Introduction
Non-small cell lung cancer (NSCLC) is a malignant
tumor with one of the highest morbidity rates worldwide, and is the
most common cause of malignant tumor-related death (1). With the improvements in living
standards and health, the morbidity of NSCLC has been decreased in
recent years (2). However, its
morbidity in male patients still ranks second out of all types of
malignant tumors. Furthermore, the incidence of NSCLC appears to be
rising in females (3). Although the
treatment of NSCLC has been constantly improving, the survival rate
has not increased substantially. This may be due to common late
diagnosis, late treatment and complexity (4). Therefore, it is necessary to explore
the pathogenetic mechanisms underlying NSCLC and the potential
modulatory mechanisms involved to facilitate its early diagnosis
and early treatment.
In recent years, molecular diagnosis and targeted
therapies have provided a new approach to the integrated control of
NSCLC (5). Individual gene
expression patterns, individual neoplasm staging and their
relevance to patient prognosis have gradually become an area of
interest in NSCLC research (6). The
pathogenetic mechanism of NSCLC is associated with frequent
mutation, amplification and epigenetic changes of tumor-related
genes (7). Epigenetic alterations
do not affect genomic sequence, but can cause protein expression
changes that result in tumor development and progression (7). Studies of epigenetics mainly focus on
DNA methylation, chromatin rearrangement, and changes to RNA
editing. Furthermore, miRNAs are currently a hotspot for research
into tumor-related changes (5,6).
Recent research indicates that alterations to the regulation of
miRNAs may to some extent contribute to the occurrence of malignant
tumors.
miRNAs are small non-coding RNA molecules that are
processed by specific incision enzymes; transcriptional precursors
consisting of double-stranded RNA (pri-miRNAs) are cleaved by
Drosha and Dicer enzymes (8).
miRNAs have complementary sequence to their target mRNA and base
pair with the target sequence resulting in mRNA degradation or
decreased translation, inducing gene silencing (9). Gene expression is negatively regulated
by the interaction of a miRNA with the 3 non-coding region of the
target gene mRNA. This regulation of protein translation by miRNAs
influences a wide variety of physiological and abnormal cell
processes (10). miRNAs are also
highly specific; as biomarkers, they may have an important role in
tumor prevention (11). In NSCLC,
miRNAs have been shown to affect tumor formation, occurrence and
development (11). Although in many
cases the precise functions of specific miRNAs are not clear,
miRNAs are regarded as important contributors to various
physiological and developmental processes (12).
Myocyte enhancer factor 2D (MEF2D) is a gene of the
MEF2 family, and contains a structural domain of the regulatory
factor MCM1 (13). Its main
function is to regulate the survival and differentiation of
multiple cell types. Early studies on MEF2 were predominantly
limited to its role in muscle and the nervous system (14). However, numerous studies have
demonstrated that chromosome translocation in NSCLC may result in
abnormally high expression of MEF2D and promote the formation and
development of NSCLC (15).
Meanwhile, MEF2D may play an important role in the NSCLC formative
process (13). In the present
study, we investigated the function of miR-1244 in
cisplatin-treated NSCLC.
Materials and methods
Patient samples and follow-up
In total, 83 fresh tissue samples from
cisplatin-induced NSCLC patients and 49 fresh tissue samples from
non-cisplatin-induced NSCLC patients were collected at the
Department of Thoracic Surgery of the Tumor Hospital of Yunnan
Province (The Third Affiliated Hospital of Kunming Medical
University) from April 2006 to December 2006. The present study was
performed with all the patients written informed consent and in
accordance to the procedures approved by the Ethics Review Board at
The Third Affiliated Hospital of Kunming Medical University. All
patients underwent surgery, and subsequently received cisplatin or
no treatment, and were monitored by chest, abdominal and pelvic
computed tomography (CT). The overall survival (OS) time was
assessed between surgery and cisplatin treatment.
Quantitative real-time RT-PCR
(qRT-PCR)
Total RNAs were extracted from the NSCLC tissues
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer's instructions. Total RNA (2 µg) was
reversely-transcribed to cDNA using a First-Strand cDNA synthesis
kit (OriGene Technologies, MD, USA). qRT-PCR was conducted using
SYBR Premix Taq (CWbio, Beijing, China) on a 7500 Fast PCR
instrument (Applied Biosystems, Carlsbad, CA, USA). The relative
miR-1244 expression was normalized to U6, which was calculated
using the 2−ΔΔCt method.
Cell culture and transfection
The human NSCLC cell lines A549 and NCI-H522 were
purchased from the Shanghai Cell Bank of the Chinese Academy of
Sciences, and cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS; both from Gibco, Rockville, MD, USA), 100
U/ml penicillin G and 100 mg/ml streptomycin sulfate at 37°C in a
humidified 5% CO2 atmosphere. miR-1244 mimics, si-MEF2D
or the negative control were constructed by Sangon Biological
Engineering Co., Ltd. (Shanghai, China). miR-1244 mimics, si-MEF2D
(30 nM) and the negative control (30 nM) were transfected into
cells with Lipofectamine™ 2000 (Invitrogen).
MTT and LDH assays
For the MTT assay, transfected A549 and H522 cells
were seeded (2.5×103 cells/well) in 96-well plates and
allowed to attach overnight. A549 and H522 cells were cultured with
10 µM of cisplatin for 0, 24, 48 and 72 h. MTT (20 µl at 5 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) was added to the medium after
treatment at 37°C for 4 h. The supernatant was aspirated, and 150
µl of dimethyl sulfoxide (DMSO) was added to each well to dissolve
the precipitate at 37°C for 20 min. The absorbance at 490 nm was
assessed using a microplate reader (Molecular Devices, Sunnyvale,
CA, USA).
For the LDH assay, transfected A549 and H522 cells
were seeded (2.5×103 cells/well) in 96-well plates and
allowed to attach overnight. A549 and H522 cells were cultured with
10 µM of cisplatin for 48 h. The supernatant was aspirated and 150
µl of LDH releasing reagent was added into each well at 37°C for 1
h. The absorbance at 490 nm was assessed using a microplate reader
(Molecular Devices).
Flow cytometry
Transfected A549 and H522 cells were seeded
(5×106 cells/well) in 6-well plates and allowed to
attach overnight. A549 and H522 cells were cultured with 10 µM of
cisplatin for 48 h. The cells were then stained with an Annexin
V-FITC and propidium iodide (PI) apoptosis detection kit
(MultiSciences Biotech Co., Ltd., Hangzhou, China) at 4°C for 20
min in the dark. Cell apoptosis was assessed on an LSR2 upgraded
flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blotting
Transfected A549 and H522 cells were seeded
(5×106 cells/well) in 6-well plates and allowed to
attach overnight. A549 and H522 cells were cultured with 10 µM of
cisplatin for 48 h. Then, cells were harvested at 3,000 × g for 5
min and lysed in RIPA buffer in the presence of protease inhibitors
(Roche, Mannheim, Germany) at 4°C for 30 min. The protein
concentration in the supernatants was quantified using the Enhanced
BCA Protein Assay kit (Beyotime Biotechnology, Shanghai, China).
