Introduction
Besides genetic mutations in oncogenes and tumor
suppressor genes, the importance of epigenetic regulation for
carcinogenesis has been recognized. Epigenetic regulation includes
DNA methylation and histone modifications (the so called histone
code) that can modulate chromosome structure for gene expression
(1). Protein arginine methylation
is a post-translational modification that has been implicated in
signal transduction, gene transcription, DNA repair and RNA
processing. Recent studies have linked this modification to
carcinogenesis and metastasis (2).
According to the attachment of methyl groups to
specific guanidino nitrogen atoms of arginine, the protein arginine
methyltransferases (PRMTs) are divided into four different groups:
type I catalyzes the formation of asymmetric
ω-NG, NG dimethylarginine
(ADMA), type II symmetric ω-NG,
NG dimethylarginine (SDMA), type III
ω-NG monomethylarginine (MMA), and type IV
enzymes catalyzing the transfer of the methyl group to the
δ-nitrogen of arginine residues are only reported in yeast
(3). Nine different human PRMTs
have been identified to catalyze protein arginine methylation
(4,5). PRMT1, 2, 3, 4, 6 and 8 belong to the
type I while PRMT5 and PRMT9 (6) to
the type II PRMT. Type III activity have been reported for PRMT7
(7).
PRMTs can affect gene expression through their
coactivator/corepressor activity to modify histones and regulate
transcription (5). Furthermore,
PRMTs can also exert their impacts by adjusting the modified
substrates for different interactions, localization, function, or
signaling pathways (3). Each PRMT
can modify various non-histone substrate proteins including many
RNA binding proteins (3). Critical
proteins involved in various cancers such as p53 (8), estrogen receptor (9), BRCA1 (10) and EGF receptor (11) have been reported to be methylated by
PRMTs.
DNA damage response (DDR) is critical for the
maintenance of genome integrity to prevent cancer development.
Numerous PRMT substrates such as MRE11, Rad 9, 53BP1 and DNA
polymerase β, FEN1 and BRCA1 are involved in DDR (12). Protein arginine methyltransferases
thus, can influence cancer development through their regulation of
DDR. Protein arginine methylation also participates in many signal
transduction pathways that are critical in cancer development. For
example, both PRMT1 and CARM1 have been reported to play roles in
the Wnt/β-catenin pathway (13–16).
PRMT1 methylation of Axin, a key scaffold protein for β-catenin
degradation, can stabilize Axin and thus negatively regulates WNT
signaling (13–16). Overall it is apparent that protein
arginine methylation is important for carcinogenesis.
Overexpression of PRMTs is often associated with
various types of cancer. For example, the expression level of PRMT1
and PRMT6 in cancer cells of various tissues including bladder,
lung and breast are significantly higher than that in normal
(17). For melanoma, only
PRMT4/CARM1 was significantly induced whereas PRMT6 was reduced
(18). Understanding the
involvement of certain PRMTs in a specific cancer will help to make
them as therapeutic targets.
Most studies related to arginine methylation and
cancer are from the studies of breast (19–23)
and in prostate (24,25), lung (17,26),
bladder (17) and colorectal cancer
(27,28). Head and neck cancer is very
heterogeneous with malignancies arising in the oral cavity and
oropharynx, nasopharynx, hypopharynx, larynx, nasal cavity and
paranasal sinuses, ear, salivary glands and thyroid. Ninety percent
of the HNC malignancies are squamous cell carcinomas (HNSCC). Risk
factors associated with HNSCC carcinogenesis include tobacco and
alcohol use, poor diet and human papilloma virus (HPV) infection
(29). There have been no reports
focusing on protein arginine methylation in head and neck
cancer.
Oral cancer accounts for the largest group in head
and neck cancers. In the present study, we thus investigated the
involvement of the modification in HNC using an immortalized oral
cell (S-G) and oral cancer cell lines (SAS, OECM-1 and HSC-3).
PRMT1 is the enzyme that accounts for 85% of protein arginine
methylation activity (30) and has
been implicated in many different cancer types. We thus first
examined the levels of the predominant type I enzyme PRMT1 and the
modified ADMA-containing proteins in oral cancer cells. We then
treated the oral cancer cells with an indirect methyltransferase
inhibitor adenosine dialdehyde (AdOx) to follow the effects of
arginine methylation. AdOx can accumulate the methyltransferase
product inhibitor S-adenosine homocysteine (SAH) through the
inhibition of SAH hydrolase. Protein arginine methylation stands
for the majority of the AdOx-blocked protein methylation (31,32).
Furthermore, AdOx has been shown to affect the proliferation and
metastasis of various cancer cells (33–37).
We showed that the AdOx treatment decrease the level of
ADMA-containing proteins and can reduce growth and migration in
oral cancer cells that express a high level of PRMT1. Knockdown of
PRMT1 protein had similar effects on cell growth and migration in
the SAS oral cancer cells. We further examined PRMT1 protein in HNC
patient tissue sections and observed elevated PRMT1 levels in
tumors.
Materials and methods
Cell culture and cell extract
preparation
Oral squamous carcinoma cancer cell lines SAS and
OECM1 and immortalized human gingival keratinocyte S-G cells were
used in the present study. The SAS cell line was established from a
poorly differentiated squamous cell carcinoma of the tongue. The
OECM-1 cell line was established from OSCC cells surgically excised
from a Taiwanese patient. The S-G cell is an epithelial-like cell
line established from an area of clinically normal adult human
attached gingiva and thus is used as a normal control. The S-G and
SAS cells were grown in Dulbeccos modified Eagles medium (DMEM;
Gibco/Life Technologies), the OECM1 cells in RPMI-1640 medium
(Gibco/Life Technologies), and HSC-3 cells were in DMEM/F-12 medium
(Gibco/Life Technologies) with 10% fetal bovine serum (FBS; HyClone
Laboratories, Inc., Logan, UT, USA) and antibiotics (100 U/ml
penicillin and 100 ml/ml streptomycin) at 37°C in an incubator with
5% CO2. L-glutamine (2 mM) was supplemented to all cell
lines except HSC-3 and pyruvate (2 mM) was supplemented to the S-G
cells. After reaching confluency, the cells were harvested and
washed with phosphate-buffered saline (PBS, 10 mM
Na2HPO4; 1.8 mM KH2PO4
140 mM NaCl and 2.7 mM KCl, pH 7.4), resuspended and lysed in
buffer A [50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 1% Triton
X-100; 1x complete protease inhibitor (Roche); 10 mM NaF] by
sonication. The lysate was centrifuged and the supernatant was
designated as the cell extract. The protein concentration was
determined by the BCA protein assay (Thermo Fisher Scientific,
Waltham, MA, USA) using bovine serum albumin (BSA) as the
standard.
SDS-PAGE and western blot
analyses
Protein samples were separated by SDS-polyacrylamide
gel electrophoresis (PAGE) and then transferred to nitrocellulose
membranes (Hybond-C Extra, Gelman Sciences; Amersham Biosciences,
Little Chalfont, UK) as previously described (38). The membranes were blocked in 7%
skimmed dry milk in TTBS (10 mM Tris-HCl, pH 7.5; 100 mM NaCl; 0.1%
Tween-20) for 1 h, incubated with primary antibodies (1:1,000 for
anti-PRMT1, UPSTATE and 1:1,000 for anti-methylarginine antibodies
ADMA from Cell Signaling, Danvers, MA, USA) overnight, washed three
times in TTBS, then incubated with secondary antibody for 1 h.
