Introduction
Acute lymphocytic leukemia (ALL), also called acute
lymphoblastic leukemia, is a kind of lymphoid neoplasm. Lymphoid
neoplasms have been divided into two major categories: neoplasms
derived from B- and T-lineage lymphoid precursors and those derived
from mature B, T or NK cells in recent WHO classification (1). Also, ALL belongs to the first group
which is B/T-precursor-stage lymphoid cell malignancies that block
lymphoid differentiation and drive aberrant cell proliferation and
survival. In addition, ALL is the most common leukemia in
pediatrics, among which there are ≤80% of leukemia in this group
and 20% of leukemia in adults (2).
With the developments in theoretical knowledge and techniques,
novel treatments in increasing number such as chemotherapy,
steroids, radiation therapy and intensive combined treatments have
been adopted for ALL treatment. Nevertheless, relapse among ALL
patients is still the leading problem causing children's death.
Despite the high cure rate in children with ALL, patients
accounting for 10–20% are forecasted to relapse and outcomes of
salvage therapy have been disappointing and only one-third of
children survive long-term after recurrence of disease (3). In addition, the outcomes of adults
with ALL are usually markedly worse than those of pediatric ones.
Therefore, novel methods and effective drugs are urgently
needed.
Arsenic is known as one of the toxic metalloids and
its oxidized form, arsenic trioxide (As2O3)
has been successfully used as a medicine for patients with acute
promyelocytic leukemia (APL) via intravenous injection. However,
the anticancer effect of arsenic trioxide has not been proven in
solid tumors or other hematologic malignancies (4). KML001 (NaAsO2, sodium
metaarsenite, KOMINOX), a sodium salt of arsenous acid, is
water-soluble, therefore, orally bioavailable, trivalent arsenical
compound. KML001 has shown a potent antitumor effect on various
human cancers, including solid tumors such as prostate cancer,
ovarian cancer and hematologic malignancy like acute myeloid
leukemia (AML) (5–7). In addition, its half maximal
inhibitory concentration (IC50) has been found much
lower than arsenic trioxide in various NHL cell lines, which may
make it more suitable for clinical applications (8).
Based on epidemiological research (9,10) and
in vitro studies (11–13),
vitamin D derivatives (VDDs) have been considered as a promising
anticancer drug. As one of the most typical VDDs, 1,
25-dihydroxyvitamin D3 is known to control cell proliferation and
differentiation as well as calcium related actions in leukemia
cells (14–16). However, the side effect of
hypercalcemia has limited its clinical application in hematologic
malignancies. Doxercalciferol, a low calcemic vitamin D2 derivative
with similar effect as 1, 25-dihydroxyvitamin D3, can be safely
given to patients and was investigated in phase II studies of the
myelodysplastic syndrome (17) and
androgen-independent prostate cancer (18).
In this study, the antileukemic effect of KML001 on
ALL was investigated, which was focused on apoptosis and cell
cycle. In addition, the combination effect of KML001 with
doxercalciferol was studied. We provided evidence of synergistic
effect of KML001 and doxercalciferol in ALL cell lines and possible
explanations of this synergy.
Materials and methods
Cells and cell culture
Human acute lymphoid leukemia cell line CCRF-CEM and
Molt-4 were kindly presented by Dr Y.Y. Lee (Hanyang University,
Seoul, Korea). The cells were cultured in tissue flasks or plates
in RPMI-1640 medium supplemented with 10% fetal bovine serum
(Gibco-BRL, Gaithersburg, MD, USA), 100 U/ml penicillin, 100 µg/ml
streptomycin and incubated at 37°C in a humidified atmosphere with
5% CO2.
Chemicals and antibodies
KML001 was obtained from Komipharm International
(Siheung-Si, Gyeonggi-Do, Korea). As2O3 was
purchased from Sigma-Aldrich (St. Louis, MO, USA). Doxercalciferol
was purchased from Selleck Chemicals (Houston, TX, USA). Antibodies
of Cdk1, p-Cdk1, cyclin B, p-p53, p-p21, Bcl-2, Bax, Bcl-xL were
from Abcam (Cambridge, UK).
Growth inhibition assay
Cell viability was determined by Cell Counting Kit-8
(CCK-8, Dojindo Molecular Technologies, Gaithersburg, MD, USA).
Cells (5×103 cells/well) were seeded in 96-well
microtiter plates (Falcon) and then incubated at 37°C for 48 h.
After adding 10 µl of the CCK-8 solution to each well of the plate,
the plate was incubated for ≥1 h in the incubator. Finally,
absorbance at 450 nm was measured by Multiskan Spectrum microplate
reader (Thermo Labsystems, USA).
Western blot analysis
Cells were lysed with lysis buffer [25 mM Tris-HCl
(pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS
with Halt™ Protease, Phosphatase Inhibitor Cocktail and
Benzonase® Nuclease] on ice for 20 min. Briefly, protein
samples were resolved in SDS-polyacrylamide gel, transferred to
polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica,
MA, USA), and then probed with antibodies overnight. Finally, the
blots were developed using the Pierce™ ECL Western Blotting
Substrate (Thermo Fisher Scientific, USA).