Proteins (50–80 µg) were separated using 8–12% SDS-PAGE, and then
transferred to polyvinylidene fluoride (PVDF) membranes (Millipore,
Bedford, MA, USA). The membranes were blocked using 5% non-fat milk
in Tris-buffered saline with Tween-20 (TBST) for 1 h at 37°C, and
incubated with the primary antibodies Bax, MEF2D, cyclin D1, p53
(all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) and GAPDH
overnight at 4°C. Subsequently the membranes were incubated with
anti-rabbit horseradish peroxidase-conjugated secondary antibodies
(Santa Cruz Biotechnology) and developed using an enhanced
chemiluminescence (ECL) detection system (Invitrogen-Life
Technologies, Carlsbad, CA, USA).
Statistical analysis
The values in the present study are expressed as the
means ± SD, and were analyzed by t-test or one-way ANOVA. Data were
analyzed using SPSS software 19.0 for Windows (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant result.
Results
Expression of miR-1244 in
cisplatin-treated NSCLC
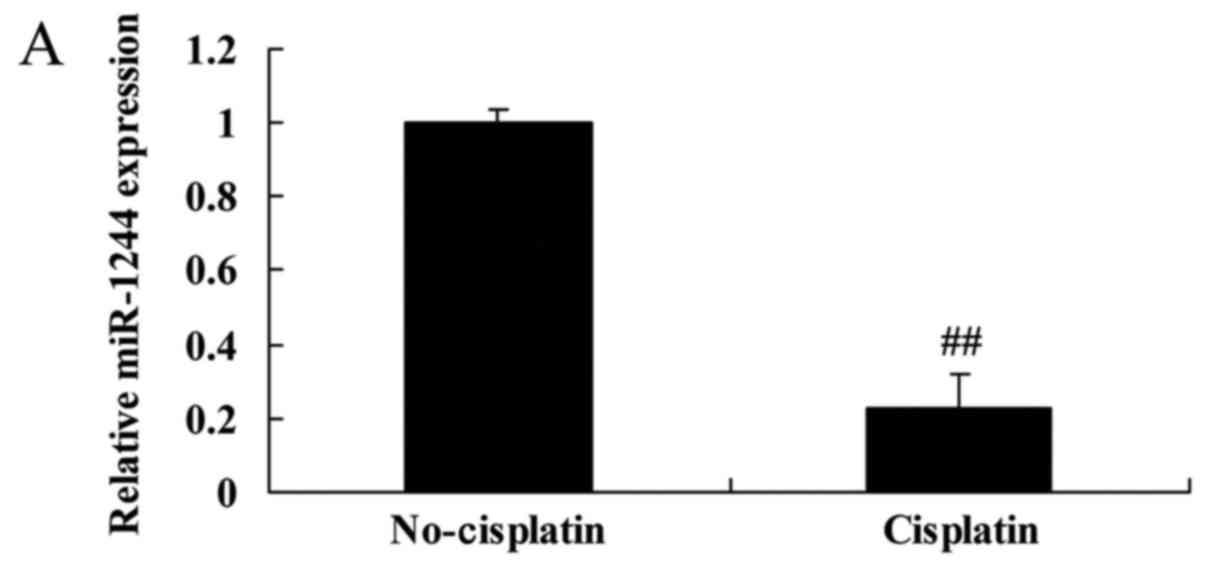
Initially, we surveyed the expression of miR-1244 in
cisplatin-treated or non-cisplatin-treated NSCLC tissues and cell
lines. The miR-1244 expression of cisplatin-treated NSCLC patient
tissues was lower than that of non-cisplatin-treated NSCLC patients
(Fig. 1A). Consistently, the
miR-1244 expression of cisplatin-treated A549 and NCI-H522 cells
was also lower than those of the control A549 and NCI-H522 cells
(Fig. 1B and C).
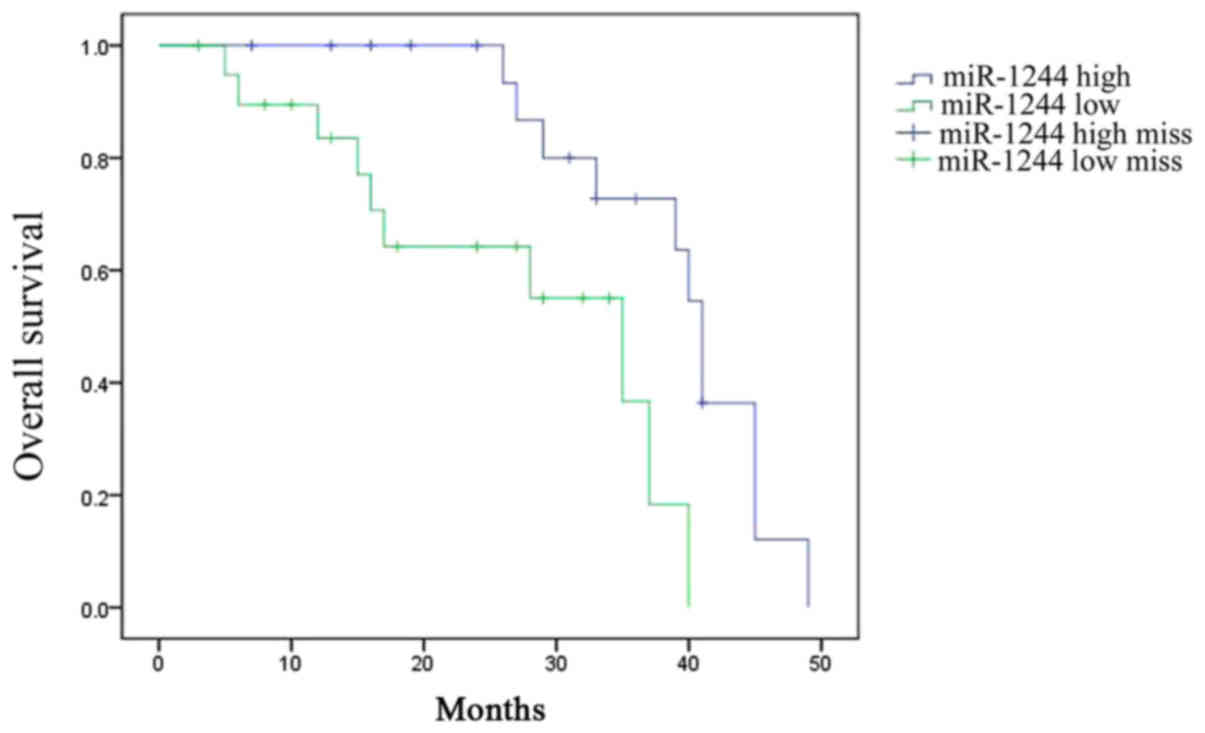
Overall survival (OS) of
cisplatin-treated NSCLC patients
The OS of cisplatin-treated NSCLC patients was
assessed. Among cisplatin-treated NSCLC patients, the OS time of
patients with high miR-1244 expression was greater than that of
patients with low miR-1244 expression (Fig. 2).
