Introduction
T-cell lymphoma is the main aggressive malignancy of
T lymphocytes, which is characterized by highly heterogeneous
molecular and clinical characteristics. This type of lymphoma is
generally resistant to chemotherapy (1). Identification of the molecular
abnormalities involved in the development of T-cell lymphoma will
improve the classification and provide novel therapeutic
strategy.
Klotho was considered an anti-aging gene when it was
originally identified (2). Klotho
homozygous mutant mice (kl−/−) developed multiple
premature aging syndromes, including hypogonadism, skin atrophy,
and pulmonary emphysema (2,3). Klotho gene is located in chromosome
13q12 in human with the size of 50 kb (2). Klotho protein consists of an
extracellular domain, a single transmembrane domain and an
intracellular domain. The extracellular domain (secreted Klotho),
consisting of KL1 and KL2, could be released into the serum to
function as a circulating hormone to regulate the activity of
oxidative stress and multiple growth factor receptors (4,5).
Accumulating evidence has implicated that Klotho is
extensively downregulated in several solid malignancies (6–9).
Klotho was revealed to modulate the activity of several signaling
pathways, including the FGF signaling, insulin-like growth factor-1
receptor (IGF-1R) and Wnt pathways (5,10–12).
These pathways also play crucial roles in the development of T-cell
lymphoma (13,14). However, the role of Klotho in T-cell
lymphoma has not been reported.
In the present study, we hypothesized that Klotho
was aberrantly expressed and involved in the pathogenesis in T-cell
lymphoma. We observed decreased expression of Klotho in the patient
tissues and cell lines of T-cell lymphoma. The anti-proliferative
and pro-apoptotic effect of Klotho were further identified in
T-cell lymphoma cells. Klotho could act as an upstream regulator of
IGF-1R signaling in this disease. The data elucidated the potential
tumor suppressive role of Klotho in the development of T-cell
lymphoma. It may serve as a potent candidate for the target therapy
of T-cell lymphoma.
Materials and methods
Patients and samples
The paraffin-embedded lymph node samples were
collected from 35 newly diagnosed cases of T-cell lymphoma (21
males and 14 females; age range, 19–81 years; median age, 53 years)
and 20 normal lymph nodes. Histological diagnoses were established
according to the WHO classification (15). Normal peripheral blood mononuclear
cells (PBMCs) from healthy volunteers were isolated by Ficoll
centrifugation (TBD Science, Tianjin, China). T cells were isolated
with Nylon Wool Fiber Columns. This study was approved by the
Medical Ethics Committee of Shandong Provincial Hospital Affiliated
to Shandong University. All samples were obtained with informed
consent in accordance with the Declaration of Helsinki.
Cell lines
Jurkat and Molt-3 (T-cell acute lymphocytic leukemia
cell lines) were available from Typical Culture Preservation
Commission Cell Bank (Chinese Academic of Science, Shanghai,
China). MyLa 3676 (cutaneous T-cell lymphoma, lymphoblast, Sezary
syndrome) was retained by our laboratory. Karpas 299
(ALK+ ALCL cell line) was obtained from Shanghai Bioleaf
Biotech Co., Ltd. All the above cell lines were maintained in
PRMI-1640 (Gibco, Life Technologies, Rockville, MD, USA) containing
10% fetal bovine serum (FBS; Gibco, Life Technologies) and 1%
penicillin/streptomycin mixture.
Cell transfection
Lentivirus vectors either encoding Klotho (LV-KL) or
an empty lentiviral vector (LV-Con) were generated by GeneChem
(Shanghai, China). Lentivirus transfection of T-cell lymphoma cells
were performed according to the manufacturer's instructions.
Infection efficiencies were assessed by green fluorescent protein
(GFP) fluorescence through flow cytometry. The stably transfected
cells were selected with 5 µg/ml puromycin (Sigma-Aldrich,
USA).
Immunohistochemistry (IHC)
IHC was carried out with primary rabbit anti-Klotho
(Abcam, Cambridge, USA). 4-µM-paraffin sections were deparaffinized
and hydrated. Antigen retrieval was performed in 0.01 M sodium
citrate (pH 6.0) buffer under high-pressure. Endogenous peroxidase
was blocked with 3% H2O2 for 15 min, followed
by incubation overnight at 4°C with primary anti-Klotho (1:100).
Then the tissue sections were treated with the second antibody from
SP reagent kit (Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) for 30 min at room temperature. Bound antibody was
detected by secondary antibody and diaminobenzidine (DAB) kit
(Zhongshan Golden Bridge Biotechnology). The stained slices were
counterstained with hematoxylin and mounted. Negative control was
performed with the primary antibody replaced by PBS. IHC staining
was scored by two independent observers who were blinded to the
patient clinical data. Cases with ≥10% positive cells were
considered as positive.
Real-time quantitative polymerase
chain reaction (qRT-PCR)
For the qRT-PCR, total RNA was extracted with RNAiso
Plus (Takara, Dalian, China). The mRNA level was detected by
Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Reverse
transcription reaction was performed with reverse transcription
reagents (Takara). Expression level of mRNA was quantified using
SYBR Green Premix Ex Taq II kit (Takara) in LightCycler 480
real-time quantitative PCR system (Roche, Basel, Swizerland).