Cell cycle distribution
Cells were fixed with 66% ethanol for >2 h at 4°C
and then stained with 50 µg/ml of propidium iodide (PI) containing
550 U/ml of RNaseA (ab139418 PI Flow Cytometry kit for cell cycle
analysis (Abcam, MA, USA). The DNA content of the cells was gated
and analyzed using a FACSCanto II (Becton-Dickinson, San Jose, CA,
USA) equipped with an FACSDiva software 8.0.1
(Becton-Dickinson).
Evaluation of apoptosis
Apoptosis was evaluated by FITC Annexin V apoptosis
detection kit according to the manufacturer's instructions. Cells
were washed twice with cold PBS, then resuspended in the 1X binding
buffer, containing 0.01 M HEPES/NaOH, 0.14 M NaCl and 2.5 mM
CaCl2, pH 7.4. Next, a total of 100 µl of the mixtures
was transferred to a 5-ml culture tube and then incubated with 5 µl
Annexin V-FITC and 5 µl propidium iodide for 15 min at room
temperature in the dark. Subsequently, 400 µl of 1X binding buffer
was added to each tube and then cells were immediately analyzed by
flow cytometry. In analysis process, Annexin V-positive or
PI-negative cells were considered as early apoptotic, both Annexin
V and PI-positive cells were considered as late apoptotic, and
Annexin V-negative but PI-positive cells were considered as
necrotic.
Intracellular calcium analysis
Cells treated with KML001, doxercalciferol or medium
(control) for 48 h were washed three times with the PBS and then
incubated with 4 µM Fluo-4 AM (Thermo Fisher Scientific) and 0.1%
Pluronic F-127 (Thermo Fisher Scientific) in PBS for 60 min at
37°C. After removing the medium in time, each well was washed with
PBS and 200 µl PBS was used to cover all cells in the trough.
Finally, we used laser confocal scanning microscopy (LCSM) to
measure the fluorescence intensity (FI) of the cells at an
excitation wavelength of 488 nm.
Statistical analysis
The combined effects of KML001 and doxercalciferol
were analyzed by CalcuSyn software (Biosoft Ferguson, MO, USA)
using the Chou-Talalay method (19). The combination index (CI) values of
<1, 1 and >1 represent synergy, additive effect, and
antagonism, respectively. All experiments have been repeated at
least 3 times. Statistical significance was determined by using
Student's t-test. P-value <0.05 was considered statistically
significant.
Results
The superiority of KML001 compared
with arsenite trioxide
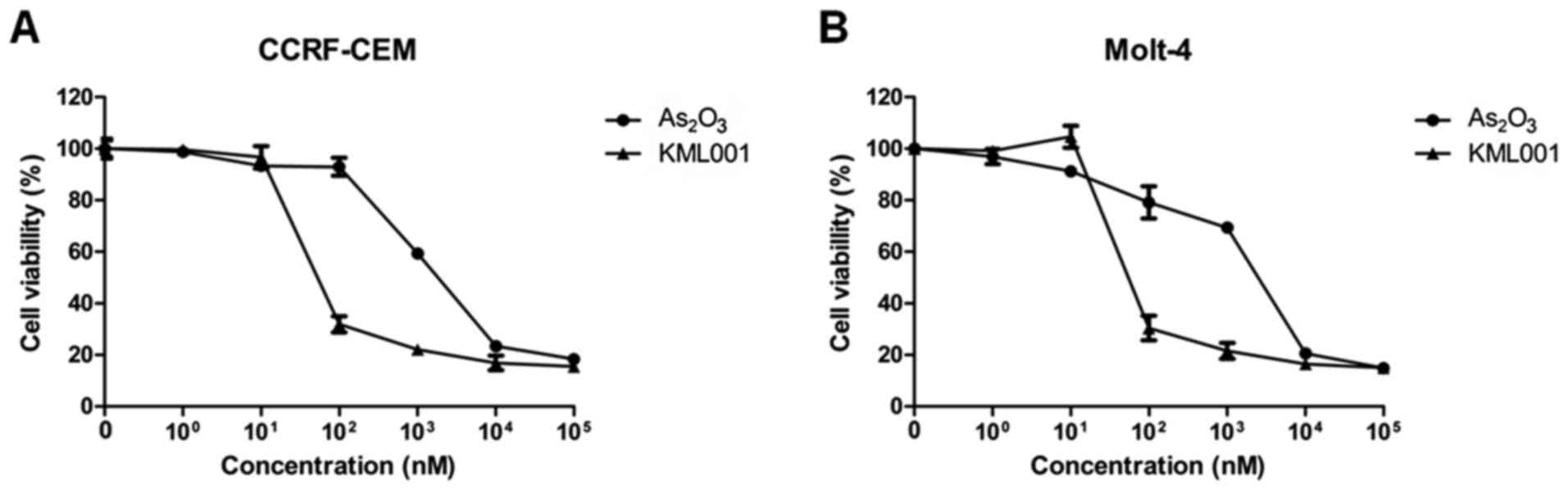
The current experiments were initiated by treating
CCRF-CEM and Molt-4 cells, in in vitro models of human ALL,
for 48 h with two types of arsenic compounds, arsenic trioxide and
KML001. Results showed that KML001 inhibited cell proliferation of
the cell lines at a fairly lower concentration (>100-fold) than
As2O3 (Fig.
