Introduction
Gastric cancer (GC) is a type of common and fatal
digestive malignant disease worldwide. Despite recent advances in
diagnosis and treatment as well as declining incidence in some
developed countries, it remains a major cause of cancer-related
deaths in Eastern Asia (including China, Japan, and South Korea)
(1,2). GC in early stages is curable by using
endoscopic procedures. In China, approximately two-thirds of
patients develop advanced or metastatic disease, and >50% have
recurrent disease following curative surgery. Effective therapeutic
approaches are still limited (3).
Systematic chemotherapy is an extremely important therapeutic
strategy for advanced GC. Among the patients, many are resistant to
chemotherapy agents, including cisplatin (DDP), adriamycin, and
5-fluorouracil. Multidrug resistance (MDR) is largely responsible
for ineffective chemotherapy (4).
The mechanism of MDR remains unclear. Increased expression of
MDR-associated protein (MRP), P-glycoprotein (P-gp), increased DNA
damage repair, cell cycle arrest, and resistance of tumor cells to
apoptosis are the mechanisms that might account for MDR (5). A large number of studies have shown
that the dysfunction of signaling pathways in cancer cells is the
major reason for tumorigenesis and metastasis (6). Therefore, finding novel therapeutic
strategies and key target molecules for reversing resistance and
diminishing the side effects of chemotherapy agents are the main
goals of drug resistance cancer treatment protocol.
Mammalian target of rapamycin complex 1 (mTORC1), a
complex composed of mTOR, Raptor and mSIN1, controls protein
translation, cell growth, and metabolism (7). mTORC1 over-activation is detected in
GC and many other cancers (7–11).
mTORC1 activation could induce transcription of a number of key
oncoproteins and MDR-associated proteins including cyclin D1 and
hypoxia-inducible factor 1α (HIF-1α) (12). Activation of mTORC1 results in
phosphorylation of important effectors [i.e., ribosomal protein S6
kinase (S6K) and eukaryotic translation initiation factor
4E-binding protein 1 (4E-BP1)] involved in cancer progression and
apoptosis resistance. In addition, mTORC1 is also the major
negative regulator of autophagy (7). Therefore, targeting mTORC1 may be an
attractive therapeutic strategy for cancer drug resistance
treatment.
The activation of mTORC1 is induced by numerous
oncoproteins and mitogenic factors via the class I
phospho-inositide3 kinase (PI3K)/Akt pathway (13). Cancerous inhibitor of protein
phosphatase 2A (CIP2A) is a human oncoprotein that stabilizes c-Myc
and activates Akt by inhibiting protein phosphatase 2A
(PP2A)-mediated dephosphorylation of c-Myc and Akt (14). CIP2A can associate with mTORC1
directly and act as an allosteric inhibitor of mTORC1-associated
PP2A, thereby enhancing mTORC1-dependent growth signaling and
inhibiting autophagy (7). An
increased expression of CIP2A protein was found in GC and several
other cancers (14–18). In 2015, Zhang et al reported
that CIP2A expression is associated with DDP resistance (19). Thus, effective and discerning
CIP2A-mTORC1 axis inhibitors would be beneficial for therapy to DDP
resistance and induce autophagy in GC.
Cucurbitacin B (CuB) is a natural product that is
extensively distributed in medicinal plants Cucurbitaceae family is
shown to inhibit the growth of numerous human cancer cell lines
such as colon, breast, leukemia, pancreatic hepatic, and
glioblastoma (20–22). The effect of CuB on DDP-resistant GC
cells previously has not been evaluated. The aim of this work was
to investigate antitumor effects and possible mechanisms of CuB on
DDP resistant human GC cells.
Materials and methods
Reagents
CuB with a purity of up to 98% was purchased from
Shanghai Yuanye Bio-Technology Co., Ltd. CuB was dissolved in
dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) at a
stock solution of 40 mM and stored at −20°C. DDP and
3-methyladenine (3-MA, #M9281) were purchased from
Sigma-Aldrich.
Cell culture
Human DDP-resistant gastric cancer cell line
SGC7901/DDP and human GC cell line SGC7901 were purchased from the
Shanghai Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China). SGC7901/DDP cells were grown
in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with fetal bovine serum (FBS; HyClone; GE Healthcare Life
Sciences, Chalfont, UK), 500 ng/ml DDP, and antibiotics and
incubated in a humidified atmosphere without CO2 at
37°C. SGC7901 cells were grown in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) with FBS and
antibiotics and incubated in a humidified atmosphere with
CO2 at 37°C.
Cytotoxic assay and cell
viability
Cells were seeded into 96-well plate and
pre-cultured for 24 h, then treated with CuB for 24 h. Cell
cytotoxicity was determined by MTT assay. The absorbance was
measured at 490 nm by automated microplated reader (BioTek
Instruments, Inc., Winooski, VT, USA), and the inhibition rate was
calculated as followed: Inhibition rate (%) = (average A490 of the
control group - average A490 of the experimental group) / (average
A490 of the control group - average A490 of the blank group) ×100%.
Cell viability was estimated by trypan blue dye exclusion (23,24).
Soft-agar colony formation assay
Cells were suspended in 1 ml of RPMI-1640 containing
0.3% low-melting-point agarose (Amresco, Cleveland, OH, USA) and
10% FBS, and plated on a bottom layer containing 0.6% agarose and
10% FBS in 6-well plate in triplicate. After 2 weeks, plates were
stained with 0.2% gentian violet and the colonies were counted
under a light microscope (IX70; Olympus Corp., Tokyo, Japan)
(25).
Apoptosis determination by DAPI
staining
Approximately 2×105 cells/well of cells
in a 12-well plate was treated with CuB for 24 h. Then cells in
each treatment and control were stained by DAPI and examined and
photographed under fluorescence microscopy (IX73; Olympus Corp.) as
described elsewhere (26).
Western blotting
Cell pellets were lysed in RIPA buffer containing 50
mM Tris pH 8.0, 150 mM NaCl, 0.1% SDS, 0.5% deoxycholate, 1% NP-40,
1 mM DTT, 1 mM NaF, 1 mM sodium vanadate, 1 mM PMSF (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany), and 1% protease inhibitors
cocktail (Merck Millipore). Lysates were normalized for total
protein (25 µg) and loaded on 8–12% sodium dodecyl sulfate
polyacrylamide gel, electrophoresed, and transferred to a PVDF
membrane (Merck & Co., Inc., Kenilworth, NJ, USA), followed by
blocking with 5% skimmed milk at room temperature for 1 h. The
membrane was incubated with primary antibodies overnight at 4°C and
rinsed with Tris-buffered saline with Tween-20.
