Introduction
Renal cell carcinoma (RCC) is the most common
neoplasm of the kidney in adults accounting for ~2–3% of all adult
malignancies and 80–90% of primary malignant renal tumors (1,2).
Currently, surgical resection is considered the first choice for
treating RCC when possible and multiple treatments can be used
together. However, relapse occurs in 20–40% of patients after
curative nephrectomy, and most patients with RCC are relatively
refractory to both systemic chemotherapy and radiotherapy (2). Moreover, the absence of biomarkers for
the early detection and follow-up of the disease complicate the
promptness and accuracy of diagnosis. Therefore, it is important to
develop novel tumor markers that have higher sensitivity and
reliability and effective therapeutic methods for treating RCC.
Tumor angiogenesis has been indicated to be a
promising target for developing effective treatments for cancer
patients. To date, many pro-angiogenic factors and angiogenic
inhibitors have been identified, including growth factors,
cytokines and proteases (3). Among
the pro-angiogenic factors, vascular endothelial growth factor
(VEGF), platelet-derived growth factor (PDGF) and hypoxia-inducible
factor (HIF) families have been demonstrated to play important
roles in mediating tumor angiogenesis, and are associated with
tumor progression, invasion, metastasis and poor survival of
patients with RCC (4–7). However, the role of angiogenic
inhibitors in RCC development is poorly understood.
Vasohibin-1 (VASH1), as a novel endothelium-derived
inhibitor of angiogenesis, has been recently identified (8). VASH1 expression is induced in response
to angiogenic stimuli such as VEGF-A and fibroblast growth factor
(FGF)-2 and can inhibit angiogenesis in an autocrine manner
(8,9). Studies have demonstrated that VASH1
plays important roles in disease-induced angiogenesis (10,11),
and malignancies including breast cancer, gynecological and
hepatocellular carcinoma (HCC), gastric, lung and colon cancer
(12–18). In addition, various preliminary
studies have demonstrated that VASH1 exerts an antitumor effect by
inhibiting angiogenesis in the tumor environment (19–22).
However, the expression pattern of VASH1 and its potential clinical
and biological roles in RCC have not been elucidated.
In our previous study, we firstly investigated the
expression pattern of VASH1 in RCC samples by immunohistochemistry.
We found that VASH1 was expressed in both RCC tissues and adjacent
non-tumorous renal tissues (ANRT). VASH1 expression was reduced in
the RCC tissues compared to that in ANRT, and its expression showed
a correlation with clinicopathological features of RCC (23). Based on the expression pattern of
VASH1 in RCC, we postulated that VASH1 may not only act as an
intrinsic angiogenesis inhibitor produced by ECs, but also plays a
critical role in regulating angiogenesis as an extrinsic factor
secreted by other cells in RCC.
In the present study, we sought to determine whether
VASH1 decreased angiogenesis and suppressed tumor growth in RCC. We
demonstrated that overexpression of VASH1 effectively inhibited
cell proliferation, arrested the cell cycle in the G0/G1 phase and
promoted cell apoptosis in HUVECs and 786–0 cells in vitro
and inhibited the subcutaneous growth of 786–0 tumors in
vivo. Therefore, according to knowledge based on other
angiogenesis inhibitors, our findings have implications for the
potential use of VASH1 as a candidate molecular-targeted therapy
for patients with RCC.
Materials and methods
Cell culture and cell
transfection
Human umbilical vein endothelial cells (HUVECs) were
obtained from the Tianjin Institute of Urology. HUVECs were
maintained at 37°C in a humidified atmosphere of 5% CO2
in RPMI-1640 medium (Gibco, Grand Island, NY, USA), supplemented
with 10% fetal bovine serum (FBS) (Gibco, Montevideo, Uruguay),
penicillin (100 U/ml) and streptomycin (100 µg/ml). The human renal
carcinoma cell line 786–0 was obtained from Tianjin Institute of
Urology. 786–0 cells were maintained at 37°C in a humidified
atmosphere of 5% CO2 in RPMI-1640 medium, supplemented
with 10% FBS (both from Gibco), penicillin (100 U/ml) and
streptomycin (100 µg/ml). Plasmid pReceiver-M61-VASH1 was
successfully constructed in our laboratory at the Tianjin Institute
of Urology. Each cell line was transiently transfected using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), and an empty
vector was used to ensure equal content in transfections.
Western blot analysis
Forty-eight hours after transfection, the VASH1
protein expression in HUVECs and 786–0 cells was determined using
western blot analysis. Cells were rinsed twice with cold
phosphate-buffered saline (PBS), then, homogenized in RIPA cell
lysate and centrifuged. The protein concentration of each sample
was quantified by the Bradford assay. Total cell extract protein
(50 mg) was separated by 10% SDS-PAGE and transferred onto
polyvinylidene fluoride (PVDF) membrane. After being blocked with
5% skimmed milk for 1 h at room temperature, the membrane was
incubated with goat anti-vasohibin-1 (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) antibody overnight at 4°C. After extensive
washes, blots were incubated with a dilution (1:2,000) of
horseradish peroxidase-conjugated anti-goat or anti-goat IgG (Santa
Cruz Biotechnology) for 90 min at 37°C. The bands were developed
with 3,3′-diaminobenzidine (DAB). GAPDH (Santa Cruz Biotechnology)
was used as an internal control. We used the ratio of the grey
value of VASH1 and GAPDH as a variable for statistical
analysis.
Cell proliferation assay
The effect of VASH1 on the proliferation of HUVECs
and 786–0 cells was measured using MTT assay. Briefly, the cells
were seeded in 96-well plates at a density of 1×105/well
and cultured for 24 h. Then, the cells were divided into
pReceiver-M61-VASH1, pReceiver-M61 or blank control group. After
transfection at different time points, 20 µl of 5 mg/ml MTT
solution was added to each well. After 4 h of incubation at 37°C,
the supernatant was removed and 150 µl of dimethyl sulfoxide (DMSO)
was added to each well, and shaken for 10 min. Absorbance of each
well was measured on a microplate reader at a wavelength of 490 nm.
All experiments were carried out in triplicate.
Cell cycle assay
The effect of VASH1 on the cell cycle distribution
of HUVECs and 786–0 cells was identified using flow cytometric
analysis. The cells were plated in 6-well plates at a density of
1×105/well and cultured at 37°C in a 5% CO2
incubator for 24 h. Then, the cells were divided into a
pReceiver-M61-VASH1, pReceiver-M61 or blank control group.