Chemiluminescent detection using the VisGlow substrate for HRP
(Visual Protein, Taipei City, Taiwan) was performed according to
the manufacturers instructions.
Cell growth assay
Aliquots of cultured cells were mixed with trypan
blue and the cell numbers were counted using a hemocytometer. The
MTT assay was used to evaluate cell proliferation. Cells seeded in
6-well plates (10,000 cells/well) were incubated with the MTT
solution (0.25 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) for 3 h.
The resulting formazan crystals were dissolved in dimethyl
sulfoxide (DMSO) and measured by spectrophotometry at the
absorbance of 570 nm.
Cell migration assay
Cells (5×104) were seeded into wells of
the Oris Cell Migration Assembly Kit-FLEX (Platypus Technologies
Llc, Fitchburg, WI, USA) and migration assays were conducted in
accordance with the manufacturers instructions. After attached for
10 h, cells were allowed to migrate into the clear field with the
removal of the well inserts. After migration for a period of time,
cells were fixed with formaldehyde, stained with crystal violet and
photographed. The pre-migration and post-migration images were
analyzed using ImageJ software (http://rsb.info.nih.gov/ij/).
Stable shRNA-mediated PRMT1 knockdown
in SAS cells
Lentiviral particles produced in packaging vector
pLKO_TRC005 with short hairpin RNA (shRNA) targeting human PRMT1
(A1 with the target sequences: 5′- GTGTTCCAGTAT CTCTGATTA-3′; B1
with the target sequences: 5′-CCGGCAGTACAAAGACTACAA-3′) and a
negative control were obtained from the National RNAi Core Facility
(Academia Sinica, Taipei City, Taiwan). Cells were infected in
complete growth medium supplemented with polybrene (Sigma-Aldrich).
After 24 h, cells were grown and selected in medium containing in 5
µg/ml puromycin (Sigma-Aldrich). The knockdown effects were
examined by PRMT1 protein expression using immunoblotting.
Oral cancer specimens and
immunohistochemical (IHC) studies
Paraffin embedded specimens (Pathological blocks)
were from patients with head and neck cancers of different stages.
The patients recruited in the Department of Otolaryngology,
Chung-Shan Medical University Hospital (Taichung, Taiwan) between
2001 and 2010 were de-linked from their identification information
and were randomly numbered. The present study was approved by the
Institutional Research Board of Chung-Shan Medical University
Hospital. IHC was conducted with the ultraView Universal DAB
Detection kit (Roche) using the Ventana BenchMark XT automated
IHC/ISH slide staining system (Roche) at the short CC antigen
retrieval condition. Anti-PRMT1 antibodies from Upstate
Biotechnologies were diluted (1:200) and incubated at 37°C for 16
min.
Results
Protein arginine methyltransferase
PRMT1 and methylarginine containing proteins in different oral
cancer cell lines
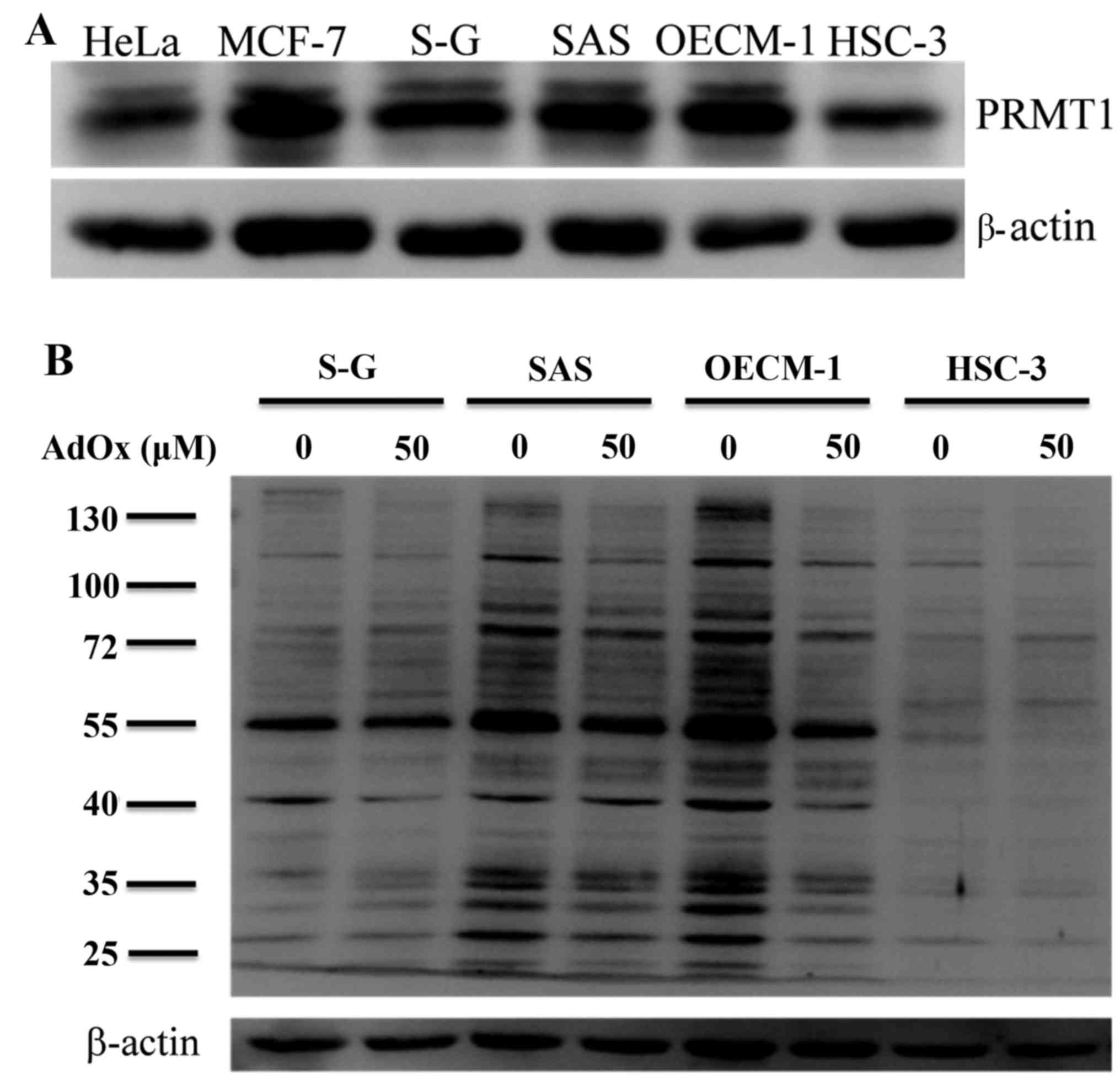
We first examined protein arginine methylation in
three oral squamous carcinoma cancer cell lines (SAS, OECM-1 and
HSC-3). Immortalized S-G cells established from clinically normal
gingiva was used as a normal control. In comparison, cell extracts
prepared from other human cancer cell lines such as HeLa (cervical
cancer cell line) and MCF-7 (breast cancer cell line) cells that
have been investigated for PRMTs or arginine methylation (39–41)
were included. The major type I protein arginine methyltransferase
PRMT1 and ADMA-containing proteins were detected using specific
antibodies. Western blot analyses showed that the expression
patterns of PRMT1 in oral cancer cells were similar to that of HeLa
and MCF-7 cells with the major signals of about the same molecular
masses (Fig. 1A). The expression
levels of PRMT1 was lowest in HSC-3.