The primary antibodies used were anti-MRP1 (ratio of
1:500 dilution; catalog no. sc-13960), anti-CIP2A (1:500; catalog
no. sc-80662), anti-phospho-Akt (S473) (1:500; catalog no.
sc-7985), anti-Akt (1:500; catalog no. sc-8312), (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), anti-caspase-9 (1:1000;
catalog no. 9508), anti-caspase-3 (1:1,000; catalog no. 9662),
anti-PARP (1:1,000; catalog no. 9542), anti-PP2A (1:1,000; catalog
no. 2038), anti-LC3B (1:1,000; catalog no. 2775), anti-HIFα
(1:1,000; catalog no. 5537), anti-p4E-BP1 (Thr37/46) (1:1,000;
catalog no. 2855), anti-mTOR (1:1,000; catalog no. 2983),
anti-pmTOR (Ser2448) (1:1,000; catalog no. 5536), anti-P70S6K
(1:1,000; catalog no. 9202), anti-pP70S6K (Thr389) (1:1,000;
catalog no. 92775) (Cell Signaling Technology, Danvers, MA, USA),
anti-Beclin1 (1:2,000; catalog no. 11306-1-AP), anti-4E-BP1
(1:2,000; catalog no. 60624-1-Ig) (Proteintech Group, Inc.,
Rosemont, USA), anti-P-gp (1:2,000; catalog no. ab170904) (Abcam,
Cambridge, UK), and anti-GAPDH (1:5,000; catalog no. M20006;
Abmart, Shanghai, China).
The blots were then washed, and incubated with
horseradish peroxidase (HRP)-conjugated secondary antibody
(1:10,000; catalog no. E030120-01 and E030110-01; EarthOx, LLC, San
Francisco, CA, USA) at room temperature for 1.5 h. Detection was
performed by using a SuperSignal® West Pico Trial kit
(catalog no. QA210131; Pierce Biotechnology, Inc., Rockford, IL,
USA) (25). The defined sections of
the film were scanned for image capture and quantification using
Adobe Photoshop software (CS4, Adobe Systems Inc., San Jose, CA,
USA) and ImageJ software (National Institutes of Health, Bethesda,
MD, USA).
Autophagy assays
The cells were transfected with pQCXIP-GFP-LC3
plasmid using the Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the recommended protocol by the
manufacturer and then fixed in 4% paraformaldehyde. The percentage
of cells with fluorescent dots representing GFP-LC3 translocation
was counted by Olympus confocal laser scanning microscope.
PP2A activity assay
PP2A immunoprecipitation phosphatase assay kit
(Upstate, Temecula, CA, USA) was used to measure phosphate release
as an index of phosphatase activity according to the manufacturer's
instructions. Briefly, 100 µg protein isolated from cells was
incubated with 4 µg anti-PP2A monoclonal antibody overnight.
Protein A agarose beads (40 µl) were added and the mixture was
incubated at 4°C for 2 h. Subsequently, the beads were collected
and washed three times with 700 µl of ice-cold TBS and one time
with 500 µl Ser/Thr Assay Buffer. The beads were further incubated
with 750 mM phosphopeptide in assay buffer for 10 min at 30°C with
constant agitation. 100 µl of Malachite Green Phosphate Detection
Solution was added and the absorbance at 650 nm was measured on a
microplate reader (27).
Transfection of DNA and siRNA
The pOTENT-1-CIP2A expression plasmid was purchased
from Youbio Co. (Changcha, China). Transfection of the
pOTENT-1-CIP2A plasmid into GC cells were carried out using
Lipofectamine 3000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. Two siRNAs
targeting CIP2A were designed and synthesized by Shanghai
GenePharma Co., referred to as siRNA1 and siRNA2. The siRNA
sequences were as follows: 5′-CUGUGGUUGUGUUUGCACUTT-3′ (CIP2A
siRNA1), 5′-ACCAUUGAUAUCCUUAGAATT-3′ (CIP2A siRNA2),
5′-UUCUCCGAACGUGUCACGUTT-3′ [negative control (NC) siRNA].
Using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol,
SGC7901/DDP cells were transfected with 100 nM siRNA. At 48 h after
transfection, the cells were then harvested for western blotting,
and cell viability (28).
Statistical analysis
All experiments were repeated at least three times
and the data are presented as the mean ± SD unless noted otherwise.
Differences between data groups were evaluated for significance
using Student's t-test of unpaired data or one way analysis of
variance and Bonferroni post hoc test. P-values <0.05 indicate
statistical significance.
Results
Chemical structure of CuB and
characterization of SGC7901 and SGC7901/DDP GC cells
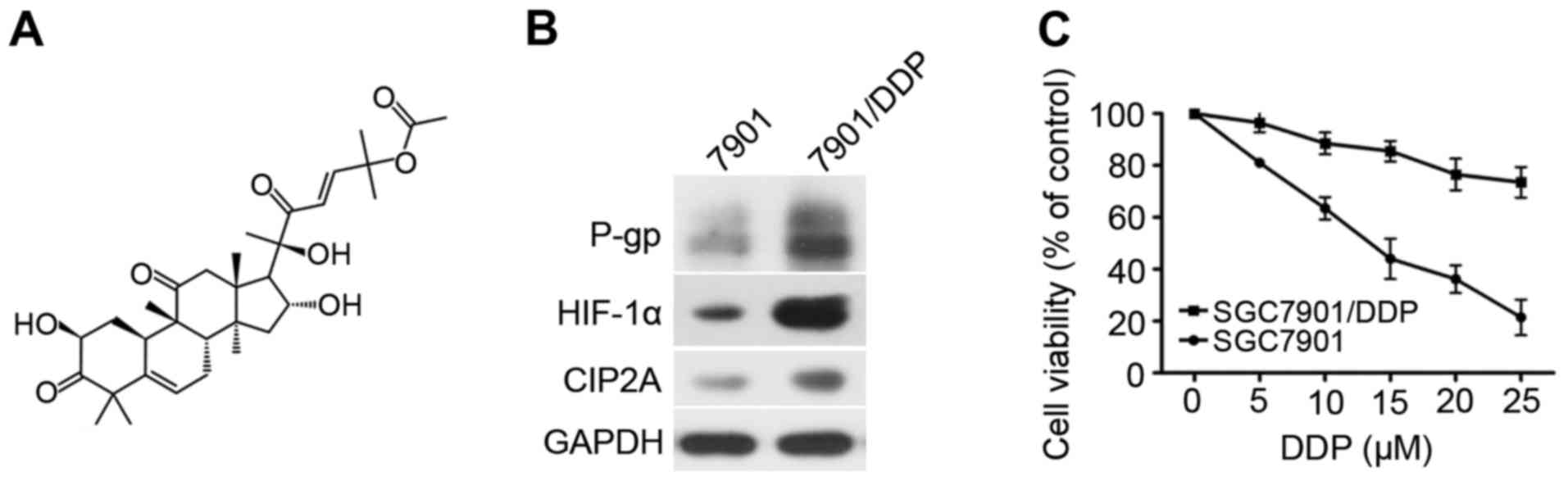
The chemical structure of CuB is shown in Fig. 1A. Firstly, the P-gp, HIF-1α, and
CIP2A expression was compared between SGC7901 and SGC7901/DDP
cells, which confirmed P-gp, HIF-1α, and CIP2A were overexpressed
in SGC7901/DDP cells (Fig. 1B).
