Introduction
Angiogenesis, a process involving the formation of
new blood vessels from pre-existing vessels, is an essential event
in a variety of physiological processes such as embryonic
development, ovulation and wound healing, as well as pathological
conditions such as cancer, chronic inflammation, arthritis,
aneurysms and arteriovenous malformations (1,2). It is
now well-known that angiogenesis is vital for tumor growth,
invasion and metastasis, which contribute to over 90% of deaths in
various types of cancers, including human breast cancer (3,4).
Modulating tumor-associated angiogenesis thus represents a
promising strategy for the development of anticancer therapies
(5,6). In the last decades, several drugs that
target tumor vascularization and inhibit tumor angiogenesis have
been developed and approved by the US Food and Drug Administration
for clinical use, such as the humanized anti-VEGF-A antibody
bevacizumab, and the tyrosine kinase inhibitors sorafenib and
sunitinib (6,7).
The vascular endothelial growth factor (VEGF) family
of proteins play a pivotal role in tumor angiogenesis by increasing
vascular permeability and endothelial cell proliferation, migration
and invasion into surrounding tissues (8). Cellular responses to VEGF are mainly
mediated by the receptor tyrosine kinase VEGFR2 (also known as
Flk-1) on the surface of endothelial cells (9). The activation of Akt/mTOR/p70S6K
mediated by the HIF-1α/VEGF-receptor (VEGFR) alliance triggers many
functions in tumorigenesis such as tumor cell proliferation,
angiogenesis and metastasis (10–12).
Consequently, the discovery of novel HIF-1α/VEGF and
Akt/mTOR/p70S6K pathway inhibitors shows great promise for
anticancer therapeutics.
Panax ginseng (P. ginseng) is a
traditional herbal medicine popular in China, Korea and Japan. It
has a wide range of beneficial effects in the treatment of
cardiovascular or cerebrovascular diseases, immune deficiency,
aging, as well as cancer (13,14).
Saponins, commonly known as ginsenosides, are the main active
ingredients in P. ginseng. Among more than 150 ginsenosides
that have been identified (15),
ginsenoside Rd (Rd) (Fig. 1A) has
attracted increasing attention. It displays a remarkable
neuroprotective effect on cerebral ischemia (16), and can attenuate myocardial
ischemia-reperfusion injury (17).
Moreover, increasing evidence indicates that Rd exerts significant
antiproliferative/pro-apoptotic effects on diverse cancers,
including breast, gastric, liver and cervical cancers (18–20),
through the negative regulation of various oncogenic molecules such
as the melastatin type transient receptor potential 7 (TRPM7)
channel, cell cycle progression or the induction of caspase
activity. However, there is no evidence on its anti-angiogenic
potential and effect on the Akt/mTOR/p70S6K signaling cascade. In
the present study, we reported for the first time that Rd
suppressed VEGF-induced angiogenesis and the Akt/mTOR/p70S6K
signaling cascade under both normoxic and hypoxic conditions in
human umbilical vascular endothelial cells (HUVECs), an extensively
used in vitro model for angiogenesis research (21). Our findings may contribute to the
potential use of Rd as an anticancer drug.
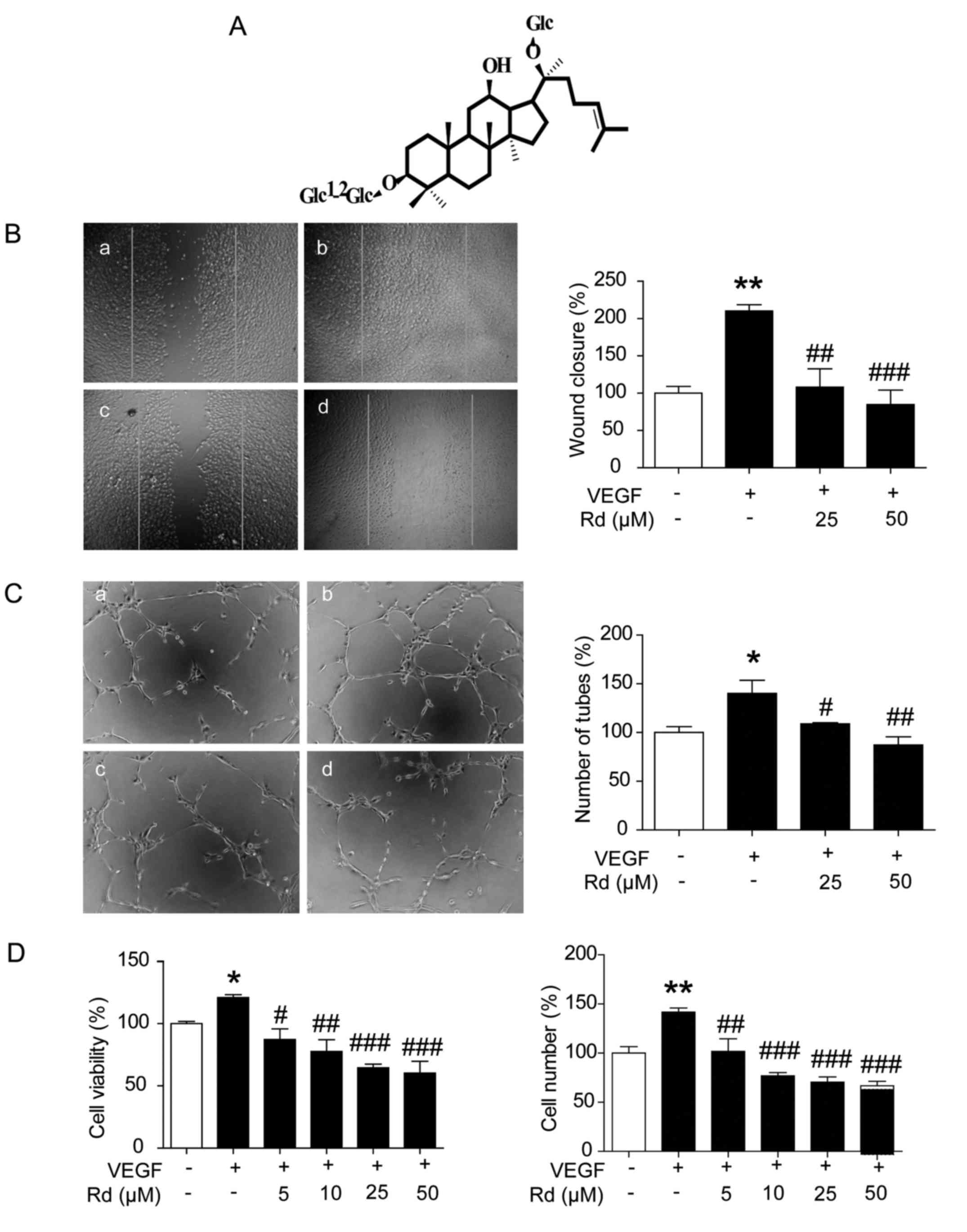 | Figure 1.Rd inhibits the VEGF-induced
proliferation, migration and capillary-structure formation of
HUVECs. (A) The molecular structure of ginsenoside Rd. (B) Rd
inhibited HUVEC migration. HUVECs were scratched by pipette tips
and treated with or without VEGF (10 ng/ml) and Rd. The migrated
cells were quantified by assessing the width of the scratches and
are expressed as the percentage to that of the untreated cells. a,
control; b, VEGF; c, VEGF + Rd (25 µM); d, VEGF + Rd (50 µM). (C)
Rd inhibited VEGF-induced tube formation in Matrigel. Tubes forming
intact networks were quantified by counting the number of branch
points from 5 random fields/well in a blinded manner under an
inverted microscope. a, control; b, VEGF; c, VEGF + Rd (25 µM); d,
VEGF + Rd (50 µM). (D) Rd inhibited VEGF-induced cell viability and
the number of HUVECs; *P<0.05, **P<0.01 vs. the control;
#P<0.05, ##P<0.01,
###P<0.001 vs. VEGF alone. Rd, ginsenoside Rd;
HUVECs, human umbilical vascular endothelial cells; VEGF, vascular
endothelial growth factor. |
Materials and methods
Reagents
Rd was obtained from the Shanghai Research Center
for Standardization of Chinese Medicines (Shanghai, China). Its
structure was confirmed using 1HNMR and 13C
NMR spectral analysis, and its purity was >98% as determined by
high pressure liquid chromatography (HPLC) analysis.
