Introduction
Lung cancer is the most lethal type of cancer
accounting for nearly 1,600,000 deaths worldwide in 2012 (1). Non-small cell lung cancer (NSCLC) is
the predominant histological type, accounting for 80–85% of all
cases (2). Histological type and
extension of disease at the time of diagnosis influence prognosis.
Yet, even with the advent of new targeted therapies and improved
methods for early diagnosis, patients with lung cancer have
short-term survival (1).
Highlighting biologic markers is essential to clarify malignant
lung processes, to identify patient subtypes and thereby to improve
the poor prognosis of lung cancer.
The apoptosis pathway has been extensively studied
in cancer research since malignant cells have the ability to evade
this pathway. The disruption in the balance between pro-survival
and pro-death cascades could break the barrier to cancer (3). In this context, the inhibitors of
apoptosis proteins comprise a range of molecules, which play a
critical role in cell-acquired resistance to death. In many types
of tumors, the levels of expression of certain molecules commonly
appear upregulated, and one example is the X-linked inhibitor of
apoptosis protein (XIAP) protein (4). XIAP-associated factor 1 (XAF1) is a
pro-apoptotic protein and it was primarily identified as an
antagonist of XIAP, counteracting XIAP anti-caspase activity
(5). Data suggest a plethora of
possible XAF1 functions favoring apoptosis either through survivin
degradation (6), mitochondrial
cytochrome c release (7,8), p53
stabilization (9,10), as a modulator of cell cycle
G2/M phase (11), as an
inductor of autophagy by upregulating Beclin 1 (12), inhibition of the AKT pathway
(12) or also inhibition of VEGF
(13).
Loss of XAF1 has been observed in many types of
tumors and is associated with increased malignant potential
(9,13–21).
Exceptionally, testicular germ cell tumors show XAF1
overexpression, which could be one of the reasons behind their
extraordinary sensitivity to chemotherapy (22). In vitro assays have shown
that restoration of XAF1 expression sensitizes cancer cells to
apoptotic stimuli (5,9).
Understanding the mechanism of XAF1 downregulation
in tumors could be a promising strategy by which to introduce new
agents capable of restoring XAF1 expression and improving drug
response. There are a variety of processes under investigation. For
example, epigenetic patterns altered within the promoter region of
XAF1 genes have been identified in tumors. Hypermethylation of CpGs
sites located in the 5′ proximal region was found to be associated
with transcriptional silencing of XAF1 in gastric cancer (14,23),
urogenital (24) and colon
(25) cancer cell lines. However,
epigenetic modifications are extremely complex and other XAF1
associations have been observed. Heat-shock factor 1 (HSF1) belongs
to a family of transcriptional factors involved in cellular
response under stress conditions. Once activated, HSF1
homotrimerizes, translocates to the nucleus and binds to heat shock
response elements (HSEs) to regulate the transcription of target
genes (26). Notably, a functional
HSF1-binding element was recognized in the XAF1 promoter gene and
was found to be associated with the repression of XAF1
transcription (27). Wang et
al reported that gastrointestinal cancer (GI) overexpressed
HSF1 compared to normal tissues and the XAF1 expression was
inversely correlated with HSF1 in GI cancer cell lines (27).
The tumor microenvironment can also provide abnormal
conditions for cells. Dai et al reported that HSF1 may
promote oncogenesis by facilitating cellular adaptation to the
malignant lifestyle (26). HSF1 was
also reported as a coadjuvant at the p53 transcriptional machinery.
In response to DNA damage, HSF1 can complex with p53 and regulate
p53-responsive genes, but only in wild-type p53 cells (28).
Recently, a link between XAF1 and p53 was
demonstrated. The p53 responsive element was found within the
promoter of the XAF1 gene and p53 was found to suppress the
transcription of XAF1 (29). Zou
et al confirmed that there is a direct and functional
interaction between p53 protein and the XAF1 promoter (10). This interaction was found in
wild-type p53, but not mutant p53 cells. Furthermore, there are no
data integrating XAF1 and HSF1 expression and the p53 status in
patient cohorts. Since there are limited studies performed in lung
cancer patients (21), we
investigated the XAF1 expression in a prospective cohort of NSCLC
patients, and also lung cancer cell lines. Our data may contribute
to the understanding of the potential role of XAF1 as a
tumor-suppressor gene and its suggestive regulations.
Patients and methods
Primary tumor tissues and cancer cell
lines
A total of 39 primary non-small cell lung tumor and
their adjacent non-tumor tissues, were prospectively obtained by
surgical resection at the Brazilian National Cancer Institute
(INCA). Patients were recruited from January 2006 to January 2008.
Inclusion criteria were primary resectable lung tumors,
non-metastatic and non-small cell subtype. Pancoast tumors were
excluded from our cohort. All patients enrolled provided informed
consent before surgery. Tissue specimens were snap-frozen
immediately and stored (until used) in liquid nitrogen according to
the Brazilian National Tumor and DNA Bank (BNT-INCA) guidelines.
Data regarding clinical and histopathological characteristics were
collected from medical records based on the World Health
Organization and 2009 tumor-node-metastasis (TNM) classification
guidelines. Patients were classified according to smoking status:
never-smokers, individuals who had smoked 100 cigarettes in their
lifetime; former smokers, who had quit smoking for at least one
year; current smokers, those who smoke >100 cigarettes and have
also smoked for less than one year. As an indication of cumulative
smoking exposure, pack-years were defined as the average number of
packs smoked/day multiplied by years smoked. All patients received
a detailed explanation concerning the study aims and procedures and
a signed informed consent form was provided. The protocol was
previously accepted by the Instituto Nacional de Cancer
Institutional Review Board (Protocol 31/05).
Cell culture
NSCLC cell lines with different mutational statuses
were kindly provided by Dr Giuseppe Giaccone (Free University
Medical Center, Amsterdam, The Netherlands), H460 and A549;
ACC-LC-94, ACC-LC-319 and Calu-1 cells were from Dr Takashi
Takahashi (Center for Neurological Diseases and Cancer, Nagoya
University Graduate School of Medicine, Japan); H820 and H1975
cells were from Dr Michael Peyton (Human Center for Therapeutic
Oncology Research, University of Texas Southwestern Medical Center,
TX, USA). Mutant cells for p53 included ACC-LC-94,
ACC-LC-319, Calu-1 and H820. Except for H1975, all cell lines
present K-ras mutation. For EGFR status only H1975
and H820 were mutated. Cells were cultured in RPMI-1640 medium
supplemented with 10% fetal calf serum, containing
penicillin/streptomycin/glutamine (Gibco-BRL, Grand Island, NY,
USA) at 37°C under 10% CO2.
RNA extraction and cDNA synthesis
Total RNA was isolated from cell lines or frozen
tissues using TRIzol® (Invitrogen Life Technologies) and
purified with Qiagen RNeasy kit (Qiagen, Hilden, Germany) according
to the manufacturer's protocol. RNA concentrations were determined
using NanoDrop® ND-1000 spectrophotometer. The RNA was
reverse transcribed using SuperScript II Reverse Transcriptase
(Invitrogen Life Technologies) according to the manufacturer's
instructions. In summary, 1 µg of total RNA was used to synthesize
cDNA using oligo(dT)15 (Promega, Madison, WI, USA) at
65°C for 5 min, followed by incubation with the reverse
transcriptase enzyme at 42°C for 50 min, and inactivation at 70°C
for 15 min. The resulting complementary DNA was diluted in
ultra-pure water and used in the real-time amplification
reaction.