SGC7901 and SGC7901/DDP cells were exposed to various
concentrations of DDP (2.5–80 µM) for 24 h. The half-maximal
inhibitory concentration (IC50) of DDP against SGC7901
cells is 6.2 µM. while IC50 of DDP against SGC7901/DDP
cells is 37.78 µM. As shown in Fig.
1C, the DDP cytotoxicity was higher in SGC7901 cells than in
SGC7901/DDP cells.
Effects of CuB on SGC7901 and
SGC7901/DDP GC cells
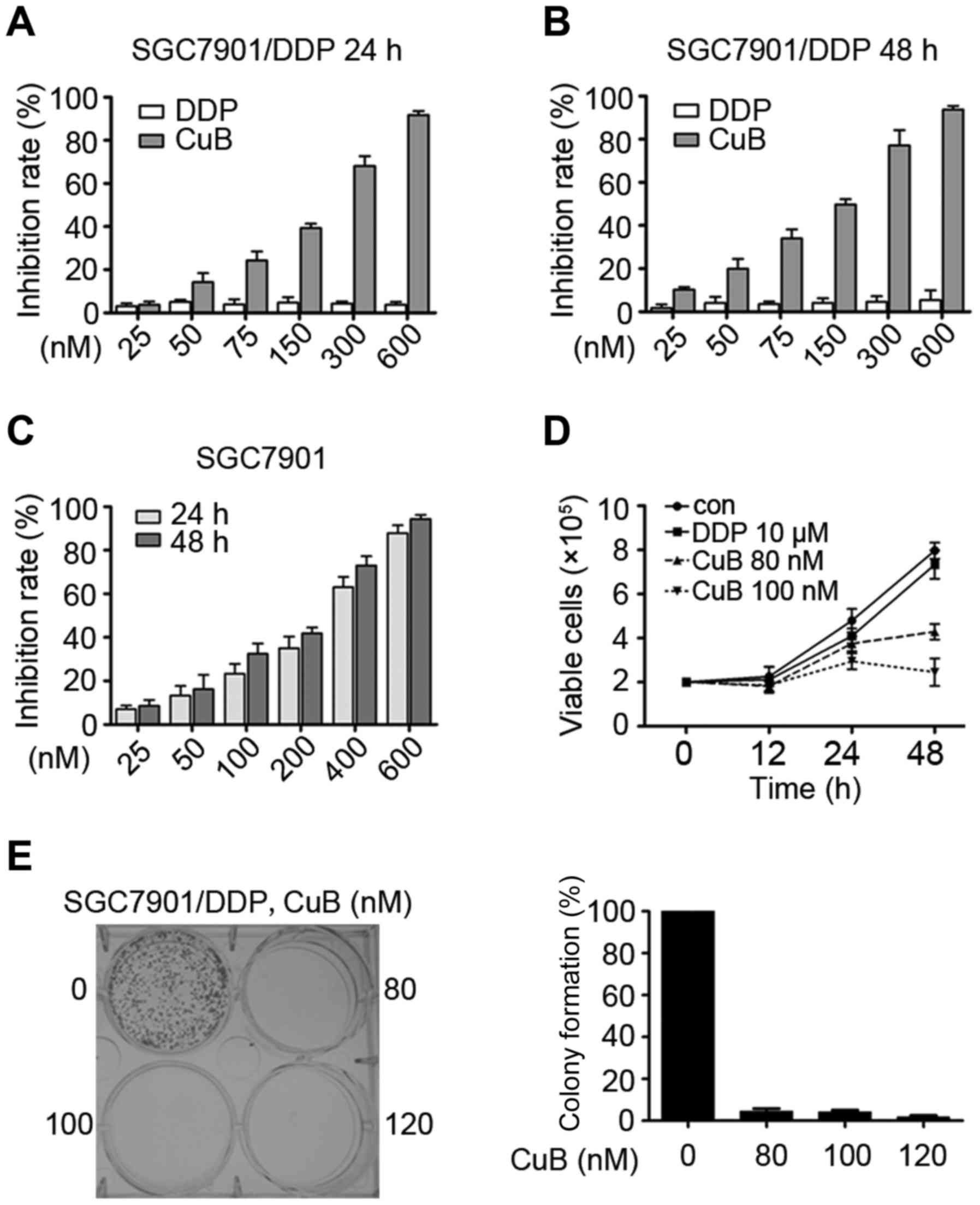
SGC7901 and SGC7901/DDP cells were seeded in 96-well
plates for 24 h, 48 h and then treated with different
concentrations of CuB and DDP (Fig.
2A-C). After 24 or 48 h, the cell viability was evaluated by
the MTT assay according to the manual. Absorbance at 490 nm was
measured on an automated microplate reader. We found that CuB had
moderate cytotoxicity to SGC7901 and SGC7901/DDP cells with an
IC50 of 216.70 and 170.25 nM (Table I). By trypan blue exclusion assay,
we found that CuB rapidly reduced viable SGC7901/DDP cells
(Fig. 2D) in a dose- and
time-dependent manner. We investigated the CuB effect on cell
colony formation activity, and the results showed that CuB
significantly inhibited the clonogenic ability of SGC7901/DDP
(Fig. 2E). These results suggested
that CuB inhibited the anchorage-dependent (cell proliferation) and
anchorage-independent (colony formation) growth of SGC7901/DDP
cells.
 | Table I.IC50 of CuB on gastric
cancer cell lines. |
Table I.