Primers for qRT-PCR are listed in Table
I. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as
an internal control and relative quantification was determined by
the 2−∆∆Ct method.
 | Table I.Primer sequences for qRT-PCR. |
Table I.
Primer sequences for qRT-PCR.
| Gene | Primer sequence |
|---|
| Klotho | F,
5′-AGCAATCTGGTCTGAATAACACTGG-3′ |
|
| R,
5′-CATGTTTCAGCGTGAAAGTTCAAAG-3′ |
| GAPDH | F,
5′-GGGAAACTGTGGCGTGAT-3′ |
|
| R,
5′-GAGTGGGTGTCGCTGTTGA-3′ |
Western blotting
For western blot analysis, cell lysates were
extracted by radio-immunoprecipitation assay (RIPA) buffer
(Shenergy Biocolor, Shanghai, China) together with phosphatase
inhibitor cocktail (PhosSTOP; Roche, Mannheim, Germany). The BCA
assay (Shenergy Biocolor) was performed to detect protein
concentration. Proteins (30 µg) were electrophoresed on SDS-PAGE
and transferred to PVDF membranes (Millipore, Billerica, MA, USA).
Membranes were blocked with 5% skim milk in Tris-buffered saline.
After incubation with primary antibodies, membranes were incubated
with HRP-conjugated secondary antibodies (Zhongshan Golden Bridge).
Proteins were visualized by electro-chemi-luminescence detection
reagents (Amersham Imager; General Electric, USA). The following
antibodies used for western blotting were purchased from Cell
Signaling Technology: p-IGF-1R, t-IGF-1R, p-AKT, t -AKT, p-ERK1/2,
t-ERK1/2, Mcl-1 and caspase-3. Klotho antibody was purchased from
Abcam. The experiments were performed in triplicate with GAPDH
(Zhongshan Golden Bridge) as endogenous control.
CCK-8 proliferation assay
The influence of Klotho on viability of T-cell
lymphoma cells were assessed by Cell Counting Kit-8 (CCK-8;
Dojindo, Kumamoto, Japan). Briefly, T-cell lymphoma cell lines
stably transfected with LV-KL or LV-Con were seeded into 96-well
plates (5,000 cells/100 µl/well, respectively) with PRMI-1640
medium supplemented with 10% FBS. Then the cells were incubated
with 10 µl/well of CCK-8 and cell viability was detected by light
absorption at 450 nm by Multiskan GO Microplate Reader (Thermo
Scientific, Rockford, IL, USA).
Cell apoptosis assay
Apoptosis of T-cell lymphoma cells were tested by
Annexin V-PE/7-aminoactinomycin (7AAD) kit (BD Biosciences,
Bedford, MA, USA) according to the manufacturer's instructions.
Cells with designed treatment were harvested and washed twice in
clod PBS and resuspended in 1X binding buffer at a concentration of
1×106 cells/ml, then 100 µl was taken and incubated with
5 µl Annexin V-PE and 5 µl 7AAD for 15 min at room temperature in
the dark for each tube. Afterwards, 400 µl of 1X binding buffer was
added and cell apoptotic rates were detected by Navios Flow
Cytometer (Beckman Coulter, CA, USA).
Immunofluoresence
T-cell lymphoma cells with designed treatment were
seeded onto glass slides by liquid thin layer cell smear (Xiaogan
Aohua, Xiaogan, China). Thereafter, the T-cell lymphoma cells were
fixed in 4% PFA and permeabilized with 0.1% Triton X-100. The cells
were blocked with normal goat serum for 1 h. Then the slides were
incubated with primary antibodies at 4°C overnight. After washing
with PBS, the DyLight 488-conjugated secondary antibodies (Abbkine,
CA, USA) were added. Slides were washed and mounted with
4′6-diamino-2-phenylindole (DAPI; Boster, Wuhan, China). Images
were examined and recorded by Nikon IX73 fluorescent
microscope.
Statistical analysis
All statistical analyses were performed by using
statistic software SPSS17.0 (SPSS Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation (mean ± SD) from three
separate experiments. Differences between groups were analyzed by
one-way analysis of variance (ANOVA) or t-tests. P<0.05 was
considered statistically significant.
