Introduction
Breast cancer is the most common type of tumor among
women and is the second leading cause of cancer-related mortality
worldwide. It is estimated that, in 2016, approximately 250,000 new
cases of breast cancer will be diagnosed and 40,890 deaths will
result from breast cancer (1).
Breast cancer is regarded as a systemic disease, with
micrometastatic involvement at diagnosis in many patients. Although
targeted therapies, including hormonal therapy in hormone
receptor-positive and trastuzumab therapy in human epidermal growth
factor receptor 2 (HER2)-positive patients are well established,
chemotherapy remains an important mainstay in systemic treatment,
especially in patients with high risk of relapse (2–4).
Anthracycline-based chemotherapy regimens with the addition of
taxanes into chemotherapy has improved survival outcome in the
adjuvant setting (5).
Unfortunately, long-term adverse effects and chemoresistance, pose
challenges to further improving the efficacy of adjuvant breast
cancer chemotherapy (6). Therefore,
there is an urgent need to identify new reliable predictive
biomarkers for chemosensitivity in breast cancer to improve disease
management and patient survival.
PRKDC is a nuclear protein serine/threonine kinase
that is activated upon association with DNA (7). It is a critical component of DNA
repair machinery that plays a pivotal role in the DNA damage
response (DDR), primarily double stranded break (DSB) response, and
maintenance of genomic stability (8). In response to DSB formation, PRKDC is
recruited to DSBs by the Ku70/80 heterodimer where it is rapidly
activated after phosphorylation at multiple serine and threonine
residues (9). Both aberrant
expression or genetic mutations of PRKDC have been observed in
various cancer types or pre-malignant cells, indicating that PRKDC
may have paradoxically opposing roles in carcinogenesis, depending
on the cell context or the tissue type (9–11).
Moreover, due to its role in DDR, increased activity of PRKDC has
been found to be associated with resistance to chemotherapeutic
drugs and radiotherapy in glioma cells and oral squamous cell
carcinoma, respectively (12,13).
Therefore, it is plausible to hypothesize that inhibitors of PRKDC
might be useful in sensitizing cancer cells to chemotherapy and
radiotherapy (9,13).
In the present study, we evaluated the expression
level of PRKDC in breast cancer patients receiving NAC and explored
its potential as a prognostic biomarker as well as predictor of
chemosensitivity in breast cancer.
Materials and methods
Patient and tissue samples
This study used archived material from Affiliated
Cancer Hospital of Xinjiang Medical University admitted between
March 2011 and December 2015, including breast cancer tissue
samples from patients with stage IIIA-C disease for whom matching
biopsies were available for pathological and immunohistochemical
analysis. All tissues were immediately snap-frozen in liquid
nitrogen and stored at −80°C until use. The patients with any other
tumor were excluded from the study. A total of 159 breast cancer
tissues and 59 matched non-tumor adjacent tissues (NATs) were
examined in the study. None of the subjects had received any
therapeutic procedures prior to this study, including surgery,
chemotherapy, and radiotherapy. In addition, a total of 89 patients
who received six cycles of an anthracycline based-therapy (FEC:
5-fluorouracil (5-FU) 500 mg/m2, epirubicin 75–100
mg/m2, cyclophosphamide 500 mg/m2, on day 1
of a 21-day cycle) were also included. All patients underwent
surgery (mastectomy and axillary node clearance) 4 weeks after the
last cycle of chemotherapy, followed by radiotherapy to the chest
wall. The pathological complete response (pCR) was defined as the
absence of any residual invasive carcinoma at both the primary site
and in axillary lymph nodes. Patients with pCR were defined as
responders whereas others were defined as non-responders. The
prognosis was evaluated in all breast cancer patients in April
2015. Overall survival was defined as the time from cancer onset
until death or by censoring at the last follow-up date. The study
was approved by Affiliated Cancer Hospital of Xinjiang Medical
University, and informed consent was obtained from all
patients.
Total RNA extraction
Tissue sections were minced with scissors into small
fragments (1–2 mm3) and homogenized with TRIzol™ reagent
(Takara Bio, Inc., Otsu, Japan). Chloroform (200 µl; Sigma-Aldrich,
Santa Clara, CA, USA) was added to the TRIzol homogenate. The
preparations were then centrifuged at 12,000 × g for 15 min at 4°C,
and the upper aqueous layer was transferred to a clean Eppendorf
tube, containing an equal volume of isopropanol (Sigma-Aldrich).
The mixed suspensions were centrifuged at 12,000 × g for a further
15 min at 4°C. The precipitations were then collected. After
washing with 70% ethanol, total RNA was dissolved in RNase-free
water and the quality of RNA was evaluated by gel electrophoresis.
RNA concentrations were measured by optical density (260 nm, Q5000,
Quawell, San Jose, CA, USA) and the preparations stored at −80°C
for subsequent analysis.
RT-qPCR analysis
cDNA was reverse transcribed on the Bio-Rad S1000
Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA) using
oligo (dT) as primers. Briefly, the total RNA (1 µg) from each
sample was reverse transcribed in a 20 µl reaction volume,
containing 0.5 µg of oligo (dT) and 200 units M-MLV (MBI Fermentas,
Vilnius, Lithuania). All samples were amplified in triplicate under
the following conditions: 95°C for 2 min, 35 cycles of 95°C for 15
sec, 60°C for 30 sec and 72 C for 20 sec. qPCR reaction was
performed on the Bio-Rad C1000 Real-time fluorescence thermal
cycler (Bio-Rad Laboratories), using the following cycling
conditions: Initiation at 95°C for 10 min; amplification for 35
cycles, with denaturation at 95°C for 30 sec; annealing at 56°C for
30 sec; and elongation at 72°C for 30 sec. A final extension at
72°C was performed for 10 min. GAPDH mRNA level was used for
normalization.
