Instruction
Non-Hodgkin lymphoma (NHL) is thought as the most
common hematologic malignancy arising from lymphatic cells
worldwide (1). Approximately 90% of
lymphoma patients have non-Hodgkin lymphoma (NHL). Most NHL
patients have a B-cell type of NHL (85%) and the other have a
T-cell type or an NK-cell type of NHL (2–4). In
younger patients, the most common subtypes of NHL are Burkitt
lymphoma, diffuse large B-cell lymphoma, lymphoblastic lymphoma,
primary mediastinal B-cell lymphoma and anaplastic large cell
lymphoma (2,5,6). NHL
often arises from genetic alterations such as gene translocations.
It has been found that translocation of MYC oncogene on chromosome
8 with IGH gene on chr14, 22 or 2 in adolescents Burkitt lymphoma
patients (7). In diffuse large
B-cell lymphoma, aberrant increased incidence of MYC translocation
and MYC expression and higher incidence of BCL2 translocations have
also been observed (7). PMBL
(primary mediastinal large B cell lymphoma) and nodular sclerosis
Hodgkin lymphoma have biological features of expression of the CD30
antigen (8). Several checkpoint
inhibitors are used for immune checkpoint blockade and other immune
therapies such as pidilizumab, nivolumab and pembrolizumab
(9). These reports still are not
enough to explain the pathogenesis of NHL. Therefore, new molecular
biomarkers of NHL and its mechanism need to be further
explored.
Reelin, also known as RELN, is a 420-kDa secreted
extracellular glycoprotein which is involved in regulation of
neuronal migration during the process of brain development
(10–12). Reelin guides neuron migration by
interacting with two cell surface receptors, VLDLR (very low
density lipoprotein receptor) and ApoER2 (apolipoprotein E receptor
2) and by activating downstream signaling pathway which instructs
neurons to reach the proper laminar position in cortex (13). Most studies focused on the function
of Reelin in neural system development. Interestingly, it has been
reported that Reelin is aberrantly expressed in various cancer
tissues, including esophageal, pancreatic, prostate, breast cancer
and mouse medulloblastoma (14–18).
Expression of Reelin was associated with high-grade prostate cancer
(17). These are some controversial
reports. Epigenetic silence of Reelin was related to poor outcomes
of gastric and pancreatic cancer (14,19).
Epigenetical controlled loss of Reelin is involved in the poor
prognosis of breast cancer (18).
Mutation and expression spectrum of adult T-ALL (T-cell acute
lymphoblastic leukemia) revealed aberrant expression level of
Reelin (20). Silence of Notch1
results in downregulation of glutamatergic transmission and leads
to inhibition of cAMP signaling pathway. cAMP intracellular level
depends on synthesis by adenylate cyclase and cAMP participate in
the Notch1 mediated downstream signaling pathway. The mechanism of
Reelin in cancer is still unclear and we assume that function of
Reelin is associated with cAMP signaling pathway.
In the present study, we detected the differential
expression of Reelin in normal lymph and lymphoma and altered the
expression of Reelin to explore the function in cell proliferation,
migration and invasion and apoptosis in vitro and in
vivo, as well the underlying molecular mechanism in NHL.
Materials and methods
Tissue samples
Fresh samples from NHL and corresponding normal
injury induced lymphoid tissue were obtained from patients after
informed consents at the Department of Pathology, the Second
Hospital of Shandong University. The research was conducted with
the approval of the Ethics Committee of the Second Hospital of
Shandong University. The samples were stored at −80°C.
Cell culture, transfection and
treatment
The mouse B cell lymphoma cell line A20 (#0046;
Hongshun, Shanghai, China), and normal lymphocyte cell lines were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The two cell lines were cultured in RPMI-1640
medium (Invitrogen, Carlsbad, CA, USA) supplemented with 90% 0.05
mM 2-mercaptoethanol and 10% fetal bovine serum (FBS). Both of the
cell lines were incubated in a humidified aseptic condition at 37°C
with 5% CO2.
Knockdown of Reelin in A20 cells was
performed by lipofection transfection
The plasmid vectors containing short hairpin RNA
(shRNA) for Reelin (shReelin) and the corresponding negative
control (NC) were obtained from Thermo Fisher Scientific (Waltham,
MA, USA). Quantitative real-time PCR (qRT-PCR) and western blotting
were performed to analyze the expression of Reelin. Adenylate
cyclase (AC) inhibitor SQ22536 were purchased from Selleck
Chemicals (Houston, TX, USA). All the cells were cultured in
SQ22536 (1×10−5 mol/l) for signaling inhibition.
RNA extraction and qRT-PCR
Total RNA was extracted from cells and fresh tissues
using TRIzol (Takara Bio, Tokyo, Japan) according to the
manufacturers instructions. After reverse transcription using qPCR
Bestar® SYBR-Green kit (DBI, Shanghai, China), qRT-PCR
was performed using SYBR-Green method. Primers for GAPDH: F,
5′-TGTTCGTCATGGGTGTGAAC-3′ and R, 5′-ATGGCATGGACTGTGGTCAT-3′.
Primers for Reelin: F, 5′-AAGGGAGAAGAAACTGAGAA GC-3′ and R,
5′-TGGGAAGGTCGTGACTGAAA-3′. Primers for CDK5: F,
5′-GTCGTGCCCAAACTCAATG-3′ and R, 5′-GCGGACAGAAGTCGGAGAA-3′. Primers
for IL-10: F, 5′-AGAACCAAGACCCAGACATCA-3′ and R,
5′-GCATTCTTCACCTGCTCCAC-3′. Primers for caspase-3: F,
5′-TGGTTCATCCAGTCGCTTTG-3′ and R, 5′-AATTCTGTGCCACCTTTCG-3′. The
total volume was 20 µl and the reaction system as follow:
Bestar® SYBR-Green qPCR Master Mix 10 µl, PCR forward
primer (10 µM) 0.5 µl, PCR reverse primer (10 µM) 0.5 µl, cDNA 1
µl, ddH2O 8 µl. The qRCR procedure was: initial
denaturation at 94°C for 2 min, denaturation at 94°C for 20 sec,
annealing extension at 58°C for 20 sec 40 cycles was performed on
Agilent Stratagene Real-Time PCR platform (Mx3000P). The absorbance
value was then detected. The evaluation of samples was performed
three times and the fold-change of gene expression was calculated
by the 2−∆∆CT method.