Results
Klotho is downregulated in T-cell
lymphoma
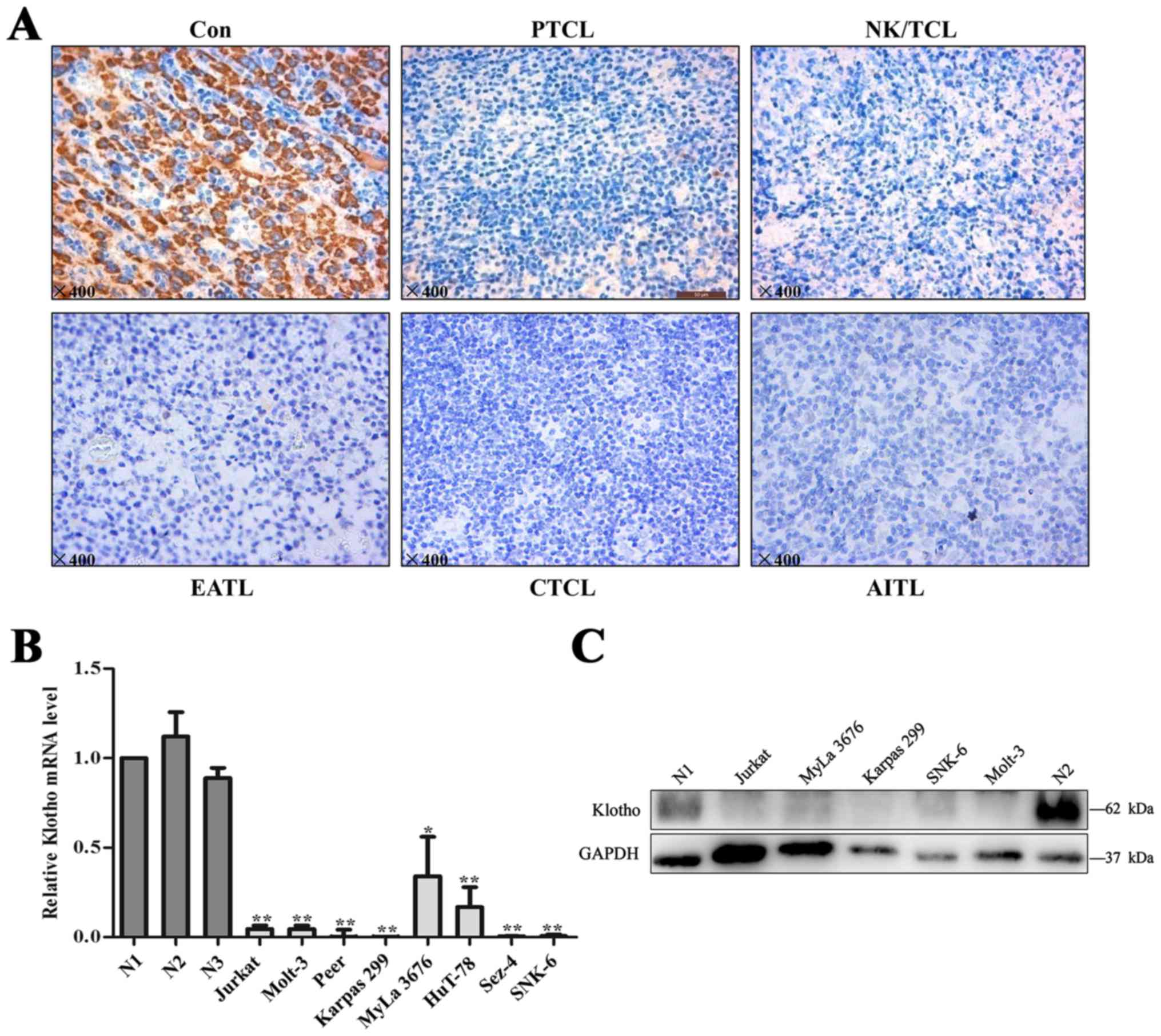
We examined Klotho expression for primary T-cell
lymphoma samples including NK/T-cell lymphoma (n=12), peripheral
T-cell lymphoma-not otherwise specified (PTCL-NOS, n=7),
angioimmunoblasic T-cell lymphoma (AITL, N=6), cutaneous T-cell
lymphoma (CTCL, n=6), and enteropathy associated T-cell lymphoma
(EATL, n=4). Compared with normal lymph nodes, expression levels of
Klotho were significantly lower in T-cell lymphoma tissues
(Fig. 1A). Klotho positive rate was
14% (5 of 35) in T-cell lymphoma tissues whereas 75% (15 of 20) in
normal lymph nodes. Cases with ≥10% positive cells were considered
as positive. More detailed data are summarized in Table II.
 | Table II.Immunohistochemical expression of
Klotho in T-cell lymphoma tissues. |
Table II.
Immunohistochemical expression of
Klotho in T-cell lymphoma tissues.
| Klotho
expression | NK/TCL (n=12) | AITL (n=6) | CTCL (n=6) | EATL (n=4) | PTCL-NOS (n=7) |
|---|
| Positive | 0
(0%) | 1 (16.7%) | 2 (33.3%) | 1 (25%) | 1 (14.3%) |
| Negative | 12 (100%) | 5 (83.3%) | 4 (66.7%) | 3 (75%) | 6 (85.7%) |
We next confirmed the expression of Klotho in T-cell
lymphoma cell lines. As shown in Fig.
1B, T-cell lymphoma cells, exhibited remarkably lower mRNA
levels of Klotho compared to the peripheral blood T lymphocytes
from healthy donors. Decreased protein levels of Klotho expression
were also noted in T-cell lymphoma cell lines (Fig. 1C).