1).
Antileukemic effects of KML001 on ALL
cells
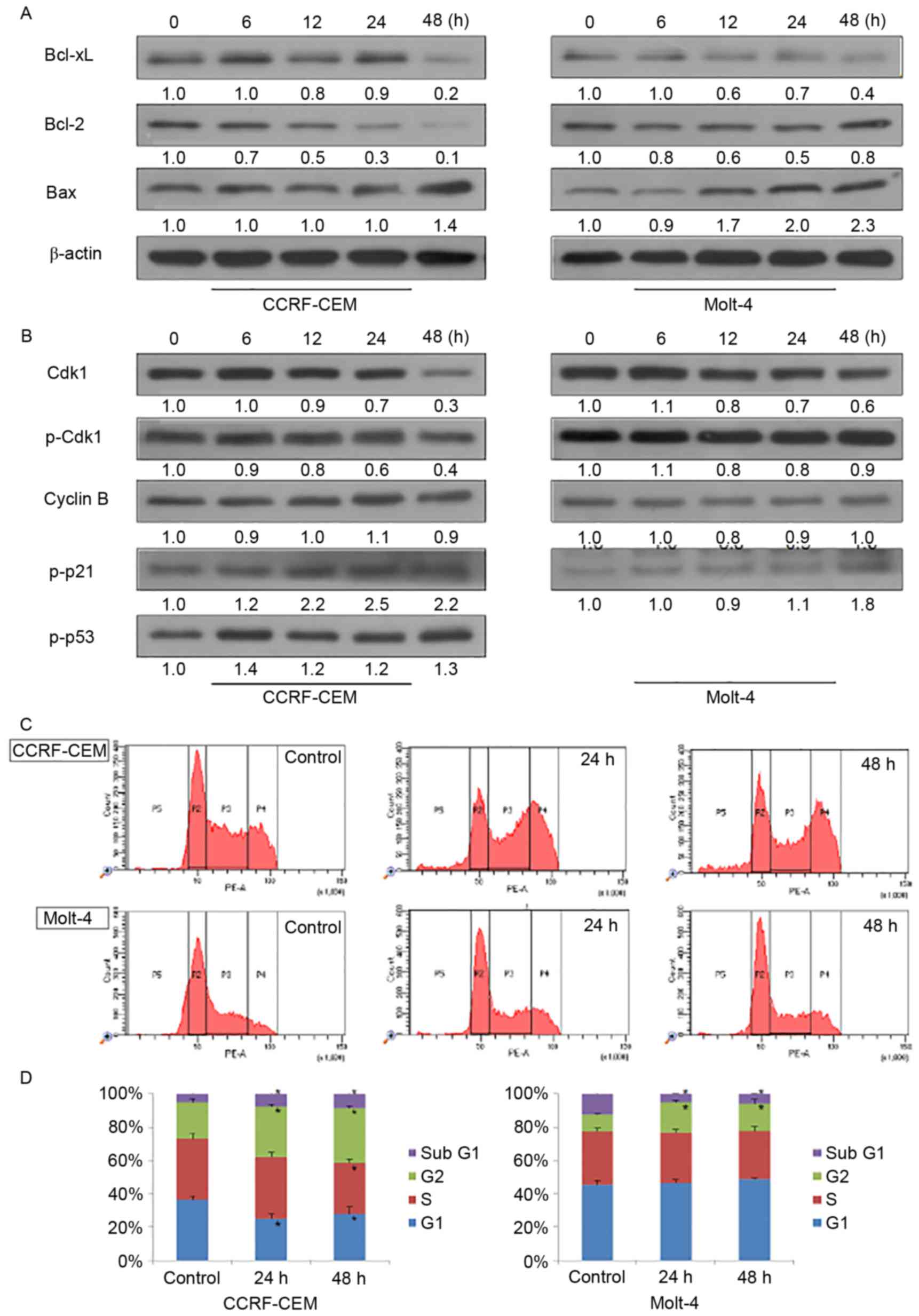
To further investigate the mechanism of action of
KML001, western blot analysis was performed for apoptosis-related
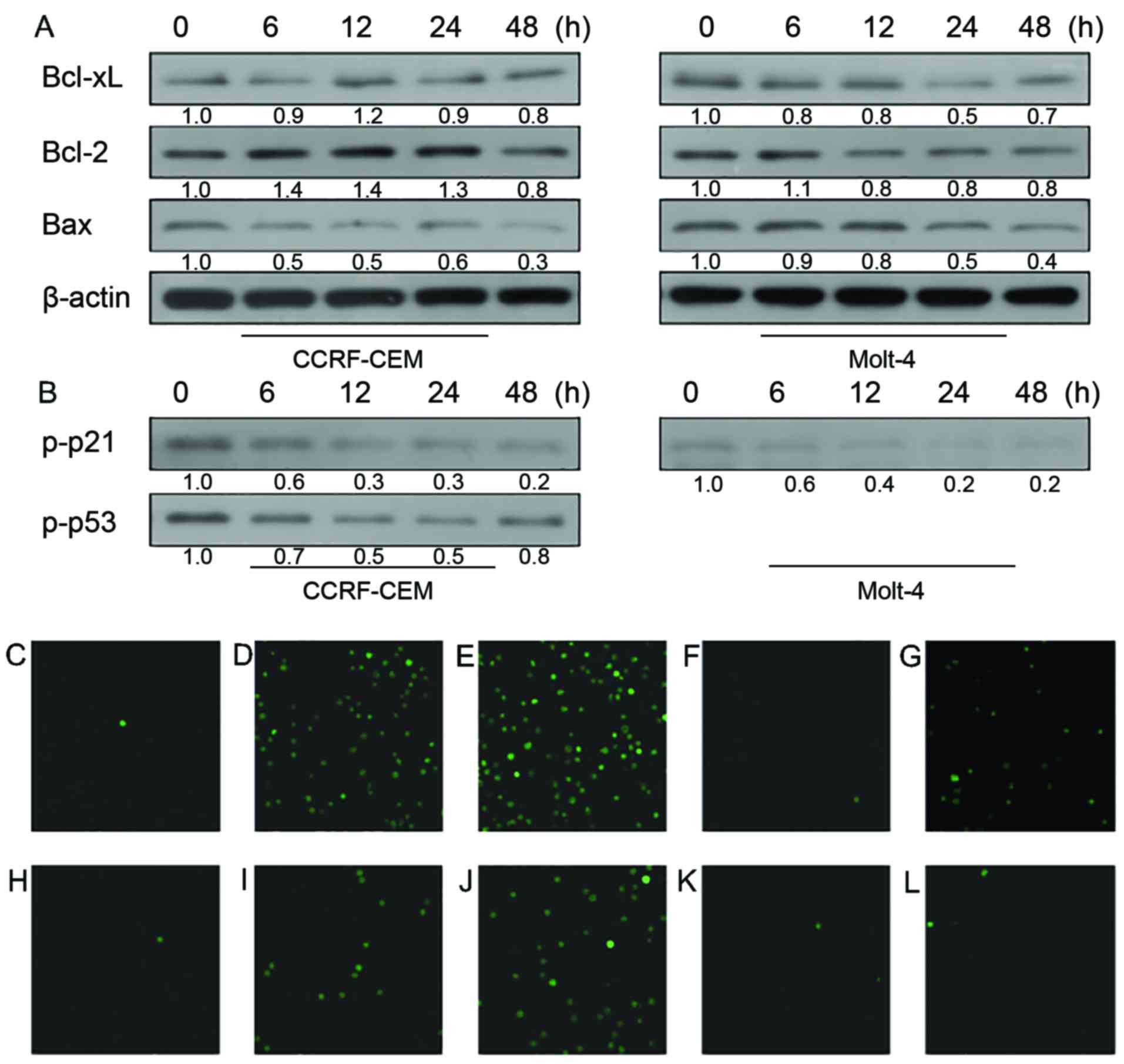
proteins in CCRF-CEM and Molt-4 cells (Fig. 2A). In both cell lines, decreased
Bcl-xL and increased Bax were observed after 48-h treatment of
KML001. Expression of anti-apoptotic protein Bcl-2 decreased
markedly in CCRF-CEM cell line while its expression increased a
little in the Molt-4 cell line.
Cdk1/cyclin B1 complex, the critical regulator of
G2/M checkpoint, plays important roles in mitosis and mitotic
catastrophe (20,21). As shown in Fig. 2B, the expression of Cdk1 kept
decreasing after treatment of KML001 while cyclin B1 expression did
not show any change. Lack of Cdk1 may cause cell arrest at G2/M
phase. To confirm this point, we conducted cell cycle analysis by
propidium iodide staining of the treated cells followed by flow
cytometry (Fig. 2C). As shown by
the G2/M ratios in both cell lines (Fig. 2D), KML001 did induce cell cycle
arrest both in CCRF-CEM and Molt-4 cells. Previous studies suggest
that p53 and p21 were involved in both cell cycle arrest and cell
apoptosis (22,23). Here, we found p53/p21WAF1
pathway was involved in KML001-induced G2/M phase arrest. Fig. 2B indicates that p-p21 expression
increased in a time-dependent manner in CCRF-CCEM and Molt-4 cells
after KML001 treatment. In addition, increased p53 phosphorylation
was observed in CCRF-CEM cells but not in Molt-4 cells which are
p53-deficient.
Synergistic antileukemic effect of
KML001 combined with doxercalciferol
We also investigated whether KML001 and
doxercalciferol could cause synergistic effects in terms of reduced
cell viability in ALL cell lines. CCRF-CEM and Molt-4 cell lines
were incubated for 48 h with one drug alone or with a combination
treatment of KML001 and doxercalciferol at a constant ratio of
1:312.5 or 1:625 (KML001:doxercalciferol). CI and cell viability
were then calculated. As shown in Table
I, in the two cell lines, the combined treatments were
synergistic, as indicated by CI <1.
 | Table I.MTT assay for synergistic effect of
KML001 and doxercalciferol. |
Table I.
