According to the World Health Organization's World
Cancer Report 2014, cancer caused 8.2 million deaths worldwide in
2012, and this number is expected to rise to 22 million by 2035
(1). Along with surgery and
radiotherapy, chemotherapy is a mainstay of cancer treatment.
Chemotherapy is the most frequently used systemic treatment for
suppressing cancer cell proliferation, disease progression and
metastasis. However, chemotherapeutic drugs not only kill
proliferating cancer cells but also inevitably attack normal cells,
causing adverse effects. Therefore, antitumor drug vehicles that
maintain or improve the efficacy of chemotherapy while reducing the
severity of reactions and side effects are urgently needed.
Nanoparticles, which can be adapted to have various
biological properties and can be used in a range of settings,
provide a safer and effective means of delivering chemotherapy
(2–4). In the past decade, approximately
12,000 reports on the topic of nanomaterials as drug carriers in
cancer treatment have been published. However, there remains a gap
between technological advances and clinical applications. Many
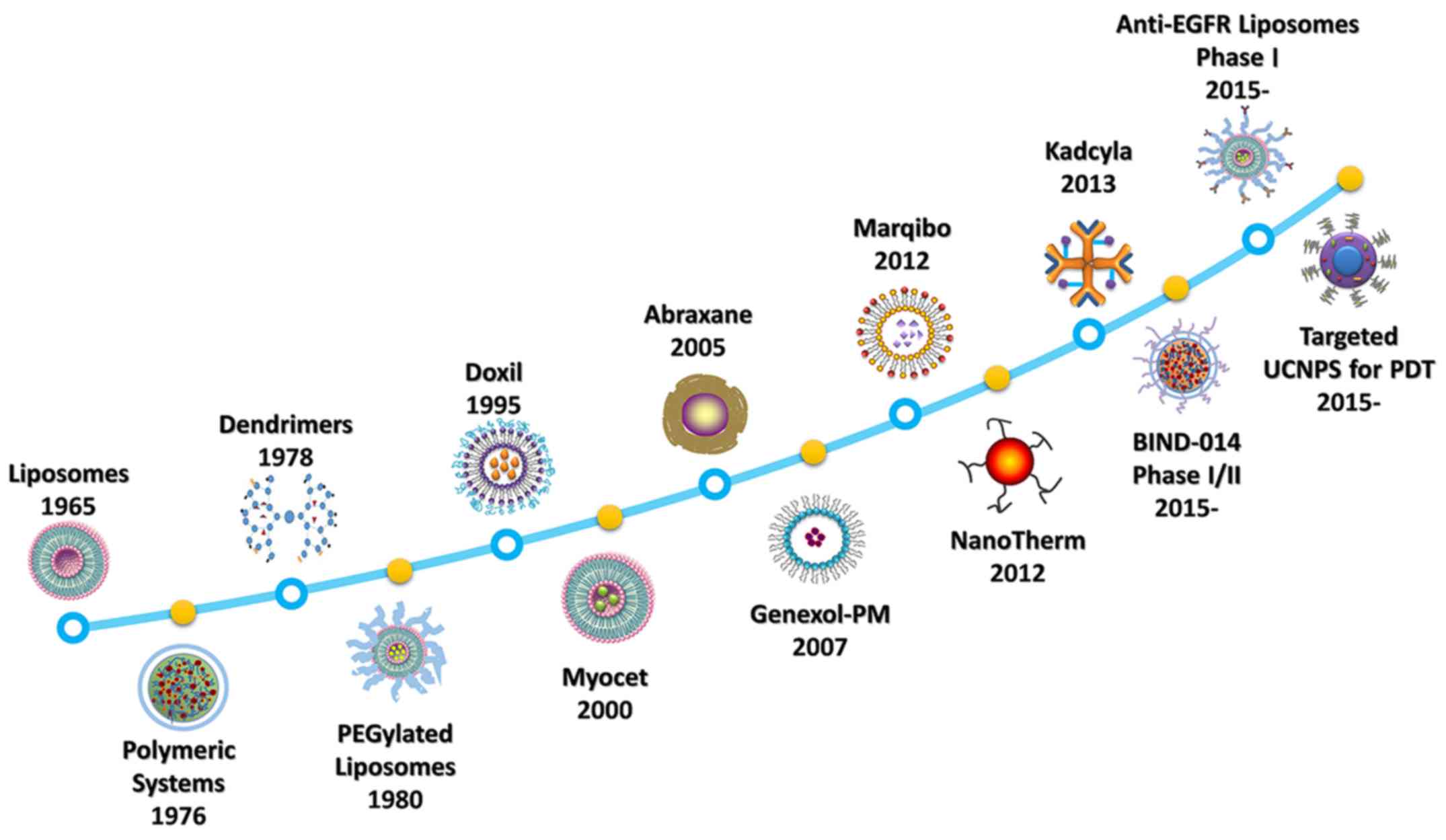
nanodrugs have been developed over the last 50 years (Fig. 1). In 1965, a group led by Bangham
discovered liposomes (5). A
liposomal formulation of doxorubicin (Doxil), was approved by the
US Food and Drug Administration (FDA) in 1995 for treating
AIDS-related Kaposi sarcoma (6). In
2005, an albumin-based nanoparticle, protein-bound paclitaxel
(Abraxane) (7), has been approved
by the FDA for clinical use in the treatment of breast cancer,
non-small cell lung cancer, and pancreatic cancer. More recently,
in 2013, targeted ado-trastuzumab emtansine (DM1) (Kadcyla) was
approved for use in patients with human epidermal growth factor
receptor 2-positive breast cancer (8).
Nanomaterials have a number of advantages as drug
carriers. Nanocarriers can: i) increase water solubility and
protect drugs dissolved in the bloodstream, improving the
pharmacokinetic and pharmacological properties of the drugs; ii)
target the delivery of drugs in a tissue- or cell-specific manner,
thereby limiting drug accumulation in the kidneys, liver, spleen,
and other non-targeted organs and enhancing therapeutic efficacy;
and iii) deliver a combination of imaging and therapeutic agents
for real-time monitoring of therapeutic efficacy (9,10).
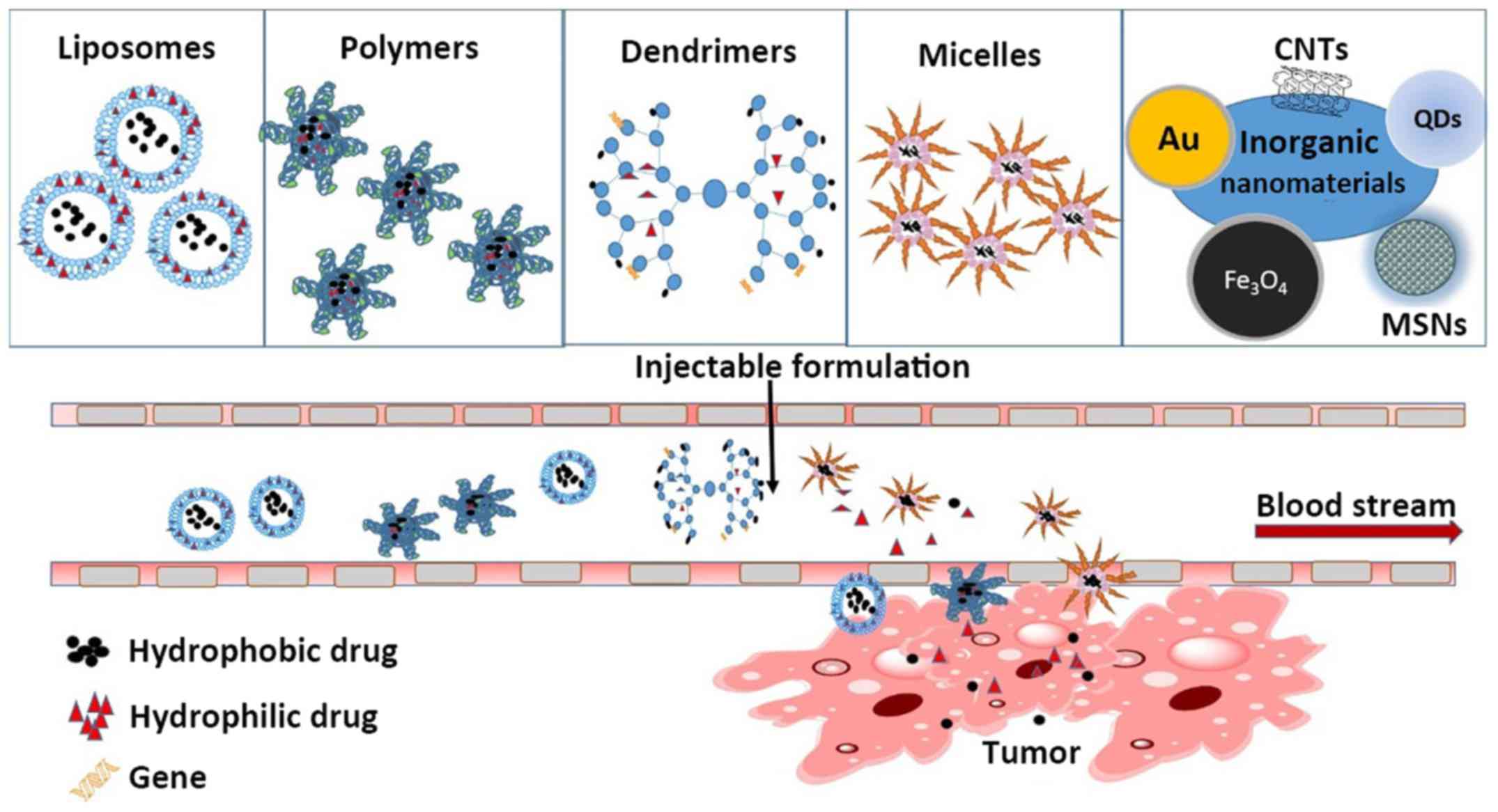
This review summarizes recent developments in the
use of nanomaterials in cancer therapy. Specifically, we discuss
the use of liposomes, polymer nanoparticles, dendritic polymers,
and micelles as drug carriers (Fig.
2). Each category of nanomaterials has unique strengths and
limitations; thus, a major goal of this review is to unveil the
emerging possibilities of different nanovectors for different
therapeutic applications, their relevant molecular targets, and
their advantages and disadvantages.
Liposomes consist of an aqueous core surrounded by
one or several layers of phospholipids and cholesterol that form a
lipid bilayer. Because of this unique structure, liposomes can load
and hold hydrophilic agents in the aqueous compartment and
hydrophobic agents in the lipid space (11). Because their composition is similar
to that of the cell membrane, liposomes are more biocompatible than
other synthetic materials. In addition, distinct surface
modification with functional ligands and differences in size and
charge make liposomes coat with polyethylene glycol (PEG) useful
for specific drug delivery tasks.
Liposomes have several additional advantages as
nanocarriers for drug delivery applications. Liposomes protect the
loaded drug from degradation and prevent undesirable exposure of
the drug to the environment, which may slow the rate of drug
release (12–14). Specific lipid species, such as
cholesterol and rigid saturated lipids, stabilize the lipid bilayer
to resist attack from plasma proteins and reduce drug leakage
(13,14). However, the present challenge facing
the development of liposomes as drug carriers is how to control
their distribution and removal in vivo.
