Introduction
Hepatocellular carcinoma (HCC), a primary malignancy
of the liver, is one of the most prevalent cancers, with an
increasing incidence and mortality rate around the world (1,2). The
most effective therapy is liver resection or transplantation for
patients with early-stage disease, however, most patients are
diagnosed in later or inoperable stages (3). Although the diagnosis and therapies
for HCC have advanced in recent years, the prognosis for HCC
patients remains poor (4,5). Therefore, it is imperative to clarify
the molecular mechanisms underlying HCC, and to discover valuable
diagnostic and prognostic biomarkers for HCC. Furthermore, new
therapeutic agents to treat this malignancy must be explored.
Cadherin is a calcium-dependent adhesion protein
that is a member of a large family of cell adhesion molecules.
Cadherins have been identified by the presence of extracellular
cadherin repeats of about 110 amino acid residues, and can be
classified into: the classical cadherins, desmosomal cadherins, and
protocadherins (PCDHs) (6,7). PCDHs are predominantly expressed in
the nervous system, and are reported to participate in the circuit
formation and maintenance of the brain (8,9).
However, in past decades accumulating evidence has revealed that
PCDH family members act as tumor-suppressor genes in multiple
carcinomas (10–14).
The protocadherin10 (PCDH10) gene is located on
human chromosome 4q28.3. The PCDH10 protein belongs to the PCDH
subfamily, and is expressed on the plasma membrane. Previous
research regarding PCDH10 focused on neuronal diseases, such as
autism (15). However, recent
studies have demonstrated that PCDH10 is frequently downregulated
by promoter DNA methylation, and functions as a tumor-suppressor
gene in gastric, colorectal and lung cancer, as well as in many
other carcinomas (16–19). Previous studies have indicated that
the expression of PCDH10 was notably downregulated in HCC tissue
and cells, compared to that in normal liver tissue (20). Furthermore, decreased PCDH10
expression was found to correlate with the methylation status of
the PCDH10 promoter (20). However,
the biological functions and mechanism of PCDH10 in HCC have yet to
be elucidated. Therefore, the aim of the present study was to
identify the biological function and molecular mechanism of PCDH10
in HCC, thus aiding the discovery of valuable diagnostic and
prognostic biomarkers for HCC, as well as the development of new
therapeutic agents to treat this malignancy.
Materials and methods
Cell culture and transfection
HCC cell lines (HepG2, HuH7, HuH1 and SNU387) and a
normal liver cell line (L02) were purchased from the American Type
Culture Collection (ATCC; Mannasas, VA, USA). The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone
Laboratories, Inc., Logan, UT, USA) with 10% fetal bovine serum
(FBS; Gibco, Grand Island, NY, USA). All the cells were maintained
at 37°C in an incubator with 95% air and 5% CO2.
The plasmid pcDNA3.1-PCDH10 and pcDNA3.1-vector were
purchased from GeneChem Co., Ltd. (Shanghai, China). The
transfection was performed in 6-well plates. Cells (HepG2 and HuH7)
were seeded into 6-well plates and allowed to culture overnight.
The wells were then filled with 1 ml of fresh, serum-free medium
after washing the cells twice with serum-free medium. Four
micrograms of plasmid (pcDNA3.1-PCDH10 or pcDNA3.1-vector) and 5 µl
of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) were diluted
in 500 µl of serum-free medium respectively, and allowed to
incubate for 5 min at room temperature. Following this, plasmid and
Lipofectamine 2000 diluent were mixed and incubated for 20 min at
room temperature, then 1 ml of the aforementioned mixture was added
to each well. Renewal of the medium with 2 ml of fresh medium with
10% FBS was conducted after culturing 4 h at 37°C. The efficiency
of transfection was detected with RT-qPCR and western blotting
assays.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol (Invitrogen) was used to isolate the total
RNA from cells. The concentration and purity of total RNA were
assessed, with absorbance ratio OD(260)/OD(280) values with a
purity range of 1.8–2.0. Total RNA was reverse-transcribed into
cDNA using the GoScript reverse transcription kit (Promega,
Madison, WI, USA). RT-qPCR amplification primers that were
synthesized by Takara Bio (Dalian, China) are as follows: target
gene PCDH10 forward, 5′-ACTGCTATCAGGTATGCCTG-3′ and reverse,
5′-GTCTGTCAACTAGATAGCTG-3′; internal control β-actin forward,
5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse, 5′-GCTGTCACCTTCACCGTTCC-3′.
RT-qPCR was performed with the SYBR® Premix ExTaq™ II
(Tli RNaseH Plus; Takara Biotechnology Co., Ltd.) and according to
the manufacturer's protocol on the Light-cycler 480® II
real-time PCR system (Roche Diagnostics, Basel, Switzerland). The
2−ΔΔCt method was used to determine the relative
quantitation of gene expression levels.
Western blot assay
Cells were scraped and collected after washing with
phosphate-buffered saline (PBS). RIPA lysate buffer (Beyotime
Institute of Biotechnology, Shanghai, China) was used to extract
total protein, and the concentration of the protein was assessed
using a BCA kit (Beyotime Institute of Biotechnology). Proteins
were separated by polyacrylamide gel electrophoresis, transferred
onto polyvinylidene fluoride (PVDF) membranes (Beyotime Institute
of Biotechnology), blocked with 5% skim milk for 2 h at room
temperature, and then incubated with primary antibodies for PCDH10
[mouse anti-human monoclonal antibody (Abnova, Taipei, Taiwan)] and
β-actin [mouse anti-human monoclonal antibody (Cell Signaling
Technology, Inc., Danvers, MA, USA)] at 4°C overnight.
Subsequently, the membranes were washed with TBST buffer three
times, and then incubated with the secondary antibodies (goat
anti-mouse; Cell Signaling Technology) with horseradish peroxidase
(HRP) for 2 h at room temperature. The blots were washed again and
detected with BeyoECL Plus kits (Beyotime Institute of
Biotechnology). Fusion software was used to analyze the
results.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8; Dojindo) was used to
evaluate cell proliferation. Cells (HepG2 and HuH7) were seeded in
a 96-well plate with a density of 5×103 cells/well,
allowed to culture for 24 h, and then transfected with plasmid
pcDNA3.1-PCDH10 or pcDNA3.1-vector as previously described.
According to the manufacturer's protocol, the CCK-8 reagent was
added to each plate at 0, 24, 48 and 72 h after transfection.
Subsequently, the reaction was incubated for 2 h in the incubator,
and then the OD was detected at 450 nm generating a cell
proliferation curve.