Klotho restraines T-lymphoma cell
proliferation
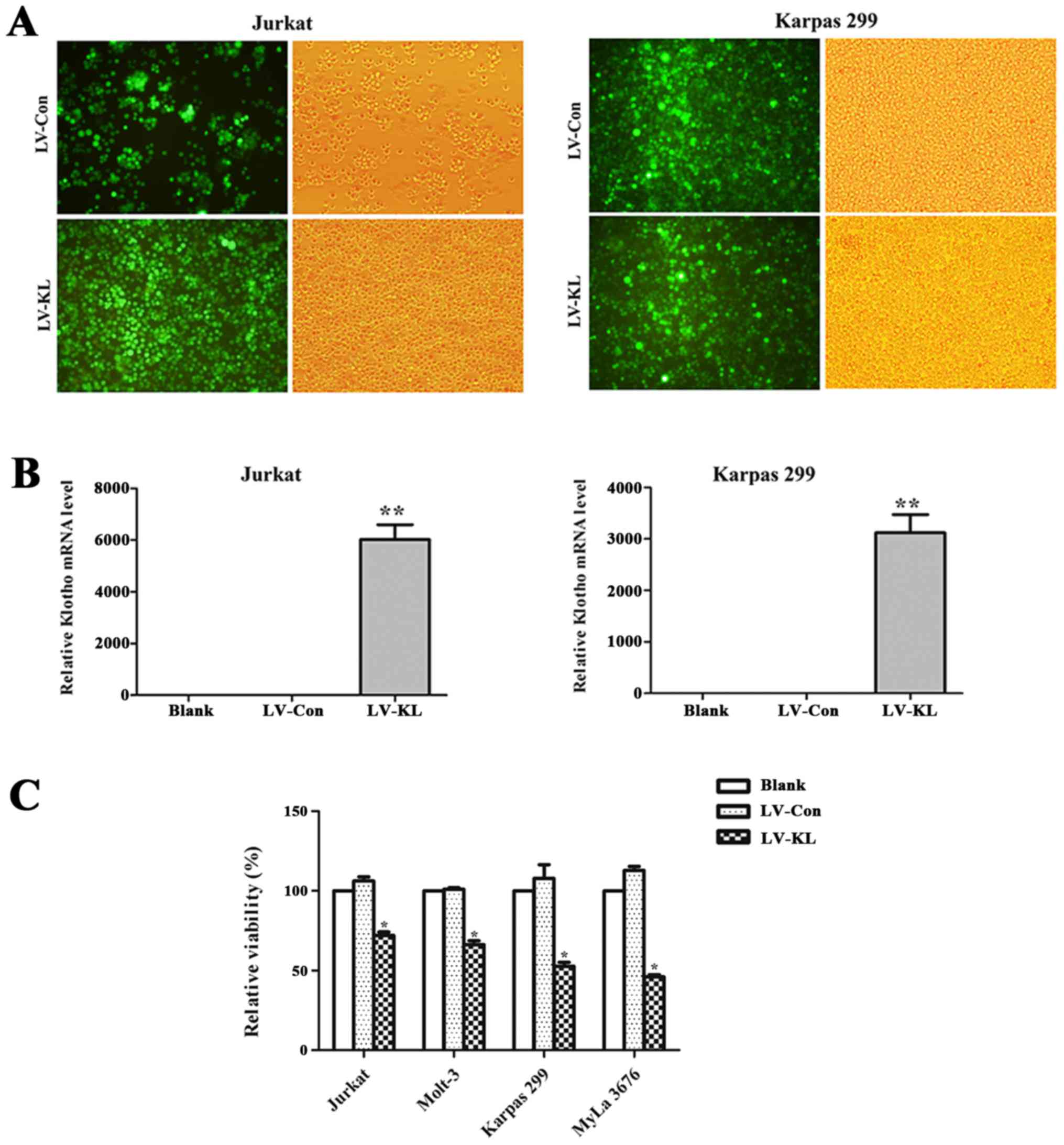
To explore the function relevance of Klotho in the
progression of T-cell lymphoma, T-cell lymphoma cell lines (Jurkat,
Molt-3, Karpas 299 and MyLa 3676) were stably transfected with
either Klotho-overexpression lentivirus vectors or empty vector
control (Fig. 2A). Upregulated
level of Klotho mRNA was confirmed by quantitative PCR (Fig. 2B). CCK-8 assay was employed to
examine the viability of the above cells. Significantly decreased
cell viability was noted in T-cell lymphoma cells transfected with
LV-KL, compared with those transfected with LV-Con (Fig. 2C). These results indicate that
Klotho inhibits the proliferation of T-cell lymphoma cells.
