Introduction
HCC is one of the most common malignancies
worldwide, with a rapidly progressive clinical course (1). Patients with advanced HCC have an
average life span of just a few months (2). At present, progress in the early
diagnosis and treatment of HCC have increased the mean survival
time, but the prognosis is still poor and novel therapeutic
strategies are urgently needed (3).
As known, the antitumor immune response is functionally impaired in
HCC patients and the function of T cell is inhibited (4,5). Thus,
it is important to develop a treatment method to enhance the immune
response in HCC patients.
Since found in 1973, dendritic cells (DC) pulsed
with tumor-associated antigens have been applied as a therapeutic
vaccine to tumor patients and they elicited an antitumor immune
response (6,7). In addition, DC loaded with tumor cell
lysate (TCL), prepared by the artificial lysis of tumor cells,
significantly inhibited tumor growth, increased the survival of
tumor-born mice and strengthened antitumor cytotoxic activity
(8–10). However, it was reported that the
application of TCL-loaded DC only elicited weak T cell responses
(9). Furthermore, in tumor
patients, most DCs are immature cells that do not express
costimulatory signals required to promote T cell development.
It was demonstrated that the activation of signal
transducer and activator of transcription 3 (STAT3) inhibits DC
maturity (11,12). The STATs are related to
tumorigenesis and tumor progression, and STAT3 belongs to an
important member of the STAT family that is excessively activated
in many tumors, including hematological malignancies and solid
malignancies (13). The abnormal
expression of STAT3 in tumor tissues leads to tumor cell
proliferation, suppression of cell apoptosis and impaired host
antitumor immunity (14,15). Thus, blocking STAT3 activation might
have antitumor effects that inhibit the growth of human HCC cells
including HepG2, PLC/PRF/5 and H7402 and strengthen the function of
natural killer (NK) cells in a mouse model of HCC (11,12).
Therefore, it is important to develop a new, safer and more
effective agent to inhibit the activation of STAT3, which might be
applied to treat the HCC patients in the clinic. Nifuroxazide
directly inhibited STAT3 thereby inhibiting the survival of
multiple myeloma cells (16).
Recently, nifuroxazide was also reported to induce apoptosis of
breast cancer cells and inhibit pulmonary metastasis in a breast
cancer model (17).
Thus, in this study, we observed the effect of
combining nifuroxazide and DC pulsed with TCL on the survival rate
and lymphocyte infiltration in tumor tissues of an orthotopically
implanted hepatocarcinoma model. We found that the combination
applied of nifuroxazide and DC pulsed with TCL improved the
survival rate, inhibited the tumor growth, but also increased the
CD4+ and CD8+ T cell infiltration in tumor
tissues.
Materials and methods
Reagents and antibodies
Nifuroxazide was purchased from Shanghai Seebio
Biotech, Inc and was preserved at 20 mg/ml solution in dimethyl
sulfoxide (DMSO) and stored at −20°C. The cytokines of
granulocyte-macrophage colony-stimulating factor (GM-CSF),
interleukin (IL)-4 and tumor necrosis factor (TNF)-α were purchased
from PeproTech Inc. (Rocky Hill, NJ, USA).
Mice
Male C57BL/6 mice, aged 6–8 weeks, were purchased
from Vital River Laboratory Animal Technology Co. Ltd. (Beijing,
China). All the mice were maintained at 22±2°C within pathogen-free
conditions according to the Care and Use of Laboratory Animals of
the National Institute of Health Guide.
Cell culture
Human HepG2 cells and the mouse hepatoma cell line
H22 were obtained from Professor Xuejian Zhao (Department of
Pathophysiology, Prostate Diseases Prevention and Treatment
Research Centre, Norman Bethune College of Medicine, Jilin
University, Changchun, China). All the cells were propagated in
RPMI-1640 media supplemented with 10% heat-inactivated fetal bovine
serum (FBS, Gibco) and cultured in a 5% CO2 humidified
incubator at 37°C.
Cell viability assay
HepG2 cells were plated into 96-well flat-bottom
dishes (Corning-Costar, Corning, NY, USA) (2×104
cells/well) and cultured for 16 h. Then the cells were treated with
different concentrations of nifuroxazide. After 48 and 72 h, 10 µl
of CCK8 was added to each well and the cells were incubated for
another 4 h, respectively. The viability effects were recorded
using a multiwell microtiter plate reader (Thermo Fisher
Scientific). Each experiment contained three wells and was repeated
three times.
Wound healing assay
Cell migration activity was assessed using a wound
healing assay. HepG2 cells (3×105 cells/well) were
plated into 6-well flat-bottom dishes (Corning-Costar). After
culturing for 16 h, the initial gap was established using a
micropipette tip and the gap length was measured. At the same time,
nifuroxazide was added into the well at doses of 0.25, 0.5, 1, 2
and 4 µg/ml. At 24 h, and 48 h after cultured with nifuroxazide,
the residual gap length was recorded, respectively. Each experiment
contained three wells and was repeated three times.
Isolation of mouse bone-marrow-derived
DCs
Mouse bone-marrow-derived DCs (BMDCs) were isolated
from C57BL/6 mice as previously described (18,19).
Briefly, on day 0, mice were sacrificed and bone marrow cells were
sluiced out from the femurs and tibiae and added into ACK lysis
buffer (Beyotime Biotechnology, Shanghai, China) to remove
erythrocytes. Then the cells were cultured in a 6-well plate using
RPMI 1640 medium supplemented with 20 ng/ml GM-CSF and 10% FBS. On
day 2, the original medium was replaced with fresh medium including
20 ng/ml GM-CSF. On day 5, half of the medium was replaced with
fresh medium including 20 ng/ml IL-4 and 20 ng/ml GM-CSF. On day 7,
the cells in plates were harvested. At this point, DCs mainly
possessed the characteristic of immature DCs (iDC) and their purity
was ≥85%.
Preparation of the tumor cell
lysate
TCL of H22 was prepared. In brief, cultured H22
cells were collected in phosphate buffered saline (PBS) buffer and
lysed by a freeze-thaw cycle five times. Then, TCL were centrifuged
for 10 min at 12,000 rpm and the supernatant was gathered with the
tumor antigen and stored in a −70°C refrigerator. Finally, the
concentration of protein was evaluated using a BSA kit.
Preparation of TCL-pulsed DCs
iDCs (3×106) were loaded with TCL
containing 100 µg protein/ml and incubated for 6 h. Then the wells
were super-induced by TNF-α at a concentration of 50 ng/ml and then
cultured for another 72 h. Finally, the DCs were harvested and the
surface molecules of CD80 and CD86 were detected to determine
whether DCs were mature.
Mouse experiments
In this study, male C57BL/6 mice were used to
establish an orthotopically implanted HCC model and maintained at
22±2°C with a 12 h light/dark cycle. All mice had free access to
food and water during the experiments. Briefly, two mice were
injected subcutaneously with 1×106 H22 cells. After 2
weeks, the tumors were isolated and cut into small pieces with an
equal volume of 1 mm3. The C57/BL6 mice were
anesthetized using pentobarbitone at dose of 70 mg/kg body weight.