Recently, a number of studies have focused on
modifying liposome drug-releasing mechanisms. For example, drug
release from liposomes can be triggered by ultrasound (15,16),
enzymes (17,18), light (19,20),
magnetism (21–23), or hyperthermia (24). Drug-releasing liposomes may also be
combined with ligand-mediated targeted delivery of nucleic acids
(25–28).
Liposomes not only increase the intracellular uptake
of drugs but also can be used to modify anticancer agents,
antibiotics, and DNA. Using an AAN-TAT-liposome platform, Liu et
al (31) created a doxorubicin
carrier that enhanced the drug tumoricidal effect and reduced
systemic adverse effects. The RNA liposome platform is another
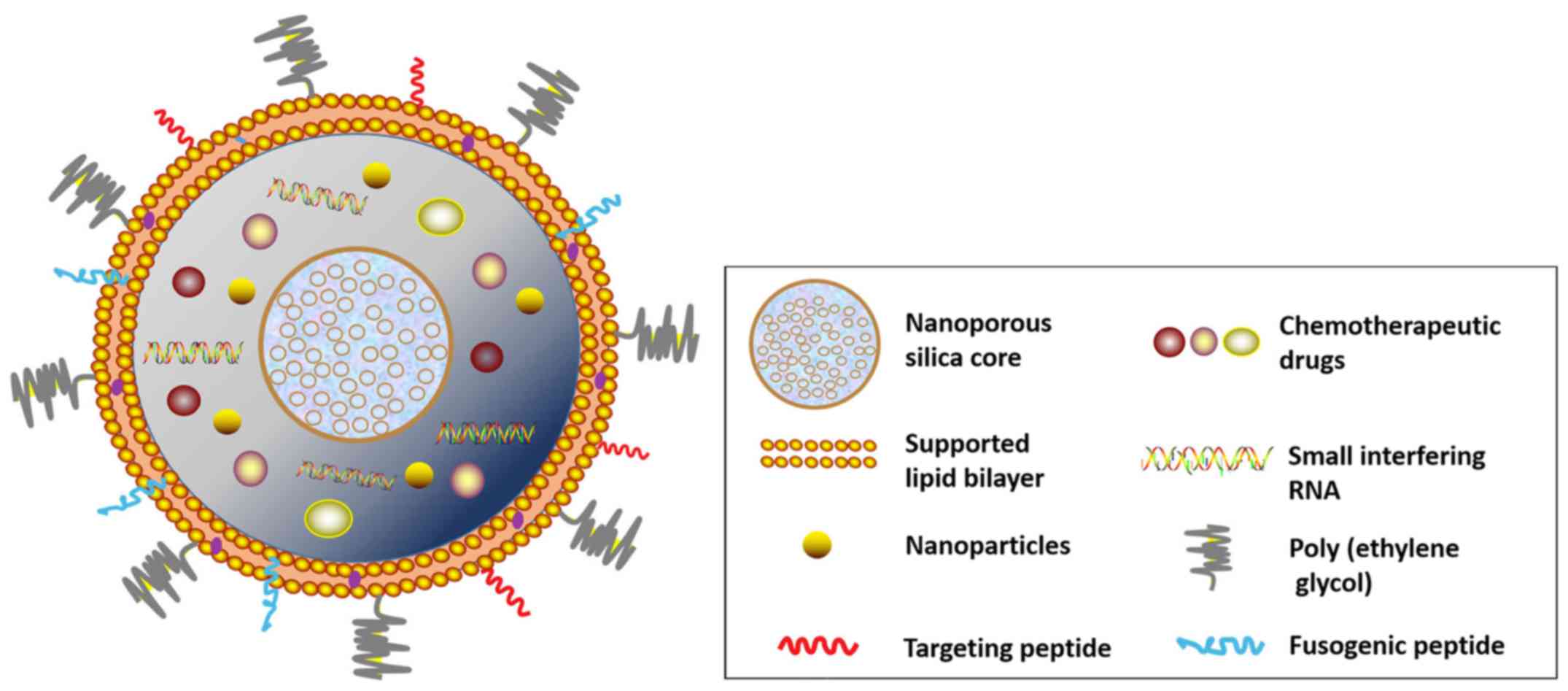
promising strategy for boosting therapeutic efficacy (32). Recently, protocells have been
designed to incorporate various types of modification to achieve a
comprehensive nanodrug delivery system (Fig. 3). Chemotherapy agents, short
interfering RNA, and nanoparticles, for instance, can be coupled
with or encapsulated in a nanoporous silica core for simulating
chemotherapy treatment with site-specific drug delivery. The
supporting lipid bilayer can also be decorated with
surface-targeting molecules, such as fusogenic peptide and
polyethylene glycol, according to tumor type or vasculature.
Liposomes can also be used as a nonviral vector for
gene delivery, making the liposome/DNA complex one of the most
promising tools for cancer gene therapy (33). For example, Felgner and colleagues
(34) developed cationic
liposome-mediated gene delivery, in which a liposome was
incorporated with an antisense oligodeoxynucleotide specific for
growth factor receptor-bound protein 2 (Grb2) mRNA (L-Grb2). These
liposomes inhibited Grb2 protein expression, reduced proliferation
of bcr-abl-positive leukemia cells, and extended survival durations
in mice bearing bcr-abl-positive leukemia xenografts (35) (Table
I).
Polymers can be categorized as: i) natural polymers,
such as proteins, peptides, glycans, starches, and cellulose; ii)
synthetic polymers, which are synthesized from natural monomers,
for instance, polylactic acid (PLA) and poly (lactic-co-glycolic
acid) (PLGA); and iii) microbial fermentation polymers, such as
polyhydroxybutyrate (36). Natural
and synthetic polymers constitute a diversified platform for
synthesis of a variety of nanoparticles, including liposomes,
dendrimers, and micelles (Fig.
2).
Nanosponges, which are made from biocompatible,
biodegradable polymer nanoparticles, are prepared by fusing
erythrocyte membrane vesicles onto PLGA nanoparticles by means of
extrusion. Nanosponges are composed of hyper-cross-linked
cyclodextrins connected in a three-dimensional network. Nanosponges
form porous nanoparticles with sizes <500 nm, so they easily
circulate in the bloodstream. As ‘sponges’, they can absorb toxins,
secretions, and fragments produced by tumor cells themselves
(37,38,60).
Their spherical shape and negative surface charge give them a good
capacity for incorporating small molecules, macromolecules, ions,
and gases within their structure. Therefore, nanosponges have been
designed to improve chemotherapeutic efficacy by targeting
drug-resistant cells (60–62). The erythrocyte membrane can be used
as a cloak containing >3,000 nanosponges. Once they are fully
loaded with toxins, nanosponges are safely disposed of by the liver
with low toxicity. Therefore, nanosponges are designed to work with
any type of cancer or poisoning that exhibits dysregulation of, or
abnormalities in, cellular membranes.
Among the polymer-based delivery systems, only one
albumin-based nanoparticle, protein-bound paclitaxel (Abraxane)
(63), has been approved by the FDA
for clinical use in the treatment of breast cancer, non-small cell
lung cancer, and pancreatic cancer (Table II). Albumin nanoparticle that
incorporates paclitaxel has improved the water solubility of the
drug and reduced its dose-limiting toxicity by modifying its
pharmacokinetic formulation (64).
Given these successes, various albumin-based nanoparticles, such as
ABI-008 (65), ABI-009 (66), and ABI-011 (67), are currently undergoing clinical
trials. BIND-014 (68) is the first
PEG-PLGA targeted polymeric nanoparticle to reach phase I/II
studies for the treatment of metastatic cancer and KRAS-positive or
squamous cell non-small cell lung cancer. Its pharmaceutical
activity is 10-fold higher than that of conventional docetaxel in
tumor sites, and it prolongs the time the drug is maintained in the
circulation. Also, a targeted cyclodextrin-polymer hybrid
nanoparticle (CALAA-01), a short interfering RNA inhibitor designed
to inhibit tumor growth and/or reduce tumor size (69), was tested in phase I clinical trial.
Current research on polymer nanocarriers focuses on elucidating
their mechanisms of action, environmental responses, active
targeting, and composite materials. Relevant diagnostic and
therapeutic platforms still need to be constructed and
evaluated.
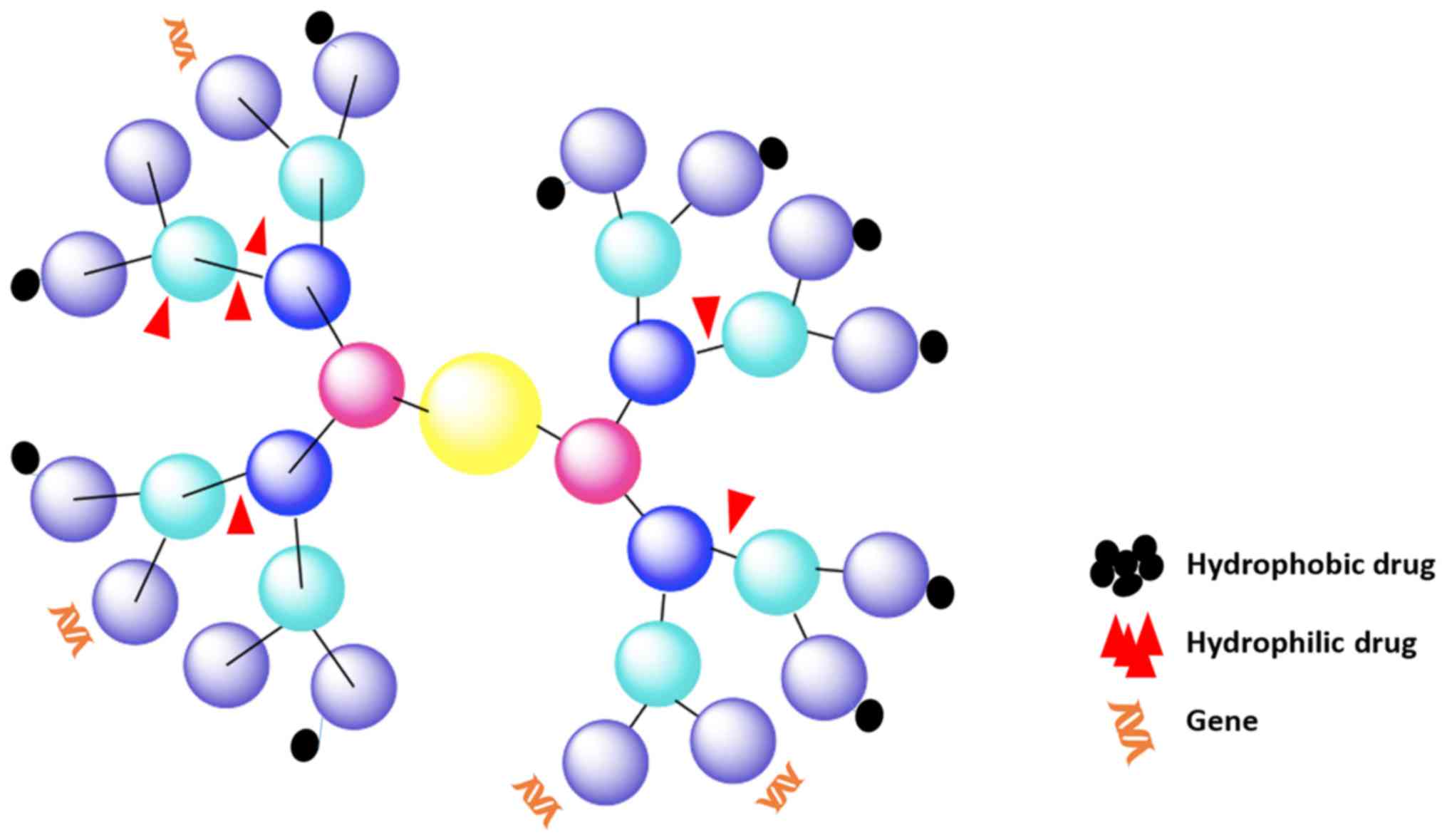
Dendrimers are a unique class of polymeric
macromolecules found in nature. Dendrimers began to be synthesized
during the period 1970–1990 by Buhleier et al (70) and Tomalia et al (71). They are globular, nanosized (1–100
nm) macromolecules with complex spherical structures. Dendrimers
are characterized by: i) a central core; ii) branches, called
‘generations’, emanating from the core; iii) repeat units with at
least one branch junction; and iv) many terminal functional groups
(Fig. 4) (72,73).
