Introduction
Allogeneic bone marrow transplant is an effective
treatment for many advanced and high-risk hematological
malignancies (1). During the
procedure, the hematopoietic and immune system of the recipient is
destroyed by chemotherapy or radiotherapy, followed by the
administration of hematopoietic stem cells harvested from the
donor. Hematopoietic stem cells of the donor engraft, proliferate,
and finally reconstitute hematopoiesis in the recipient. Thus,
white blood cells from allogeneic bone marrow transplant (allo-BMT)
recipients are usually entirely derived from the bone marrow of the
donor (1). According to National
Comprehensive Cancer Network (NCCN) guidelines, the DNA of allo-BMT
recipients should be extracted from a fibroblast culture derived
from skin fibroblasts of the allo-BMT recipients, when available.
When this is not possible, buccal cells may be considered as an
alternative source for DNA (2).
Since various studies have reported that over time, buccal
epithelial cells are replaced by donor-derived cells in allo-BMT
recipients (3,4), molecular genetic testing in allo-BMT
patients performed using DNA derived from blood as well as from
buccal samples may reflect the genetic status of the transplant
donor and not the recipient. For genetic counseling it is extremely
important to ensure that detected DNA variants (mutations) reflect
the germline DNA of the patient, since they impact the subsequent
clinical management of the patient and his/her family members
(5). Therefore, biological samples
other than blood or buccal swabs should be considered for DNA
isolation.
The aim of the present study was to present the
germline testing and counseling approach utilized in a rare case of
a chimeric donor-related BRCA1-positive woman who received
an allogenic bone marrow transplant from a sibling with a germline
BRCA1 pathogenic mutation.
Patient and methods
Ethical approval and consent to
participate
All tested family members provided written informed
consent to participate and attended genetic counseling sessions
before and after testing. A copy of the written consent can be
obtained by contacting the authors of the present study. Genetic
counseling and testing at the Institute of Oncology Ljubljana was
approved by the Commission for Medical Ethics at the Ministry of
Health, Republic of Slovenia in 1999.
Case and family presentation
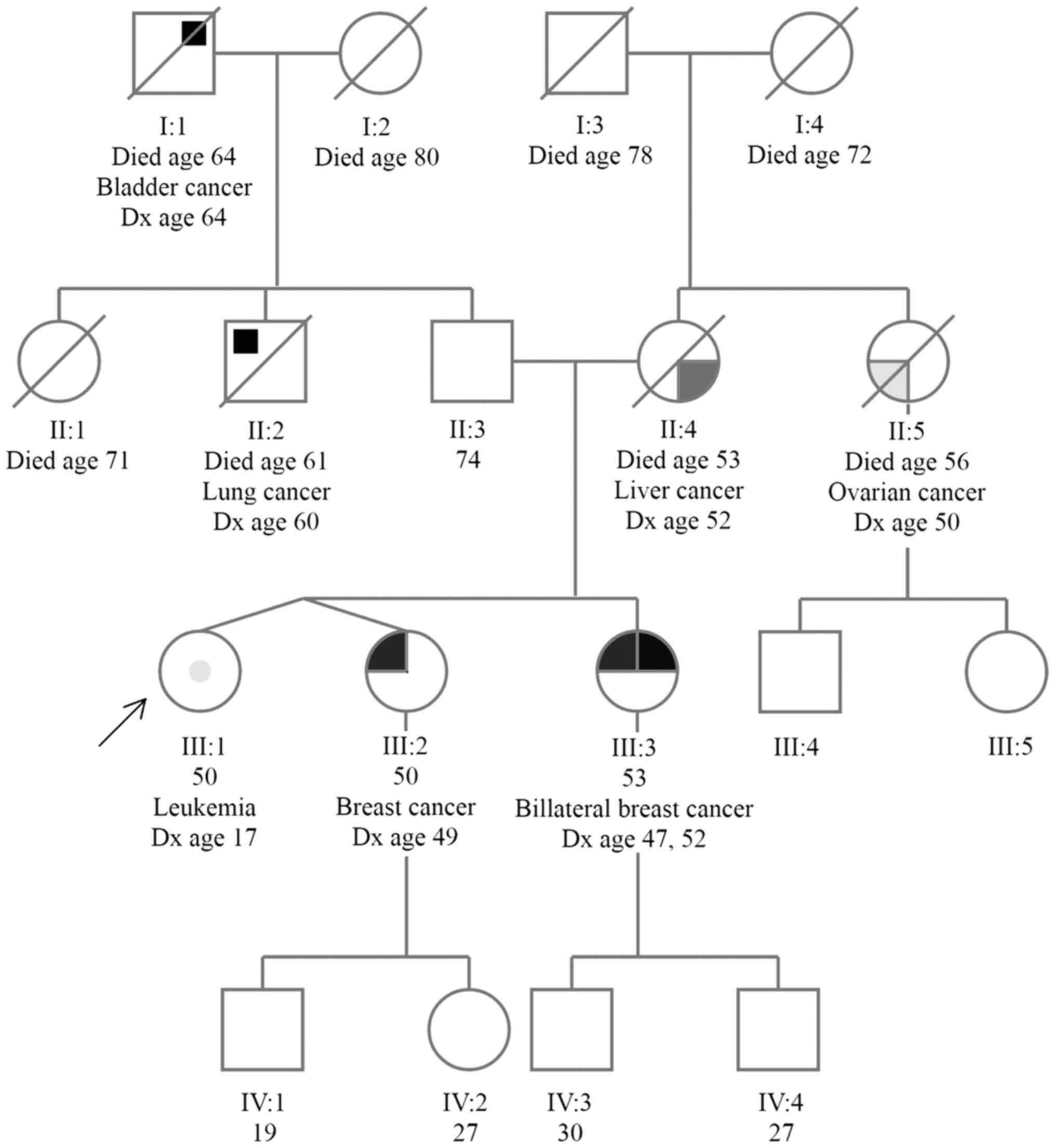
The patient we are presenting is a 50-year-old
female, who approached our clinic, since her non-identical twin
sister had been diagnosed with breast cancer at the age of 49 and
her older sister had been diagnosed with bilateral breast cancer
(at age 47 and 52). Her mother and maternal aunt had also been
diagnosed with cancer (liver cancer, at the age of 52 and ovarian
cancer at the age of 50, respectively), and on her father's side of