Then, the mice were laparotomized and the fragments of tumor tissue
were thrusted into livers by forming a 3-mm-long hole.
After 7 days, the mice were randomly divided into
four groups including PBS, nifuroxazide, TCL-pulsed DC and
nifuroxazide combination with TCL-pulsed DC group. The mice in the
PBS group were intraperitoneally injected with 100 µl PBS, mice in
the nifuroxazide group were intraperitoneally injected with
nifuroxazide at a dose of 200 µg per mouse, mice in the TCL-loaded
DC group were intravenously injected with DCs and mice in the
combination treatment group were intraperitoneally injected with
nifuroxazide at a dose of 200 µg per mouse plus intravenously
injection of DCs. Nifuroxazide was injected once daily and
maintained for 7 days. TCL-loaded DCs were injected, respectively,
on 7 and 14 days after tumor challenge. The survival rate was
recorded each day and the tumor was weighed at 21 days after tumor
challenge.
Immunohistochemistry assay
At 14 days after treatment, tumor tissues were fixed
in 10% neutral formalin for 24 h, and then the tissues were
embedded in paraffin and cut into 5 µm-thick sections for next
assay as previously described. To analyze the lymphocyte
infiltration of tumor tissues, immunohistochemical analyses were
carried out using antibodies against CD4and CD8 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA).
Flow cytometry assay
The ratios of CD4, CD8 and NK cells in splenocytes
were analyzed using flow cytometry. Briefly, at 14 days after
treatment, 3 mice from group were randomly sacrificed. The spleens
were isolated and homogenized in RPIM-1640, and centrifuged at 2000
rpm for 5 min at 4°C. The precipitates were harvested and lysed the
red blood cell using ACK buffer. The cells were harvested and
counted. Then, the splenocytes were incubated with the following
antibodies: CD3-FITC, CD4-PE, CD8-APC and NK1.1-PE (BioLegend) at
4°C for 30 min. Finally, the cells were washed and detected by flow
cytometry. Staining score was made according to the standards of
grading: 0, no positive cells; 1, population of positive cells
<1%; 2, population of positive cells >1%, <10%; 3,
population of positive cells >10%, <20%; 4, population of
positive cells >20%.
Detection of cytokines
Mouse serum was separated at 21 days after tumor
challenge. The samples were centrifuged at 6,000 rpm for 10 min at
4°C. The levels of interferon (IFN)-γ, TNF-α or vascular
endothelial growth factor (VEGF) were determined using
enzyme-linked immunosorbent assay (ELISA) kit (RayBiotech, Inc.)
according to the manufacturer's directions.
Detection of protein expression
To analyze protein expression, tumor tissues were
lysed using RIPA Lysis Buffer (Beyotime Institute of Biotechnology,
Shanghai, China) and the expression of various proteins was
detected by western blotting (WB) as previously described (20). The antibodies were against STAT3,
p-STAT3, cleaved caspase-3, MMP2 and PARP. All the antibodies were
purchased from cell signaling technology except the α-tubulin,
which was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Statistical analysis
Data are shown as the mean ± standard deviation
(SD). The survival of mice in different groups was determined by
the Kaplan-Meier test. Other data were determined by one-way ANOVA.
All statistical analysis was performed using SPSS software and the
statistical differences were considered at P<0.05.
Results
Nifuroxazide inhibits hepatocellular
carcinoma cell invasion and migration
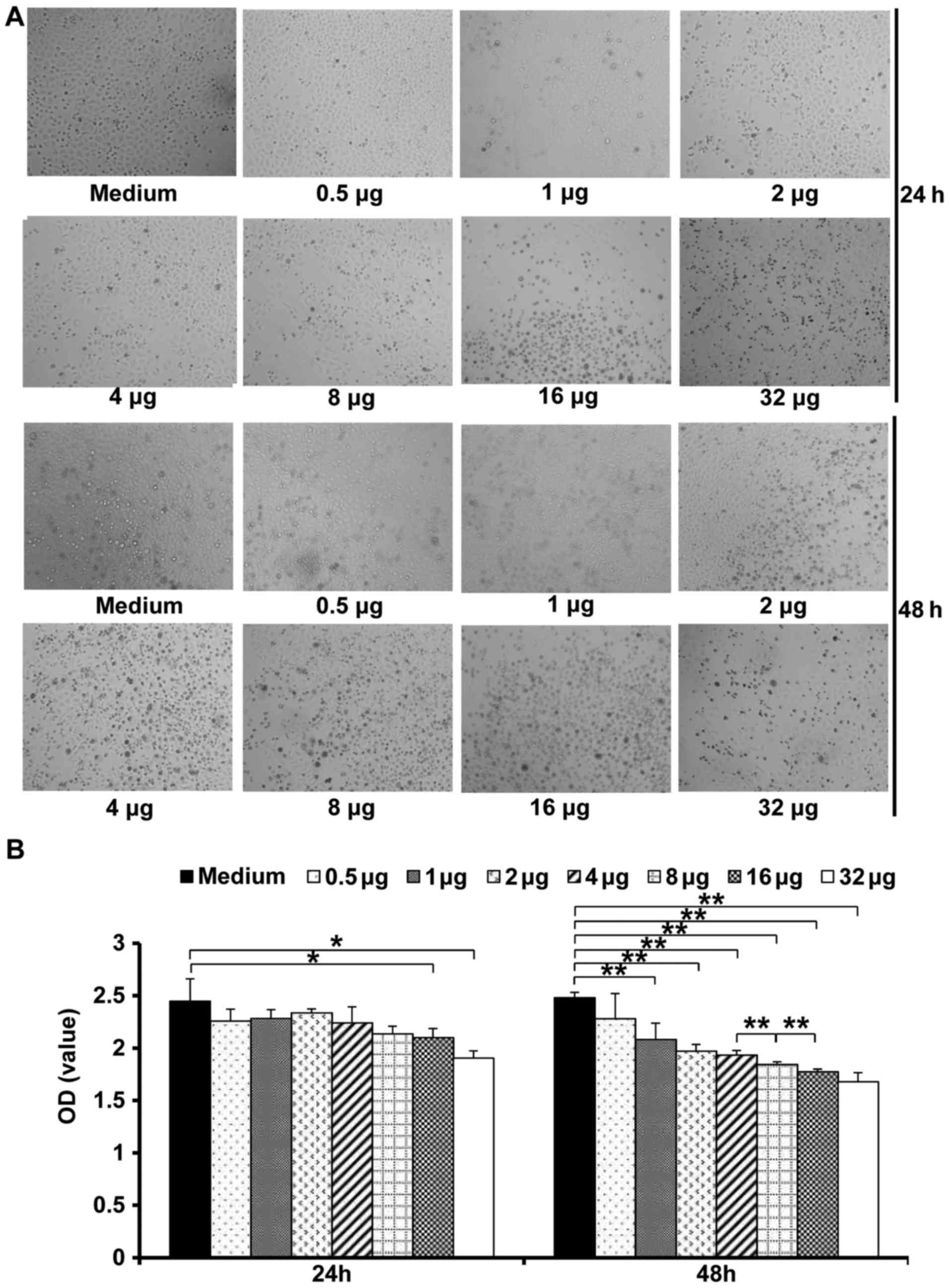
HepG2 cells were treated with different
concentrations (0.5, 1, 2, 4, 8, 16 and 32 µg/ml) of nifuroxazide
to investigate its effect on HCCs. Cell proliferation was detected
at 24 and 48 h post-treatment, respectively. The results showed
that 24 h after treatment with nifuroxazide, the cell viability
only was significantly inhibited at the concentration of 32 µg/ml
compared with treatment of medium alone, while nifuroxazide
inhibited cell viability at all tested concentration at 48 h. These
data suggested that nifuroxazide inhibited HepG2 cell viability
time and concentration dependently. However, nifuroxazide at the
doses of 16 and 32 µg/ml showed similar cell viability (Fig. 1).
