Introduction
Conventional cancer treatments include chemotherapy,
surgery and radiation. They are not very effective in controlling
cancers and bring huge suffering to the patients. Novel therapies
need to be developed for cancer treatment (1,2).
Most tumor cells express specific antigens that are
not found on normal cells. Those so-called tumor-associated
antigens allow tumor cells to be recognized and destroyed by the
immune system (3). Triggering
antitumor immunity by specific vaccination is a safe and effective
way to control tumor growth. Comparing with conventional
vaccinations such as whole tumor cells, proteins or derived
peptides, DNA vaccination is a relatively new method (4). DNA vaccination can generate both
humoral and cellular immune responses. Cytotoxic T lymphocyte (CTL)
response is regarded critical for tumor cell killing. Furthermore,
plasmid DNA is relatively easy to be manipulated to encode desired
tumor associated antigens and can be manufactured in large scale
without stringent condition requirements compared with protein
vaccines, which provides a more practical approach for vaccine
development (5).
Although plasmid DNA vaccines are safe and easy to
prepare, they are poorly immunogenic molecules. Thus, in order to
augment immune responses, a variety of adjuvants have been utilized
(6). CpG oligodeoxynucleotides (CpG
ODN) are small DNA molecules mimicking the unmethylated CpG motifs
which frequently present in bacterial DNA. In mammals, these
specific DNA motifs bind and activate toll-like receptor 9 (TLR9),
leading to activation, maturation, and proliferation of immune
cells. TLR9 is localized in endoplasmatic reticulum, late endosomal
and lysosomal compartments of the intracellular milieu. Thus,
internalization of pathogen-derived DNA is required for TLR9
triggering, an outcome that results from either intracellular
infection or uptake of bacterial/viral particles by immune cells
(7). Once stimulated, TLR9
initiates a response biased towards proinflammatory/Th1 immunity
(8). Extensive animal experiments
showed that CpG ODN could support the induction of Ag-specific
immunity against co-administered peptides and vaccines (9). The early phase I trials showed that
CpG ODN was safe and could improve the immunogenicity of
co-administered vaccines (10). To
increase their DNase resistance, CpG-ODN can be synthesized with a
phosphorothioate backbone (11–13).
Folate receptor α (FRα) is a 38 kDa
glycosylphosphatidylinositol (GPI)-anchored glycoprotein. It binds
folic acid and 5-methyltetrahydrofolate (5-MTHF) with high affinity
(14). FRα expression in normal
tissues is highly restricted and inaccessible to the normal
circulation. High expressions of FRα have been described in some
cancers, such as non-mucinous ovarian, endometrial, non-small cell
lung carcinomas and to a lesser extent in clear cell renal,
colorectal and breast cancers. Moreover, FRα expression has been
observed in nearly 90% of non-mucinous ovarian cancer and
correlated with tumor grade, stage, and aggressiveness.
Furthermore, FRα expression remains unchanged in epithelial ovarian
and endometrial cancer after chemotherapy. Based on its highly
tumor restricted expression profile, FRα represents an attractive
candidate for cancer diagnostics and therapeutics (15–19).
Several FR-targeted agents are currently in development,
representing a promising approach for relevant cancer treatments
(20–22).
In this study, we assembled a cytomegalovirus
promoter expression vector containing human FRα cDNA, and we
evaluated its ability to induce an immune response in mice. We
detected both FRα-specific antibodies and cytotoxic T lymphocyte
responses, which significantly reduced the ‘in vivo’ growth
of FRα expressing tumor cells. In addition, the adjuvant effect of
CpG ODN was confirmed.
Materials and methods
Reagents, cell lines and animals
CpG ODN was custom-synthesized by Sangon Biotech
(Shanghai, China). The sequence of stimulatory phosphorothioate CpG
ODN was: 5′-TCCATGACGTTCCTGACGTT-3′. Recombinant human folate
receptor α protein, rabbit polyclonal anti-FRα antibody (antigen
affinity purified), HRP conjugated goat anti-mouse IgG secondary
antibody and HRP conjugated goat anti-rabbit IgG secondary antibody
were purchased from Sino Biological Inc. (Beijing, China). G418
sulfate and plasmid purification kits were from Sangon Biotech and
Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA,
USA). Lactate dehydrogenase (LDH) kits were purchased from
Jiancheng Bioengineering Institute (Nanjing, China).
The ovarian cancer cell line SKOV3 and metastatic
melanoma B16 cell line were from Shanghai Cell Biology Institutes
(Academia Sinica, Shanghai, China) and were maintained in
Dulbecco's modified Eagle's medium (Gibco, Carlsbad, CA, USA) with
10% fetal bovine serum and antibiotics.
Female C57BL/6 (6 weeks, 18–20 g) were purchased
from the Yangzhou University Animal Center and used under the
experimental animal production license 2121922. All animals were
housed in a controlled environment (25°C; 12 h light-dark cycle),
with water and food provided freely. The authors confirm that
experiments involving animals adhered to the ethical standards of
China Pharmaceutical University and the care of animals was in
accordance with the licensing guidelines of China Pharmaceutical
University.
DNA vaccine construction
Total RNA was isolated from human ovarian cancer
SKOV3 cells. The DNA fragment encoding FRα was amplified using
RT-PCR. Reverse transcription was performed at 42°C using oligo
d(T)15 as a primer and PCR amplification was carried out
for 30 cycles (1 min at 94°C, 1 min at 55°C, 1 min at 72°C) using
the following primers specific for FRα gene amplification:
CAGTAAGCTTGC CATGGCTCAGCGGATGA (HindIII); CCGGAATTCTCA
GCTGAGCAGCCACAGC (EcoRI). The gene was cloned into the
eukaryotic vector pcDNA3.1 and the constructed recombinant plasmid
was identified by restriction endonuclease digestion and DNA
sequencing.
Expression of recombinant plasmid
encoding FRα
B16 cells were transfected with a recombinant
plasmid pcDNA3.1/FRα or a control plasmid pcDNA3.1 using
Lipofectamine 2000 according to the manufacturer's instructions.
After incubation for 72 h, the cells were harvested and tested for
FRα expression by RT-PCR, western blotting and immunofluorescence.
For RT-PCR, the total RNA was isolated and reverse transcribed into
cDNA. FRα gene was amplified using previously described primers and
analyzed by electrophoresis. For western blotting, collected cell
lysates were resolved by polyacrylamide gel electrophoresis and the
protein bands were transferred onto a membrane. The membrane was
blocked with 5% nonfat dry milk and FRα was detected with rabbit
polyclonal anti-FRα antibodies (1:5000) followed by HRP conjugated
second antibodies (1:5000). The protein band was visualized with an
enhanced ECL chemiluminescent reagent using a Bio-Rad detection
system. For cell immunofluorescence staining, the cells were fixed
with 4% polyoxymethylene for 20 min. After washing with PBS, the
cells were treated with Triton X-100 for 10 min and blocked with 5%
BSA for 1 h. Then the cells were incubated with rabbit polyclonal
anti-FRα antibodies (1:100) at 4°C overnight. After being washed
with PBS, the cells were incubated with FITC-conjugated goat
anti-rabbit IgG secondary antibodies (1:100) for 2 h and visualized
with a fluorescent microscope (Olympus) and photographed.