Unlike linear polymers, dendrimers have a precisely controllable
architecture with tailor-made surface groups. The branches of
dendrimers can be decorated with a wide variety of molecules that
can be utilized for passive entrapment and eventual release of
drugs or other cargoes. The molecular structure of dendrimers can
be fine-tuned, and because they are geometrically symmetrical and
have many peripheral functional groups, an internal molecular
cavity, controlled molecular weight, and nanometer size, they are
excellent nanocarriers with good fluid mechanic performance,
versatility, and strong adsorption ability.
Dendrimers are self-assembled and stabilize by
forming organic or inorganic hybrid nanoparticles. Dendrimers can
be linked to liposomes (74–76),
nanoparticles (77,78), and carbon nanotubes (79–81) to
modulate their solubility for use as drug carriers (74,82)
and target-specific carriers (82–84) of
detecting agents (such as dye molecules), affinity ligands,
radioligands, imaging agents, or pharmaceutically active anticancer
compounds.
Thanks to recent advances in synthetic chemistry and
characterization techniques, novel dendritic carriers are rapidly
being developed. Dendrimers are being widely investigated as gene
delivery vectors. For example, polyamidoamine (PAMAM) dendrimers
have the ability to condense DNA for transfection. Liu et al
(85) used five fluorinated
polypropylenimine (PPI) dendrimers to improve DNA transfection
efficacy. The heptafluorobutyric acid modified on the PPI dendrimer
improved the efficacy of enhanced green fluorescent protein
transfection in all five fluorinated PPI dendrimers by 89% over
that of regular PPIs. The uptake efficacy achieved with PPI
dendrimers (as indicated by both the percentage of positively
stained cells and the mean fluorescence intensity) was superior to
that of G5-Arg110, bPEI 25K, and four commercial transfection
reagents, including Lipofectamine 2000 (with as high as 71%
improvement).
Highly branched dendrimer-amplified aptamer probes
can be easily rebuilt and have high affinity and specificity for a
wide range of targets. They are able to reach various targets with
such high sensitivity, reliability, and selectivity because of
their novel optical, magnetic, electric, chemical, and biological
properties (86). For instance,
surface-functionalized PAMAM dendrimers with carboxyl groups, whose
particles are spherical colloidal crystal clusters decorated with
dendrimer-amplified aptamer probes, are designed to immobilize DNA
aptamers; thus, they can serve as high-efficacy probes that target
cancer cells. Malik et al (87) showed that conjugates of cisplatin
with the negatively charged 4th-generation PEGylated PAMAM
dendrimer exhibited antitumor activity against B16F10 solid
melanoma tumors. Methotrexate conjugated to PEGylated poly-L-lysine
(PLL) dendrimers (G5, PEG1100) has been shown to accumulate in
HT1080 fibrosarcoma tumors in rats and mice (88). Al-Jamal et al (89) reported that the complexation of
doxorubicin with the novel 6th-generation cationic PLL dendrimer
Gly-Lys63 (NH2)64 (molecular weight 8149 kDa) produced systemic
anti-angiogenic activity in tumor-bearing mice. Dendrimer
nanotechnology has also been used to produce contrast agents,
including agents used in molecular imaging (90). Qiao and Shi (86), and Yang et al (91), for instance, successfully
synthesized ultrasmall iron oxide nanoparticles by conjugating them
with Arg-Gly-Asp-modified dendrimers
(G5.NHAc-RGD-Fe3O4 NPs) for targeted magnetic
resonance imaging of C6 glioma cells.
Dendrimers have the advantages of being
biocompatible and easily eliminated from the body. PAMAM dendrimer
nanoparticles, with their large number of surface amino groups, are
more biocompatible and circulate for longer in the serum than do
small-molecule drugs. Dendrimer nanoparticles are eventually
eliminated from the human body through the kidneys along the same
metabolic pathways taken by folate (84,92),
growth factors (93), peptides
(94,95), and antibodies (96). However, dendrimers also have the
drawbacks of being cytotoxic to normal cells, and that the end
groups present on their peripheries (97) such as PAMAM, PPI, and PLL are
cationic groups with physiological stability. This stability
increases their cytotoxicity that can inevitably attack normal
cells.
Micellar nanoparticles possess a core and a shell
structure. PEG is often used as a hydrophilic shell; shells with
hydrophobic domains include PLA (52), PLGA (44,45),
polystyrene, poly (cyanoacrylate), poly (vinylpyrrolidone), and
polycaprolactone (56). These
copolymers are widely used owing to their natural biodegradability
and biocompatibility as well as their ability to entrap hydrophobic
drugs. A primary mPEG-PLA polymeric micelle loaded with paclitaxel
(Genexol-PM) was approved by the FDA in 2007 (98,99).
It is loaded with a free-Taxol formulation and has been shown to
reduce the severity of toxic effects such as hypersensitivity
reactions, hyperlipidemia, and peripheral neuropathy.
Micellar nanoparticles are obtained from
self-assembly of amphiphilic block copolymers in aqueous media
above the critical micelle concentration (100). The core, consisting of the
hydrophobic domain, acts as a reservoir and protects the drug from
being dissolved, whereas the hydrophilic shell mainly confers
aqueous solubility and steric stability to the micellar structure
(27). With this technique,
undissolvable drugs, such as paclitaxel and docetaxel, can be
covered with a water-solute layer to enhance their hydrophilicity
and ultimately facilitate their bioavailability. The hydrophilic
shell affords protection and lengthens circulation in vivo,
providing enhanced permeability and retention. In recent years, a
number of nanomicellar drugs have advanced to clinical trials or to
the market (Table III).
With the rise of precision medicine, micellar
nanoparticles have become increasingly important for passive
targeted cancer therapy. Peptide modification on the surface of the
micelle can be used effectively for precise targeting.
Integrin-binding sequence peptides with covalent bonds to the
micelle can actively target tumors (101). Block copolymers are environmental
response modifiers that display a physico-chemical response to
stimuli such as temperature (102–104), pH (105), light (106), or electricity (107). Some block copolymers can produce
functional signals and higher levels of signaling (103,108); thus, micelles made from them are
called ‘intelligent’ block copolymer micelles. The self-assembly of
such polypeptide-based copolymers can be triggered by temperature
and pH changes (105). Poly
(N-isopropylacrylamide) (PNIPAM) is a temperature-sensitive polymer
segment with a lowest critical solution temperature of 31–32°C
(105). It quickly switches from a
hydrated to a dehydrated state, using PNIPAM-OH and the
ring-opening polymerization reaction synthesis of PLA
(PNIPAM-b-PLA) (104) and
self-assembles into dual-response micelle carriers. A series of
dual-stimuli responsive polymers such as PNIPAM-b-PGA and
PNIPAM-b-PLL have been synthesized as copolymer micelle
materials (108). Doxorubicin can
be effectively encapsulated in PNIPAM-block-poly
(L-histidine) (PNIPAM-b-PLH) micelle carriers as a
controlled delivery system for the treatment of hepatocellular
carcinoma (109). Light-sensitive
groups, including the azide, cinnamon acyl, screw pyran, coumarin,
and 2-nitrobenzyl groups, have also been widely used in cancer
therapeutic settings (106,110,111).
Photodynamic therapy (PDT) is a non-invasive treatment modality for
a variety of diseases including cancer (112). PDT based on upconversion
nanoparticles (UCNPs) has received much attention in recent years.
Under near-infrared (NIR) light excitation, UCNPs are able to emit
high-energy visible light, which can activate surrounding
photosensitizer (PS) molecules to produce singlet oxygen and kill
cancer cells (113,114) also represent a promising direction
in future research (115,116).
The greatest benefit of biodegradable drug delivery
systems is the controlled release of the drug payload to a specific
site and the degradation into nontoxic materials for elimination
from the body via metabolic pathways (117). Organelle-targeted biodegradable
copolymers, mitochondria-targeting gold-peptide, and
radiation-hyperthermia nanoassembly-copolymers (118,119) are used to evaluate
micro-environmental change by taking advantage of the sensitivity
of mitochondria to temperature elevation. In the presence of a
thermal stimulus, the passive targeted biodegradable micellar
nanoparticles of a copolymer-controlled drug release system are
activated, resulting in slow degradation of the nanoparticles into
smaller fragments and the release of carried products, which
eventually enhance the drug's cytotoxic effects on cancer cells.
Currently, new biocompatible and/or biodegradable
stimuli-responsive copolymers that form stable micellar systems
capable of encapsulating a broad range of chemotherapeutic agents
are being developed (120,121).
It is generally accepted that nonviral vectors are
safer than viral vectors for gene transfer (122). Biodegradable copolymers based on
polylysine were the first nanoparticles used for gene transfer.
Currently, PEG-grafted PLGA-PLL (123), pluronic polyethylenimine (PEI),
polyphosphoric acid (124), and
phosphate (125) micelles are
being used as gene carriers for biological separation and cancer
diagnosis. However, applications of cationic polymer-based gene
delivery systems are limited because the polymers interact with the
cell membrane and produce increased toxicity (122).
Various forms of inorganic nanoparticles, including
quantum dots, superparamagnetic iron oxides, gold nanoparticles,
carbon nanotubes, and other metallic and non-metallic nanoparticles
or nanoclusters, enhance the efficiency of radiotherapy and improve
tumor imaging (119,126). Several of these inorganic
nanoparticles are sufficiently small (10–100 nm) to penetrate the
capillaries and can be taken up in distinct tissues. Others are
larger and need to be delivered at disease-specific anatomic sites
for passive targeting. Multifunctional nanodevices are also
emerging as tools to target cancer (42,43,127).