The levels of ADMA-containing proteins were high in
OECM-1 and SAS, and lower in S-G cells (Fig. 1B). HSC-3 only showed few weak
ADMA-containing signals. To analyze the effects of arginine
methylation in the oral cancer cells, we treated the cells with an
indirect methyltransferase inhibitor AdOx that has been widely used
in protein methylation studies. AdOx treatment clearly decreased
ADMA signals in oral cancer cell line SAS and OECM-1 that express
high level of PRMT1, the major type I PRMT catalyzing the formation
of ADMA (Fig. 1B; 50 mM AdOx).
Decreased ADMA signals following the methylation inhibition by AdOx
also confirmed that the signals detected by the ADMA-specific
antibodies are from methylation modification. However,
methylarginine levels reduced less significantly for HSC-3 and S-G
cells.
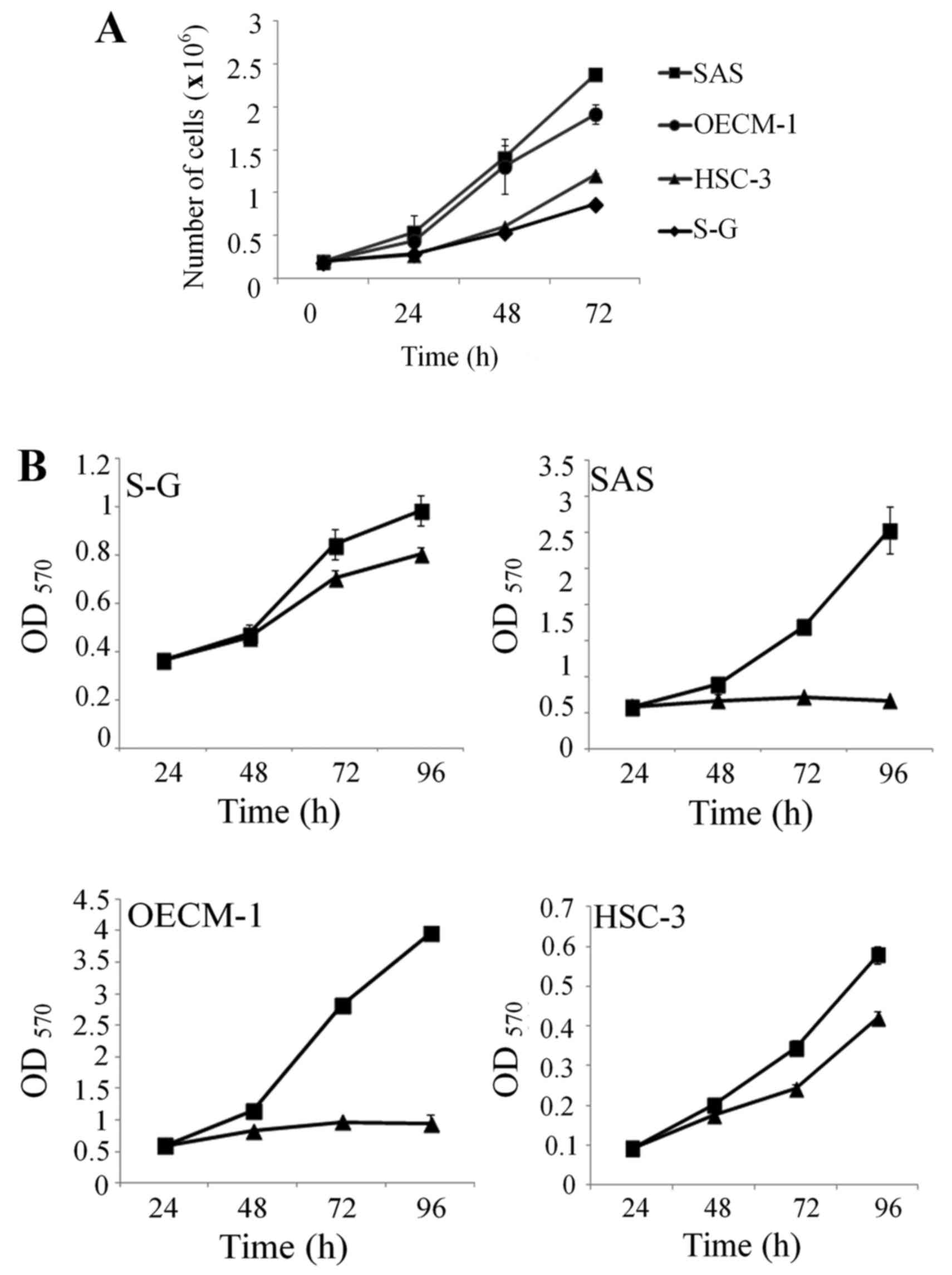
Reduced growth rate of oral cancer
cell lines, but not immortalized oral cells, after treatment with
the methylation inhibitor AdOx
As a general transmethylation inhibitor, AdOx has
been shown to block proliferation of different cancer cells
(33,36,37).
To examine whether AdOx can affect growth of oral cancer cells, we
first determined the growth curves of the four cell lines. SAS and
OECM-1 proliferated at a higher growth rate compared to the S-G
cells (Fig. 2A). On the other hand,
HSC-3 cells that expressed low level of PRMT1 and ADMA-containing
proteins, proliferated at a slower rate similar to the S-G cells.
We then determined the effects of AdOx treatment on the
proliferation of these cells. Addition of AdOx at the concentration
that could effectively reduce the ADMA level (50 mM) also blocked
the proliferation of SAS and OECM-1 cells significantly (Fig. 2B). In contrast, cell growth of the
S-G cells was only slightly inhibited by the same AdOx treatment.
Limited growth inhibition of the HSC-3 cells by AdOx treatment was
observed (Fig. 2B).