Quantitative real-time PCR
The mRNA levels of XAF1 were measured by real-time
RT-PCR based on TaqMan® chemistry and quantified with an
ABI PRISM 7500 Fast Sequence Detection system (Applied Biosystems,
Foster City, CA, USA). An internal control gene, GAPDH, was used to
normalize the mRNA levels. The XAF1 and GAPDH mRNA levels were
assessed using pre-developed TaqMan® Gene Expression
Assays part no. Hs00213882_m1 and 4310884E, respectively
(Assay-on-Demand™; Applied Biosystems). A commercially available
cDNA derived from normal human lung tissue was used to compare
normal lung XAF1 mRNA expression levels with the ones from
different cell lines (Ambion's FirstChoice® PCR-Ready
Human Lung cDNA; Applied Biosystems). The patient tumor samples
were compared to their adjacent normal tissues. All primers and
probes were allocated in exon-exon regions, thus, preventing the
amplification of residual genomic DNA. Real-time PCR was performed
in duplicate reactions using TaqMan® Fast Universal PCR
Master Mix (Applied Biosystems). Instrument raw data (fluorescence)
of all the samples was converted to threshold cycles by SDS 1.2
software (Applied Biosystems). All target gene amplification was
normalized with their internal control gene expression (ΔCt). To
calculate the relative expression level, ΔCt tumor was compared
with the ΔCt adjacent non-tumor tissue for patient samples. For the
cell lines, comparison was made to a commercially normal human lung
control. The relative expression value was log2-transformed for
statistical analysis. The fold-change between samples and the human
lung control represents the relative XAF1 expression in the cell
lines. The fold-change between non-tumoral and tumoral tissues
represents the relative expression in the patient cohort. Patients
with loss of XAF1 expression were defined as tumors expressing a
fold-change 1/5 below the non-tumoral value.
Immunohistochemistry
The XAF1 and HSF1 proteins were evaluated by
immunohistochemistry. Formalin-fixed paraffin-embedded serial
sections (3 µm) were mounted on glass slides (ImmunoSlide;
EasyPath® São Paulo, SP, Brazil), dewaxed with xylene
and gradually hydrated in ethanol. The slides were heated in a
pressure cooker with a citrate buffer (pH 6,0) for antigen
activation. After cooling, endogenous hydrogen peroxidase and
unspecific proteins were blocked. Anti-XAF1 antibody (1:500; Abcam,
Cambridge, MA, USA) and anti-HSF1 antibody (1:100; Cell Signaling
Technology, Beverly, MA, USA) were incubated at 37°C for 45 min and
at 4°C overnight. Then, the immunoslides were blocked with Primary
Block and secondary antibody treatment (Novolink Polymer;
Novocastra, Newcastle, UK). Immunostaining was carried out with
diaminobenzidine solution and counterstained with hematoxylin.
In both analyses, the score was determined using
nuclear staining. XAF1 expression was scored using two measures:
the rate of cells stained classified as 1 (<5%), 2 (5–24%), 3
(25–49%), 4 (50–74%) and 5 (>75%); and staining intensity graded
as 1 (weak) and 2 (strong). The final score was calculated by
multiplying the area stained and the intensity resulting in three
expression levels: low (1–3); moderate (4–6); and
high (8–10) (30).
Reduction in XAF1 protein was considered when samples with high
expression levels became moderate or low.
HSF1 scoring was based on staining intensity with 0
indicating no staining, 1 indicating low-level, and 2 indicating
strong staining. Cases with no detectable HSF1, or only
cytoplasmatic reaction were defined HSF1-negative and cases with
low or strong nuclear staining were defined HSF1-positive (36).
Mutation analysis
TP53 mutation analysis was performed according to
the standard protocol at the International Agency for Research on
Cancer (IARC) (http://www-p53.iarc.fr/Download/TP53_DirectSequencing_IARC.pdf).
Approximately 25 mg of fresh tissue was processed to obtain DNA
using the QIAamp DNA Mini kit (Qiagen) according to the
manufacturer's protocol. TP53 mutation was assessed by direct
sequencing using primers described as follows: 5′-tgttcactt
gtgccctgact-3′ and 5′-tctctgggaggaggggttaa-3′ (exon 5–6); 5′-ctt
gccacaggtctccccaa-3′ and 5′-tctgcttgccgctgacccct-3′ (exon 7);
5′-ttgggagtagatggagccct-3′ and 5′-aaagtttccagtctaacact-3′ (exon
8–9). Sequencing reaction was carried out using BigDye®
Terminator v1.1 Cycle Sequencing kit. Before analysis, purification
of the sequencing reaction products was carried out using the
sequencing service (IARC) with 96-well MultiScreen filtration
plates (G50; Pharmacia/Millipore, Billerica, MA, USA). PCR products
were analyzed using a 16-capillary automated sequencer (ABI
PRISM® 3100 Genetic Analyzer; Applied Biosystems), based
on the Sanger method. Chromatograms were semi-automatically
analyzed by visual inspection of sequences imported into the
sequence analysis software using the reference sequence,
NC_000017.9, from GenBank (http://www-p53.iarc.fr/TP53sequence_NC_000017-9.html).
Variations were checked with the mutation validation tool available
at IARC at http://www-p53.iarc.fr/Mutation
ValidationCriteria.asp. This tool allowed us to check whether
the variation is a known polymorphism or a mutation, and provided
frequency and functional data as reported in the IARC TP53 database
(http://www-p53.iarc.fr).
Statistical analysis
Data analysis was conducted using Statistical
Package for Social Sciences (SPSS) version 13.0 software.
Differences between XAF1 expression in cell samples were evaluated
by Students t-test. Paired adjacent non-tumoral and tumoral samples
were compared by McNemar test. The Chi-square test was applied to
evaluate the univariative correlations between XAF1 expression and
the clinicopathological parameters. Kaplan-Meier estimates were
calculated to detail differences in recurrence-free and overall
survival by XAF1 expression and were tested for statistical
significance using the log-rank test. All p-values were two-tailed
and p<0.05 was considered to indicate a statistically
significant result.
Results
Depletion of XAF1 mRNA expression
occurs in different lung cancer cell lines
To investigate the potential role of XAF1 as a
tumor-suppressor gene in lung tumorigenesis, we characterized the
XAF1 mRNA expression levels in a variety of lung cancer cell lines.
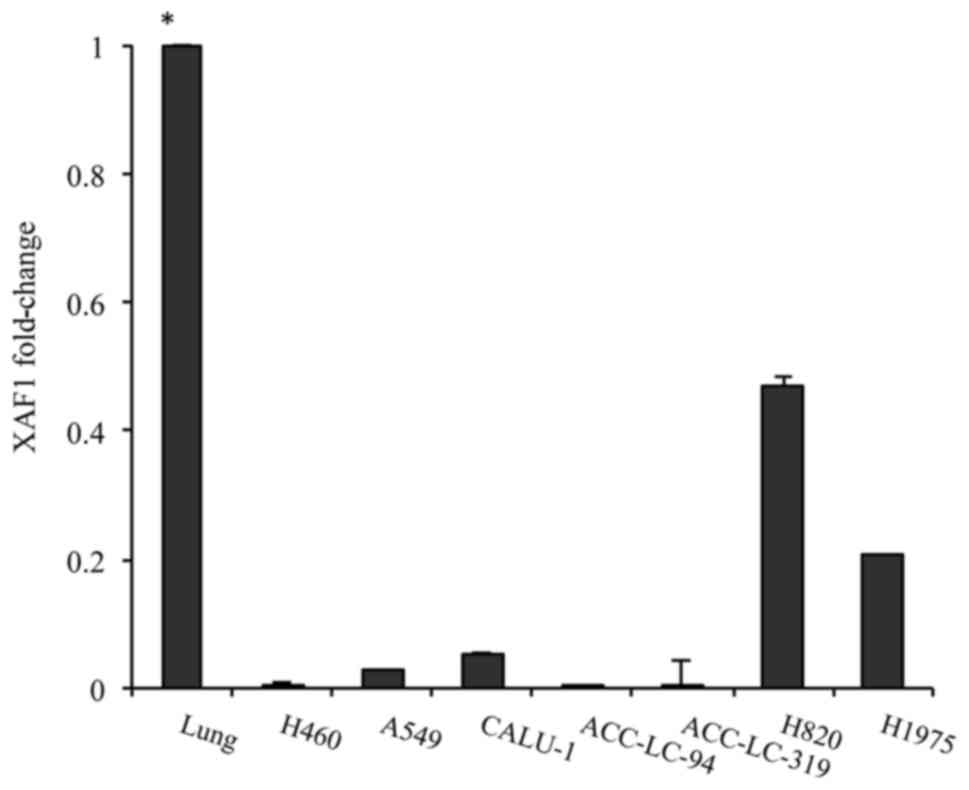
The cDNA derived from normal lung human tissue was used as a
reference to compare XAF1 expression in cell lines in relation to
normal lung tissue. All cells showed a significant reduction in the
XAF1 mRNA expression level when compared to the normal lung control
(Fig. 1). The levels of XAF1 mRNA
were low to non-existent in the H460, A549, ACC-LC-94 and
ACC-LC-319 cells, while in the mutant EGFR cells (H820 and H1975)
and TP53-deleted cell (Calu-1), the levels were higher than that
noted in the other cells (Fig.
