and Sugimoto H: Microglia-derived interleukin-6 and
leukaemia inhibitory factor promote astrocytic differentiation of
neural stem/progenitor cells. Eur J Neurosci. 25:649–658. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Terry J and Nielsen T: Expression of CD133
in synovial sarcoma. Appl Immunohistochem Mol Morphol. 18:159–165.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okamoto H, Fujishima F, Nakamura Y,
Zuguchi M, Ozawa Y, Takahashi Y, Miyata G, Kamei T, Nakano T,
Taniyama Y, et al: Significance of CD133 expression in esophageal
squamous cell carcinoma. World J Surg Oncol. 11:512013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang D, Sun B, Zhao X, Ma Y, Ji R, Gu Q,
Dong X, Li J, Liu F, Jia X, et al: Twist1 expression induced by
sunitinib accelerates tumor cell vasculogenic mimicry by increasing
the population of CD133+ cells in triple-negative breast
cancer. Mol Cancer. 13:2072014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bouvier C, Bertucci F, Métellus P, Finetti
P, de Paula A Maues, Forest F, Mokhtari K, Miquel C, Birnbaum D,
Vasiljevic A, et al: ALDH1 is an immunohistochemical diagnostic
marker for solitary fibrous tumours and haemangiopericytomas of the
meninges emerging from gene profiling study. Acta Neuropathol
Commun. 1:102013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
England DM, Hochholzer L and McCarthy MJ:
Localized benign and malignant fibrous tumors of the pleura. A
clinicopathologic review of 223 cases. Am J Surg Pathol.
13:640–658. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Lv DL, Duan JJ, Xu SL, Zhang JF,
Yang XJ, Zhang X, Cui YH, Bian XW and Yu SC: ALDH1A1 expression
correlates with clinicopathologic features and poor prognosis of
breast cancer patients: A systematic review and meta-analysis. BMC
Cancer. 14:4442014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuroda T, Hirohashi Y, Torigoe T, Yasuda
K, Takahashi A, Asanuma H, Morita R, Mariya T, Asano T, Mizuuchi M,
et al: ALDH1-high ovarian cancer stem-like cells can be isolated
from serous and clear cell adenocarcinoma cells, and ALDH1 high
expression is associated with poor prognosis. PLoS One.
8:e651582013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsika A, Srinivasan B, Day C, Mader SA,
Kiernan DM, Broomfield A, Fu J, Hooper JD, Kench JG and Samaratunga
H: Cancer stem cell markers in prostate cancer: An
immunohistochemical study of ALDH1, SOX2 and EZH2. Pathology.
47:622–628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishida S, Hirohashi Y, Torigoe T, Inoue
R, Kitamura H, Tanaka T, Takahashi A, Asanuma H, Masumori N,
Tsukamoto T, et al: Prostate cancer stem-like
cells/cancer-initiating cells have an autocrine system of
hepatocyte growth factor. Cancer Sci. 104:431–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ieni A and Tuccari G: Comments on the
‘Prognostic impact and clinicopathological correlation of CD133 and
ALDH1 expression in invasive breast cancer’. J Breast Cancer.
19:96–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vassilopoulos A, Chisholm C, Lahusen T,
Zheng H and Deng CX: A critical role of CD29 and CD49f in mediating
metastasis for cancer-initiating cells isolated from a
Brca1-associated mouse model of breast cancer. Oncogene.
33:5477–5482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Naka N, Takenaka S, Araki N, Miwa T,
Hashimoto N, Yoshioka K, Joyama S, Hamada K, Tsukamoto Y, Tomita Y,
et al: Synovial sarcoma is a stem cell malignancy. Stem Cells.
28:1119–1131. 2010.PubMed/NCBI
|
|
38
|
Brett D, Whitehouse S, Antonson P, Shipley
J, Cooper C and Goodwin G: The SYT protein involved in the t(X;18)
synovial sarcoma translocation is a transcriptional activator
localised in nuclear bodies. Hum Mol Genet. 6:1559–1564. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hayakawa K, Ikeya M, Fukuta M, Woltjen K,
Tamaki S, Takahara N, Kato T Jr, Sato S, Otsuka T and Toguchida J:
Identification of target genes of synovial sarcoma-associated
fusion oncoprotein using human pluripotent stem cells. Biochem
Biophys Res Commun. 432:713–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cironi L, Provero P, Riggi N, Janiszewska
M, Suva D, Suva ML, Kindler V and Stamenkovic I: Epigenetic
features of human mesenchymal stem cells determine their
permissiveness for induction of relevant transcriptional changes by
SYT-SSX1. PLoS One. 4:e79042009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Garcia CB, Shaffer CM, Alfaro MP, Smith
AL, Sun J, Zhao Z, Young PP, VanSaun MN and Eid JE: Reprogramming
of mesenchymal stem cells by the synovial sarcoma-associated
oncogene SYT-SSX2. Oncogene. 31:2323–2334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saito T: The SYT-SSX fusion protein and
histological epithelial differentiation in synovial sarcoma:
Relationship with extracellular matrix remodeling. Int J Clin Exp
Pathol. 6:2272–2279. 2013.PubMed/NCBI
|