Forty-eight hours after transfection, the adherent cells were
collected by 0.25% trypsinization, washed in PBS and centrifugated.
Cells were resuspended at 1×106 cells/ml in PBS and
fixed in ice-cold ethanol overnight at 4°C. Fixed cells were
centrifuged and washed once with PBS. Each sample was resuspended
in propidium iodide (PI) solution (33 µg/ml PI, 0.13 mg/ml RNase A,
10 mmol/l EDTA, 0.5% Triton X-100). Samples were analyzed using a
fluorescence-activated cell sorting (FACS) flow cytometer (BD
Biosciences, San Jose, CA, USA), and DNA histograms were analyzed
with modified software. Each test was repeated in triplicate.
Cell apoptosis assay
The effect of VASH1 on the apoptosis of HUVECs and
786–0 cells was identified using flow cytometric analysis. Cells
were plated in 6-well plates at a density of 1×105/well
and cultured at 37°C in a 5% CO2 incubator for 24 h.
Then, they were divided into a pReceiver-M61-VASH1, pReceiver-M61
or blank control group. Forty-eight hours after transfection, the
adherent cells were collected by 0.25% trypsinization, washed in
PBS and centrifuged and resuspended at 1×106 cells/ml in
PBS. Then, the cells were stained with FITC-labeled Annexin V (10
µl) (20 µg/ml) and PI (5 µl) (50 µg/ml) and immediately analyzed
using a fluorescence-activated cell sorting (FACS) flow cytometer
(BD Biosciences). The results were analyzed with modified software.
Each test was repeated in triplicate.
Cell invasion assay
The effect of VASH1 on 786–0 cell invasion was
assessed using a 24-well Transwell insert (pore size, 8 µm;
Corning, Corning, NY, USA). The cells were divided into
pReceiver-M61-VASH1, pReceiver-M61 or blank control group.
Forty-eight hours after transfection, the cells were collected by
0.25% trypsinization, washed in PBS and centrifuged and resuspended
at 1×106 cells/ml. Cells were starved in serum-free
medium overnight, and 2×104 cells/well were resuspended
in 200 µl serum-free medium and placed in the upper chamber with
8-µm filter pores. The membrane undersurface was coated with
Matrigel (BD Biosciences) mixed with RPMI-1640 serum-free medium in
a 1:5 dilution for 30 min at 37°C. The lower chamber was filled
with 600 µl 10% FBS as the chemoattractant. After incubation for 48
h, non-migrated/non-invaded cells were removed from the upper well
with cotton swabs while the migrated/invaded cells were then fixed
with methanol, stained with 0.1% crystal violet, and photographed
(magnification, ×200) in five independent fields for each well.
Each test was repeated in triplicate.
In vivo antitumoral activity
Six-week-old male BALB/c nude mice were purchased
from the Animal Resources Centre, Military Medical Sciences
(Beijing, China). All animal experiments were approved by the
Tianjin Medical University (Tianjin, China) Ethics Committee, and