Phospho-p85 PI3K (Tyr458), PI3K, phospho-Akt
(Thr308), Akt, phospho-mTOR (Ser2481), mTOR, cleaved caspase-3,
Bax, Bcl-2, VEGFR2, GAPDH, goat anti-rabbit horseradish peroxidase
(HRP)-conjugated, and goat anti-mouse HRP antibodies were obtained
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Other
antibodies against Ki67, HIF-1α and CD31 were provided by Abcam
(Cambridge, UK). The Pierce BCA protein assay kit was purchased
from Thermo Fisher Scientific (Waltham, MA, USA). Human recombinant
VEGF was supplied by PeproTech (Rocky Hill, NJ, USA). All of the
other reagents were obtained from Sigma-Aldrich (St. Louis, MO,
USA) unless otherwise indicated.
Cell lines
HUVECs were cultured in endothelial cell medium
(ECM; ScienCell, San Diego, CA, USA) supplemented with 5% fetal
bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin
(both from Gibco, Gaithersburg, MD, USA). Breast cancer cell line
MDA-MB-231 obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA) was maintained in Dulbeccos modified
Eagles medium (DMEM) containing 10% FBS, 100 U/ml of penicillin and
100 µg/ml streptomycin. All the cells were cultured at 37°C with
95% humidity and a 5% CO2 gas environment.
Cell viability assay
HUVECs or MDA-MB-231 cells were treated with or
without VEGF (10 ng/ml) and Rd for 48 h. The cell viability was
determined using MTT assay (Sigma-Aldrich). The number of cells
were counted after trypsinizing HUVECs. In addition, the final cell
viability and the numbers of the treated cells were expressed as a
percentage relative to that of the untreated control cells.
Flow cytometric analysis
MDA-MB-231 cells were treated with Rd at 0, 25 and
50 µM for 24 h. The cells were collected by centrifugation at 400 ×
g, and stained with propidium iodide (PI) (50 µg/ml) and Annexin
V-FITC (2 µg/ml) for 15 min in the dark. The staining was then
immediately analyzed by flow cytometry using the FACScan and
CellQuest program. The FCS Express program (BD Biosciences, San
Jose, CA, USA) was used to determine the percentage of apoptotic
cells.
Wound healing migration assay
The wound healing migration assay was performed as
previously described (22).
Briefly, HUVECs were treated with mitomycin C to inactivate cell
proliferation. Scratches were drawn with sterile pipette tips.
Fresh ECM was added with or without VEGF (10 ng/ml) and different
concentrations of Rd. Images of the cells were captured using an
inverted microscope (Olympus CKX41; Olympus, Tokyo, Japan) after
incubation at 37°C for 10 h. The width of the scratches was
evaluated and used as the indicator for the assessment of cell
migration ability.
Capillary-like tube formation
assay
After incubation with ECM containing 1% FBS for 4 h,
HUVECs were seeded at a density of 1×104 cells/well into
Matrigel (BD Biosciences, Bedford, MA, USA) coated 96-well plates
followed by treatment with Rd at different concentrations for 4 h.
Tubes forming intact networks were quantified by counting the
number of branch points from 5 random fields/well in a blinded
manner under an inverted microscope.
Rat aortic ring assay
Rat aortic ring assay was performed as previously
described (23). In brief, aortas
isolated from Sprague-Dawley rats were cleaned of fibroadipose
tissue and collateral vessels, and cut into rings of 1–1.5 mm of
thickness. The aortic rings were randomly placed into growth factor
reduced Matrigel-coated 48-well plates and further overlayed with
100 µl of Matrigel. Medium with or without VEGF (10 ng/ml)
supplemented with different concentrations of Rd was added to the
wells and incubated with the aortic rings for 6 days. At the end of
the incubation period, the microvessel sprouts that had formed were
fixed and photographed using an inverted microscope. After images
were acquired, the outgrowth area was delineated and measured using
Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA),
and used for the assessment of angiogenesis.
Matrigel plug assay
Matrigel plug assay is a widely used method to
assess the in vivo anti-angiogenic effect of drugs (24). To examine the anti-angiogenic
property of Rd, Matrigel (0.5 ml) containing 100 ng VEGF and 20 U
of heparin with or without Rd (25 and 50 µM) were subcutaneously
injected into the ventral area of female C57BL/6 mice (5 weeks old,
n=6/group). After 7 days, the mice were sacrificed and the intact
Matrigel plugs were isolated and photographed. The hemoglobin in
the Matrigel plugs was quantified using Drabkin's reagent kit
(Sigma-Aldrich) according to the manufacturer's instructions. The
concentration of hemoglobin was calculated based on a set of
hemoglobin standards. Blood vessels in the Matrigel were visualized
with an antibody against CD31.