Such devices can contain not only the drug payload but also
specific receptor-targeting agents, such as antibodies or ligands,
as well as magnetic resonance imaging contrast agents. Quantum dots
and gold nanoparticles exhibit unique optical, electrical, and
magnetic properties (128) that
are beneficial for imaging the intracellular localization and
trafficking of multifunctional carriers. Drugs can also be
delivered at specific sites after they are attached, encapsulated,
absorbed, entrapped, or dissolved in the nanomaterial matrix.
However, in early-stage clinical trials, some inorganic
nanomaterials, such as gold nanoparticles (129) and silica nanoparticles (130), have encountered obstacles,
including toxicity and a lack of stability. Of the iron oxide
nanoparticles, NanoTherm (131),
used for the treatment of glioblastoma, is the only one that has
obtained approval for clinical use. With NanoTherm, tumors can be
thermally ablated by magnetic hyperthermia induced by entrapped
superparamagnetic iron oxides.
Many solid tumors develop several biological
features distinguished from those of normal tissues (132). Abnormal tumor structures including
physically compromised vasculature, abnormal extracellular matrix
(ECM), and high interstitial fluid pressure (IFP), can create
constraints that compromise effective delivery of nanotherapeutics
(133,134). There are also extravascular
barriers to overcome, whereby nanoparticles can extravasate but
cannot penetrate through the ECM of the tumors (135). It is well recognized that the
irregularity of the tumor vasculature with its abnormal blood flow
and impaired venous and lymphatic drainage creates high
interstitial fluid pressure, making the diffusion of nutrients and
chemotherapeutics throughout the tumor very inefficient, thus
presenting challenges to effective diffusion of nanocarriers as
well (136).
Liposomes and polymers are the most widely used
biodegradable nanocarriers because of their biocompatibility,
biodegradability, and mechanical properties. However, because of
adverse effects and the still-unclear mechanisms of interaction
among nanoparticles, the tumor microenvironment, and tumor cells,
these nanocarriers may offer only brief extension of patient
survival (Table IV). Despite
numerous achievements in liposomal drug delivery, current liposomal
formulations have primarily reduced systemic toxicity rather than
increasing efficacy. For instance, hydrophilic drugs such as
cisplatin are decorated with liposomal bilayers to reduce drug
internal toxicity. However, it needs time to degrade the liposome
vehicle for the release of the embedded pharmaceutical. Therefore,
long systemic circulation and minimal side effects could result in
poor efficacy in vivo. Nevertheless, it is still challenging
to achieve an optimal balance between high and specific drug
bioavailability in tumor tissue and prolonged liposome stability in
systemic circulation (137).
Despite many advances in the production of more
stable, efficient, and safe biopolymers, there remain controversies
regarding the safety of polymeric nanomaterials. Some polymers are
themselves cytotoxic (41,138). It has been demonstrated, for
example, that PEI destabilizes the plasma membrane and activates
effector caspase-3; thus, PEI appears to be a proapoptotic agent
(138). Inflammatory and immune
responses have also been reported (139–141). However, PLGA can be formulated as
an acidic product to provoke inflammatory responses, and it has
shown minimal systemic toxicity and excellent biocompatibility
in vitro and in vivo (142). Thus, advancements in formulating,
synthesizing, and modifying biodegradable polymers promise to
improve treatment efficacy and reduce adverse effects.
Compared to other types of nanocarriers, dendrimers
provide more opportunities for design and adaptation owing to their
peculiar tailor-made surfaces. Toxicity associated with dendrimers
is primarily attributed to the end groups present on their
peripheries (97). Cationic
dendrimers with high charge density and high molecular weight, such
as PAMAM, PPI, and PLL, are more stable in physiological
conditions. This stability increases their cytotoxicity, owing to
the excess positive charges on the periphery, which destabilize the
cell membrane. However, stability may also cause several adverse
effects (143–145). Fortunately, neutral or anionic
groups such as sulfonated, carboxylated, and phosphonated groups
have been shown to be less toxic (73). In light of this progress, the next
step will be to modify the surface groups of dendrimers with
minimally toxic reagents in order to adapt them to physiological
conditions.
Other nanoparticles of particularly urgent concern
are micelles and inorganic nanomaterials, which present challenges
with instability, potential toxicity, cytotoxicity, immune
response, and chronic inflammation (146,147). For specific targeted therapy,
micelles and inorganic nanomaterials can be decorated with
receptor-stimuli agents such as PH, light and magnetic resonance
imaging contrast agents, one major limitation of this treatment
methodology in clinical applications is the poor tissue penetration
ability (148,149).
Research aimed at overcoming these drawbacks will
facilitate the use of nanomaterials as drug delivery vehicles and
eventually improve patient survival. Ideally, an anticancer
nanotherapeutic should be able to reach tumors without systemic
loss, easily penetrate into the core of the tumor mass, enter tumor
cells where their target molecules reside, and completely eradicate
the tumors.
Nanotechnology receives extraordinary attention, and
its applications in cancer treatment are relatively new and
ever-evolving. Nonetheless, it is clear that nanomaterials are
promising tools for cancer treatment. In spite of the progress
being made in developing drug delivery systems for cancer therapy,
a number of critical issues still need to be addressed. Molecularly
targeted drugs preferentially modulate functional proteins, so they
can be used to treat diseases (150), like cancers, that are
characterized by abnormal protein expression and activation.
However, such targeting mechanisms can be challenged by the
stability of nanomaterials, the development of multi-drug
resistance, and the dysregulated accumulation of cancer cells. The
ability to decorate nanomaterial shells with multiple chemically or
physically active components permits the delivery of different
drugs. Therefore, nanomaterial drug carriers can be organized and
optimized for site-specific chemotherapy, thermotherapy,
photodynamic therapy, and radiotherapy. Although the benefits of
metal-based nanoparticles are remarkable, toxicity remains a
critical issue. Nano-toxicological issues also need to be addressed
so that more effective cancer therapeutic strategies can be
developed. Notably, combination therapeutic regimens for different
cancer types remain a challenge because of the diverse mechanisms
of cancer development. Combination therapy with nanoparticle drug
carriers, therefore, warrants further study at the preclinical and
clinical levels. Other challenges exist for modified and
functionalized nanomaterials with well-established formulations,
including improving the localization, biodistribution,
biocompatibility, and efficacy of nanodrug systems in vivo,
to meet the requirements of precision cancer diagnosis and
therapy.
We thank Ms. Yazmin Salina at the Department of
Clinical Cancer Prevention, and Dr Amy Ninetto, ELS at the
Department of Scientific Publications, The University of Texas MD
Anderson Cancer Center for proofreading and editing of this
manuscript. This study was supported by the Scholarship Award for
Excellent Doctoral Student Granted of Yunnan Province (6011418150
to Z. Li), the Foundation of Leading Talent Program of Health and
Family Planning Commission of Yunnan Province (no. L-201205 to K.
Wang), and the Foundation of Institute of Gastroenterology,
Research institutions attached to Health and Family Planning
Commission of Yunnan Province (2014NS122 to K. Wang).
|
1
|
Shanthi M: Global Status Report on
Noncommunicable Diseases 2014. Geneva: WHO Press, World Health
Organization; 2014
|
|
2
|
LaVan DA, McGuire T and Langer R:
Small-scale systems for in vivo drug delivery. Nat Biotechnol.
21:1184–1191. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi J, Xiao Z, Kamaly N and Farokhzad OC:
Self-assembled targeted nanoparticles: Evolution of technologies
and bench to bedside translation. Acc Chem Res. 44:1123–1134. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Langer R: New methods of drug delivery.
Science. 249:1527–1533. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bangham AD, Standish MM and Watkins JC:
Diffusion of univalent ions across the lamellae of swollen
phospholipids. J Mol Biol. 13:238–252. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
James ND, Coker RJ, Tomlinson D, Harris
JR, Gompels M, Pinching AJ and Stewart JS: Liposomal doxorubicin
(Doxil): An effective new treatment for Kaposi's sarcoma in AIDS.
Clin Oncol (R Coll Radiol). 6:294–296. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Green MR, Manikhas GM, Orlov S, Afanasyev
B, Makhson AM, Bhar P and Hawkins MJ: Abraxane, a novel
Cremophor-free, albumin-bound particle form of paclitaxel for the
treatment of advanced non-small-cell lung cancer. Ann Oncol.
17:1263–1268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamaly N, Xiao Z, Valencia PM,
Radovic-Moreno AF and Farokhzad OC: Targeted polymeric therapeutic
nanoparticles: Design, development and clinical translation. Chem
Soc Rev. 41:2971–3010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burgess P, Hutt PB, Farokhzad OC, Langer
R, Minick S and Zale S: On firm ground: IP protection of
therapeutic nanoparticles. Nat Biotechnol. 28:1267–1270. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farokhzad OC and Langer R: Impact of
nanotechnology on drug delivery. ACS Nano. 3:16–20. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gulati M, Grover M, Singh S and Singh M:
Lipophilic drug derivatives in liposomes. Int J Pharm. 165:129–168.
1998. View Article : Google Scholar
|
|
12
|
Scherphof G, Roerdink F, Waite M and Parks
J: Disintegration of phosphatidylcholine liposomes in plasma as a
result of interaction with high-density lipoproteins. Biochim
Biophys Acta. 542:296–307. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allen TM and Cleland LG: Serum-induced
leakage of liposome contents. Biochim Biophys Acta. 597:418–426.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Senior J and Gregoriadis G: Is half-life
of circulating liposomes determined by changes in their
permeability? FEBS Lett. 145:109–114. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang S-L and MacDonald RC: Acoustically
active liposomes for drug encapsulation and ultrasound-triggered
release. Biochim Biophys Acta. 1665:134–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueno Y, Sonoda S, Suzuki R, Yokouchi M,
Kawasoe Y, Tachibana K, Maruyama K, Sakamoto T and Komiya S:
Combination of ultrasound and bubble liposome enhance the effect of
doxorubicin and inhibit murine osteosarcoma growth. Cancer Biol
Ther. 12:270–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pak CC, Erukulla RK, Ahl PL, Janoff AS and
Meers P: Elastase activated liposomal delivery to nucleated cells.
Biochim Biophys Acta. 1419:111–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meers P: Enzyme-activated targeting of
liposomes. Adv Drug Deliv Rev. 53:265–272. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerasimov OV, Boomer JA, Qualls MM and
Thompson DH: Cytosolic drug delivery using pH- and light-sensitive
liposomes. Adv Drug Deliv Rev. 38:317–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bondurant B, Mueller A and O'Brien DF:
Photoinitiated destabilization of sterically stabilized liposomes.
Biochim Biophys Acta. 1511:113–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du B, Han S, Li H, Zhao F, Su X, Cao X and
Zhang Z: Multi-functional liposomes showing
radiofrequency-triggered release and magnetic resonance imaging for
tumor multi-mechanism therapy. Nanoscale. 7:5411–5426. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arie AA and Lee JK: Effect of boron doped
fullerene C 60 film coating on the electrochemical characteristics
of silicon thin film anodes for lithium secondary batteries. Synth
Met. 161:158–165. 2011. View Article : Google Scholar
|
|
23
|
Dao TT, Matsushima T and Murata H: Organic
nonvolatile memory transistors based on fullerene and an
electron-trapping polymer. Org Electron. 13:2709–2715. 2012.