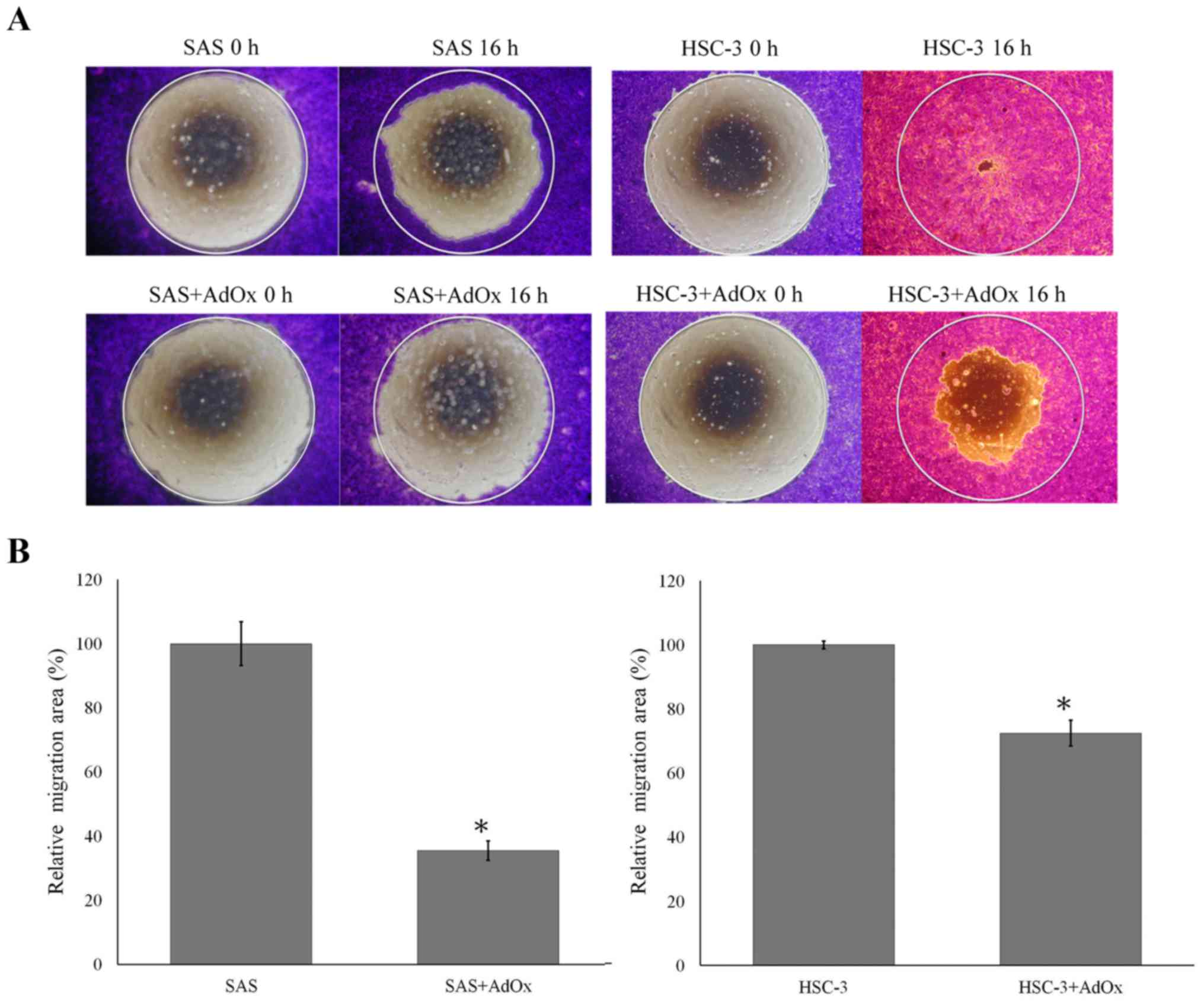
Migration activity of SAS oral cancer
cells decreases after AdOx treatment
AdOx treatment has also been demonstrated to
suppress tumor cell invasion and migration (35). We thus evaluated the effects of AdOx
on migration of the oral cancer cells. Analyses of the migration
activity of the oral cancer cells showed migration activity of SAS
and HSC-3 but limited migration ability of OECM-1 (data not shown).
We thus used SAS and HSC-3 cells to study the effects of AdOx on
migration. It is apparent that HSC-3 migrated faster than SAS
(Fig. 3A, 16 h). Treatment with 50
mM of AdOx reduced the migration of SAS and HSC-3 cells (Fig. 3A). To analyze the effects of AdOx
treatment on the migration activity of the cells, migration area of
the AdOx-treated cells was compared with that of the untreated
ones. After the treatment, the area covered by the migrated SAS
cells was only about 30% of the area covered by the migrated
untreated cells. The migration activity of HSC-3 cells was slightly
suppressed with the migration area close to 70% of that of
untreated cells (Fig. 3B).
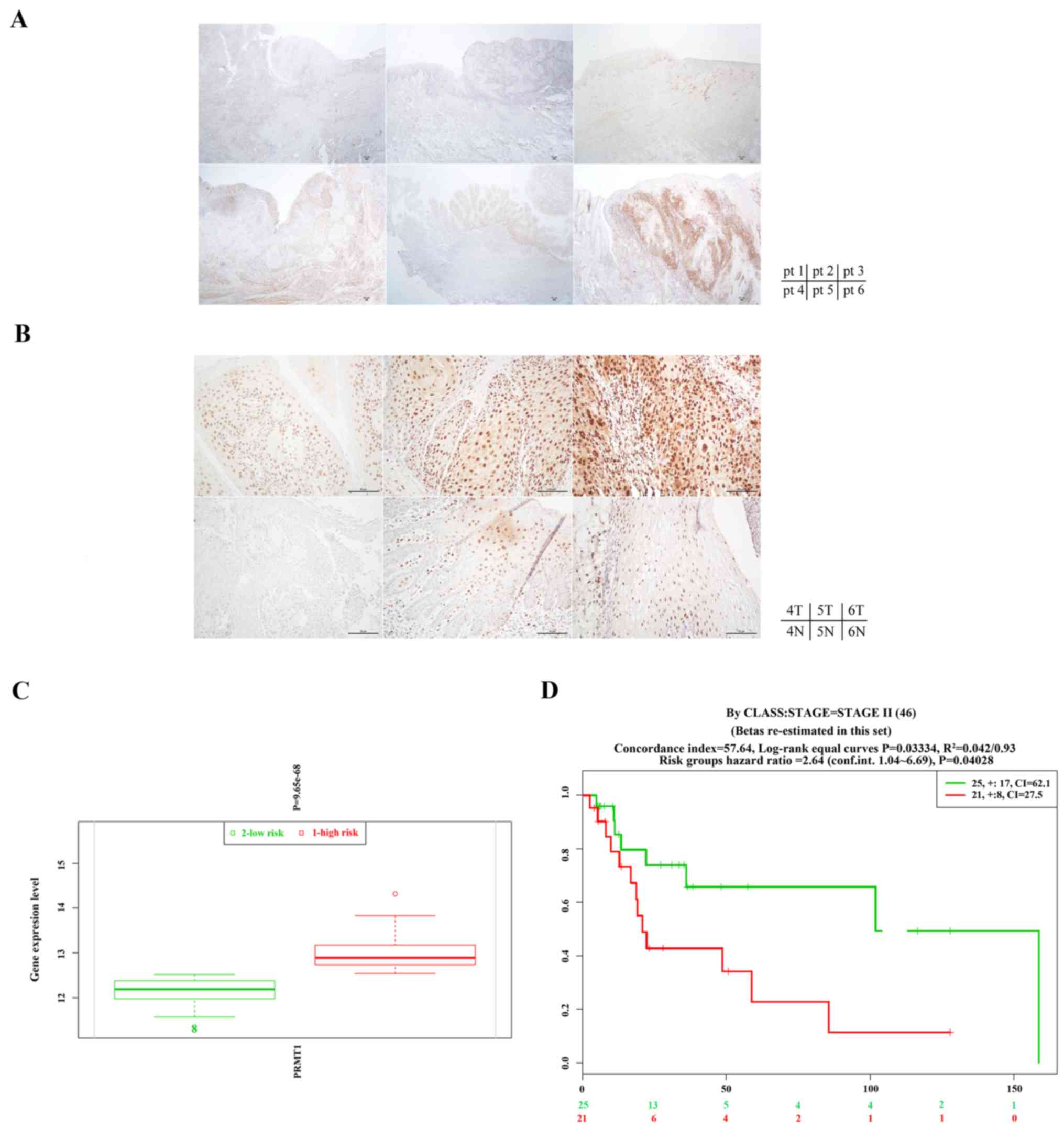
Elevated expression of PRMT1 in oral
cancer specimens
To further investigate the clinical involvement of
PRMT1 in HNC, we analyzed PRMT1 expression in oral cancer specimens
by immunohistochemistry. Six patients were included in the present
study as described in Table I. We
included tissue sections from patients of the late (IVA) or early
(I) oral cavity SCC stages and the subside from tongue or buccal
mucosa. Representative IHC stains of all six patients are shown in
Fig. 4A. To better illustrate the
PRMT1 expression in tumor and normal cells, representative regions
containing tumor or normal cells from patients 4, 5 and 6 are shown
in Fig. 4B with higher
magnification. It is clear that PRMT1 stained weakly in the normal
cells but its expression were stronger in the poorly differentiated
tumor cells. The majority of the intense PRMT1 signals were
concentrated in the nucleus. As illustrated in patients 4–6, the
IHC stain of PRMT1 revealed strong nuclear staining in cancer cells
(upper panels) and weak nuclear staining in non-neoplastic squamous
cells (lower panels). Even though the samples were limited in the
present study, the expression level of PRMT1 in the specimens
appeared to have strong correlation with the tumors. The PRMT1
level did not appear to be correlated with the cancer stage,
subside or sex.
 | Table I.HNC patients included in the PRMT1
IHC studies. |
Table I.
HNC patients included in the PRMT1
IHC studies.