IC50 of CuB on gastric
cancer cell lines.
| Cell lines | SGC7901 | SGC7901/DDP |
|---|
| IC50
(nM) | 216.70±34.23 | 170.25±26.78 |
CuB induces apoptosis and autophagic
death of SGC7901/DDP GC cells
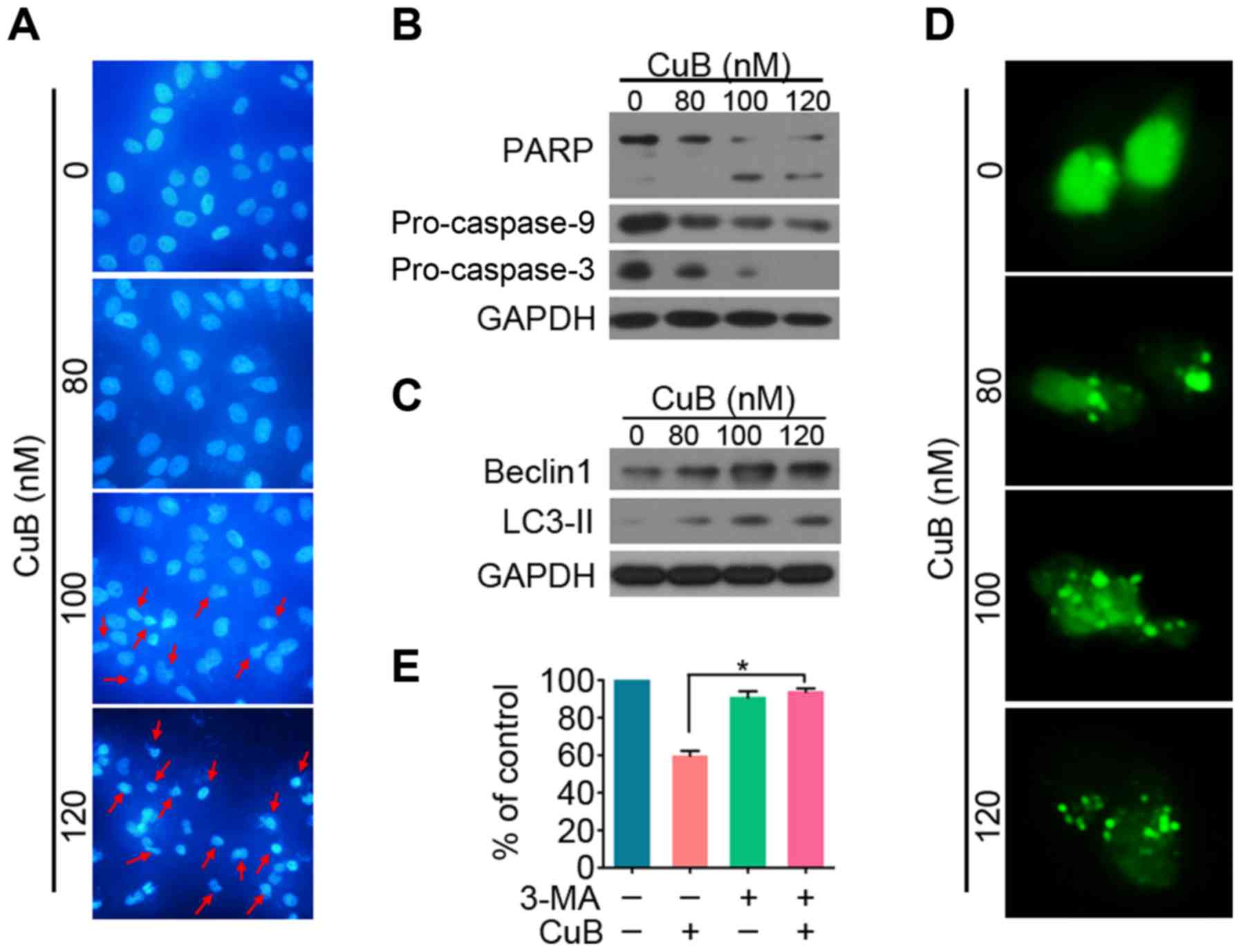
To investigate whether CuB induced apoptosis in
SGC7901/DDP GC cells, microscopy with DAPI staining was carried
out. As shown in Fig. 3A,
cytoplasmic shrinkage occurred after treatment with 100 nM CuB,
supporting that apoptosis was induced. To determine which cell
death pathways was induced by CuB, SGC7901/DDP cells were treated
with increasing concentrations of CuB for 24 h. Western blotting
results showed that PARP cleavage, as well as a significant
dose-dependent decrease in pro-caspases-3 and −9 (pro-casp-3 and
pro-casp-9), were detected in CuB-treated cells (Fig. 3A and B). These results indicate that
CuB induces cell death via caspase-dependent apoptosis.
Autophagy is a lysosomal degradation process for
cytoplasmic constituents during the stress condition. To assess
whether autophagy is also involved in CuB-induced cell death, we
subsequently evaluated the expression level of LC3 II and Beclin1
of cells treated with CuB. A marked increase of LC3 II and Beclin1
was observed in CuB treated cells (Fig.
3C). When autophagy is initiated, LC3 is cut on the C-terminal
and produces LC3 II protein, which is then transferred on the
autophagosomes (LC3-positive vesicle) (29). To detect the autophagosome
formation, the fluorescent autophagy marker GFP-LC3 plasmid was
transfected into SGC7901/DDP cells which were then treated with CuB
for 24 h, followed by confocal microscopy assessment. We showed
that while control cells displayed a diffuse staining, SGC7901/DDP
cells upon CuB exhibited a speckled fluorescent staining pattern,
indicating the redistribution of LC3 to autophagosomes (Fig. 3D). Autophagy has been reported to
play contradictory roles in tumor progression or suppression
(30). To demonstrate CuB induced
autophagic cell death, CuB and autophagy inhibitor 3-MA was
combined to treat SGC7901/DDP cells (Fig. 3E). Our results showed that 3-MA
significantly reversed the cell proliferation inhibited by CuB.
Taken together, these findings suggest that CuB induces
caspase-dependent apoptosis and autophagic death of SGC7901/DDP
cells.