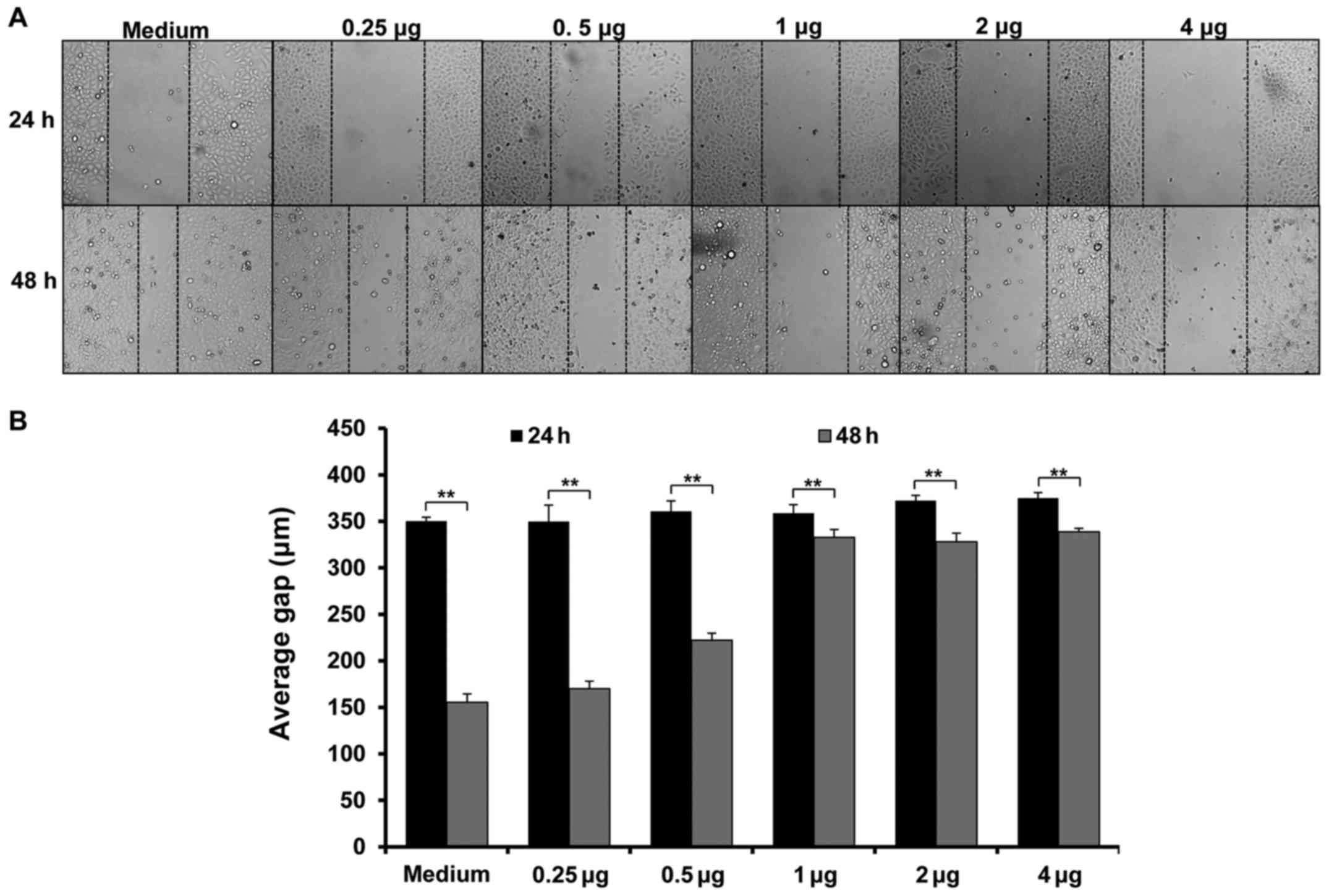
Next, we investigated whether nifuroxazide could
inhibit cell migration. As shown in Fig. 2, cell migration was significantly
inhibited at 24 h after treatment with nifuroxazide at
concentrations of 2 or 4 µg/ml. Although nifuroxazide significantly
inhibited cell migration at the concentrations of 0.50, 1, 2 and 4
µg/ml, respectively, at 48 h, nifuroxazide at the concentrations of
1, 2 or 4 µg/ml had a similar effect on cell migration (Fig. 2).