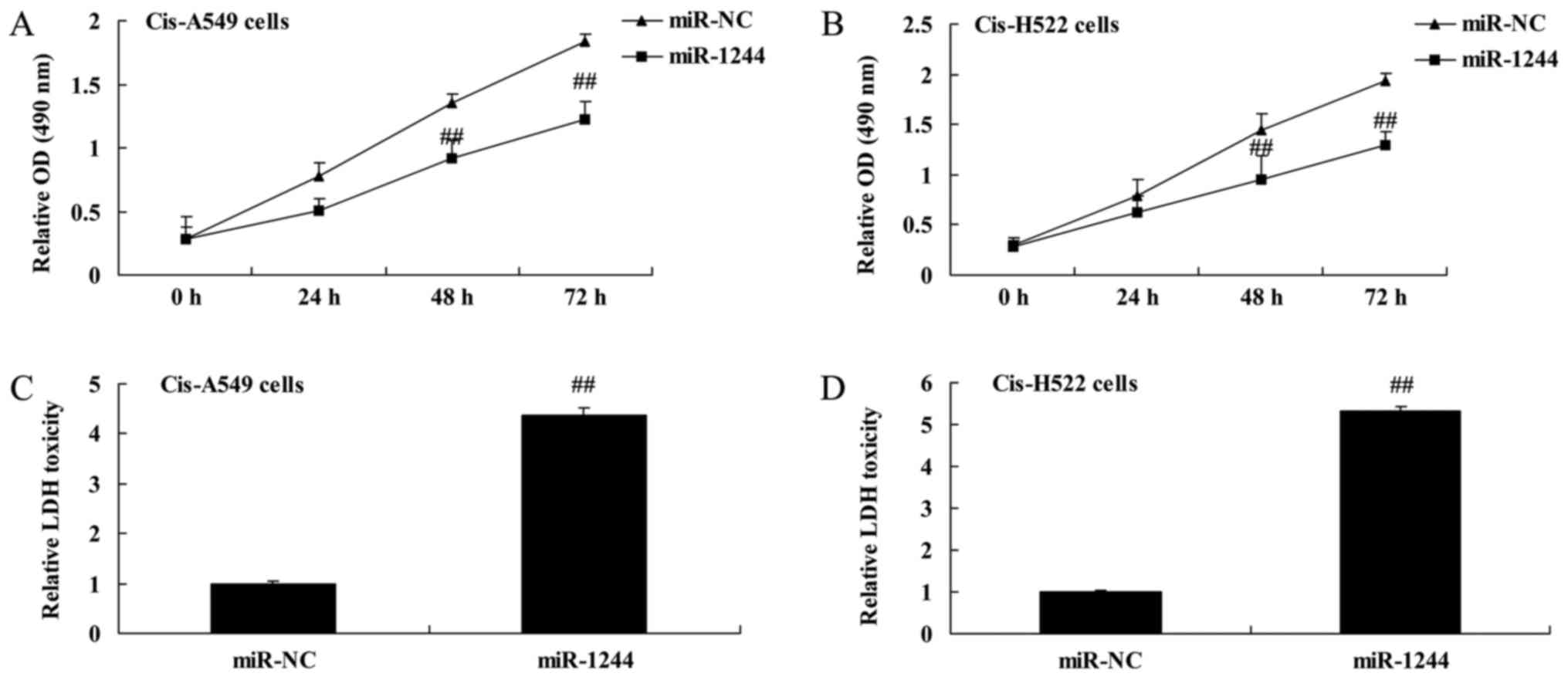
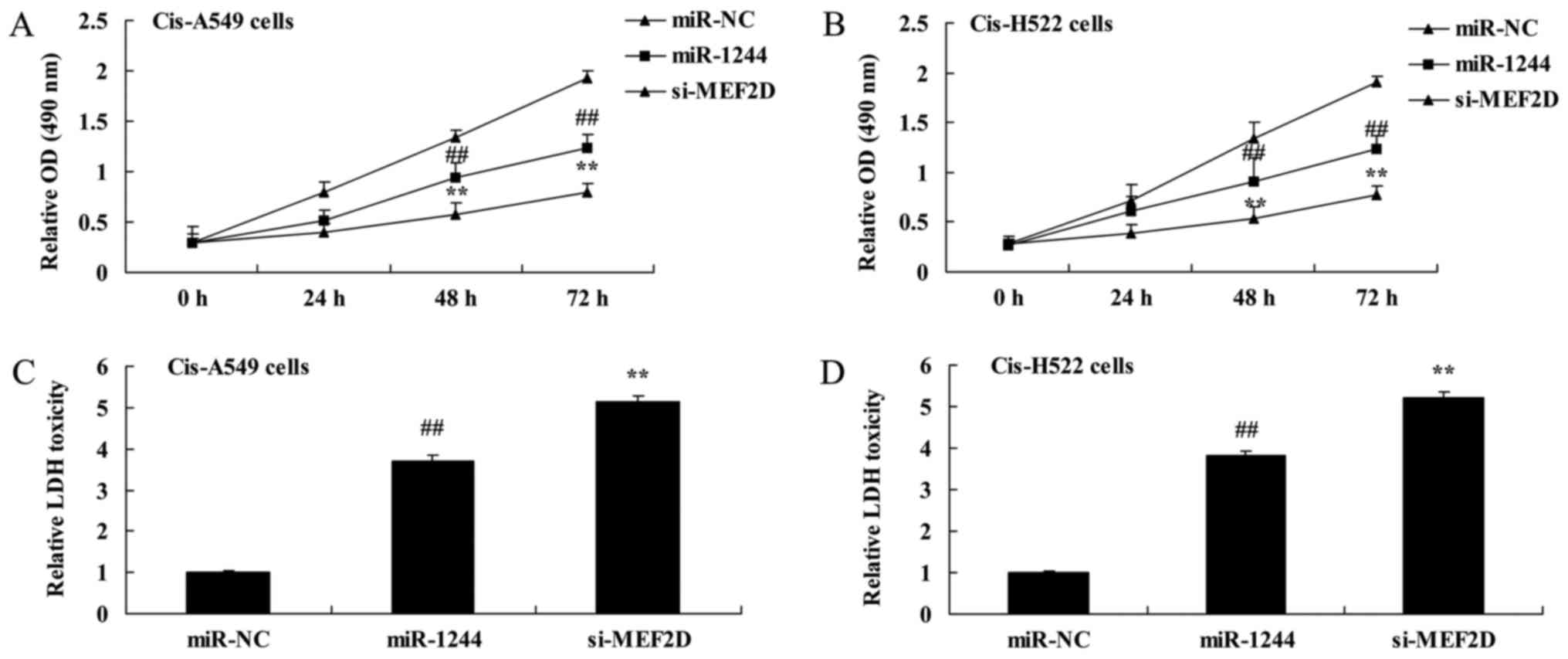
Overexpression of miR-1244 inhibits
the growth of cisplatin-treated NSCLC cells
MTT and LDH assays were used to investigate the
effect of miR-1244 on the growth of cisplatin-treated NSCLC cells.
The results demonstrated that the overexpression of miR-1244
significantly inhibited cell proliferation in cisplatin-treated
A549 and NCI-H522 cells (Fig. 3A and
B). Additionally, overexpression of miR-1244 significantly
increased the LDH activity of cisplatin-treated A549 and NCI-H522
cells (Fig. 3C and D).