Plasmid DNA preparation
Plasmid DNA was propagated in E. coli and was
isolated using endonuclease-free plasmid purification kits
according to the supplier's protocol. The purified plasmids were
dissolved in sterile PBS and used for injection or stored at −80°C
until use.
Preparation of FRα-expressing tumor
cell lines
B16 cells were transfected with pcDNA3.1/FRα using
Lipofectamine 2000 as described by the manufacturer. After
incubation with DNA-lipid complex for 24 h, cells were cultured in
fresh growth medium (RPMI-1640 containing 10% fetal bovine serum)
with 1000 µg/ml antibiotics G418 for 2 weeks. A G418 dose-response
curve was established prior to the selection of the cells. The
resistant cells were obtained and serially diluted. Single cells
were picked and cultured in presence of G418 for another two weeks
to obtain cells that stably express FRα.
Immunization
Wild-type female C57BL/6 mice (4–6 weeks old) were
randomly divided into 4 groups with 6 mice in each group.
Preliminary experiments were performed to compare the stimulating
effect of CpG ODN at different dosages and it was found that 10 µg
CpG ODN was a proper dosage (data not shown). In the following
immunization, we used 10 µg CpG ODN per mice. Mice receiving a
blank vector or 10 µg CpG ODN served as the control groups. In the
third group, mice were administered with 100 µg recombinant plasmid
pcDNA3.1/FR. In the fourth group mice were injected with 100 µg
pcDNA3.1/FR Plus 10 µg CpG ODN. All the reagents were injected in
the rectus femoris muscle of both hind legs. Four identical
injections were given at one week intervals.
Antibody detection
One week after the fourth immunization, blood
samples were collected through the canthus and were kept at 4°C for
30 min. Then the blood samples were centrifuged at 1500 × g for 10
min, and the supernatants were taken and stored at −80°C until
detection. Microtiter plates (96-well) were coated with 100 µl of 1
µg/ml recombinant human FRα in 0.05 M sodium bicarbonate (pH 9.6)
and the plate was kept overnight at 4°C. After washing three times,
the plate was blocked with 0.1 M PBS (pH 7.4) containing 10% (V/V)
skim milk at 37°C for 1.5 h. Then, serial dilutions of mouse sera
(diluted in PBS/0.1% BSA/0.05% Tween-20) were added and incubated
for 2 h at 37°C. After washing three times, 100 µl of
HRP-conjugated sheep anti-mouse IgG (1:5000) was added and
incubated for 1 h at 37°C. After washing, tetramethylbenzidine
(TMB) substrate (100 µl/well) was added and incubated for 15 min.
The reactions were stopped with 2M sulfuric acid (50 µl/well). The
absorbance of each well at 450 nm was detected with an automated
ELISA reader.
Cytotoxic T-lymphocyte (CTL)
assays
One week after the last immunization, spleens were
isolated from three sacrificed mice of each immunized group. The
spleens were ground and passed through a 100 µm filter under
sterile conditions. Erythrocytes were lysed using
Tris-NH4Cl (pH 7.2). Splenocytes were washed by PBS and
resuspended in RMPI-1640 containing 10% FBS. Then splenocytes of
each group were cultured in the presence of 10 µg/ml recombinant
human FRα for five days and used as effector cells. The FRα
expressing tumor cells were used as target cells. Cytotoxic
activity was determined using a lactate dehydrogenase kits.
Effector cells were mixed with target cells (5×104
cells) in triplicate with E:T (effector cells : target cells)
ratios of 50:1, 25:1 and 12.5:1. The mixture cells were co-cultured
for 4 h at 37°C in an atmosphere containing 5% CO2. LDH
release under each condition was evaluated according to the
instructions of the manufacturer. Cytotoxicity was calculated using
the following equation: cytotoxicity (%) =
[(ODexperiment - ODeffector spontaneous -
ODtarget spontaneous) / (ODtarget maximum -
ODtarget spontaneous)] ×100%.
Lymphocyte proliferation assay
One week after the last immunization, the
splenocytes were isolated from each immunized group as described
above. Splenocytes (1×105) were cultured in 100 µl
culture medium as blank, or co-cultured with different stimulants
including BSA (100 µg/ml) as non-relevant peptide control,
recombinant human FRα (100 µg/ml) or 100 µg/ml ConA. Cells were
cultured in triplicates in 96-well, flat-bottom plates at 37°C for
72 h in a 5% CO2 incubator. MTT dissolved in PBS was
added to the cultures at a final concentration of 0.5 mg/ml and
incubated at 37°C for 4 h to form formazan crystals, which were
later dissolved in DMSO. The optical density was measured at 540 nm
on a Multimode plate Reader. The results were analyzed as the
stimulate index (SI) defined as ODexperiment /
ODblank / pcDNA3.1.
Evaluation of the protective effect in
C57BL/6 mice
Female C57BL/6 mice (4–6 weeks) were used to
evaluate tumor growth inhibition. Immunization procedure was as
described above. One week after the final immunization (week 5),
the mice were challenged intradermally in the right flank with
2×105 FRα expressing B16 cells. Tumor width and length
were measured with a caliper periodically and tumor volume was
calculated as V = (length × width2) / 2. In the survival
experiment, the animals were kept for 50 days or until death after
tumor challenge.
Evaluation of the therapeutic effect
in C57BL/6 mice
Female C57BL/6 mice (4–6 weeks) were challenged with
FRα expressing B16 cells on day 0. Four times immunizations with
one week intervals were followed as described. Tumor growth was
monitored and tumor volume was calculated. In the survival
experiment, the animals were kept for 50 days or until death after
tumor challenge.
Analysis of FRα protein expression in
tumor tissues
Tumor tissues from experimental mice were collected,
ground and lysed in RAPI buffer. The proteins were extracted and
resolved by SDS-PAGE. Then western blotting was used to detect FRα
expression in tumor tissues.
Statistical analysis
Data were expressed as mean ± SD. A two-tailed
Student's t-test was used to analyze significance among the groups.
A value of P<0.05 was considered statistically significant;
P<0.01 was considered highly statistically significant.