Chemosensitivity assay
Cells were seeded at a density of 5×103
cells/well in 96-well microtiter plates and allowed to attach
overnight. Chemo drugs were then added and cultured for an
additional 72 h. Cell viability was assessed using
CellTiter-Glo® assay (Promega, Madison, WI, USA). Each
value was normalized to cells treated with DMSO and the
IC50 values are calculated using Graphpad Prism
software.
Viral transductions and stable
selections
For lentivirus production, 1 µg of shPRKDC (Origene)
together with 1 µg of helper plasmids (0.4 µg pMD2G and 0.6 µg
psPAX2) were transfected into 293FT cells with Effectene reagent
(Qiagen, Valencia, CA, USA). Viral supernatants were collected 48 h
after transfections and cleared through a 0.45-µm filter. Cells
were infected with viral supernatants containing 4 µg/ml polybrene
(Sigma, St. Louis, MO, USA) and selected with puromycin for 7
days.
Immunohistochemistry (IHC)
staining
The paraffin-embedded sections were subjected to
antigen retrieval by heating the slides in a microwave at 100°C for
10 min in 0.1 M citric acid buffer (pH 6.0), and then incubated
with corresponding antibodies at 4°C overnight. After secondary
antibody incubation at room temperature for 1 h, the slides were
developed in 0.05% diaminobenzidine containing 0.01% hydrogen
peroxidase. For negative controls, specific antibodies were
replaced with normal goat serum by co-incubation at 4°C overnight
preceding the immunohistochemical staining procedure.
Xenograft experiments
All animal experiments were approved by
Institutional Animal Care and Use Committee of National Cancer
Center. MCF-7 cells (3×106 cells/injection) expressing
control shRNA or PRKDC shRNA-2 were subcutaneously injected into
both flanks of 5-week-old female nude mice. Vehicle or 5-FU (25
mg/kg) was injected i.p. into mice daily for 12 days. Tumor volumes
were measured using caliper and determined by a formula [volume =
(length × width2)/2] from day 6 to day 18
post-implantation. The results were expressed as mean tumor volumes
with SD.
Statistical analysis
For cell culture and mouse experiments, quantitative
data are expressed as mean ± SD. Statistical significance was
assessed by the Student's t-test. Differences were considered to be
significant when P<0.05. For patient sample data, statistical
analysis was performed using IBM SPSS Statistics version 16 (SPSS
Inc., Chicago, IL, USA) and GraphPad Prism v5.0 (Graphpad Software
Inc.). The Wilcoxon test was used to compare PRKDC expression in
paired tumor tissue samples and NATs. The Mann-Whitney U test was
used to perform statistical analysis of PRKDC mRNA level between
unpaired groups. The Pearson's Chi-squared test and Fisher's exact
test were used to evaluate the association between tissue PRKDC
mRNA level and clinicopathological parameters. In addition,
survival curves were constructed with the Kaplan-Meier method and
compared using log-rank test. Cox proportional hazards regression
analysis was used for univariate and multivariate analyses of
prognostic values. P-value of two-sided <0.05 was considered
statistically significant.
Results
PRKDC expression is upregulated in
breast cancer tissues
The expression level of PRKDC was evaluated in 59
pairs of breast cancer tissues and the NATs using quantitative
real-time PCR. The relative mean expression value of PRKDC mRNA in
cancer tissues (4.25±2.68, normalized to GAPDH gene expression) was
significantly higher than that (1.96±1.42) in the corresponding
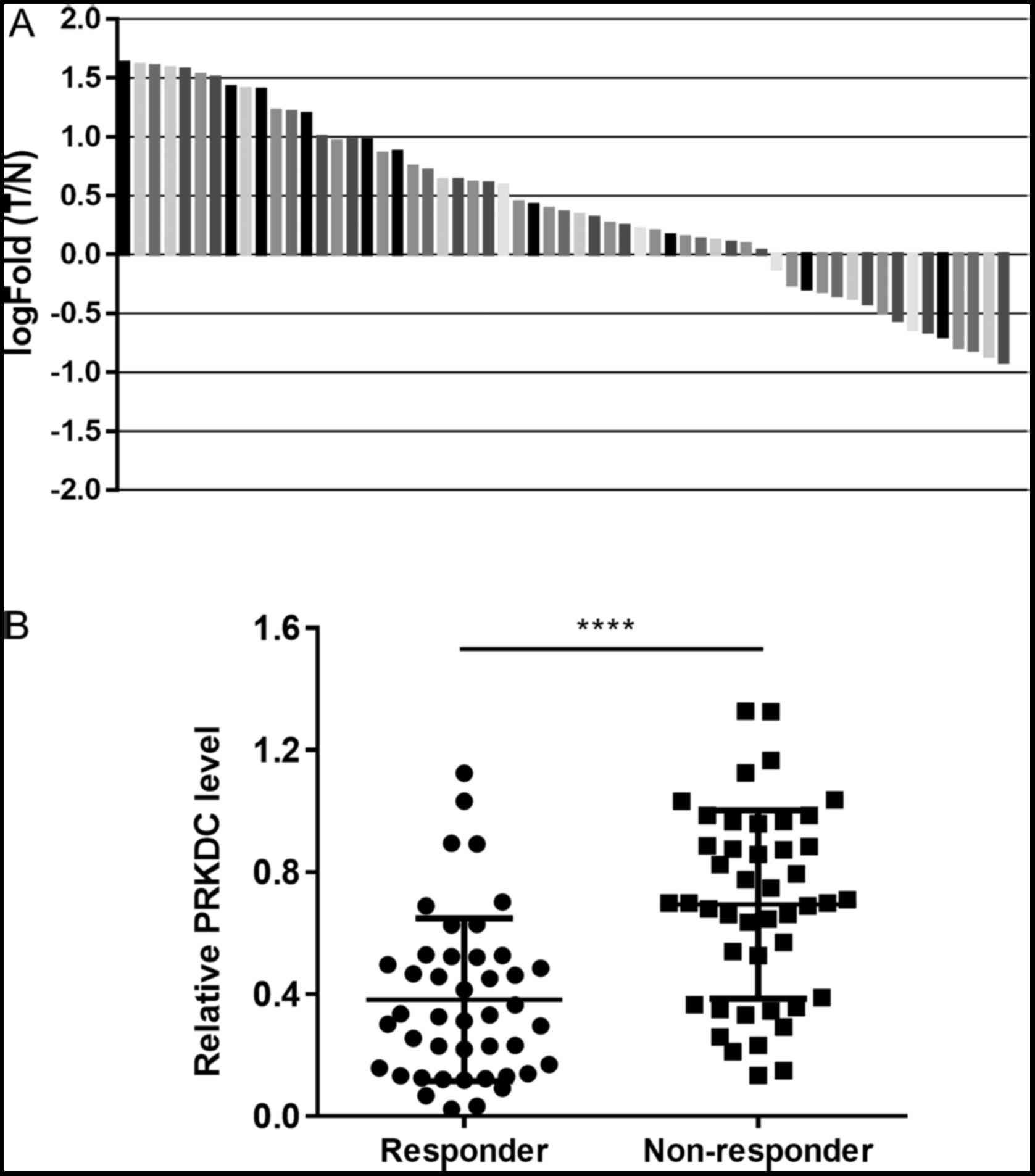