CuB influences the expression of P-gp,
HIF-1α and inhibits CIP2A/PP2A/mTORC1 signaling axis in SGC7901/DDP
cells
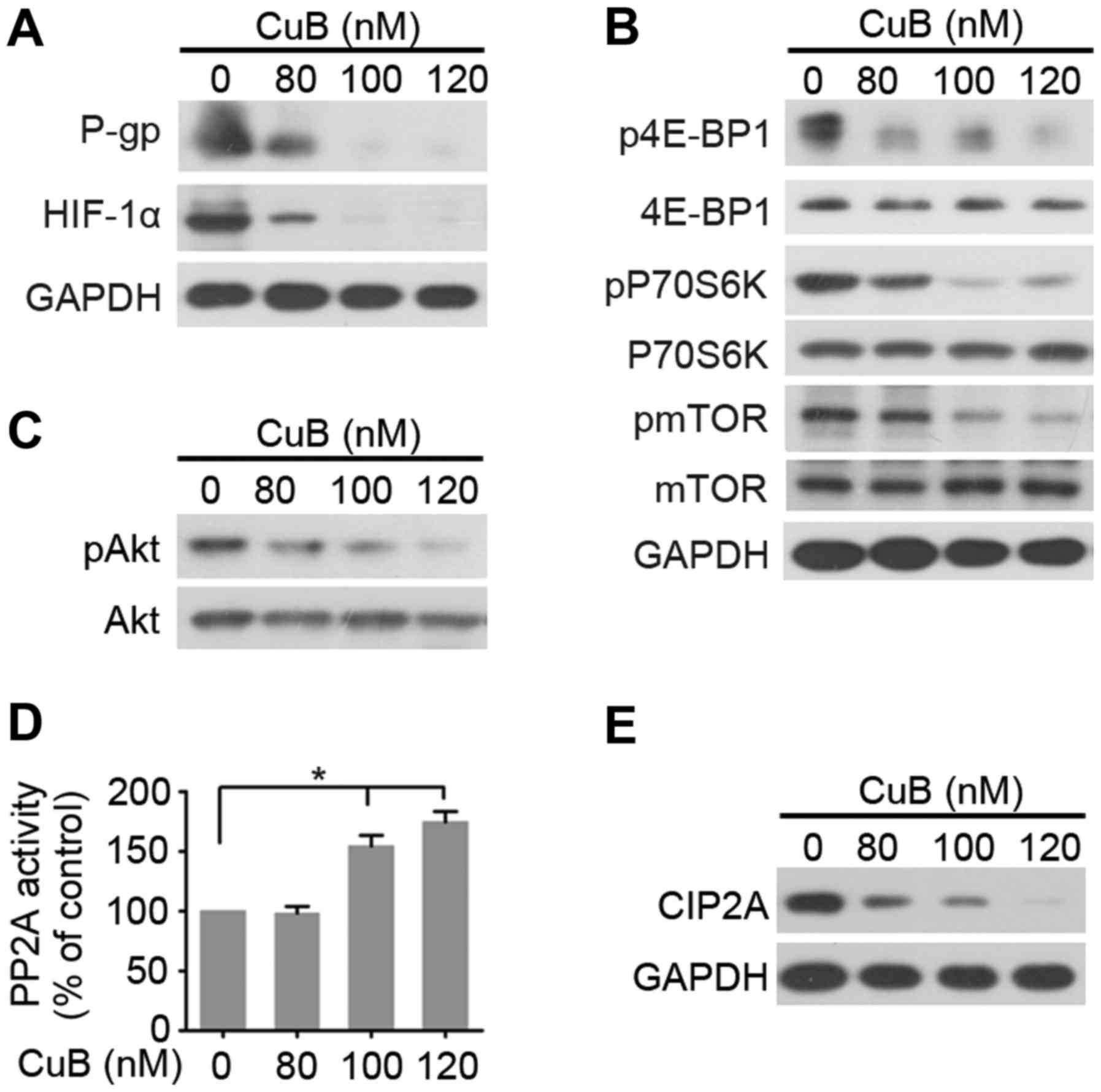
We detected expression levels of MDR related factors
P-gp, and HIF-1α by western blotting analysis (Fig. 4A). The results indicated that P-gp,
and HIF-1α expression of SGC7901/DDP cells were downregulated by
treatment with increasing concentration of CuB. The decreased
expression of P-gp and HIF-1α in SGC7901/DDP cells may in part
contribute to the reversal of MDR. Considering that mTORC1 is a
major negative regulator of autophagy (7), and CuB actives autophagy in
SGC7901/DDP cells, we next determined whether CuB inhibited the
activation of mTORC1. As shown in Fig.
4B, CuB decreased the phosphorylation of mTORC1 effectors
(mTOR, p70S6K and 4E-BP1). Akt is an upstream regulator of mTORC1
and a downstream substrate of serine-threonine phosphatase PP2A
(9). We tested effects of CuB on
Akt phosphorylation (pAkt) and PP2A activity, and found that CuB
downregulated pAkt and upregulated PP2A activity (Fig. 4C and D). These results suggested
that CuB-induced mTORC1 inactivation may be mediated by PP2A/Akt.
CIP2A is an oncogenic PP2A inhibitor protein that is highly
expressed in malignant cancers including GC (31). Furthermore, we tested effects of CuB
on CIP2A expression and found that the protein level of CIP2A
decreased (Fig. 4E), indicating
that CuB may target CIP2A to reactivate PP2A, then inhibits
Akt/mTORC1 signal pathway.