1).
Depletion of XAF1 expression is
correlated with increased malignant potential in patient
samples
After obtaining the expression levels of XAF1 in the
cell lines, we conducted analysis using our patient cohort.
Clinicopathological characteristics of 39 patients with NSCLC
treated at our institute are described in Table I. The median follow-up was 63
months. During this period, 16 patients died, and 19 had
progressive disease.
 | Table I.Clinicopathological characteristics
of the study cohort. |
Table I.
Clinicopathological characteristics
of the study cohort.
|
Characteristics | Data |
|---|
| NSCLC patients, n
(%) | 39 (100) |
| Age (years) |
| Median
(range) | 62 (41–79) |
| Sex, n (%) |
|
Male | 22 (56.4) |
|
Female | 17 (43.6) |
| Pathological TNM
stage, n (%) |
| I | 22 (56.4) |
| II | 9 (23.1) |
|
IIIA | 8 (20.5) |
| Tumor size
(cm) |
| Median
(range) | 5.0 (2.3–16.0) |
| Nodal invasion, n
(%) |
|
N0 | 28 (71.8) |
|
N1 | 5 (12.8) |
|
N2 | 6 (15.4) |
| Histologic subtype,
n (%) |
|
Squamous cell carcinoma | 15 (38.5) |
|
Adenocarcinoma | 24 (61.5) |
| Tumor grade, n
(%) |
|
Well-differentiated | 4 (10.3) |
|
Moderately differentiated | 25 (64.1) |
| Poorly
differentiated | 8 (20.5) |
|
Missing | 2 (5.1) |
| Adjuvant therapy, n
(%) | 15 (38.5) |
|
Chemotherapy | 4 (10.2) |
|
Radiotherapy |
| No | 20 (51.3) |
| Smoking status, n
(%) |
|
Smoker | 21 (53.8) |
|
Former-smoker | 15 (38.5) |
|
Never-smoker | 3 (7.7) |
| Median follow-up
(months) | 63 |
| Recurrence, n
(%) | 19 (48.7) |
| Deaths, n (%) | 16 (41.0) |
| Missing, n (%) | 4 (10.3) |
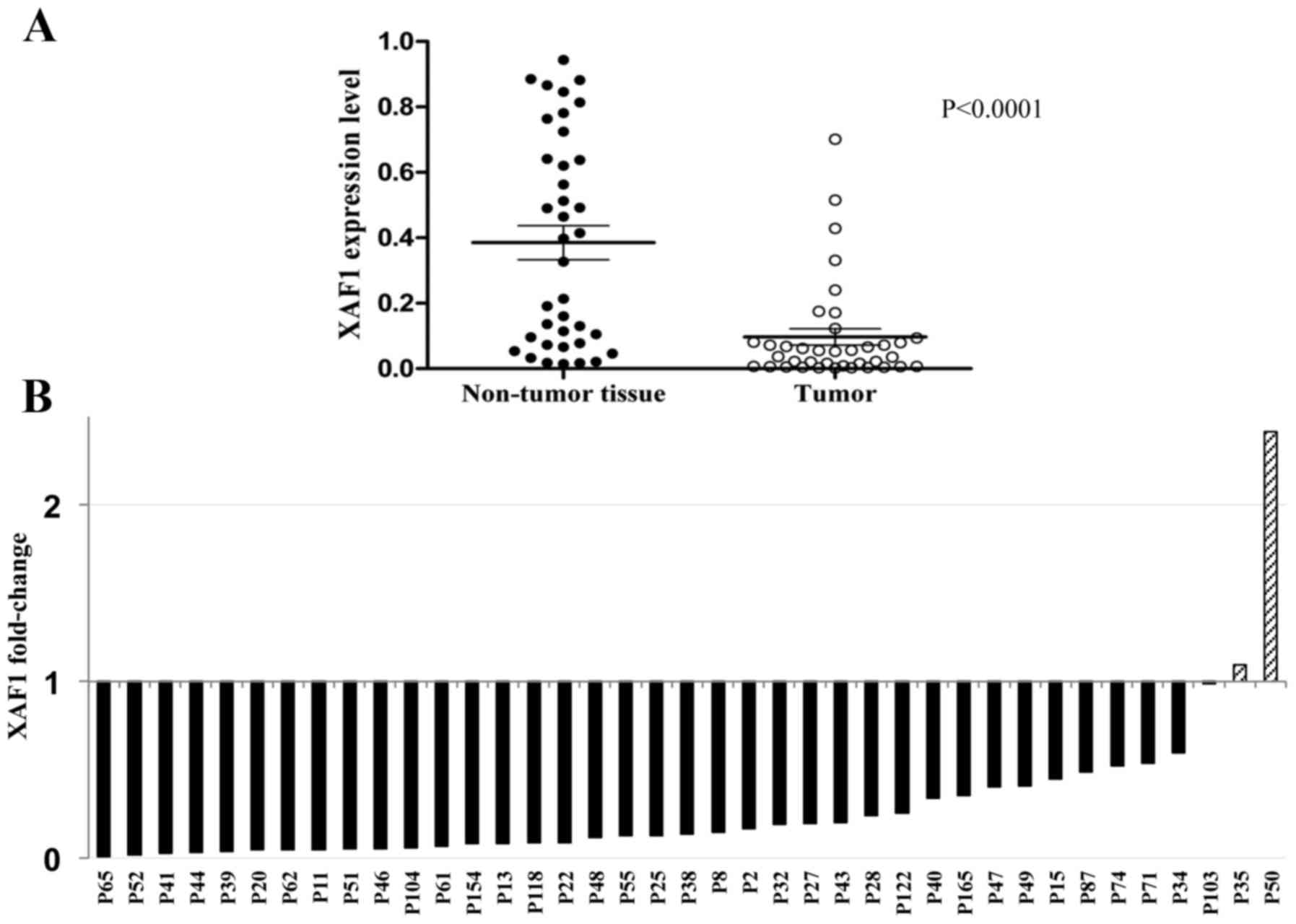
Primary lung specimens were evaluated in 39 matched
sets of adjacent non-tumor and tumor tissues. All patients had a
markedly tumor-specific low expression of XAF1 mRNA with a mean of
0.39 [95% confidence interval (CI), 0.28–0.49) in the non-tumor and
0.09 (95% CI, 0.05–0.15) in the tumor tissue group (Fig. 2A). The relative expression values
indicated that almost all patients had a reduction in XAF1
expression values in the tumor and only two showed tumor expression
higher than that noted in the matched tissue (Fig. 2B).