normal tissues (P<0.010). Expression fold changes above 1 was
considered as upregulation of PRKDC mRNA in the cancer tissues. The
results revealed that 72.9% (43/59) of breast cancer tissues
expressed a higher level of PRKDC compared with the matched normal
tissues. We also measured the PRKDC expression levels in tissue
samples from 44 breast cancer patients who responded to NAC and 45
patients who did not. We found that the expression levels of PRKDC
were significantly higher in the non-responder group than the
responder group (P<0.0001) (Fig.
1B).
Association between tissue PRKDC
expression level and clinicopathological factors or chemotherapy
response
The correlation between PRKDC expression level and
clinicopathological factors or chemotherapy response was assessed
using χ2 test. As shown in Table I, a total of 62 of 102 (60.7%)
patients who were Grade II or III had significantly higher
expression level of PRKDC than Grade I patients (P=0.001). We
detected high PRKDC level in patients who were lymph node
metastasis positive (P=0.0357). High PRKDC expression level was
observed in 34% and 71.1% of patients who were responders or
non-responders to neoadjuvant chemotherapy (NAC), respectively
(P=0.0006). However, no significant correlation was observed
between PRKDC expression levels and age (P=0.1541), tumor size
(P=0.8736), tumor stage (P=0.3691), ER status (P=0.1354), PR status
(P=0.191) or Her-2 status (P=0.4189).
 | Table I.Correlation between tissue PRKDC
expression level and clinicopathological factors or
chemosensitivity. |
Table I.
Correlation between tissue PRKDC
expression level and clinicopathological factors or
chemosensitivity.
| Characteristics | No. of patients | PRKDC low
expression | PRKDC high
expression | P-value |
|---|
| Age (years) |
|
|
| 0.1541 |
| ≤50 | 117 | 57 | 60 |
|
|
>50 | 42 | 26 | 16 |
|
| Tumor size (cm) |
|
|
| 0.8736 |
| ≤2 | 83 | 39 | 44 |
|
|
>2 | 76 | 34 | 42 |
|
| Tumor stage |
|
|
| 0.3691 |
| I,
II | 118 | 62 | 56 |
|
|
III | 41 | 25 | 16 |
|
| Tumor grade |
|
|
|
0.0010a |
| I | 57 | 38 | 19 |
|
| II,
III | 102 | 40 | 62 |
|
| Lymph node
metastasis |
|
|
|
0.0357a |
|
Negative | 65 | 40 | 25 |
|
|
Positive | 94 | 41 | 53 |
|
| ER status |
|
|
| 0.1354 |
|
Negative | 56 | 23 | 33 |
|
|
Positive | 103 | 56 | 47 |
|
| PR status |
|
|
| 0.1910 |
|
Negative | 74 | 50 | 24 |
|
|
Positive | 85 | 48 | 37 |
|
| Her-2 status |
|
|
| 0.4189 |
|
Negative | 96 | 48 | 48 |
|
|
Positive | 63 | 27 | 36 |
|
| Clinical response
to NAC |
|
|
|
0.0006a |
|
Responder | 44 | 29 | 15 |
|
|
Non-responder | 45 | 13 | 32 |
|
High expression of PRKDC predicts poor
prognosis in breast cancer patients with or without receiving
NAC
Univariate and multivariate survival analyses were
performed to examine the effects of clinicopathological factors and
PRKDC expression on prognosis in patients receiving NAC. As shown
in Table II, lymph node metastasis
(P=0.044), tumor grade (P=0.038), PRKDC expression (P=0.011) and
response to NAC (P=0.008) were significantly associated with
overall survival (OS). Tumor grade (P=0.037, HR=2.16, 95%
confidence interval: 1.35–3.04), PRKDC expression (P=0.022,
HR=2.69, 95% confidence interval: 1.81–3.84) and response to NAC
(P=0.014, HR=3.96, 95% confidence interval: 2.61–5.47) were
independent predictors of OS in these patients (Table II).
 | Table II.Univariate and multivariate analysis
of factors associated with overall survival for patients with
breast cancer (stage IIIA-C) who received NAC prior to surgery. |
Table II.