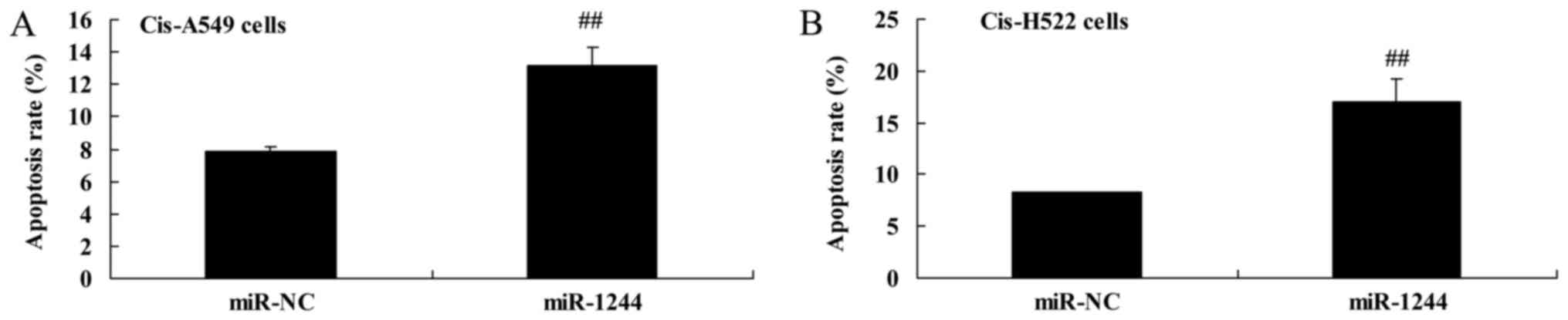
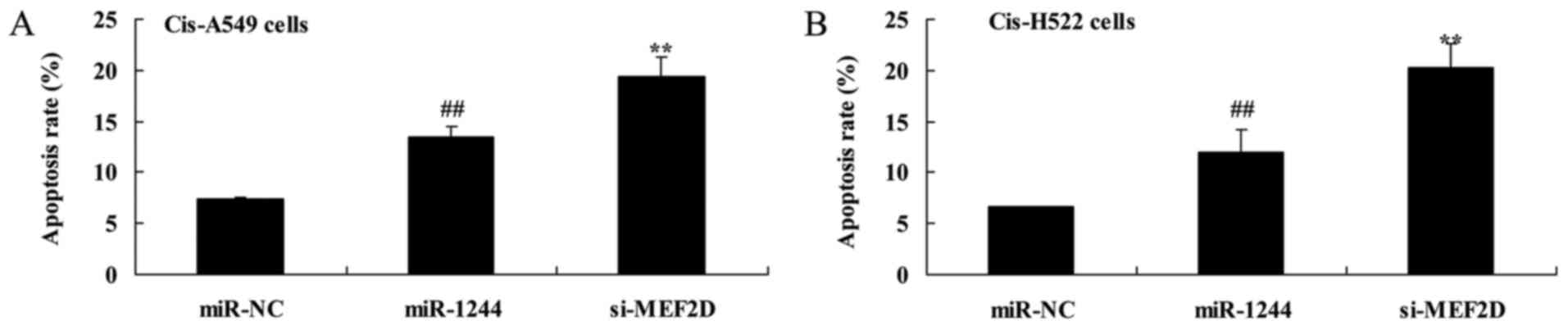
Overexpression of miR-1244 increases
cisplatin-treated NSCLC cell death
The effect of miR-1244 on cisplatin-induced NSCLC
cell death was explored using flow cytometry. There was a
significant increase in apoptosis in cisplatin-treated A549 and
NCI-H522 cells compared with untreated cells (Fig. 4A and B).
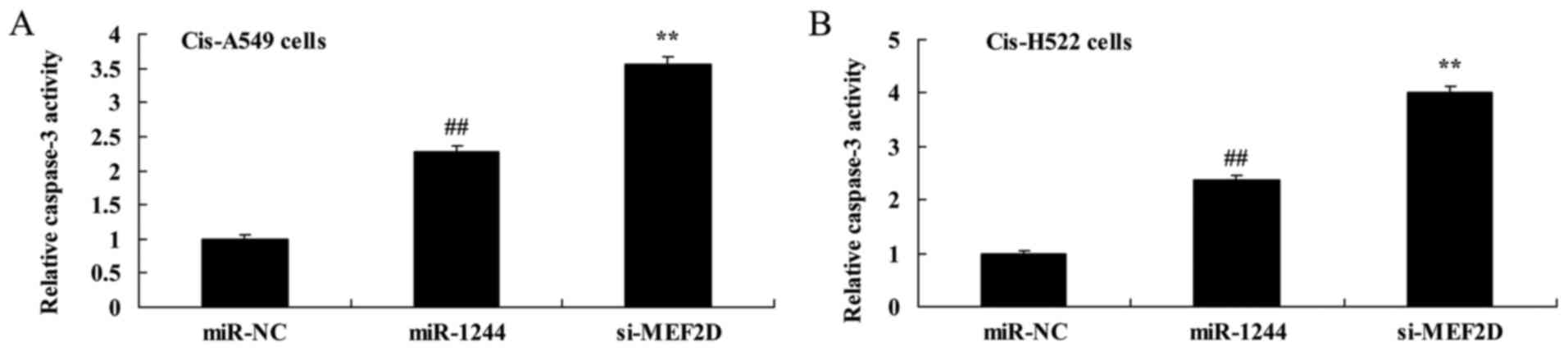
Overexpression of miR-1244 increases
caspase-3 and Bax protein expression in cisplatin-treated NSCLC
cells
We next explored the apoptosis-promoting mechanism
of miR-1244 in cisplatin-treated NSCLC cells by assessing caspase-3
activity and Bax protein expression. As demonstrated in Fig. 5A and B, increased caspase-3 activity
was observed in cisplatin-treated A549 and NCI-H522 cells compared
with untreated cells. Furthermore, the induction of Bax protein
expression was observed in cisplatin-treated A549 and NCI-H522
cells (Fig. 5C and D and H and I,
respectively).