View Article : Google Scholar
|
|
24
|
Papahadjopoulos D, Jacobson K, Nir S and
Isac T: Phase transitions in phospholipid vesicles. Fluorescence
polarization and permeability measurements concerning the effect of
temperature and cholesterol. Biochim Biophys Acta. 311:330–348.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Landen CN Jr, Chavez-Reyes A, Bucana C,
Schmandt R, Deavers MT, Lopez-Berestein G and Sood AK: Therapeutic
EphA2 gene targeting in vivo using neutral liposomal small
interfering RNA delivery. Cancer Res. 65:6910–6918. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miller CR, Bondurant B, McLean SD,
McGovern KA and O'Brien DF: Liposome-cell interactions in vitro:
Effect of liposome surface charge on the binding and endocytosis of
conventional and sterically stabilized liposomes. Biochemistry.
37:12875–12883. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wolfrum C, Shi S, Jayaprakash KN,
Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K,
Ndungo EM, et al: Mechanisms and optimization of in vivo delivery
of lipophilic siRNAs. Nat Biotechnol. 25:1149–1157. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Yu Y, Dai W, Lu J, Cui J, Wu H,
Yuan L, Zhang H, Wang X, Wang J, et al: The use of a tumor
metastasis targeting peptide to deliver doxorubicin-containing
liposomes to highly metastatic cancer. Biomaterials. 33:8451–8460.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Irvine DJ: Drug delivery: One
nanoparticle, one kill. Nat Mater. 10:342–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Jiang H, Wu Y, Li Y and Gao Y: A
novel glycyrrhetinic acid-modified oxaliplatin liposome for
liver-targeting and in vitro/vivo evaluation. Drug Des Devel Ther.
9:2265–2275. 2015.PubMed/NCBI
|
|
31
|
Liu Z, Xiong M, Gong J, Zhang Y, Bai N,
Luo Y, Li L, Wei Y, Liu Y, Tan X, et al: Legumain
protease-activated TAT-liposome cargo for targeting tumours and
their microenvironment. Nat Commun. 5:4280–4291. 2014.PubMed/NCBI
|
|
32
|
Davis ME, Zuckerman JE, Choi CHJ, Seligson
D, Tolcher A, Alabi CA, Yen Y, Heidel JD and Ribas A: Evidence of
RNAi in humans from systemically administered siRNA via targeted
nanoparticles. Nature. 464:1067–1070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee JH and Lee MJ: Liposome mediated
cancer gene therapy: Clinical trials and their lessons to stem cell
therapy. Bull Korean Chem Soc. 33:433–442. 2012. View Article : Google Scholar
|
|
34
|
Felgner PL, Gadek TR, Holm M, Roman R,
Chan HW, Wenz M, Northrop JP, Ringold GM and Danielsen M:
Lipofection: A highly efficient, lipid-mediated DNA-transfection
procedure. Proc Natl Acad Sci USA. 84:7413–7417. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tari AM, Gutiérrez-Puente Y, Monaco G,
Stephens C, Sun T, Rosenblum M, Belmont J, Arlinghaus R and
Lopez-Berestein G: Liposome-incorporated Grb2 antisense
oligodeoxynucleotide increases the survival of mice bearing
bcr-abl-positive leukemia xenografts. Int J Oncol. 31:1243–1250.
2007.PubMed/NCBI
|
|
36
|
Yu L, Dean K and Li L: Polymer blends and
composites from renewable resources. Prog Polym Sci. 31:576–602.
2006. View Article : Google Scholar
|
|
37
|
Hu C-MJ, Fang RH, Copp J, Luk BT and Zhang
L: A biomimetic nanosponge that absorbs pore-forming toxins. Nat
Nanotechnol. 8:336–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu C-MJ and Zhang L, Aryal S, Cheung C,
Fang RH and Zhang L: Erythrocyte membrane-camouflaged polymeric
nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci
USA. 108:10980–10985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sinha M, Banik RM, Haldar C and Maiti P:
Development of ciprofloxacin hydrochloride loaded poly (ethylene
glycol)/chitosan scaffold as wound dressing. Nat Mater. 20:799–807.
2013.
|
|
40
|
Nguyen TTT, Ghosh C, Hwang S-G, Dai Tran L
and Park JS: Characteristics of curcumin-loaded poly (lactic acid)
nanofibers for wound healing. J Mater Sci Mater Med. 48:7125–7133.
2013. View Article : Google Scholar
|
|
41
|
Tan S, Gan C, Li R, Ye Y, Zhang S, Wu X,
Yang YY, Fan W and Wu M: A novel chemosynthetic peptide with
β-sheet motif efficiently kills Klebsiella pneumoniae in a
mouse model. Int J Nanomedicine. 10:1045–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Janib SM, Moses AS and MacKay JA: Imaging
and drug delivery using theranostic nanoparticles. Adv Drug Deliv
Rev. 62:1052–1063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grossman JH and McNeil SE: Nanotechnology
in cancer medicine. Phys Today. 65:38–42. 2012. View Article : Google Scholar
|
|
44
|
Mishra S, De A and Mozumdar S: Synthesis
of thermoresponsive polymers for drug delivery. Drug Delivery
System Clifton: Springer; pp. 77–101. 2014, View Article : Google Scholar
|
|
45
|
Gundogdu N and Cetin M: Chitosan-poly
(lactide-co-glycolide) (CS-PLGA) nanoparticles containing metformin
HCl: Preparation and in vitro evaluation. Pak J Pharm Sci.
27:1923–1929. 2014.PubMed/NCBI
|
|
46
|
Tajmir-Riahi HA, Nafisi Sh, Sanyakamdhorn
S, Agudelo D and Chanphai P: Applications of chitosan nanoparticles
in drug delivery. Methods Mol Biol. 1141:165–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Malhotra M, Tomaro-Duchesneau C, Saha S
and Prakash S: Intranasal delivery of chitosan-siRNA nanoparticle
formulation to the brain. Methods Mol Biol. 1141:233–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Raja MAG, Katas H and Wen Jing T:
Stability, intracellular delivery, and release of siRNA from
chitosan nanoparticles using different cross-linkers. PLoS One.
10:e01289632015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Malhotra M, Tomaro-Duchesneau C, Saha S
and Prakash S: Intranasal delivery of chitosan-siRNA nanoparticle
formulation to the brain. Drug Delivery System. Jain KK: New York,
NY: Springer; pp. 233–247. 2014, View Article : Google Scholar
|
|
50
|
Yang X, Wu S, Wang Y, Li Y, Chang D, Luo
Y, Ye S and Hou Z: Evaluation of self-assembled HCPT-loaded
PEG-b-PLA nanoparticles by comparing with HCPT-loaded PLA
nanoparticles. Nanoscale Res Lett. 9:24082014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun C, Wang X, Zheng Z, Chen D, Wang X,
Shi F, Yu D and Wu H: A single dose of dexamethasone encapsulated
in polyethylene glycol-coated polylactic acid nanoparticles
attenuates cisplatin-induced hearing loss following round window
membrane administration. Int J Nanomedicine. 10:3567–3579.
2015.PubMed/NCBI
|
|
52
|
Rabanel J-M, Faivre J, Tehrani SF, Lalloz
A, Hildgen P and Banquy X: Effect of the polymer architecture on
the structural and biophysical properties of PEG-PLA nanoparticles.
ACS Appl Mater Interfaces. 7:10374–10385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Diou O, Greco S, Beltran T, Lairez D,
Authelin J-R and Bazile D: A method to quantify the affinity of
cabazitaxel for PLA-PEG nanoparticles and investigate the influence
of the nano-assembly structure on the drug/particle association.
Pharm Res. 32:3188–3200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Asadi H, Rostamizadeh K, Salari D and
Hamidi M: Preparation of biodegradable nanoparticles of tri-block
PLA-PEG-PLA copolymer and determination of factors controlling the
particle size using artificial neural network. J Microencapsul.
28:406–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xie Y, Yi Y, Hu X, Shangguan M, Wang L, Lu
Y, Qi J and Wu W: Synchronous microencapsulation of multiple
components in silymarin into PLGA nanoparticles by an
emulsification/solvent evaporation method. Pharm Dev Technol.
21:672–679. 2016.PubMed/NCBI
|
|
56
|
Xiong W, Peng L, Chen H and Li Q: Surface
modification of MPEG-b-PCL-based nanoparticles via oxidative
self-polymerization of dopamine for malignant melanoma therapy. Int
J Nanomed. 10:2985–2996. 2015.
|
|
57
|
Zhang R, Luo K, Yang J, Sima M, Sun Y,
Janát-Amsbury MM and Kopeček J: Synthesis and evaluation of a
backbone biodegradable multiblock HPMA copolymer nanocarrier for
the systemic delivery of paclitaxel. J Control Release. 166:66–74.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang Q, Tang H and Wu P: Aqueous solutions
of poly (ethylene oxide)-poly (N-isopropylacrylamide):
Thermosensitive behavior and distinct multiple assembly processes.
Langmuir. 31:6497–6506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
James Priya H, John R, Alex A and Anoop
KR: Smart polymers for the controlled delivery of drugs - a concise
overview. Acta Pharm Sin B. 4:120–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Trotta F, Dianzani C, Caldera F, Mognetti
B and Cavalli R: The application of nanosponges to cancer drug
delivery. Expert Opin Drug Deliv. 11:931–941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hu C-MJ, Fang RH, Wang K-C, Luk BT,
Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll
AV, et al: Nanoparticle biointerfacing by platelet membrane
cloaking. Nature. 526:118–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hu CM, Fang RH, Luk BT and Zhang L:
Nanoparticle-detained toxins for safe and effective vaccination.
Nat Nanotechnol. 8:933–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Miele E, Spinelli GP, Miele E, Tomao F and
Tomao S: Albumin-bound formulation of paclitaxel (Abraxane ABI-007)
in the treatment of breast cancer. Int J Nanomed. 4:99–105.
2009.
|
|
64
|
Hawkins MJ, Soon-Shiong P and Desai N:
Protein nanoparticles as drug carriers in clinical medicine. Adv
Drug Deliv Rev. 60:876–885. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zheng Y-R, Suntharalingam K, Johnstone TC,
Yoo H, Lin W, Brooks JG and Lippard SJ: Pt (IV) prodrugs designed
to bind non-covalently to human serum albumin for drug delivery. J
Am Chem Soc. 136:8790–8798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cirstea D, Hideshima T, Rodig S, Santo L,
Pozzi S, Vallet S, Ikeda H, Perrone G, Gorgun G, Patel K, et al:
Dual inhibition of akt/mammalian target of rapamycin pathway by
nanoparticle albumin-bound-rapamycin and perifosine induces
antitumor activity in multiple myeloma. Mol Cancer Ther. 9:963–975.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fu Q, Sun J, Zhang W, Sui X, Yan Z and He
Z: Nanoparticle albumin-bound (NAB) technology is a promising
method for anti-cancer drug delivery. Recent Patents Anticancer
Drug Discov. 4:262–272. 2009. View Article : Google Scholar
|
|
68
|
Von Hoff DD, Mita MM, Ramanathan RK, Weiss
GJ, Mita AC, LoRusso PM, Burris HA III, Hart LL, Low SC, Parsons
DM, et al: Phase I study of PSMA-targeted docetaxel-containing
nanoparticle BIND-014 in patients with advanced solid tumors. Clin
Cancer Res. 22:3157–3163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zuckerman JE, Gritli I, Tolcher A, Heidel
JD, Lim D, Morgan R, Chmielowski B, Ribas A, Davis ME and Yen Y:
Correlating animal and human phase Ia/Ib clinical data with
CALAA-01, a targeted, polymer-based nanoparticle containing siRNA.