Clone formation assay
Cells (HepG2 and HuH7) were seeded in a 6-well plate
and transfected with plasmid pcDNA3.1-PCDH10 or pcDNA3.1-vector.
Approximately 48 h post-transfection, all cells were collected and
counted, seeded in a 6-well plate at a density of 1×104
cells/well, and cultured under selectiong by G418 (0.6 mg/ml) for
more than 2 weeks. The surviving clones (≥50 cells) were counted
after fixation with paraformaldehyde and staining with Gentian
violet.
Flow cytometric assays of the cell
cycle and cell apoptosis
For the cell cycle assay, HepG2 and HuH7 cells were
seeded into 6-well plates and transfected with plasmid
pcDNA3.1-PCDH10 or pcDNA3.1-vector. After incubation with
transfection media for 24 h, the cells were washed with PBS,
digested by trypsin without EDTA, then collected and fixed in
precooling 70% ethanol overnight at 4°C. The cells were then washed
with precooling PBS and then stained with propidium iodide (PI)
50mg/ml for 30 min at 4°C in dark. Elite ESP flow cytometry was
performed to assay the cell-cycle profiles. Data were analyzed with
CELL Quest software (BD Biosciences, San Jose, CA).
The apoptosis of HepG2 and HuH7 cells was assessed
by flow cytometry, combined with Annexin V-FITC/PI staining. The
cells were transfected as previously described, and 48 h after
transfection the cells were gently collected, and then incubated
with Annexin V-fluorescein isothiocynate and PI at room
temperature. The samples were evaluated by flow cytometry
immediately.
Molecular mechanism of PCDH10 in HCC
cells
PCDH10 plays an important role in cell-cell signal
conduction, and previous studies have demonstrated that PCDH10
could regulate pathways relating to tumorigenesis and tumor
progression (27,28). In this study, we observed a
difference of phenotype in HCC cells after overexpression of
PCDH10. Therefore, we speculated that the signaling pathway could
regulate cell proliferation and cell apoptosis. Western blot
analyses were performed to detect the target proteins. The western
blot analysis method was aforementioned. Briefly, 48–72 h
post-transfection, total proteins were extracted, separated by
polyacrylamide gel electrophoresis, and then transferred to PVDF
membranes. The membranes were blocked with 5% skim milk and then
incubated with primary antibodies for p-AKT, p-MDM2, p53, p-GSK-3β
and p21 (Abcam, Cambridge, MA, USA) and caspase-3, Bax, Bcl-2 and
cyclin D1 (Cell Signaling Technology, Inc.) overnight at 4°C.
Membranes were then incubated with HRP-conjugated secondary
antibodies, and then the blots were visualized with BeyoECL Plus
kits.
To further explore how PCDH10 affects signaling
pathways, we performed co-immunoprecipitation (Co-IP) assays to
detect the molecules interacting with PCDH10. Co-IP assays were
performed with the Co-IP kit (Life Technologies, Carlsbad, CA, USA)
according to the manufacturer's protocol. HepG2 cells were
transfected with plasmid pcDNA3.1-PCDH10. Approximately 48 h
post-transfection, total proteins were extracted from the cells,
and a PCDH10-antibody, a phosphoinositide 3-kinase (PI3K)
p85-antibody [mouse monoclonal antibody (Abcam)] and a control
mouse antibody lgG (Abcam) were used to perform
immunoprecipitations. Samples were analyzed by western blot
analysis as aforementioned.
Statistical analysis
Data are presented as the mean ± SD from independent
triplicate experiments, and analyzed with Student's t-test.
P<0.05 was considered to indicate a statistically significant
result. All data analysis was performed with SPSS version 18.0
(IBM, Armonk, NY, USA).
Results
PCDH10 expression is dysregulated in
HCC cells
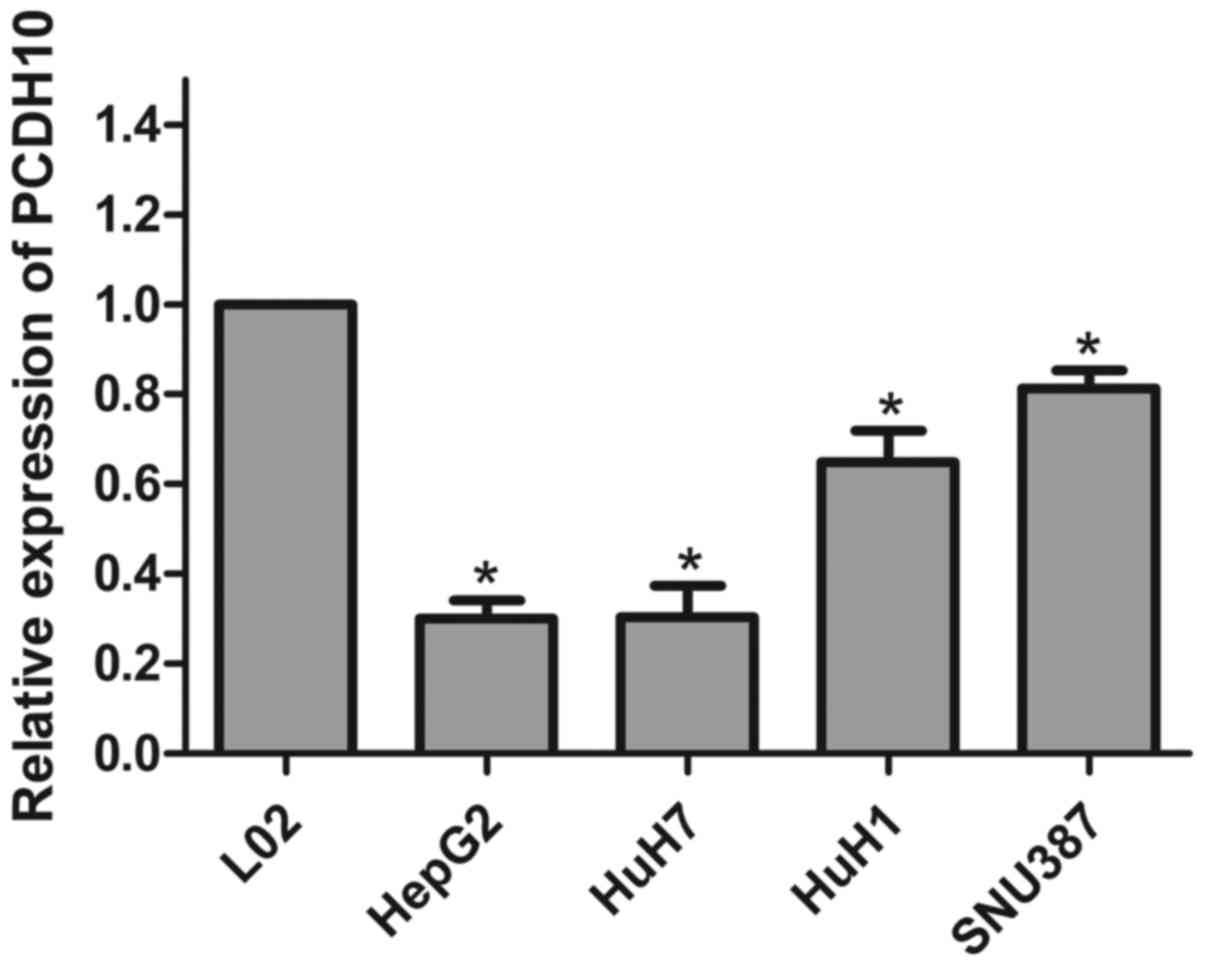
We used RT-qPCR to detect the expression of PCDH10
in HCC cells and normal liver cells. The expression of PCDH10 was
significantly less in the HCC cells when compared to that in the
normal liver cells (L02) (P<0.05; Fig. 1).