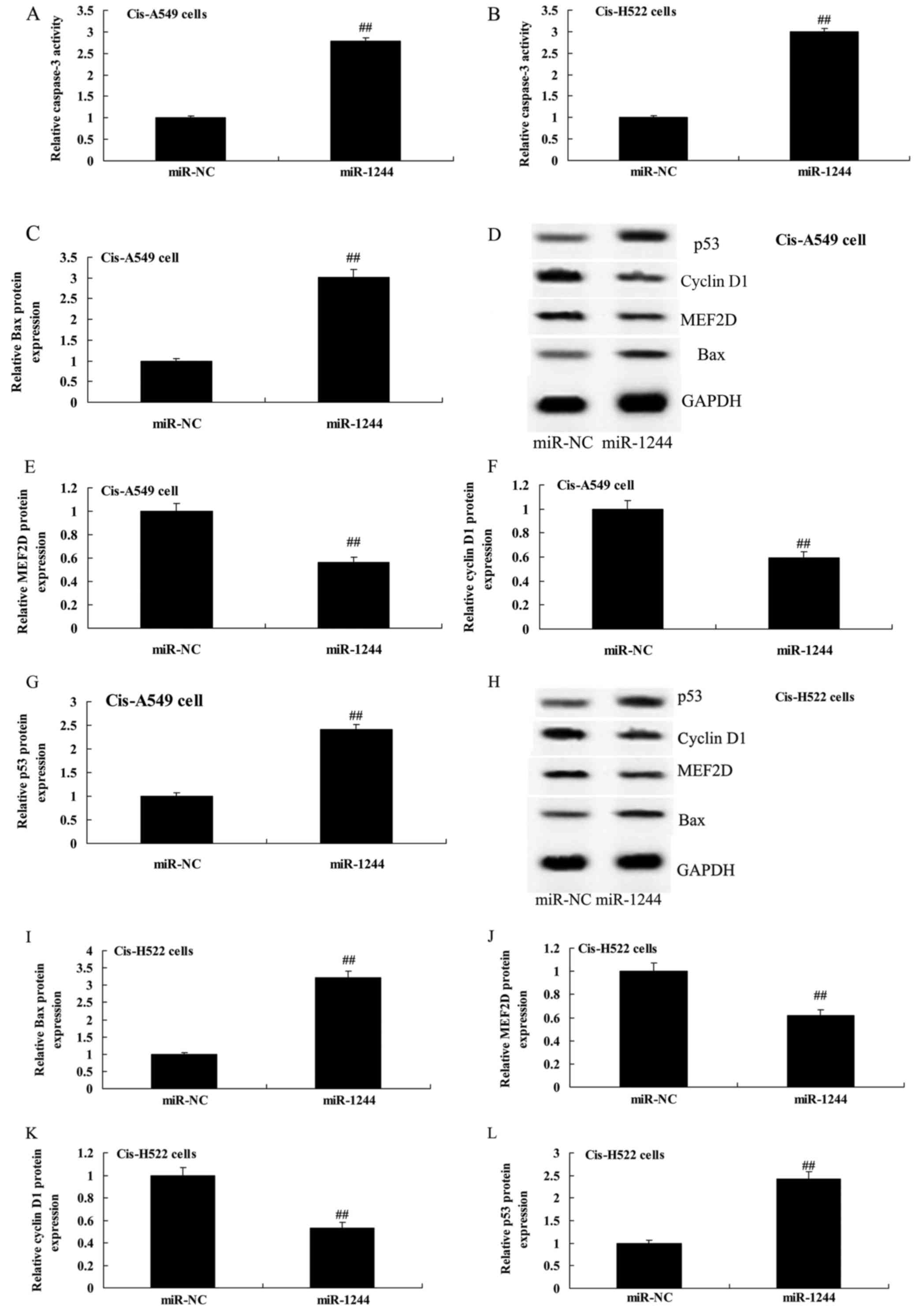 | Figure 5.Overexpression of miR-1244 affects
caspase-3, Bax, MEF2D, cyclin D1 and p53 protein expression in
cisplatin-induced NSCLC cells. (A and B) Overexpression of miR-1244
affects caspase-3 and (C, E-G and I-L) Bax, MEF2D, cyclin D1 and
p53 protein expression using statistical analysis. (D and H)
Western blot analysis of Bax, MEF2D, cyclin D1 and p53 protein
expression in cisplatin-induced A549 and H522 cells. miR-NC,
miRNA-negative control; miR-1244, miRNA-1244 group;
##p<0.01 vs. the miR-NC group. MEF2D, myocyte
enhancer factor 2D; NSCLC, non-small cell lung cancer. |
Overexpression of miR-1244 decreases
MEF2D protein expression in cisplatin-treated NSCLC cells
To study the role of MEF2D in the effect of miR-1244
on cisplatin-treated NSCLC, miR-1244 mimics were transfected into
cisplatin-treated A549 and NCI-H522 cells. As shown in Fig. 5D and E and H and J, respectively,
overexpression of miR-1244 significantly suppressed MEF2D protein
expression in cisplatin-treated A549 and NCI-H522 cells.
Overexpression of miR-1244 suppresses
cyclin D1 protein expression in cisplatin-treated NSCLC cells
Cyclin D1 protein expression levels were evaluated
to assess the apoptosis-promoting mechanism of miR-1244 in
cisplatin-treated NSCLC cells. Fig. 5D,
F, H and K demonstrate that overexpression of miR-1244
significantly suppressed cyclin D1 protein expression in
cisplatin-treated A549 and NCI-H522 cells.
Overexpression of miR-1244 increases
p53 protein expression in cisplatin-treated NSCLC cells
p53 protein expression was assessed in
cisplatin-treated NSCLC cells in which miR-1244 was overexpressed.
Overexpression of miR-1244 significantly induced p53 protein
expression in cisplatin-treated A549 and NCI-H522 cells (Fig. 5D, G, H and L).
MEF2D knockdown inhibits MEF2D protein
expression in cisplatin-treated NSCLC cells following
overexpression of miR-1244
In order to further confirm the role of MEF2D in the
effect of miR-1244 on cisplatin-treated NSCLC cells, si-MEF2D was
transfected into cisplatin-treated A549 and NCI-H522 cells
following overexpression of miR-1244. si-MEF2D effectively
inhibited MEF2D protein expression in cisplatin-treated
miR-1244-overexpressing A549 and NCI-H522 cells compared with the
negative control group (Fig. 6A and B
and F and G, respectively).
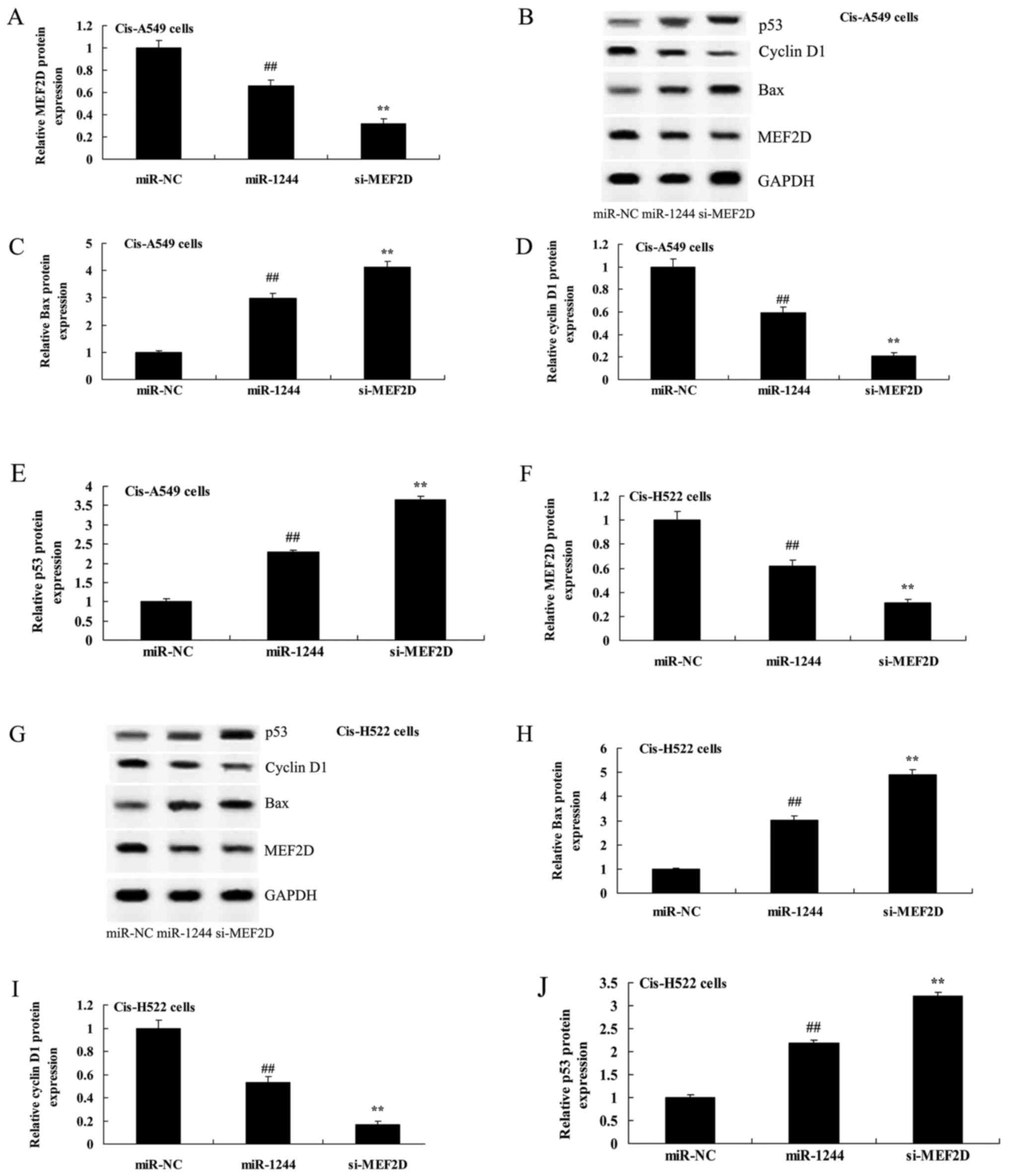 | Figure 6.Inhibition of MEF2D affects MEF2D,
Bax, cyclin D1 and p53 protein expression in cisplatin-induced
NSCLC cells following overexpression of miR-1244. (A, C-F and H-J)
Inhibition of MEF2D affects MEF2D, Bax, cyclin D1 and p53 protein
expression using statistical analysis. (B and G) Western blot
analysis of MEF2D protein expression in cisplatin-induced NSCLC
following overexpression of miR-1244. miR-NC, miRNA-negative
control; miR-1244, miRNA-1244; si-MEF2D, si-MEF2D group;
##p<0.01 vs. the miR-NC group; **p<0.01 vs. the
miRNA-1244 group. MEF2D, myocyte enhancer factor 2D; NSCLC,
non-small cell lung cancer. |
Inhibition of MEF2D decreases
cisplatin-treated NSCLC cell growth following overexpression of
miR-1244
The effects of MEF2D inhibition on the growth of
cisplatin-treated miR-1244-overexpressing NSCLC cells were next
investigated. Fig. 7 shows that the
inhibition of MEF2D significantly inhibited cell proliferation and
increased LDH activity of cisplatin-treated A549 and NCI-H522 cells
following overexpression of miR-1244, compared with the negative
control group in which MEF2D was not inhibited.
Inhibition of MEF2D promotes
cisplatin-treated NSCLC cell death following overexpression of
miR-1244
We further explored the effects of MEF2D inhibition
on cisplatin-treated NSCLC cell death following overexpression of
miR-1244. As presented in Fig. 8,
the inhibition of MEF2D significantly induced apoptosis of
cisplatin-treated A549 and NCI-H522 cells following overexpression
of miR-1244, compared with the negative control group in which
MEF2D was not inhibited.
Inhibition of MEF2D promotes caspase-3
and Bax protein expression in cisplatin-treated NSCLC cells
following overexpression of miR-1244