Western blotting
Total protein was extracted in lysis buffer and
quantified using the BCA method (# 23227, Pierce BCA protein assay
kit; Thermo Fisher Scientific). Proteins were separated on 10%
SDS-PAGE and transferred to PVDF (polyvinylidene fluoride)
membranes (Millipore, Billerica, MA, USA). After incubation with
antibodies (Reelin: 1:5,000; Sigma-Aldrich, St. Louis, MO, USA;
CDK5: 1:1,000; Sigma-Aldrich; IL-10: 1:1,500; Sigma-Aldrich;
caspase-3: 1:1,000; Sigma-Aldrich), the membranes were washed and
incubated with HRP goat anti-rabbit IgG (#BA1054, 1:20,000; Wuhan
Boster Biological Technology, Ltd., Wuhan, China) or HRP goat
anti-mouse IgG (#BA1051, 1:20,000; Wuhan Boster Biological
Technology) for 40 min. Densitometric analysis was performed on the
auto-radiograms and relative protein levels were quantified using
ImageJ.
H&E staining
Tumor specimens were fixed and cut into 4-µm-thick
slices. The sections were deparaffinized in xylene and rehydrated
in graded alcohol. The sections were stained using Hematoxylin and
Eosin Staining kit (#C0105; Beyotime Institute of Biotechnology,
Shanghai, China). Nuclear and ribosome were stained blue and
cytoplasm was stained red.
Immunohistochemistry
Tumor specimens were fixed and cut into 4-µm-thick
slices. The tumor and normal tissues sections were deparaffinized
in xylene and rehydrated in graded alcohol. The sections were
incubated with non-immune serum after being boiled in 0.01 M
citrate buffer for 2 min. Immunostaining was performed using DAB
Plus kit (Fuzhou Maixin Biotechnology, Co., Ltd., Fuzhou, China)
after incubating in Reelin human or mouse antibody (1:5,000;
Sigma-Aldrich) at 4°C overnight.
Immunofluorescence staining
The cell lines grown on coverclips were washed with
cold phosphate-buffered saline (PBS) and fixed with
methanol/acetone for 10 min. After washing with PBS three times,
the cells were blocked with 2% serum in PBS for 40 min. Then the
cells were incubated with Reelin antibody (Sigma-Aldrich), followed
by staining with FITC conjugated secondary antibodies. Finally,
cells were double stained with DAPI and images were collected by a
laser scanning confocal microscopy.
Cell proliferation assay
Cell Counting kit-8 (CCK-8; Dojindo Laboratories,
Kumamoto, Japan) was used to detect cell proliferation according to
the guidance of the product instructions. The cell lines were
cultured for six overnight and CCK-8 liquids were added to the
medium at time-points of 0, 24, 48 and 72 h for 1–2 h each time.
The absorbance of cells was measured at 450 nm with the microplate
reader (Thermo Fisher Scientific).
Transwell assay
Transwell assay was performed to measure the
invasion ability with 8-µm pore membrane filter chamber kit
(Corning, Inc., Corning, NY, USA). After infection with plasmid
vectors for 48 h, the cells were resuspended with serum-free medium
and the bottoms were filled with complete medium with 10% FBS.
After 24 h, the chambers were fixed with methyl alcohol and
strained with 800 µl Giemsa, transmembraned cells were stained
using 46-diamidino-2-phenylindole dye and counted under a
fluorescence microscope to count the cell numbers.
Flow cytometry assay
The effect of Reelin depletion on cell cycle
progression was determined by flow cytometric analysis of cells
incubated with PI staining according to the manufacturers
instructions. Cell apoptosis analysis was performed using Annexin V
and PI double staining. Briefly, the cells were harvested when the
density reach to 80% and then fixed in 75% ice-cold ethanol for 1
day. After being washed with cold PBS, the collected cells were
stained with 500 µl PI buffer (propidium iodide, #C1052; Beyotime
Institute of Biotechnology) for 1 h in 37°C in the dark to analyze
cell cycle distribution. The collected cells were stained by 5 µl
Annexin V-APC and 5 µl PI to detect apoptosis for 15 min in the
dark. The suspension cells were filtered and were analyzed by a
flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA,
USA).
Hoechst 33258 staining
The A20-shReelin cells and A20-NC cells were stained
using Hoechst 33258. The cells were fixed in fixing solution for 10
min and subsequently washed twice using PBS. The cells were then
stained in Hoechst 33258 solution for 5 min and washed in PBS
twice. The images were collected by fluorescence microscope.
ELISA for cAMP
To investigate the content of cAMP in the
supernatants, ELISA was performed in A20 cells. Briefly, A20 cells
treated with shReelin or SQ22536 were seeded in 6-well plates. The
plates were incubated in 50 µl/well PBS with 1% BSA, and the
supernatants were collected to detect the concentration of cAMP
according to the manufacturers instructions.
BALB/c mouse model
A20 cell lines were cultured and infected shReelin
and NC. Stable transfected cell lines of two groups were cultured
under the treatment of G418. Cells (2×106) were injected
into BALB/c mice (5-weeks old, 6 mice per group). After 30 days,
the tumor tissues were collected to perform detection. The size of
tumor in mice were measured as the long diameter (a) and short
diameter (b) every two days, the average diameter = (a + b)/2
(21). The average diameters were
collected for evaluate the tumor size.
Statistical analysis
The Graphpad Prism was used to analyze the related
data. All data were expressed as mean ± SD of at least three
independent assays, each one was performed in triplicate. The
difference was evaluated using the Students t-test and P<0.05
was considered statistically significant.