carried out in accordance with the NIH Guide for the Care and Use
of Laboratory Animals. All animals were kept under specific
pathogen-free, temperature-controlled conditions and handled in
accordance with the Institutional Animal Welfare Guidelines. Mice
were randomly divided into three groups (n=6): one group received
an injection of 786–0 cells transfected with pReceiver-M61-VASH1;
one group received an injection of 786–0 cells transfected with
pReceiver-M61 as the negative control; another group received an
injection of 786–0 cells as a non-treated control group. Cells
(5×106) in a volume of 200 µl were injected into the
left flank area of the nude mice. The tumor volume was measured
with a caliper at day 7, 14, 21, 28 and 35, respectively. The tumor
volume (V)was calculated at regular intervals according to the
formula: V = π/6 × length × width2. These three groups
were treated for 35 days. Afterward, the mice were sacrificed, and
tumors were extracted.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 16.0). The significance of differences between
multiple groups was determined by one-way analysis of variance.
Data are expressed as means ± standard deviation. Statistical
significance was set at P<0.05.
Results
Expression of VASH1 after transfection
of the HUVECs and 786–0 cells
To evaluate whether the recombinant plasmids were
successfully transfected into the HUVECs and 786–0 cells and
whether VASH1 protein was expressed, we investigated the protein
expression of VASH1 in HUVECs and 786–0 cells after transfection
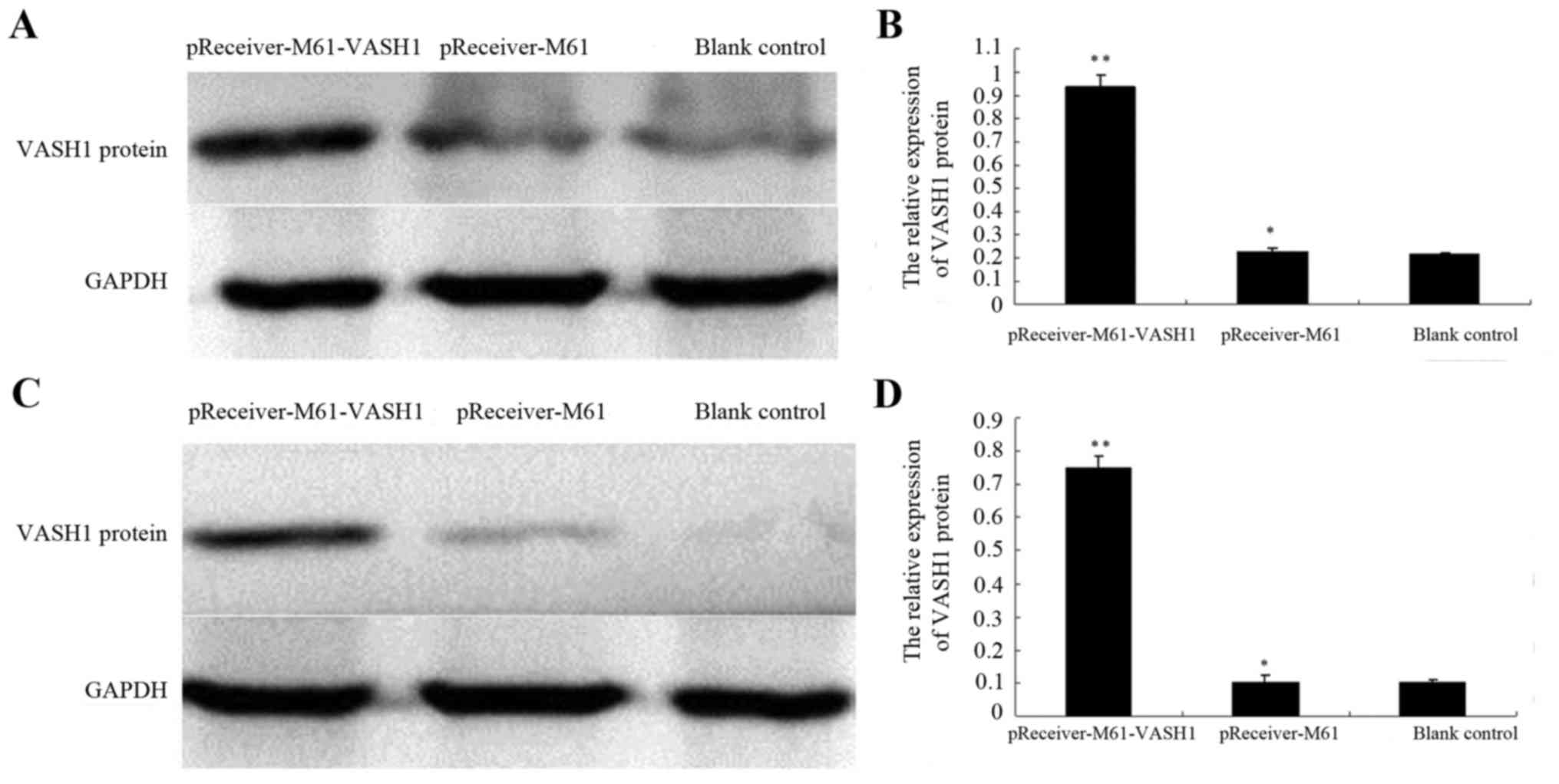
with pReceiver-M61-VASH1. In the HUVECs, the relative protein
expression of VASH1 (0.936±0.053) was significantly higher in the
pReceiver-M61-VASH1 group, when compared with the level in the
blank control (0.214±0.008) and pReceiver-M61 group (0.227±0.013)
(P<0.05) (Fig. 1A and B). In the
786–0 cells, the relative protein expression of VASH1 (0.751±0.035)
was significantly higher in the pReceiver-M61-VASH1 group, when
compared with the level in the blank control (0.104±0.007) and
pReceiver-M61 group (0.102±0.024) (P<0.05) (Fig. 1C and D). These results showed that
transfection with pReceiver-M61-VASH1 increased the VASH1 protein
level in the HUVECs and 786–0 cells.
Effect of VASH1 on the cell growth of
HUVECs and 786–0 cells
To investigate the effect of VASH1 overexpression on
the cell growth of HUVECs and 786–0 cells, the level of cell
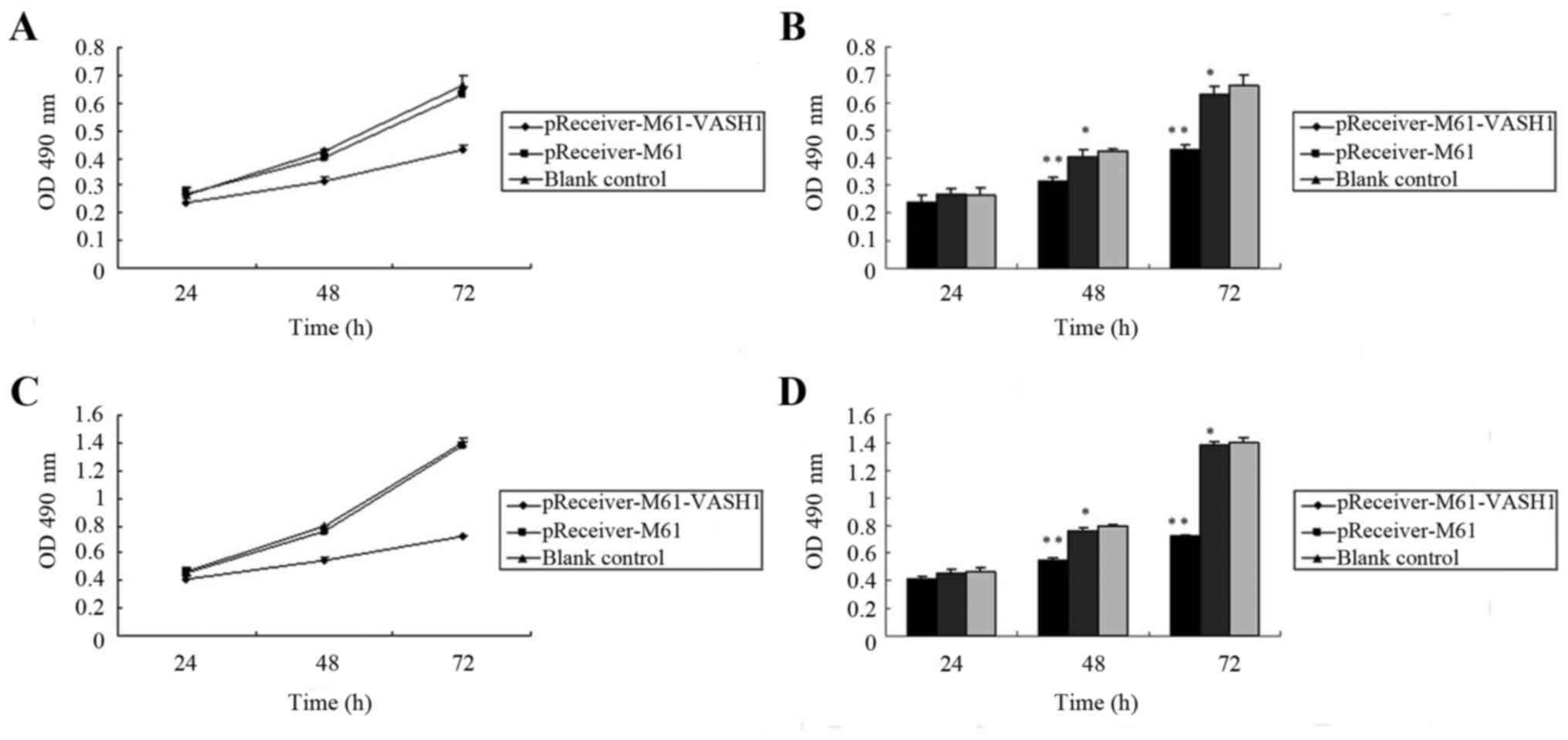
proliferation was assessed by MTT assay after transfection. In
HUVECs, the optical density (OD) of the pReceiver-M61-VASH1,
pReceiver-M61 and blank control group at 24 h after transfection
was 0.239±0.026, 0.269±0.021 and 0.265±0.028, respectively. There
was no significant difference among the three groups (P>0.05).