Xenograft mouse model
Healthy 5-week-old female athymic nude mice (BALB/c)
were obtained from Shanghai Laboratory Animal Center. All studies
were performed in accordance with the guidelines approved by the
Animal Ethics Committee of Shanghai University of TCM (SHUTCM).
MDA-MB-231 cells were subcutaneously injected (1×107
cells/mouse) into the right flank of each mouse. Treatments were
started 4 days after tumor cell implantation and lasted for 4
weeks. After the tumors grew to ~50 mm3, the
tumor-bearing mice were randomly assigned into 5 groups
(n=10/group): the vehicle control, the doxorubicin (DOC; 10 mg/kg,
once a week for 4 weeks), and the Rd groups (1, 3 and 10 mg/kg).
The vehicle control group received the vehicle solvent [0.1% v/v
dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS)]. The
Rd groups were intraperitoneally administered with Rd diluted in
vehicle solvent daily. The body weight of the mice was monitored
once a week. The tumors were assessed every day using a digital
caliper. The tumor volume was calculated using the formula:
V (mm3) = [ab2] × 0.5, where
a is the length, and b is the width of the tumor. At
the end of treatment, the mice were sacrificed and the tumors of
the mice from the different groups were collected for further
analysis.
Immunohistochemical analysis
Solid tumors were fixed with 10% phosphate-buffered
formalin, embedded in paraffin and longitudinally sectioned at 5-µm
of thickness. The sections were incubated with 3%
H2O2 for 10 min to deactivate the endogenous
peroxidase. For antigen retrieval, the sections were soaked in 10
mM citrate buffer solution (pH 6.0), and heated twice in the
microwave oven. The slides were then washed thoroughly with PBS (pH
7.4). After being blocked with 5% bovine serum albumin (BSA;
Sigma-Aldrich) in TBS for 20 min, the sections were incubated with
primary antibodies against CD31 and Ki67 at 4°C overnight followed
by a thorough wash with PBS. Afterwards, the slides were
sequentially incubated with a biotinylated secondary antibody for
20 min and streptavidin-HRP for another 20 min. The staining was
visualized after incubation with a DAB-H2O2
solution. The slides were then counterstained with hematoxylin for
1 min, dehydrated with ethanol and sealed in resin for microscopic
observation.
Western blot analysis
Cell and tissue homogenates were lysed in lysis
buffer containing 50 mM Tris (pH 7.5), 1 mM EDTA, 150 mM NaCl, 20
mM NaF, 0.5% NP-40, 10% glycerol, 1 mM phenylmethylsulfonyl
fluoride, 10 µg/ml aprotinin, 10 µg/ml leupeptin and 10 µg/ml
pepstatin A on ice. After centrifugation at 12,000 × g for 15 min
at 4°C, the supernatant was collected and the protein concentration
was determined using the BCA method. Total proteins, 30 µg for each
sample, were separated on 12% SDS-PAGE and transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA,
USA). Blocking was performed in 5% BSA (Sigma-Aldrich) in 0.1%
Tween-20 in PBS (PBST) for 1 h. The membranes were probed with
respective primary antibodies overnight at 4°C. Binding of the
primary antibody was detected using a peroxidase-conjugated
secondary antibody (Pierce, Rockford, IL, USA) for 1 h at room
temperature. The blots were developed using ECL detection reagents
(GE Healthcare, Waukesha, WI, USA). The gray intensity of the
protein bands was quantified using ImageJ and normalized to that of
GAPDH in each sample.
Statistical analysis
To examine the difference among multiple groups,
one-way ANOVA followed by Tukey's multiple comparison test were
conducted with GraphPad Prism 5.0. The unpaired t-test was used to
assess the difference between two groups. All data are presented as
the mean ± SEM. A value of p<0.05 was considered as a
significant difference.
Results
Rd inhibits VEGF-induced migration,
vascularization and viability of HUVECs
As endothelial cell migration is one of the most
important and early events during the process of angiogenesis
(25), the wound-healing migration
assay was performed to determine the effects of Rd on HUVEC
migration. Upon stimulation with VEGF, HUVECs migrated much faster
and the wounds healed faster compared with the control (Fig. 1B; p<0.01). Rd treatment at 25 and
50 µM significantly prevented VEGF-induced migration of HUVECs as
the wound healing was delayed compared to the VEGF-treated cells
(p<0.01 and p<0.001). Tube formation assay represents a
simple, reliable and powerful model for studying inhibitors of
angiogenesis. As shown in Fig. 1C,
cells stimulated with VEGF formed robust tubular structures when
seeded on growth factor-reduced two-dimensional Matrigel
(p<0.05). The addition of Rd suppressed the formation of the
capillary-like network (p<0.05 and p<0.01). The process of
angiogenesis also requires the proliferation of endothelial cells.
VEGF alone promoted the cell viability and increased the number of
HUVECs (Fig. 1D; p<0.05,
p<0.01). Rd treatment (5, 10, 25 and 50 µM) mitigated the
VEGF-induced cell viability and number of HUVECs in a
dose-dependent manner (p<0.05, p<0.01 and p<0.001).
Overall, these findings clearly demonstrated that Rd exerted an
anti-angiogenic effect through the inhibition of cell
proliferation, migration and tube formation of endothelial
cells.
Rd mitigates VEGF-induced angiogenesis
ex vivo and in vivo
To study whether Rd affected VEGF-induced
angiogenesis ex vivo, an aortic ring assay was conducted. As
shown in Fig. 2A, VEGF treatment
significantly stimulated microvessel sprouting, leading to the
formation of a network of vessels around the aortic rings
(p<0.001). The addition of Rd at 25 and 50 µm significantly
counteracted the VEGF-induced microvessel sprouting which appeared
to be achieved in a dose-dependent manner (p<0.05 and
p<0.01).