Results
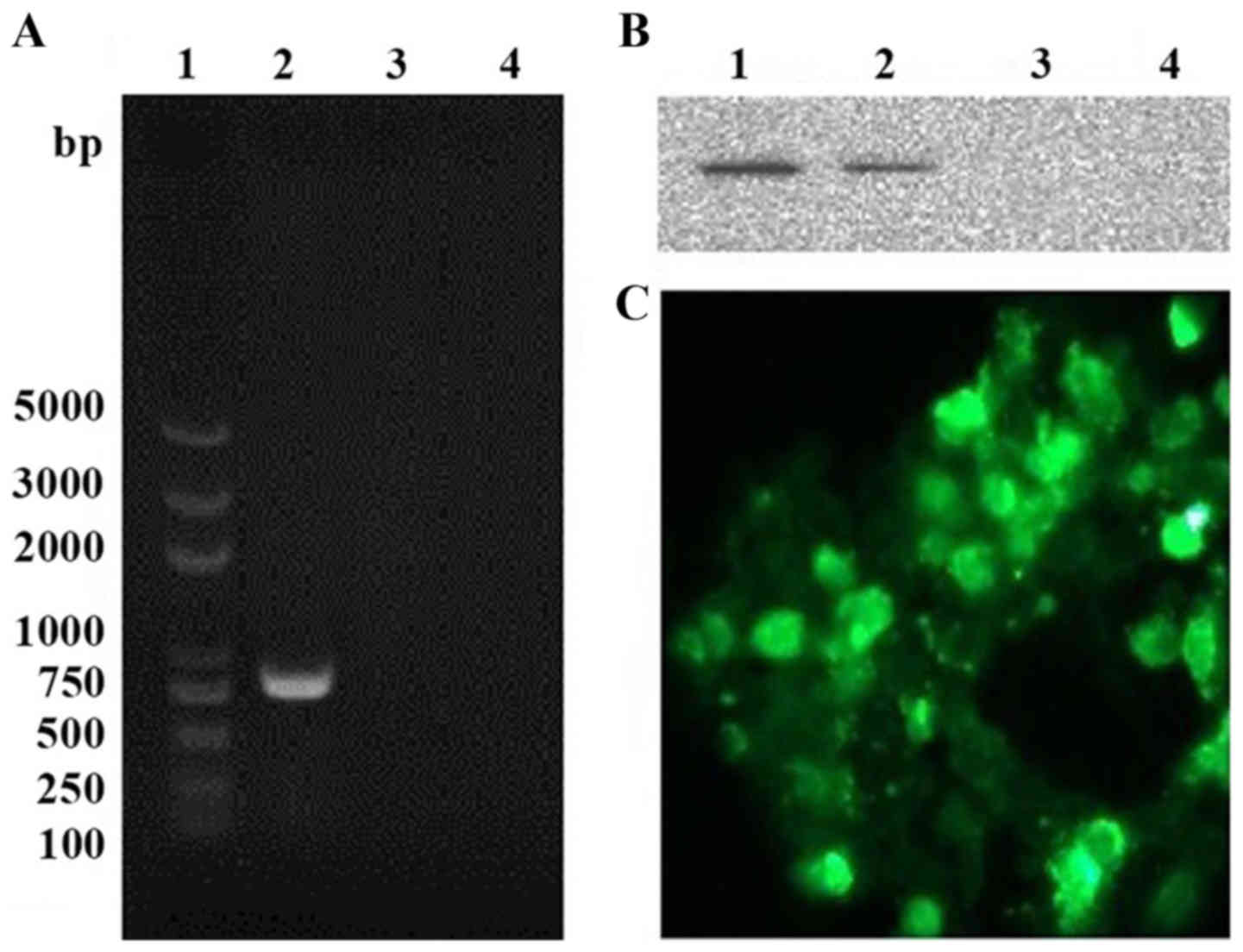
DNA vaccine construction
Human FRα gene was assembled into the pcDNA3.1
expression plasmid under the transcriptional control of a
cytomegalovirus promoter. The resulting plasmid (pcDNA3.1/FRα) was
assessed for its ability to drive protein synthesis by transient
transfection of B16 cells. FRα expression on mRNA level was
confirmed with RT-PCR (Fig. 1A) and
its expression on a protein level was detected with western blot
analysis (Fig. 1B) and
immunofluorescence staining (Fig.
1C). These data indicated that the plasmid was functional and
capable of inducing expression of the encoded antigen.
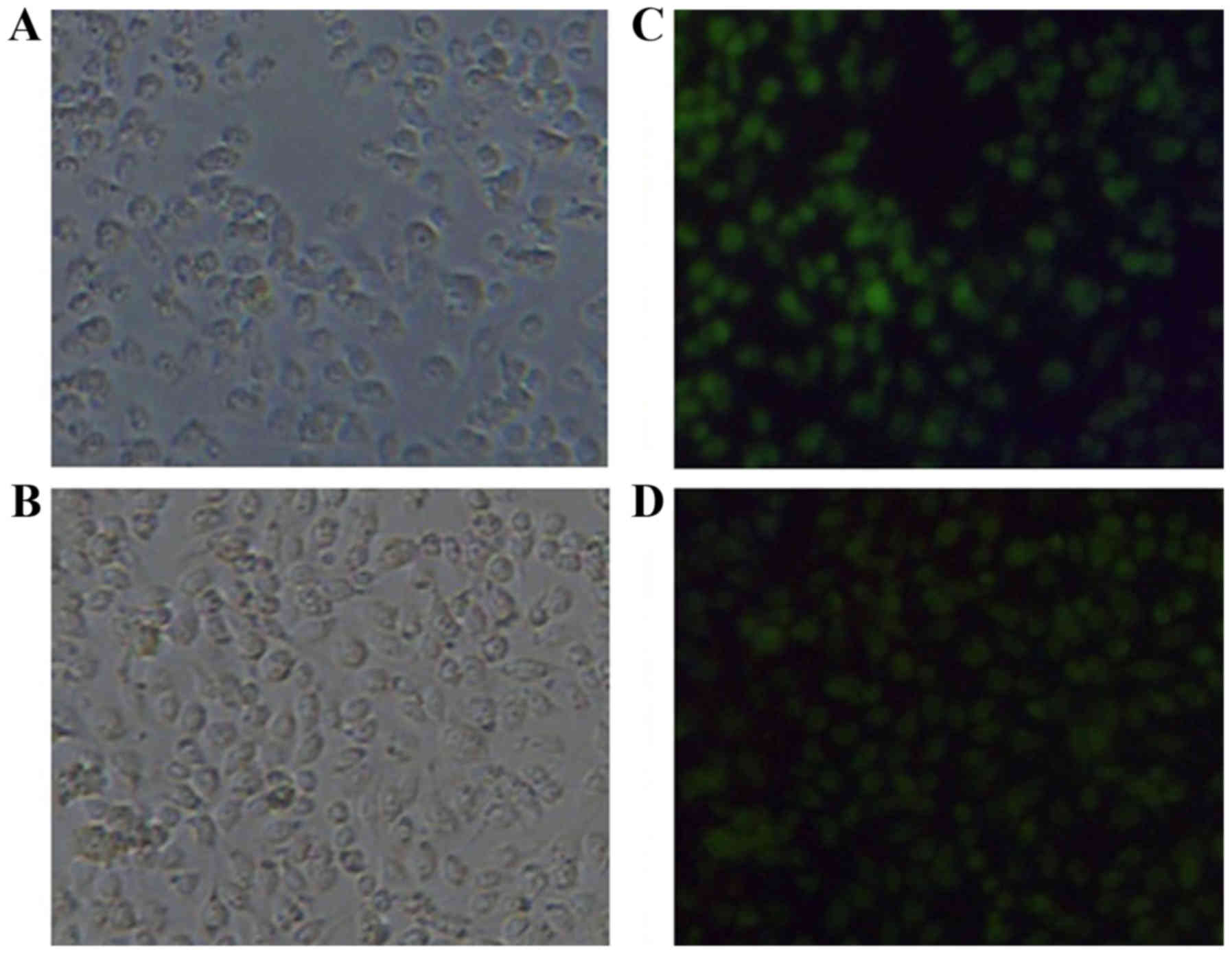
Establishment of FRα expressing tumor
cell lines
Cells were transfected with pcDNA3.1/FRα. FRα
expressing cells were selected with G418 for two weeks. Only the
cells with the foreign gene integrated into their genomes could
proliferate in the presence of G418. By serial dilution, two FRα
expressing B16 cell lines (B62 and C411) were established.