the family, there were cases of lung and bladder cancer (Fig. 1).
A genetic test was first performed in her older
sister (diagnosed with bilateral breast cancer), who was identified
as a carrier of BRCA1 mutation. Subsequently, genetic
testing for a known family BRCA1 pathogenic mutation was
performed in her twin sister (diagnosed with breast cancer), who
was identified as a carrier of a known family BRCA1
pathogenic mutation.
At the pre-test counseling session, the patient
informed her consultant that she had been diagnosed with chronic
myeloid leukemia (CML), Ph1-positive, at the age of 17 (in 1984)
and that she had been systemically treated over the next 8 years.
At the age of 25, she was still in the first chronic phase of CML,
and her older sister was found to be a human leukocyte-associated
antigen (HLA) identical and mixed lymphocyte culture (MLC)
non-reactive donor. Hence, allogeneic bone marrow transplantation
was performed with her older sister's marrow stem cells in 1992.
Since then, she has been on yearly follow-up.
The BRCA test result was of great importance for our
patient, since in the case of a positive result, she may be
eligible for high risk screening and may be offered preventive
surgeries for breast and ovarian cancer at our institution. In
contrast, in the case her test result is negative, she may be
relieved of a great psychological burden and may be enrolled in a
national breast cancer screening programme.
Sample collection, DNA extraction and
sequencing
All tested family members provided written informed
consent and attended genetic counseling sessions before and after
testing.
In the case of our patient, three different types of
biological samples were obtained: peripheral blood, buccal swabs
and 5–7 plucked head hair samples.
Blood samples were collected in EDTA-tubes, and DNA
was extracted using QIAamp DNA Blood Mini kit (Qiagen, Hilden,
Germany) according to the manufacturer's instructions.
Buccal swab sampling was performed by softly
brushing the mucosal cheek using a cotton swab (ForensiX SafeDry
Evidence Collection Tube; Prionics AG, Schlieren-Zürich, CH,
Switzerland). DNA was extracted using QIAamp DNA Blood Mini kit
according to the manufacturer's instructions for buccal swabs.
Five to seven full-length hairs with follicles were
placed into one 1.5-ml tube. The presence of a hair follicle was
visually confirmed. Hairs were cut into ~1 cm including the
follicle, and the distal part was discarded. Buffer ATL and
proteinase K (both from Qiagen) were added and tubes were incubated
overnight at 56°C with shaking at 900 rpm. DNA was extracted using
QIAamp DNA Blood Mini kit according to the manufacturer's
instructions with a modification including additional incubation
with AL buffer (Qiagen) at 70°C for 10 min after overnight
incubation.