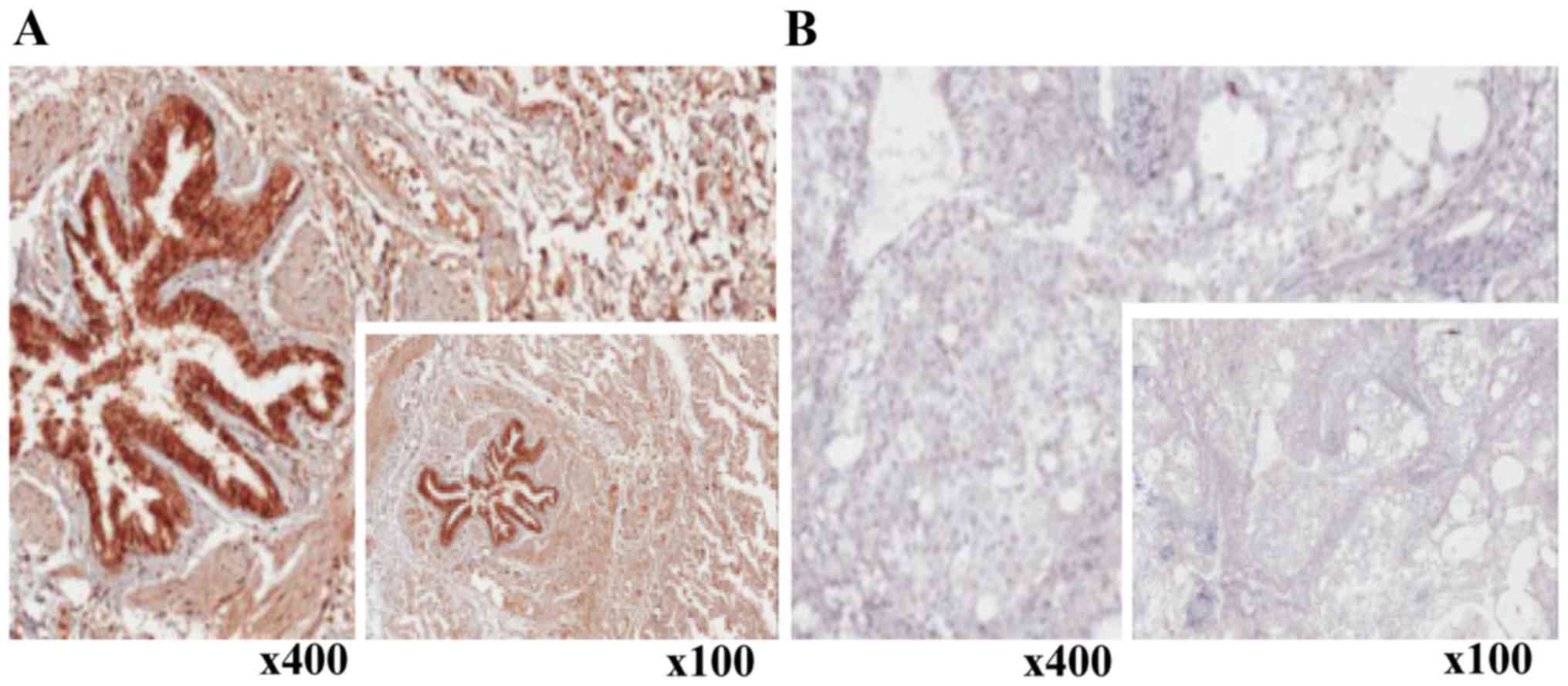
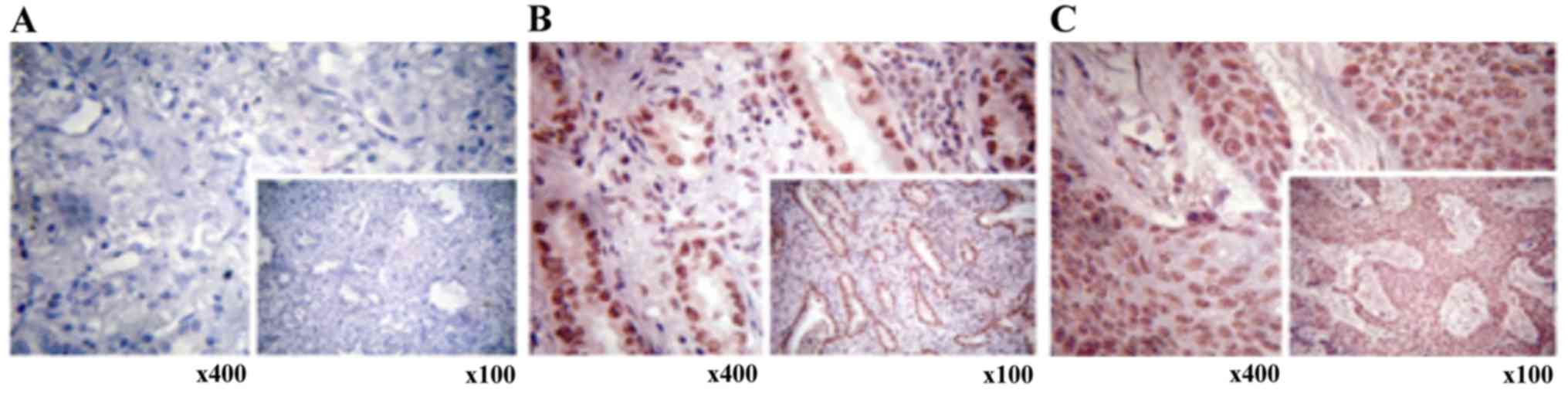
Regarding protein levels, a reduction in XAF1
expression was observed in lung tumor samples upon
immunohistochemical analyses performed using the anti-XAF1
antibody. In contrast, high XAF1 protein expression was found
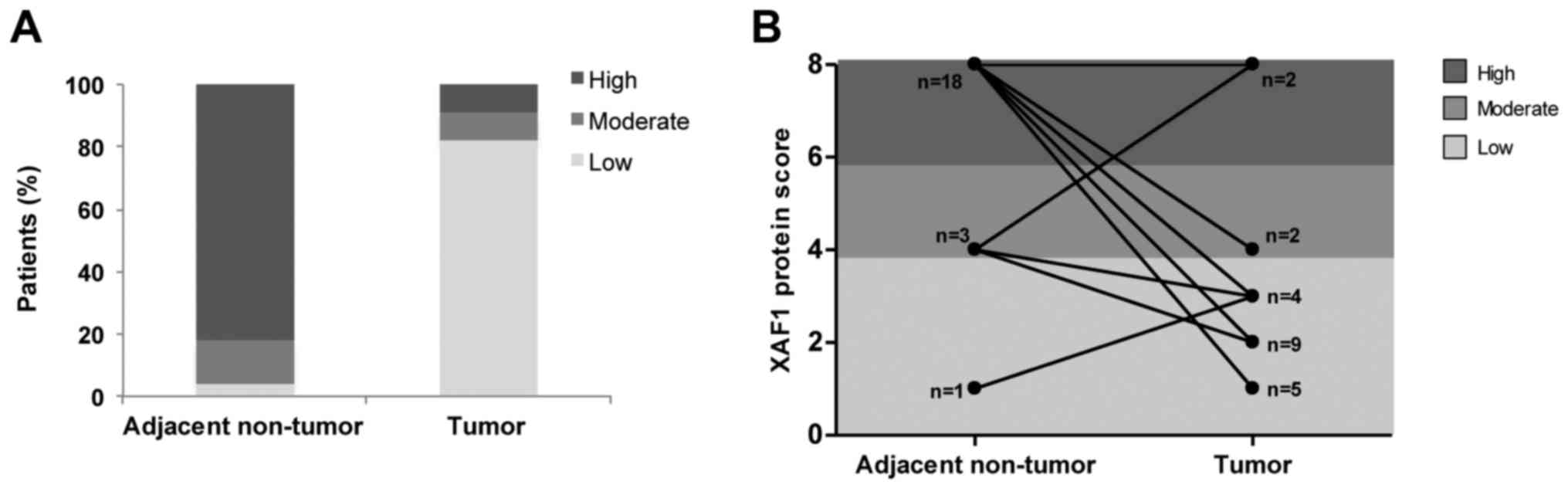
predominantly in non-tumor tissues (Fig. 3A and B). When paired samples were
compared, with exception of one sample in which increased XAF1
protein expression was observed and another which maintained a high
score, all other samples significantly showed a decrease in the
expression of XAF1 protein levels after malignant transformation
(McNemar test; p=0.001) (Fig. 4A and
B). In order to summarize and better visualize the results
obtained from the XAF1 expression experiments according to IHC
score, two different plots are shown in Fig. 4.