| Patient | Sex | Subside | Age (years) | Tumor T status | Lymph node
metastasis | Metastasis | Stage | Cell
differentiation |
|---|
| 1 | Male | Tongue | 51 | 2 | 2 | 0 | IVA | Poorly
differentiated |
| 2 | Male | Tongue | 55 | 1 | 2 | 0 | IVA | Moderately
differentiated |
| 3 | Female | Buccal mucosa | 72 | 1 | 0 | 0 | I | Moderately
differentiated |
| 4 | Male | Buccal mucosa | 57 | 1 | 0 | 0 | I | Well
differentiated |
| 5 | Male | Buccal mucosa | 71 | 1 | 0 | 0 | I | Verrucous
carcinoma |
| 6 | Female | Tongue | 52 | 1 | 0 | 0 | I | Poorly
differentiated |
We also analyzed the head and neck squamous
carcinoma database from The Cancer Genome Atlas (TCGA; 273 samples)
by SurvExpress (http://bioinformatica.mty.itesm.mx/SurvExpress)
(42). In SurvExpress, all datasets
from TCGA were obtained at the gene level (level 3) which were the
data of RNA-Seq (42). It is
apparent that the high-risk group express higher level of PRMT1
than the low-risk group (Fig. 4C).
Similar results can be obtained from another database (TCGA Head
and Neck squamous cell carcinoma June 2016, 502 samples).
Furthermore, extended survival of the PRMT1 low expression group is
statistically significant (P<0.05) compared with the high
expression ones in either the stage II (Fig. 4D) or grade II samples (data not
shown).
Knockdown of PRMT1 in SAS oral cancer
cells decreases proliferation and migration
As arginine methylation stands for the majority of
the AdOx-blocked protein methylation (38) and PRMT1 is the predominant type I
PRMT, AdOx might affect mostly through PRMT1 for decreased
migration activity. Furthermore, PRMT1 expression was strongest in
the poorly differentiated cells in the clinical samples. We thus
suspect that PRMT1 is critical for the proliferation and
migration/invasion activity of HNC. To specifically analyze the
function of PRMT1 in oral cancer cells, we knocked down PRMT1 gene
in SAS cells with shRNA by lentivirus (shPRMT1A1, B1 or combined)
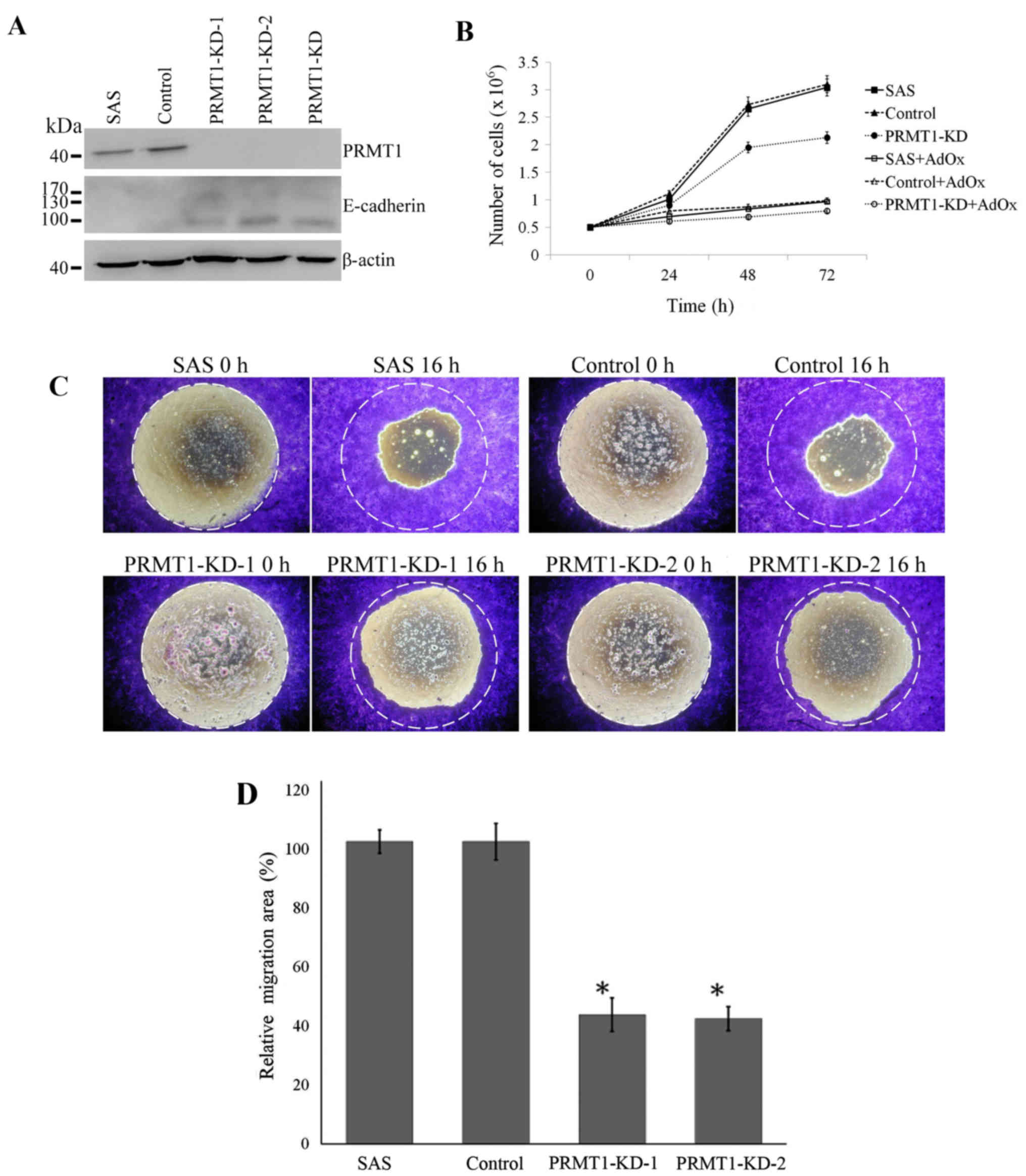
infection. The protein level of PRMT1 decreased almost completely
in cells that stably express either or both of the two different
PRMT1 shRNA (PRMT1-KD-1, PRMT1-KD-2 or PRMT1-KD) compared to that
in the non-infected or control shRNA infected SAS cells (SAS and
Control; Fig. 5A). As PRMT1 is the
major type I PRMT, the level of proteins containing asymmetric
dimethylarginine (ADMA) also decreased significantly after the
PRMT1 knockdown (data not shown). We then examined the growth of
SAS cells with PRMT1 knockdown. The PRMT1 KD cells apparently grew
slower than the non-infected or control shRNA infected cells.
