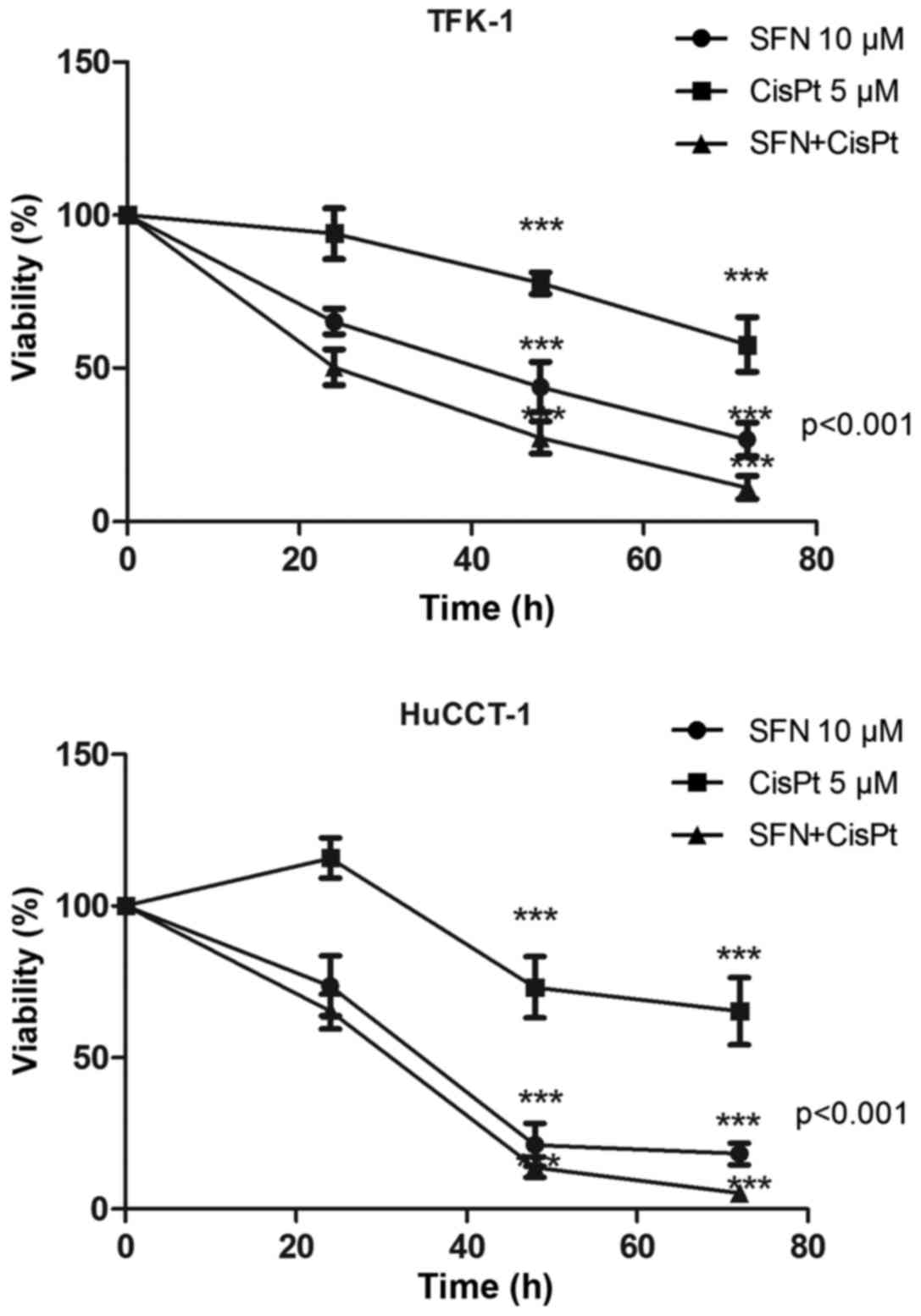
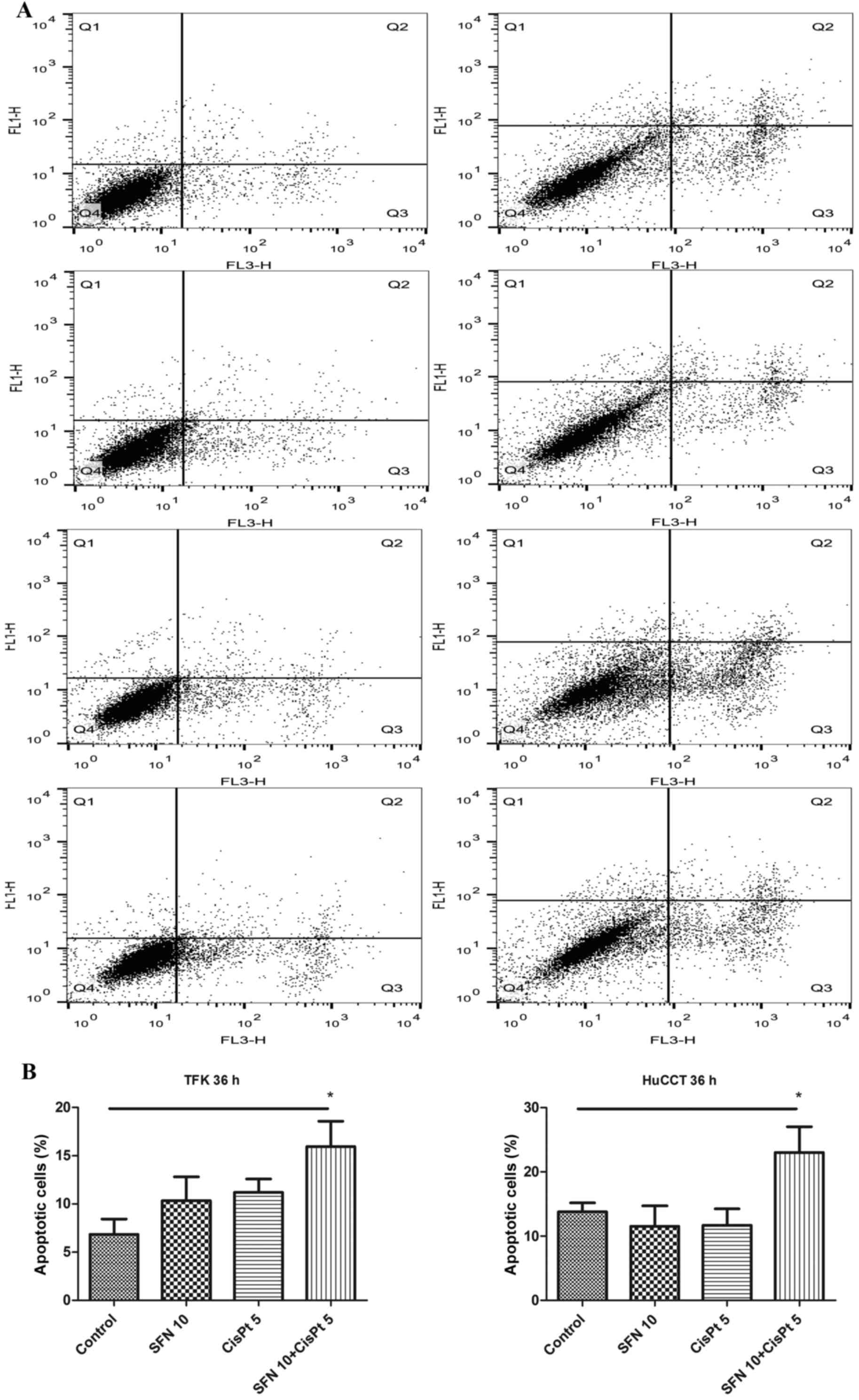
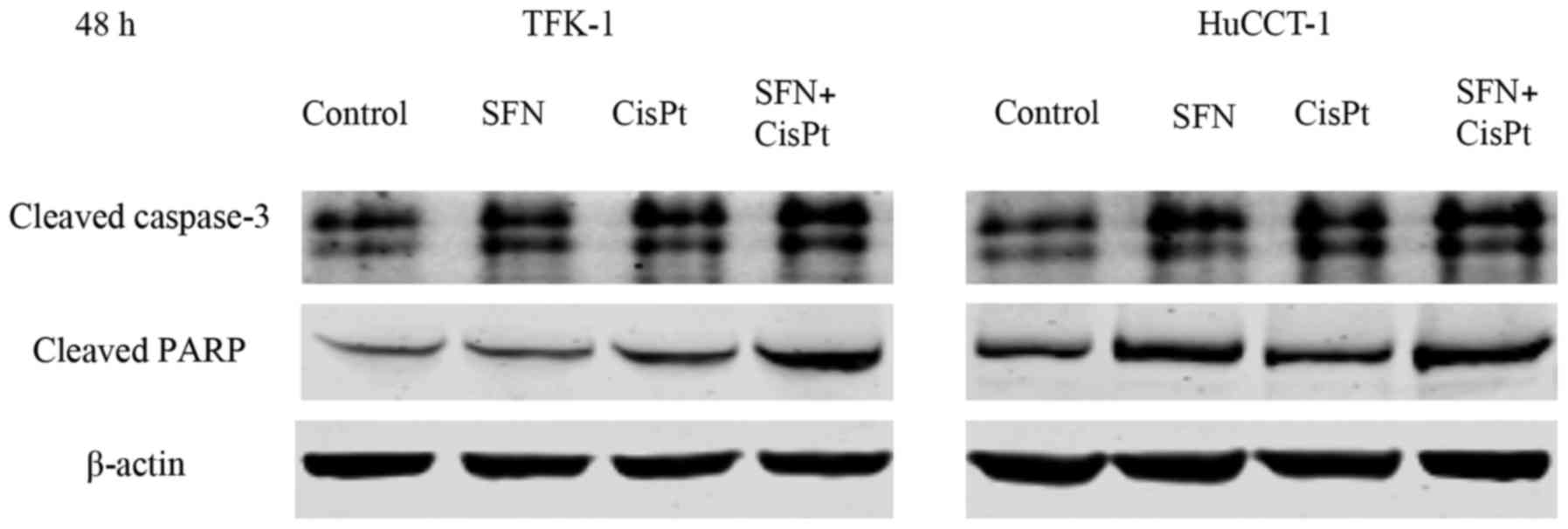
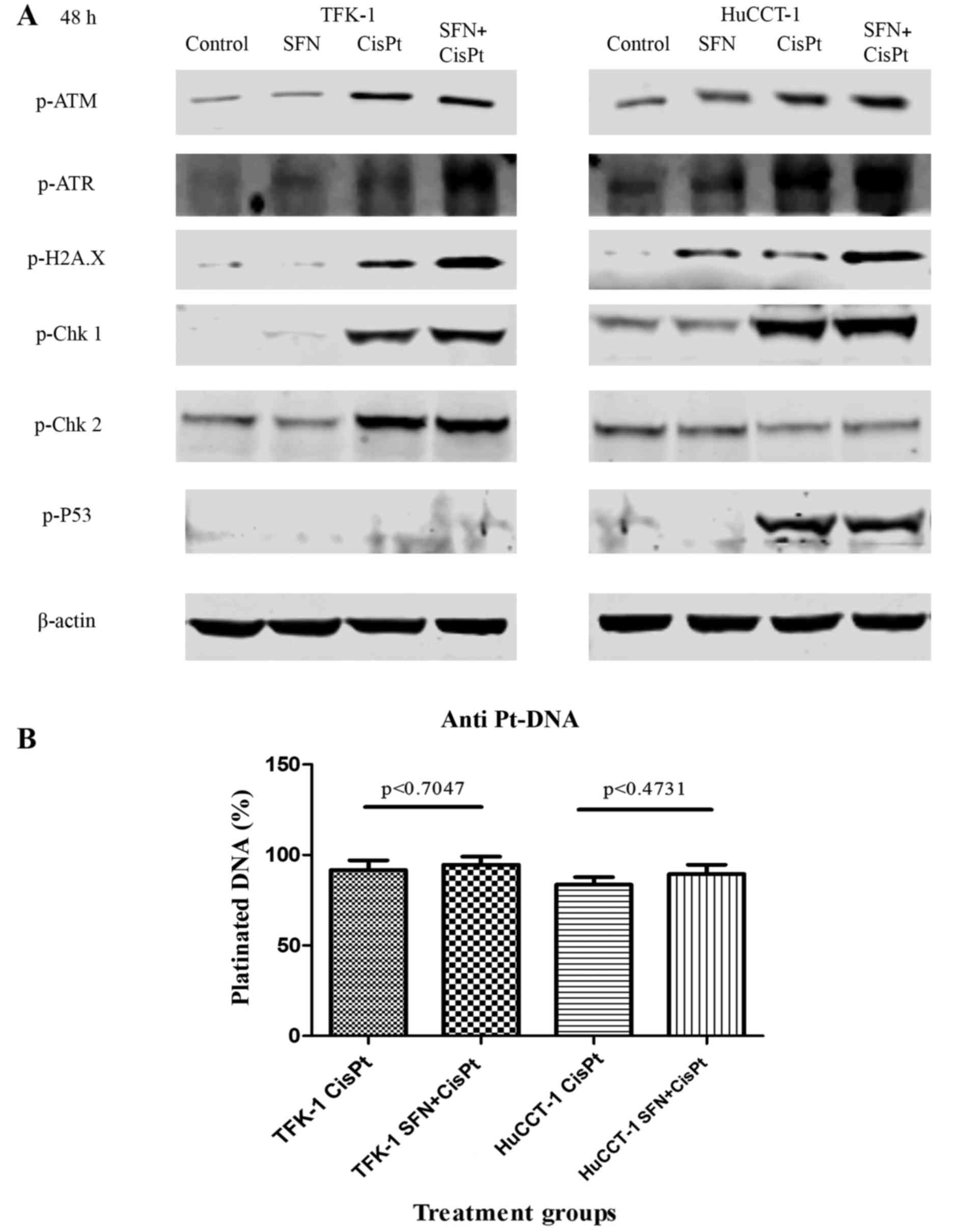
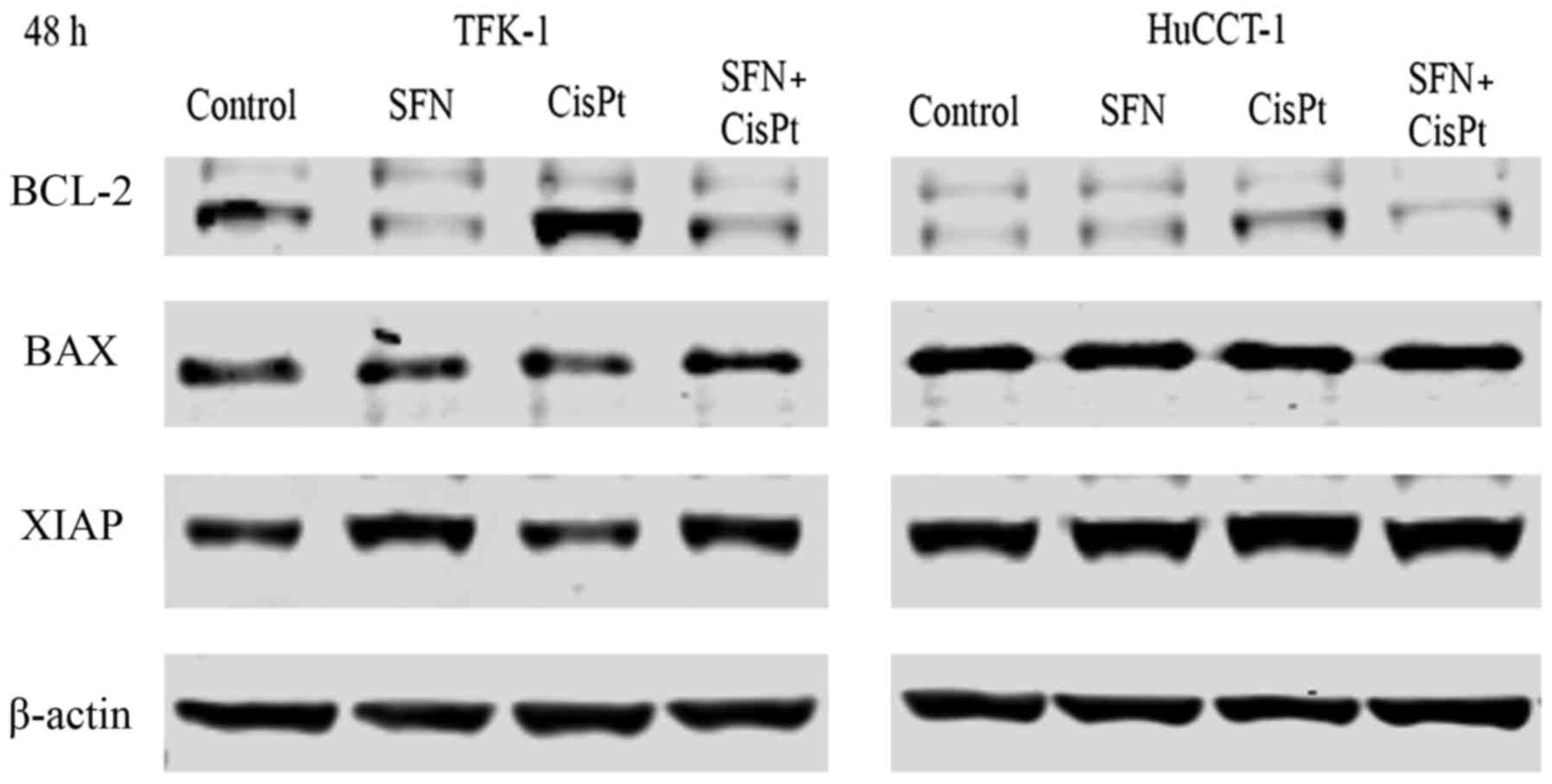
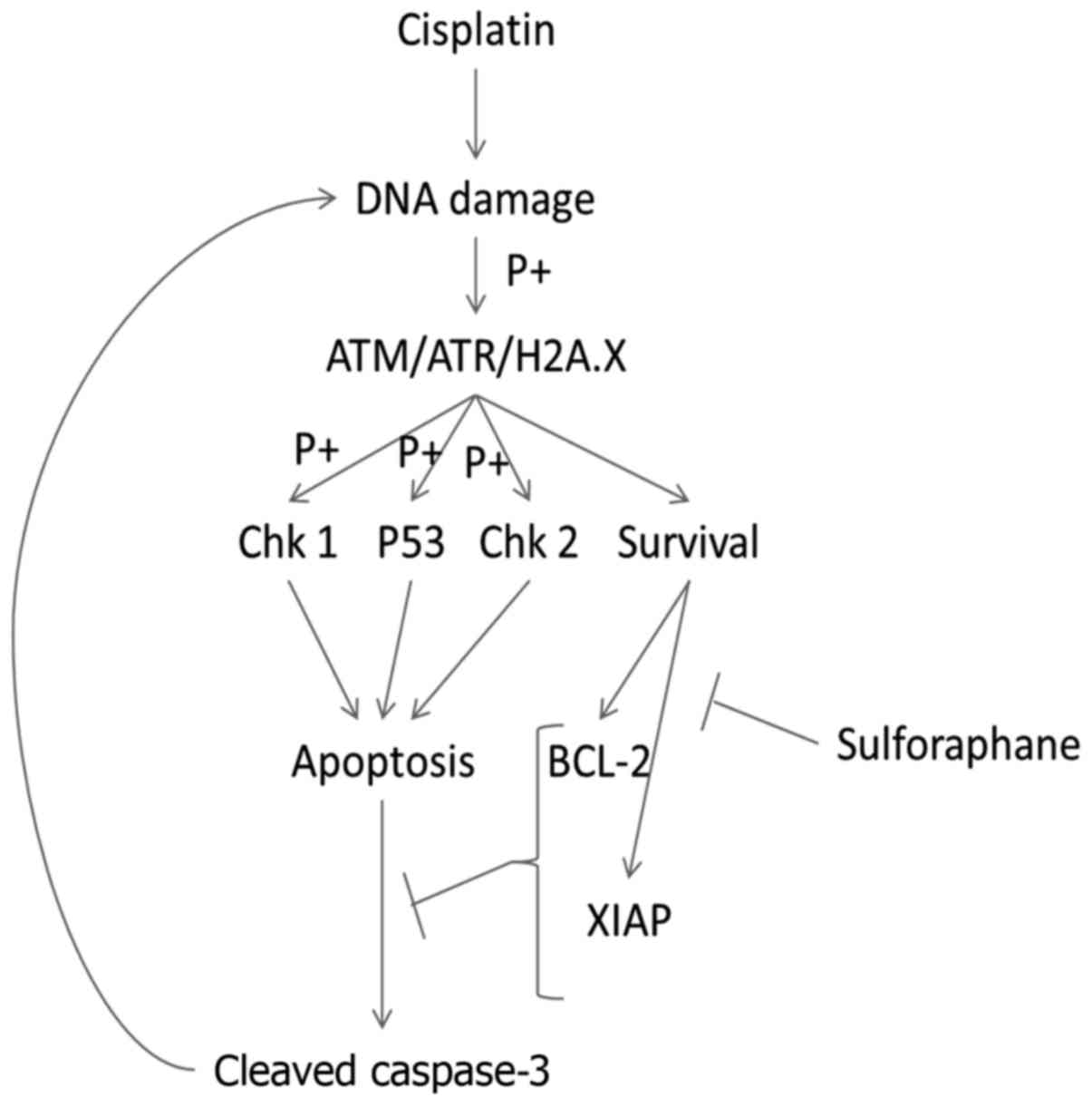
|
1
|
Macias RI: Cholangiocarcinoma: Biology,
clinical management, and pharmacological perspectives. ISRN
Hepatol. 2014:8280742014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakeeb A, Pitt HA, Sohn TA, Coleman J,
Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ and Cameron
JL: Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and