XAF1 depletion is correlated with HSF1
protein expression in lung tissues
A functional HSF1-binding element was recognized in
the XAF1 promoter gene and was related to the repression of XAF1
transcription (27). Therefore, in
order to investigate whether XAF1 downregulation in lung tumors is
associated with activated HSF1, the HSF1 protein was evaluated in
all tumoral/adjacent non-tumoral matched tissues via
immunohistochemistry. HSF1 protein can appear in both the cytoplasm
and nucleus, but the active form is localized only in the nucleus.
In all tumor specimens, strong nuclear staining of HSF1 was
observed. In contrast, we did not observe HSF1 expression in the
adjacent non-tumoral tissues (Fig.
5).
XAF1 protein reduction occurs in
wild-type p53 tumors
Published data (29)
have shown that XAF1 is a target gene of p53, since it carries a
p53 responsive element within the XAF1 promoter region. In
addition, the presence of wild-type p53 was shown to influence the
transcriptional repression of XAF, whereas in mutated p53 cells
this did not occur (29). Following
these results, we decided to analyze whether XAF1 reduction could
have an association with TP53 mutational status, by comparing the
score levels of XAF1 protein expression noted in Fig. 4 with the p53 mutational status in
our patient cohort. We observed that in all wild-type p53 patients,
except one, there was a loss in XAF1 protein expression in the
tumor tissue compared to that noted in the adjacent non-tumor
tissue (Table II). Nevertheless,
we did not observe the same pattern when comparing the mutant p53
status and XAF1 mRNA level between specimens. Regarding mutation
types, we did not observe a specific pattern. The patients from our
cohort showed different TP53 mutations and also deletions (data not
shown). Not all 39 patients had obtained results due to technical
difficulties related to the sample quality.
 | Table II.Correlation between XAF1 protein
losses and mutational status of the TP53 gene in NSCLC tumor
tissues. |
Table II.
Correlation between XAF1 protein
losses and mutational status of the TP53 gene in NSCLC tumor
tissues.
|
| Loss of XAF1
protein |
|---|
|
|
|
|---|
| TP53 status | Yes | No |
|---|
| Wild-type | 13 | 1 |
| Mutated | 3 | 4 |
Correlation between
clinicopathological characteristics, prognosis and XAF1
expression
Clinicopathological characteristics and prognosis
were correlated according to XAF1 tumor expression as shown in
Table III. Expression levels of
XAF1 appeared to be independent of sex, pathological stage, tumor
size, nodal invasion and histological subtype. However, a
correlation was noted between XAF1 reduction and age and
undifferentiated tumor grade (p<0.01). Patients in the group of
XAF1 depletion tended to have a poor differentiated histological
grade.
 | Table III.Correlation between XAF1 mRNA losses
and clinicopathological parameters in the NSCLC patients. |
Table III.
Correlation between XAF1 mRNA losses
and clinicopathological parameters in the NSCLC patients.
|
| XAF1 losses |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristics | Yes, n (%) | No, n (%) | P-value |
|---|
| Age (years) |
|
| 0.01a |
|
≤60 | 14 (93.3) | 1 (6.7) |
|
|
>60 | 11 (45.8) | 13 (54.2) |
|
| Sex |
|
| 0.19 |
|
Male | 12 (54.5) | 10 (45.5) |
|
|
Female | 13 (76.5) | 4 (23.5) |
|
| Smoking status |
|
| 0.05a |
|
Never-smoker | 0 (0) | 3 (100) |
|
|
Former-smoker | 11 (73.3) | 4 (26.7) |
|
|
Smoker | 14 (66.7) | 7 (33.3) |
|
| Pathological TNM
stage |
|
| 0.48 |
| I | 14 (63.6) | 8 (38.1) |
|
| II | 6 (66.7) | 3 (33.3) |
|
|
III | 6 (75.0) | 2 (25) |
|
| Tumor size
(cm) |
|
| 0.80 |
| ≤5 | 14 (63.6) | 8 (36.4) |
|
|
>5 | 11 (73.3) | 6 (26.7) |
|
| Nodal invation |
|
| 0.42 |
|
Positive | 8 (72.7) | 3 (27.3) |
|
|
Negative | 18 (64.3) | 10 (35.7) |
|
| Histological
subtype |
|
| 0.49 |
|
Squamous cell | 10 (66.7) | 5 (33.3) |
|
|
Adenocarcinoma
(differentiated) | 14 (58.3) | 10 (41.7) |
|
| Tumor grade |
|
| 0.01a |
|
Well | 0 (0) | 4 (100) |
|
|
Moderate | 19 (76) | 6
(24) |
|
|
Poor | 5 (62.5) | 3 (37.5) |
|
The levels of XAF1 expression were also analyzed
according to smoking behavior. Tumors from 2 out of 3 never-smoker
patients, presented the highest XAF1 expression in the entire tumor
cohort, both at the mRNA and protein levels. In addition, the
frequency of XAF1 reduction was higher in the group of smoker
patients than in the group of former- and-never smokers
(p=0.05).
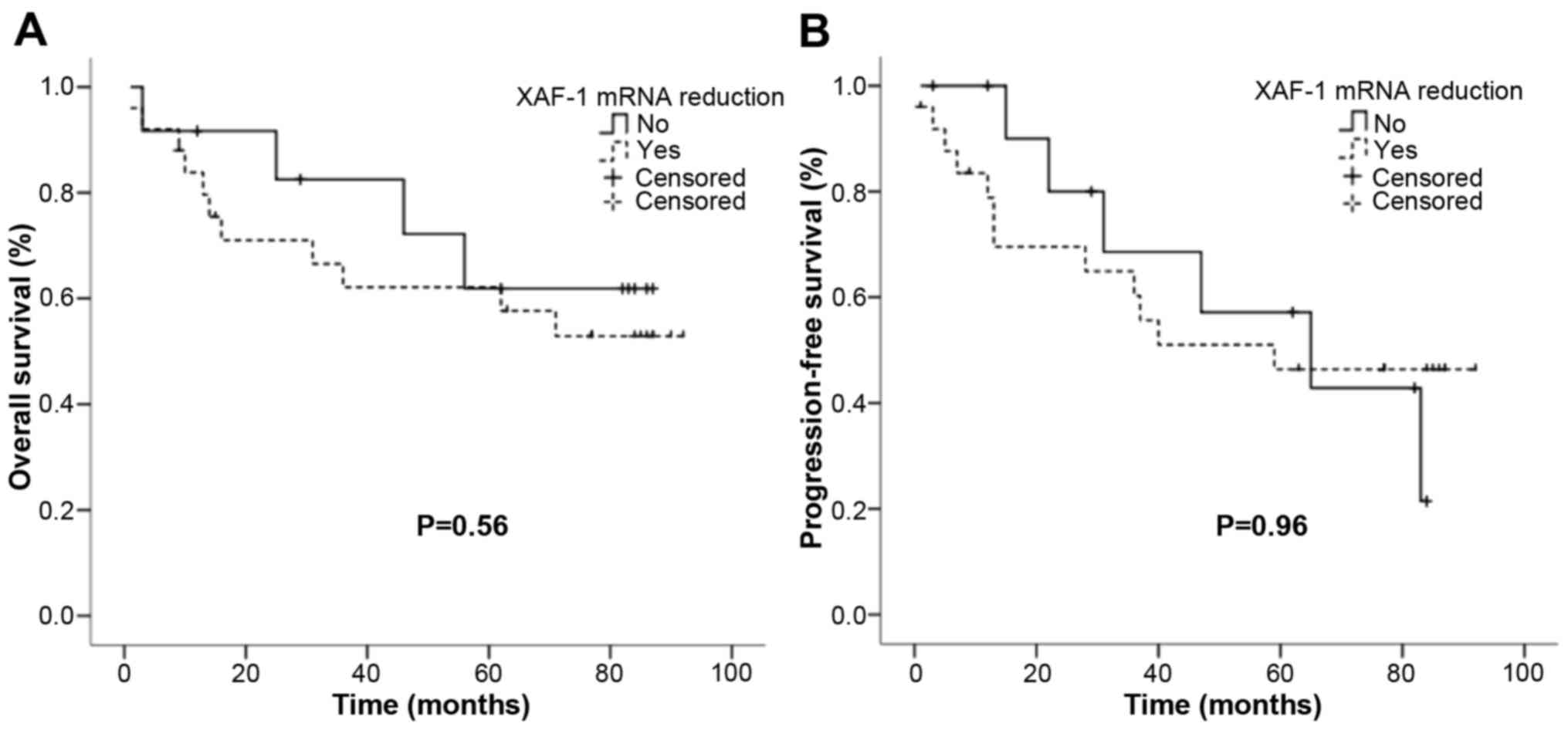
In order to further investigate the
clinicopathological characteristics, we compared XAF1 expression
levels with the risk of tumor-recurrence or tumor-related death and
the data were analyzed by Kaplan-Meier estimates. The result
indicated that there were no significant associations in higher
XAF1 losses and recurrence-free survival and overall survival
(Fig. 6).