Univariate and multivariate analysis
of factors associated with overall survival for patients with
breast cancer (stage IIIA-C) who received NAC prior to surgery.
|
|
| Multivariate |
|---|
|
|
|
|
|---|
| Variables | Univariate
P-value | HR | 95% CI | P-value |
|---|
| Age | 0.446 |
|
| NA |
| Tumor size | 0.645 |
|
| NA |
| Tumor stage | 0.297 |
|
| NA |
| Lymph node
metastasis |
0.044a |
|
| 0.079 |
| Tumor grade |
0.038a | 2.16 | 1.35–3.04 | 0.037a |
| ER status | 0.058 |
|
| NA |
| PR status | 0.112 |
|
| NA |
| Her-2 status | 0.265 |
|
| NA |
| PRKDC
expression |
|
Positive vs. negative |
0.011a | 2.69 | 1.81–3.84 | 0.022a |
| Response to
NAC |
|
Non-responder vs.
responder |
0.008a | 3.96 | 2.61–5.47 | 0.014a |
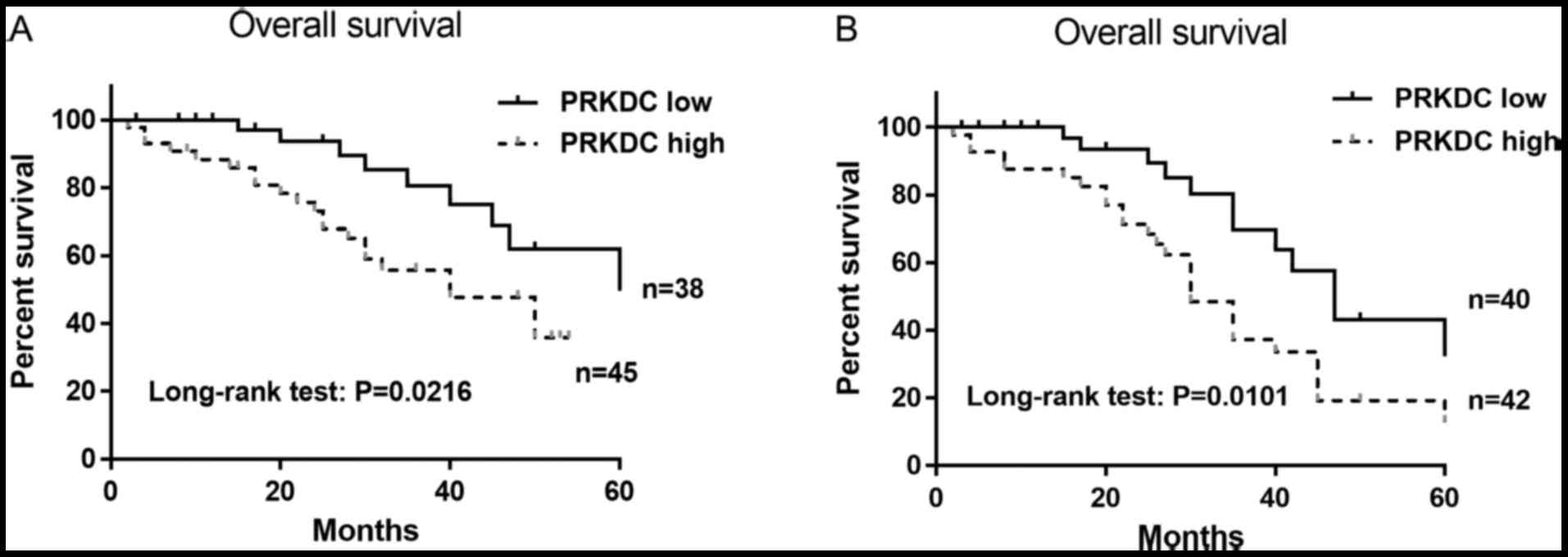
A survival analysis of OS was performed in patients
with or without NAC treatment to determine whether PRKDC expression
level can predict prognosis. The estimated Kaplan-Meier OS curves
showed that high expression of PRKDC was significantly correlated