DNA quality and quantity of all samples were
spectrophotometrically evaluated using NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Genetic testing for a known family BRCA1
(LRG_292t1) pathogenic mutation c.5266dupC p.(Gln1756Profs*74) was
performed by Sanger sequencing on the ABI3500 sequencer (Applied
Biosystems, Foster City, CA, USA) as previously described (6,7). All
results were confirmed by the analysis of an independent second DNA
sample obtained from blood, buccal swab and hair.
Results and Discussion
In the present study, we studied a rare case of a
woman with a prior history of leukemia, who received an allogenic
bone marrow transplant 25 years ago from a sibling harboring a
germline BRCA1 pathogenic mutation c.5266dupC
p.(Gln1756Profs*74). At the time of allo-transplantation the donor
(sister) was healthy and unaware of having a BRCA
mutation.
When an allo-BMT recipient requires genetic testing,
the first question that arises is what may be the appropriate and
reliable biological sample for DNA isolation. For a long-time it
was believed that, in contrast to blood cells which may often be
replaced by those with the donor genotype, other cells remain of
recipient origin (3). Recently,
bone marrow and peripheral blood stem cells have been noted to have
the potential to transdifferentiate or dedifferentiate into neural,
bone, muscular, cartilage, liver, gut, alveolar, buccal, epidermal
or endothelial cells. This process is called ‘adult stem cell
plasticity phenomenon’. The frequency of appearance of these
exogenous cells is reported to be between 0.1 and 10% of
tissue-specific cells (8–12).
Tran et al (4) were the first to report that allogenic
human bone marrow-derived cells migrate into the cheek and
differentiate into epithelial cells. Since it was reported that
over time, buccal epithelial cells of the recipient could be
replaced by donor-derived cells, genetic testing based on DNA
isolated from buccal swab samples has become questionable given
this known risk of donor DNA contamination (4). Therefore, in allo-BMT recipients.
other sources of DNA should also be considered. It has been shown
that hair follicles lack adult stem cell plasticity and remain of
recipient origin for more than 20 years after allogenic
hematopoietic stem cell transplantation (3). Taking into account all of these facts,
we collected peripheral blood, buccal swabs and 5–7 plucked head
hair samples in our case of an allo-BMT recipient. The sequencing
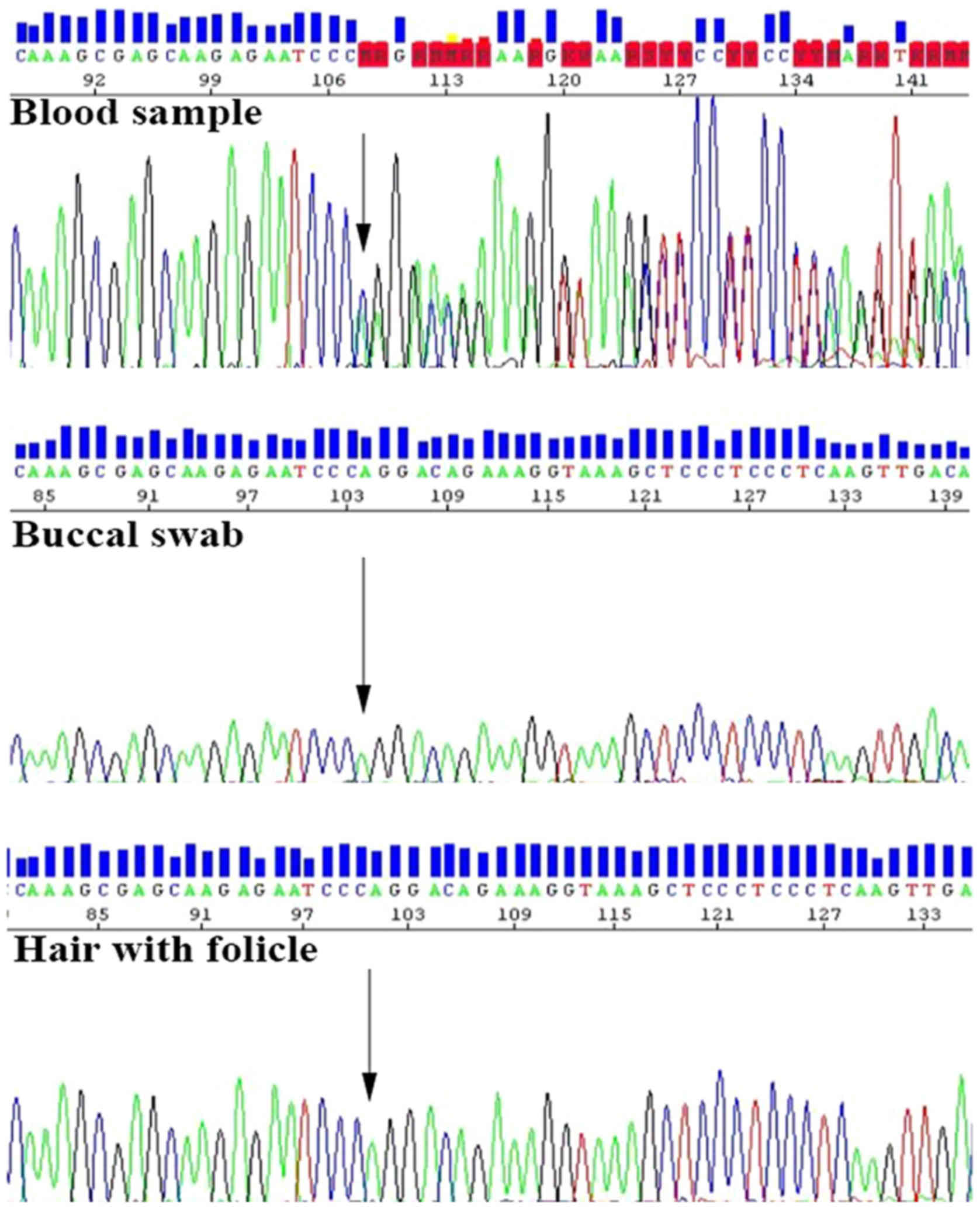
analysis of the DNA sample isolated from her blood showed a
positive result for a known family BRCA1 mutation c.5266dupC
p.(Gln1756Profs*74). However, the DNA samples extracted from her