Discussion
The process of lung carcinogenesis is still not well
understood and several studies have tried to investigate target
molecules to improve therapy and survival. A particular molecule
called XAF1, which belongs to the apoptosis pathway, was
investigated by us. The putative tumor-suppressor gene XAF1 has
been found underexpressed in various cancer tissues and cell lines
and, in addition, data generated elsewhere showed that restoration
of its expression could increase sensitivity to apoptotic stimuli
(14–21,30).
Low expression of XAF1 was observed in squamous cell
lung cancer (21). In the present
study, we identified low expression of XAF1 in NSCLC cells lines
compared to that noted in normal lung (Fig. 1). However, to the best of our
knowledge, this is the first study to describe the same pattern in
both lung adenocarcinoma cell lines and patient specimens.
Despite no significant evidence in prognosis
(Fig. 6), our cohort showed an
association between XAF1 and increased malignancy. An association
between reduced XAF1 and a higher histopathological grade was
observed in our tumor specimens (Fig.
2) corroborating the results of previous studies (15,21,31–33).
This may reinforce the potential role of XAF1 in lung tumor
progression. Restoration of XAF1 could be a target mechanism to
suppress malignant progression in the lung, as described in the
literature for other tissue types. To that end a better
understanding of the mechanisms leading to its downregulation is
warranted. The first evidence for a possible cause of
downregulation has been focused in hypermethylated regions at the
XAF1 promoter gene (14).
Therefore, we explored hypermethylation of CpG sites near the 5′
proximal region (−2 to −246 nt) that appeared strongly correlated
with transcriptional silencing. We frequently observed the same
profile in both non-tumor and tumor tissues by the
methylation-specific PCR technique (data not shown). In order to
investigate specific CpG sites and quantify the methylated
percentage, we performed quantitative real-time PCR. One primer
covered proximal CpGs sites −3 and −4 and the second one covered −5
to −7 CpG sites. After evaluating the methylation pattern in all
matched paired lung tissues, we observed that 13 (31%) tumor
tissues were hypermethylated (data not shown). The percentage of
methylation varied from 2 to 30% in the CpG site −3 and −4 and from
2 to 88% in CpG site −5 to −7. When CpG −3 and −4 site appeared
hypermethylated, the CpG site −5 to −7 was usually highlighted too.
Almost all cases of non-tumor adjacent tissues appeared
unmethylated or <1% methylated (data not shown). Our intention
was to explore methylated areas with possible useful techniques
applicable to the clinic setting, such as PCR. Since the XAF1
promoter region does not contain defined CpG islands but instead it
contains CpG sites too closely, we found it difficult to deeply
explore methylated areas using these techniques.
Meanwhile, the silencing of XAF1 in tumors has been
investigated by other groups. Wang et al observed a
functional HSF1-binding element (HSE/XAF1) located at −862/-821 of
the XAF1 gene. They showed that HSF1 could repress XAF1
transcription and this could be reversed by blocking HSF1 binding
(23). Under oncogenic stimulus,
HSF1 could affect cell transformation, proliferation, signal
transduction and metabolic process in favor of survival. Therefore,
HSF1 could respond to oncogenic stimuli and add on the global
network to support tumorigenesis (26). HSF1 can also have a dual action
under stressful conditions. In normal cells it was described as a
molecule, which protects cells from pathophysiological conditions
such as hypoxia, thermal injury and age-related neurodegeneration
(34,35). Since normal physiology is altered in
the tumor microenvironment, HSF1 could also enhance malignancy
levels. Notably, breast cancer containing high HSF1 was associated
with poor prognosis (36). Another
study showed that HSF1−/− mice presented superior
survival to HSF1+/+ mice after tumor induction, as well
as enhanced resistance to tumor formation (26).
In the present study, we investigated HSF1 protein
and we observed that higher levels of HSF1 protein were
translocated to the nucleus (where HSF1 is active) in tumor tissues
(Fig. 5). Moreover, absence of
expression was detected in non-tumor paired tissues indicating that
HSF1 may be activated during the lung carcinogenesis process. This
is consistent with the findings in gastric and colon cancer, where
Li et al observed higher expression of HSF1 in
gastrointestinal cancer tissues than in normal tissues (37). They also showed that stress stimuli
of gastrointestinal cancer cell lines could upregulate HSF1
expression, but inversely downregulate XAF1 expression (37). In our immunohistochemical results
(Figs. 3 and 5), a reduction in XAF1 expression was
observed in lung tumor samples (Fig.
3), whereas a strong nuclear staining of HSF1 was observed in
the tumor tissues (Fig. 5). Since
HSF1 was shown to downregulate XAF1 expression in tumors, it may be
informative to develop a selective HSF1 inhibitor in order to
observe whether the levels of XAF1 could be restored in cancer
cells. One difficulty which must be overcome is the fact that HSF1
is highly versatile, involved in numerous stress and non-stress
related cell processes (38).
Furthermore, it is also an important link for the translational
machinery, since it was shown that a block in translation in cancer
cells resulted in inhibition of HSF1 binding to its target genes
(39). Thus, another possible way
to block HSF1 activity may be to interfere with the ribosomal
function in the cancer cells.
Logan et al supports the idea of HSF1
implicated in cell conjecture via p53-regulated transcription. It
is well known that p53 can predominantly act in favor of cell cycle
arrest and apoptosis under DNA damage. However it can also act as a
pro-cell survival factor depending on the extent of damage or
stress duration. Under genotoxic stress, evidence shows that p53
transcriptional activity is dependent upon HSF1 interaction and
that HSF1 also facilitates phosphorylation of p53 serine residue 6
and 15 via the Chk1/ATR complex (28). Although these findings indicate
pro-apoptotic effects, the author advocates that in response to
oncogenic activation, HSF1 may redirect its function via p53 to
promote cell survival and tumorigenesis (28).