Results
Reelin was highly expressed in human
NHL tissue and cell lines
To investigate the role of Reelin in NHL, the
expression of Reelin was first determined in lymphoma tissue and
normal tissue using H&E staining and immunohistochemistry
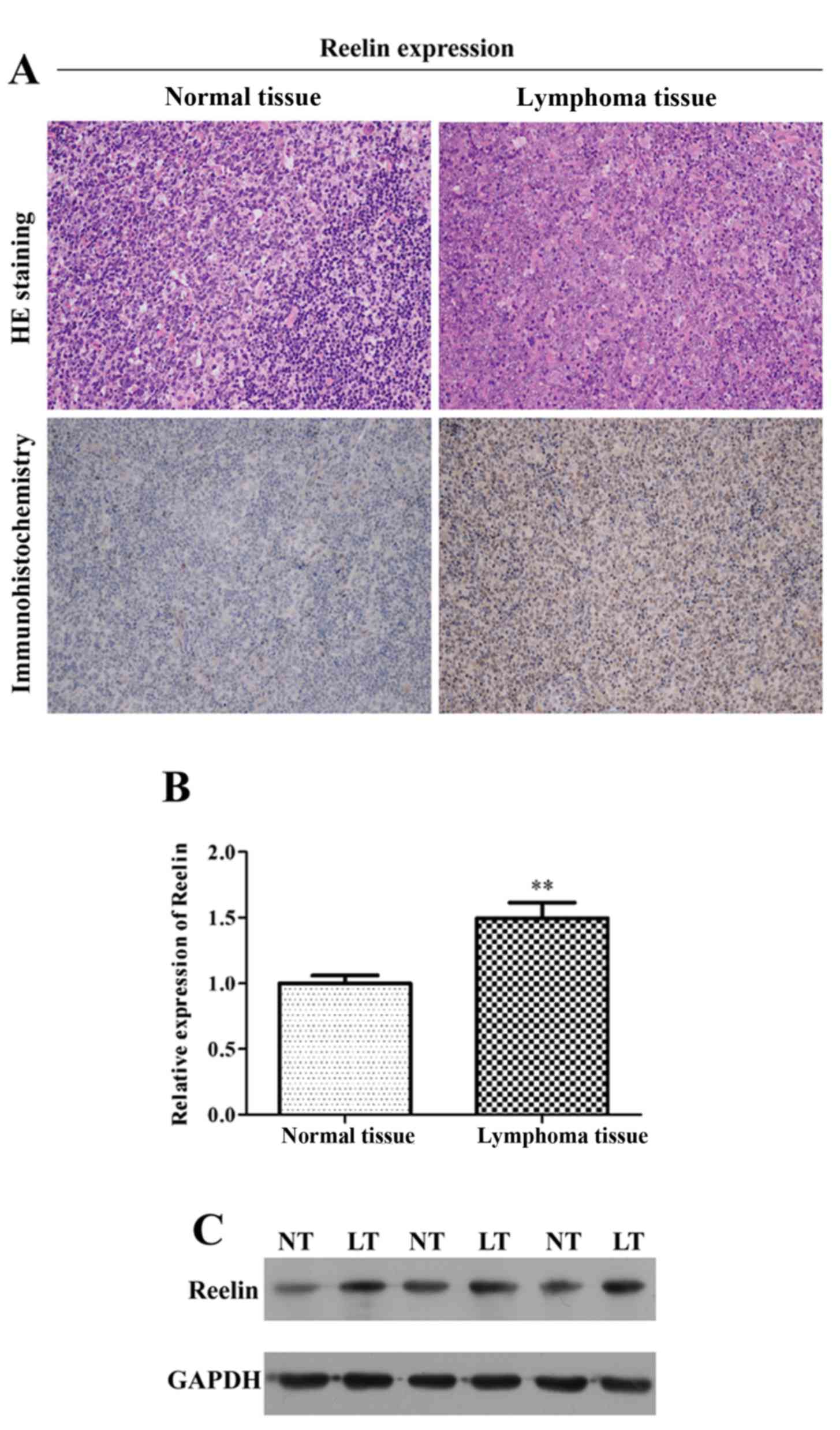
assays. As depicted in Fig. 1A, we
found that Reelin showed positive cytoplasmic and nuclear
immunostaining in tumor tissues. Furthermore, the mRNA expression
of Reelin in lymphoma tissue was significantly higher than that in
normal tissue (Fig. 1B; P<0.01).
The results of western blotting revealed that the protein level of
Reelin was also elevated in lymphoma tissue (Fig. 1C). Collectively, it suggested that
aberrant higher expression of Reelin occurred in lymphoma tissue
comparing to the normal tissue.
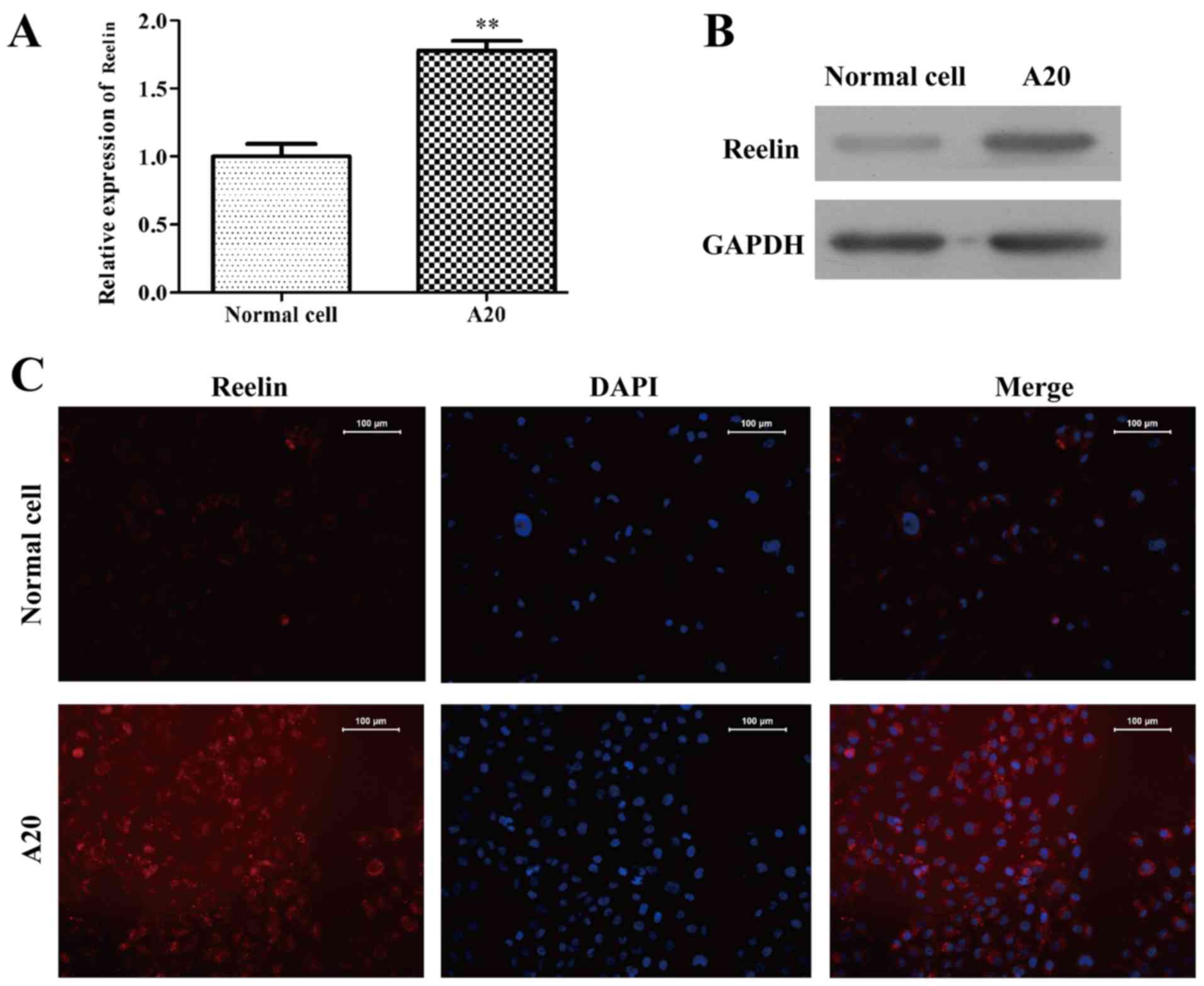
To address the expression of Reelin in the lymphoma
A20 cell line was used to detect the mRNA level using RT-PCR and
protein level in western blotting. The mRNA expression of Reelin
was increased in A20 cells (1.78±0.07), as compared with normal
cells (1.00±0.09) (Fig. 2A;
P<0.01). Similarly, the protein expression of Reelin (Fig. 2B) was markedly higher than that of
the normal cells. The immunofluorescence staining showed that
Reelin protein mainly located in the cell cytoplasm and presented a
higher level expression than that of normal cells (Fig. 2C). Taken together, Reelin might play
an important role in the progression of lymphoma.