Klotho promotes apoptosis of
T-lymphoma cells
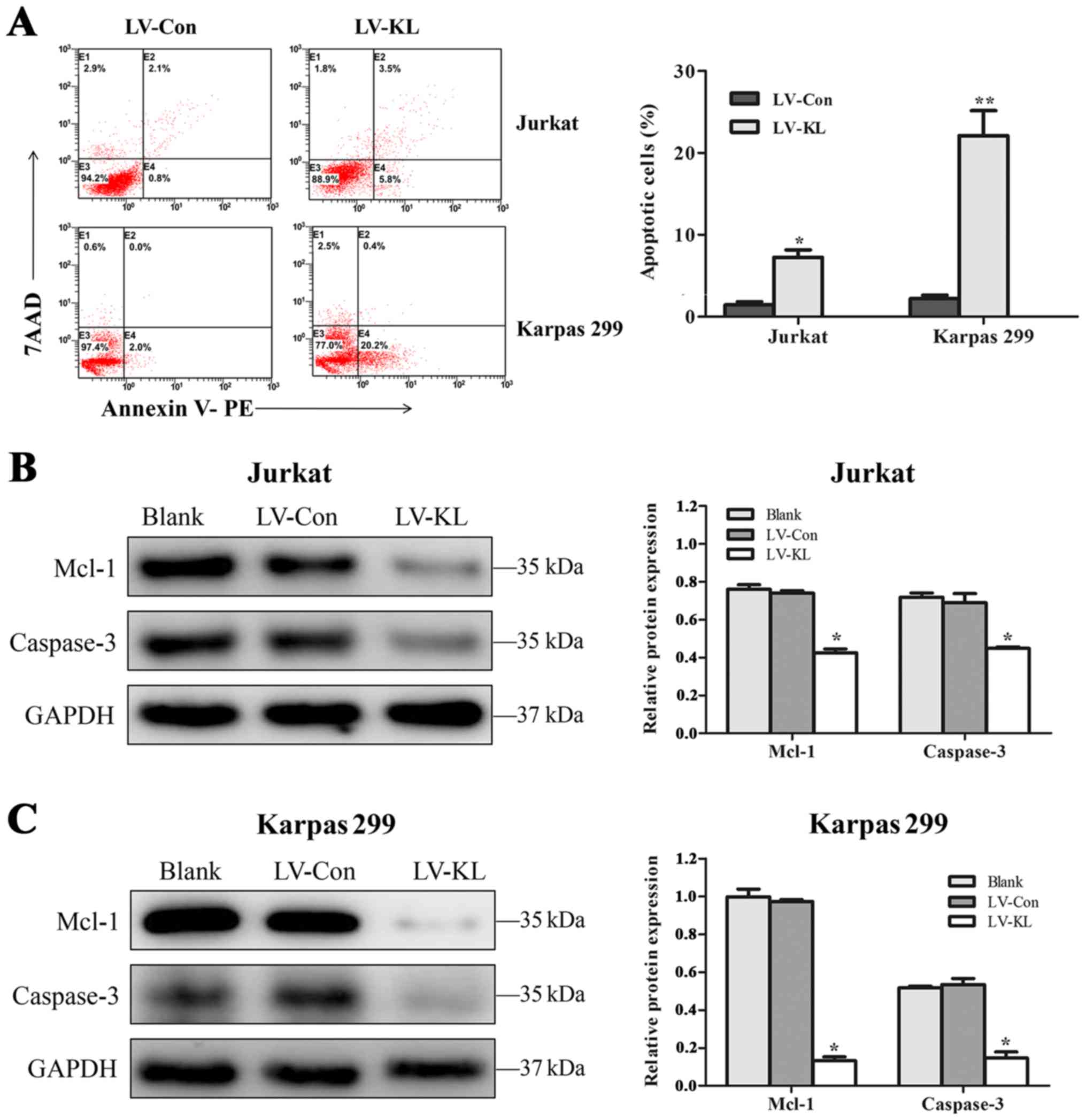
To further investigate the mechanisms underlying the
suppression of Klotho in cell proliferation by ectopic Klotho
expression, Annexin V-based apoptotic assays were performed in
Jurkat and Karpas 299 cell lines with stable LV-KL or LV-Con
transfection. Cells transfected with LV-KL exhibited enforced
apoptosis rates in T-cell lymphoma cell lines (Fig. 3A). In addition, apoptotic promotion
effect of Klotho was confirmed by western blot analysis. Remarkable
reduction of anti-apoptotic protein Mcl-1 and total caspase-3
protein was observed in Jurkat and Karpas 299 cell lines (Fig. 3B and C).
Klotho suppresses the activation of
IGF-1R signaling in T-cell lymphoma
Having elucidated that Klotho could interfere with
the viability and apoptosis of T-cell lymphoma cell lines, we next
explored the involved molecular mechanisms responsible for the
function of Klotho. The IGF-1R signaling plays significant role in
the development of T-cell lymphoma and proliferation of T-cell
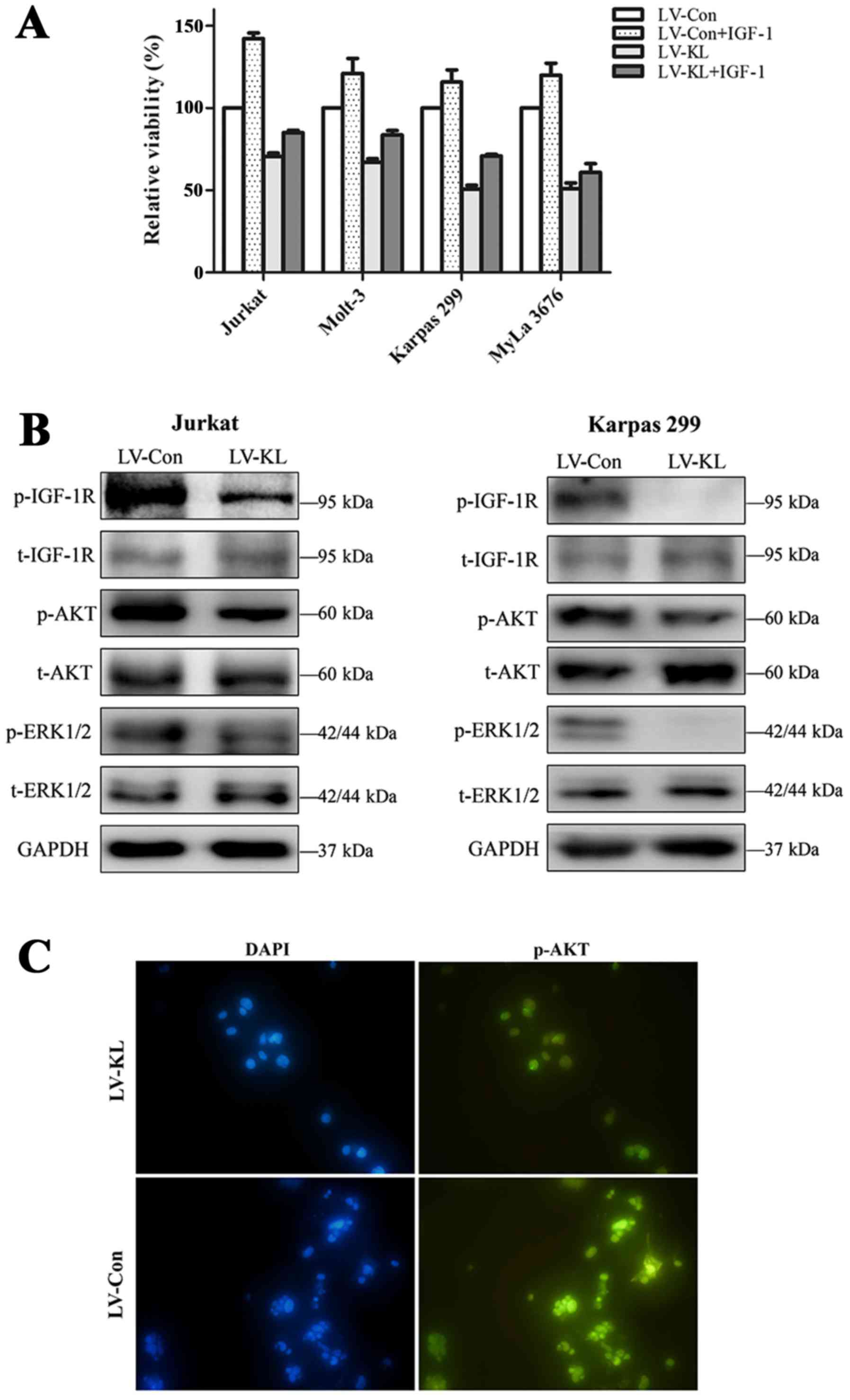
lymphoma cell lines could be induced by IGF-1 exploration. CCK-8
assay was performed to determine the effect of Klotho
overexpression on IGF-1-induced cell proliferation. T-cell lymphoma
cell lines stably transfected with either Klotho overexpressing
vector or empty control vector were incubated with IGF-1 or vehicle
control in 0.5% FBS culture medium for 48 h. In the group without
IGF-1 treatment, LV-KL transfection induced declined viability of
T-cell lymphoma cell lines compared to the empty-vector group.
Furthermore, we noted that the Klotho-induced inhibition of T-cell
viability was less reversed by IGF-1 treatment. With IGF-1
addition, cell proliferation of LV-Con treated cells increased by
up to 50%, whereas the only up to 30% enhancement of cell
proliferation was found in cells with Klotho overexpression
(Fig. 4A).
We next investigated the modulation effect of Klotho
on IGF-1R signaling in T-cell lymphoma cell lines. Jurkat and