MTT assay for synergistic effect of
KML001 and doxercalciferol.
| Cell lines | KML001 (nM) | Doxercalciferol
(µM) | Fraction of cell
death | CI |
|---|
| CCRF-CEM | 10 | 3.125 | 0.183 | 1.529 |
|
| 20 | 6.25 | 0.367 | 1.242 |
|
| 40 | 12.5 | 0.556 | 1.195 |
|
| 50 | 15.625 | 0.723 | 0.741 |
|
| 60 | 18.75 | 0.775 | 0.682 |
|
| 80 | 25 | 0.801 | 0.786 |
| Molt-4 |
5 | 3.125 | 0.082 | 1.226 |
|
| 10 | 6.25 | 0.107 | 1.906 |
|
| 20 | 12.5 | 0.280 | 1.394 |
|
| 40 | 25 | 0.683 | 0.675 |
|
| 80 | 50 | 0.809 | 0.782 |
|
| 160 | 100 | 0.777 | 1.824 |
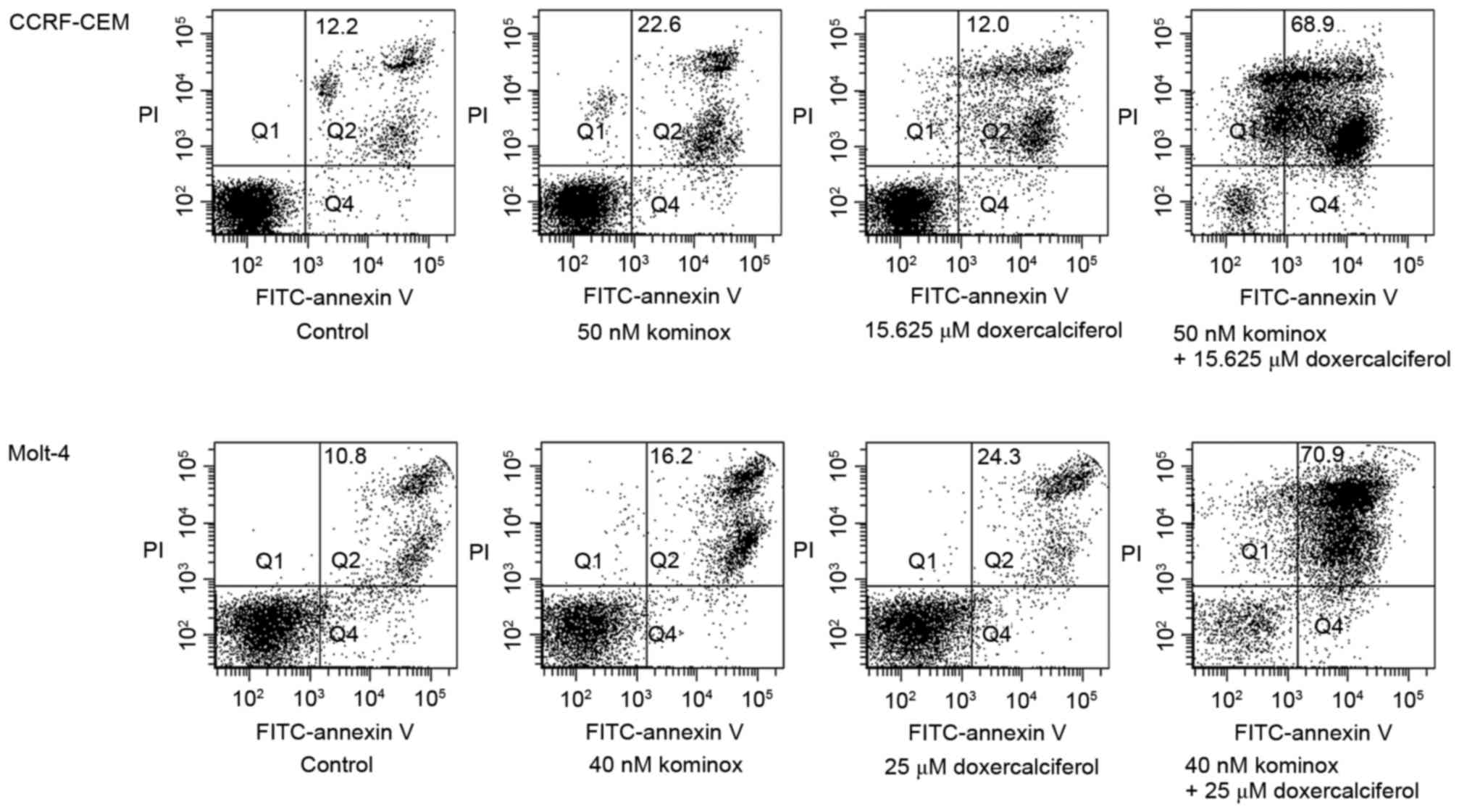
To further evaluate its synergy, flow
cytometry analysis was performed
Also, we observed a significant increase of late
apoptotic cells (double-positive for Annexin V and PI) after
combination treatment compared with single treatment of KML001 or
doxercalciferol (Fig. 3).