Combined application of nifuroxazide
and DC pulsed with TCL inhibits tumor growth and increases the
survival rate of mice implanted with hepatocarcinoma
It has been demonstrated that nifuroxazide inhibited
myeloid-derived suppressor cell (MDSCs) proliferation (17), thus we detected whether nifuroxazide
could enhance the antitumor immune response of TCL-loaded DC in
mice with implanted hepatocarcinoma. First, we detected the effect
of different dose of nifuroxazide on mice with implanted
hepatocarcinoma. We found that nifuroxazide at the dose of 200 µg
per mouse showed a preferable treatment effect compared with doses
of 100 or 300 µg per mouse (data not shown). Next, we detected
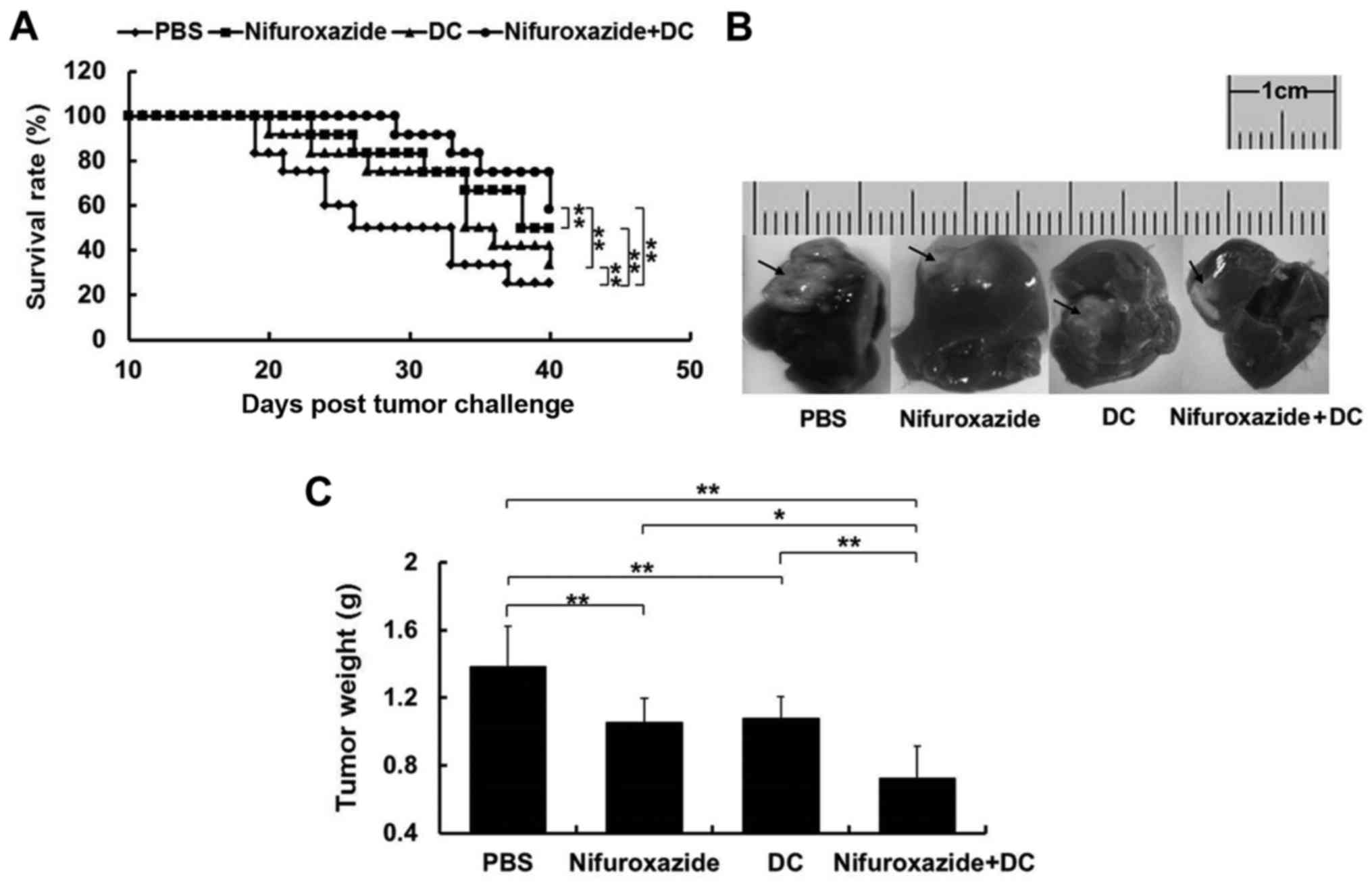
whether nifuroxazide at 200 µg per mouse could prompt the antitumor
effect of TCL-loaded DC. The results showed that at 40 days
post-tumor challenge, both of the survival rates of mice in the
nifuroxazide group (50%, n=12) and DC group (33.3%, n=12) were
significantly enhanced compared with the control group (25%, n=12).
Furthermore, the survival rate of mice in the combination treatment
group was highest (58.3%, n=12) compared with the nifuroxazide
group or DC group (Fig. 3A).
To further compare the antitumor
effect of the combined application of nifuroxazide and TCL-loaded
DC, we detected the tumor weight of mice at 21 days after tumor
challenge
As shown in Fig. 3B and
C, though the tumor growth was significantly inhibited in mice
treated with nifuroxazide or TCL-loaded DC, the combined
application with nifuroxazide and TCL-loaded DC had a more potent
effect on inhibiting tumor growth.
Combined application of nifuroxazide
and TCL-loaded DC increases lymphocyte infiltration in tumor
tissues
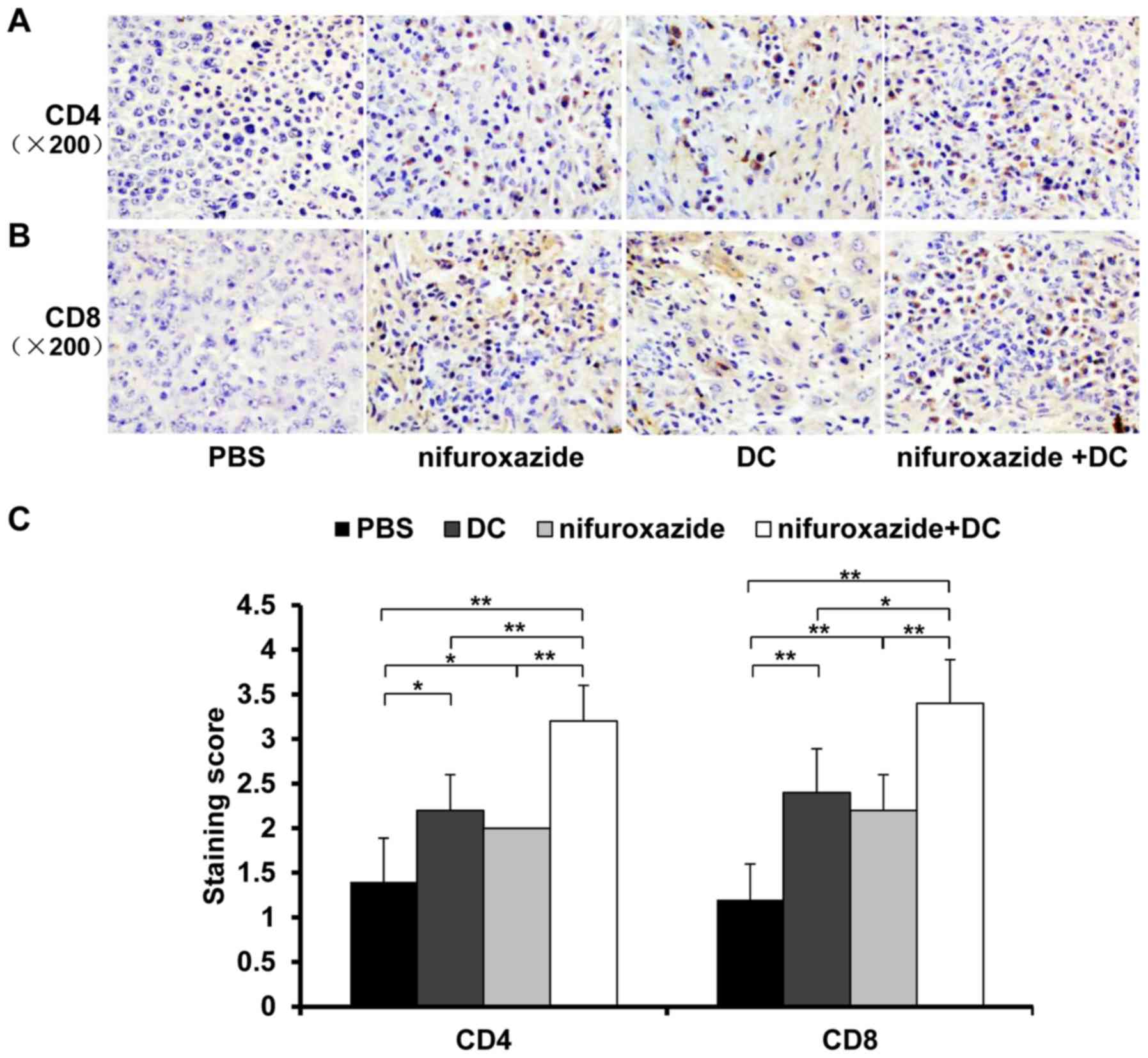
Next, we detected CD4+ and
CD8+ T lymphocyte infiltration in tumor tissues at 14
days post-tumor-bearing by immunohistochemistry. As shown in
Fig. 4, treatment with nifuroxazide
or TCL-loaded DC increased the amount of CD4+ and
CD8+ T lymphocyte infiltration in tumor tissues compared
with control group, respectively. Furthermore, the combination
treatment resulted in the greatest amount of lymphocyte
infiltration in tumor tissues (Fig.