Immunofluorescence staining showed that all the cells present in
the picture expressed FRα (Fig.
2).
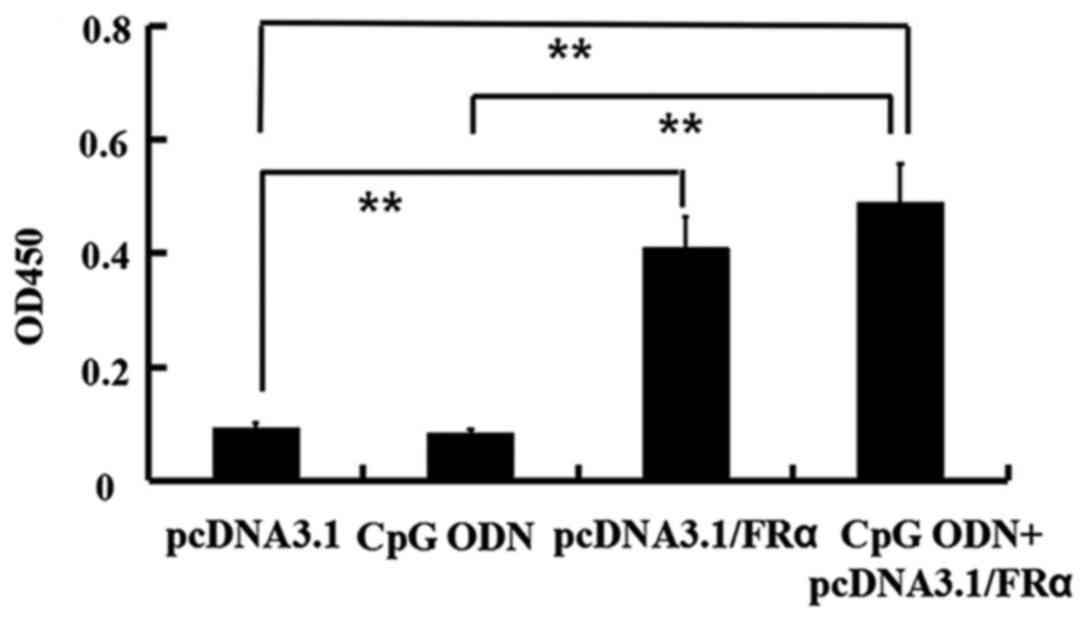
Humoral immunity induced by the DNA
vaccine
To investigate humoral immune response in the mice
vaccinated with pcDNA3.1/FRα, mice were immunized four times at one
week intervals by intramuscular injections. Serum samples were
collected one week after the last immunization and tested by ELISA
for their reactivity with recombinant FRα. As shown in Fig. 3, pcDNA3.1/FRα vaccine elicited
antibodies against FRα, displaying a very significant difference
compared with the pcDNA3.1 group (P=0.00756). The group injected
with pcDNA3.1/FRα in combination with CpG ODN showed a very
significant difference compared with the pcDNA3.1 group (P=0.00726)
and with the CpG ODN group (P=0.00651) whereas there was not a
significant difference compared with the pcDNA3.1/FRα group
(P=0.7119).
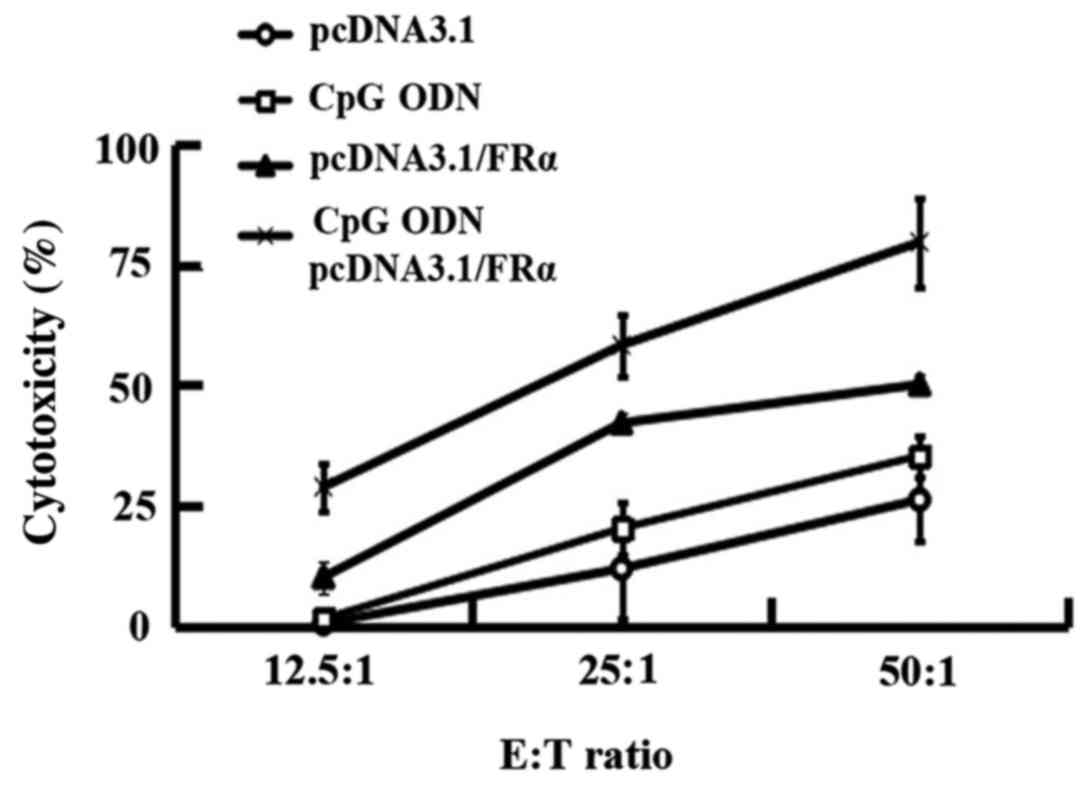
Cytotoxic T-lymphocyte (CTL)
assays
The FRα specific CTL activities of splenocytes from
immunized C57BL/6 mice were assessed with FRα expressing B16 cells
as target cells using a LDH release method. The effect of
pcDNA3.1/FRα plus CpG ODN was tested in comparison with the groups
treated with pcDNA3.1, CpG ODN or pcDNA3.1/FRα (Fig. 4). Four injections of pcDNA3.1/FRα
resulted in a mean specific killing rate of 10% (E:T ratio of
12.5:1), 42% (E:T ratio of 25:1) or 50% (E:T ratio of 50:1). These
killing rates were significantly higher than those of pcDNA3.1
treatment group with P-values of 0.013, 0.012 and 0.016 for the E:T
ratio of 12.5:1, 25:1 and 50:1. The killing rates of combined
immunization group with pcDNA3.1/FRα and CpG ODN were 28% (E:T
ratio of 12.5:1), 58% (E:T ratio of 25:1) or 79% (E:T ratio of
50:1), which were much higher than those of the pcDNA3.1 group
(P<0.001) and the CpG ODN group (P<0.001) at the
corresponding E:T ratio. Furthermore, the killing rates of combined
immunization group (pcDNA3.1/FRα and CpG ODN) were higher than the
pcDNA3.1/FRα immunized group with P-values of 0.011, 0.024 and
0.010 at the E:T ratio of 12.5:1, 25:1 and 50:1.