Efficiency of transfection in HCC
cells, HepG2 and HuH7
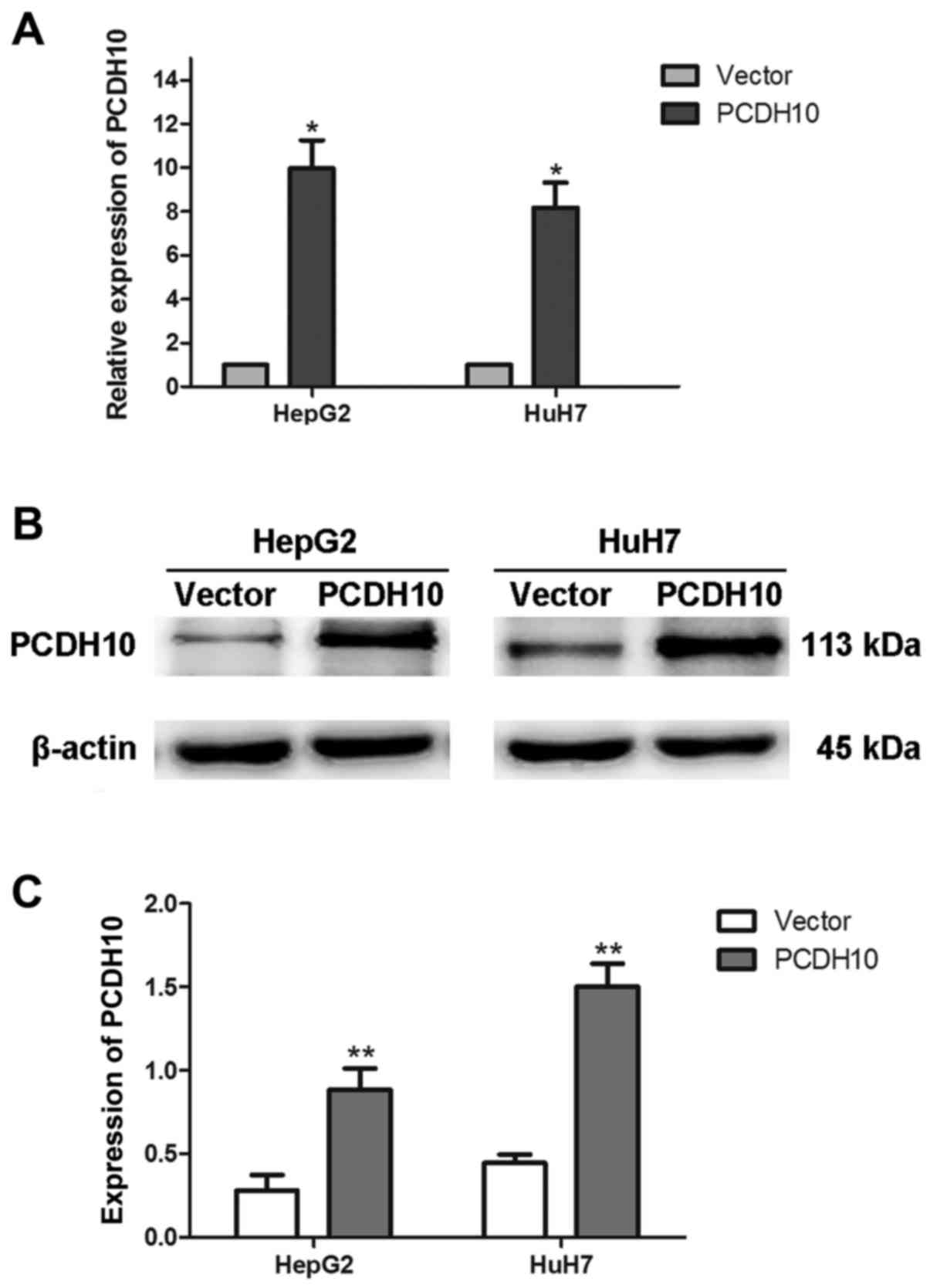
HepG2 and HuH7 were transfected with plasmid
pcDNA3.1-PCDH10 or pcDNA3.1-vector. Approximately 48 h later, the
total RNA and proteins were extracted, and RT-qPCR and western blot
analysis assays were used to assess the efficiency of transfection.
The results indicated that PCDH10 expression was increased in the
cells transfected with plasmid pcDNA3.1-PCDH10 when compared to
that in the cells transfected with the pcDNA3.1-vector (P<0.05;
Fig. 2).