Inhibition of Reelin suppressed
lymphoma cell proliferation, migration and invasion
To further investigate the biological behavior of
Reelin in lymphoma, the expression of Reelin was downregulated in
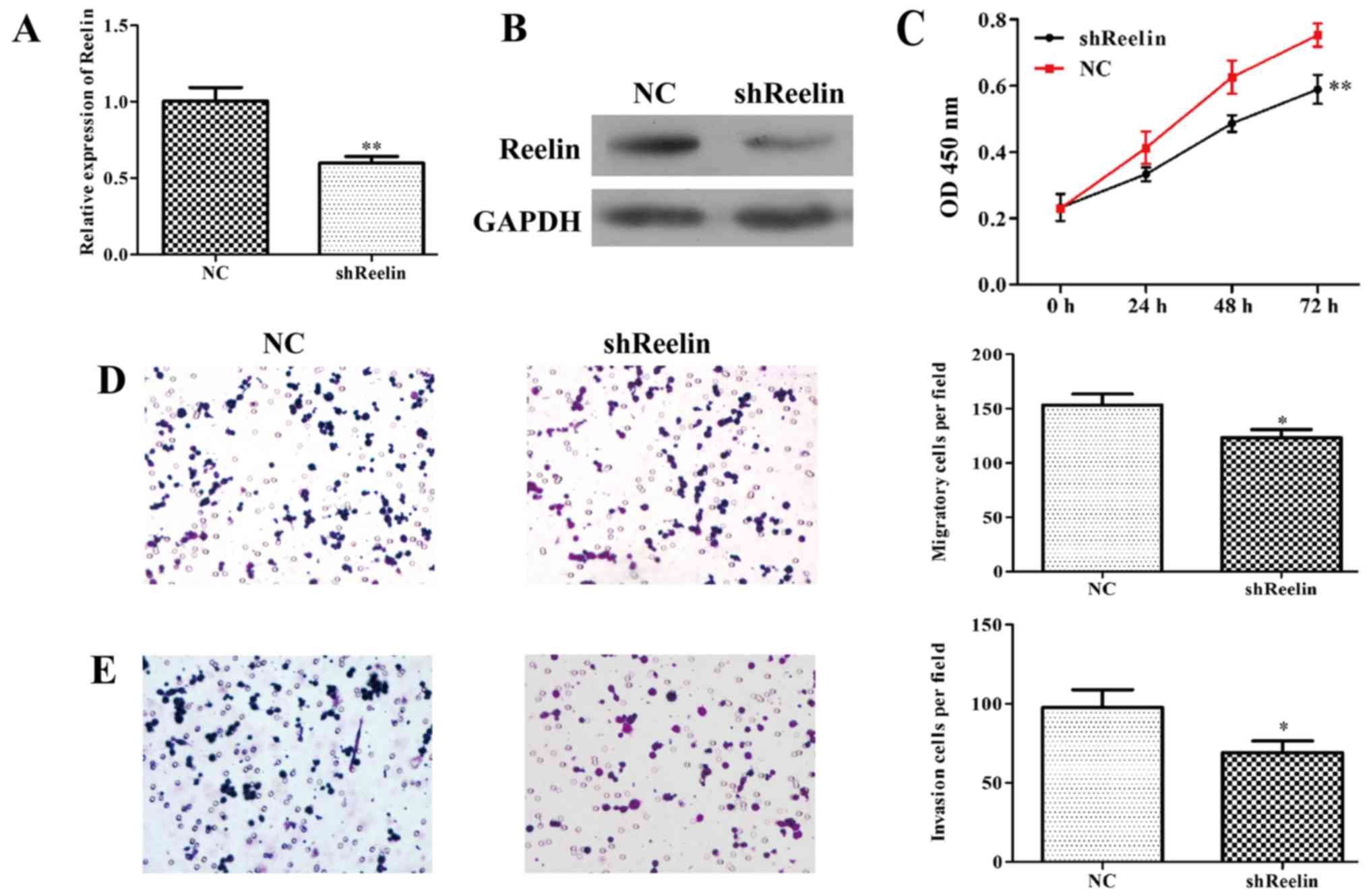
the A20 cells using RNA interference. The results of qRT-PCR
indicated that the mRNA level of Reelin was significantly reduced
by 43% in shReelin group (Fig. 3A).
Consitently, the protein level of Reenlin was also obviously
downregulated in A20 cells infected with shReelin (Fig. 3B). These results suggested stably
Reelin-silenced A20 cells were successfully constructed.
To better understand the role of Reelin in NHL, we
examined the variation tendency of cell proliferation after
infection with shReelin or NC using MTT assay. As shown in Fig. 3C, the growth curve of
shReelin-infected cells was remarkably lower than that of controls
(P<0.01). In addition, the Transwell assay results showed the
number of migratory A20 cells was markedly decreased from 154±12 in
shReelin treated group to 118±9 in NC group (Fig. 3D; P<0.05). The invasion ability
of shReelin group was also significantly decreased after Reelin
knockdown in A20 cells (Fig. 3E;
P<0.05). Collectively, above data indiate that Reelin
prominently favors cell proliferation, migration and invasion in
lymphoma.
Inhibition of Reelin induces cell
cycle arrest and apoptosis
To determine whether downregulation of Reelin
inhibited cell proliferation via direct regulation of cell cycle or
apoptosis, we evaluated the distribution of cell cycle and
apoptosis of A20 infected with shReelin using flow cytometry assay.