Treatment of the cells with AdOx further reduced the growth of all
three cells with or without PRMT1 KD (Fig. 5B).
We also determined the migration abilities of the
SAS cells. The migration of the SAS cells with PRMT1 knockdown
showed significantly decreased migration compared with the cells
with control shRNA or SAS cells (Fig.
5C and D). E-cadherin is a calcium-dependent adhesion molecule
expressed on normal epithelium (43). The elevated expression of E-cadherin
has been reported to suppress cell migration (44). We further examined the expression of
E-cadherin in PRMT1 knockdown SAS cells and the results showed that
PRMT1 knockdown upregulated the epithelial marker E-cadherin
(Fig. 5A).
Discussion
Besides as players of epigenetic modifiers, protein
arginine methyltransferases are known to methylate a plethora of
substrates involved in transcriptional regulation, signal
transduction and DNA methylation. The involvement of protein
arginine methylation or PRMTs has been analyzed in different
cancers but not in head and neck cancer. We thus started the
investigation using oral cancer cells.
We observed that two oral cancer cell lines SAS and
OECM-1 expressed high ADMA level while another oral cancer cell
line HSC-3 contained low level of ADMA-containing proteins.
Consistently, the expression level of the major type I protein
arginine methyltransferase PRMT1 is high in SAS and OECM-1 but low
in HSC-3 cells. The expression level of PRMT1 and ADMA-containing
proteins in HSC-3 cells were even lower than that in the
immortalized normal S-G cells. The results might reflect the
heterogeneous nature of oral cancer formation and suggests that
oral cancer cell lines expressing different levels of PRMTs might
have diverse PRMT involvement in their carcinogenesis.
The indirect methyltransferase inhibitor AdOx has
been shown to decrease cancer cell survival and induce apoptosis
(33,36,37).
SAS and OECM-1, the two oral cancer cells with high level of
ADMA-containing polypeptides, showed more significant decrease of
the ADMA level than the S-G and HSC-3 cells when treated with AdOx.
Treatment of AdOx at the concentration (50 mM) that could
effectively reduce ADMA levels in SAS and OECM1 oral cancer cells
also inhibited their cell growth. The same AdOx treatment that
reduced less ADMA level in S-G and HSC-3 cells also only decreased
the growth rate slightly. It is likely that cancer cells with high
PRMT1/ADMA levels have a higher proliferation rate and AdOx
treatment can reduce their growth more significantly than the ones
with low ADMA levels.
We also showed that AdOx treatment can reduce the
migration activity of the oral cancer cells SAS and HSC-3. In
comparison, migration of SAS can be inhibited to a greater extent
than that of the HSC-3 cells. AdOx treatment can effectively block
cell proliferation in SAS and OECM1 and inhibit migration in SAS
and HSC-3. Thus, AdOx can have the potential to reduce growth or
migration of the oral cancer cells depending on PRMT1/ADMA levels
and the nature of the cells.
We suspect that inhibition of PRMT1 might be
responsible for most of the AdOx effects. Besides as an epigenetic
modifier, PRMT1 is known to methylate a plethora of substrates
involved in transcriptional regulation, signal transduction and DNA
methylation. Indeed knockdown of PRMT1 phenocopied the effects of
AdOx treatment on SAS cells. Furthermore, PRMT1 has been shown to
be highly expressed in various cancers including bladder (17), liver (45) and esophageal cancer (46). We showed overexpression of PRMT1 in
the tumor cells compared with the neighboring normal cells in
tissue sections from head and neck cancer patients. The PRMT1
staining is highly concentrated in the nucleus and is thus likely
to play roles in transcriptional regulation. Even though the tissue
samples were limited, increased high PRMT1 expression level
appeared to be typical in the tumor cells. We included stage I and
stage IV patients for IHC stains but the expression levels of PRMT1
in the specimens were not correlated with the cancer stage.
Nevertheless, HNSCC data from TCGA confirmed the high expression of
PRMT1 in the high-risk group and extended survival of the low PRMT1
expression patients especially of grade II or stage II.
We knocked down the expression of PRMT1 in the SAS
cells that express high level of PRMT1 and show high migration
activity. After knockdown of PRMT1 in SAS, we observed reduced
mobility of the cells. PRMT1 has been shown to induce
epithelial-mesenchymal transition (EMT) in lung cancer cells
(47) and breast cancer cells
(48). Upregulated PRMT1 catalyzed
the methylation of Twist1 for E-cadherin repression in lung cancer
cell lines (47). PRMT1 also
activated the transcription of another E-cadherin repressor ZEB1 in
breast cancer cells through epigenetic regulation (48). Increased expression of the
epithelial marker E-cadherin after PRMT1 knockdown in SAS cells was
detected. The mechanism of the inhibitory effect of PRMT1 KD to the
mobility of SAS cells requires further investigation.
Recently methylation of epidermal growth factor
receptor (EGFR) by PRMT1 has been shown to be related to the
activation of the EGF signaling and might resulted in resistance to
cetuximab, a targeted therapeutic monoclonal antibody to EGFR.