PCDH10 inhibits cell proliferation of
HCC
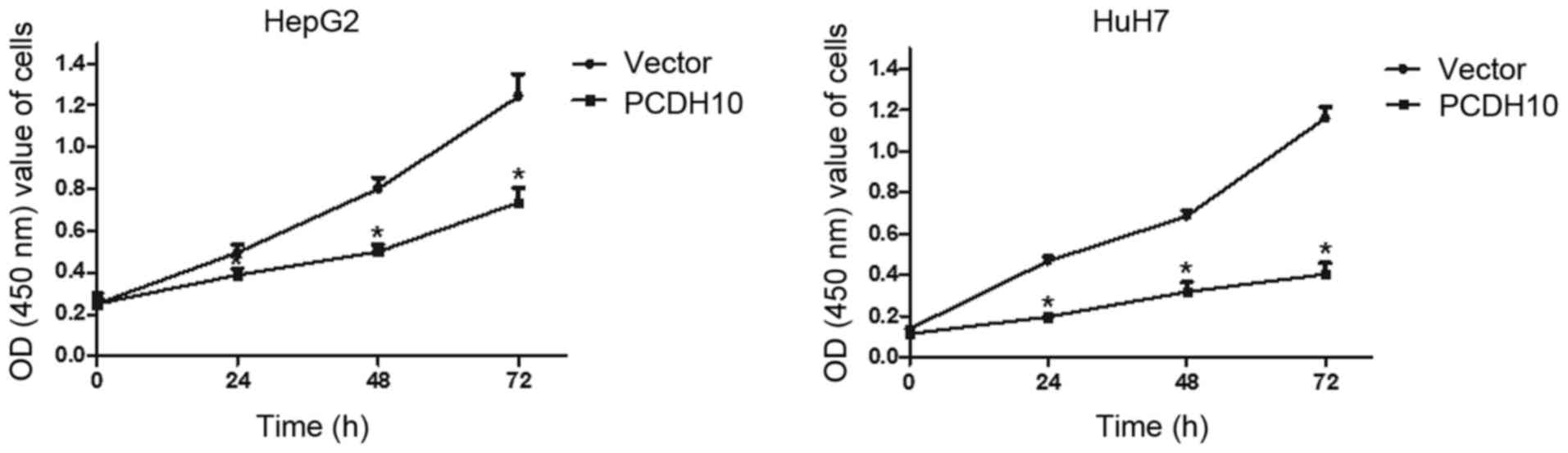
To assess the influence of PCDH10 on cell
proliferation in HCC, the CCK-8 assay was performed. Cells were
transfected with plasmid PCDH10 or vector and monitored for
proliferation. The proliferation ability of the PCDH10-transfected
cells was lower than that of the vector-transfected cells at 24, 48
and 72 h (P<0.05; Fig. 3).
PCDH10 inhibits clone formation in HCC
cells
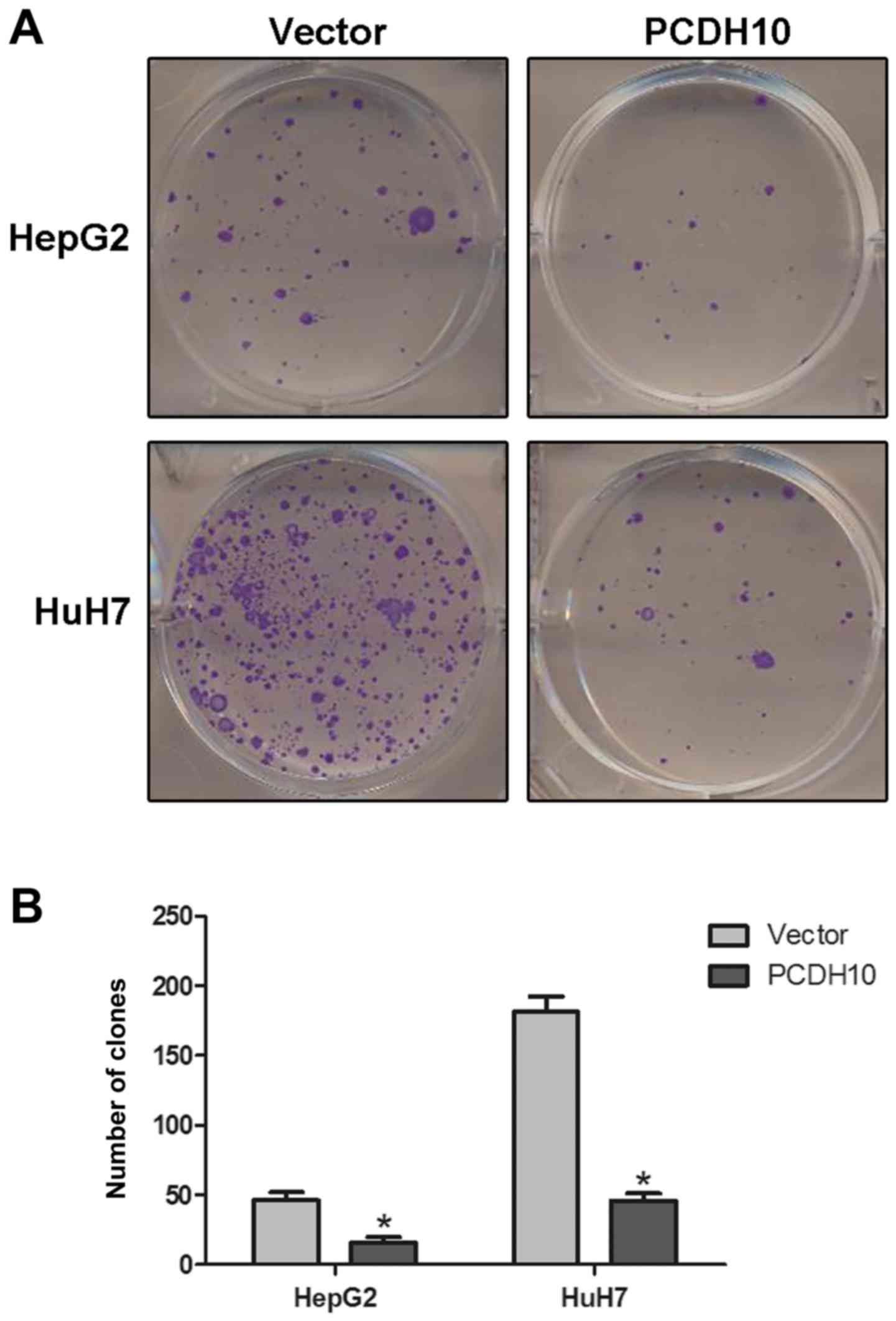
The clone formation assay was performed to evaluate
the suppressive effect of PCDH10 in HCC cells. As we expected,
there was a strong decrease in the number of clones, and a
significant decrease in the size of cells transfected with PCDH10
when compared to the cells transfected with the vector (P<0.05;
Fig. 4).