XAF1 has been reported as a target gene for p53
since it carries a p53 responsive element (−86 to −95 nt) within
the XAF1 promoter region (29). In
cancer cells, the presence of wild-type p53 could lead to
transcriptional repression of XAF1 while in mutated p53 cells this
did not occur (29). Byun et
al reported for the first time the inverse correlation between
loss of XAF1 and p53 mutations in gastric cells and tumors
(14), but to date there are scarce
studies investigating other cohorts. In the present study,
p53-deleted cells had higher XAF1 expression than wild-type ones
indicating a possible feedback loop mechanism (Fig. 1). In lung tumor specimens, we
observed a reduction in XAF1 expression compared to the paired
non-tumoral adjacent tissue indicating that XAF1 expression could
be influenced by the level of tumorigenesis (Fig. 2B). In our wild-type 53 patients, we
observed a reduction in XAF1 protein levels in tumor samples when
compared to adjacent non-tumor tissue (Table II). In contrast, we did not observe
the same pattern in mutant p53 specimens (Table II). Although this could be further
confirmed by increasing the cohort number, one possible reason for
this result could be that XAF1 may be upregulated in order to
compensate for mutant p53, indicating a possible feedback-loop
mechanism. It was recently observed that XAF1 forms a positive
feedback loop with p53 by acting as a molecular switch in
p53-decision making and that different isoforms of XAF1 have
different functions (10,40).
We also observed that all never-smoker patients did
not lose XAF1 expression and among them, two exhibited a higher
level of XAF1 in the tumor tissue than that in the adjacent tissue
(Table III). Conversely, XAF1
expression was reduced in smokers, showing that XAF1 expression
could be associated with smoking (Table III; p=0.05). Moreover, cells
harboring EGFR mutation (an alteration more commonly observed in
never-smokers) also had higher XAF1 expression when compared to
EGFR wild-type cells. Altogether, these findings are congruent with
the current distinction between tumors from smokers and
never-smokers. In the mid 2000′s, non-smoking-related lung cancer
became an independent disease from the well-established lung cancer
in smokers (41). Different studies
indicated a heavier mutation burden and a greater perturbation of
gene expression levels in smoking-related lung cancer than in
non-smoking, and different driver genes were identified in
non-smoker vs. smoker tumors (42,43).
In addition, distinct characteristics were observed in non-smoker
patients, which could vary according to sex, histological type and
ethnic origin. For example, most occurrences were in woman, Asian
people and the predominant histological type was adenocarcinoma
(41). Our findings should be
further validated in a larger cohort of never-smoker patients.
In conclusion, our results indicated that XAF1
expression is altered in NSCLC tumor samples when compared to
normal tissue and that the XAF1 status was influenced by different
clinicopathological characteristics, such as smoking, EGFR and TP53
mutation status. However, no significant associations were observed
for both tumor-recurrence or tumor-related death. The limited
sample size could have influenced these results, since patients in
the group of with lack of XAF1 tended to have poorly differentiated
histological grade. It may be interesting to validate our findings
in a larger cohort, including stage IV patients. In addition, XAF1
not only plays a role as a tumor suppressor but it is also
important in the entire cell stress mechanism where the p53 status
is crucial. It remains to be determined which molecules belonging
to different signaling cascades may mediate XAF1 activation or
downregulation in lung cancer. Moreover, further understanding of
the molecular mechanisms underlying HSF1 must to be increased, and
it may be valuable to determine whether HSF1 may be a therapeutic
target by which to mediate XAF1 levels in lung cancer cells.
Acknowledgements
The present study was supported by the Ary Frauzino
Cancer Foundation and the Coordination for the Improvement of
Higher Education Personnel (CAPES). We would like to thank all the
patients involved.
Glossary
Abbreviations
Abbreviations:
|
XAF1
|
XIAP-associated factor 1
|
|
HSF1
|
heat-shock transcription factor 1
|
|
NSCLC
|
non-small cell lung cancer
|
|
XIAP
|
X-linked inhibitor of apoptosis
protein
|
|
HSEs
|
heat shock response elements
|
|
GI
|
gastrointestinal cancer
|
|
CI
|
confidence interval
|
|
Nt
|
nucleotides
|
|
INCA
|
Instituto Nacional de Câncer
|
|
BNT-INCA
|
Brazilian National Tumor and DNA
Bank
|
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC CancerBase No. 11 (Internet). International Agency
for Research on Cancer. Lyon, France: 2013 http://globocan.iarc.frJanuary 23–2015.
|
|
2
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holcik M, Gibson H and Korneluk RG: XIAP:
Apoptotic brake and promising therapeutic target. Apoptosis.
6:253–261. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liston P, Fong WG, Kelly NL, Toji S,
Miyazaki T, Conte D, Tamai K, Craig CG, McBurney MW and Korneluk
RG: Identification of XAF1 as an antagonist of XIAP anti-caspase
activity. Nat Cell Biol. 3:128–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arora V, Cheung HH, Plenchette S, Micali
OC, Liston P and Korneluk RG: Degradation of survivin by the
X-linked inhibitor of apoptosis (XIAP)-XAF1 complex. J Biol Chem.
282:26202–26209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia Y, Novak R, Lewis J, Duckett CS and
Phillips AC: Xaf1 can cooperate with TNFalpha in the induction of
apoptosis, independently of interaction with XIAP. Mol Cell
Biochem. 286:67–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Straszewski-Chavez SL, Visintin IP,
Karassina N, Los G, Liston P, Halaban R, Fadiel A and Mor G: XAF1
mediates tumor necrosis factor-alpha-induced apoptosis and X-linked
inhibitor of apoptosis cleavage by acting through the mitochondrial
pathway. J Biol Chem. 282:13059–13072. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee MG, Huh JS, Chung SK, Lee JH, Byun DS,
Ryu BK, Kang MJ, Chae KS, Lee SJ, Lee CH, et al: Promoter CpG
hypermethylation and downregulation of XAF1 expression in human
urogenital malignancies: Implication for attenuated p53 response to
apoptotic stresses. Oncogene. 25:5807–5822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou B, Chim CS, Pang R, Zeng H, Dai Y,
Zhang R, Lam CS, Tan VP, Hung IF, Lan HY, et al: XIAP-associated
factor 1 (XAF1), a novel target of p53, enhances p53-mediated
apoptosis via post-translational modification. Mol Carcinog.
51:422–432. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Gu Q, Li M, Zhang W, Yang M, Zou
B, Chan S, Qiao L, Jiang B, Tu S, et al: Identification of XAF1 as
a novel cell cycle regulator through modulating G2/M checkpoint and
interaction with checkpoint kinase 1 in gastrointestinal cancer.
Carcinogenesis. 30:1507–1516. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun PH, Zhu LM, Qiao MM, Zhang YP, Jiang
SH, Wu YL and Tu SP: The XAF1 tumor suppressor induces autophagic
cell death via upregulation of Beclin-1 and inhibition of Akt
pathway. Cancer Lett. 310:170–180. 2011.PubMed/NCBI
|
|
13
|
Zhu LM, Shi DM, Dai Q, Cheng XJ, Yao WY,
Sun PH, Ding Y, Qiao MM, Wu YL, Jiang SH, et al: Tumor suppressor
XAF1 induces apoptosis, inhibits angiogenesis and inhibits tumor
growth in hepatocellular carcinoma. Oncotarget. 5:5403–5415. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Byun DS, Cho K, Ryu BK, Lee MG, Kang MJ,
Kim HR and Chi SG: Hypermethylation of XIAP-associated factor 1, a
putative tumor suppressor gene from the 17p13.2 locus, in human
gastric adenocarcinomas. Cancer Res. 63:7068–7075. 2003.PubMed/NCBI
|
|
15
|
Chung SK, Lee MG, Ryu BK, Lee JH, Han J,
Byun DS, Chae KS, Lee KY, Jang JY, Kim HJ, et al: Frequent
alteration of XAF1 in human colorectal cancers: Implication for
tumor cell resistance to apoptotic stresses. Gastroenterology.