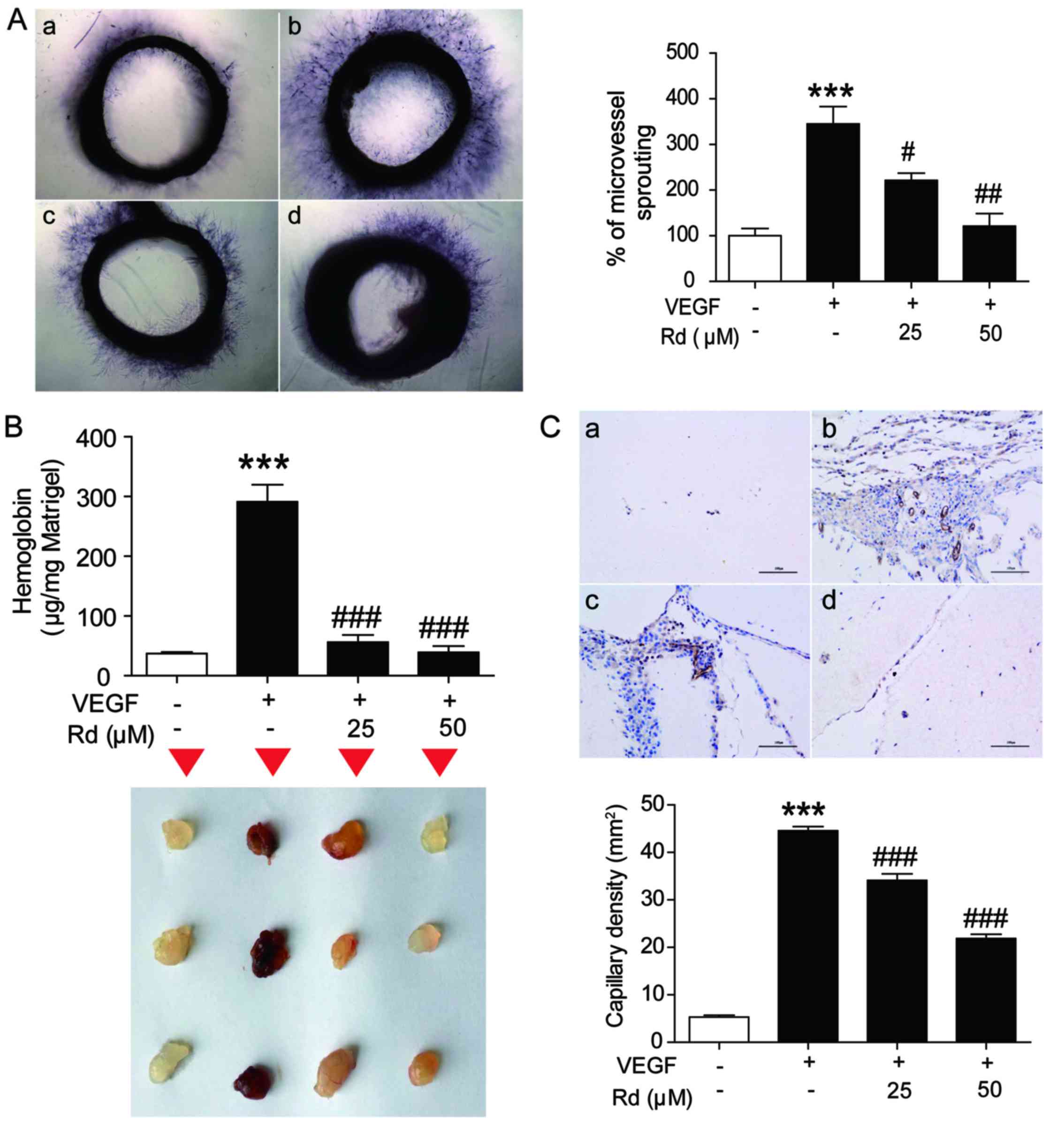 | Figure 2.Rd mitigates VEGF-induced
angiogenesis ex vivo and in vivo. Aortic segments
isolated from Sprague-Dawley rats were placed in the
Matrigel-covered wells and treated with VEGF (10 ng/ml) in the
presence or absence of Rd. (A) Representative images and the
average microvessel area of sprouts from the margins of aortic
rings (n=4/group). a, control; b, VEGF; c, VEGF + Rd (25 µM); d,
VEGF + Rd (50 µM). (B) Upper panel, the hemoglobin content of
Matrigel plugs from the indicated groups (n=3/group). Lower panel,
the representative images of the Matrigel plugs from the indicated
groups. (C) Rd inhibited blood vessel formation in Matrigel plugs.
The Matrigel plugs were fixed, sectioned and stained with the
anti-CD31 antibody (n=3/group). Upper panel, immunostaining of
CD31. Scale bar, 100 µm. Lower panel, CD31 positive capillary
density; ***P<0.001 vs. the control; #P<0.05;
##P<0.01; ###P<0.001 vs. VEGF alone.Rd,
ginsenoside Rd; VEGF, vascular endothelial growth factor. |
To further verify the inhibitory effect of Rd on
angiogenesis in vivo, the Matrigel plug assay was carried
out. As shown in Fig. 2B, Matrigel
plugs containing VEGF alone appeared reddish-brown, inside of which
increased hemoglobin was found (p<0.001), indicating the
formation of functional vasculatures. Accordingly, more CD31
immunoreactive capillaries were found within the VEGF-treated
Matrigel plugs (Fig. 2C) and the
capillary density was significantly higher (p<0.001), compared
with the vehicle-treated control. In contrast, Rd at 25 and 50 µM
markedly inhibited VEGF-induced hemoglobin accumulation in the
Matrigel plugs as the color of the Rd-treated Matrigel plugs became
bleached (Fig. 2B; both
p<0.001). Meanwhile, CD31 immunoreactive capillaries were
decreased in Rd-treated Matrigel plugs (Fig. 2C; p<0.001). All of these results
demonstrated that Rd effectively inhibited angiogenesis in
vivo.
Rd inhibits the VEGF-mediated
signaling cascade for angiogenesis
Interaction of VEGFR2 with VEGF leads to the
activation of various downstream signaling molecules responsible
for endothelial cell migration, proliferation and survival
(26). HIF-1α is a key regulatory
protein in hypoxic response, which is downstream of mTOR signaling
and is an important modulator of VEGF (27). To further elucidate the underlying
mechanism of the anti-angiogenic effect of Rd, the activation of
the signaling molecules in HUVECs were examined under both normoxic
and hypoxic conditions.