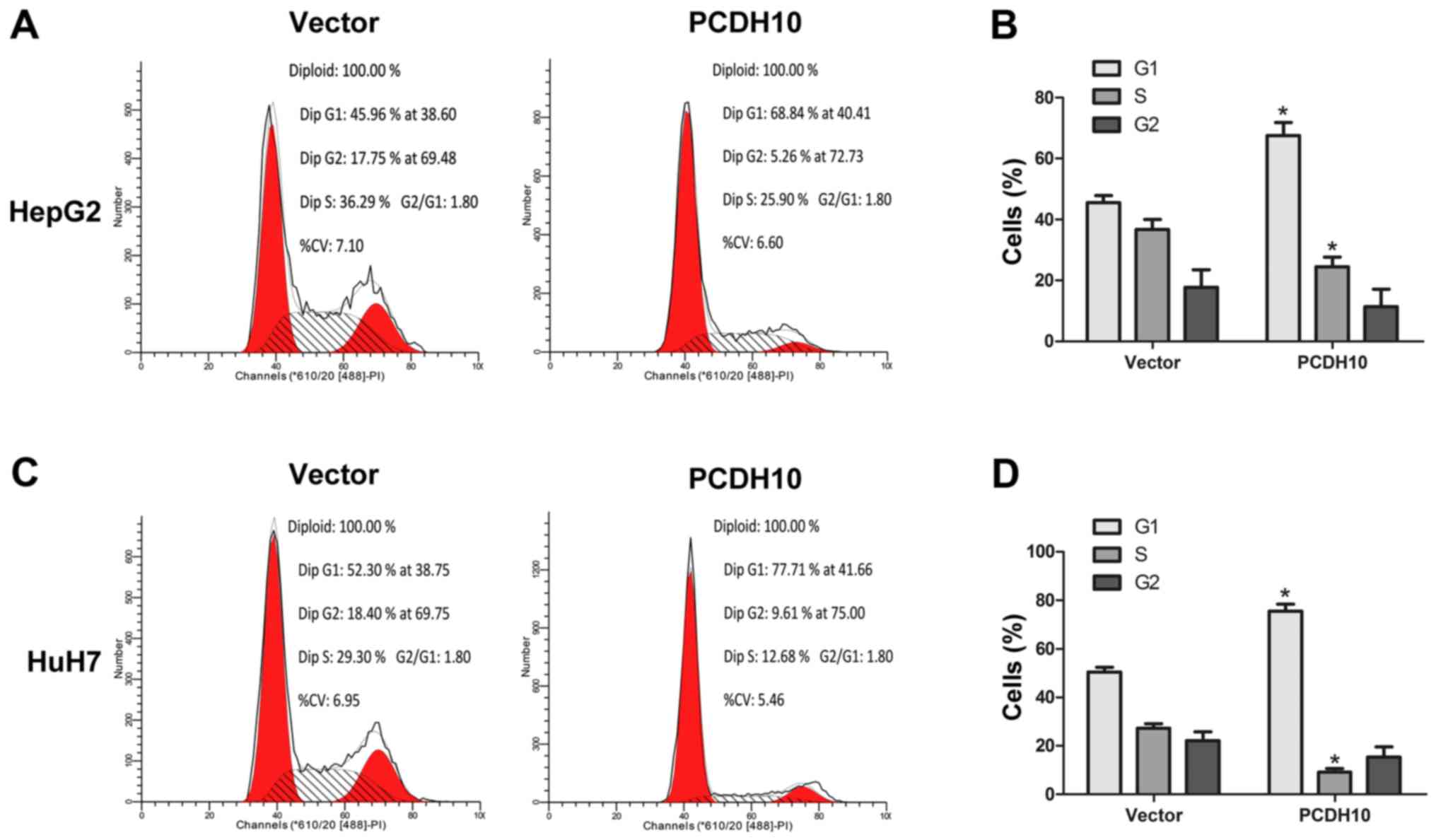
PCDH10 arrests the cell cycle at the
G1 phase and induces cell apoptosis in HCC
Cell proliferation and clone formation assays were
performed and it was determined that PCDH10 could inhibit HCC cell
growth. In order to further understand how PCDH10 restricts cell
growth, we used flow cytometry to analyze the cell cycle and cell
apoptosis. The results indicated that in cells transfected with
PCDH10, the number of cells in the G1 phase was increased, and the
number of cells in the G2 phase were decreased, as compared to the
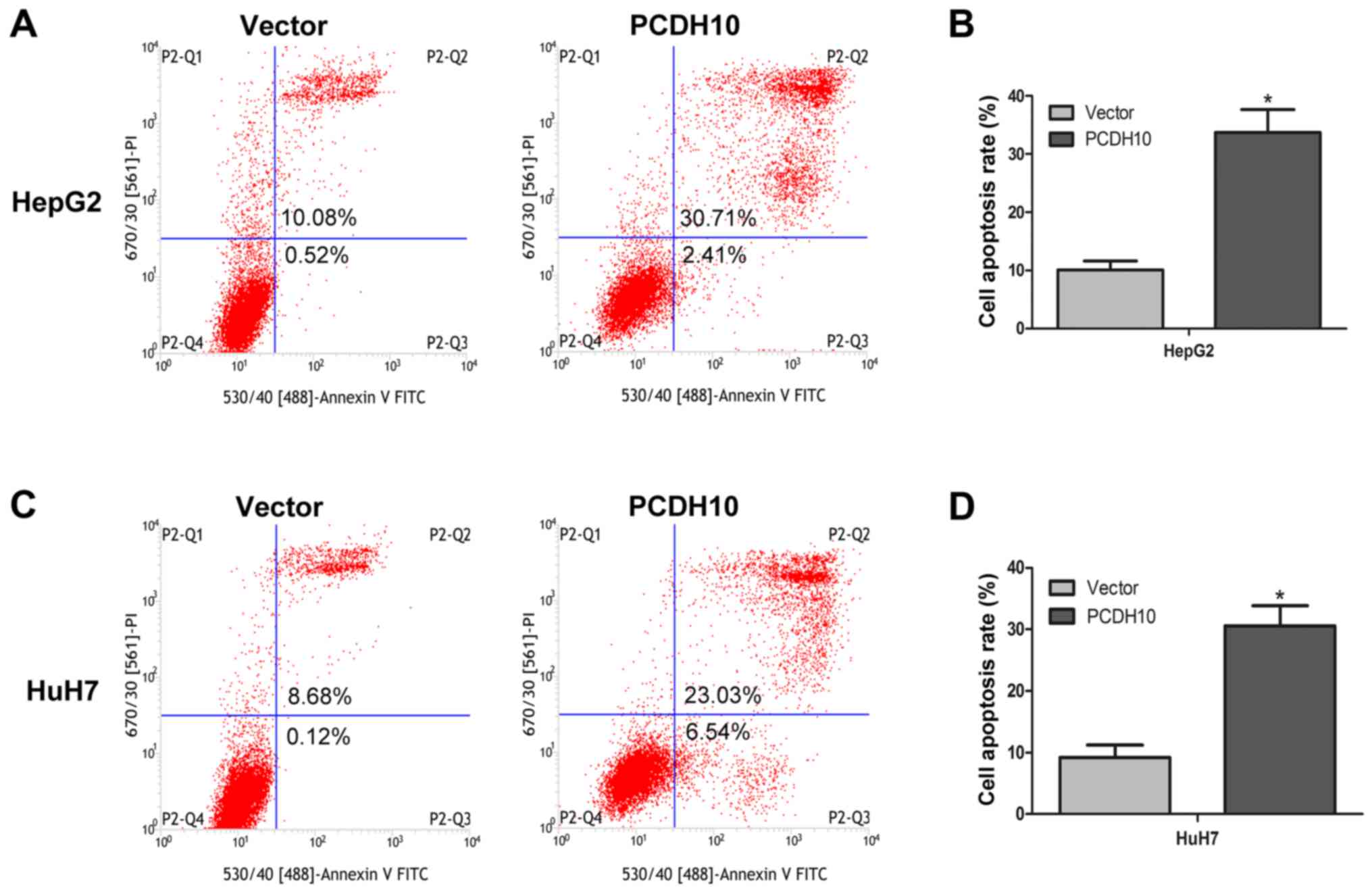
control groups transfected with the vector (P<0.05; Fig. 5). Notably, flow cytometry combined
with Annexin V-FITC/PI staining was used to assess cell apoptosis,
and these results indicated that cell apoptosis was markedly
increased in the cells transfected with PCDH10, when compared to
the control groups (P<0.05; Fig.