Proc Natl Acad Sci USA. 111:11449–11454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Buhleier E, Wehner W and Vögtle F:
‘Cascade’- and ‘nonskid-chain-like’ syntheses of molecular cavity
topologies. Synthesis (Mass). 155–158:19781978.
|
|
71
|
Tomalia DA, Baker H, Dewald J, Hall M,
Kallos G, Martin S, Roeck J, Ryder J and Smith P: A new class of
polymers: Starburst-dendritic macromolecules. Polym J. 17:117–132.
1985. View Article : Google Scholar
|
|
72
|
Gillies ER and Fréchet JM: Dendrimers and
dendritic polymers in drug delivery. Drug Discov Today. 10:35–43.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kesharwani P, Jain K and Jain NK:
Dendrimer as nanocarrier for drug delivery. Prog Polym Sci.
39:268–307. 2014. View Article : Google Scholar
|
|
74
|
Khopade AJ, Caruso F, Tripathi P, Nagaich
S and Jain NK: Effect of dendrimer on entrapment and release of
bioactive from liposomes. Int J Pharm. 232:157–162. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Papagiannaros A, Dimas K, Papaioannou GT
and Demetzos C: Doxorubicin-PAMAM dendrimer complex attached to
liposomes: Cytotoxic studies against human cancer cell lines. Int J
Pharm. 302:29–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Purohit G, Sakthivel T and Florence AT:
Interaction of cationic partial dendrimers with charged and neutral
liposomes. Int J Pharm. 214:71–76. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Karadag M, Geyik C, Demirkol DO, Ertas FN
and Timur S: Modified gold surfaces by
6-ferrocenyl)hexanethiol/dendrimer/gold nanoparticles as a platform
for the mediated biosensing applications. Mater Sci Eng C.
33:634–640. 2013. View Article : Google Scholar
|
|
78
|
Tao X, Yang Y-J, Liu S, Zheng Y-Z, Fu J
and Chen J-F: Poly (amidoamine) dendrimer-grafted porous hollow
silica nanoparticles for enhanced intracellular photodynamic
therapy. Acta Biomater. 9:6431–6438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yoshioka H, Suzuki M, Mugisawa M, Naitoh N
and Sawada H: Synthesis and applications of novel fluorinated
dendrimer-type copolymers by the use of fluoroalkanoyl peroxide as
a key intermediate. J Colloid Interface Sci. 308:4–10. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zeng Y-L, Huang Y-F, Jiang J-H, Zhang X-B,
Tang C-R, Shen G-L and Yu R-Q: Functionalization of multi-walled
carbon nanotubes with poly (amidoamine) dendrimer for mediator-free
glucose biosensor. Electrochem Commun. 9:185–190. 2007. View Article : Google Scholar
|
|
81
|
Tang L, Zhu Y, Yang X and Li C: An
enhanced biosensor for glutamate based on self-assembled carbon
nanotubes and dendrimer-encapsulated platinum
nanobiocomposites-doped polypyrrole film. Anal Chim Acta.
597:145–150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Prajapati RN, Tekade RK, Gupta U, Gajbhiye
V and Jain NK: Dendimer-mediated solubilization, formulation
development and in vitro-in vivo assessment of piroxicam. Mol
Pharm. 6:940–950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chauhan AS, Sridevi S, Chalasani KB, Jain
AK, Jain SK, Jain NK and Diwan PV: Dendrimer-mediated transdermal
delivery: Enhanced bioavailability of indomethacin. J Control
Release. 90:335–343. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Quintana A, Raczka E, Piehler L, Lee I,
Myc A, Majoros I, Patri AK, Thomas T, Mulé J and Baker JR Jr:
Design and function of a dendrimer-based therapeutic nanodevice
targeted to tumor cells through the folate receptor. Pharm Res.
19:1310–1316. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu H, Wang Y, Wang M, Xiao J and Cheng Y:
Fluorinated poly (propylenimine) dendrimers as gene vectors.
Biomaterials. 35:5407–5413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Qiao Z and Shi X: Dendrimer-based
molecular imaging contrast agents. Prog Polym Sci. 44:1–27. 2015.
View Article : Google Scholar
|
|
87
|
Malik N, Evagorou EG and Duncan R:
Dendrimer-platinate: A novel approach to cancer chemotherapy.
Anticancer Drugs. 10:767–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kaminskas LM, Kelly BD, McLeod VM, Boyd
BJ, Krippner GY, Williams ED and Porter CJ: Pharmacokinetics and
tumor disposition of PEGylated, methotrexate conjugated
poly-l-lysine dendrimers. Mol Pharm. 6:1190–1204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Al-Jamal KT, Al-Jamal WT, Wang JT-W, Rubio
N, Buddle J, Gathercole D, Zloh M and Kostarelos K: Cationic
poly-L-lysine dendrimer complexes doxorubicin and delays tumor
growth in vitro and in vivo. ACS Nano. 7:1905–1917. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Huang Z, Sengar RS, Nigam A, Abadjian MC,
Potter DM, Grotjahn DB and Wiener EC: A fluorinated dendrimer-based
nanotechnology platform: New contrast agents for high field
imaging. Invest Radiol. 45:641–654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yang J, Luo Y, Xu Y, Li J, Zhang Z, Wang
H, Shen M, Shi X and Zhang G: Conjugation of iron oxide
nanoparticles with RGD-modified dendrimers for targeted tumor MR
imaging. ACS Appl Mater Interfaces. 7:5420–5428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shi X, Wang SH, Van Antwerp ME, Chen X and
Baker JR Jr: Targeting and detecting cancer cells using
spontaneously formed multifunctional dendrimer-stabilized gold
nanoparticles. Analyst (Lond). 134:1373–1379. 2009. View Article : Google Scholar
|
|
93
|
Thomas TP, Shukla R, Kotlyar A, Liang B,
Ye JY, Norris TB and Baker JR Jr: Dendrimer-epidermal growth factor
conjugate displays superagonist activity. Biomacromolecules.
9:603–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hill E, Shukla R, Park SS and Baker JR Jr:
Synthetic PAMAM-RGD conjugates target and bind to odontoblast-like
MDPC 23 cells and the predentin in tooth organ cultures. Bioconjug
Chem. 18:1756–1762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lesniak WG, Kariapper MS, Nair BM, Tan W,
Hutson A, Balogh LP and Khan MK: Synthesis and characterization of
PAMAM dendrimer-based multifunctional nanodevices for targeting
alphavbeta3 integrins. Bioconjug Chem. 18:1148–1154. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Thomas TP, Patri AK, Myc A, Myaing MT, Ye
JY, Norris TB and Baker JR Jr: In vitro targeting of synthesized
antibody-conjugated dendrimer nanoparticles. Biomacromolecules.
5:2269–2274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen H-T, Neerman MF, Parrish AR and
Simanek EE: Cytotoxicity, hemolysis, and acute in vivo toxicity of
dendrimers based on melamine, candidate vehicles for drug delivery.
J Am Chem Soc. 126:10044–10048. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ahn HK, Jung M, Sym SJ, Shin DB, Kang SM,
Kyung SY, Park JW, Jeong SH and Cho EK: A phase II trial of
Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in
patients with advanced non-small cell lung cancer. Cancer Chemother
Pharmacol. 74:277–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Park S and Healy KE: Nanoparticulate DNA
packaging using terpolymers of poly
(lysine-g-(lactide-b-ethylene glycol)). Bioconjug
Chem. 14:311–319. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Sun T-M, Du J-Z, Yan L-F, Mao H-Q and Wang
J: Self-assembled biodegradable micellar nanoparticles of
amphiphilic and cationic block copolymer for siRNA delivery.
Biomaterials. 29:4348–4355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gao Y, Zhou Y, Zhao L, Zhang C, Li Y, Li
J, Li X and Liu Y: Enhanced antitumor efficacy by cyclic
RGDyK-conjugated and paclitaxel-loaded pH-responsive polymeric
micelles. Acta Biomater. 23:127–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kohori F, Sakai K, Aoyagi T, Yokoyama M,
Sakurai Y and Okano T: Preparation and characterization of
thermally responsive block copolymer micelles comprising poly
(N-isopropylacrylamide-b-DL-lactide). J Control
Release. 55:87–98. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
He C, Zhao C, Chen X, Guo Z, Zhuang X and
Jing X: Novel pH- and temperature-responsive bock copolymers with
tunable pH-responsive range. Macromol Rapid Commun. 29:490–497.
2008. View Article : Google Scholar
|
|
104
|
Xu F, Yan T-T and Luo Y-L: Studies on
micellization behavior of thermosensitive PNIPAAm-b-PLA
amphiphilic block copolymers. J Nanosci Nanotechnol. 12:2287–2291.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhao C, Zhuang X, He C, Chen X and Jing X:
Synthesis of novel thermo-and pH-responsive poly (L-lysine)-based
copolymer and its micellization in water. Macromol Rapid Commun.
29:1810–1816. 2008. View Article : Google Scholar
|
|
106
|
Saravanakumar G, Lee J, Kim J and Kim WJ:
Visible light-induced singlet oxygen-mediated intracellular
disassembly of polymeric micelles co-loaded with a photosensitizer
and an anticancer drug for enhanced photodynamic therapy. Chem
Commun (Camb). 51:9995–9998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bae J, Maurya A, Shariat-Madar Z, Murthy
SN and Jo S: Novel redox-responsive amphiphilic copolymer micelles
for drug delivery: Synthesis and characterization. AAPS J.
17:1357–1368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Liu Y, Li C, Wang HY, Zhang XZ and Zhuo
RX: Synthesis of thermo- and pH-sensitive polyion complex micelles
for fluorescent imaging. Chemistry. 18:2297–2304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Johnson RP, Jeong YI, John JV, Chung CW,
Kang DH, Selvaraj M, Suh H and Kim I: Dual stimuli-responsive poly
(N-isopropylacrylamide)-b-poly (L-histidine) chimeric
materials for the controlled delivery of doxorubicin into liver
carcinoma. Biomacromolecules. 14:1434–1443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhou F, Zheng B, Zhang Y, Wu Y, Wang H and
Chang J: Construction of near-infrared light-triggered reactive
oxygen species-sensitive (UCN/SiO2-RB + DOX)@PPADT nanoparticles
for simultaneous chemotherapy and photodynamic therapy.
Nanotechnology. 27:2356012016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wu HQ and Wang CC: Biodegradable smart
nanogels: A new platform for targeting drug delivery and biomedical
diagnostics. Langmuir. 32:6211–6225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Moan J and Peng Q: An outline of the
hundred-year history of PDT. Anticancer Res. 23A:3591–3600.