The inhibition of MEF2D significantly promoted
caspase-3 activity in cisplatin-treated A549 and NCI-H522 cells
following overexpression of miR-1244, compared with the negative
control group (Fig. 9).
Furthermore, as shown in Fig. 6B and C
and G and H, respectively, the inhibition of MEF2D
significantly increased Bax protein expression in cisplatin-treated
A549 and NCI-H522 cells following overexpression of miR-1244
compared with the cells in which MEF2D was not inhibited.
Inhibition of MEF2D suppresses cyclin
D1 protein expression in cisplatin-treated NSCLC cells following
overexpression of miR-1244
As shown Fig. 6B, D, G
and I, in cisplatin-treated miR-1244-overexpressing A549 and
NCI-H522 cells, inhibition of MEF2D significantly suppressed cyclin
D1 protein expression compared with the negative control group.
Inhibition of MEF2D increases p53
protein expression in cisplatin-treated NSCLC cells following
overexpression of miR-1244
As shown in Fig. 6B, E,
G and J, in cisplatin-treated miR-1244-overexpressing A549 and
NCI-H522 cells, the inhibition of MEF2D significantly induced p53
protein expression compared with the negative control group.
Discussion
NSCLC is one of the most common malignant tumors,
and accounts for 80–85% of all lung cancers (3). It has a high mortality rate and
presents a significant threat to human life and health worldwide,
with ~1.1 million patients each year succumbing to NSCLC (16). Despite extensive research efforts,
the overall effects of NSCLC treatment remain unsatisfactory. In
recent years, NSCLC has exhibited a rising morbidity rate.
Furthermore, the pathogenesis of NSCLC has not been established
(17,18). Currently, it is believed that cell
cycle control abnormalities are associated with the cancerous
transformation of cells; malignant tumors may have a dysregulated
cell cycle (17). In the present
study, we observed that the expression levels of miR-1244 in the
cisplatin-treated A549 and NCI-H522 cells were lower than those of
the untreated A549 and NCI-H522 cells, and that the OS time of the
cisplatin-treated NSCLC patients with high miR-1244 expression was
greater than the patients with low miR-1244 expression. Thus,
miR-1244 may play an essential role in NSCLC development.
NSCLC is the most common cause of death resulting
from a malignant tumor (19).
Although diagnosis and treatment methods are improving constantly,
the prognosis of patients with NSCLC remains poor (19). At present, clinical and pathological
staging of NSCLC is the ideal method to evaluate NSCLC prognosis
and select optimal treatment. The identification of miRNAs in
studies of tumors and other diseases has led to much research into
the alterations in miRNA expression associated with NSCLC (20). In tumor subtype classification,
abnormal miRNA expression profiles have been detected (8). Such reports are few in number at
present; however, many miRNA alterations are being discovered
constantly. The integration of miRNA expression analysis with NSCLC
staging may improve the diagnosis and prognosis of NSCLC, and it
may aid in the individual treatment of NSCLC (11). To the best of our knowledge, the
present study is the first to show that the overexpression of
miR-1244 can suppress cell viability, increase LDH toxicity, induce
apoptosis, and promote caspase-3 activity and Bax protein
expression in cisplatin-treated A549 and NCI-H522 cells. Notably,
for the first time, we established the link between miR-1244
expression and cisplatin-treated NSCLC cell growth.
MEF2D, a member of the myocyte enhancer factor 2
family of transcription factors, has been shown to be expressed in
NSCLC cells and to increase the proliferation rate of cells by
increasing G2/M transition (14).
MEF2D is a target of miR-122 (13).
Thus, MEF2D may be a potential target for use in cancer treatments.
This is consistent with our observation that the overexpression of
miR-1244 significantly suppressed MEF2D protein expression in
cisplatin-treated A549 and NCI-H522 cells. These results indicated
that miR-1244/MEF2D play an important role in the development of
cisplatin resistance in NSCLC.