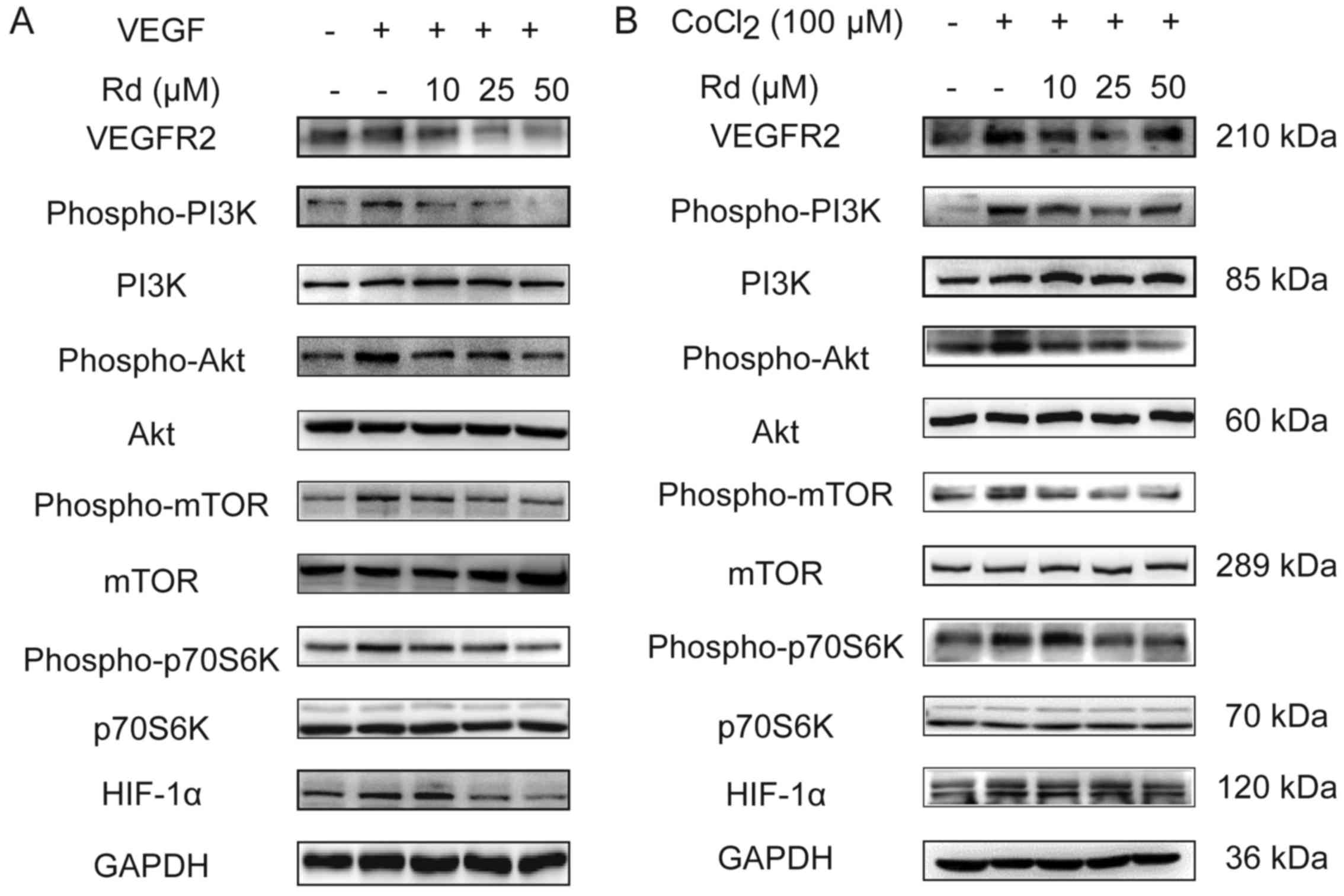
As shown in Fig. 3A,
under normoxic conditions, VEGF induced the expression of VEGFR2,
thereby, enhancing the phosphorylation of the PI3K/Akt/mTOR
signaling molecules. As a result, downstream p70S6K and HIF-1α that
are crucial to the regulation of protein synthesis and angiogenesis
(28) were also phosphorylated.
Conversely, VEGF-induced VEGFR2 was suppressed by Rd in a
dose-dependent manner. Meanwhile, the PI3K/Akt/mTOR signaling
pathway molecules as well as p70S6K and HIF-1α activated by VEGF
were all inhibited with Rd treatment. We next examined the effect
of Rd on the VEGF signaling cascade under hypoxic conditions using
CoCl2, a reagent used widely for the induction of
hypoxia (29,30). Not surprisingly, CoCl2
treatment enhanced the activation of the PI3K/Akt/mTOR/p70S6K
signals and increased the expression of HIF-1α and VEGFR2 (Fig. 3B). However, similar to its effect
under normoxic conditions, Rd treatment diminished the angiogenic
signals induced by CoCl2 on HUVECs. Therefore, Rd
inhibited the VEGF-mediated PI3K/Akt/mTOR/p70S6K signaling cascade
activation in both normoxic and hypoxic conditions.
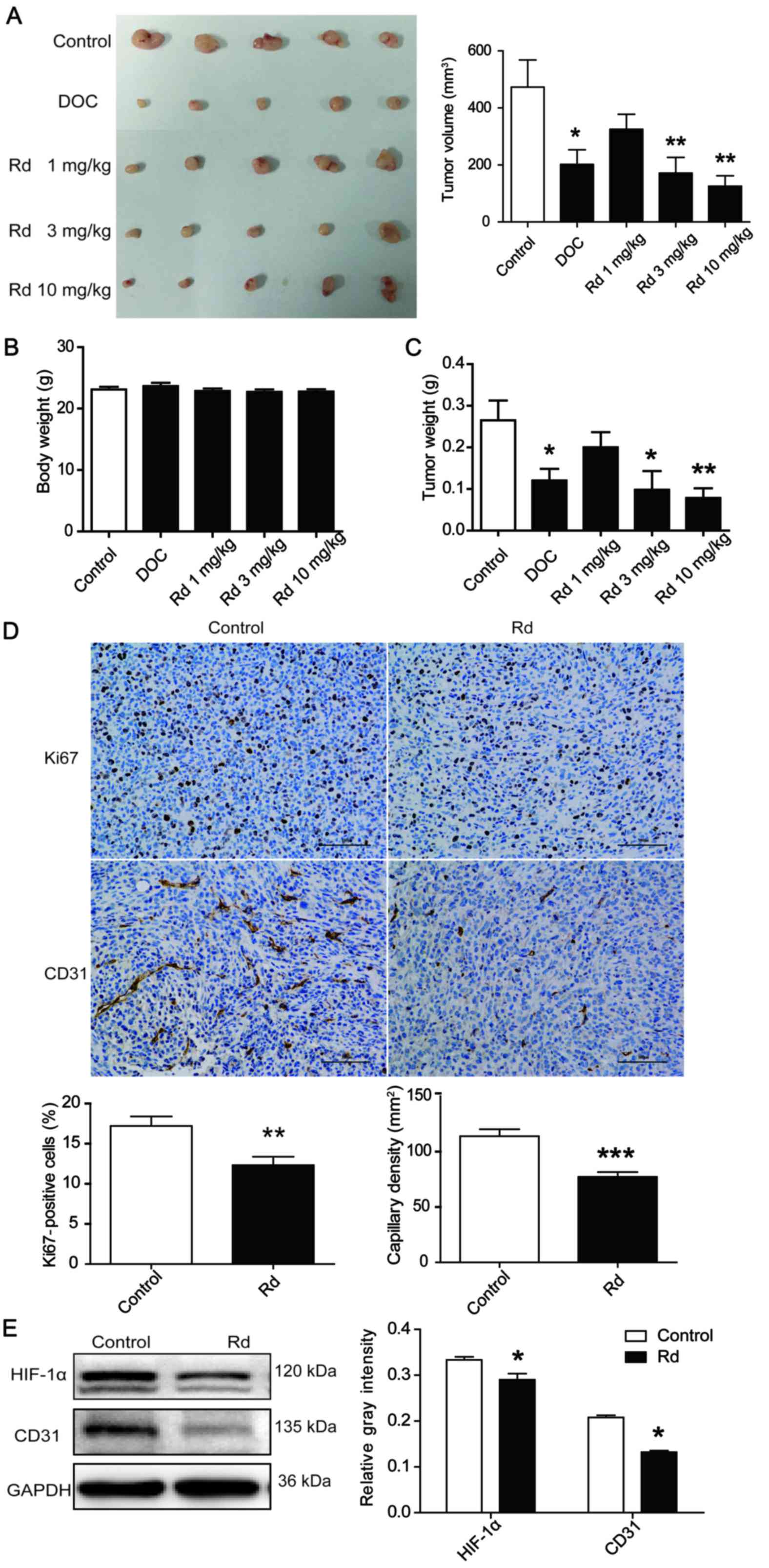
Rd inhibits tumor growth and tumor
angiogenesis in a xenograft mouse model
To investigate the effect of Rd on tumor growth and
tumor angiogenesis in vivo, a human breast tumor-bearing
xenograft mouse model was employed. As shown in Fig. 4A, the administration of Rd at 3 and
10 mg/kg for 28 days substantially suppressed the tumor volume
(Fig. 4A; p<0.01) and decreased
the tumor weight (Fig. 4C;
p<0.05 or p<0.01 in a dose-dependent manner. Notably,
administration of Rd at all experimental doses exhibited no obvious