with poor OS in patients without receiving NAC (P=0.0216) (Fig. 2A). In patients receiving NAC
treatment, the OS was significantly worse in patients with high
levels of PRKDC (P=0.0101) with a median survival of 30 months
(Fig. 2B). These results indicate
that high PRKDC expression was associated with poor OS in patients
with or without NAC treatment and was an independent prognostic
factor.
Downregulation of PRKDC sensitizes
breast cancer cells to chemo-drugs in vitro and in vivo
To examine the regulatory role of PRKDC in
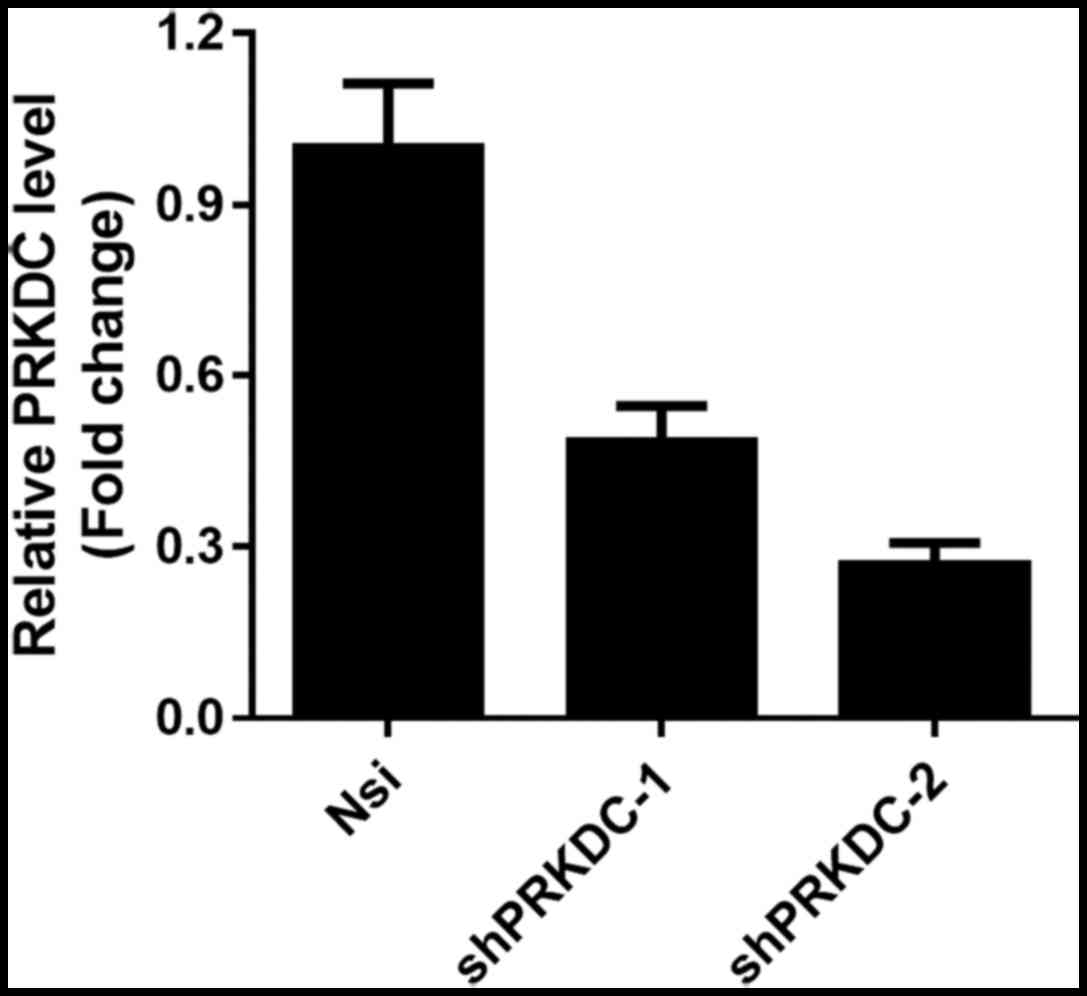
chemoresistance in breast cancer cells, we generated stable PRKDC
knockdown MCF-7 cell lines using two independent shRNAs targeting
PRKDC. The knockdown efficiency was confirmed by qPCR (Fig. 3) and MCF-7 cells expressing
shPRKDC-2 which exhibited better knockdown was used in the
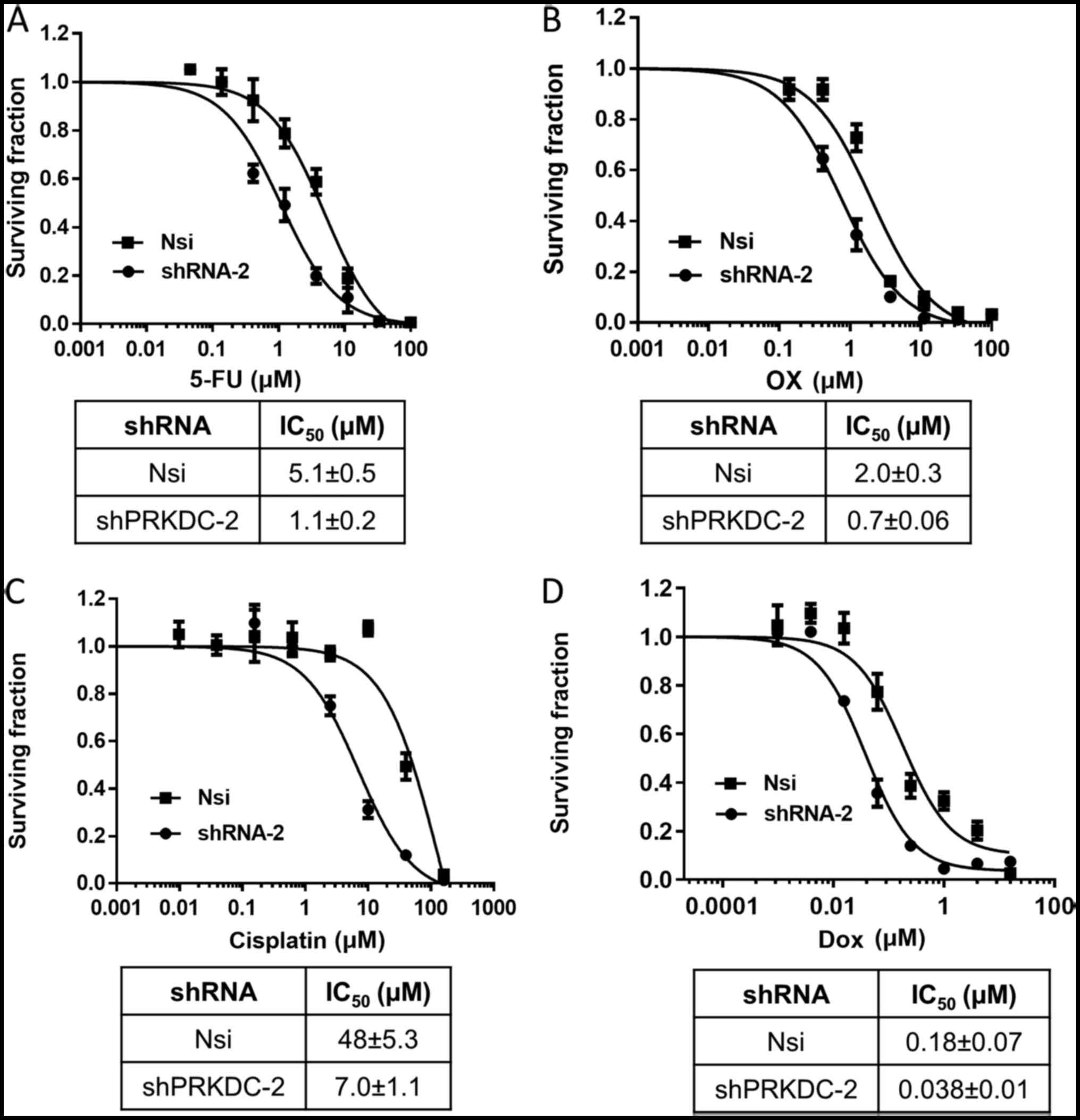
following experiments. Cytotoxicity of 4 commonly used chemo-drugs
was evaluated in these cells by performing dose response analysis.