buccal swab and hair were negative for this mutation (Fig. 2). According to the results of
genetic testing, she was found not to be a carrier of a germline
BRCA1 pathogenic mutation known in her family, but she
represents a chimeric donor-related BRCA1-positive carrier.
At the post-test genetic counseling session she was advised to take
part in the national breast cancer screening programme, where
mammographies are performed every two years (for women between
50–69 years). She is also followed up yearly for CML.
In our case, the genetic testing strategy was
greatly facilitated since the BRCA1 mutation was known in
this family [c.5266dupC p.(Gln1756Profs*74)]. Therefore, the
genetic testing from blood was expected to be positive for a known
family mutation. To determine whether the mutation was of germline
origin, other DNA sources were successfully used. In the ‘vice
versa’ situation, when the bone marrow donor is negative for a
known family mutation, a false-negative result could be reported,
when additional sources of DNA are not tested.
An additional question that arises from these
results is what cancer risk is expected for such chimeric
donor-related BRCA1 carriers. Notably, after the allogenic
bone marrow transplant, our 50-year-old allo-BMT recipient has not
been diagnosed with breast or ovarian cancer or any hematologic
malignancies. As mentioned previously, bone marrow and peripheral
blood stem cells transdifferentiate into different types of cells
including buccal, epidermal or endothelial cells. However, to the
best of our knowledge, there are no data in the literature clearly
describing the risk for breast or ovarian cancer in
BRCA-negative patients receiving BRCA-positive
allogenic bone marrow transplants. There are several studies
supporting the hypothesis that BRCA1 mutations affect the
development of hematologic malignancies through defective DNA
repair pathways (13). There is an
evident increase in the risk of hematologic malignancies in
patients with a detected mutation in genes involved in the BRCA
pathway, including mantle cell lymphoma, acute myeloid, acute and
chronic lymphocytic and prolymphocytic leukemia (14). Various epidemiologic studies have
also found increased risks for leukemia and lymphoma in identified
BRCA1/BRCA2 mutation carriers (15,16).
Unfortunately, there are no studies of a possible risk for a second
hematologic malignancy for chimeric donor-related BRCA1
carriers with a prior history of leukemia. The significance of a
donor-related BRCA1 mutation in such carriers should be
further evaluated. Therefore, careful genetic counseling and
follow-up for such patients should be considered.