The OD of the pReceiver-M61-VASH1, pReceiver-M61 and blank control
group 48 h after transfection was 0.315±0.012, 0.403±0.026 and
0.423±0.010, respectively. The OD of the pReceiver-M61-VASH1,
pReceiver-M61 and blank control group at 72 h after transfection
was 0.431±0.017, 0.632±0.025, 0.661±0.039, respectively. The OD of
the pReceiver-M61-VASH1 group was significantly lower compared to
that of the pReceiver-M61 or blank control group at 48 and 72 h
after transfection (P<0.05). There was no significant difference
between the pReceiver-M61 and blank control group (P>0.05)
(Fig. 2A and B). In the 786–0
cells, the OD of the pReceiver-M61-VASH1, pReceiver-M61 and blank
control group at 24 h after transfection was 0.411±0.016,
0.453±0.029 and 0.469±0.028, respectively. There was no significant
difference among the three groups (P>0.05). The OD of the
pReceiver-M61-VASH1, pReceiver-M61 and blank control group at 48 h
after transfection was 0.547±0.022, 0.756±0.026 and 0.792±0.010,
respectively. The OD of the pReceiver-M61-VASH1, pReceiver-M61 and
blank control group at 72 h after transfection was 0.718±0.017,
1.384±0.025 and 1.396±0.039. The OD of the pReceiver-M61-VASH1
group was significantly lower compared to that of the pReceiver-M61
or blank control group at 48 and 72 h after transfection
(P<0.05). There was no significant difference between the
pReceiver-M61 and blank control group (P>0.05) (Fig. 2C and D). These results showed that
overexpression of VASH1 significantly reduced the growth of HUVECs
and 786–0 cells in vitro.
Effect of VASH1 on the cell cycle
distribution of HUVECs and 786–0 cells
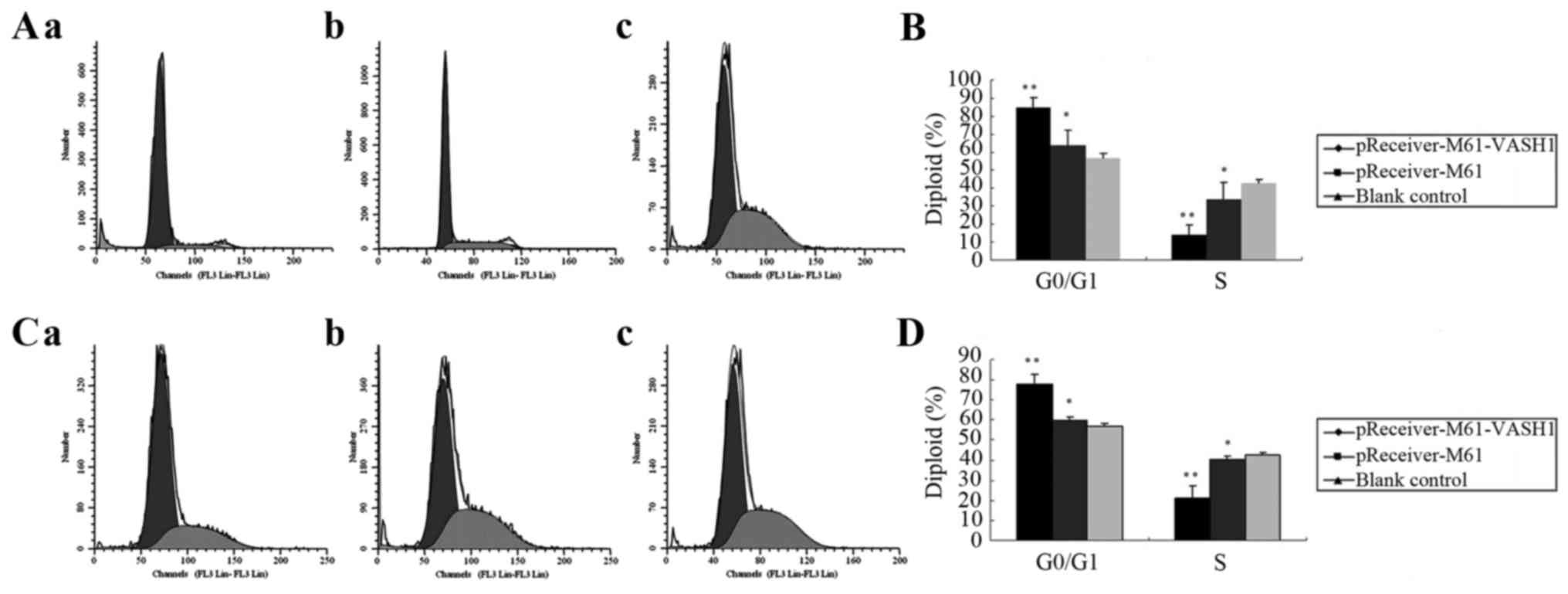
The effect of VASH1 on the cell cycle distribution
of HUVECs and 786–0 cells after transfection was analyzed by flow
cytometric analysis. At 48 h after transfection of HUVECs, the
pReceiver-M61-VASH1 transfection group had an increase in the G0/G1
phase cells (84.90±5.42%) (P<0.05) as compared with the
pReceiver-M61 (63.68±8.62%) and blank control group (56.89±2.35%),
and a decrease in S phase cells (13.99±5.39%) (P<0.05) as
compared with the pReceiver-M61 (33.90±9.34%) and blank control
group (42.52±2.45%), respectively (Fig.
3A and B). At 48 h after transfection in the 786–0 cells, the
pReceiver-M61-VASH1 group exhibited an increase in G0/G1 phase
cells (77.91±4.89%) (P<0.05) as compared with the pReceiver-M61
(59.55±2.00%) and blank control group (57.05±1.33)%, and a decrease
in S phase cells (21.27±5.67%) (P<0.05) as compared with the
pReceiver-M61 (40.13±1.73%) and blank control group (42.60±1.28%),
respectively (Fig. 3C and D). These
results showed that overexpression of VASH1 had an effect on the
cell cycle distribution of HUVECs and 786–0 cells. VASH1 arrested
the cell cycle of the HUVECs and 786–0 cells in the G0/G1 phase
in vitro.