toxicity on solid tumor model animals as no significant loss of
body weight occurred during the course of the experiment (Fig. 4B). We next evaluated the effect of
Rd on cell proliferation and angiogenesis in the solid tumors by
immunohistochemical analysis. As shown in Fig. 4D, the number of Ki67 (a marker of
cell proliferation) immunoreactive cells in tumor tissues of
Rd-treated (3 mg/kg) mice was less than that in the control group
of mice (p<0.01). Moreover, CD31 immunoreactive capillaries were
decreased in the Rd-treated tumors (p<0.001). In addition, Rd
treatment led to a decrease in the expression of HIF-1α and CD31
protein (Fig. 4E; p<0.05). All
of these results revealed that Rd prevented angiogenesis and tumor
growth in mice.
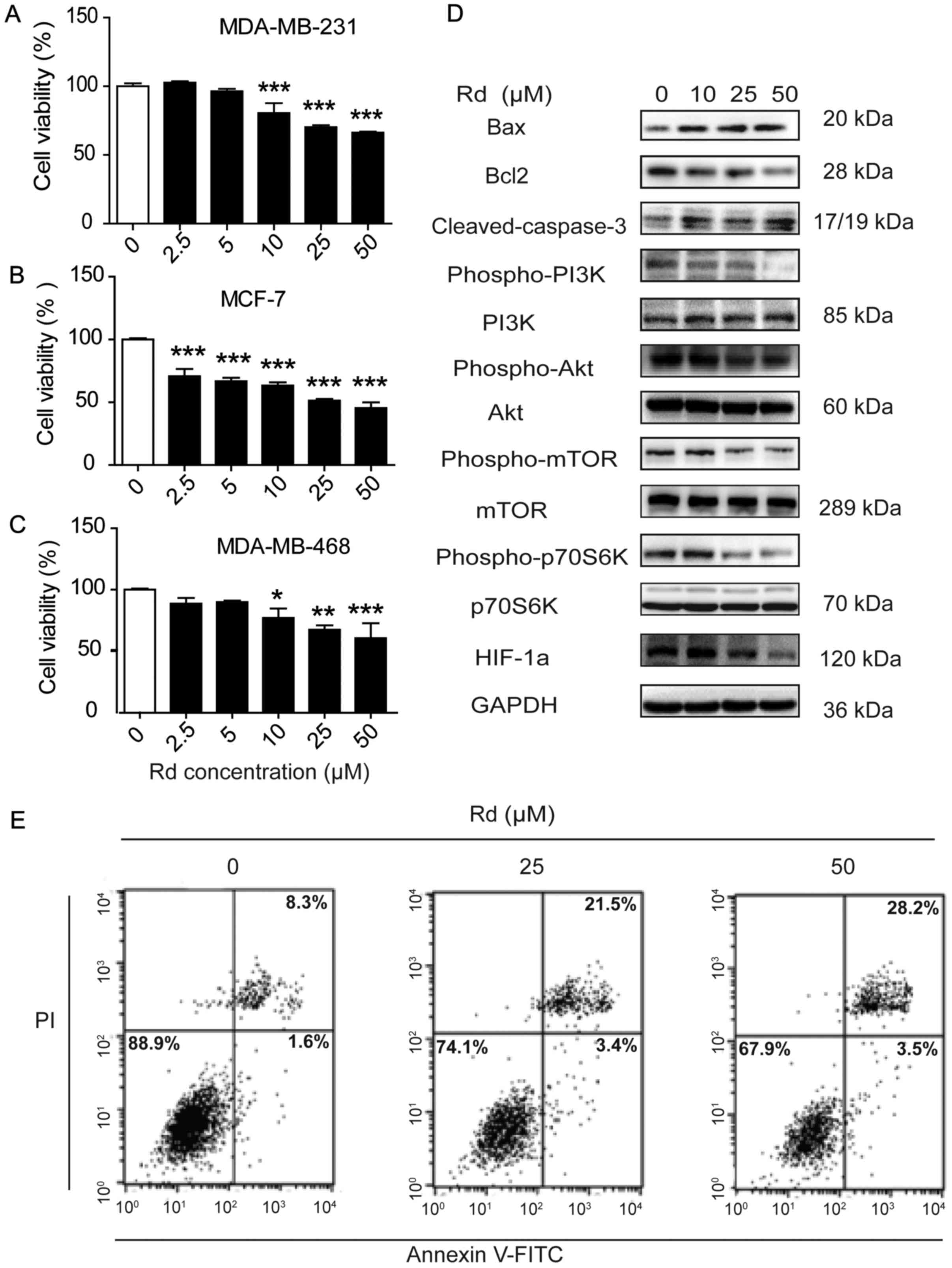
Rd induces apoptosis in breast cancer
cells
Since Rd effectively decreased cell proliferation in
the xenografted breast tumors, we next examined whether it also had
a direct influence on breast cancer cells. As shown in Fig. 5A-C, Rd treatment dose-dependently
decreased the cell viability of cancer cell lines, such as
MDA-MB-231, MCF-7 and MDA-MB-468. Flow cytometric analysis revealed
that Rd significantly increased the percentage of the apoptotic
cells (Fig. 5D). Furthermore, Rd
treatment increased Bax and cleaved caspase-3 while it decreased
Bcl-2 expression in MDA-MB-231 cells, implicating its pro-apoptotic
effect. Rd treatment also inhibited the activation of PI3K, Akt,
mTOR and p70S6K and mitigated the expression of HIF1-α in
MDA-MB-231 cells (Fig. 5E). All of
these results indicated that Rd induced direct apoptosis of breast
cancer cells, which might be mediated through the inhibition of
PI3K/Akt/mTOR signaling pathway.