distal tumors. Ann Surg. 224:463–475. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogel A, Wege H, Caca K, Nashan B and
Neumann U: The diagnosis and treatment of cholangiocarcinoma. Dtsch
Arztebl Int. 111:748–754. 2014.PubMed/NCBI
|
|
4
|
DeOliveira ML, Cunningham SC, Cameron JL,
Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ and Schulick
RD: Cholangiocarcinoma: Thirty-one-year experience with 564
patients at a single institution. Ann Surg. 245:755–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eckel F and Schmid RM: Chemotherapy in
advanced biliary tract carcinoma: A pooled analysis of clinical
trials. Br J Cancer. 96:896–902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Y, Hu Y, Hu X, Li X, Lin L and Han X:
Cisplatin combined with irinotecan or etoposide for untreated
extensive-stage small cell lung cancer: A multicenter randomized
controlled clinical trial. Thorac Cancer. 6:785–791. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Velasquez WS, Cabanillas F, Salvador P,
McLaughlin P, Fridrik M, Tucker S, Jagannath S, Hagemeister FB,
Redman JR, Swan F, et al: Effective salvage therapy for lymphoma
with cisplatin in combination with high-dose Ara-C and
dexamethasone (DHAP). Blood. 71:117–122. 1988.PubMed/NCBI
|
|
8
|
O'Kane GM, Cadoo KA, Walsh EM, Emerson R,
Dervan P, O'Keane C, Hurson B, O'Toole G, Dudeney S, Kavanagh E, et
al: Perioperative chemotherapy in the treatment of osteosarcoma: A
26-year single institution review. Clin Sarcoma Res. 5:172015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stein A, Arnold D, Bridgewater J,
Goldstein D, Jensen LH, Klümpen HJ, Lohse AW, Nashan B, Primrose J,
Schrum S, et al: Adjuvant chemotherapy with gemcitabine and
cisplatin compared to observation after curative intent resection
of cholangiocarcinoma and muscle invasive gallbladder carcinoma
(ACTICCA-1 trial) - a randomized, multidisciplinary, multinational
phase III trial. BMC Cancer. 15:5642015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Talalay P, Cho CG and Posner GH:
A major inducer of anticarcinogenic protective enzymes from
broccoli: Isolation and elucidation of structure. Proc Natl Acad
Sci USA. 89:pp. 2399–2403. 1992; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fahey JW, Zalcmann AT and Talalay P: The
chemical diversity and distribution of glucosinolates and
isothiocyanates among plants. Phytochemistry. 56:5–51. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Juge N, Mithen RF and Traka M: Molecular
basis for chemoprevention by sulforaphane: A comprehensive review.
Cell Mol Life Sci. 64:1105–1127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin CY, Moon DO, Lee JD, Heo MS, Choi YH,
Lee CM, Park YM and Kim GY: Sulforaphane sensitizes tumor necrosis
factor-related apoptosis-inducing ligand-mediated apoptosis through
downregulation of ERK and Akt in lung adenocarcinoma A549 cells.