4).
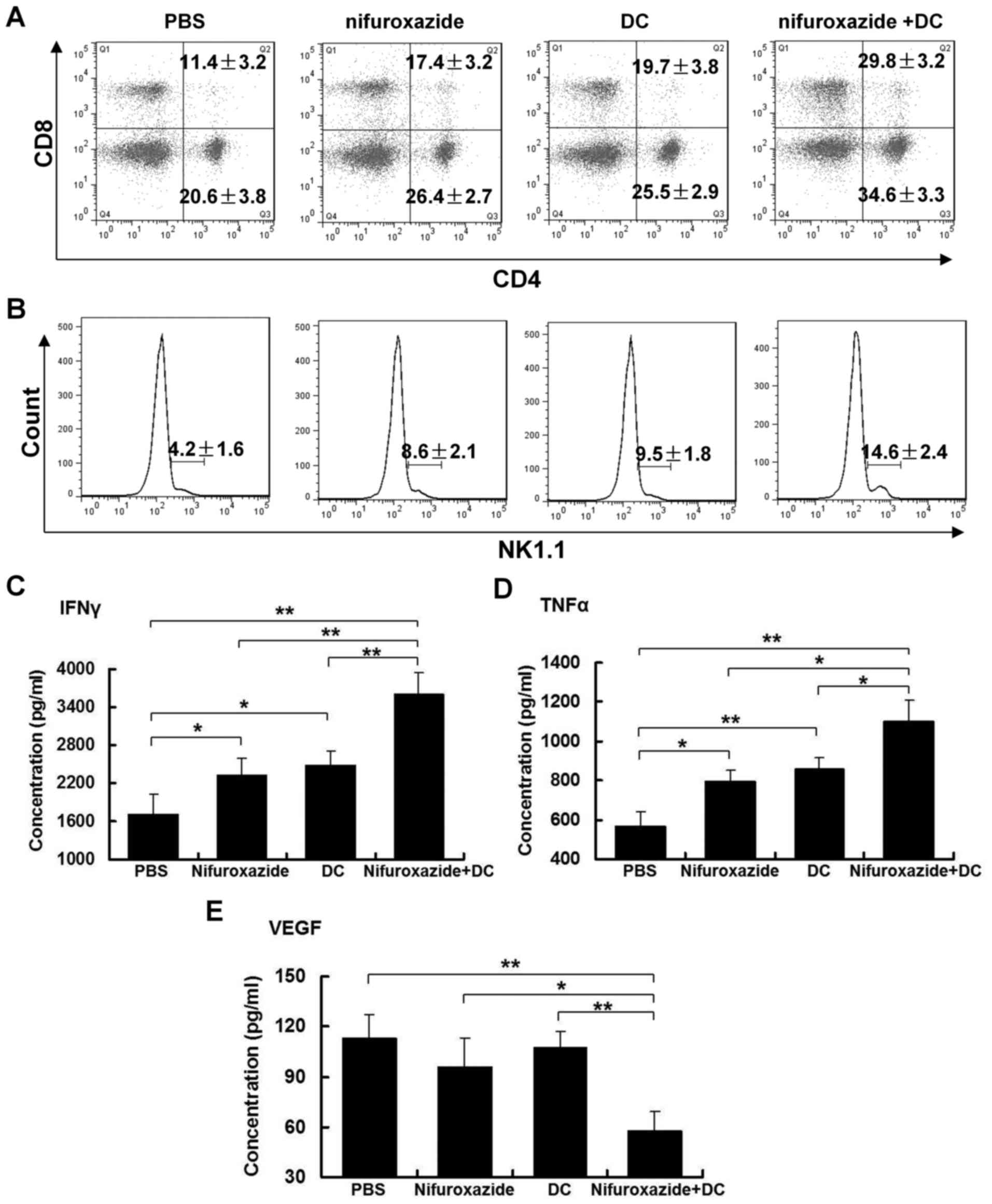
Combined application of nifuroxazide
and TCL-loaded DC elevated the immune cell response in spleen
Spleen, as the largest peripheral immune organ, is
known to play an irreplaceable role in antitumor immunity. Thus, we
assumed that the difference of lymphocyte infiltration in tumor
tissues was related to the percentage change of lymphocyte numbers
in the spleen. Excitedly, application of nifuroxazide or TCL-loaded
DC significantly increased the numbers of CD4+ and
CD8+ T lymphocytes in the spleen. Importantly, the
combination treatment induced the greatest number of lymphocytes in
the spleen, especially CD8+ T lymphocytes compared with
the control group, and nifuroxazide or TCL-loaded DC group
(Fig. 5A).
In addition, we determined the number of NK cells,
which play an important effect on fighting tumors. A similar
situation was observed as for T lymphocytes in the spleen. The
combination treatment led to the most marked observable increment
of NK cells in the spleen (Fig.
5B).
Combined application of nifuroxazide
and TCL-loaded DC influences the concentration of cytokines in
serum
The cytokines could play a role of eliciting
beneficial antitumor effects. Thus, levels of IFN-γ, TNF-α and VEGF
in sera were detected by ELISA kits. The results showed that the
application of nifuroxazide or TCL-loaded DC increased the levels
of IFN-γ and TNF-α, but did not influence the concentration of
VEGF. The combined application of nifuroxazide and TCL-loaded DC
not only significantly increased the levels of IFN-γ and TNF-α, but
also inhibited the release of VEGF compared with the nifuroxazide
or TCL-loaded DC group (Fig.