As shown in Fig. 4, stable
knockdown of PRKDC sensitized MCF-7 cells to all the drugs. In
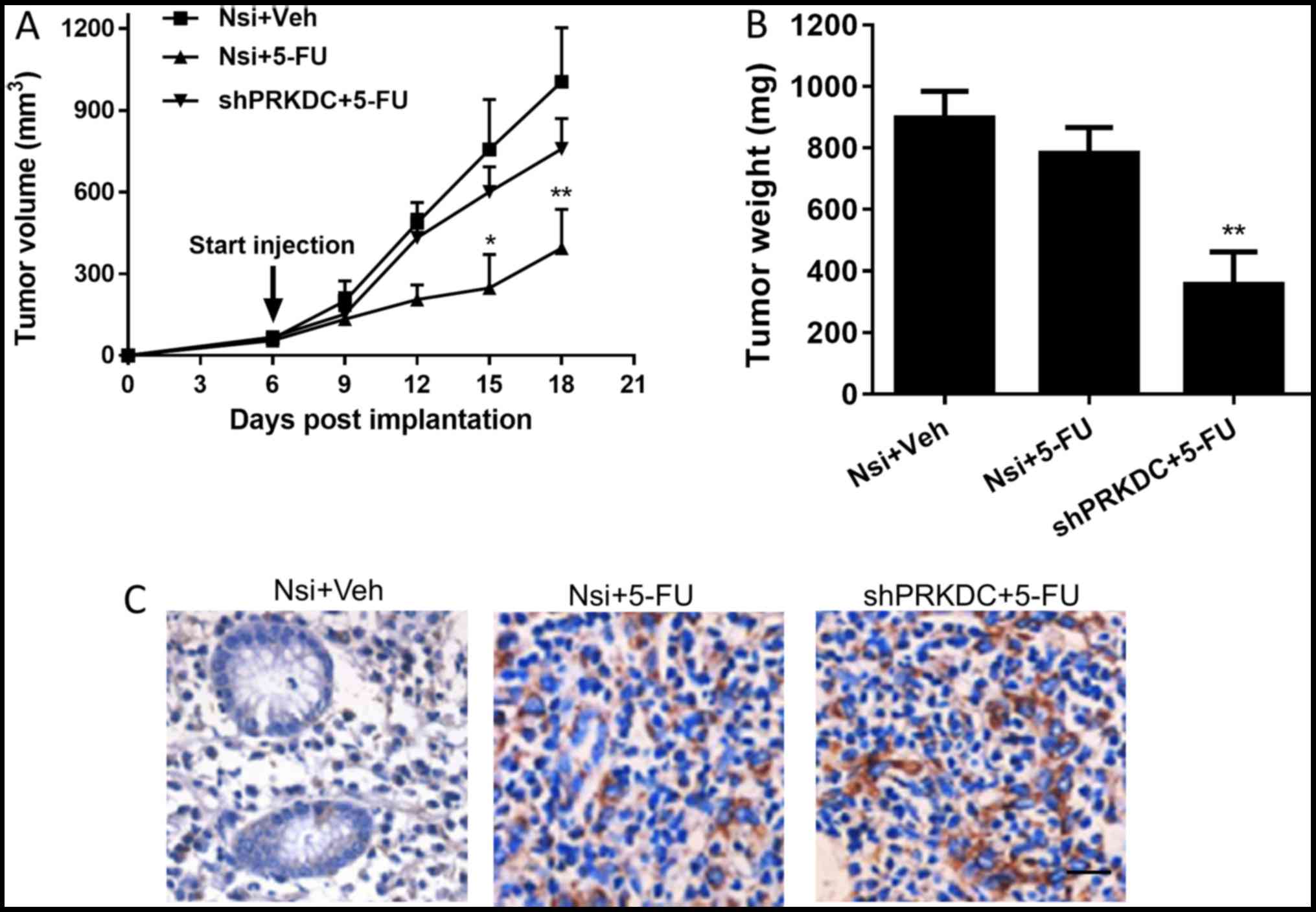
addition, downregulation of PRKDC significantly reduced tumor
growth rate as well as tumor weight in MCF-7 xenografted mice
receiving 5-FU whereas injection of 5-FU alone had negligible
effects on tumor growth or tumor weight (Fig. 5A and B). Immunohistochemical
staining showed that 5-FU treatment led to more induction of
cleaved caspase-3 in PRKDC knockdown xenografts compared with
control xenografts (Fig. 5C).
Collectively, the results showed that PRKDC directly regulates
chemosensitivity of breast cancer cells both in vitro and
in vivo by enhancing drug-induced apoptotic cell death.
Discussion
PRKDC, a serine/threonine-protein kinase, is a
member of the phosphatidylinositol 3-kinase-related kinase (PIKK)
family and is abundantly expressed in almost all mammalian cells
(14). It forms the DNAPK complex
with DNA-binding Ku70/80 heterodimer which serves as a key
regulator of the non-homologous end-joining (NHEJ) pathway
(15). Additionally, PRKDC plays an
important role in maintaining proper cell cycle progression by
coordinating mitosis, microtubule dynamics and chromosomal
segregation and has been shown to prevent mitotic catastrophe after
ionization (9,16,17).
Interestingly, both up- and down-regulation of PRKDC have been
observed in various cancer types. For example, loss of PRKDC
expression was found in gastric cancer, and was associated with
advanced tumor stage, lymphatic invasion, lymph node metastasis and
poor patient survival (18).