In conclusion, according to our results and a study
mentioned above, hairs with follicles are a reliable and ready
source of DNA as they appear to be completely of allo-BMT recipient
origin. Compared with a fibroblast culture, which is more difficult
to obtain, the hair follicles are much more accessible and hair
sampling is less invasive for the patient. Evaluation of the family
history is important before a bone marrow transplant, and patients
who fulfill the criteria for genetic testing may be referred for
genetic counseling before a bone marrow transplant. Since the
family history can be unremarkable at the time of transplantation,
blood or DNA samples from all allo-BMT patients must be routinely
stored in case they are needed for future genetic testing.
Acknowledgements
The authors would like to thank Simona Traven for
her substantial laboratory technical assistance. We are grateful to
the family for participation. The present study was partially
supported by the Slovenian Research Agency (grant no. P3-0352).
References
|
1
|
Waterhouse M, Themeli M, Bertz H, Zoumbos
N, Finke J and Spyridonidis A: Horizontal DNA transfer from donor
to host cells as an alternative mechanism of epithelial chimerism
after allogeneic hematopoietic cell transplantation. Biol Blood
Marrow Transplant. 17:319–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Network NCCN: NCCN Clinical Practice
Guidelines in Oncology - Genetic/Familial high-risk assessment:
Breast and ovarian. Version. 2016.
|
|
3
|
Hong YC, Liu HM, Chen PS, Chen YJ, Lyou
JY, Hu HY, Yi MF, Lin JS and Tzeng CH: Hair follicle: A reliable
source of recipient origin after allogeneic hematopoietic stem cell
transplantation. Bone Marrow Transplant. 40:871–874. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tran SD, Pillemer SR, Dutra A, Barrett AJ,
Brownstein MJ, Key S, Pak E, Leakan RA, Kingman A, Yamada KM, et
al: Differentiation of human bone marrow-derived cells into buccal
epithelial cells in vivo: A molecular analytical study. Lancet.
361:1084–1088. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mancini-DiNardo D, Landon M, Abbott B,
Elias M, Rinsky J and Roa B: Complexities in genetic testing for
allogenic bone marrow transplant recipients and patients with
hematologic malignancies. Journal. 2016.http://www.ashg.org/2014meeting/abstracts/fulltext/f140122408.htm
|
|
6
|
Novaković S, Milatović M, Cerkovnik P,
Stegel V, Krajc M, Hočevar M, Zgajnar J and Vakselj A: Novel
BRCA1BRCA2 pathogenic mutations in Slovene hereditary breast and
ovarian cancer families. Int J Oncol. 41:1619–1627. 2012.PubMed/NCBI
|
|
7
|
Stegel V, Krajc M, Zgajnar J, Teugels E,
De Grève J, Hočevar M and Novaković S: The occurrence of germline
BRCA1BRCA2 sequence alterations in Slovenian population. BMC Med
Genet. 12:92011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Körbling M, Katz RL, Khanna A, Ruifrok AC,
Rondon G, Albitar M, Champlin RE and Estrov Z: Hepatocytes and
epithelial cells of donor origin in recipients of peripheral-blood
stem cells. N Engl J Med. 346:738–746. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krause DS, Theise ND, Collector MI,
Henegariu O, Hwang S, Gardner R, Neutzel S and Sharkis SJ:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mezey E, Chandross KJ, Harta G, Maki RA
and McKercher SR: Turning blood into brain: Cells bearing neuronal
antigens generated in vivo from bone marrow. Science.
290:1779–1782. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HS, Lee SW, Choi YJ, Shin SW, Kim YH,
Cho MS, Lee SN and Park KH: Novel germline mutation of BRCA1 gene
in a 56-year-old woman with breast cancer, ovarian cancer, and
diffuse large B-cell lymphoma. Cancer Res Treat. 47:534–538. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedenson B: The BRCA1/2 pathway prevents
hematologic cancers in addition to breast and ovarian cancers. BMC
Cancer. 7:1522007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Risch HA, McLaughlin JR, Cole DE, Rosen B,
Bradley L, Kwan E, Jack E, Vesprini DJ, Kuperstein G, Abrahamson
JL, et al: Prevalence and penetrance of germline BRCA1BRCA2
mutations in a population series of 649 women with ovarian cancer.
Am J Hum Genet. 68:700–710. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shih HA, Nathanson KL, Seal S, Collins N,
Stratton MR, Rebbeck TR and Weber BL: BRCA1 and BRCA2 mutations in
breast cancer families with multiple primary cancers. Clin Cancer
Res. 6:4259–4264. 2000.PubMed/NCBI
|