Effect of VASH1 on the apoptosis of
HUVECs and 786–0 cells
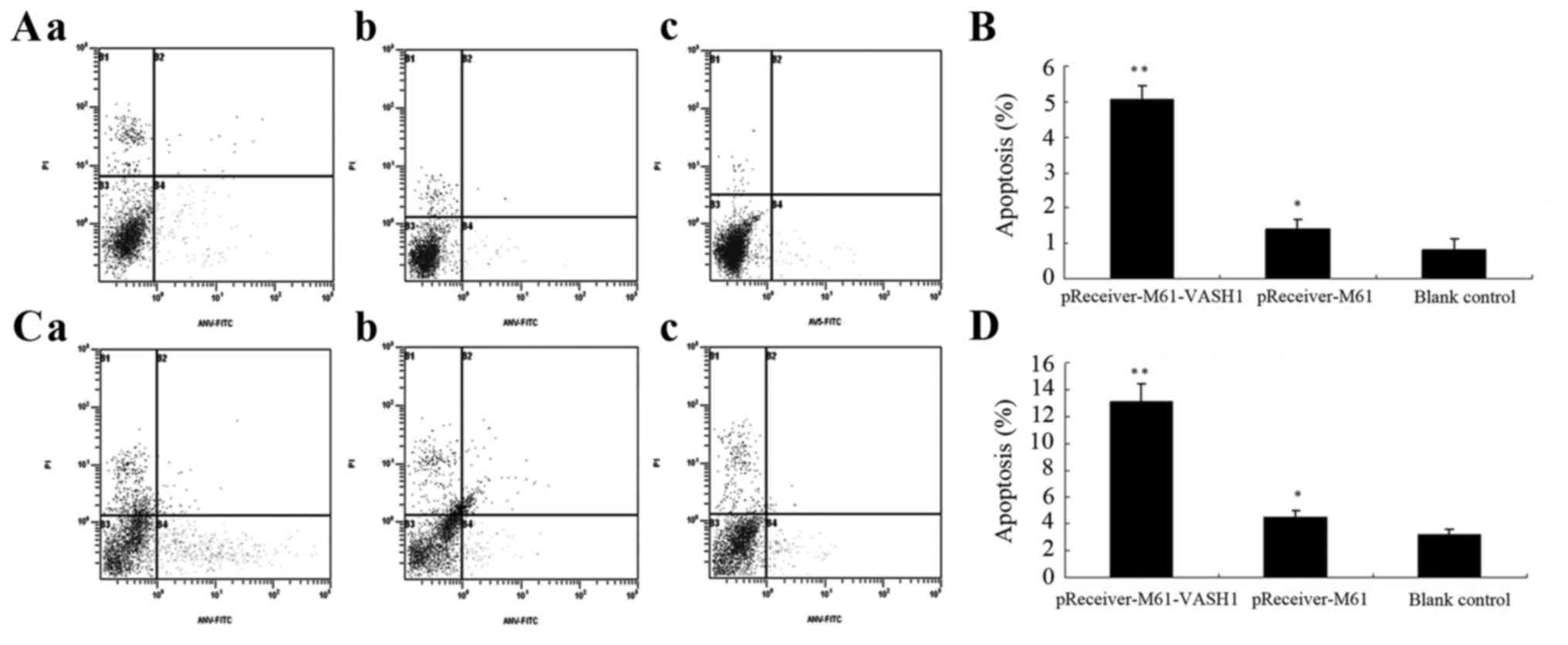
The effect of VASH1 on cell apoptosis in the HUVECs
and 786–0 cells after transfection was examined by FCM. At 48 h
after transfection of the HUVECs, the percentage of apoptotic cells
in the pReceiver-M61-VASH1 group was significantly higher
(5.06±0.39%) (P<0.05) than that in the pReceiver-M61
(1.41±0.26%) and blank control group (0.83±0.31%), while there was
no statistically significant difference between that in the
pReceiver-M61 and the blank control group (P>0.05) (Fig. 4A and B). At 48 h after transfection
in the 786–0 cells, the percentage of apoptotic cells in the
pReceiver-M61-VASH1 group was significant higher (13.09±1.39%)
(P<0.05) than that in the pReceiver-M61 (4.47±0.48%) and blank
control group (3.24±0.39%), while there was no statistically
significant difference between that in the pReceiver-M61 and blank
control group (P>0.05) (Fig. 4C and
D). These results showed that overexpression of VASH1
significantly promoted cell apoptosis in the HUVECs and 786–0 cells
in vitro.
Effect of VASH1 on 786–0 cell
invasion
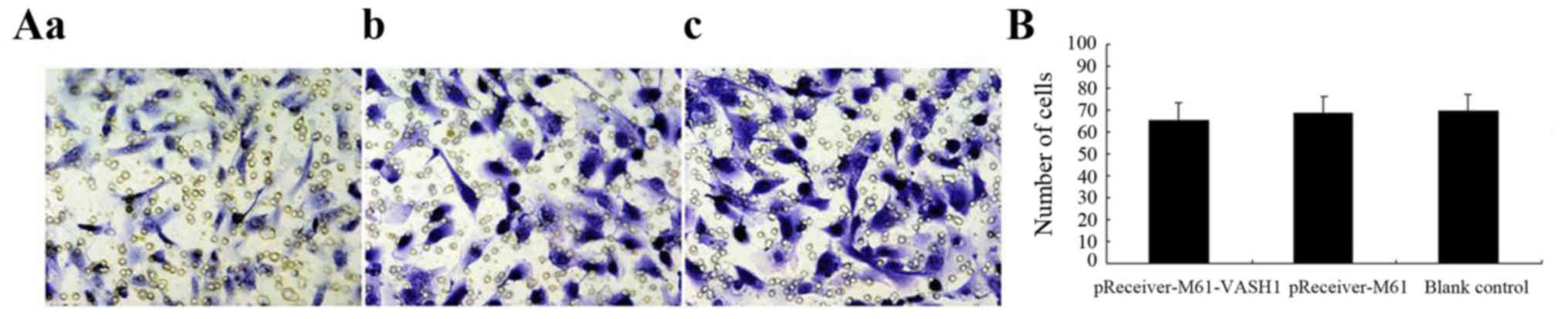
The effect of VASH1 on the invasive ability of the
786–0 cells after transfection was assessed by Transwell assay. At
48 h after transfection, the number of 786–0 cells passing through
the EC Matrix gel in the pReceiver-M61-VASH1 group did not show
significant differences (65.33±8.16) compared with that in the
pReceiver-M61 (68.87±7.44) and blank control group (69.53±7.59)
(P>0.05) (Fig. 5A and B). These
results showed that overexpression of VASH1 did not reduce the
invasiveness of the 786–0 cells in vitro.