132:2459–2477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shibata T, Noguchi T, Takeno S, Gabbert
HE, Ramp U and Kawahara K: Disturbed XIAP and XAF1 expression
balance is an independent prognostic factor in gastric
adenocarcinomas. Ann Surg Oncol. 15:3579–3587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang F, Wu LM, Zhou L, Chen QX, Xie HY,
Feng XW and Zheng SS: Predictive value of expression and promoter
hypermethylation of XAF1 in hepatitis B virus-associated
hepatocellular carcinoma treated with transplantation. Ann Surg
Oncol. 15:3494–3502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakemi R, Yano H, Ogasawara S, Akiba J,
Nakashima O, Fukahori S, Sata M and Kojiro M: X-linked inhibitor of
apoptosis (XIAP) and XIAP-associated factor-1 expressions and their
relationship to apoptosis in human hepatocellular carcinoma and
non-cancerous liver tissues. Oncol Rep. 18:65–70. 2007.PubMed/NCBI
|
|
19
|
Pinho MB, Costas F, Sellos J, Dienstmann
R, Andrade PB, Herchenhorn D, Peixoto FA, Santos VO, Small IA,
Guimarães DP, et al: XAF1 mRNA expression improves progression-free
and overall survival for patients with advanced bladder cancer
treated with neoadjuvant chemotherapy. Urol Oncol. 27:382–390.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Yao WY, Zhu Q, Tu SP, Yuan F,
Wang HF, Zhang YP and Yuan YZ: XAF1 as a prognostic biomarker and
therapeutic target in pancreatic cancer. Cancer Sci. 101:559–567.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen YB, Shu J, Yang WT, Shi L, Guo XF,
Wang FG and Qian YY: XAF1 as a prognostic biomarker and therapeutic
target in squamous cell lung cancer. Chin Med J. 124:3238–3243.
2011.PubMed/NCBI
|
|
22
|
Kempkensteffen C, Hinz S, Jäger T, Weikert
S, Krause H, Schostak M, Christoph F, Strenziok R, Miller K and
Schrader M: Expression levels of the IAP antagonists XAF1,
Smac/DIABLO and HtrA2 in testicular germ cell tumours. Aktuelle
Urol. 39:436–441. 2008.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ling ZQ, Lv P, Lu XX, Yu JL, Han J, Ying
LS, Zhu X, Zhu WY, Fang XH, Wang S, et al: Circulating methylated
XAF1 DNA indicates poor prognosis for gastric cancer. PLoS One.
8:e671952013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang X, Liu Z, Fan Y, Zheng C, Nilson S,
Egevad L, Ekman P and Xu D: Switch to full-length of XAF1 mRNA
expression in prostate cancer cells by the DNA methylation
inhibitor. Int J Cancer. 118:2485–2489. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zou B, Chim CS, Zeng H, Leung SY, Yang Y,
Tu SP, Lin MC, Wang J, He H, Jiang SH, et al: Correlation between
the single-site CpG methylation and expression silencing of the
XAF1 gene in human gastric and colon cancers. Gastroenterology.
131:1835–1843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai C, Whitesell L, Rogers AB and
Lindquist S: Heat shock factor 1 is a powerful multifaceted
modifier of carcinogenesis. Cell. 130:1005–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, He H, Yu L, Xia HH, Lin MC, Gu Q,
Li M, Zou B, An X, Jiang B, et al: HSF1 down-regulates XAF1 through
transcriptional regulation. J Biol Chem. 281:2451–2459. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Logan IR, McNeill HV, Cook S, Lu X, Meek
DW, Fuller-Pace FV, Lunec J and Robson CN: Heat shock factor-1
modulates p53 activity in the transcriptional response to DNA
damage. Nucleic Acids Res. 37:2962–2973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang W, Guo Z, Jiang B, Niu L, Xia G,
Wang X, Cheng T, Zhang Y and Wang J: Identification of a functional
p53 responsive element within the promoter of XAF1 gene in
gastrointestinal cancer cells. Int J Oncol. 36:1031–1037.
2010.PubMed/NCBI
|
|
30
|
Tu SP, Liston P, Cui JT, Lin MC, Jiang XH,
Yang Y, Gu Q, Jiang SH, Lum CT, Kung HF, et al: Restoration of XAF1
expression induces apoptosis and inhibits tumor growth in gastric
cancer. Int J Cancer. 125:688–697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Augello C, Caruso L, Maggioni M, Donadon
M, Montorsi M, Santambrogio R, Torzilli G, Vaira V, Pellegrini C,
Roncalli M, et al: Inhibitors of apoptosis proteins (IAPs)
expression and their prognostic significance in hepatocellular
carcinoma. BMC Cancer. 9:1252009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kempkensteffen C, Fritzsche FR, Johannsen
M, Weikert S, Hinz S, Dietel M, Riener MO, Moch H, Jung K, Krause
H, et al: Down-regulation of the pro-apoptotic XIAP associated
factor-1 (XAF1) during progression of clear-cell renal cancer. BMC
Cancer. 9:2762009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Mao H, Hao Q, Wang Y, Yang Y, Shen
L, Huang S and Liu P: Association of expression of XIAP-associated
factor 1 (XAF1) with clinicopathologic factors, overall survival,
microvessel density and cisplatin-resistance in ovarian cancer.
Regul Pept. 178:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Christians ES, Yan LJ and Benjamin I: Heat
shock factor 1 and heat shock proteins: Critical partners in
protection against acute cell injury. Crit Care Med. 30 Suppl
1:S43–S50. 2002. View Article : Google Scholar
|
|
35
|
Westerheide SD and Morimoto RI: Heat shock
response modulators as therapeutic tools for diseases of protein
conformation. J Biol Chem. 280:33097–33100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Santagata S, Hu R, Lin NU, Mendillo ML,
Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM,
Lindquist S, et al: High levels of nuclear heat-shock factor 1
(HSF1) are associated with poor prognosis in breast cancer. Proc
Natl Acad Sci USA. 108:18378–18383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li T, Chen CL, Wang JD, Cui SD, Cui DY and
Guo W: Expression of heat-shock transcription factor 1 and X-linked
inhibitor of apoptosis protein-associated factor-1 in
gastrointestinal cancer. Nan Fang Yi Ke Da Xue Xue Bao. 28:487–490.
2008.(In Chinese). PubMed/NCBI
|
|
38
|
Vihervaara A and Sistonen L: HSF1 at a
glance. J Cell Sci. 127:261–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Santagata S, Mendillo ML, Tang YC,
Subramanian A, Perley CC, Roche SP, Wong B, Narayan R, Kwon H,
Koeva M, et al: Tight coordination of protein translation and HSF1
activation supports the anabolic malignant state. Science.
341:12383032013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee MG, Han J, Jeong SI, Her NG, Lee JH,
Ha TK, Kang MJ, Ryu BK and Chi SG: XAF1 directs apoptotic switch of
p53 signaling through activation of HIPK2 and ZNF313. Proc Natl
Acad Sci USA. 111:15532–15537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Toh CK, Gao F, Lim WT, Leong SS, Fong KW,
Yap SP, Hsu AA, Eng P, Koong HN, Thirugnanam A, et al:
Never-smokers with lung cancer: Epidemiologic evidence of a
distinct disease entity. J Clin Oncol. 24:2245–2251. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Govindan R, Ding L, Griffith M,
Subramanian J, Dees ND, Kanchi KL, Maher CA, Fulton R, Fulton L,
Wallis J, et al: Genomic landscape of non-small cell lung cancer in
smokers and never-smokers. Cell. 150:1121–1134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gou LY, Niu FY, Wu YL and Zhong WZ:
Differences in driver genes between smoking-related and
non-smoking-related lung cancer in the Chinese population. Cancer.
121 Suppl 17:S3069–S3079. 2015. View Article : Google Scholar
|