6).
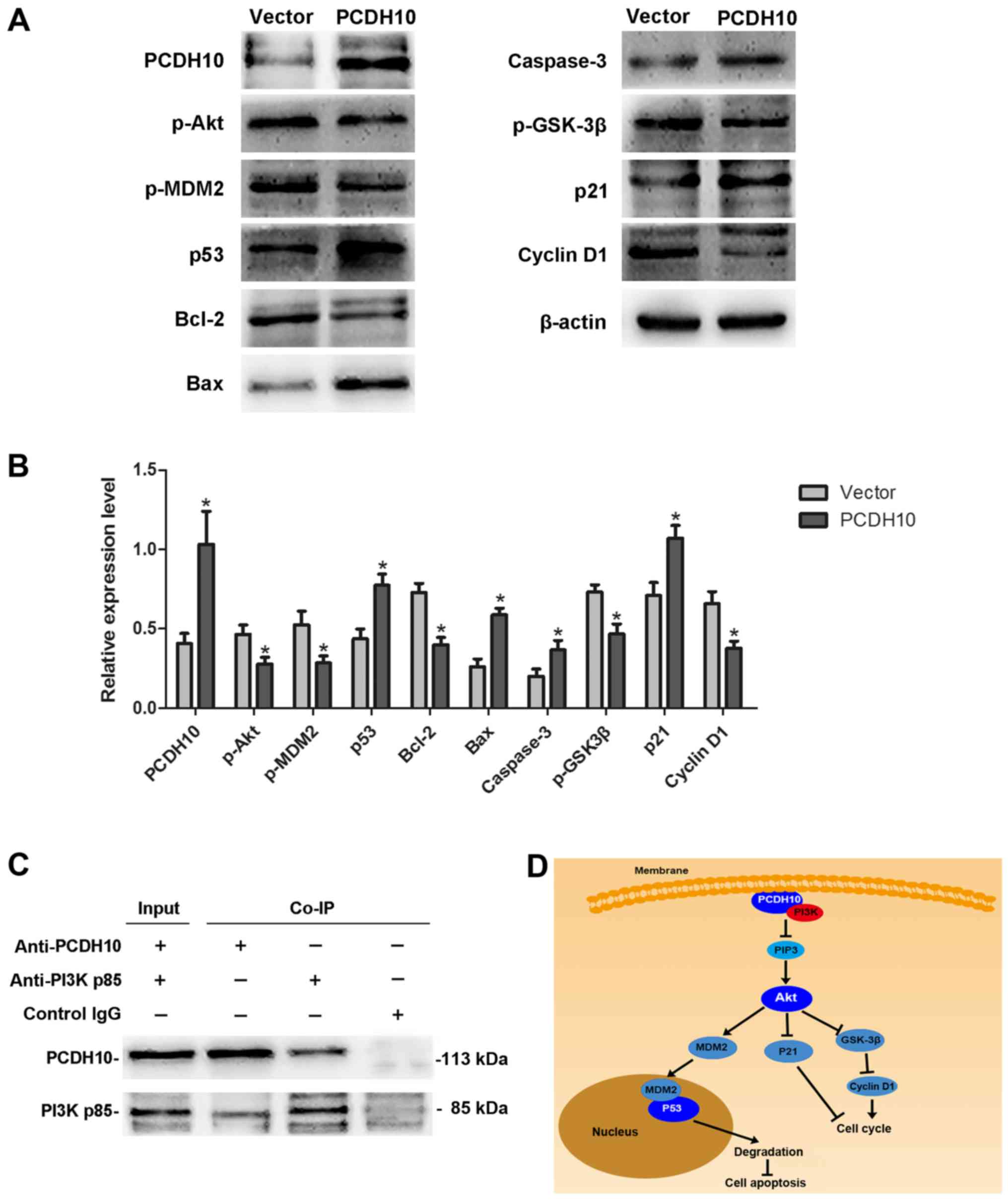
PCDH10 alters the PI3K/Akt signaling
pathway in HCC cells
Dysregulation of the PI3K/Akt signaling pathway is
implicated in a number of human diseases, including cancer. The
PI3K/Akt pathway can promote cell proliferation and inhibit cell
apoptosis via a series of complicated phosphorylation reactions. In
this study, western blot analyses were performed to detect changes
in the PI3K/Akt signaling pathway after transfecting PCDH10 into
HepG2 HCC cells. We demonstrated that the protein levels of p-Akt,
p-MDM2, p-GSK-3β, Bcl-2, and cyclin D1 were decreased, while
caspase-3, p53, p21, and Bax were increased, in cells transfected
with PCDH10 as compared to the control groups (P<0.05; Fig. 7A and B). Our results suggested that
the Akt signaling pathway was impaired. To explore how PCDH10
regulates the pathway, we hypothesized that PCDH10 could affect the
proteins upstream of Akt. Fortunately, the Co-IP assay demonstrated
interaction between PCDH10 and PI3K (Fig. 7C). These results indicate that
PCDH10 inhibits cell proliferation and induces cell apoptosis via
suppression of the PI3K/Akt signaling pathway in HCC cells
(Fig. 7D).