2003.
|
|
113
|
Castano AP, Mroz P and Hamblin MR:
Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer.
6:535–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Krammer B: Vascular effects of
photodynamic therapy. Anticancer Res. 21B:4271–4277. 2001.
|
|
115
|
Monge-Fuentes V, Muehlmann LA and de
Azevedo RB: Perspectives on the application of nanotechnology in
photodynamic therapy for the treatment of melanoma. Nano Rev.
5:24381–24395. 2014. View Article : Google Scholar
|
|
116
|
Wang C, Cheng L and Liu Z: Upconversion
nanoparticles for photodynamic therapy and other cancer
therapeutics. Theranostics. 3:317–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Marin E, Briceño MI and Caballero-George
C: Critical evaluation of biodegradable polymers used in nanodrugs.
Int J Nanomed. 8:3071–3090. 2013.
|
|
118
|
Ma X, Wang X, Zhou M and Fei H: A
mitochondria-targeting gold-peptide nanoassembly for enhanced
cancer-cell killing. Adv Healthc Mater. 2:1638–1643. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Jung HS, Han J, Lee J-H, Lee JH, Choi JM,
Kweon HS, Han JH, Kim JH, Byun KM, Jung JH, et al: Enhanced NIR
radiation-triggered hyperthermia by mitochondrial targeting. J Am
Chem Soc. 137:3017–3023. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Biswas S, Kumari P, Lakhani PM and Ghosh
B: Recent advances in polymeric micelles for anti-cancer drug
delivery. Eur J Pharm Sci. 83:184–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Dewit MA and Gillies ER: A cascade
biodegradable polymer based on alternating cyclization and
elimination reactions. J Am Chem Soc. 131:18327–18334. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Deming TJ: Synthetic polypeptides for
biomedical applications. Prog Polym Sci. 32:858–875. 2007.
View Article : Google Scholar
|
|
123
|
Sun J, Chen X, Lu T, Liu S, Tian H, Guo Z
and Jing X: Formation of reversible shell cross-linked micelles
from the biodegradable amphiphilic diblock copolymer poly
(L-cysteine)-block-poly (L-lactide). Langmuir. 24:10099–10106.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dourado ER, Pizzorno BS, Motta LM, Simao
RA and Leite LF: Analysis of asphaltic binders modified with PPA by
surface techniques. J Microsc. 254:122–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kim IB, Han MH, Phillips RL, Samanta B,
Rotello VM, Zhang ZJ and Bunz UH: Nano-conjugate fluorescence probe
for the discrimination of phosphate and pyrophosphate. Chemistry.
15:449–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chen PC, Mwakwari SC and Oyelere AK: Gold
nanoparticles: From nanomedicine to nanosensing. Nanotechnol Sci
Appl. 1:45–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Huang H-C, Barua S, Sharma G, Dey SK and
Rege K: Inorganic nanoparticles for cancer imaging and therapy. J
Control Release. 155:344–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Namiki Y, Fuchigami T, Tada N, Kawamura R,
Matsunuma S, Kitamoto Y and Nakagawa M: Nanomedicine for cancer:
Lipid-based nanostructures for drug delivery and monitoring. Acc
Chem Res. 44:1080–1093. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Libutti SK, Paciotti GF, Byrnes AA,
Alexander HR Jr, Gannon WE, Walker M, Seidel GD, Yuldasheva N and
Tamarkin L: Phase I and pharmacokinetic studies of CYT-6091, a
novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res.
16:6139–6149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Benezra M, Penate-Medina O, Zanzonico PB,
Schaer D, Ow H, Burns A, DeStanchina E, Longo V, Herz E, Iyer S, et
al: Multimodal silica nanoparticles are effective cancer-targeted
probes in a model of human melanoma. J Clin Invest. 121:2768–2780.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Sanchez C, Belleville P, Popall M and
Nicole L: Applications of advanced hybrid organic-inorganic
nanomaterials: From laboratory to market. Chem Soc Rev. 40:696–753.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Jain RK and Stylianopoulos T: Delivering
nanomedicine to solid tumors. Nat Rev Clin Oncol. 7:653–664. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kamaly N, Yameen B, Wu J and Farokhzad OC:
Degradable controlled-release polymers and polymeric nanoparticles:
Mechanisms of controlling drug release. Chem Rev. 116:2602–2663.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Holback H and Yeo Y: Intratumoral drug
delivery with nanoparticulate carriers. Pharm Res. 28:1819–1830.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Jain RK, Martin JD and Stylianopoulos T:
The role of mechanical forces in tumor growth and therapy. Annu Rev
Biomed Eng. 16:321–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kim KY: Nanotechnology platforms and
physiological challenges for cancer therapeutics. Nanomedicine
(Lond). 3:103–110. 2007. View Article : Google Scholar
|
|
137
|
Liu D, He C, Wang AZ and Lin W:
Application of liposomal technologies for delivery of platinum
analogs in oncology. Int J Nanomed. 8:3309–3319. 2013.
|
|
138
|
Moghimi SM, Symonds P, Murray JC, Hunter
AC, Debska G and Szewczyk A: A two-stage poly
(ethylenimine)-mediated cytotoxicity: Implications for gene
transfer/therapy. Mol Ther. 11:990–995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Lü J-M, Wang X, Marin-Muller C, Wang H,
Lin PH, Yao Q and Chen C: Current advances in research and clinical
applications of PLGA-based nanotechnology. Expert Rev Mol Diagn.
9:325–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kim J, Dadsetan M, Ameenuddin S, Windebank
AJ, Yaszemski MJ and Lu L: In vivo biodegradation and
biocompatibility of PEG/sebacic acid-based hydrogels using a cage
implant system. J Biomed Mater Res A. 95:191–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Nicolete R, dos Santos DF and Faccioli LH:
The uptake of PLGA micro or nanoparticles by macrophages provokes
distinct in vitro inflammatory response. Int Immunopharmacol.
11:1557–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ceonzo K, Gaynor A, Shaffer L, Kojima K,
Vacanti CA and Stahl GL: Polyglycolic acid-induced inflammation:
Role of hydrolysis and resulting complement activation. Tissue Eng.
12:301–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Madaan K, Kumar S, Poonia N, Lather V and
Pandita D: Dendrimers in drug delivery and targeting:
Drug-dendrimer interactions and toxicity issues. J Pharm Bioallied
Sci. 6:139–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Jain K, Kesharwani P, Gupta U and Jain NK:
Dendrimer toxicity: Let's meet the challenge. Int J Pharm.
394:122–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ma Y, Mou Q, Wang D, Zhu X and Yan D:
Dendritic polymers for theranostics. Theranostics. 6:930–947. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Dreaden EC, Austin LA, Mackey MA and
El-Sayed MA: Size matters: Gold nanoparticles in targeted cancer
drug delivery. Ther Deliv. 3:457–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Gu Y, Zhong Y, Meng F, Cheng R, Deng C and
Zhong Z: Acetal-linked paclitaxel prodrug micellar nanoparticles as
a versatile and potent platform for cancer therapy.
Biomacromolecules. 14:2772–2780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Idris NM, Gnanasammandhan MK, Zhang J, Ho
PC, Mahendran R and Zhang Y: In vivo photodynamic therapy using
upconversion nanoparticles as remote-controlled nanotransducers.
Nat Med. 18:1580–1585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yang Y, Shao Q, Deng R, Wang C, Teng X,
Cheng K, Cheng Z, Huang L, Liu Z, Liu X, et al: In vitro and in
vivo uncaging and bioluminescence imaging by using photocaged
upconversion nanoparticles. Angew Chem Int Ed Engl. 51:3125–3129.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Chen H, Yang Z, Ding C, Chu L, Zhang Y,
Terry K, Liu H, Shen Q and Zhou J: Fragment-based drug design and
identification of HJC0123, a novel orally bioavailable STAT3
inhibitor for cancer therapy. Eur J Med Chem. 62:498–507. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Langer R and Folkman J: Polymers for the
sustained release of proteins and other macromolecules. Nature.
263:797–800. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Lee CC, MacKay JA, Fréchet JMJ and Szoka
FC: Designing dendrimers for biological applications. Nat
Biotechnol. 23:1517–1526. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Torchilin VP: Immunoliposomes and
PEGylated immunoliposomes: Possible use for targeted delivery of
imaging agents. Immunomethods. 4:244–258. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Immordino ML, Dosio F and Cattel L:
Stealth liposomes: Review of the basic science, rationale, and
clinical applications, existing and potential. Int J Nanomed.
1:297–315. 2006.
|
|
155
|
Batist G, Ramakrishnan G, Rao CS,
Chandrasekharan A, Gutheil J, Guthrie T, Shah P, Khojasteh A, Nair
MK, Hoelzer K, et al: Reduced cardiotoxicity and preserved
antitumor efficacy of liposome-encapsulated doxorubicin and
cyclophosphamide compared with conventional doxorubicin and
cyclophosphamide in a randomized, multicenter trial of metastatic
breast cancer. J Clin Oncol. 19:1444–1454. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Silverman JA and Deitcher SR:
Marqibo® (vincristine sulfate liposome injection)
improves the pharmacokinetics and pharmacodynamics of vincristine.
Cancer Chemother Pharmacol. 71:555–564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Verma J, Lal S and Van Noorden CJ:
Inorganic nanoparticles for the theranostics of cancer. Eur J
Nanomed. 7:271–287. 2015. View Article : Google Scholar
|
|
158
|
Kim MT, Chen Y, Marhoul J and Jacobson F:
Statistical modeling of the drug load distribution on trastuzumab
emtansine (Kadcyla), a lysine-linked antibody drug conjugate.
Bioconjug Chem. 25:1223–1232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Mamot C, Ritschard R, Wicki A, Stehle G,
Dieterle T, Bubendorf L, Hilker C, Deuster S, Herrmann R and
Rochlitz C: Tolerability, safety, pharmacokinetics, and efficacy of
doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid
tumours: A phase 1 dose-escalation study. Lancet Oncol.
13:1234–1241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Swiss Group for Clinical Cancer Research:
Anti-EGFR-immunoliposomes loaded with doxorubicin in patients with
advanced triple negative EGFR positive breast cancer. NCT02833766.
https://clinicaltrials.gov/ct2/show/NCT02833766Accessed.
October 20–2016.
|
|
161
|
Markman M: Pegylated liposomal doxorubicin
in the treatment of cancers of the breast and ovary. Expert Opin
Pharmacother. 7:1469–1474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Listed N: Kaposi's sarcoma: DaunoXome
approved. AIDS Treat News. 79:3–4. 1996.
|
|
163
|
Rau K-M, Lin Y-C, Chen Y-Y, Chen JS, Lee
KD, Wang CH and Chang HK: Pegylated liposomal doxorubicin
(Lipo-Dox®) combined with cyclophosphamide and
5-fluorouracil is effective and safe as salvage chemotherapy in
taxane-treated metastatic breast cancer: An open-label,
multi-center, non-comparative phase II study. BMC Cancer.