Colon cancer as well as HNC patients with higher levels of
methyl-EGFR in tumors have been shown to have a higher recurrence
rate after cetuximab treatment (11). A gastric cancer study showed that
low PRMT1 and thus low-nuclear FOXO1 levels were associated with
higher recurrence after adjuvant chemotherapy and poor prognosis
(49). Furthermore, they showed
that the reduction in PRMT1 expression in gastric cancer cell lines
significantly inhibited sensitivity to cisplatin and 5-fluorouracil
(49). Chemotherapy with both
agents is also the standard treatment of choice for HNSCC. The
effects of PRMT1 on these agents can be evaluated and as PRMT1
expression is heterogeneous in HNC patients and oral cancer cell
lines, it should be helpful to analyze the PRMT1 level for proper
treatment.
Though elevated PRMT1 expression in various cancer
has been suggested to function in tumorigenesis, PRMT1 might still
play some roles in tumor suppression. For example, PRMT1 was shown
to have ordered cooperative roles with p300 and CARM1 as a
coactivator for the master tumor suppressor p53 (50). Moreover, repression of the NF-κB
pathway critical for inflammation and cancer by PRMT1 through
arginine methylation of the RelA subunit was reported recently
(51). The effects of PRMT1 on
specific cancer might have to consider the major signals in
conjunction with the microenvironments leading to the tumor
formation. The heterogeneous nature of oral cancers and the origin
of the cancer cell lines might explain the variable levels of ADMA
and PRMT1 in different oral cancer cells in this study.
Interestingly, the efficiency of a methylation inhibitor AdOx to
inhibit cell proliferation or migration was correlated with the
level of ADMA-containing protein and the type I PRMT proteins in
the oral cancer cells. Thus, while AdOx treatment can greatly
inhibit growth and migration of SAS, it worked limitedly with HSC-3
in comparison. Further application of methylation inhibition in
therapeutic use should require analyses of PRMT expression and ADMA
levels on pathological oral cancer specimens. The present study
thus provides fundamental background for future evaluation of the
PRMT genes as the therapeutic targets of oral cancer as well as
using AdOx or related compounds for oral cancer treatment.
Acknowledgements
The present study was supported by the
CSH-2014-C-021 from the Chung Shan Medical University Hospital to
C.C. and MOST 103-2320-B-040-022-MY3 from the Ministry of Science
and Technology to C.L. We thank Professor Shun-Fa Yang for
providing the HSC-3 cells.
References
|
1
|
Chen QW, Zhu XY, Li YY and Meng ZQ:
Epigenetic regulation and cancer (Review). Oncol Rep. 31:523–532.
2014.PubMed/NCBI
|
|
2
|
Yang Y and Bedford MT: Protein arginine
methyltransferases and cancer. Nat Rev Cancer. 13:37–50. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bedford MT and Clarke SG: Protein arginine
methylation in mammals: Who, what, and why. Mol Cell. 33:1–13.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krause CD, Yang ZH, Kim YS, Lee JH, Cook
JR and Pestka S: Protein arginine methyltransferases: Evolution and
assessment of their pharmacological and therapeutic potential.
Pharmacol Ther. 113:50–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang YC and Li C: Evolutionarily conserved
protein arginine methyltransferases in non-mammalian animal
systems. FEBS J. 279:932–945. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Y, Hadjikyriacou A, Xia Z, Gayatri S,
Kim D, Zurita-Lopez C, Kelly R, Guo A, Li W, Clarke SG, et al:
PRMT9 is a type II methyltransferase that methylates the splicing
factor SAP145. Nat Commun. 6:64282015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zurita-Lopez CI, Sandberg T, Kelly R and
Clarke SG: Human protein arginine methyltransferase 7 (PRMT7) is a
type III enzyme forming ω-NG-monomethylated arginine residues. J
Biol Chem. 287:7859–7870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jansson M, Durant ST, Cho EC, Sheahan S,
Edelmann M, Kessler B and La Thangue NB: Arginine methylation
regulates the p53 response. Nat Cell Biol. 10:1431–1439. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Le Romancer M, Treilleux I, Leconte N,
Robin-Lespinasse Y, Sentis S, Bouchekioua-Bouzaghou K, Goddard S,
Gobert-Gosse S and Corbo L: Regulation of estrogen rapid signaling
through arginine methylation by PRMT1. Mol Cell. 31:212–221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guendel I, Carpio L, Pedati C, Schwartz A,
Teal C, Kashanchi F and Kehn-Hall K: Methylation of the tumor
suppressor protein, BRCA1, influences its transcriptional cofactor
function. PLoS One. 5:e113792010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao HW, Hsu JM, Xia W, Wang HL, Wang YN,
Chang WC, Arold ST, Chou CK, Tsou PH, Yamaguchi H, et al:
PRMT1-mediated methylation of the EGF receptor regulates signaling
and cetuximab response. J Clin Invest. 125:4529–4543. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Auclair Y and Richard S: The role of
arginine methylation in the DNA damage response. DNA Repair (Amst).
12:459–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bikkavilli RK, Avasarala S, Vanscoyk M,
Sechler M, Kelley N, Malbon CC and Winn RA: Dishevelled3 is a novel
arginine methyl transferase substrate. Sci Rep. 2:8052012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bikkavilli RK and Malbon CC: Arginine
methylation of G3BP1 in response to Wnt3a regulates β-catenin mRNA.
J Cell Sci. 124:2310–2320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blythe SA, Cha SW, Tadjuidje E, Heasman J
and Klein PS: beta-Catenin primes organizer gene expression by
recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev
Cell. 19:220–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cha B, Kim W, Kim YK, Hwang BN, Park SY,
Yoon JW, Park WS, Cho JW, Bedford MT and Jho EH: Methylation by
protein arginine methyltransferase 1 increases stability of Axin, a
negative regulator of Wnt signaling. Oncogene. 30:2379–2389. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshimatsu M, Toyokawa G, Hayami S, Unoki
M, Tsunoda T, Field HI, Kelly JD, Neal DE, Maehara Y, Ponder BA, et
al: Dysregulation of PRMT1 and PRMT6, Type I arginine
methyltransferases, is involved in various types of human cancers.
Int J Cancer. 128:562–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Limm K, Ott C, Wallner S, Mueller DW,
Oefner P, Hellerbrand C and Bosserhoff AK: Deregulation of protein
methylation in melanoma. Eur J Cancer. 49:1305–1313. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Dhaheri M, Wu J, Skliris GP, Li J,
Higashimato K, Wang Y, White KP, Lambert P, Zhu Y, Murphy L, et al:
CARM1 is an important determinant of ERα-dependent breast cancer
cell differentiation and proliferation in breast cancer cells.
Cancer Res. 71:2118–2128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baldwin RM, Morettin A, Paris G, Goulet I
and Côté J: Alternatively spliced protein arginine
methyltransferase 1 isoform PRMT1v2 promotes the survival and
invasiveness of breast cancer cells. Cell Cycle. 11:4597–4612.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dowhan DH, Harrison MJ, Eriksson NA,
Bailey P, Pearen MA, Fuller PJ, Funder JW, Simpson ER, Leedman PJ,
Tilley WD, et al: Protein arginine methyltransferase 6-dependent
gene expression and splicing: Association with breast cancer
outcomes. Endocr Relat Cancer. 19:509–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goulet I, Gauvin G, Boisvenue S and Côté
J: Alternative splicing yields protein arginine methyltransferase 1
isoforms with distinct activity, substrate specificity, and
subcellular localization. J Biol Chem. 282:33009–33021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mathioudaki K, Scorilas A, Ardavanis A,
Lymberi P, Tsiambas E, Devetzi M, Apostolaki A and Talieri M:
Clinical evaluation of PRMT1 gene expression in breast cancer.