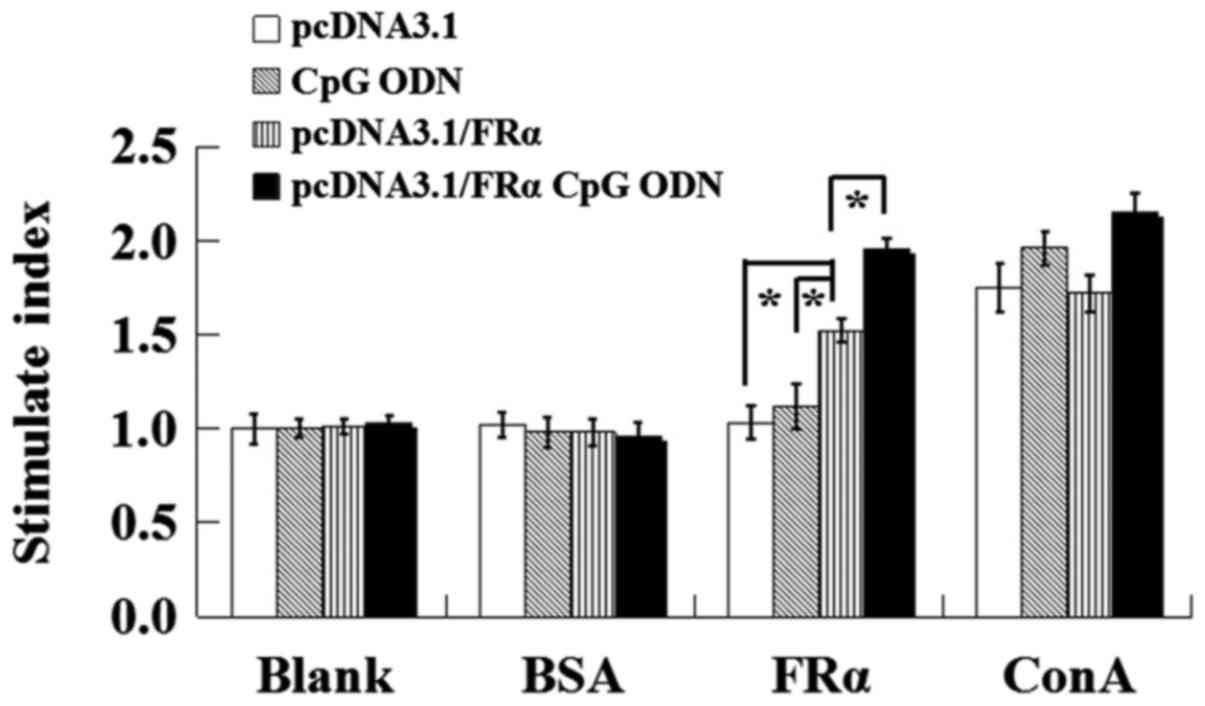
Lymphocyte proliferation assays
By treating the isolated lymphocytes with
recombinant human FRα, the antigen specific lymphocyte
proliferation of immunized C57BL/6 mice was measured using a MTT
method and compared with the non-relevant peptide group and the
mitogen ConA group. As shown in Fig.
5, there was no proliferation in the blank group or the
non-relevant peptide group. With FRα as a stimulant, the stimulate
index of mice immunized with pcDNA3.1/FRα was significantly higher
than that of pcDNA3.1 group (P=0.013) and CpG ODN group (P=0.035).
While CpG ODN can further increase this antigen specific lymphocyte
proliferation comparing with pcDNA3.1/FRα group (P=0.037). As a
mitogen for T cells, ConA stimulated non-specific T cell
proliferation and CpG ODN enhanced the stimulating effect of
ConA.