15:4232015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
DiGiulio S: DiGiulio, S. FDA approves
onivyde combo regimen for advanced pancreatic cancer. Oncol Times.
37:82015. View Article : Google Scholar
|
|
165
|
Stathopoulos G and Boulikas T: Lipoplatin
formulation review article. J Drug Deliv. 2012:5813632012.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Ohyanagi F, Horai T, Sekine I, Yamamoto N,
Nakagawa K, Nishio M, Senger S, Morsli N and Tamura T: Safety of
BLP25 liposome vaccine (L-BLP25) in Japanese patients with
unresectable stage III NSCLC after primary chemoradiotherapy:
Preliminary results from a Phase I/II study. Jpn J Clin Oncol.
41:718–722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Dou Y, Hynynen K and Allen C: To heat or
not to heat: Challenges with clinical translation of
thermosensitive liposomes. J Control Release. 249:63–73. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Lancet JE, Uy GL, Cortes JE, Newell LF,
Lin TL, Ritchie EK, Stuart RK, Strickland SA, Hogge D, Solomon SR,
et al: Final results of a phase III randomized trial of CPX-351
versus 7+3 in older patients with newly diagnosed high risk
(secondary) AM. J Clin Oncol. 34:70002016.
|
|
169
|
Dragovich T, Mendelson D, Kurtin S,
Richardson K, Von Hoff D and Hoos A: A Phase 2 trial of the
liposomal DACH platinum L-NDDP in patients with therapy-refractory
advanced colorectal cancer. Cancer Chemother Pharmacol. 58:759–764.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Chinsriwongkul A, Chareanputtakhun P,
Ngawhirunpat T, Rojanarata T, Sila-on W, Ruktanonchai U and
Opanasopit P: Nanostructured lipid carriers (NLC) for parenteral
delivery of an anticancer drug. AAPS PharmSciTech. 13:150–158.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Strumberg D, Schultheis B, Traugott U,
Vank C, Santel A, Keil O, Giese K, Kaufmann J and Drevs J: Phase I
clinical development of Atu027, a siRNA formulation targeting PKN3
in patients with advanced solid tumors. Int J Clin Pharmacol Ther.
50:76–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Awada A, Bondarenko IN, Bonneterre J,
Nowara E, Ferrero JM, Bakshi AV, Wilke C and Piccart M: CT4002
study group: A randomized controlled phase II trial of a novel
composition of paclitaxel embedded into neutral and cationic lipids
targeting tumor endothelial cells in advanced triple-negative
breast cancer (TNBC). Ann Oncol. 25:824–831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Slingerland M, Guchelaar H-J, Rosing H,
Scheulen ME, van Warmerdam LJ, Beijnen JH and Gelderblom H:
Bioequivalence of Liposome-Entrapped Paclitaxel Easy-To-Use
(LEP-ETU) formulation and paclitaxel in polyethoxylated castor oil:
A randomized, two-period crossover study in patients with advanced
cancer. Clin Ther. 35:1946–1954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Bala V, Rao S, Boyd BJ and Prestidge CA:
Prodrug and nanomedicine approaches for the delivery of the
camptothecin analogue SN38. J Control Release. 172:48–61. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Senzer NN, Matsuno K, Yamagata N, Fujisawa
T, Wasserman E, Sutherland W, Sharma S and Phan A: Abstract C36:
MBP-426, a novel liposome-encapsulated oxaliplatin, in combination
with 5-FU/leucovorin (LV): Phase I results of a Phase I/II study in
gastro-esophageal adenocarcinoma, with pharmacokinetics. Mol Cancer
Ther. 8:(Suppl. 1). C36. 2009.doi: 10.1158/1535-7163.TARG-09-C36.
View Article : Google Scholar
|
|
176
|
Seiden MV, Muggia F, Astrow A, Matulonis
U, Campos S, Roche M, Sivret J, Rusk J and Barrett E: A phase II
study of liposomal lurtotecan (OSI-211) in patients with topotecan
resistant ovarian cancer. Gynecol Oncol. 93:229–232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Noble GT, Stefanick JF, Ashley JD,
Kiziltepe T and Bilgicer B: Ligand-targeted liposome design:
Challenges and fundamental considerations. Trends Biotechnol.
32:32–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Wetzler M, Thomas DA, Wang ES, Shepard R,
Ford LA, Heffner TL, Parekh S, Andreeff M, O'Brien S and Kantarjian
HM: Phase I/II trial of nanomolecular liposomal annamycin in adult
patients with relapsed/refractory acute lymphoblastic leukemia.
Clin Lymphoma Myeloma Leuk. 13:430–434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Hwang JH, Lim MC, Seo S-S, Park S-Y and
Kang S: Phase II study of belotecan (CKD 602) as a single agent in
patients with recurrent or progressive carcinoma of uterine cervix.
Jpn J Clin Oncol. 41:624–629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Hough B, Posner M, Chung C, et al: A phase
II study of single agent OSI-7904L in patients with metastatic or
recurrent squamous cell carcinoma of the head and neck (SCCHN).
Journal. 27:e170052009.
|
|
181
|
Pattni BS, Chupin VV and Torchilin VP: New
developments in liposomal drug delivery. Chem Rev. 115:10938–10966.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Semple SC, Leone R, Wang J, Leng EC,
Klimuk SK, Eisenhardt ML, Yuan ZN, Edwards K, Maurer N, Hope MJ, et
al: Optimization and characterization of a
sphingomyelin/cholesterol liposome formulation of vinorelbine with
promising antitumor activity. J Pharm Sci. 94:1024–1038. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Ugwu S, Zhang A, Parmar M, Miller B,
Sardone T, Peikov V and Ahmad I: Preparation, characterization, and
stability of liposome-based formulations of mitoxantrone. Drug Dev
Ind Pharm. 31:223–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
McMurtry V, Nieves-Alicea R, Donato N and
Tari A: Liposome-incorporated Grb2 antisense oligonucleotides as a
novel therapy against drug resistant chronic myelogenous leukemia.
Cancer Res. 68:1503. 2008.
|
|
185
|
Stathopoulos GP, Boulikas T, Kourvetaris A
and Stathopoulos J: Liposomal oxaliplatin in the treatment of
advanced cancer: A phase I study. Anticancer Res. 262B:1489–1493.
2006.
|
|
186
|
Oncology Venture: Oncology Venture
presents LiPlaCis on AACR in New Orleans. https://www.aktietorget.se/NewsItem.aspx?ID=77244
|
|
187
|
Barraud L, Merle P, Soma E, Lefrançois L,
Guerret S, Chevallier M, Dubernet C, Couvreur P, Trépo C and
Vitvitski L: Increase of doxorubicin sensitivity by
doxorubicin-loading into nanoparticles for hepatocellular carcinoma
cells in vitro and in vivo. J Hepatol. 42:736–743. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Zhou Q, Sun X, Zeng L, Liu J and Zhang Z:
A randomized multicenter phase II clinical trial of
mitoxantrone-loaded nanoparticles in the treatment of 108 patients
with unresected hepatocellular carcinoma. Nanomedicine (Lond).
5:419–423. 2009. View Article : Google Scholar
|
|
189
|
Pang X, Du H-L, Zhang H-Q, Zhai Y-J and
Zhai G-X: Polymer-drug conjugates: Present state of play and future
perspectives. Drug Discov Today. 18:1316–1322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
QUILT-3.014: A Trial of ABI-011
administered weekly in patients with advanced solid tumors or
lymphomas. NCT02582827. https://clinicaltrials.gov/ct2/show/NCT02582827Accessed.
March 6–2017.
|
|
191
|
Giglio V, Sgarlata C and Vecchio G: Novel
amino-cyclodextrin cross-linked oligomer as efficient carrier for
anionic drugs: A spectroscopic and nanocalorimetric investigation.
RSC Advances. 5:16664–16671. 2015. View Article : Google Scholar
|
|
192
|
Svenson S: Clinical translation of
nanomedicines. Curr Opin Solid State Mater Sci. 16:287–294. 2012.
View Article : Google Scholar
|
|
193
|
Williamson SK, Johnson GA, Maulhardt HA,
Moore KM, McMeekin DS, Schulz TK, Reed GA, Roby KF, Mackay CB,
Smith HJ, et al: A phase I study of intraperitoneal nanoparticulate
paclitaxel (Nanotax®) in patients with peritoneal
malignancies. Cancer Chemother Pharmacol. 75:1075–1087. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Vergote I, Brize A, Lisyanskaya AS and
Lichinitser M: Randomized phase III study comparing
paclical-carboplatin with paclitaxel-carboplatin in patients with
recurrent platinum-sensitive epithelial ovarian cancer. J Clin
Oncol. 33:55172015.
|
|
195
|
Pitto-Barry A and Barry NP:
Pluronic® block-copolymers in medicine: From chemical
and biological versatility to rationalisation and clinical
advances. Polym Chem. 5:3291–3297. 2014. View Article : Google Scholar
|
|
196
|
Kato K, Chin K, Yoshikawa T, Yamaguchi K,
Tsuji Y, Esaki T, Sakai K, Kimura M, Hamaguchi T, Shimada Y, et al:
Phase II study of NK105, a paclitaxel-incorporating micellar
nanoparticle, for previously treated advanced or recurrent gastric
cancer. Invest New Drugs. 30:1621–1627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Endo K, Ueno T, Kondo S, Wakisaka N,
Murono S, Ito M, Kataoka K, Kato Y and Yoshizaki T: Tumor-targeted
chemotherapy with the nanopolymer-based drug NC-6004 for oral
squamous cell carcinoma. Cancer Sci. 104:369–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Tsuji A, Hamaguchi T, Yamaguchi K, et al:
A phase II study of NK012, a polymeric micelle formulation of
SN-38, in colorectal cancer patients who had received prior
oxaliplatin-based regimen. Journal. 33:35272015.
|
|
199
|
Ghamande S, Lin C-C, Cho DC, Coleman T,
Chaudhary I, Shapiro GI, Silverman M, Kuo M-W, Mach WB, Tseng Y, et
al: Abstract A89: A phase I study of the novel DNA topoisomerase-1
inhibitor, TLC388 (Lipotecan®), administered
intravenously to patients with advanced solid tumors. Mol Cancer
Ther. 10:(Supplement 1). 892011. View Article : Google Scholar
|
|
200
|
Ueno T, Endo K, Hori K, Ozaki N, Tsuji A,
Kondo S, Wakisaka N, Murono S, Kataoka K, Kato Y, et al: Assessment
of antitumor activity and acute peripheral neuropathy of
1,2-diaminocyclohexane platinum (II)-incorporating micelles
(NC-4016). Int J Nanomed. 9:3005–3012. 2014. View Article : Google Scholar
|
|
201
|
Takahashi A, Yamamoto Y, Yasunaga M, Koga
Y, Kuroda J, Takigahira M, Harada M, Saito H, Hayashi T, Kato Y, et
al: NC-6300, an epirubicin-incorporating micelle, extends the
antitumor effect and reduces the cardiotoxicity of epirubicin.
Cancer Sci. 104:920–925. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Owen SC, Chan DP and Shoichet MS:
Polymeric micelle stability. Nano Today. 7:53–65. 2012. View Article : Google Scholar
|