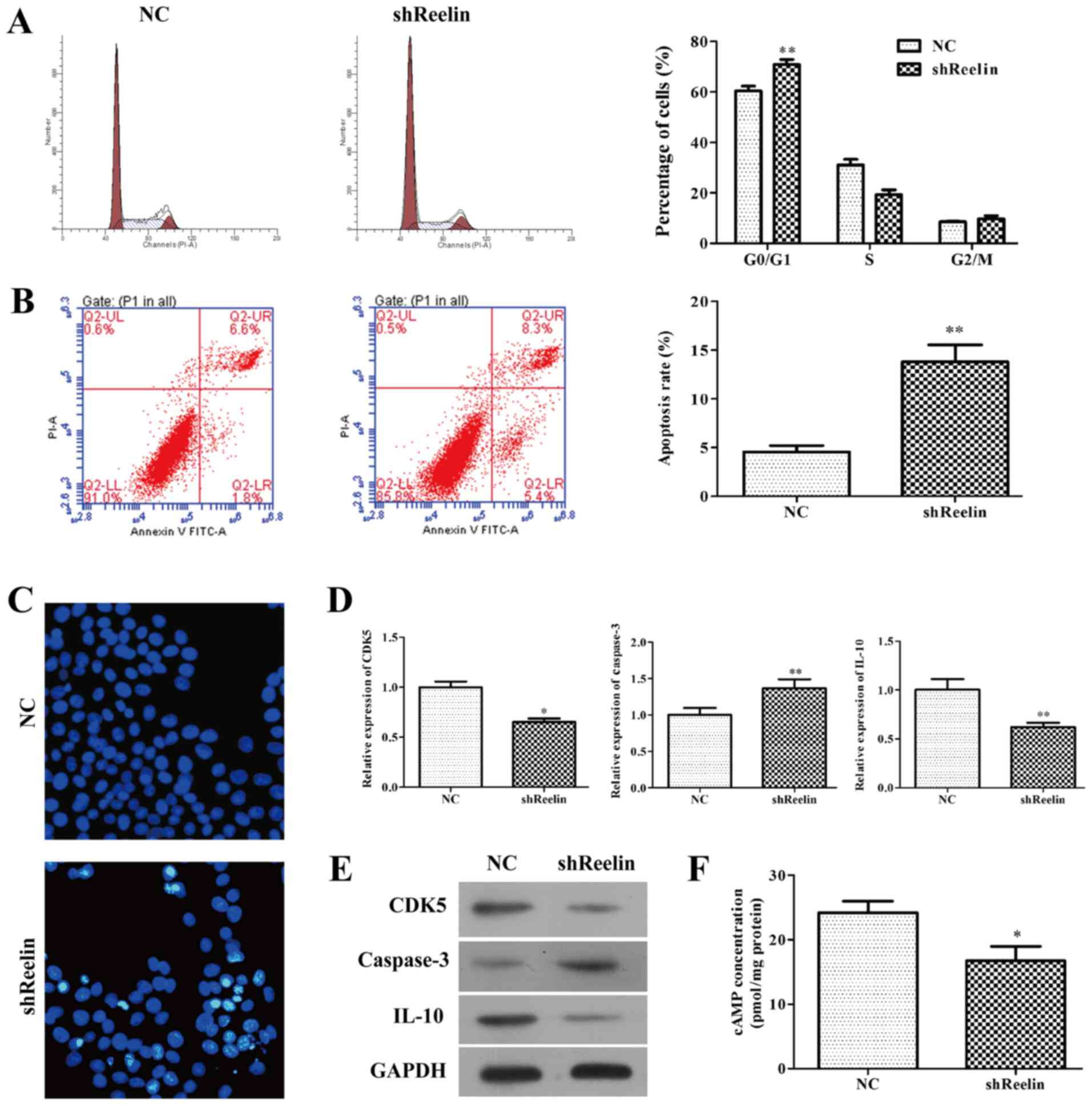
As shown in Fig. 4A, the percentage
of cells in G0/G1 phase remarkably increased in shReelin group
(70.9±1.95), when compared with NC groups (60.39±1.96), suggesting
cell cycle was arrested in G0/G1 phase induced by shReelin
(P<0.01). Moreover, we found knockdown of Reelin obviously
promoted overall cell apoptosis in A20 cells (Fig. 4B, P<0.01, NC group, 4.57±0.37;
shReelin group, 13.80±1.1). Consistently, Hoechst 33258 staining
revealed obvious decrease in the nuclei of live cells (blue color)
(Fig. 4C) in A20 cells infected
with shReelin. Our data strongly suggest that knockdown of Reelin
has anticancer potential by inducing cell cycle arrest and
apoptosis.
Furthermore, we investigated the regulatory
mechanism of Reelin in A20 cells using qRT-PCR and western blot
assays. As shown in Fig. 4D, the
mRNA levels of CDK5 (NC group, 1.00±0.06; shReelin group,
0.65±0.04) and IL-10 (NC group, 1.00±0.11; shReelin group,
0.62±0.04) were signficantly reduced in A20 cells infected with
shReelin. Moreover, knockdown of Reelin obviously upregulated the
mRNA level of caspase-3 (NC group, 1.00±0.1; shReelin group,
1.37±0.12) in A20 cells. Similar trends of Reelin protein levels
were observed in both shReelin group and NC group by western blot
analysis (Fig. 4E). In addition,
ELISA was performed to measure the concentration of intracellular
second messenger cAMP. The results showed that intracellular level
of cAMP was significantly decreased in Reelin knockdown (Fig. 4F, P<0.05, NC group, 24.19±1.045;
shReelin group, 16.75±1.269).
Effects of adenylate cyclase (AC)
inhibitor on lymphoma cell proliferation
It was previously shown that increased cAMP could
elevate the level of cyclin E and activate the MEK/ERK pathway
(22), suggesting it might play an
important role in regulating cell cycle progression and apoptosis.
To clarify the effects of cAMP level on A20 cells, its
intracellular level was strongly decreased by AC inhibitor SQ22536,
as confirmed by RT-PCR analysis (Fig.
5A, NC group, 26.23±0.7636; shReelin group, 14.33±0.5633).
CCK-8 assay showed that downregulation of cAMP significantly
suppressed cell growth rate in A20 cells compared with that of the
controls (Fig. 5B; P<0.01).
Moreover, cells presented reduced expression of Reelin, CDK5, and
IL-10 and elevated expression of caspase-3 after SQ22536 treatment
(Fig. 5C), which might all be
associated with decreased cAMP levels. Collectively, these findings
suggest that activation of Reelin/cAMP pathway might play a pivotal
role in both survival and proliferation of NHL cells.