Karpas 299 cells transfected with LV-KL revealed significantly
decreased phosphorylation level of IGF-1R. Moreover, the downstream
targets of IGF-1R signaling, including AKT and ERK1/2, were also
inhibited by Klotho overexpression (Fig. 4B and C).
Discussion
In the present study, we provide the first evidence
that Klotho as a potential tumor suppressor in the tumorigenesis of
T-cell lymphoma. The decline of Klotho initiates anti-proliferative
and pro-apoptotic effect through inhibiting IGF-1R signaling
activation. This study illuminates the tumor suppressive effect of
Klotho and highlights the potential application of Klotho in the
targeted therapy in T-cell lymphoma.
The tumor suppressive function of Klotho has been
reported in several human solid malignancies, but less in
hematological cancers, especially in lymphoma (16–19).
Lower Klotho expression was detected in T-cell lymphoma tissues
whereas high expression level of Klotho was observed in normal
lymph nodes, in accordance with recent studies showing decreased
Klotho expression in human malignancies. Recently, accumulating
evidence demonstrated several mechanisms involved in the aberrant
expression of Klotho in solid cancers, including epigenetic
mechanisms and autophagy (17,20,21).
Additionally, Klotho decline has a significant prognostic value in
cancers, such as large cell neuroendocrine carcinoma (LCNEC), small
cell lung cancer (SCLC) and hepatocellular carcinoma (22–24).
Usuda et al (22) reported
that Klotho staining provided a novel biomarker for prognosis in
patients with LCNEC, especially for those without
lymphangioinvasion or lymph node metastasis. It suggested that
evaluation of Klotho in cancer may improve the personalized
treatment in human malignancies. T-cell lymphoma is characterized
by heterogeneity in clinical, molecular and biological
presentations. The clinical diagnosis of T-cell lymphoma is primary
based on the biopsy histopathology (25). With the deepening of further
investigations, lower Klotho expression may serve as a potential
marker for the pathological diagnosis of T-cell lymphoma.