Discussion
PCDH10 belongs to the PCDH family, a subfamily of
the cadherin superfamily. This family member contains 6
extracellular cadherin domains, a transmembrane domain, and a
cytoplasmic tail differing from those of the classical cadherins.
The encoded protein of PCDH10 is a cadherin-related neuronal
receptor thought to function in the establishment of specific
cell-cell connections in the brain. Although PCDH10 is mainly
expressed in the brain, it can be found in other organs, and a
multitude of epigenetic studies have found that the PCDH10 promoter
has high DNA methylation, which results in silencing of gene
expression in many tumors. The methylation status of PCDH10 could
predict the prognosis of cancers (21–23).
Further studies have demonstrated that re-expression of PCDH10
inhibited cellular proliferation, invasion ability, and increased
cell apoptosis in multiple cancers (19,22,24).
These studies demonstrated that PCDH10 plays an important role in
tumorigenesis and tumor development. Previous studies demonstrated
that the expression of PCDH10 was notably downregulated in HCC
tissues and cells when compared to normal liver tissue, and
decreased PCDH10 expression in HCC was correlated with the
methylation status of the PCDH10 promoter (20). However, the role of PCDH10 in HCC
has not yet been elucidated. In this study, we restored PCDH10
expression in HCC cells and observed that PCDH10 inhibited cell
growth of HCC. This effect occured through cell cycle arrest at the
G1 phase and induction of apoptosis of HCC cells. When we assessed
the expression levels of the cell cycle-related protein cyclin D1
and the apoptosis-related protein caspase-3, the amount of cyclin
D1 protein was decreased and the amount of caspase-3 protein was
increased. This indicates that PCDH10 inhibits HCC cellular
proliferation via cell cycle arrest and induction of apoptosis.
Combined with results from previous studies, we propose that PCDH10
is a tumor-suppressor gene frequently downregulated with promoter
methylation in HCC, which is consistent with the role of PCDH10 in
other cancers.
PCDHs have been identified as regulators of other
molecules. PCDHs lack a cytoplasmic β-catenin binding site present
in classical cadherins. The cytoplasmic domains of non-clustered
PCDHs are different from each other, and their homology ranges from
low to moderate (7,9). Therefore, non-clustered PCDHs could
act as regulators via interaction with a variety of intracellular
binding partners. It has been reported that PCDH10/OL-PC interacts
with Nck-associated protein 1 (Nap1)/WAVE1 and that the
PCDH10/Nap1/WAVE1 complex affects actin assembly and subsequently
regulates cell migration (25,26).
Recent studies demonstrated that PCDH10 is involved in some
signaling pathways, including the nuclear factor-κB signaling
pathway and the Wnt/β-catenin/BCL-9 signaling pathway (27,28).
These results demonstrate that PCDH10 carries out the suppressive
function via interference with the signaling pathways that are
involved in malignancy.
The Akt signaling pathway has become a major focal
point because of its critical role in the regulation of diverse
cellular functions including metabolism, growth, proliferation,
survival, transcription, and protein synthesis. Dysregulation of
the Akt signaling pathway can result in many diseases, including
cancers (29). It is well known
that the Akt signaling pathway is activated by receptor tyrosine
kinases, integrins, B and T cell receptors, cytokine receptors,
G-protein-coupled receptors, and other stimuli that induce
production of phosphatidylinositol (3,4,5)-trisphosphates
(PIP3) by PI3K, and PIP3 via PDK1 phosphorylating Akt at Thr308
leading to partial activation of Akt. Activated Akt contributes to
cell proliferation via phosphorylation of the CDK inhibitor p21, or
by inhibiting the activity of GSK-3β by phosphorylation, and GSK-3β
can regulate cyclin D1 proteolysis and subcellular localization.
Akt inhibits cell apoptosis through the phosphorylation of the
downstream molecule MDM2. Phosphorylation of MDM2 mediates
ubiquitination and degradation of p53, a well-known molecule with
various biological functions (30,31).
In our study, we demonstrated that PCDH10 inhibited HCC cell
proliferation and increased cell apoptosis, and in view of the
research on PCDH10 in other tumors (19,28),
we put forward the hypothesis that PCDH10 may regulate the Akt
signaling pathway in HCC. We detected the Akt signaling pathway and
found that the protein levels of p-Akt, p-MDM2, Bcl-2 and cyclin D1
were decreased while p53, p-GSK3β, p21, Bax and caspase-3 were
increased in cells transfected with PCDH10 when compared to the
control groups. This evidence indicates that PCDH10 can inhibite
the Akt signaling pathway in HCC.
To illuminate the mechanism by which PCDH10
regulates the Akt signaling pathway, we detected the level of PI3K,
a key upstream molecule of Akt. However, we found that the protein
level of PI3K exhibited no significant change after PCDH10
overexpression (data not shown). The Co-IP assays demonstrated that
there was an interaction between PCDH10 and PI3K. As previously
described, PI3K, a phosphoinositide-kinase, catalyzes the
production of phosphatidylinositol (3,4,5)-triphosphate
(PIP3) which is necessary for the activation of Akt, and this
phosphorylation event is triggered by growth factors and hormones.
We inferred that PCDH10 acts as a competitive substrate binding
with PI3K to obstruct the catalytic reaction of PIP3 synthesis.
Consequently, the activation of Akt is inhibited.
In summary, in the present sutdy, we explored the
function and mechanism of PCDH10 in HCC cells. Our results
indicated that PCDH10 can inhibit cell proliferation and induce
cell apoptosis by negative regulation of the PI3K/Akt signaling
pathway. Combined with the results of previous research, we
conclude that PCDH10 acts as a tumor-suppressor gene in HCC by
inhibiting the PI3K/Akt signaling pathway. This helps us to better
understand the tumorigenesis and progression of HCC. Notably, these
experiments provide novel insights and put forward the hypothesis
that PCDH10 could be a new biomarker for HCC, or could possibly be
used with other molecular markers to increase the specificity and
sensitivity of diagnostic tests for HCC. Finally, restoration of
PCDH10 could be a potentially valuable therapeutic target for HCC
therapy.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Block TM, Mehta AS, Fimmel CJ and Jordan
R: Molecular viral oncology of hepatocellular carcinoma. Oncogene.