Doxercalciferol induces cell arrest at
G2/M phase
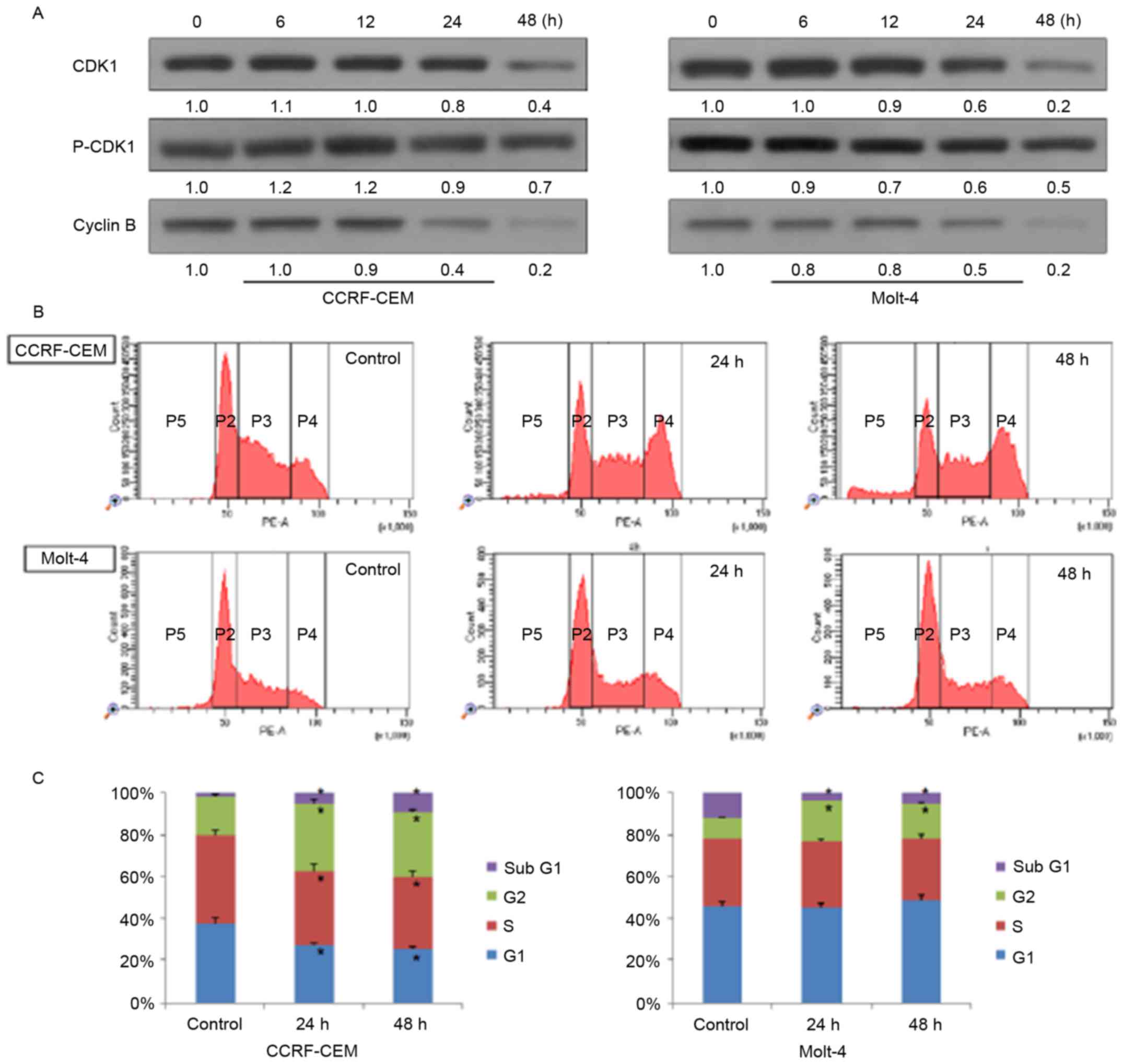
Western blot analysis and flow cytometry were
performed to know whether doxercalciferol induces cell cycle arrest
in ALL cells. As shown in Fig. 4A,
expression of Cdk1 and cyclin B decreased in a time-dependent
manner after treatment, which indicates that doxercalciferol may
also lead to G2/M phase arrest of these two cell lines. Flow
cytometry analysis indicates that doxercalciferol was able to
induce cell cycle arrest at G2/M phase in CCRF-CEM and Molt-4 cells
(Fig. 4B and C).
Doxercalciferol induces cell apoptosis
and cell arrest via different mechanism of action
To further identify mechanisms underlying this
synergy, we also tried to understand mechanisms of action of
doxercalciferol in ALL models in vitro. The pro-apoptotic
protein Bax and anti-apoptotic protein Bcl-xL did not change as we
expected (Fig. 5A). Considering the
late apoptotic cells observed in flow cytometry results,
doxercalciferol may cause cell apoptosis without the involvement of
Bcl-2 family pathway.
In addition, we tried to understand mechanisms of
doxercalciferol-induced G2/M phase arrest by studying intracellular
signaling pathway through western blot analyses. However,
doxercalciferol neither induce p53 activation nor p21 activation in
the two cell lines (Fig. 5B).