The cell cycle refers to the entire process between
cell fission and the end of the next mitosis process, in which a
parent cell is divided into two daughter cells (22). The cell cycle is composed of two
main stages: interphase, which can be further divided into gap 1
(G1), DNA synthesis (S), and gap 2 (G2) phases (23); and mitosis, which is comprised of
prophase, metaphase, anaphase and telophase, and is responsible for
the division of a cells genetic material prior to cytokinesis
(24). The mechanisms involved in
the control of the cell cycle have become an important area of
research (23). In the present
study, it was demonstrated that the overexpression of miR-1244
significantly suppressed cyclin D1 protein expression in
cisplatin-treated A549 and NCI-H522 cells, indicating that
miR-1244/MEF2D may target cyclin D1, and that miR-1244 exerts its
antitumor effect by suppressing the development of cisplatin
resistance in NSCLC. Our study indicated that oncogenic MEF2D is
also a target of miR-1244, and at least partially, miR-1244 exerts
its antitumor effect by suppressing MEF2D expression.
Cyclins are proteins that contain a conserved cyclin
box structure, and they participate in the regulation of the cell
cycle (25). Cyclins and
cyclin-dependent kinases form complexes that play roles in
regulating different phases of cell division and allowing
transitions at each checkpoint. Cyclin D is a subtype of the cyclin
family (26). Cyclins B1 and D1 are
involved in cell division and are important regulatory factors in
the growth phases. The cyclin D family may play a role in cell
cycle control and DNA repair and participate in apoptosis (27). In different types of tissue and
tumor cells, its expression and biological functions vary (28).
In the present study, we also found that the
overexpression of miR-1244 significantly induced p53 protein
expression in cisplatin-treated A549 and NCI-H522 cells. Therefore,
our study adds p53 to the list of miR-1244/MEF2D-regulated
molecules that are involved in the development of cisplatin
resistance in NSCLC. Further studies are required to explore the
underlying mechanisms of miR-1244/MEF2D in cyclin D1-p53 signaling
in cisplatin-treated NSCLC.
Finally, to explore the possible regulatory
mechanism of MEF2D inhibition in cisplatin resistance in NSCLC, we
found that siRNA-mediated knockdown suppressed the protein
expression of MEF2D, and was able to decrease cell proliferation,
promote caspase-3 activity, increase p53 and Bax protein
expression, and inhibit cyclin D1 protein expression in
cisplatin-treated A549 and NCI-H522 cells following overexpression
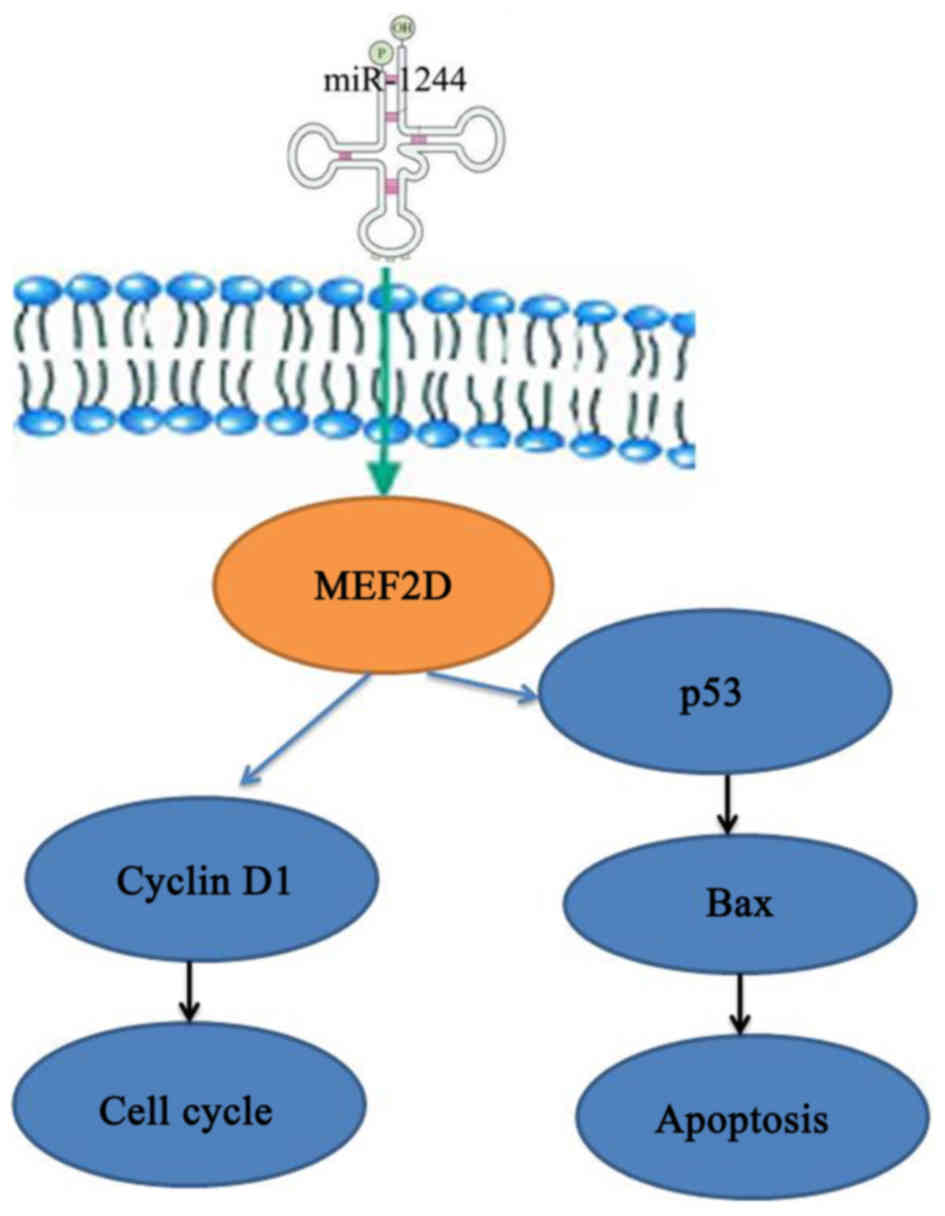
of miR-1244. In conclusion, our study demonstrates that a
miR-1244/MEF2D/cyclin D1-p53 signaling network contributes to the
regulation of NSCLC cell growth, and provides a novel potential
molecular target for future NSCLC cancer therapy (Fig. 10).
Acknowledgements
The present study was supported by The Key Program
of The National Natural Science Foundation-Yunnan United Foundation
(U1202224).