5C-E).
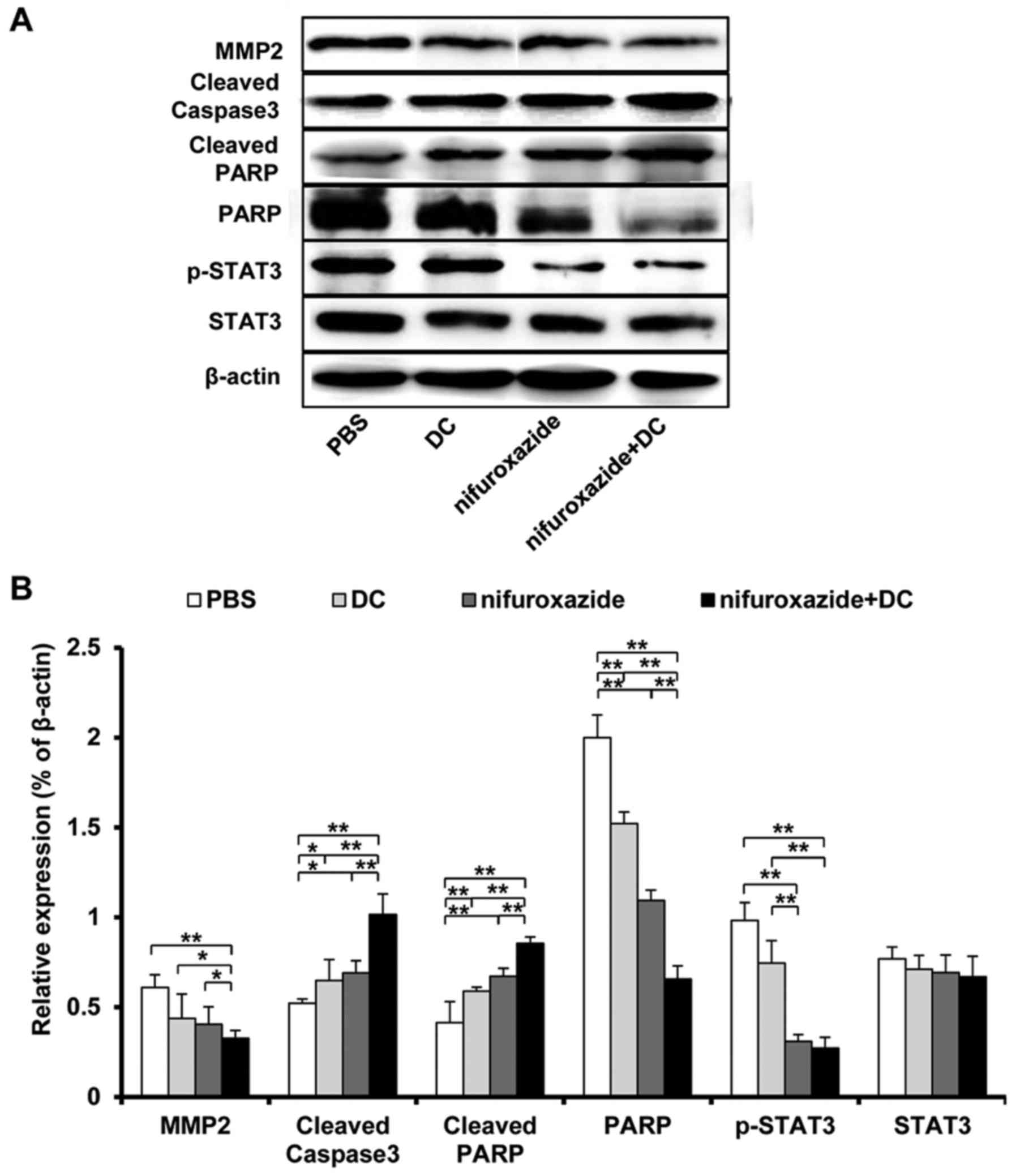
Combined application of nifuroxazide
and TCL-loaded DC influences the protein expression in tumor
tissues
Many proteins participate in the nascence and
development of tumor, therefore, we investigated the expression of
some classical proteins related to proliferation, apoptosis and
migration in tumor tissues. As shown in Fig. 6, the combined application of
nifuroxazide and TCL-loaded DC significantly inhibited the
expression of matrix metalloproteinase 2 (MMP-2) that is related
with the tumor development and the formed tumor microvasculature,
increased the expression of casepase 3 that is known to relate with
the tumor cell apoptosis, and reduced the level of poly ADP-ribose
polymerase (PARP) which is a substrate incised by caspase protein.
In addition, nifuroxazide has been proven to inhibit the STAT3
expression, so we also detected the influence of the combined
application of nifuroxazide and TCL-loaded DC on STAT3 expression
in tumor tissues. The result showed that the STAT3 expression did
not significantly lessen in the nifuroxazide group and combined
group, however the phosphorylation STAT3 was obviously
inhibited.
Discussion
HCC is a frequent human malignancy globally with a
poor prognosis due to the absence of effective treatment methods.
The antitumor immune responses are often impaired because of the
presentation of immunosuppressive factors in the microenvironment
of tumor tissues (21). Thus,
improving antitumor immune responses would be a useful method to
treat HCC. DCs, known as a professional antigen-presenting cells,
play an important role in T cell activation. However, in the body
of tumor patients exist a large number of immature DC that could
not provide the costimulation signals to help T cell development
and proliferation (22). Activation
of STAT3 has been proved to inhibit the function of immune cells
including DCs, NK cells, macrophages and T cells (23–26).
Furthermore, the activation of STAT3 also played a key role in the
progress of HCC (27,28). In a previous study, nifuroxazide, a
STAT3 inhibitor, induced tumor cell apoptosis, suppressed the tumor
migration and reduced the MDSC infiltration in the lungs of mice in
a breast cancer model (17).
Furthermore, because single treatment strategy does not have
satisfactory effect, combination treatments are increasingly
studied. Thus, in this study, we showed that combined application
of nifuroxazide and TCL-loaded DC significantly enhanced the
survival rate, and inhibited the tumor growth in mice with
orthotopically-implanted hepatocarcinomas. Importantly, treatment
with nifuroxazide and TCL-loaded DC improved the antitumor immune
response by increasing the number of T cells in spleen and
enhancing T cell infiltration in tumor tissues.
CD8+ T and NK cells, which have the major
antitumor effects in vivo, the function, included directly
killing the tumor cells and indirectly damaging the tumor cells by
secreting cytokines. It was shown that DC-based vaccine enhanced T
cell-mediated cytotoxicity and cytokine secretion (29,30).