Finally, to investigate whether
doxercalciferol-induced apoptosis is associated with
Ca2+, the effect of doxercalciferol on intracellular
calcium ion concentration in ALL cells was observed by using LSCM
after staining with the fluorescent probe, Fluo-4/AM. As shown in
Fig. 5C-L, the depth of the green
signal, which indirectly reflects intracellular Ca2+
concentration, increased as the time increased after treatment of
doxercalciferol while there was relatively less change observed in
groups treated with KML001.
Discussion
Acute lymphoblastic leukemia occurs when primitive
or immature lymphoid cells grow uncontrollably, disturbing normal
hematopoiesis. Until now, it is still one of the most aggressive
hematologic malignancies. With the progress and availability of
combination chemotherapy, radiotherapy or hematopoietic cell
transplantation, the outcomes of ALL patients have improved
markedly. However, there are still many who cannot survive after
treatment due to problems such as relapse, drug resistance or side
effects like toxicity. Here, we evaluated antileukemic activity of
KML001, a kind of arsenic compound alone and KML001 combined with
doxercalciferol in ALL cells.
Previous studies have demonstrated that antileukemia
activity of KML001 is mainly due to its ability to induce apoptosis
and cell cycle arrest via regulation of MAPK and PI3K pathways or
telomere shortening (6,24). Also, our study showed that KML001
induced apoptosis both in CCRF-CEM and Molt-4 cell lines. Gene
expression of Bcl-xL, Bax, Bcl-2, members of Bcl-2 family, has been
known to control the apoptotic machinery (25). KML001 increased expression of
pro-apoptotic protein Bax, and suppressed the expression of
anti-apoptotic protein Bcl-xl in both cell lines.
Cdk1/cyclin B1 complex is one of the pivotal
signaling molecules driving cell progression, which is involved in
G2/M checkpoint. Downregulation of Cdk1 or cyclin B1 expression may
lead to cell cycle arrest at G2/M phase. In our experiments,
constant decreasing expression of Cdk1 in western blotting plus
flow cytometry results identified that treatment with KML001
induced cell cycle arrest mainly at G2/M phase rather than at G0/G1
phase which was shown in AML research (6). To investigate the specific mechanism
of KML001-induced G2/M phase arrest, we checked the expression of
p-p53 and p-p21 by western blot analyses. Results showed a constant
increase of expression of p-p53 or p-p21 after treatment of KML001.
The activation of p21 and p53, which are the main regulator of
CDK1, might be precedent to the change of Cdk1 after KML001
treatment in CCRF-CEM and Molt-4 cells. In addition, KML001 may
cause cell arrest and apoptosis in Molt-4 cells through non-p53
involved pathway since these cells are p53-deficient. This
difference may be associated with the reality that Molt-4 cell line
was created from a relapsed patient but CCRF-CEM cell line was
not.
In order to overcome the possible resistance of
KML001, we tried to evaluate the additional benefit of
doxercalciferol in combination with KML001 in vitro models.
As far as we know, this is the first study demonstrating the
combination effect of KML001 with doxercalciferol against ALL
cells. Also, in our results, the combinatorial treatment of KML001
with doxercalciferol showed a synergistic antileukemic activity in
CCRF-CEM and Molt-4 cell lines, which suggests that the different
mechanisms might work in ALL cells co-treated with KML001 and
doxercalciferol.
Vitamin D and vitamin D analogs exert its anticancer
effects mainly through anti-proliferation, pro-differentiation and
pro-apoptotic effect (26). Unlike
KML001 treatment, doxercalciferol treatment induced increment of
intracellular calcium ion level. Furthermore, doxercalciferol
induced apoptosis and G2/M phase cell cycle arrest but without the
involvement of Bcl-2 family pathways or activation of p53 and
p21.
Collectively, KML001 suppressed Cdk1 via p53 and p21
activation. These changes led to cell cycle arrest at G2/M phase in
ALL cells, and its apoptosis mechanisms also includes p53, p21
activation and other mechanisms such as telomere length shortening.
However, doxercalciferol induced cell apoptosis and cell cycle
arrest in a totally different way, which involves calcium related
pathway but without activation of proteins p53, p21 or Bcl-2 family
involved pathway. This study provided evidence that KML001 could be
an effective anti-leukemic agent especially with doxercalciferol in
ALL treatment in the terms of cell level. Its availability on
clinical application, however, should be further investigated.
Acknowledgements
This study was supported by Seoul National
University College of Medicine (800-20130310 and 800-20140171),
Komipharm International Co., Ltd., Seoul, Korea and the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education
(NRF-2016-0024046).
References
|
1
|
Swerdllow S, Campo E and Harris NL: WHO
Classification of Tumours of Haematopoietic and Lymphoid Tissues.
4th. IARC Press; Lyon: 2008
|
|
2
|
Zuckerman T and Rowe JM: Pathogenesis and
prognostication in acute lymphoblastic leukemia. F1000Prime Rep.
6:592014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raetz EA and Bhatla T: Where do we stand
in the treatment of relapsed acute lymphoblastic leukemia?