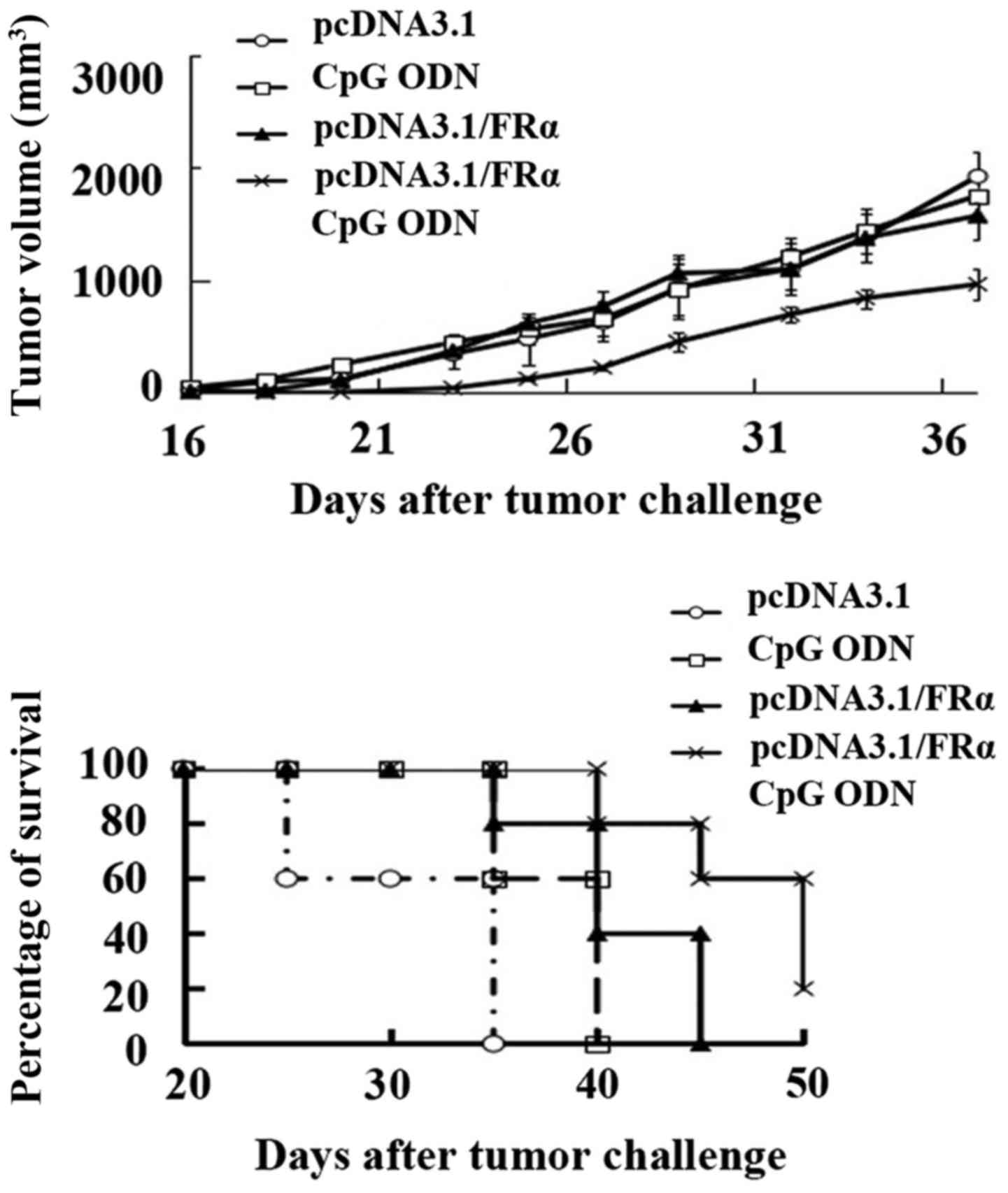
Protective effect of DNA vaccination
in C57BL/6 mice
One week after the final immunization (week 5), the
female C57BL/6 mice (4–6 weeks, 10 mice per group) were challenged
subcutaneously with 2×105 FRα expressing B16 cells.
Tumor growth was monitored after tumor challenge. As shown in
Fig. 6, compared with the empty
vector pcDNA3.1 control group, the mice immunized with CpG ODN did
not show statistically reduced tumor growth whereas the
pcDNA3.1/FRα immunized mice showed significantly reduced tumor
growth (P=0.017). The mice injected with pcDNA3.1/FRα plus CpG ODN
had reduced tumor growth with a very significant difference
compared with the pcDNA3.1 control group (P=0.000281), the CpG ODN
group (P=0.001) and the pcDNA3.1/FRα group (P=0.00579).
Furthermore the mice in pcDNA3.1 control group and
CpG ODN group all died before day 38, whereas the mice immunized
with pcDNA3.1/FRα died before day 45, showing a significant
protective effect (P=0.0344). Mice immunized with pcDNA3.1/FRα plus
CpG ODN (20%) still survived at day 50, showing a very significant
difference compared with pcDNA3.1 control group (P=0.00569) and CpG
ODN group (P=0.00453), and showing a significant difference
compared with pcDNA3.1/FRα group (P=0.046).
Therapeutic effect of DNA vaccination
in C57BL/6 mice
To evaluate the therapeutic effect of this DNA
vaccine on an existing tumor, mice (10 per group) were inoculated
with FRα expressing B16 cells on day 0, and the mice were immunized
with different reagents four times at one week intervals. Tumor
growth was monitored daily after tumor inoculation. Fig. 7 shows that mice immunized with CpG
ODN did not show reduced tumor growth compared with the empty
vector pcDNA3.1 control group. The mice receiving pcDNA3.1/FRα did
not show significantly reduced tumor growth (P=0.314) compared with
the control group. The group injected with pcDNA3.1/FRα in
combination with CpG ODN showed reduced tumor growth with a very
significant difference compared with the pcDNA3.1 control group
(P=0.000337) with the CpG ODN group (P=0.00579), and a significant
difference compared with the pcDNA3.1/FRα group (P=0.0251).
The therapeutic experiment was followed up with
survival as the end point. The mice in the pcDNA3.1 control group
all died before day 34 and all the mice in the CpG ODN group died
before day 38. The mice immunized with pcDNA3.1/FRα died before day
42, without a statistically significant prolonged survival compared
with pcDNA3.1 control group (P=0.13). For the mice immunized with
pcDNA3.1/FRα plus CpG ODN, 20% of the mice survived until day 50,
showing a significant difference compared with the pcDNA3.1 control
group (P=0.028), the CpG ODN group (P=0.031), and the pcDNA3.1/FRα
group (P=0.0265).
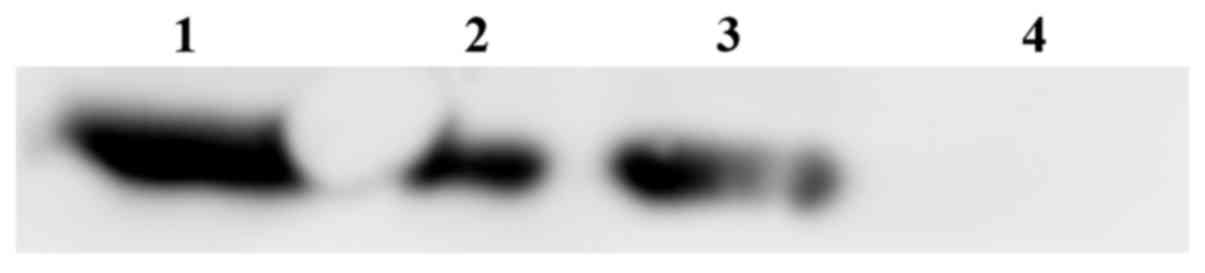
Analysis of FRα protein expression in
tumor tissues
After DNA vaccine treatment, tumors of mice from
different treatment groups were obtained and western blotting was
used to detect FRα expression in tumors. Fig. 8 shows that FRα expression was
maintained in all the tested tumors.
Discussion
FRα is a tumor associated antigen. Because of its
high expression in tumor cells and very limited expression in
normal tissues, it is regarded as a promising target for cancer
therapy (6).