However, TCL-loaded DC might play a dual role by inducing low
antitumor tumor responses and attenuating T cell immune responses
(31). In addition, the activation
of STAT3 has been demonstrated not only to suppress the DC
maturity, but also to inhibit the proliferation of T lymphocytes,
the infiltration of NK cells and the antitumor immune response
(23–26). In our experiments, treatment with
nifuroxazide or TCL-loaded DC increased the T lymphocyte
infiltration in tumor tissues and the ratio of CD8+ T
and NK cells in the spleen. Importantly, combination application of
nifuroxazide and TCL-loaded DC had the strongest effect on the
raising lymphocyte infiltration and the proliferation of T
lymphocytes and NK cells. This might be related to the inhibition
of p-STAT3 expression. We found that combined application of
nifuroxazide and TCL-loaded DC significantly inhibited the
expression of p-STAT3 compared with PBS group and nifuroxazide
group. Inhibition of the expression of STAT3 increased the T cell
infiltration and proliferation (32). Moreover, application of nifuroxazide
inhibited the proliferation of MDSC that had an immunosuppressive
effect on antitumor responses (17). This might be the method to
strengthen the antitumor immune response of TCL-loaded DC by
combination application with nifuroxazide.
Furthermore, several immune cells were shown to be
involved in antitumor response through secreting some cytokines
including IFN-γ and TNF-α (33–35).
So the increased number of T lymphocytes and NK cells would lead to
higher level of cytokines in the serum. Similar with the antitumor
effect, our results showed the combination application of
nifuroxazide and TCL-loaded DC significantly raised the
concentration of IFN-γ and TNF-α. In addition, VEGF, secreted by
tumor cells, induced the hyperplasia of tumor vessels, facilitated
the proliferation of tumor cells, and inhibited the tumor apoptosis
(36,37). Noteworthy, we found that treatment
with nifuroxazide or TCL-loaded DC did not significantly inhibit
the level of VEGF, but the combination application of nifuroxazide
and TCL-loaded DC had the maximum inhibition effect on the release
of VEGF compared with nifuroxazide or TCL-loaded DC group. Since
STAT3 signal could promote angiogenesis, we considered that this
finding might be related to the lowest expression level of p-STAT3
(38), and the lowest tumor
weight.
In addition, the activation of STAT3 signals also
promoted tumorigenesis, migration and inhibited cell apoptosis by
dysregulating the expression of key proteins including caspase 3,
bcl2, MMP2 and MMP9 (39–42). In this study, combination therapy
showed a significant inhibitory effect on the expression of MMP2,
which promotes tumor migration and progress, and increased the
expression of caspase 3, an apoptosis-related protein (43). Apoptosis participates in
pathogenesis and progression of cancer. Inducing the expression of
apoptosis-related proteins might have antitumor effects and inhibit
tumor growth (36,44).
In summary, we demonstrated that combination
treatment of nifuroxazide and TCL-loaded DC significantly inhibited
tumor growth and prompted the antitumor immune responses in a mouse
model of orthotopically-implanted hepatocarcinoma. Nifuroxazide and
TCL-loaded DC might be a novel method for treatment of HCC, but
further in-depth research is still needed.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81301947 and 81300442), the
Scientific Research Fund of Xinxiang Medical University (no.
2013QN112), the Doctor Launch Fund of Xinxiang Medical University
(no. 505016), the platform of collaborative innovation center of
Molecular Diagnosis and Laboratory Medicine- Momentous Science and
Technology Project of Xinxiang in 2014 and Natural Science Fund for
colleges and universities in Jiangsu Province (no.
13KJB320028).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
TCL
|
tumor cell lysate
|
|
DC
|
dendritic cells
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
NK
|
natural killer
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM: Treatment of hepatocellular
carcinoma. Curr Treat Options Gastroenterol. 7:431–441. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Reig M and Sherman M:
Evidence-based diagnosis, staging, and treatment of patients with
hepatocellular carcinoma. Gastroenterology. 150:835–853. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao F, Korangy F and Greten TF: Cellular
immune suppressor mechanisms in patients with hepatocellular
carcinoma. Dig Dis. 30:477–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harding JJ, El Dika I and Abou-Alfa GK:
Immunotherapy in hepatocellular carcinoma: Primed to make a
difference? Cancer. 122:367–377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steinman RM and Cohn ZA: Pillars Article:
Identification of a novel cell type in peripheral lymphoid organs
of mice. I. Morphology, quantitation, tissue distribution. J. Exp.
Med.1973. 137: 1142–1162. J Immunol. 178:5–25. 2007.PubMed/NCBI
|
|
7
|
Sun K, Wang L and Zhang Y: Dendritic cell
as therapeutic vaccines against tumors and its role in therapy for
hepatocellular carcinoma. Cell Mol Immunol. 3:197–203.
2006.PubMed/NCBI
|
|
8
|
Ge C, Xing Y, Wang Q, Xiao W, Lu Y, Hu X,
Gao Z, Xu M, Ma Y, Cao R, et al: Improved efficacy of therapeutic
vaccination with dendritic cells pulsed with tumor cell lysate
against hepatocellular carcinoma by introduction of 2 tandem
repeats of microbial HSP70 peptide epitope 407–426 and OK-432. Int
Immunopharmacol. 11:2200–2207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee WC, Wang HC, Hung CF, Huang PF, Lia CR
and Chen MF: Vaccination of advanced hepatocellular carcinoma
patients with tumor lysate-pulsed dendritic cells: A clinical
trial. J Immunother. 28:496–504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JH, Lee Y, Lee M, Heo MK, Song JS, Kim
KH, Lee H, Yi NJ, Lee KW, Suh KS, et al: A phase I/IIa study of
adjuvant immunotherapy with tumour antigen-pulsed dendritic cells
in patients with hepatocellular carcinoma. Br J Cancer.
113:1666–1676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun X, Zhang J, Wang L and Tian Z: Growth
inhibition of human hepatocellular carcinoma cells by blocking
STAT3 activation with decoy-ODN. Cancer Lett. 262:201–213. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sui Q, Zhang J, Sun X, Zhang C, Han Q and
Tian Z: NK cells are the crucial antitumor mediators when
STAT3-mediated immunosuppression is blocked in hepatocellular
carcinoma. J Immunol. 193:2016–2023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murone M, Chessex A Vaslin, Attinger A,
Ramachandra R, Shetty SJ, Daginakatte G, Sengupta S, Marappan S,
Dhodheri S, Rigotti S, et al: Debio 0617B inhibits growth of
STAT3-driven solid tumors through combined inhibition of JAK, SRC,
and class III/V receptor tyrosine kinases. Mol Cancer Ther.
15:2334–2343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hillmer EJ, Zhang H, Li HS and Watowich
SS: STAT3 signaling in immunity. Cytokine Growth Factor Rev.