Hematology. Am Soc Hematol Educ Program. 2012:129–136. 2012.
|
|
4
|
Litzow MR: Arsenic trioxide. Expert Opin
Pharmacother. 9:1773–1785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
You D, Kim Y, Jang MJ, Lee C, Jeong IG,
Cho YM, Hwang JJ, Hong JH, Ahn H and Kim CS: KML001 induces
apoptosis and autophagic cell death in prostate cancer cells via
oxidative stress pathway. PLoS One. 10:e01375892015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon JS, Kim ES, Park BB, Choi JH, Won YW,
Kim S and Lee YY: Anti-leukemic effect of sodium metaarsenite
(KML001) in acute myeloid leukemia with breaking-down the
resistance of cytosine arabinoside. Int J Oncol. 46:1953–1962.
2015.PubMed/NCBI
|
|
7
|
Muenyi CS, Trivedi AP, Helm CW and States
JC: Cisplatin plus sodium arsenite and hyperthermia induces
pseudo-G1 associated apoptotic cell death in ovarian cancer cells.
Toxicol Sci. 139:74–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoon JS, Hwang DW, Kim ES, Kim JS, Kim S,
Chung HJ, Lee SK, Yi JH, Uhm J, Won YW, et al: Anti-tumoral effect
of arsenic compound, sodium metaarsenite (KML001), in non-Hodgkin's
lymphoma: An in vitro and in vivo study. Invest New Drugs. 34:1–14.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grant WB and Garland CF: The association
of solar ultraviolet B (UVB) with reducing risk of cancer:
Multifactorial ecologic analysis of geographic variation in
age-adjusted cancer mortality rates. Anticancer Res. 26A:2687–2699.
2006.
|
|
10
|
Giovannucci E, Liu Y, Rimm EB, Hollis BW,
Fuchs CS, Stampfer MJ and Willett WC: Prospective study of
predictors of vitamin D status and cancer incidence and mortality
in men. J Natl Cancer Inst. 98:451–459. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Colston K, Colston MJ and Feldman D:
1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of
receptors and inhibition of cell growth in culture. Endocrinology.
108:1083–1086. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyaura C, Abe E, Kuribayashi T, Tanaka H,
Konno K, Nishii Y and Suda T: 1α,25-Dihydroxyvitamin D3 induces
differentiation of human myeloid leukemia cells. Biochem Biophys
Res Commun. 102:937–943. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gardner JP, Zhang F, Uskokovic MR and
Studzinski GP: Vitamin D analog
25-(OH)-16,23E-Diene-26,27-hexafluoro-vitamin D3 induces
differentiation of HL60 cells with minimal effects on cellular
calcium homeostasis. J Cell Biochem. 63:500–512. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCarthy DM, San Miguel JF, Freake HC,
Green PM, Zola H, Catovsky D and Goldman JM: 1,25-dihydroxyvitamin
D3 inhibits proliferation of human promyelocytic leukaemia (HL60)
cells and induces monocyte-macrophage differentiation in HL60 and
normal human bone marrow cells. Leuk Res. 7:51–55. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Zhang J and Studzinski GP: AKT
pathway is activated by 1, 25-dihydroxyvitamin D3 and participates
in its anti-apoptotic effect and cell cycle control in
differentiating HL60 cells. Cell Cycle. 5:447–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sergeev IN: Calcium as a mediator of
1,25-dihydroxyvitamin D3-induced apoptosis. J Steroid Biochem Mol
Biol 89–90. 419–425. 2004. View Article : Google Scholar
|
|
17
|
Petrich A, Kahl B, Bailey H, Kim K, Turman
N and Juckett M: Phase II study of doxercalciferol for the
treatment of myelodysplastic syndrome. Leuk Lymphoma. 49:57–61.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu G, Wilding G, Staab MJ, Horvath D,
Miller K, Dresen A, Alberti D, Arzoomanian R, Chappell R and Bailey
HH: Phase II study of 1α-hydroxyvitamin D(2) in the treatment of
advanced androgen-independent prostate cancer. Clin Cancer Res.
9:4077–4083. 2003.PubMed/NCBI
|
|
19
|
Chou T-C and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castedo M, Perfettini J-L, Roumier T,
Andreau K, Medema R and Kroemer G: Cell death by mitotic
catastrophe: A molecular definition. Oncogene. 23:2825–2837. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castedo M, Perfettini J-L, Roumier T,
Valent A, Raslova H, Yakushijin K, Horne D, Feunteun J, Lenoir G,
Medema R, et al: Mitotic catastrophe constitutes a special case of
apoptosis whose suppression entails aneuploidy. Oncogene.
23:4362–4370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Woo SR, Ham Y, Kang W, Yang H, Kim S, Jin
J, Joo KM and Nam DH: KML001, a telomere-targeting drug, sensitizes
glioblastoma cells to temozolomide chemotherapy and radiotherapy
through DNA damage and apoptosis. BioMed Res Int. 2014:7474152014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guyton KZ, Kensler TW and Posner GH:
Cancer chemoprevention using natural vitamin D and synthetic
analogs. Annu Rev Pharmacol Toxicol. 41:421–442. 2001. View Article : Google Scholar : PubMed/NCBI
|