Overexpression of VASH1 inhibits 786–0
tumor growth in vivo
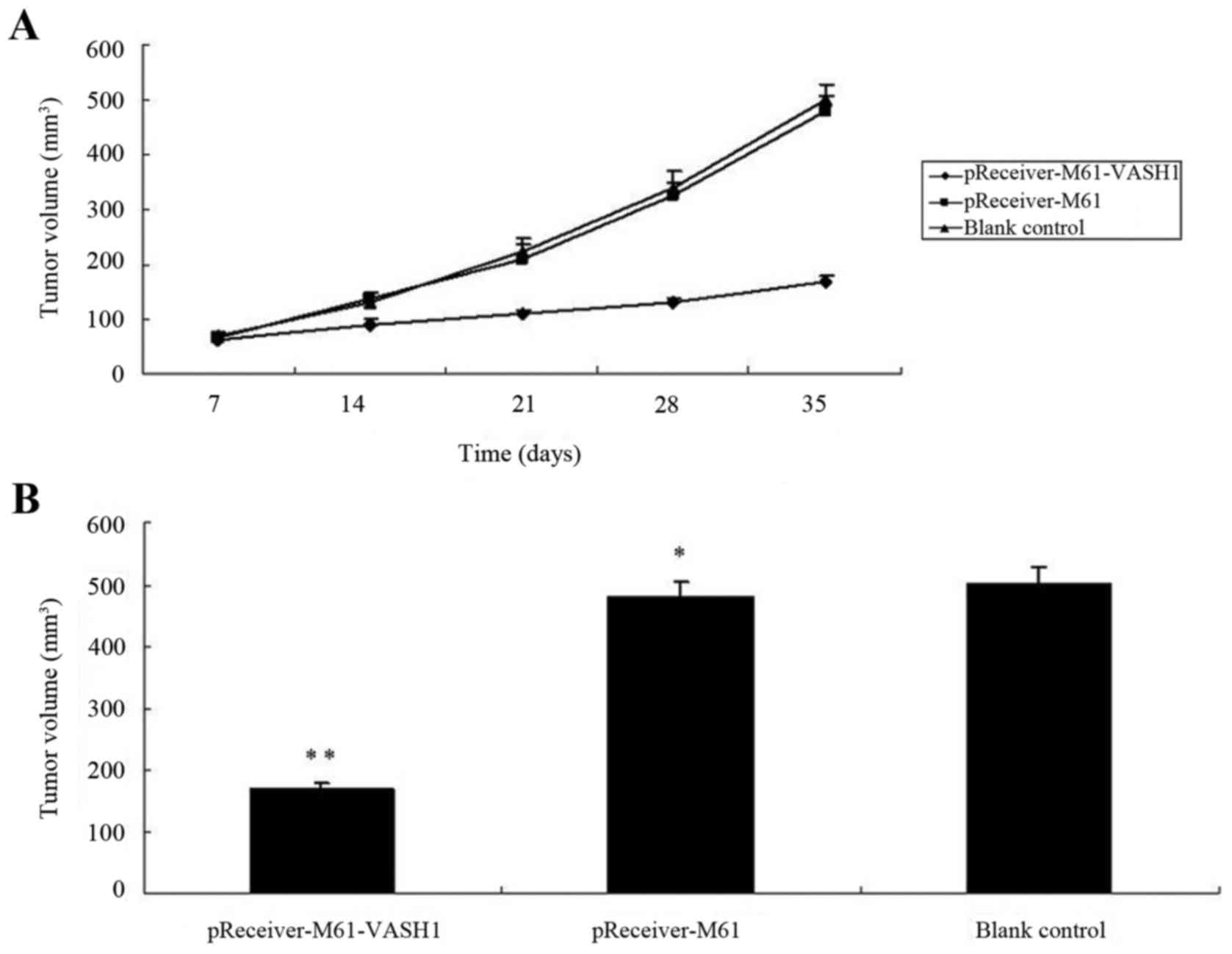
To evaluate the efficacy of VASH1 overexpression in
suppressing 786–0 tumor growth in an animal model, the tumor volume
was measured. All mice survived during the period of treatment.
After 35 days, the tumor volume of the pReceiver-M61-VASH1 group
(168.23±25.33 mm3) was significantly smaller than that
for the pReceiver-M61 and blank control group (478.83±27.32 and
500.67±28.21 mm3, respectively) (P<0.05), while there
was no statistically significant difference between the
pReceiver-M61 and blank control group (P>0.05). The tumor growth
curves indicated significant growth inhibition in the
pReceiver-M61-VASH1 group after 21 days, but no difference between
the pReceiver-M61 and blank control group was noted (Fig. 6A and B). These results showed that
overexpression of VASH1 inhibited the subcutaneous growth of 786–0
tumors in vivo.
Discussion
Recently, angiogenesis has been indicated to be a
pivotal event in various biological processes, and it plays a
critical role in physiological and pathological processes
particularly in multiple types of cancers (24,25).
Renal cell carcinoma (RCC) is a solid tumor that arises from the
proximal convoluted tubules of the kidney and is characterized by
abundant angiogenesis (26). In
view of the resistance of RCC to most existing cytotoxic drugs or
radiotherapy, therapies targeting the various molecules involving
angiogenesis may be highly desirable. Tumor angiogenesis is induced
when ‘angiogenic homeostasis’ is disrupted in tumor tissues
(24). Therefore, it is reasonable
to hypothesize that when we reconstruct the balance by applying
agents to block stimulators or by applying exogenous angiogenesis
inhibitors directly, the process of angiogenesis may be
inhibited.
Vasohibin-1 (VASH1), as a novel endothelium-derived
inhibitor of angiogenesis, has been recently identified (8). Its expression is induced in response
to angiogenic stimuli such as VEGF-A, fibroblast growth factor
(FGF)-2 and can inhibit angiogenesis in an autocrine manner
(8,9). Several previous studies have
demonstrated that VASH1 as an angiogenesis inhibitor plays
important roles in disease-induced angiogenesis such as nephropathy
(10), retinal disease (11) and malignancies including breast
cancer, gynecological and hepatocellular carcinoma (HCC), gastric,
colon and lung cancer (12–18). In addition, various preliminary
studies have demonstrated that VASH1 exerts an antitumor effect by
inhibiting angiogenesis in the tumor environment (19–22).