Tumour Biol. 32:575–582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim YR, Lee BK, Park RY, Nguyen NT, Bae
JA, Kwon DD and Jung C: Differential CARM1 expression in prostate
and colorectal cancers. BMC Cancer. 10:1972010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Majumder S, Liu Y, Ford OH III, Mohler JL
and Whang YE: Involvement of arginine methyltransferase CARM1 in
androgen receptor function and prostate cancer cell viability.
Prostate. 66:1292–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zakrzewicz D, Zakrzewicz A, Preissner KT,
Markart P and Wygrecka M: Protein arginine methyltransferases
(PRMTs): Promising targets for the treatment of pulmonary
disorders. Int J Mol Sci. 13:12383–12400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mathioudaki K, Papadokostopoulou A,
Scorilas A, Xynopoulos D, Agnanti N and Talieri M: The PRMT1 gene
expression pattern in colon cancer. Br J Cancer. 99:2094–2099.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Papadokostopoulou A, Mathioudaki K,
Scorilas A, Xynopoulos D, Ardavanis A, Kouroumalis E and Talieri M:
Colon cancer and protein arginine methyltransferase 1 gene
expression. Anticancer Res. 29:1361–1366. 2009.PubMed/NCBI
|
|
29
|
Park BJ, Chiosea SI and Grandis JR:
Molecular changes in the multistage pathogenesis of head and neck
cancer. Cancer Biomark. 9:325–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang J, Frankel A, Cook RJ, Kim S, Paik
WK, Williams KR, Clarke S and Herschman HR: PRMT1 is the
predominant type I protein arginine methyltransferase in mammalian
cells. J Biol Chem. 275:7723–7730. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Najbauer J and Aswad DW: Diversity of
methyl acceptor proteins in rat pheochromocytoma (PC12) cells
revealed after treatment with adenosine dialdehyde. J Biol Chem.
265:12717–12721. 1990.PubMed/NCBI
|
|
32
|
Najbauer J, Johnson BA and Aswad DW:
Analysis of stable protein methylation in cultured cells. Arch
Biochem Biophys. 293:85–92. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong S, Heo J, Lee S, Heo S, Kim SS, Lee
YD, Kwon M and Hong S: Methyltransferase-inhibition interferes with
neuronal differentiation of P19 embryonal carcinoma cells. Biochem
Biophys Res Commun. 377:935–940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan L, Zhao HY, Zhang Y and Shen YF:
Differential effects of AdOx on gene expression in P19 embryonal
carcinoma cells. BMC Neurosci. 13:62012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JH, Kim JH, Kim SC, Yi YS, Yang WS,
Yang Y, Kim HG, Lee JY, Kim KH, Yoo BC, et al: Adenosine dialdehyde
suppresses MMP-9-mediated invasion of cancer cells by blocking the
Ras/Raf-1/ERK/AP-1 signaling pathway. Biochem Pharmacol.
86:1285–1300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim JH, Lee YG, Yoo S, Oh J, Jeong D, Song
WK, Yoo BC, Rhee MH, Park J, Cha SH, et al: Involvement of Src and
the actin cytoskeleton in the antitumorigenic action of adenosine
dialdehyde. Biochem Pharmacol. 85:1042–1056. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schwerk C and Schulze-Osthoff K:
Methyltransferase inhibition induces p53-dependent apoptosis and a
novel form of cell death. Oncogene. 24:7002–7011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li C, Ai LS, Lin CH, Hsieh M, Li YC and Li
SY: Protein N-arginine methylation in adenosine dialdehyde-treated
lymphoblastoid cells. Arch Biochem Biophys. 351:53–59. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen DH, Wu KT, Hung CJ, Hsieh M and Li C:
Effects of adenosine dialdehyde treatment on in vitro and in vivo
stable protein methylation in HeLa cells. J Biochem. 136:371–376.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hung CJ, Lee YJ, Chen DH and Li C:
Proteomic analysis of methylarginine-containing proteins in HeLa
cells by two-dimensional gel electrophoresis and immunoblotting
with a methylarginine-specific antibody. Protein J. 28:139–147.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Herrmann F, Pably P, Eckerich C, Bedford
MT and Fackelmayer FO: Human protein arginine methyltransferases in
vivo - distinct properties of eight canonical members of the PRMT
family. J Cell Sci. 122:667–677. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aguirre-Gamboa R, Gomez-Rueda H,
Martínez-Ledesma E, Martínez-Torteya A, Chacolla-Huaringa R,
Rodriguez-Barrientos A, Tamez-Peña JG and Treviño V: SurvExpress:
An online biomarker validation tool and database for cancer gene
expression data using survival analysis. PLoS One. 8:e742502013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Perl AK, Wilgenbus P, Dahl U, Semb H and
Christofori G: A causal role for E-cadherin in the transition from
adenoma to carcinoma. Nature. 392:190–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li B, Liu L, Li X and Wu L: miR-503
suppresses metastasis of hepatocellular carcinoma cell by targeting
PRMT1. Biochem Biophys Res Commun. 464:982–987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou W, Yue H, Li C, Chen H and Yuan Y:
Protein arginine methyltransferase 1 promoted the growth and
migration of cancer cells in esophageal squamous cell carcinoma.
Tumour Biol. 37:2613–2619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Avasarala S, Van Scoyk M, Rathinam
Karuppusamy MK, Zerayesus S, Zhao X, Zhang W, Pergande MR, Borgia
JA, DeGregori J, Port JD, et al: PRMT1 is a novel regulator of
epithelial-mesenchymal-transition in non-small cell lung cancer. J
Biol Chem. 290:13479–13489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gao Y, Zhao Y, Zhang J, Lu Y, Liu X, Geng
P, Huang B, Zhang Y and Lu J: The dual function of PRMT1 in
modulating epithelial-mesenchymal transition and cellular
senescence in breast cancer cells through regulation of ZEB1. Sci
Rep. 6:198742016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Altan B, Yokobori T, Ide M, Mochiki E,
Toyomasu Y, Kogure N, Kimura A, Hara K, Bai T, Bao P, et al:
Nuclear PRMT1 expression is associated with poor prognosis and
chemosensitivity in gastric cancer patients. Gastric Cancer.
19:789–797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
An W, Kim J and Roeder RG: Ordered
cooperative functions of PRMT1, p300, and CARM1 in transcriptional
activation by p53. Cell. 117:735–748. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Reintjes A, Fuchs JE, Kremser L, Lindner
HH, Liedl KR, Huber LA and Valovka T: Asymmetric arginine
dimethylation of RelA provides a repressive mark to modulate
TNFα/NF-κB response. Proc Natl Acad Sci USA. 113:4326–4331. 2016.
View Article : Google Scholar : PubMed/NCBI
|