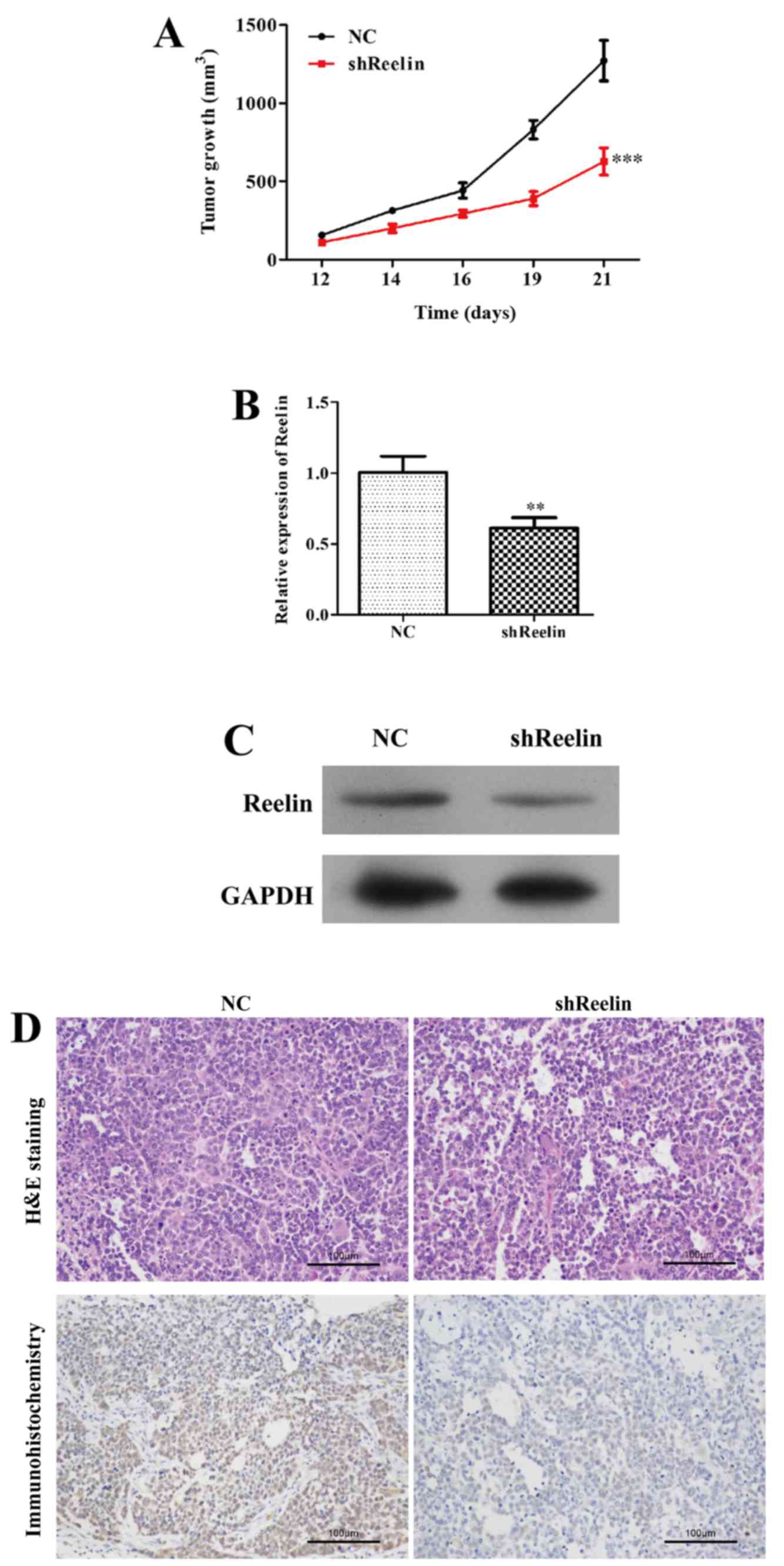
The lymphoma mouse model
To address the carcinogenicity of Reelin in an
animal model, we injected shReelin cells and shRNA-NC cells into
5-week-old BAL/B mice. As shown in Fig.
6A, the volume of tumors was significantly suppressed in mice
inoculated with Reelin-silenced A20 cells (P<0.05), while the
size of the mice in control groups was steadily increased during
day 12–21. These results were confirmed by analysis of expression
of Reelin in tissue of xenograft murine model using qRT-PCR,
western blot analysis, H&E staining and immunohistochemistry
assays. The expression level of Reelin group was strongly
suppressed (Fig. 6B and C, NC
group, 1.00±0.11; shReelin group, 0.61±0.07) compared to the NC
groups. The number of Reelin positive cells was significantly less
in shReelin group than that of control groups (Fig. 6D). These results indicated that
Reelin depletion could inhibit NHL tumorigenesis.
Discussion
NHL constitutes 90% of lymphoma and causes
significant high morbidity and mortality (2,3). The
incidence of NHL increases with age and higher in males (23). Although molecular investigators have
provided evidence that NHL involves a number of genetic
alterations, it is not enough to explain the mechanism of NHL. The
treatment of NHL patients is according to the type of NHL, the
stage and category of NHL and the patients overall health. Types of
treatment include radiation therapy, stem cell transplantation and
drug therapy such as Rituxan, Adcetris, Folotyn, Lstodax, Treanda,
Velcade and Zevalin (2–4). Recently, overexpression of Reelin was
reported to be associated with poor prognosis and survival in
multiple myeloma cells (24).
Previous studies also showed that high level of Reelin was
correlated with poor outcomes of esophageal cancer and
retinoblastoma (16,25). Therefore, we speculated that Reelin
might play essential roles in NHL. In the present study, we found
Reelin was aberrantly upregulated in lymphoma tissue and cell
lines. Therefore, in order to investigate the role of Reelin in
NHL, shRNA was used to efficiently downregulate the expression of
Reelin. When infected with shReelin, it was observed that A20 have
lower proliferation, suppressed invasion and increased apoptosis,
together with arrested cell cycle in the G0/G1 phase. Mice bearing
tumor analysis further revealed that the size of tumor in mice
injected shReelin A20 cells was smaller than that of the controls.
These results indicated that Reelin may play prominent roles in
regulating NHL cell growth and invasion.
In order to explore the mechanism underlying Reelin
may contribute to NHL, we detected the apoptosis markers in the
shReelin infected A20 cell lines and control cell lines. In the
present study, we found that Reelin, as a secreted extracellular
glycoprotein that functions to affect cAMP signaling pathway. cAMP
(activating cyclic adenosine monophosphate) is an intracellular
second messenger which can activate downstream factor PKA and
NF-κB. These proteins regulate the transcription of apoptotic
target genes including Bcl-2 and Bax (26–29).