Previous studies have shown that VASH1 is
selectively expressed in the ECs of tumor tissue, and the
expression of VASH1 in tumor tissue was found to be significantly
increased when compared to that noted in normal tissue.
Furthermore, the increase in expression of VASH1 strongly
correlates with the advancement of the degree of malignancy
(12–14,16,17).
However, we investigated the expression pattern of VASH1 and the
association with clinicopathological features in RCC in our
previous study (23). We found that
VASH1 was expressed mainly in the cytoplasm and membrane of tumor
cells and partly in vascular endothelial cells in RCC. In ANRT, it
was mainly expressed in the cytoplasm and membrane of renal tubular
epithelial cells and partly in vascular endothelial cells and
glomerular mesangial cells. Based on the above findings and the
literature which indicated that VASH1 mRNA is also expressed in a
wide range of tissues and organs (27,28),
we hypothesized that VASH1 may not only act as an intrinsic
angiogenesis inhibitor produced by ECs, but also may function as an
extrinsic factor secreted by other cells to regulate the process of
angiogenesis in RCC. In addition, we found that the expression
level of VASH1 in RCC tissue was significant lower than that in
ANRT and was significantly reduced with the increased degree of
malignancy in RCC tissues. In addition, a significantly negative
correlation was noted between VASH1 expression and HIF-1α
expression and a significantly negative correlation was noted
between VASH1 expression and MVD in RCC (23). Therefore, VASH1 expression is
reduced and is associated with clinicopathological features in RCC.
Considering the seemingly paradoxical observations compared to that
in other types of tumors, we presume that it may result from the
difference in histological origin and the cancer type. It may also
be attributed to the complexity and distinctiveness of the
secretory pathway of VASH1 in different cancers.
In the present study, we investigated the biological
effects of VASH1 by evaluating the effects of VASH1 on cell
proliferation, cell cycle, cell apoptosis and cell invasion in
HUVECs and 786–0 cells and evaluating the effect of VASH1 on the
growth of 786–0 tumors in nude mice. MTT assay demonstrated that
overexpression of VASH1 significantly reduced the growth of HUVECs
and 786–0 cells in vitro. Flow cytometric analysis revealed
that overexpression of VASH1 had an effect on the cell cycle
distribution of HUVECs and 786–0 cells; VASH1 overexpression was
able to arrest HUVECs and 786–0 cells in the G0/G1 phase in
vitro. We also found that VASH1 overexpression significantly
promoted HUVEC and 786–0 cell apoptosis in vitro.
Nevertheless, overexpression of VASH1 did not reduce 786–0 cell
invasion in vitro. Furthermore, a strong antitumor effect of
VASH1 in vivo was observed, as tumor growth in nude mice
with xenografts was significantly suppressed. Therefore, we
hypothesized that disruption of ‘angiogenic homeostasis’ results in
abundant angiogenesis, and the reduction in VASH1 expression in RCC
tissues may partially explain the reason why there is abundant
angiogenesis in RCC. In the present study by applying exogenous
VASH1 directly, the process of angiogenesis in RCC was
inhibited.
In recent years, molecular-targeted therapy has been
clinically applied. Tyrosine kinase inhibitors (TKIs) including
bevacizumab, sunitinib, sorafenib, pazopanib, axitinib and mTOR
inhibitors such as temsirolimus and everolimus have achieved
favorable clinical responses and have been administered as
first-line or second-line therapy for patients with RCC (29). Nevertheless, since various
angiogenic factors are involved in tumor angiogenesis, only
targeting a single angiogenic factor may likely be ineffective.
Therefore, to achieve sufficient therapeutic benefit, it may be
necessary to simultaneously target multiple angiogenic factors. It
was recently reported that VASH1 inhibits angiogenesis mediated by
various angiogenic factors other than VEGF suggesting that VASH1,
which acts alone to inhibit multiple angiogenic factors, is a more
effective therapeutic agent compared with VEGF inhibitors in terms
of improving patient survival (20).
Anti-angiogenic therapy has been approved for
several types of cancers and several drugs are in clinical use.
However, this type of drugs may have side-effects including
hypertension or proteinuria due to the impairment of normal
quiescent vessels. It was recently reported that VASH1 did not
increase mean blood pressure and urinary albumin excretion in
animal experiments (30). It has
also been reported that VASH1 did not affect any morphological
changes in normal blood vessels (31), wound healing, body weight and
peripheral blood flow (32) in
adenoviral VASH1 gene-treated mice. These findings all suggest that
VASH1 could be a potential candidate anti-angiogenic therapy with
fewer or less side-effects.
In summary, here we present both in vitro and
in vivo evidence that VASH1 effectively inhibits the cell
proliferation, arrests the cell cycle in the G0/G1 phase and
promotes cell apoptosis in HUVECs and 786–0 cells and could
suppress the subcutaneous growth of 786–0 tumors. Therefore,
according to our knowledge of angiogenesis inhibitors, VASH1 could
potentially be utilized as a candidate for molecular-targeted
therapy for patients with RCC. Nonetheless, the effect of VASH1 on
RCC needs to be verified in other RCC cell lines and investigation
of the inhibitory effects of VASH1 on tumor models in situ
is warranted. In addition, further studies are required to clarify
the underlying inhibitory mechanisms and the targets of signal
transduction pathways of anti-angiogenesis in RCC.
Acknowledgements
The present study was supported by grants from the
Tianjin Medical University General Hospital Youth Foundation (award
no. ZYYFY2014034; grant recipient, G.Z.).
References
|
1
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 73:1119–1132. 2009. View Article : Google Scholar
|
|
2
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qian CN, Huang D, Wondergem B and Teh BT:
Complexity of tumor vasculature in clear cell renal cell carcinoma.