Moreover, loss of PRKDC expression was shown to be predictive
markers for poor prognosis of patients with gall bladder
malignancies (19). On the other
hand, PRKDC has been found to be upregulated associated with
advanced clinical stages and poor prognosis in numerous tumor types
(20–22). An elevated expression of PRKDC has
been observed in esophageal cancer tissues compared with adjacent
normal mucosae (21). High
tumor/normal expression ratio of PRKDC is correlated with increased
risk of death in non-small cell lung cancer (22). An increased expression of PRKDC was
observed in high-grade lymphoma lymph node samples by staining
compared with those from low-grade lymphoma patients (23). However, the expression of PRKDC and
its association with clinicopathological factors has not been
investigated in breast cancer patients. In our study, we observed
significantly higher levels of PRKDC in tumor tissues compared with
NATs in 72.9% of the patients analyzed (Fig. 1A) and the mean expression level
difference was significant (P<0.01). In addition, PRKDC
expression levels were significantly correlated with tumor grade,
lymph node metastasis, and response to NAC (Table I). Moreover, the breast cancer
patient with elevated levels of PRKDC showed significantly worse OS
than those with low PRKDC expression (Fig. 2A). These results suggest that PRKDC
may function as an oncogene in breast cancer tumorigenesis. The
potential mechanism by which PRKDC regulates metastasis is through
mediation of transcriptional network or through regulation of
secreted proteins involved in migration and invasion (24,25).
Due to its role in mediating DDR, aberrant
expression/activation of PRKDC has been associated with chemo- or
radio-resistance (26,27). A previous study showed that PRKDC
regulated AKT activation and inhibited apoptosis in ovarian cancer
cells with acquired platinum resistance (28,29).
In oral squamous cell carcinoma, a significant upregulation of
PRKDC proteins was detected after radiotherapy in the residual
cancer cells and this upregulation was associated with
radioresistance (13). On the
contrary, inhibition of PRKDC sensitized cancer cells to
radiotherapy and chemotherapy (27,30,31).
For example, inhibition of PRKDC activity by a small molecule
inhibitor, NU7441, sensitized breast cancer cells to ionizing
radiation and doxorubicin (30).
High expression of PRKDC was detected in glioma patients who were
resistant to cisplatin-based chemotherapy and was shown to be a
predictor for response to radiation therapy in esophageal cancer
and early breast cancer (12,32,33).
Our study suggested an association between PRKDC
expression and chemosensitivity in breast cancer. We found that the
expression of PRKDC was elevated in tissue samples from patients
who do not respond to NAC compared to those who respond (Fig. 1B). There was a significant
correlation between PRKDC expression level and chemosensitivity in
these patients (Table I). In
addition, high PRKDC expression level predicted poor survival in
patients receiving NAC treatment (Fig.
2B). Moreover, multivariate analysis demonstrated that PRKDC
was an independent prognostic factor for OS in breast cancer
patients receiving NAC (Table II).
Importantly, knockdown of PRKDC sensitized MCF-7 breast cancer
cells to chemo-drugs in vitro and in vivo with
increased induction of apoptosis (Figs.
4 and 5), demonstrating the
direct involvement of PRKDC in regulating chemosensitivity in
breast cancer cells. These results also highlight the potential of
PRKDC as a drug target for chemosensitization or as an anti-cancer
therapeutic strategy.
In summary, our study showed that PRKDC regulates
chemosensitivity in breast cancer cells and its expression was
significantly associated with chemoresistance in patients receiving
NAC. PRKDC may serve as prognostic biomarker for poor survival and
a predictor for NAC response in breast cancer patients. Further
studies are warranted to understand the precise mechanism
underlying the role of PRKDCs in regulation of chemosensitivity
which may lend support to the development of new therapeutic
strategies to overcome chemoresistance in breast cancer.
Acknowledgements
The present study was funded by Natural Science Foundation
of China (NSFC grant no. 81360391) and Youth Science and
Technology Innovative Talent Training Project of Xinjiang
(grant no. 2014721043).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cuzick J, Sestak I, Baum M, Buzdar A,
Howell A, Dowsett M and Forbes JF: ATAC/LATTE investigators: Effect
of anastrozole and tamoxifen as adjuvant treatment for early-stage
breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol.
11:1135–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perez EA, Romond EH, Suman VJ, Jeong JH,
Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA and
Wolmark N: Four-year follow-up of trastuzumab plus adjuvant
chemotherapy for operable human epidermal growth factor receptor
2-positive breast cancer: Joint analysis of data from NCCTG N9831
and NSABP B-31. J Clin Oncol. 29:3366–3373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slamon D, Eiermann W, Robert N, Pienkowski
T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et
al: Breast Cancer International Research Group: Adjuvant
trastuzumab in HER2-positive breast cancer. N Engl J Med.
365:1273–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palmieri C and Jones A: The 2011 EBCTCG
polychemotherapy overview. Lancet. 379:390–392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gonzalez-Angulo AM, Morales-Vasquez F and
Hortobagyi GN: Overview of resistance to systemic therapy in
patients with breast cancer. Adv Exp Med Biol. 608:1–22. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jackson SP: DNA-dependent protein kinase.
Int J Biochem Cell Biol. 29:935–938. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goodwin JF and Knudsen KE: Beyond DNA
repair: DNA-PK function in cancer. Cancer Discov. 4:1126–1139.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu FM, Zhang S and Chen BP: Role of
DNA-dependent protein kinase catalytic subunit in cancer
development and treatment. Transl Cancer Res. 1:22–34.
2012.PubMed/NCBI
|
|
10
|
Zhang L, Komurov K, Wright WE and Shay JW:
Identification of novel driver tumor suppressors through functional
interrogation of putative passenger mutations in colorectal cancer.
Int J Cancer. 132:732–737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Kim S, Jia G, Buhmeida A, Dallol
A, Wright WE, Fornace AJ, Al-Qahtani M and Shay JW: Exome
sequencing of normal and isogenic transformed human colonic
epithelial cells (HCECs) reveals novel genes potentially involved
in the early stages of colorectal tumorigenesis. BMC Genomics. 16
Suppl 1:S82015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukherjee B, McEllin B, Camacho CV,
Tomimatsu N, Sirasanagandala S, Nannepaga S, Hatanpaa KJ, Mickey B,
Madden C, Maher E, et al: EGFRvIII and DNA double-strand break
repair: A molecular mechanism for radioresistance in glioblastoma.