Furthermore, our data elucidated that the cell
growth of T-cell lymphoma could be restrained by Klotho.
Overexpression of Klotho inhibited the proliferation and induced
apoptosis of T-cell lymphoma cells. The results are consistent with
the studies in lung cancer (7,17).
Moreover, it has been reported that soluble Klotho and conditioned
medium from Klotho-overexpressing cells could suppress the growth
of pancreatic cancer and breast cancer cells (16,26).
However, investigations with soluble Klotho and in vivo
studies are still needed to be conducted to explore the effective
domain of Klotho protein and evaluate the safety and efficacy, and
pharmacokinectic mechanisms of Klotho in T-cell lymphoma.
Interestingly, we observed the suppressive effect of
Klotho on the activation of IGF-1R signaling in T-cell lymphoma.
Our findings are consistent with recent reports which elucidated
that Klotho could serve as a regulator of IGF-1R signaling in
cancer. Aberrant activation of IGF-1R signaling was involved in the
development and progression of T-cell lymphoma (14,27).
Moreover, structure-function analysis indicated that Klotho could
interact with the IGF-1R (28).
Klotho-induced suppression of IGF-1R pathway may acts as a novel
mechanism involved in the pathogenesis of T-cell lymphoma.
Additionally, several signaling pathways, such as Wnt signaling,
FGF23 signaling and PI3K signaling, are also reported to
participate in the biological mechanism of Klotho (16,29,30).
Further investigations are still needed to clarify the detailed
mechanism involved in the development different types of
tumors.
In conclusion, this study identified that Klotho
acted as a novel tumor suppressor in T-cell lymphoma. We elucidated
the crucial role of Klotho in inhibiting tumor cell proliferation
and inducing cell apoptosis in T-cell lymphoma. The tumor
suppressive effect of Klotho may be mediated by inhibiting the
activation IGF-1R signaling pathway. Being an endogenous
circulating hormone, the secreted Klotho could function as an
active form and inhibit the tumor growth safely and effectively in
mice. These data suggested that Klotho may serve as a hopeful
target for the development of novel therapeutic strategy of T-cell
lymphoma.
Acknowledgements
This study was supported by the National Natural
Science Foundation (nos. 81473486 and 81270598), National Public
Health Grand Research Foundation (no. 201202017), Natural Science
Foundations of Shandong Province (nos. ZR2012HZ003 and
2009ZRB14176), Technology Development Projects of Shandong Province
(nos. 2014GSF118021, 2010GSF10250 and 2008GG2NS02018), Program of
Shandong Medical Leading Talent, and Taishan Scholar Foundation of
Shandong Province.
References
|
1
|
Zhao WL: Targeted therapy in T-cell
malignancies: Dysregulation of the cellular signaling pathways.
Leukemia. 24:13–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi
H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et
al: Mutation of the mouse klotho gene leads to a syndrome
resembling ageing. Nature. 390:45–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurosu H, Yamamoto M, Clark JD, Pastor JV,
Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M,
Kawaguchi H, et al: Suppression of aging in mice by the hormone
Klotho. Science. 309:1829–1833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuro-o M: Klotho and aging. Biochim
Biophys Acta. 1790:1049–1058. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto M, Clark JD, Pastor JV, Gurnani
P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt
KP, et al: Regulation of oxidative stress by the anti-aging hormone
klotho. J Biol Chem. 280:38029–38034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou X and Wang X: Klotho: A novel
biomarker for cancer. J Cancer Res Clin Oncol. 141:961–969. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai D, Wang Q, Li X, Liu J, Ma X and Xu W:
Klotho inhibits human follicular thyroid cancer cell growth and
promotes apoptosis through regulation of the expression of
stanniocalcin-1. Oncol Rep. 35:552–558. 2016.PubMed/NCBI
|
|
8
|
Rubinek T, Shulman M, Israeli S, Bose S,
Avraham A, Zundelevich A, Evron E, Gal-Yam EN, Kaufman B and Wolf
I: Epigenetic silencing of the tumor suppressor klotho in human
breast cancer. Breast Cancer Res Treat. 133:649–657. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang B, Kim J, Jeong D, Jeong Y, Jeon S,
Jung SI, Yang Y, Kim KI, Lim JS, Kim C, et al: Klotho inhibits the
capacity of cell migration and invasion in cervical cancer. Oncol
Rep. 28:1022–1028. 2012.PubMed/NCBI
|
|
10
|
Yaktapour N, Ubelhart R, Schuler J, Aumann
K, Dierks C, Burger M, Pfeifer D, Jumaa H, Veelken H, Brummer T, et
al: Insulin-like growth factor-1 receptor (IGF1R) as a novel target
in chronic lymphocytic leukemia. Blood. 122:1621–1633. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vishwamitra D, Shi P, Wilson D, Manshouri
R, Vega F, Schlette EJ and Amin HM: Expression and effects of
inhibition of type I in sulin-like growth factor receptor tyrosine
kinase in mantle cell lymphoma. Haematologica. 96:871–880. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mathur R, Sehgal L, Braun FK, Berkova Z,
Romaguerra J, Wang M, Rodriguez MA, Fayad L, Neelapu SS and
Samaniego F: Targeting Wnt pathway in mantle cell
lymphoma-initiating cells. J Hematol Oncol. 8:632015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Groen RW, Oud ME, Schilder-Tol EJ,
Overdijk MB, ten Berge D, Nusse R, Spaargaren M and Pals ST:
Illegitimate WNT pathway activation by beta-catenin mutation or
autocrine stimulation in T-cell malignancies. Cancer Res.