CIP2A is essential for CuB-induced
proliferation, autophagy, and apoptosis
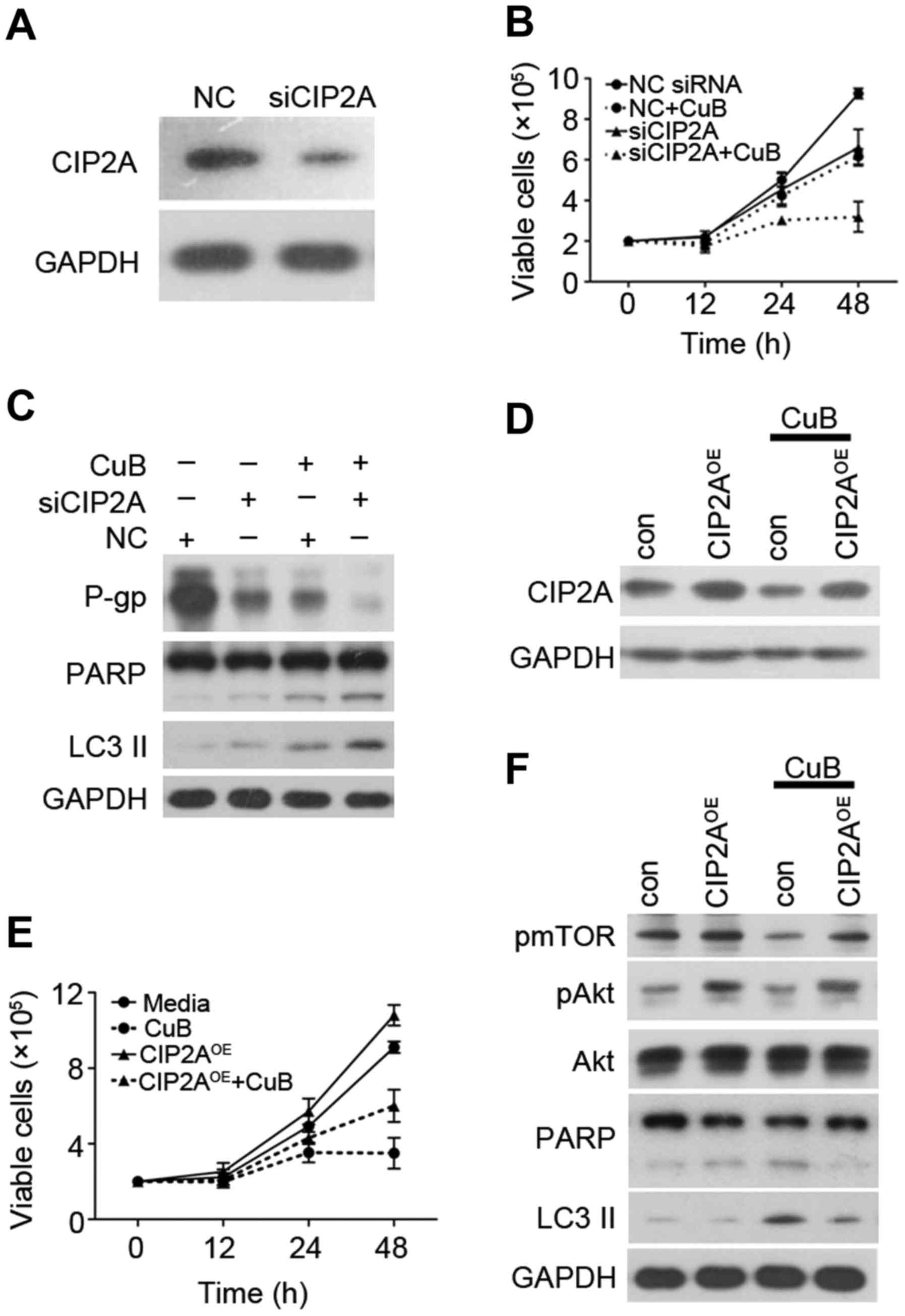
We next examined whether CIP2A depletion would alter
cellular sensitivity to CuB. Two siRNAs targeting CIP2A were
synthesized and used. As shown in Fig.
5A, knockdown by siRNA markedly decreased CIP2A protein in
SGC7901/DDP cells. These data showed that CIP2A siRNA was specific
and efficient in reducing CIP2A expression. To evaluate the role
CIP2A plays in CuB-induced proliferation inhibition, SGC7901/DDP
cells were transfected with siRNA targeting CIP2A, followed by CuB
treatment. Cell viability and western blotting were used to detect
cell growth and protein expression. CIP2A depletion enhanced
CuB-induced growth inhibition (Fig.
5B), promoted CuB-induced autophagy and apoptosis effect
(Fig. 5C). Of note, CIP2A depletion
also enhanced CuB-induced MDR inhibition. In addition, we examined
whether an increase in CIP2A expression would alter cellular
sensitivity to CuB. SGC7901/DDP cells were transfected with CIP2A
plasmid and CIP2A expression was confirmed by western blotting
(Fig. 5D). Notably, CIP2A
overexpression antagonized CuB-induced growth inhibition (Fig. 5E) and inhibited CuB-induced
autophagy and apoptosis effects by upregulating pAkt and pmTOR
(Fig. 5F). These data demonstrate
that CIP2A plays a critical role in CuB-induced SGC7901/DDP
autophagy and apoptosis.
Discussion
DDP, as a first-line chemotherapeutic drug for GC
patients, especially for advanced stage, activated apoptosis by
inducing DNA damage through crosslinking of the DNA (32). However, cancer cells often develop
several mechanisms to overcome DDP-induced apoptosis and DNA
damage, leading to DDP resistance (33–35).
Therefore, investigation of the molecular mechanism conferring DDP
resistance is urgently needed.
CuB is a representative natural compound for
anti-cancer activities. Recently, CuB was reported to enhance the
anticancer effects of DDP in lung, ovarian, cutaneous squamous cell
carcinoma, and laryngeal squamous cell carcinoma (20,36–38).
However, the relationship between CuB and DDP-resistance in GC
cells has yet to be firmly established. In the present study, CuB
alone showed an inhibitory effect on both DDP-sensitive and
DDP-resistant GC cell lines (Fig.
2).
Apoptosis, which consists of extrinsic and intrinsic
pathways, is the main cell death response to chemotherapy.
Furthermore, evading apoptosis is one of the hallmarks of
chemoresistance, and targeting apoptosis has become a cancer
therapeutic strategy (39).
Apoptosis is accompanied by various morphological changes,
including nuclear condensation, DNA fragmentation, and apoptotic
bodies. Nuclear morphology of SGC7901/DDP cells was analyzed using
DAPI staining; significant nucleus condensation change in CuB
treated SGC7901/DDP was observed, which are typical characteristics
of apoptosis (Fig. 3A). The
extrinsic and intrinsic apoptotic pathways that ultimately lead to
activation of effector casp-3 have been characterized (40,41).
In SGC7901/DDP cells, treatment with CuB for 24 h caused
downregulation of pro-casp-9 with generation of activated casp-9
(Fig. 3B). The intrinsic apoptotic
signal led to activation of casp-3, reflected by a decrease of
pro-casp-3 and increase of active casp-3 with cleavage of its
substrate PARP (Fig. 3B). Thus, CuB
may trigger apoptosis by activating the intrinsic apoptosis pathway
which results in activation of effector casp-9. Autophagy is a
programmed cell death that plays an important role in tumor
progression and chemoresistance (42). In autophagy, LC3 II plays
indispensable roles in autophagosome formation. Beclin1 functions
as the key factor in the formation of autophagosomes (10). Our present results illustrated that
CuB activated autophagy by the changes of LC3 II and Beclin 1
levels (Fig. 3C). In our further
study, drug resistance is largely mediated through overexpression
of MDR, HIF-1α, drug resistance protein, and proteasome subunits,
increases in antioxidant defenses, and TOP2 activity; these results
have been widely verified (33–35).