Substantial evidence has shown that cAMP promoted apoptosis of
lymphocytes in rats and humans (30–33).
In addition, cAMP is involved in the control and development of the
inflammatory process and cellular proliferation (34,35).
Elevating cAMP suppress T cell activation, proliferation and
production of IL2, IL-12, IFN-γ and TNF-α through inhibition of
NF-κB activity (36–38). In cell cycle distribution and
apoptosis analysis, more A20 cells infected with shReelin were
arrested in the G0/G1 phase and induced apoptosis. We propose that
Reelin plays essential roles in cell cycle regulation and
apoptosis. In apoptosis process, IL-10 and caspase-3 are major
markers. IL-10, as a Th2 cytokine, exerts immunomodulatory capacity
and can be induced by cAMP through phosphorylation of CREB
(cAMP-response element binding protein) in monocytes (39,40).
In A20 cells, knockdown of Reelin downregulated the expression of
IL-10 and upregulated the expression of cleaved caspase-3. The
evidence indicates that Reelin promotes tumor cell proliferation
and inhibits apoptosis. It was reported that Reelin has two major
receptors: ApoER2 (apolipoprotein E receptor 2) and NMDAR
(N-methyli-D aspartate receptor) to transfer signals. Notch1 could
function as a postsynaptic receptor with functional interactions
with these two receptors (41,42).
Loss of Notch1 results in suppressing glutamatergic transmission
and leads to decreased cAMP response element-binding signaling
(42). Increased intracellular
content of cAMP is closely related with the apoptosis of
lymphocytes (30,31). Injection of Reelin increased
cAMP-response element binding protein after 15 min and these
changes correlated with higher dendritic spine density and
hippocampal CA1 LTP (long-term potentiation) in vivo
(43). As an intracellular second
messenger, cAMP can inhibit NF-κB, which regulates the
transcription of downstream apoptotic target genes (26–29).
In the present study, secreted cAMP was suppressed in A20 cells
infected with shReelin, which indicates Reelin suppresses tumor
cell apoptosis through regulating the activity of cAMP. These
results further prove that Reelin has carcinogenic function in
lymphoma and cAMP signaling pathway inhibitor SQ22536 could be a
potential chemotherapy drug for NHL.
In conclusion, this study demonstrated that
knockdown of Reelin could inhibit lymphoma cell proliferation,
migration and invasion. Suppressed cell proliferation is closely
associated with cell cycle arrest at G0/G1 phase and induced
apoptosis. Furthermore, cAMP signaling inhibitor might be a
potential therapeutic approach in NHL. Further investigation may
help to better understand NHL progression.
Acknowledgements
The present study is supported by grants from the
Shandong Provincial Medical and Health Science and Technology
Development Plan (no. 2014WS0159).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, Ji X, Liu X, Yao R, Chi J, Liu S,
Wang Y, Cao W and Zhou Q: Lentivirus-mediated inhibition of USP39
suppresses the growth of breast cancer cells in vitro. Oncol Rep.
30:2871–2877. 2013.PubMed/NCBI
|
|
3
|
SEER Stat Fact Sheets. NCI2014.
|
|
4
|
Pinnix CC, Smith GL, Milgrom S, Osborne
EM, Reddy JP, Akhtari M, Reed V, Arzu I, Allen PK, Wogan CF, et al:
Predictors of radiation pneumonitis in patients receiving intensity
modulated radiation therapy for Hodgkin and non-Hodgkin lymphoma.
Int J Radiat Oncol Biol Phys. 92:175–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pulte D, Jansen L and Brenner H: Survival
disparities by insurance type for patients aged 15–64 years with
non-Hodgkin lymphoma. Oncologist. 20:554–561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rossi C, Jégu J, Mounier M, Dandoit M,
Colonna M, Daubisse-Marliac L, Trétarre B, Ganry O, Guizard AV,
Bara S, et al: Risk assessment of second primary cancer according
to histological subtype of non-Hodgkin lymphoma. Leuk Lymphoma.
56:2876–2882. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hochberg J, El-Mallawany NK and Abla O:
Adolescent and young adult non-Hodgkin lymphoma. Br J Haematol.
173:637–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Mallawany NK and Cairo MS: Advances in
the diagnosis and treatment of childhood and adolescent B-cell
non-Hodgkin lymphoma. Clin Adv Hematol Oncol. 13:113–123.
2015.PubMed/NCBI
|
|
9
|
Matsuki E and Younes A: Checkpoint
inhibitors and other immune therapies for Hodgkin and non-Hodgkin
lymphoma. Curr Treat Options Oncol. 17:312016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frotscher M: Cajal-Retzius cells, Reelin,
and the formation of layers. Curr Opin Neurobiol. 8:570–575. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Groen JL, Ritz K, Jalalzadeh H, van der
Salm SM, Jongejan A, Mook OR, Haagmans MA, Zwinderman AH,
Motazacker MM, Hennekam RC, et al: RELN rare variants in
myoclonus-dystonia. Mov Disord. 30:415–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sommen M, van Camp G, Liktor B, Csomor P,
Fransen E, Sziklai I, Schrauwen I and Karosi T: Genetic association
analysis in a clinically and histologically confirmed otosclerosis
population confirms association with the TGFB1 gene but suggests an
association of the RELN gene with a clinically indistinguishable
otosclerosis-like phenotype. Otol Neurotol. 35:1058–1064. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tissir F and Goffinet AM: Reelin and brain
development. Nat Rev Neurosci. 4:496–505. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato N, Fukushima N, Chang R, Matsubayashi
H and Goggins M: Differential and epigenetic gene expression
profiling identifies frequent disruption of the RELN pathway in
pancreatic cancers. Gastroenterology. 130:548–565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wetmore C, Eberhart DE and Curran T: The
normal patched allele is expressed in medulloblastomas from mice
with heterozygous germ-line mutation of patched. Cancer Res.
60:2239–2246. 2000.PubMed/NCBI
|
|
16
|
Wang Q, Lu J, Yang C, Wang X, Cheng L, Hu
G, Sun Y, Zhang X, Wu M and Liu Z: CASK and its target gene Reelin
were co-upregulated in human esophageal carcinoma. Cancer Lett.
179:71–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perrone G, Vincenzi B, Zagami M, Santini
D, Panteri R, Flammia G, Verzì A, Lepanto D, Morini S, Russo A, et
al: Reelin expression in human prostate cancer: a marker of tumor
aggressiveness based on correlation with grade. Mod Pathol.
20:344–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stein T, Cosimo E, Yu X, Smith PR, Simon
R, Cottrell L, Pringle MA, Bell AK, Lattanzio L, Sauter G, et al:
Loss of reelin expression in breast cancer is epigenetically
controlled and associated with poor prognosis. Am J Pathol.