Though DNA vaccination is an easy method based on
its preparation, storage and safety compared with protein and
peptide vaccines, its immunogenicity is usually low (11). Adjuvant is often needed to enhance
its efficacy. Herein, we chose CpG ODN. It is known that CpG
oligonucleotides are excellent adjuvants in murine models. When
used in combination with peptide vaccines, it was as potent as the
complete Freund's adjuvant regarding the induction of B cell and T
cell responses. Furthermore, it is less toxic and it induces a T
helper 1 (Th1) response (23).
Mineral oil used with Freund's adjuvant kept a sustained release of
antigen and at the same time made a local antigen depot (by
entrapment of antigen in the mineral oil emulsion) where primed
CD8+ T cells may accumulate instead of tumor targeting
(24). Alum, the adjuvant that is
used routinely in human vaccination, induces the less favorable Th2
response (25).
In this study, we constructed a recombinant plasmid
encoding FRα as a DNA vaccine and detected its protective and
therapeutic effect in mice models when it was used alone or in
combination with CpG ODN. The DNA vaccine by itself or coinjected
with CpG ODN both elicited humoral and cellular immune responses.
As we expected, CpG ODN as an adjuvant enhanced both humoral and
cellular immune reactivity. From the data of the humoral
reactivity, although there was not a statistically significant
increase in antibody titer after CpG ODN inclusion, we observed
that serum from mice injected with pcDNA3.1/FRα plus CpG ODN always
had a higher ELISA value compared with the pcDNA3.1/FRα group (data
not shown). The pcDNA3.1/FRα and pcDNA3.1/FRα plus CpG ODN vaccines
both elicited FRα specific CTL response (Fig. 4) and lymphocyte proliferation
(Fig. 5). CTL is a typical
CD8+ T cell reaction and antigen specific lymphocyte
proliferation is a hallmark of CD4+ cell immunity
together with antigen presenting cells. Normally, CD4+
and CD8+ T cells perform their immune functions not in a
parallel manner, but together with B cells and other immune cells,
they form an immunological network. After activation by DC cells,
FRα specific CD4+ T cells helped FRα specific B cells to
activate and become plasma cells to produce FRα specific
antibodies. The activated CD4+ T cells also helped FRα
specific CD8+ T cells to be activated to elicit their
CTL function. Furthermore, the CD4+ T cells might also
secret cytokines to activate macrophages or other immune cells. All
the above may contribute to the antitumor effect of the DNA
vaccine.
In the mouse protective model, the DNA vaccine
(pcDNA3.1/FRα) showed a significant protecting effect against FRα
expressing tumor in tumor growth and animal survival (Fig. 6). Whereas pcDNA3.1/FRα plus CpG ODN
displayed a more potent effect than DNA vaccine alone,
demonstrating the stimulating effect of CpG ODN on the immune
system.
In the therapeutic model (Fig. 7), although vaccination by
pcDNA3.1/FRα alone did not show significant effects on tumor growth
and animal survival, pcDNA3.1/FRα with CpG ODN did show a
significant therapeutic effect. This demonstrated a slow immune
reactivity that DNA vaccine can elicit. It took some time for the
FRα specific immunity to set up in mice. CpG ODN is an excellent
adjuvant in mice and stimulation via TLR9 results in the rapid
activation of the innate immune system that in turn supports the
induction of an adaptive immune response. CpG ODN accelerated the
induction of protective antibodies and generated higher and more
persistent antibody titers with protein vaccines (26). Peptide based vaccines by themselves
generally failed to elicit strong immune responses (27–30).
In an early phase I trial that focused on CpG ODN as an adjuvant,
10-fold more antigen specific T cells were generated by patients
with malignant melanoma immunized with the vaccine containing CpG
versus the same vaccine lacking CpG (31). It was reported that recipients of
the CpG ODN adjuvant vaccine developed Ag-specific CD8 T cells
earlier and with significant higher frequency than the non-CpG
group and the antitumor immunity arose more rapidly in patients
vaccinated with CpG ODN (32). This
is in line with our result. Actually, in immunotherapy of tumors,
the situation of therapeutic group is closer to clinical practice.
Our result confirmed that combined treatment of DNA vaccine and CpG
ODN had potential in growth inhibition of FR-expressing tumors.
The B16 cell clones selected were stably transfected
with pcDNA3.1/FRα. It was reported that FRα-transduced C26 cells
gradually lost FRα expression and the remaining tumor cells without
FRα expression were not attacked by FRα specific immune reactivity
(33). Therefore, the tumor growth
showed similar growth kinetics with the control group at a later
stage. In the present protective and therapeutic animal
experiments, mice treated with pcDNA3.1/FRα plus CpG ODN had a
tumor which grew slower than that of the pcDNA3.1 or CpG ODN
treatment group all through the experiment (37 days). Their growth
rate neither speeded up nor showed a similar kinetics with the
control group (Figs. 6 and 7). This indicated that the FRα expressing
tumor cells did not lose their expression of FRα (Fig. 8), which is important in testing the
effect of antitumor agents targeting FRα. Our results also
correlate with the clinical research that FRα expression remains
unchanged in different cancer after chemotherapy (19).
These work confirmed that CpG ODN was an excellent
adjuvant even when administered in solution together with the DNA
vaccine. It also confirmed that FRα represents an attractive
candidate for cancer immunotherapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301902) and
Priority Academic Program Development of Jiangsu Higher Education
Institutions (PAPD).
Glossary
Abbreviations
Abbreviations:
|
FRα
|
folate receptor α
|
|
CpG ODN
|
CpG oligodeoxynucleotides
|
|
CTL
|
cytotoxic T lymphocyte
|
|
TLR9
|
toll-like receptor 9
|
|
GPI
|
glycosylphosphatidylinositol
|
|
5-MTHF
|
5-methyltetrahydrofolate
|
|
i.m.
|
intramuscular
|
|
LDH
|
lactate dehydrogenase
|
|
E:T ratio
|
effector cells : target cells
ratio
|
References
|
1
|
Delany I, Rappuoli R and De Gregorio E:
Vaccines for the 21st century. EMBO Mol Med. 6:708–720.
2014.PubMed/NCBI
|
|
2
|
Vanneman M and Dranoff G: Combining
immunotherapy and targeted therapies in cancer treatment. Nat Rev
Cancer. 12:237–251. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Houghton AN: Cancer antigens: Immune
recognition of self and altered self. J Exp Med. 180:1–4. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolff JA, Malone RW, Williams P, Chong W,
Acsadi G, Jani A and Felgner PL: Direct gene transfer into mouse
muscle in vivo. Science. 247:1465–1468. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fioretti D, Iurescia S, Fazio VM and
Rinaldi M: DNA vaccines: Developing new strategies against cancer.