Cancer Res. 69:4252–4259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shintani S, Mihara M, Li C, Nakahara Y,
Hino S, Nakashiro K and Hamakawa H: Up-regulation of DNA-dependent
protein kinase correlates with radiation resistance in oral
squamous cell carcinoma. Cancer Sci. 94:894–900. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hartley KO, Gell D, Smith GC, Zhang H,
Divecha N, Connelly MA, Admon A, Lees-Miller SP, Anderson CW and
Jackson SP: DNA-dependent protein kinase catalytic subunit: A
relative of phosphatidylinositol 3-kinase and the ataxia
telangiectasia gene product. Cell. 82:849–856. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davis AJ, Chen BP and Chen DJ: DNA-PK: A
dynamic enzyme in a versatile DSB repair pathway. DNA Repair
(Amst). 17:21–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee KJ, Lin YF, Chou HY, Yajima H, Fattah
KR, Lee SC and Chen BP: Involvement of DNA-dependent protein kinase
in normal cell cycle progression through mitosis. J Biol Chem.
286:12796–12802. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shang ZF, Huang B, Xu QZ, Zhang SM, Fan R,
Liu XD, Wang Y and Zhou PK: Inactivation of DNA-dependent protein
kinase leads to spindle disruption and mitotic catastrophe with
attenuated checkpoint protein 2 Phosphorylation in response to DNA
damage. Cancer Res. 70:3657–3666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee HS, Yang HK, Kim WH and Choe G: Loss
of DNA-dependent protein kinase catalytic subunit (DNA-PKcs)
expression in gastric cancers. Cancer Res Treat. 37:98–102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren F, Yang ZL, Tan X, Liu D, Zou Q, Yuan
Y, Li J, Liang L, Zeng G and Chen S: DNA-PKcs and Ku70 are
predictive markers for poor prognosis of patients with gall bladder
malignancies. Appl Immunohistochem Mol Morphol. 22:741–747. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hosoi Y, Watanabe T, Nakagawa K, Matsumoto
Y, Enomoto A, Morita A, Nagawa H and Suzuki N: Up-regulation of
DNA-dependent protein kinase activity and Sp1 in colorectal cancer.
Int J Oncol. 25:461–468. 2004.PubMed/NCBI
|
|
21
|
Tonotsuka N, Hosoi Y, Miyazaki S, Miyata
G, Sugawara K, Mori T, Ouchi N, Satomi S, Matsumoto Y, Nakagawa K,
et al: Heterogeneous expression of DNA-dependent protein kinase in
esophageal cancer and normal epithelium. Int J Mol Med. 18:441–447.
2006.PubMed/NCBI
|
|
22
|
Xing J, Wu X, Vaporciyan AA, Spitz MR and
Gu J: Prognostic significance of ataxia-telangiectasia mutated,
DNA-dependent protein kinase catalytic subunit, and Ku
heterodimeric regulatory complex 86-kD subunit expression in
patients with nonsmall cell lung cancer. Cancer. 112:2756–2764.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holgersson A, Erdal H, Nilsson A,
Lewensohn R and Kanter L: Expression of DNA-PKcs and Ku86, but not
Ku70, differs between lymphoid malignancies. Exp Mol Pathol.
77:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kotula E, Berthault N, Agrario C, Lienafa
MC, Simon A, Dingli F, Loew D, Sibut V, Saule S and Dutreix M:
DNA-PKcs plays role in cancer metastasis through regulation of
secreted proteins involved in migration and invasion. Cell Cycle.
14:1961–1972. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goodwin JF, Kothari V, Drake JM, Zhao S,
Dylgjeri E, Dean JL, Schiewer MJ, McNair C, Jones JK, Aytes A, et
al: DNA-PKcs-mediated transcriptional regulation drives prostate
cancer progression and metastasis. Cancer Cell. 28:97–113. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burdine LJ, Burdine MS, Moreland L, Fogel
B, Orr LM, James J, Turnage RH and Tackett AJ: Proteomic
identification of DNA-PK involvement within the RET signaling
pathway. PLoS One. 10:e01279432015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elliott SL, Crawford C, Mulligan E,
Summerfield G, Newton P, Wallis J, Mainou-Fowler T, Evans P,
Bedwell C, Durkacz BW, et al: Mitoxantrone in combination with an
inhibitor of DNA-dependent protein kinase: A potential therapy for
high risk B-cell chronic lymphocytic leukaemia. Br J Haematol.
152:61–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stronach EA, Chen M, Maginn EN, Agarwal R,
Mills GB, Wasan H and Gabra H: DNA-PK mediates AKT activation and
apoptosis inhibition in clinically acquired platinum resistance.
Neoplasia. 13:1069–1080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ciszewski WM, Tavecchio M, Dastych J and
Curtin NJ: DNA-PK inhibition by NU7441 sensitizes breast cancer
cells to ionizing radiation and doxorubicin. Breast Cancer Res
Treat. 143:47–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Munck JM, Batey MA, Zhao Y, Jenkins H,
Richardson CJ, Cano C, Tavecchio M, Barbeau J, Bardos J, Cornell L,
et al: Chemosensitization of cancer cells by KU-0060648, a dual
inhibitor of DNA-PK and PI-3K. Mol Cancer Ther. 11:1789–1798. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Noguchi T, Shibata T, Fumoto S, Uchida Y,
Mueller W and Takeno S: DNA-PKcs expression in esophageal cancer as
a predictor for chemoradiation therapeutic sensitivity. Ann Surg
Oncol. 9:1017–1022. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Söderlund Leifler K, Queseth S, Fornander
T and Askmalm MS: Low expression of Ku70/80, but high expression of
DNA-PKcs, predict good response to radiotherapy in early breast
cancer. Int J Oncol. 37:1547–1554. 2010.PubMed/NCBI
|