177:232–2333. 2010. View Article : Google Scholar
|
|
19
|
Dohi O, Takada H, Wakabayashi N, Yasui K,
Sakakura C, Mitsufuji S, Naito Y, Taniwaki M and Yoshikawa T:
Epigenetic silencing of RELN in gastric cancer. Int J Oncol.
36:85–92. 2010.PubMed/NCBI
|
|
20
|
Neumann M, Vosberg S, Schlee C, Heesch S,
Schwartz S, Gökbuget N, Hoelzer D, Graf A, Krebs S, Bartram I, et
al: Mutational spectrum of adult T-ALL. Oncotarget. 6:2754–2766.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaur IP, Kaur T, Bhardwaj R, Deol PK,
Kakkar V, Vaiphei K and Sanyal SN: Sesamol induces apoptosis by
altering expression of bcl-2 and bax proteins and modifies skin
tumor development in Balb/c mice. Anticancer Agents Med Chem.
16:12016.
|
|
22
|
Ugland H, Boquest AC, Naderi S, Collas P
and Blomhoff HK: cAMP-mediated induction of cyclin E sensitizes
growth-arrested adipose stem cells to DNA damage-induced apoptosis.
Mol Biol Cell. 19:5082–5092. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bleyer A, Viny A and Barr R: Cancer in 15-
to 29-year-olds by primary site. Oncologist. 11:590–601. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin L, Wang P, Liu X, Zhao D, Zhang Y, Hao
J, Liang X, Huang X, Lu J and Ge Q: Epigenetic regulation of reelin
expression in multiple myeloma. Hematol Oncol. Jun
1–2016.https://doi.org/10.1002/hon.2311 View Article : Google Scholar
|
|
25
|
Yuan Y, Chen H, Ma G, Cao X and Liu Z:
Reelin is involved in transforming growth factor-β1-induced cell
migration in esophageal carcinoma cells. PLoS One. 7:e318022012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liberman AC, Refojo D, Antunica-Noguerol
M, Holsboer F and Arzt E: Underlying mechanisms of cAMP- and
glucocorticoid-mediated inhibition of FasL expression in
activation-induced cell death. Mol Immunol. 50:220–235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sen R: Control of B lymphocyte apoptosis
by the transcription factor NF-kappaB. Immunity. 25:871–883. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi N, Tetsuka T, Uranishi H and
Okamoto T: Inhibition of the NF-kappaB transcriptional activity by
protein kinase A. Eur J Biochem/FEBS. 269:4559–4565. 2002.
View Article : Google Scholar
|
|
29
|
Parry GC and Mackman N: Role of cyclic AMP
response element-binding protein in cyclic AMP inhibition of
NF-kappaB-mediated transcription. J Immunol. 159:5450–5456.
1997.PubMed/NCBI
|
|
30
|
Lømo J, Blomhoff HK, Beiske K, Stokke T
and Smeland EB: TGF-beta 1 and cyclic AMP promote apoptosis in
resting human B lymphocytes. J Immunol. 154:1634–1643.
1995.PubMed/NCBI
|
|
31
|
Ivanov VN, Lee RK, Podack ER and Malek TR:
Regulation of Fas-dependent activation-induced T cell apoptosis by
cAMP signaling: A potential role for transcription factor NF-kappa
B. Oncogene. 14:2455–2464. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Zambon AC, Vranizan K, Pothula K,
Conklin BR and Insel PA: Gene expression signatures of cAMP/protein
kinase A (PKA)-promoted, mitochondrial-dependent apoptosis.
Comparative analysis of wild-type and cAMP-deathless S49 lymphoma
cells. J Biol Chem. 283:4304–4313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zambon AC, Zhang L, Minovitsky S, Kanter
JR, Prabhakar S, Salomonis N, Vranizan K, Dubchak I, Conklin BR and
Insel PA: Gene expression patterns define key transcriptional
events in cell-cycle regulation by cAMP and protein kinase A. Proc
Natl Acad Sci USA. 102:pp. 8561–8566. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moore AR and Willoughby DA: The role of
cAMP regulation in controlling inflammation. Clin Exp Immunol.
101:387–389. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishida M, Mitsui T, Yamakawa K, Sugiyama
N, Takahashi W, Shimura H, Endo T, Kobayashi T and Arita J:
Involvement of cAMP response element-binding protein in the
regulation of cell proliferation and the prolactin promoter of
lactotrophs in primary culture. Am J Physiol Endocrinol Metab.
293:E1529–E1537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Snijdewint FG, Kaliński P, Wierenga EA,
Bos JD and Kapsenberg ML: Prostaglandin E2 differentially modulates
cytokine secretion profiles of human T helper lymphocytes. J
Immunol. 150:5321–5329. 1993.PubMed/NCBI
|
|
37
|
Hilkens CM, Snijders A, Snijdewint FG,
Wierenga EA and Kapsenberg ML: Modulation of T-cell cytokine
secretion by accessory cell-derived products. Eur Respir J (Suppl).
22:90s–94s. 1996.PubMed/NCBI
|
|
38
|
Aandahl EM, Aukrust P, Skålhegg BS, Müller
F, Frøland SS, Hansson V and Taskén K: Protein kinase A type I
antagonist restores immune responses of T cells from HIV-infected
patients. FASEB J. 12:855–862. 1998.PubMed/NCBI
|
|
39
|
Platzer C, Fritsch E, Elsner T, Lehmann
MH, Volk HD and Prösch S: Cyclic adenosine monophosphate-responsive
elements are involved in the transcriptional activation of the
human IL-10 gene in monocytic cells. Eur J Immunol. 29:3098–3104.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Platzer C, Meisel C, Vogt K, Platzer M and
Volk HD: Up-regulation of monocytic IL-10 by tumor necrosis
factor-alpha and cAMP elevating drugs. Int Immunol. 7:517–523.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gaiano N and Fishell G: The role of notch
in promoting glial and neural stem cell fates. Annu Rev Neurosci.
25:471–490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brai E, Marathe S, Astori S, Fredj NB,
Perry E, Lamy C, Scotti A and Alberi L: Notch1 regulates
hippocampal plasticity through interaction with the Reelin pathway,
glutamatergic transmission and CREB signaling. Front Cell Neurosci.
9:4472015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rogers JT, Rusiana I, Trotter J, Zhao L,
Donaldson E, Pak DT, Babus LW, Peters M, Banko JL, Chavis P, et al:
Reelin supplementation enhances cognitive ability, synaptic
plasticity, and dendritic spine density. Learn Mem. 18:558–564.
2011. View Article : Google Scholar : PubMed/NCBI
|