The present study identified that the treatment of CuB was able to
reverse the MDR of the SGC7901/DDP cells via the downregulation of
P-gp, and HIF-1α (Fig. 4A).
CIP2A, originally named KIAA1524 or P90, was cloned
from patients with HCC (14).
Induction of CIP2A is often associated with chemoresistance in
cancer cells, and the inhibition of CIP2A in combination with
chemotherapy may enhance the efficacy of cancer treatment (43). The functions of CIP2A in autophagy
are not fully understood yet. Puustinen et al reported that
the CIP2A/PP2A/mTORC1 signal axis is responsible for promoting cell
growth and autophagy inhibition (7). They found that CIP2A can associate
with mTORC1 and act as an allosteric inhibitor of mTORC1-associated
PP2A, thereby enhancing mTORC1-dependent growth signaling and
inhibiting autophagy. Our present study demonstrated that CuB
promoted the formation of autophagy via the inhibition of mTORC1
through dephosphorylating p70S6K and 4E-BP1 (Fig. 4B). Next, we detected CIP2A
expression and PP2A activation and found that CuB significantly
downregulated CIP2A expression and upregulated PP2A activity
(Fig. 4C-E). Furthermore, we
knocked down CIP2A expression in SGC7901/DDP cells and found that
CIP2A depletion significantly promotes CuB induced apoptosis,
autophagy, and reversed MDR (Fig.
5A-C). Moreover, our results showed that CIP2A overexpression
significantly antagonized CuB induced cell proliferation, apoptosis
and autophagy (Fig. 5D-F). Our data
validated the mechanism by which CuB-induced cancer cell apoptosis
and autophagy in SGC7901/DDP cells, that is, induction of cancer
cell apoptosis and autophagy by inhibiting CIP2A to reactivate PP2A
and enhance PP2A-dependent mTORC1 inactivation.
In conclusion, our results suggest that further
studies on the detailed molecular modification of the
CIP2A/PP2A/mTORC1 signaling axis by CuB and exploring its possible
application in other malignant diseases are warranted.
Acknowledgements
This work was supported by grants from Open Ended
Design Project from Hubei Province Key Laboratory of Conservation
Biology for Shennongjia Golden Monkey (grant no. 2016SNJ001); the
Natural Science Foundation of Hubei Province of China (grant no.
2016CFB528); the Foundation of Health and Family planning
Commission of Hubei Province (grant no. WJ2017F067); the Foundation
of Hubei University of Medicine (FDFR201605); the Foundation for
Innovative Research Team of Hubei University of Medicine
(2014CXX05); the Key Discipline Project of Hubei University of
Medicine and the National Training Program of Innovation and
Entrepreneurship for undergraduates (grant no. 201610929001).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang X, Cai H, Liang Y, Chen L, Wang X, Si
R, Qu K, Jiang Z, Ma B, Miao C, et al: Inhibition of c-Myc by
let-7b mimic reverses mutidrug resistance in gastric cancer cells.
Oncol Rep. 33:1723–1730. 2015.PubMed/NCBI
|
|
4
|
Faneyte IF, Kristel PM, Maliepaard M,
Scheffer GL, Scheper RJ, Schellens JH and van de Vijver MJ:
Expression of the breast cancer resistance protein in breast
cancer. Clin Cancer Res. 8:1068–1074. 2002.PubMed/NCBI
|
|
5
|
Hong L, Piao Y, Han Y, Wang J, Zhang X, Du
Y, Cao S, Qiao T, Chen Z and Fan D: Zinc ribbon domain-containing 1
(ZNRD1) mediates multidrug resistance of leukemia cells through
regulation of P-glycoprotein and Bcl-2. Mol Cancer Ther.
4:1936–1942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Radisavljevic Z: AKT as locus of cancer
multidrug resistance and fragility. J Cell Physiol. 228:671–674.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puustinen P, Rytter A, Mortensen M,
Kohonen P, Moreira JM and Jäättelä M: CIP2A oncoprotein controls
cell growth and autophagy through mTORC1 activation. J Cell Biol.
204:713–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demitrack ES, Gifford GB, Keeley TM,
Carulli AJ, VanDussen KL, Thomas D, Giordano TJ, Liu Z, Kopan R and
Samuelson LC: Notch signaling regulates gastric antral LGR5 stem
cell function. EMBO J. 34:2522–2536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erazo T, Lorente M, López-Plana A,
Muñoz-Guardiola P, Fernández-Nogueira P, García-Martínez JA,
Bragado P, Fuster G, Salazar M, Espadaler J, et al: The new
antitumor drug ABTL0812 inhibits the Akt/mTORC1 axis by
upregulating Tribbles-3 pseudokinase. Clin Cancer Res.
22:2508–2519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin HO, Hong SE, Park JA, Chang YH, Hong
YJ, Park IC and Lee JK: Inhibition of JNK-mediated autophagy
enhances NSCLC cell sensitivity to mTORC1/2 inhibitors. Sci Rep.
6:289452016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agarwal S, Bell CM, Taylor SM and Moran
RG: p53 deletion or hotspot mutations enhance mTORC1 activity by
altering lysosomal dynamics of TSC2 and Rheb. Mol Cancer Res.
14:66–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu WD, Hu ZM, Shang MJ, Zhao DJ, Zhang CW,
Hong DF and Huang DS: Cordycepin down-regulates multiple drug
resistant (MDR)/HIF-1α through regulating AMPK/mTORC1 signaling in
GBC-SD gallbladder cancer cells. Int J Mol Sci. 15:12778–12790.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puustinen P and Jäättelä M: KIAA1524/CIP2A
promotes cancer growth by coordinating the activities of MTORC1 and
MYC. Autophagy. 10:1352–1354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Junttila MR, Puustinen P, Niemelä M, Ahola
R, Arnold H, Böttzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, et
al: CIP2A inhibits PP2A in human malignancies. Cell. 130:51–62.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Ge Z, Liu C, Liu Z, Björkholm M, Jia
J and Xu D: CIP2A is overexpressed in gastric cancer and its
depletion leads to impaired clonogenicity, senescence, or
differentiation of tumor cells. Clin Cancer Res. 14:3722–3728.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Ma L, Wen ZS, Hu Z, Wu FQ, Li W,
Liu J and Zhou GB: Cancerous inhibitor of PP2A is targeted by
natural compound celastrol for degradation in non-small-cell lung
cancer. Carcinogenesis. 35:905–914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren J, Li W, Yan L, Jiao W, Tian S, Li D,
Tang Y, Gu G, Liu H and Xu Z: Expression of CIP2A in renal cell
carcinomas correlates with tumour invasion, metastasis and
patients' survival. Br J Cancer. 105:1905–1911. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu CY, Shiau CW, Kuo HY, Huang HP, Chen
MH, Tzeng CH and Chen KF: Cancerous inhibitor of protein
phosphatase 2A determines bortezomib-induced apoptosis in leukemia
cells. Haematologica. 98:729–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Xu B, Sun C, Wang L and Miao X:
Knockdown of CIP2A sensitizes ovarian cancer cells to cisplatin: An
in vitro study. Int J Clin Exp Med. 8:16941–16947. 2015.PubMed/NCBI
|
|
20
|
El-Senduny FF, Badria FA, El-Waseef AM,
Chauhan SC and Halaweish F: Approach for chemosensitization of
cisplatin-resistant ovarian cancer by Cucurbitacin B. Tumour Biol.