Discussion
The process of angiogenesis plays a crucial role in
cancer progression as the newly formed tumor vasculature serves
initially as feeding tubes providing nutrients and oxygen supply
for the growing tumor mass, and finally as conduits for
dissemination of tumor cells that escape from the established
primary tumor (31). The current
strategies in anticancer therapy become ineffective once tumor
cells reach favored secondary organs and generate metastatic foci.
Therefore, control of tumor angiogenesis has become a central issue
in the fight against cancer progression (32). In the present study, we demonstrated
that ginsenoside Rd (Rd), a potent angiogenic inhibitor, prevented
angiogenesis through multiple steps, including endothelial cell
viability, migration and differentiation into capillary-like
structures. In addition, it modulated the Akt/mTOR/p70S6K signaling
pathway in a relatively specific manner both in endothelial cells
and in breast cancer cells, leading to its overall anti-breast
tumor effect in tumor-bearing mice.
Proliferation, migration and formation of tubular
structures of endothelial cells are indicators for the development
of new blood vessels from the pre-existing vascular bed in
angiogenesis (33,34). As VEGF is the major mediator of
tumor-associated angiogenesis, we investigated the effect of Rd on
angiogenesis in different in vitro and in vivo models
upon VEGF stimulation. In HUVECs, Rd effectively abrogated
VEGF-induced migration, invasion and capillary-like structure
formation. Furthermore aortic ring capillary formation and Matrigel
plug assays confirmed the anti-angiogenetic effect of Rd. In mice
bearing breast tumors, Rd administration was also found to inhibit
CD31-positive capillary formation. All of our results demonstrated
that Rd exerted a robust anti-angiogenic function.
VEGF exerts its biological effects by binding to
transmembrane receptors such as VEGFR1 and VEGFR2, both of which
are specifically expressed on the surface of endothelial cells and
contain a cytoplasmic tyrosine kinase domain (35). Therapies targeting the VEGF-receptor
have been demonstrated to inhibit angiogenesis and tumor growth in
preclinical models (36–38). Therefore, the VEGF/VEGFR pathway has
become a major focus of basic research and drug development for
cancer therapy. In the present study, Rd substantially
downregulated the VEGF-induced activation of VEGFR2 in HUVECs,
thereby indicating that the anti-angiogenic effects of Rd may be
partially mediated through the inhibition of VEGFR2 activation.
The PI3K/Akt/mTOR pathway is involved in the
regulation of multiple cellular processes, including cell
proliferation, migration, invasion and survival. In numerous types
of cancers this pathway is overactive, decreasing apoptosis,
allowing proliferation, and thus, enhanced signaling through this
pathway is a significant contributor to new blood vessel formation
(39,40). Activation of p70S6K, the kinase
downstream of mTOR, frequently leads to the activation of HIFs
which regulate tumorigenesis, angiogenesis and tumor growth through
VEGF (27,41). In the present study, treatment with
Rd substantially inhibited proliferation of HUVECs and cancer
cells, and decreased the activation of Akt/mTOR/p70S6K as well as
HIF-1α in both endothelial and breast cancer cells, suggesting the
important role of the pathway in the anticancer effect of Rd.
Induction of apoptosis of tumor cells is one of the
characteristics of most anticancer drugs. Apoptosis can be
triggered by various stimuli through either extrinsic or intrinsic
pathways. Generally, the extrinsic pathway includes the signaling
transduction from death receptors and caspase-3 while the intrinsic
pathway involves mitochondrial apoptotic proteins Bcl-2, cytochrome
c and Bax (42). In the
present study, Rd treatment modulated the expression of Bcl-2, Bax
and caspase-3, and increased the percentage of apoptotic cells in
MDA-MB-231 cells, suggesting the regulatory effect of Rd on both
the extrinsic and intrinsic pathways of apoptosis.
In conclusion, our results demonstrated that Rd
administration inhibited angiogenesis both in vitro and
in vivo, which may be mediated via the inhibition of
HIF-1α/VEGF through the Akt/mTOR/p70S6K signaling pathway. Our
novel findings may facilitate the potential application of Rd
against breast cancer.
Acknowledgements
The present study was financially supported by the
National Natural Science Foundation of China (nos. 81530096,
81673626 and 81603354), the Shanghai Three-Year Plan for Advancing
Traditional Medicine (ZY3-CCCX-3-3014), the Shanghai Eastern
Scholar Program (2013-59), and the Shanghai E-research Institute of
Bioactive Constituent in TCM plan.
Glossary
Abbreviations
Abbreviations:
|
HUVECs
|
human umbilical vascular endothelial
cells
|
|
VEGF
|
vascular endothelial growth factor
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
mTOR
|
mammalian target of rapamycin
|
|
ECGS
|
endothelial cell growth supplement
|
References
|
1
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Potente M, Gerhardt H and Carmeliet P:
Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao W, Chang G, Wang J, Jin W, Wang L, Lin
Y, Li H, Ma L, Li Q and Pang T: Inhibition of K562 leukemia
angiogenesis and growth by selective Na+/H+ exchanger inhibitor
cariporide through down-regulation of pro-angiogenesis factor VEGF.
Leuk Res. 35:1506–1511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Otrock ZK, Mahfouz RA, Makarem JA and
Shamseddine AI: Understanding the biology of angiogenesis: Review
of the most important molecular mechanisms. Blood Cells Mol Dis.