J Biomed Biotechnol. 2010:1743782010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li L, Saade F and Petrovsky N: The future
of human DNA vaccines. J Biotechnol. 162:171–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Latz E, Schoenemeyer A, Visintin A,
Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T
and Golenbock DT: TLR9 signals after translocating from the ER to
CpG DNA in the lysosome. Nat Immunol. 5:190–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krieg AM: Therapeutic potential of
Toll-like receptor 9 activation. Nat Rev Drug Discov. 5:471–484.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klinman DM, Currie D, Lee G, Grippe V and
Merkel T: Systemic but not mucosal immunity induced by AVA prevents
inhalational anthrax. Microbes Infect. 9:1478–1483. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohno S, Okuyama R, Aruga A, Sugiyama H and
Yamamoto M: Phase I trial of Wilms' Tumor 1 (WT1) peptide vaccine
with GM-CSF or CpG in patients with solid malignancy. Anticancer
Res. 32:2263–2269. 2012.PubMed/NCBI
|
|
11
|
Mutwiri GK, Nichani AK, Babiuk S and
Babiuk LA: Strategies for enhancing the immunostimulatory effects
of CpG oligodeoxynucleotides. J Control Release. 97:1–17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vollmer J and Krieg AM: Immunotherapeutic
applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug
Deliv Rev. 61:195–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halperin SA, van Nest G, Smith B, Abtahi
S, Whiley H and Eiden JJ: A phase I study of the safety and
immunogenicity of recombinant hepatitis B surface antigen
co-administered with an immunostimulatory phosphorothioate
oligonucleotide adjuvant. Vaccine. 21:2461–2467. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elnakat H and Ratnam M: Role of folate
receptor genes in reproduction and related cancers. Front Biosci.
11:506–519. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parker N, Turk MJ, Westrick E, Lewis JD,
Low PS and Leamon CP: Folate receptor expression in carcinomas and
normal tissues determined by a quantitative radioligand binding
assay. Anal Biochem. 338:284–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basal E, Eghbali-Fatourechi GZ, Kalli KR,
Hartmann LC, Goodman KM, Goode EL, Kamen BA, Low PS and Knutson KL:
Functional folate receptor alpha is elevated in the blood of
ovarian cancer patients. PLoS One. 4:e62922009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kalli KR, Oberg AL, Keeney GL,
Christianson TJ, Low PS, Knutson KL and Hartmann LC: Folate
receptor alpha as a tumor target in epithelial ovarian cancer.
Gynecol Oncol. 108:619–626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toffoli G, Cernigoi C, Russo A, Gallo A,
Bagnoli M and Boiocchi M: Overexpression of folate binding protein
in ovarian cancers. Int J Cancer. 74:193–198. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Despierre E, Lambrechts S, Leunen K,
Berteloot P, Neven P, Amant F, O'Shannessy DJ, Somers EB and
Vergote I: Folate receptor alpha (FRA) expression remains unchanged
in epithelial ovarian and endometrial cancer after chemotherapy.
Gynecol Oncol. 130:192–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ebel W, Routhier EL, Foley B, Jacob S,
McDonough JM, Patel RK, Turchin HA, Chao Q, Kline JB, Old LJ, et
al: Preclinical evaluation of MORAb-003, a humanized monoclonal
antibody antagonizing folate receptor-alpha. Cancer Immun. 7:6–13.
2007.PubMed/NCBI
|
|
21
|
Armstrong DK, White AJ, Weil SC, Phillips
M and Coleman RL: Farletuzumab (a monoclonal antibody against
folate receptor alpha) in relapsed platinum-sensitive ovarian
cancer. Gynecol Oncol. 129:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walters CL, Arend RC, Armstrong DK,
Naumann RW and Alvarez RD: Folate and folate receptor alpha
antagonists mechanism of action in ovarian cancer. Gynecol Oncol.
131:493–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chu RS, Targoni OS, Krieg AM, Lehmann PV
and Harding CV: CpG oligodeoxynucleotides act as adjuvants that
switch on T helper 1 (Th1) immunity. J Exp Med. 186:1623–1631.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hailemichael Y and Overwijk WW: Cancer
vaccines: Trafficking of tumor-specific T cells to tumor after
therapeutic vaccination. Int J Biochem Cell Biol. 53:46–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lindblad EB: Aluminium compounds for use
in vaccines. Immunol Cell Biol. 82:497–505. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cooper CL, Davis HL, Angel JB, Morris ML,
Elfer SM, Seguin I, Krieg AM and Cameron DW: CPG 7909 adjuvant
improves hepatitis B virus vaccine seroprotection in
antiretroviral-treated HIV-infected adults. AIDS. 19:1473–1479.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perales MA, Yuan J, Powel S, Gallardo HF,
Rasalan TS, Gonzalez C, Manukian G, Wang J, Zhang Y, Chapman PB, et
al: Phase I/II study of GM-CSF DNA as an adjuvant for a
multipeptide cancer vaccine in patients with advanced melanoma. Mol
Ther. 16:2022–2029. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krug LM, Dao T, Brown AB, Maslak P, Travis
W, Bekele S, Korontsvit T, Zakhaleva V, Wolchok J, Yuan J, et al:
WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses
in patients with mesothelioma and non-small cell lung cancer.
Cancer Immunol Immunother. 59:1467–1479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barve M, Bender J, Senzer N, Cunningham C,
Greco FA, McCune D, Steis R, Khong H, Richards D, Stephenson J, et
al: Induction of immune responses and clinical efficacy in a phase
II trial of IDM-2101, a 10-epitope cytotoxic T-lymphocyte vaccine,
in metastatic non-small-cell lung cancer. J Clin Oncol.
26:4418–4425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vansteenkiste J, Zielinski M, Linder A,
Dahabreh J, Gonzalez EE, Malinowski W, Lopez-Brea M, Vanakesa T,
Jassem J, Kalofonos H, et al: Adjuvant MAGE-A3 immunotherapy in
resected non-small-cell lung cancer: Phase II randomized study
results. J Clin Oncol. 31:2396–2403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baumgaertner P, Jandus C, Rivals JP, Derré
L, Lövgren T, Baitsch L, Guillaume P, Luescher IF, Berthod G,
Matter M, et al: Vaccination-induced functional competence of
circulating human tumor-specific CD8 T-cells. Int J Cancer.
130:2607–2617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Speiser DE, Liénard D, Rufer N,
Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC and
Romero P: Rapid and strong human CD8+ T cell responses
to vaccination with peptide, IFA, and CpG oligodeoxynucleotide
7909. J Clin Invest. 115:739–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neglia F, Orengo AM, Cilli M, Meazza R,
Tomassetti A, Canevari S, Melani C, Colombo MP and Ferrini S: DNA
vaccination against the ovarian carcinoma-associated antigen folate
receptor alpha (FRalpha) induces cytotoxic T lymphocyte and
antibody responses in mice. Cancer Gene Ther. 6:349–357. 1999.
View Article : Google Scholar : PubMed/NCBI
|