22:5093–5107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakano M, Tanaka M, Kuromatsu R, Nagamatsu
H, Tajiri N, Satani M, Niizeki T, Aino H, Okamura S, Iwamoto H, et
al: Kurume Liver Cancer Study Group of Japan: Sorafenib for the
treatment of advanced hepatocellular carcinoma with extrahepatic
metastasis: A prospective multicenter cohort study. Cancer Med.
4:1836–1843. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harimoto N, Shirabe K, Ikegami T,
Yoshizumi T, Maeda T, Kajiyama K, Yamanaka T and Maehara Y:
Postoperative complications are predictive of poor prognosis in
hepatocellular carcinoma. J Surg Res. 199:470–477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nollet F, Kools P and van Roy F:
Phylogenetic analysis of the cadherin superfamily allows
identification of six major subfamilies besides several solitary
members. J Mol Biol. 299:551–572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Halbleib JM and Nelson WJ: Cadherins in
development: Cell adhesion, sorting, and tissue morphogenesis.
Genes Dev. 20:3199–3214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Redies C, Vanhalst K and Roy F:
delta-Protocadherins: Unique structures and functions. Cell Mol
Life Sci. 62:2840–2852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morishita H and Yagi T: Protocadherin
family: Diversity, structure, and function. Curr Opin Cell Biol.
19:584–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu JS, Koujak S, Nagase S, Li CM, Su T,
Wang X, Keniry M, Memeo L, Rojtman A, Mansukhani M, et al: PCDH8,
the human homolog of PAPC, is a candidate tumor suppressor of
breast cancer. Oncogene. 27:4657–4665. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu X, Sui X, Li L, Huang X, Rong R, Su X,
Shi Q, Mo L, Shu X, Kuang Y, et al: Protocadherin 17 acts as a
tumour suppressor inducing tumour cell apoptosis and autophagy, and
is frequently methylated in gastric and colorectal cancers. J
Pathol. 229:62–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin X, Xiang T, Mu J, Mao H, Li L, Huang
X, Li C, Feng Y, Luo X, Wei Y, et al: Protocadherin 17 functions as
a tumor suppressor suppressing Wnt/β-catenin signaling and cell
metastasis and is frequently methylated in breast cancer.
Oncotarget. 7:51720–51732. 2016.PubMed/NCBI
|
|
13
|
Imoto I, Izumi H, Yokoi S, Hosoda H,
Shibata T, Hosoda F, Ohki M, Hirohashi S and Inazawa J: Frequent
silencing of the candidate tumor suppressor PCDH20 by epigenetic
mechanism in non-small-cell lung cancers. Cancer Res. 66:4617–4626.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen T, Long B, Ren G, Xiang T, Li L, Wang
Z, He Y, Zeng Q, Hong S and Hu G: Protocadherin20 acts as a tumor
suppressor gene: Epigenetic inactivation in nasopharyngeal
carcinoma. J Cell Biochem. 116:1766–1775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin
Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, et al:
Identifying autism loci and genes by tracing recent shared
ancestry. Science. 321:218–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Chim JC, Yang M, Ye J, Wong BC and
Qiao L: Role of PCDH10 and its hypermethylation in human gastric
cancer. Biochim Biophys Acta. 1823:298–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong X, Zhu Y, Mao J, Zhang J and Zheng
S: Frequent epigenetic silencing of PCDH10 by methylation in human
colorectal cancer. J Cancer Res Clin Oncol. 139:485–490. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang X, Yin X, Xiang T, Li H, Li F, Chen L
and Ren G: Protocadherin 10 is frequently downregulated by promoter
methylation and functions as a tumor suppressor gene in non-small
cell lung cancer. Cancer Biomark. 12:11–19. 2012.2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu C, Bu X and Jiang Z: Protocadherin-10
acts as a tumor suppressor gene, and is frequently downregulated by
promoter methylation in pancreatic cancer cells. Oncol Rep.
36:383–389. 2016.PubMed/NCBI
|
|
20
|
Fang S, Huang SF, Cao J, Wen YA, Zhang LP
and Ren GS: Silencing of PCDH10 in hepatocellular carcinoma via de
novo DNA methylation independent of HBV infection or HBX
expression. Clin Exp Med. 13:127–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng QK, Lei YG, Lin YL, Ma JG and Li WP:
Prognostic value of protocadherin10 (PCDH10) methylation in serum
of prostate cancer patients. Med Sci Monit. 22:516–521. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu J, Cheng YY, Tao Q, Cheung KF, Lam CN,
Geng H, Tian LW, Wong YP, Tong JH, Ying JM, et al: Methylation of
protocadherin 10, a novel tumor suppressor, is associated with poor
prognosis in patients with gastric cancer. Gastroenterology.
136:640–651.e1. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li M, Yan DG and Liu JL: Methylation
status of PCDH10 and RASSF1A gene promoters in colorectal cancer.
Zhonghua Yi Xue Za Zhi. 96:456–459. 2016.(In Chinese). PubMed/NCBI
|
|
24
|
Jao TM, Tsai MH, Lio HY, Weng WT, Chen CC,
Tzeng ST, Chang CY, Lai YC, Yen SJ, Yu SL, et al: Protocadherin 10
suppresses tumorigenesis and metastasis in colorectal cancer and
its genetic loss predicts adverse prognosis. Int J Cancer.
135:2593–2603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakao S, Platek A, Hirano S and Takeichi
M: Contact-dependent promotion of cell migration by the
OL-protocadherin-Nap1 interaction. J Cell Biol. 182:395–410. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grove EA: Turning neurons into a nervous
system. Development. 135:2203–2206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, Yang Z, Peng X, Li Y, Liu Q and Chen
J: Nuclear factor-κB is involved in the protocadherin-10-mediated
pro-apoptotic effect in multiple myeloma. Mol Med Rep. 10:832–838.
2014.PubMed/NCBI
|
|
28
|
Xu Y, Yang Z, Yuan H, Li Z, Li Y, Liu Q
and Chen J: PCDH10 inhibits cell proliferation of multiple myeloma
via the negative regulation of the Wnt/β-catenin/BCL-9 signaling
pathway. Oncol Rep. 34:747–754. 2015.PubMed/NCBI
|
|
29
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bozulic L and Hemmings BA: PIKKing on PKB:
Regulation of PKB activity by phosphorylation. Curr Opin Cell Biol.
21:256–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|