Carcinogenesis. 28:1058–1066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hunakova L, Gronesova P, Horvathova E,
Chalupa I, Cholujova D, Duraj J and Sedlak J: Modulation of
cisplatin sensitivity in human ovarian carcinoma A2780 and SKOV3
cell lines by sulforaphane. Toxicol Lett. 230:479–486. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rausch V, Liu L, Kallifatidis G, Baumann
B, Mattern J, Gladkich J, Wirth T, Schemmer P, Büchler MW, Zöller
M, et al: Synergistic activity of sorafenib and sulforaphane
abolishes pancreatic cancer stem cell characteristics. Cancer Res.
70:5004–5013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Landen CN, Li Y, Alvarez RD and
Tollefsbol TO: Enhancement of cisplatin-mediated apoptosis in
ovarian cancer cells through potentiating G2/M arrest and p21
upregulation by combinatorial epigallocatechin gallate and
sulforaphane. J Oncol. 2013:8729572013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyagiwa M, Ichida T, Tokiwa T, Sato J and
Sasaki H: A new human cholangiocellular carcinoma cell line
(HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium.
In Vitro Cell Dev Biol. 25:503–510. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saijyo S, Kudo T, Suzuki M, Katayose Y,
Shinoda M, Muto T, Fukuhara K, Suzuki T and Matsuno S:
Establishment of a new extrahepatic bile duct carcinoma cell line,
TFK-1. Tohoku J Exp Med. 177:61–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong ZF, Zhao WX, Yin ZY, Xie CR, Xu YP,
Chi XQ, Zhang S and Wang XM: Capsaicin enhances the drug
sensitivity of cholangiocarcinoma through the inhibition of
chemotherapeutic-induced autophagy. PLoS One. 10:e01215382015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mah LJ, El-Osta A and Karagiannis TC:
gammaH2AX: A sensitive molecular marker of DNA damage and repair.
Leukemia. 24:679–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roberts JJ and Fraval HN: Cisplatin:
Current Status and New Developments. Prestayko AW, Crooke ST and
Carter SK: Academic Press; Orlando: pp. 57–77. 1980, View Article : Google Scholar
|
|
24
|
Kallifatidis G, Labsch S, Rausch V,
Mattern J, Gladkich J, Moldenhauer G, Büchler MW, Salnikov AV and
Herr I: Sulforaphane increases drug-mediated cytotoxicity toward
cancer stem-like cells of pancreas and prostate. Mol Ther.
19:188–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma C, Sadrieh L, Priyani A, Ahmed M,
Hassan AH and Hussain A: Anti-carcinogenic effects of sulforaphane
in association with its apoptosis-inducing and anti-inflammatory
properties in human cervical cancer cells. Cancer Epidemiol.
35:272–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fimognari C, Lenzi M, Sciuscio D,
Cantelli-Forti G and Hrelia P: Combination of doxorubicin and
sulforaphane for reversing doxorubicin-resistant phenotype in mouse
fibroblasts with p53Ser220 mutation. Ann NY Acad Sci.
1095:62–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Helt CE, Cliby WA, Keng PC, Bambara RA and
O'Reilly MA: Ataxia telangiectasia mutated (ATM) and ATM and
Rad3-related protein exhibit selective target specificities in
response to different forms of DNA damage. J Biol Chem.
280:1186–1192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Podhorecka M, Skladanowski A and Bozko P:
H2AX phosphorylation: Its role in DNA damage response and cancer
therapy. J Nucleic Acids. 2010:9201612010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doerks T, Copley RR, Schultz J, Ponting CP
and Bork P: Systematic identification of novel protein domain
families associated with nuclear functions. Genome Res. 12:47–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khalil HS, Tummala H and Zhelev N: ATM in
focus: A damage sensor and cancer target. Biodiscovery.
5(1)2012.doi: 10.7750/BioDiscovery.2012.5.1.
|
|
32
|
Liu X, He Y, Li F, Huang Q, Kato TA, Hall
RP and Li CY: Caspase-3 promotes genetic instability and
carcinogenesis. Mol Cell. 58:284–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|