Cancer. 115:(Suppl 10). S2282–S2289. 2009. View Article : Google Scholar
|
|
5
|
Xu L, Tong R, Cochran DM and Jain RK:
Blocking platelet-derived growth factor-D/platelet-derived growth
factor receptor beta signaling inhibits human renal cell carcinoma
progression in an orthotopic mouse model. Cancer Res. 65:5711–5719.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sulzbacher I, Birner P, Träxler M,
Marberger M and Haitel A: Expression of platelet-derived growth
factor-alpha alpha receptor is associated with tumor progression in
clear cell renal cell carcinoma. Am J Clin Pathol. 120:107–112.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dorević G, Matusan-Ilijas K, Babarović E,
Hadzisejdić I, Grahovac M, Grahovac B and Jonjić N: Hypoxia
inducible factor-1α correlates with vascular endothelial growth
factor A and C indicating worse prognosis in clear cell renal cell
carcinoma. J Exp Clin Cancer Res. 28:402009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watanabe K, Hasegawa Y, Yamashita H,
Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, et al:
Vasohibin as an endothelium-derived negative feedback regulator of
angiogenesis. J Clin Invest. 114:898–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sonoda H, Ohta H, Watanabe K, Yamashita H,
Kimura H and Sato Y: Multiple processing forms and their biological
activities of a novel angiogenesis inhibitor vasohibin. Biochem
Biophys Res Commun. 342:640–646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nasu T, Maeshima Y, Kinomura M,
Hirokoshi-Kawahara K, Tanabe K, Sugiyama H, Sonoda H, Sato Y and
Makino H: Vasohibin-1, a negative feedback regulator of
angiogenesis, ameliorates renal alterations in a mouse model of
diabetic nephropathy. Diabetes. 58:2365–2375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen J, Yang X, Xiao WH, Hackett SF, Sato
Y and Campochiaro PA: Vasohibin is up-regulated by VEGF in the
retina and suppresses VEGF receptor 2 and retinal
neovascularization. FASEB J. 20:723–725. 2006.PubMed/NCBI
|
|
12
|
Tamaki K, Moriya T, Sato Y, Ishida T,
Maruo Y, Yoshinaga K, Ohuchi N and Sasano H: Vasohibin-1 in human
breast carcinoma: A potential negative feedback regulator of
angiogenesis. Cancer Sci. 100:88–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshinaga K, Ito K, Moriya T, Nagase S,
Takano T, Niikura H, Yaegashi N and Sato Y: Expression of vasohibin
as a novel endothelium-derived angiogenesis inhibitor in
endometrial cancer. Cancer Sci. 99:914–919. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshinaga K, Ito K, Moriya T, Nagase S,
Takano T, Niikura H, Sasano H, Yaegashi N and Sato Y: Roles of
intrinsic angiogenesis inhibitor, vasohibin, in cervical
carcinomas. Cancer Sci. 102:446–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hosaka T, Kimura H, Heishi T, Suzuki Y,
Miyashita H, Ohta H, Sonoda H, Moriya T, Suzuki S, Kondo T, et al:
Vasohibin-1 expression in endothelium of tumor blood vessels
regulates angiogenesis. Am J Pathol. 175:430–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Tian X, Zhang C and Wang Q:
Upregulation of vasohibin-1 expression with angiogenesis and poor
prognosis of hepatocellular carcinoma after curative surgery. Med
Oncol. 29:2727–2736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen Z, Kauttu T, Seppänen H, Vainionpää
S, Ye Y, Wang S, Mustonen H and Puolakkainen P: Vasohibin-1 and
vasohibin-2 expression in gastric cancer cells and TAMs. Med Oncol.
29:2718–2726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang T, Yu TT, Zhang DM, Hou XM, Liu XJ,
Zhao D and Shan L: Vasohibin-1 expression detected by
immunohistochemistry correlates with prognosis in non-small cell
lung cancer. Med Oncol. 31:9632014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu S, Han B, Zhang Q, Dou J, Wang F, Lin
W, Sun Y and Peng G: Vasohibin-1 suppresses colon cancer.
Oncotarget. 6:7880–7898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi Y, Saga Y, Koyanagi T, Takei Y,
Machida S, Taneichi A, Mizukami H, Sato Y, Matsubara S and Fujiwara
H: The angiogenesis regulator vasohibin-1 inhibits ovarian cancer
growth and peritoneal dissemination and prolongs host survival. Int
J Oncol. 47:2057–2063. 2015.PubMed/NCBI
|
|
21
|
Takahashi Y, Saga Y, Koyanagi T, Takei Y,
Machida S, Taneichi A, Mizukami H, Sato Y, Matsubara S and Fujiwara
H: Vasohibin-1 expression inhibits advancement of ovarian cancer
producing various angiogenic factors. Cancer Sci. 107:629–637.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen Z, Yan Y, Ye C, Wang B, Jiang K, Ye
Y, Mustonen H, Puolakkainen P and Wang S: The effect of Vasohibin-1
expression and tumor-associated macrophages on the angiogenesis in
vitro and in vivo. Tumour Biol. 37:7267–7276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao G, Yang Y, Tang Y, Han R and Sun Y:
Reduced expression of vasohibin-1 is associated with
clinicopathological features in renal cell carcinoma. Med Oncol.
29:3325–3334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:(Suppl 16). S15–S18. 2002.
View Article : Google Scholar
|
|
26
|
Mancilla-Jimenez R, Stanley RJ and Blath
RA: Papillary renal cell carcinoma: A clinical, radiologic, and
pathologic study of 34 cases. Cancer. 38:2469–2480. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nimmagadda S, Geetha-Loganathan P, Pröls
F, Scaal M, Christ B and Huang R: Expression pattern of
Vasohibin during chick development. Dev Dyn. 236:1358–1362.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Naito H, Kidoya H, Sato Y and Takakura N:
Induction and expression of anti-angiogenic vasohibins in the
hematopoietic stem/progenitor cell population. J Biochem.
145:653–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vitale MG and Cartenì G: Clinical
management of metastatic kidney cancer: The role of new molecular
drugs. Future Oncol. 12:83–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyashita H, Suzuki H, Ohkuchi A and Sato
Y: Mutual balance between vasohibin-1 and soluble VEGFR-1 in
endothelial cells. Pharmaceuticals. 4:782–793. 2011. View Article : Google Scholar :
|
|
31
|
Heishi T, Hosaka T, Suzuki Y, Miyashita H,
Oike Y, Takahashi T, Nakamura T, Arioka S, Mitsuda Y, Takakura T,
et al: Endogenous angiogenesis inhibitor vasohibin1 exhibits
broad-spectrum antilymphangiogenic activity and suppresses lymph
node metastasis. Am J Pathol. 176:1950–1958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li D, Zhou K, Wang S, Shi Z and Yang Z:
Recombinant adenovirus encoding vasohibin prevents tumor
angiogenesis and inhibits tumor growth. Cancer Sci. 101:448–452.
2010. View Article : Google Scholar : PubMed/NCBI
|