39:212–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thairu N, Kiriakidis S, Dawson P and
Paleolog E: Angiogenesis as a therapeutic target in arthritis in
2011: Learning the lessons of the colorectal cancer experience.
Angiogenesis. 14:223–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folkman J: Angiogenesis: An organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adams RH and Alitalo K: Molecular
regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell
Biol. 8:464–478. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang BH: PI3K/AKT and mTOR/p70S6K1
signaling pathways in human cancer. Curr Cancer Drug Targets.
13:2332013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang BH and Liu LZ: AKT signaling in
regulating angiogenesis. Curr Cancer Drug Targets. 8:19–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CF, Chiou WF and Zhang JT: Comparison
of the pharmacological effects of Panax ginsengPanax quinquefolium.
Acta Pharmacol Sin. 29:1103–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ng TB: Pharmacological activity of sanchi
ginseng (Panax notoginseng). J Pharm Pharmacol. 58:1007–1019. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Christensen LP: Ginsenosides chemistry,
biosynthesis, analysis, and potential health effects. Adv Food Nutr
Res. 55:1–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Z, Shi M, Zhang C, Zhao H, Hui H and
Zhao G: Ginsenoside Rd protects against cerebral
ischemia-reperfusion injury via decreasing the expression of the
NMDA receptor 2B subunit and its phosphorylated product. Neurochem
Res. 41:2149–2159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng X, Li J and Li Z: Ginsenoside Rd
mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1
signaling pathway. Int J Clin Exp Med. 8:14497–14504.
2015.PubMed/NCBI
|
|
18
|
Kim BJ: Involvement of melastatin type
transient receptor potential 7 channels in ginsenoside Rd-induced
apoptosis in gastric and breast cancer cells. J Ginseng Res.
37:201–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SY, Kim GT, Roh SH, Song JS, Kim HJ,
Hong SS, Kwon SW and Park JH: Proteome changes related to the
anti-cancer activity of HT29 cells by the treatment of ginsenoside
Rd. Pharmazie. 64:242–247. 2009.PubMed/NCBI
|
|
20
|
Yang ZG, Sun HX and Ye YP: Ginsenoside Rd
from Panax notoginseng is cytotoxic towards HeLa cancer cells and
induces apoptosis. Chem Biodivers. 3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park HJ, Zhang Y, Georgescu SP, Johnson
KL, Kong D and Galper JB: Human umbilical vein endothelial cells
and human dermal microvascular endothelial cells offer new insights
into the relationship between lipid metabolism and angiogenesis.
Stem Cell Rev. 2:93–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yi T, Yi Z, Cho SG, Luo J, Pandey MK,
Aggarwal BB and Liu M: Gambogic acid inhibits angiogenesis and
prostate tumor growth by suppressing vascular endothelial growth
factor receptor 2 signaling. Cancer Res. 68:1843–1850. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nicosia RF and Ottinetti A: Modulation of
microvascular growth and morphogenesis by reconstituted basement
membrane gel in three-dimensional cultures of rat aorta: A
comparative study of angiogenesis in matrigel, collagen, fibrin,
and plasma clot. In Vitro Cell Dev Biol. 26:119–128. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malinda KM: In vivo Matrigel migration and
angiogenesis assay. Methods Mol Biol. 467:287–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gasparini G, Longo R, Toi M and Ferrara N:
Angiogenic inhibitors: A new therapeutic strategy in oncology. Nat
Clin Pract Oncol. 2:562–577. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujii T, Yonemitsu Y, Onimaru M, Inoue M,
Hasegawa M, Kuwano H and Sueishi K: VEGF function for upregulation
of endogenous PlGF expression during FGF-2-mediated therapeutic
angiogenesis. Atherosclerosis. 200:51–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu S, Wu P, Ye D, Huang Y, Zhou X, Li Y
and Cai L: Effects of lipoxin A4 on CoCl2-induced angiogenesis and
its possible mechanisms in human umbilical vein endothelial cells.
Pharmacology. 84:17–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Loboda A, Jazwa A, Wegiel B, Jozkowicz A
and Dulak J: Heme oxygenase-1-dependent and -independent regulation
of angiogenic genes expression: Effect of cobalt protoporphyrin and
cobalt chloride on VEGF and IL-8 synthesis in human microvascular
endothelial cells. Cell Mol Biol. 51:347–355. 2005.PubMed/NCBI
|
|
31
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reuben SC, Gopalan A, Petit DM and
Bishayee A: Modulation of angiogenesis by dietary phytoconstituents
in the prevention and intervention of breast cancer. Mol Nutr Food
Res. 56:14–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holash J, Wiegand SJ and Yancopoulos GD:
New model of tumor angiogenesis: Dynamic balance between vessel
regression and growth mediated by angiopoietins and VEGF. Oncogene.
18:5356–5362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lamalice L, Le Boeuf F and Huot J:
Endothelial cell migration during angiogenesis. Circ Res.
100:782–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stotz M, Gerger A, Haybaeck J, Kiesslich
T, Bullock MD and Pichler M: Molecular targeted therapies in
hepatocellular carcinoma: Past, present and future. Anticancer Res.
35:5737–5744. 2015.PubMed/NCBI
|
|
36
|
Holash J, Davis S, Papadopoulos N, Croll
SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et
al: VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc
Natl Acad Sci USA. 99:11393–11398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferrara N: Vascular endothelial growth
factor as a target for anticancer therapy. Oncologist. 9 Suppl
1:S2–S10. 2004. View Article : Google Scholar
|
|
38
|
Noble ME, Endicott JA and Johnson LN:
Protein kinase inhibitors: Insights into drug design from
structure. Science. 303:1800–1805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Radisavljevic Z: AKT as locus of cancer
angiogenic robustness and fragility. J Cell Physiol. 228:21–24.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ciuffreda L, Di Sanza C, Incani UC and
Milella M: The mTOR pathway: A new target in cancer therapy. Curr
Cancer Drug Targets. 10:484–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu LZ, Zheng JZ, Wang XR and Jiang BH:
Endothelial p70 S6 kinase 1 in regulating tumor angiogenesis.
Cancer Res. 68:8183–8188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bhushan S, Kumar A, Malik F, Andotra SS,
Sethi VK, Kaur IP, Taneja SC, Qazi GN and Singh J: A triterpenediol
from Boswellia serrata induces apoptosis through both the intrinsic
and extrinsic apoptotic pathways in human leukemia HL-60 cells.
Apoptosis. 12:1911–1926. 2007. View Article : Google Scholar : PubMed/NCBI
|