References
|
1
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singhal N, Vatandoust S and Brown MP:
Phase II study evaluating efficacy and safety of everolimus with
letrozole for management of advanced (unresectable or metastatic)
non-small cell lung cancer after failure of platinum-based
treatment: A preliminary analysis of toxicity. Cancer Chemother
Pharmacol. 75:325–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Langer CJ, Novello S, Park K, Krzakowski
M, Karp DD, Mok T, Benner RJ, Scranton JR, Olszanski AJ and Jassem
J: Randomized, phase III trial of first-line figitumumab in
combination with paclitaxel and carboplatin versus paclitaxel and
carboplatin alone in patients with advanced non-small-cell lung
cancer. J Clin Oncol. 32:2059–2066. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heigener DF, Pereira JR, Felip E, Mazal J,
Manzyuk L, Tan EH, Merimsky O, Sarholz B, Esser R and Gatzemeier U:
Weekly and every 2 weeks cetuximab maintenance therapy after
platinum-based chemotherapy plus cetuximab as first-line treatment
for non-small cell lung cancer: Randomized non-comparative phase
IIIb NEXT trial. Target Oncol. 10:255–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pu Q, Huang Y, Lu Y, Peng Y, Zhang J, Feng
G, Wang C, Liu L and Dai Y: Tissue-specific and plasma microRNA
profiles could be promising biomarkers of histological
classification and TNM stage in non-small cell lung cancer. Thorac
Cancer. 7:348–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pastorkova Z, Skarda J and Andel J: The
role of microRNA in metastatic processes of non-small cell lung
carcinoma. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
160:343–357. 2016.PubMed/NCBI
|
|
7
|
Wang G, Wang R, Strulovici-Barel Y, Salit
J, Staudt MR, Ahmed J, Tilley AE, Yee-Levin J, Hollmann C, Harvey
BG, et al: Persistence of smoking-induced dysregulation of miRNA
expression in the small airway epithelium despite smoking
cessation. PLoS One. 10:e01208242015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bianchi F, Nicassio F, Marzi M, Belloni E,
Dall'olio V, Bernard L, Pelosi G, Maisonneuve P, Veronesi G and Di
Fiore PP: A serum circulating miRNA diagnostic test to identify
asymptomatic high-risk individuals with early stage lung cancer.
EMBO Mol Med. 3:495–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JH, Voortman J, Dingemans AM, Voeller
DM, Pham T, Wang Y and Giaccone G: MicroRNA expression and clinical
outcome of small cell lung cancer. PLoS One. 6:e213002011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumarswamy R, Mudduluru G, Ceppi P,
Muppala S, Kozlowski M, Niklinski J, Papotti M and Allgayer H:
MicroRNA-30a inhibits epithelial-to-mesenchymal transition by
targeting Snai1 and is downregulated in non-small cell lung cancer.
Int J Cancer. 130:2044–2053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tibaldi C, D'Incecco A and Lagana A:
MicroRNAs and Targeted Therapies in Non-small Cell Lung Cancer:
Minireview. Anticancer Agents Med Chem. 15:694–700. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma R, Wang C, Wang J, Wang D and Xu J:
miRNA-mRNA interaction network in non-small-cell lung cancer.
Interdiscip Sci. 209–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Liu M and Li D: Oleanolic acid
suppresses the proliferation of lung carcinoma cells by
miR-122/Cyclin G1/MEF2D axis. Mol Cell Biochem. 400:1–7. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song L, Li D, Zhao Y, Gu Y, Zhao D, Li X,
Bai X, Sun Y, Zhang X, Sun H, et al: miR-218 suppressed the growth
of lung carcinoma by reducing MEF2D expression. Tumour Biol.
37:2891–2900. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang R, Zhang Y and Li H:
miR-1244/myocyte enhancer factor 2D regulatory loop contributes to
the growth of lung carcinoma. DNA Cell Biol. 34:692–700. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao L, Xu S, Xu J, Yang C, Wang J and Sun
D: S-1 plus cisplatin with concurrent radiotherapy versus cisplatin
alone with concurrent radiotherapy for stage III non-small cell
lung cancer: A pilot randomized controlled trial. Radiat Oncol.
10:102015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hattori Y, Satouchi M, Shimada T, Urata Y,
Yoneda T, Mori M, Nishimura T, Sunadome H, Kumagai T, Imamura F, et
al: A phase 2 study of bevacizumab in combination with carboplatin
and paclitaxel in patients with non-squamous non-small-cell lung
cancer harboring mutations of epidermal growth factor receptor
(EGFR) after failing first-line EGFR-tyrosine kinase inhibitors
(HANSHIN Oncology Group 0109). Lung Cancer. 87:136–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mariano C, Bosdet I, Karsan A, Ionescu D,
Murray N, Laskin JJ, Zhai Y, Melosky B, Sun S and Ho C: A
population-based review of the feasibility of platinum-based
combination chemotherapy after tyrosine kinase inhibition in EGFR
mutation positive non-small cell lung cancer patients with advanced
disease. Lung Cancer. 83:73–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rothschild SI: Epigenetic Therapy in Lung
Cancer - Role of microRNAs. Front Oncol. 3:1582013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buitrago DH, Patnaik SK, Kadota K,
Kannisto E, Jones DR and Adusumilli PS: Small RNA sequencing for
profiling microRNAs in long-term preserved formalin-fixed and
paraffin-embedded non-small cell lung cancer tumor specimens. PLoS
One. 10:e01215212015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim MK, Kim SC, Kang JI, Hyun JH, Boo HJ,
Eun SY, Park DB, Yoo ES, Kang HK and Kang JH:
6-Hydroxydopamine-induced PC12 cell death is mediated by MEF2D
down-regulation. Neurochem Res. 36:223–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiao WL, Hu HY, Shi BW, Zang LJ, Jin W and
Lin Q: Lentivirus-mediated knockdown of TSP50 suppresses the growth
of non-small cell lung cancer cells via G0/G1 phase arrest. Oncol
Rep. 35:3409–3418. 2016.PubMed/NCBI
|
|
23
|
Zhang XS, Zhao C, Tang WZ, Wu XJ and Zhao
YQ: Gypensapogenin H, a novel dammarane-type triterpene induces
cell cycle arrest and apoptosis on prostate cancer cells. Steroids.
104:276–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu J, Chen M, Chen N, Ma A, Zhu C, Zhao
R, Jiang M, Zhou J, Ye L, Fu H, et al: Glycyrrhetinic acid induces
G1-phase cell cycle arrest in human non-small cell lung cancer
cells through endoplasmic reticulum stress pathway. Int J Oncol.
46:981–988. 2015.PubMed/NCBI
|
|
25
|
Liao K, Li J and Wang Z:
Dihydroartemisinin inhibits cell proliferation via AKT/GSK3β/cyclin
D1 pathway and induces apoptosis in A549 lung cancer cells. Int J
Clin Exp Pathol. 7:8684–8691. 2014.PubMed/NCBI
|
|
26
|
Li Q, Dong Q and Wang E: Rsf-1 is
overexpressed in non-small cell lung cancers and regulates cyclin
D1 expression and ERK activity. Biochem Biophys Res Commun.
420:6–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Recchia AG, Musti AM, Lanzino M, Panno ML,
Turano E, Zumpano R, Belfiore A, Andò S and Maggiolini M: A
cross-talk between the androgen receptor and the epidermal growth
factor receptor leads to p38MAPK-dependent activation of mTOR and
cyclin D1 expression in prostate and lung cancer cells. Int J
Biochem Cell Biol. 41:603–614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lingfei K, Pingzhang Y, Zhengguo L,
Jianhua G and Yaowu Z: A study on p16, pRb, cdk4 and cyclin D1
expression in non-small cell lung cancers. Cancer Lett. 130:93–101.
1998. View Article : Google Scholar : PubMed/NCBI
|