68:6969–6977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Z, Fang Z, Zhen H, Zhou L, Amin HM
and Shi P: Inhibition of type I insulin-like growth factor receptor
tyrosine kinase by picropodophyllin induces apoptosis and cell
cycle arrest in T lymphoblastic leukemia/lymphoma. Leuk Lymphoma.
55:1876–1883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaffe ES: The 2008 WHO classification of
lymphomas: Implications for clinical practice and translational
research. Hematology (Am Soc Hematol Educ Program). 2009:523–531.
2009.
|
|
16
|
Abramovitz L, Rubinek T, Ligumsky H, Bose
S, Barshack I, Avivi C, Kaufman B and Wolf I: KL1 internal repeat
mediates klotho tumor suppressor activities and inhibits bFGF and
IGF-I signaling in pancreatic cancer. Clin Cancer Res.
17:4254–4266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen B, Ma X, Liu S, Zhao W and Wu J:
Inhibition of lung cancer cells growth, motility and induction of
apoptosis by Klotho, a novel secreted Wnt antagonist, in a
dose-dependent manner. Cancer Biol Ther. 13:1221–1228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li XX, Huang LY, Peng JJ, Liang L, Shi DB,
Zheng HT and Cai SJ: Klotho suppresses growth and invasion of colon
cancer cells through inhibition of IGF1R-mediated PI3K/AKT pathway.
Int J Oncol. 45:611–618. 2014.PubMed/NCBI
|
|
19
|
Aviel-Ronen S, Rubinek T, Zadok O, Vituri
A, Avivi C, Wolf I and Barshack I: Klotho expression in cervical
cancer: Differential expression in adenocarcinoma and squamous cell
carcinoma. J Clin Pathol. 69:53–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee J, Jeong DJ, Kim J, Lee S, Park JH,
Chang B, Jung SI, Yi L, Han Y, Yang Y, et al: The anti-aging gene
Klotho is a novel target for epigenetic silencing in human cervical
carcinoma. Mol Cancer. 9:1092010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen T, Ren H, Thakur A, Yang T, Li Y,
Zhang S, Wang T and Chen M: Decreased level of klotho contributes
to drug resistance in lung cancer cells: Involving in
klotho-mediated cell autophagy. DNA Cell Biol. 35:751–757. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Usuda J, Ichinose S, Ishizumi T, Ohtani K,
Inoue T, Saji H, Kakihana M, Kajiwara N, Uchida O, Nomura M, et al:
Klotho is a novel biomarker for good survival in resected large
cell neuroendocrine carcinoma of the lung. Lung Cancer. 72:355–359.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie B, Zhou J, Yuan L, Ren F, Liu DC, Li Q
and Shu G: Epigenetic silencing of Klotho expression correlates
with poor prognosis of human hepatocellular carcinoma. Hum Pathol.
44:795–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Usuda J, Ichinose S, Ishizumi T, Ohtani K,
Inoue T, Saji H, Kakihana M, Kajiwara N, Uchida O, Nomura M, et al:
Klotho predicts good clinical outcome in patients with
limited-disease small cell lung cancer who received surgery. Lung
Cancer. 74:332–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tilly H, da Gomes Silva M, Vitolo U, Jack
A, Meignan M, Lopez-Guillermo A, Walewski J, André M, Johnson PW,
Pfreundschuh M, et al: ESMO Guidelines Committee: Diffuse large
B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 26:(Suppl 5).
v116–v125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wolf I, Levanon-Cohen S, Bose S, Ligumsky
H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP,
et al: Klotho: A tumor suppressor and a modulator of the IGF-1 and
FGF pathways in human breast cancer. Oncogene. 27:7094–7105. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vishwamitra D, Curry CV, Alkan S, Song YH,
Gallick GE, Kaseb AO, Shi P and Amin HM: The transcription factors
Ik-1 and MZF1 downregulate IGF-IR expression in NPM-ALK(+) T-cell
lymphoma. Mol Cancer. 14:532015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ligumsky H, Rubinek T, Merenbakh-Lamin K,
Yeheskel A, Sertchook R, Shahmoon S, Aviel-Ronen S and Wolf I:
Tumor suppressor activity of klotho in breast cancer is revealed by
structure-function analysis. Mol Cancer Res. 13:1398–1407. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun H, Gao Y, Lu K, Zhao G, Li X, Li Z and
Chang H: Overexpression of Klotho suppresses liver cancer
progression and induces cell apoptosis by negatively regulating
wnt/β-catenin signaling pathway. World J Surg Oncol. 13:3072015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu Y, Xu L, Zhang J, Xu W, Liu Y, Yin H,
Lv T, An H, Liu L, He H, et al: Klotho suppresses tumor progression
via inhibiting PI3K/Akt/GSK3β/Snail signaling in renal cell
carcinoma. Cancer Sci. 104:663–671. 2013. View Article : Google Scholar : PubMed/NCBI
|