31:1–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu H, Kortylewski M and Pardoll D:
Crosstalk between cancer and immune cells: Role of STAT3 in the
tumour microenvironment. Nat Rev Immunol. 7:41–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nelson EA, Walker SR, Kepich A, Gashin LB,
Hideshima T, Ikeda H, Chauhan D, Anderson KC and Frank DA:
Nifuroxazide inhibits survival of multiple myeloma cells by
directly inhibiting STAT3. Blood. 112:5095–5102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang F, Hu M, Lei Q, Xia Y, Zhu Y, Song X,
Li Y, Jie H, Liu C, Xiong Y, et al: Nifuroxazide induces apoptosis
and impairs pulmonary metastasis in breast cancer model. Cell Death
Dis. 6:e17012015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang WT, Chen HM, Yin SY, Chen YH, Wen
CC, Wei WC, Lai P, Wang CH and Yang NS: Specific Dioscorea
phytoextracts enhance potency of TCL-loaded DC-based cancer
vaccines. Evid Based Complement Alternat Med. 2013:9320402013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin SY, Wang CY and Yang NS: Interleukin-4
enhances trafficking and functional activities of GM-CSF-stimulated
mouse myeloid-derived dendritic cells at late differentiation
stage. Exp Cell Res. 317:2210–2221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nefedova Y, Cheng P, Gilkes D, Blaskovich
M, Beg AA, Sebti SM and Gabrilovich DI: Activation of dendritic
cells via inhibition of Jak2/STAT3 signaling. J Immunol.
175:4338–4346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang JD, Nakamura I and Roberts LR: The
tumor microenvironment in hepatocellular carcinoma: Current status
and therapeutic targets. Semin Cancer Biol. 21:35–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalinski P: Dendritic cells in
immunotherapy of established cancer: Roles of signals 1, 2, 3 and
4. Curr Opin Investig Drugs. 10:526–535. 2009.PubMed/NCBI
|
|
23
|
Wang T, Niu G, Kortylewski M, Burdelya L,
Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola
D, et al: Regulation of the innate and adaptive immune responses by
Stat-3 signaling in tumor cells. Nat Med. 10:48–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cacalano NA: Regulation of natural killer
cell function by STAT3. Front Immunol. 7:1282016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hollander L, Guo X, Velazquez H, Chang J,
Safirstein R, Kluger H, Cha C and Desir GV: Renalase expression by
melanoma and tumor-associated macrophages promotes tumor growth
through a STAT3-mediated mechanism. Cancer Res. 76:3884–3894. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yue C, Shen S, Deng J, Priceman SJ, Li W,
Huang A and Yu H: STAT3 in CD8+ T cells inhibits their
tumor accumulation by downregulating CXCR3/CXCL10 axis. Cancer
Immunol Res. 3:864–870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghoshal S, Fuchs BC and Tanabe KK: STAT3
is a key transcriptional regulator of cancer stem cell marker CD133
in HCC. Hepatobiliary Surg Nutr. 5:201–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng X, Xu M, Yao B, Wang C, Jia Y and
Liu Q: IL-6/STAT3 axis initiated CAFs via up-regulating TIMP-1
which was attenuated by acetylation of STAT3 induced by PCAF in HCC
microenvironment. Cell Signal. 28:1314–1324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang Q, Jiang J and Liu J: CCR5 blockade
suppresses melanoma development through inhibition of IL-6-Stat3
pathway via upregulation of SOCS3. Inflammation. 38:2049–2056.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Delirezh N, Moazzeni SM, Shokri F,
Shokrgozar MA, Atri M and Kokhaei P: Autologous dendritic cells
loaded with apoptotic tumor cells induce T cell-mediated immune
responses against breast cancer in vitro. Cell Immunol. 257:23–31.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang DH, Park JS, Jin CJ, Kang HK, Nam JH,
Rhee JH, Kim YK, Chung SY, Choi SJ, Kim HJ, et al: The dysfunction
and abnormal signaling pathway of dendritic cells loaded by tumor
antigen can be overcome by neutralizing VEGF in multiple myeloma.
Leuk Res. 33:665–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia H, Li Y, Zhao T, Li X, Hu J, Yin D,
Guo B, Kopecko DJ, Zhao X, Zhang L, et al: Antitumor effects of
Stat3-siRNA and endostatin combined therapies, delivered by
attenuated Salmonella, on orthotopically implanted hepatocarcinoma.
Cancer Immunol Immunother. 61:1977–1987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McMichael EL, Jaime-Ramirez AC,
Guenterberg KD, Luedke E, Atwal LS, Campbell AR, Hu Z, Tatum AS,
Kondadasula SV, Mo X, et al: IL-21 enhances natural killer cell
response to cetuximab-coated pancreatic tumor cells. Clin Cancer
Res. 23:489–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iraolagoitia XL, Spallanzani RG, Torres
NI, Araya RE, Ziblat A, Domaica CI, Sierra JM, Nuñez SY, Secchiari
F, Gajewski TF, et al: NK cells restrain spontaneous antitumor
CD8+ T cell priming through PD-1/PD-L1 interactions with
dendritic cells. J Immunol. 197:953–961. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kozlowska AK, Tseng HC, Kaur K, Topchyan
P, Inagaki A, Bui VT, Kasahara N, Cacalano N and Jewett A:
Resistance to cytotoxicity and sustained release of interleukin-6
and interleukin-8 in the presence of decreased interferon-γ after
differentiation of glioblastoma by human natural killer cells.
Cancer Immunol Immunother. 65:1085–1097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xia Y, Song X, Li D, Ye T, Xu Y, Lin H,
Meng N, Li G, Deng S, Zhang S, et al: YLT192, a novel, orally
active bioavailable inhibitor of VEGFR2 signaling with potent
antiangiogenic activity and antitumor efficacy in preclinical
models. Sci Rep. 4:60312014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong W, Xian Y, Yuan W, Huifeng Z, Tao W,
Zhiqiang L, Shan F, Ya F, Hongli W, Jinghuan W, et al: Catalpol
stimulates VEGF production via the JAK2/STAT3 pathway to improve
angiogenesis in rats' stroke model. J Ethnopharmacol. 191:169–179.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alvarez JV, Febbo PG, Ramaswamy S, Loda M,
Richardson A and Frank DA: Identification of a genetic signature of
activated signal transducer and activator of transcription 3 in
human tumors. Cancer Res. 65:5054–5062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsieh FC, Cheng G and Lin J: Evaluation of
potential Stat3-regulated genes in human breast cancer. Biochem
Biophys Res Commun. 335:292–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miklossy G, Hilliard TS and Turkson J:
Therapeutic modulators of STAT signalling for human diseases. Nat
Rev Drug Discov. 12:611–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Walker SR, Xiang M and Frank DA: Distinct
roles of STAT3 and STAT5 in the pathogenesis and targeted therapy
of breast cancer. Mol Cell Endocrinol. 382:616–621. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheung TH, Chung TK, Lo KW, Yu MY,
Krajewski S, Reed JC and Wong YF: Apotosis-related proteins in
cervical intraepithelial neoplasia and squamous cell carcinoma of
the cervix. Gynecol Oncol. 86:14–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|