37:685–698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To
Y, Lee WH, Li M, Chu KH and Toh M: Cucurbitacin B induces apoptosis
and S phase cell cycle arrest in BEL-7402 human hepatocellular
carcinoma cells and is effective via oral administration. Cancer
Lett. 294:118–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan KT, Li K, Liu SL, Chu KH, Toh M and
Xie WD: Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway
in leukemia cell line K562. Cancer Lett. 289:46–52. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Cao W, Zhang B, Liu YQ, Wang ZY, Wu
YP, Yu XJ, Zhang XD, Ming PH, Zhou GB, et al: The natural compound
magnolol inhibits invasion and exhibits potential in human breast
cancer therapy. Sci Rep. 3:30982013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng T, Cao W, Shen W, Zhang L, Gu X, Guo
Y, Tsai H, Liu X, Li J, Zhang J, et al: Arctigenin inhibits STAT3
and exhibits anticancer potential in human triple-negative breast
cancer therapy. Oncotarget. 8:329–344. 2017.PubMed/NCBI
|
|
25
|
Cao W, Liu Y, Zhang R, Zhang B, Wang T,
Zhu X, Mei L, Chen H, Zhang H, Ming P, et al: Homoharringtonine
induces apoptosis and inhibits STAT3 via IL-6/JAK1/STAT3 signal
pathway in Gefitinib-resistant lung cancer cells. Sci Rep.
5:84772015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu
CC, Lin JP, Tang NY, Chung JG, Chou MJ, et al: Quercetin-mediated
cell cycle arrest and apoptosis involving activation of a caspase
cascade through the mitochondrial pathway in human breast cancer
MCF-7 cells. Arch Pharm Res. 33:1181–1191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao X, He Z, Cao W, Cai F, Zhang L, Huang
Q, Fan C, Duan C, Wang X, Wang J, et al: Oridonin inhibits
gefitinib-resistant lung cancer cells by suppressing
EGFR/ERK/MMP-12 and CIP2A/Akt signaling pathways. Int J Oncol.
48:2608–2618. 2016.PubMed/NCBI
|
|
28
|
Cai F, Zhang L, Xiao X, Duan C, Huang Q,
Fan C, Li J, Liu X, Li S and Liu Y: Cucurbitacin B reverses
multidrug resistance by targeting CIP2A to reactivate protein
phosphatase 2A in MCF-7/adriamycin cells. Oncol Rep. 36:1180–1186.
2016.PubMed/NCBI
|
|
29
|
Qian HR and Yang Y: Functional role of
autophagy in gastric cancer. Oncotarget. 7:17641–17651.
2016.PubMed/NCBI
|
|
30
|
Lin L and Baehrecke EH: Autophagy, cell
death, and cancer. Mol Cell Oncol. 2:e985913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rincón R, Cristóbal I, Zazo S, Arpí O,
Menéndez S, Manso R, Lluch A, Eroles P, Rovira A, Albanell J, et
al: PP2A inhibition determines poor outcome and doxorubicin
resistance in early breast cancer and its activation shows
promising therapeutic effects. Oncotarget. 6:4299–4314. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simonian PL, Grillot DA and Nuñez G: Bcl-2
and Bcl-XL can differentially block chemotherapy-induced cell
death. Blood. 90:1208–1216. 1997.PubMed/NCBI
|
|
33
|
Alcantara LM, Kim J, Moraes CB, Franco CH,
Franzoi KD, Lee S, Freitas-Junior LH and Ayong LS:
Chemosensitization potential of P-glycoprotein inhibitors in
malaria parasites. Exp Parasitol. 134:235–243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo L, Sun YJ, Yang L, Huang S and Wu YJ:
Avermectin induces P-glycoprotein expression in S2 cells via the
calcium/calmodulin/NF-κB pathway. Chem Biol Interact. 203:430–439.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Zhu ZA, Liu QH, Kong QY, Liu L, Cui
T and Wu YP: RAD001 can reverse drug resistance of SGC7901/DDP
cells. Tumour Biol. 35:9171–9177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marostica LL, Silva IT, Kratz JM, Persich
L, Geller FC, Lang KL, Caro MS, Durán FJ, Schenkel EP and Simões
CM: Synergistic antiproliferative effects of a new Cucurbitacin B
derivative and chemotherapy drugs on lung cancer cell line A549.
Chem Res Toxicol. 28:1949–1960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen W, Leiter A, Yin D, Meiring M, Louw
VJ and Koeffler HP: Cucurbitacin B inhibits growth, arrests the
cell cycle, and potentiates antiproliferative efficacy of cisplatin
in cutaneous squamous cell carcinoma cell lines. Int J Oncol.
37:737–743. 2010.PubMed/NCBI
|
|
38
|
Liu T, Peng H, Zhang M, Deng Y and Wu Z:
Cucurbitacin B, a small molecule inhibitor of the Stat3 signaling
pathway, enhances the chemosensitivity of laryngeal squamous cell
carcinoma cells to cisplatin. Eur J Pharmacol. 641:15–22. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang M, Zhang H, Tang F, Wang Y, Mo Z,
Lei X and Tang S: Doxorubicin resistance mediated by cytoplasmic
macrophage colony-stimulating factor is associated with switch from
apoptosis to autophagic cell death in MCF-7 breast cancer cells.
Exp Biol Med (Maywood). 241:2086–2093. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Come C, Laine A, Chanrion M, Edgren H,
Mattila E, Liu X, Jonkers J, Ivaska J, Isola J, Darbon JM, et al:
CIP2A is associated with human breast cancer aggressivity. Clin
Cancer Res. 15:5092–5100. 2009. View Article : Google Scholar : PubMed/NCBI
|