Introduction
Gastric cancer (GC) is the fourth most common cancer
and the second leading cause of cancer-related deaths worldwide
(1). Widespread metastasis is a
major factor accounting for the poor prognosis and outcome of GC.
Metastasis is considered a complex, multi-step process whereby
cancer cells migrate from the primary location to a distant organ
(2). This process initiates when
primary tumor cells invade their surrounding tissues, followed by
tumor cells entering into the blood system (intravasation),
translocating through the vasculature, penetrating from the blood
stream (extravasation) into the adjacent parenchyma tissue, forming
micrometastases and finally proliferating to become secondary gross
tumors (3). Many molecular
regulators involved in this process have been identified, among
which microRNAs (miRNAs) often plays vital roles (4).
miRNAs are small non-coding RNA molecules and play
important roles in regulating the expression of various genes by
targeting mRNA through translational suppression or cleavage
(5). Numerous studies have
indicated that these short RNAs (usually 19–25 nucleotides in
length) are involved in various biological processes including cell
differentiation, proliferation, apoptosis, stress resistance, fat
metabolism, tumorigenesis, as well as tumor metastasis (6–8).
Numerous studies have revealed that miR-31 expression was
specifically attenuated in metastatic breast cancer cells and could
inhibit breast cancer metastasis by targeting multiple genes
(9). Furthermore, Wang et al
found that miR-31 may play an important role in colon cancer
metastasis (10). We previously
revealed that miR-31 was significantly downregulated in GC tissues
through microarray analysis. However, its role in the metastasis of
GC has remained largely unknown (11).
In the present study, we investigated the role of
miR-31 in the metastasis of GC as well as its underlying
mechanisms. We examined the expression of miR-31 in GC and studied
its role in GC metastasis by both in vitro and in
vivo analysis. We further examined the association of miR-31
expression with lymph node metastasis and found that low expression
of miR-31 was associated with lymph node metastasis, poor pN stage
and invasion into lymphatic vessels. Furthermore, we used
bioinformatics analysis and the luciferase reporter assay to
identify the potential target of miR-31. The present study, to the
best of our knowledge, evaluated for the first time the role of
miR-31 in the metastasis of GC using detailed data.
Materials and methods
Human tissue samples
Seventy-eight pairs of human GC and non-tumor
adjacent tissues were obtained from patients who underwent surgical
resection at the Chinese PLA General Hospital between 2012 and
2014. All of the samples were clinically and pathologically
determined to be correctly labeled and frozen in liquid nitrogen
and stored at −80°C. No systemic or local treatment was given to
these patients before the surgery. The histological grade of the
tumor was evaluated based on the World Health Organization (WHO)
criteria and patients were staged according to
tumor-node-metastasis (TNM) staging of the International Union
Against Cancer (UICC)/American Joint Committee on Cancer (AJCC)
System (2002). The present study was approved by the Research
Ethics Committee of the Chinese PLA General Hospital. Informed
consent was obtained from all the patients who provided
samples.
Cell culture
The human GC cell lines BGC-823, MGC-803, SGC-7901
and AGS, as well as the normal gastric epithelium cell line GES-1
were obtained from the Chinese Academy of Sciences (Beijing, China)
and were all maintained in our own laboratory and cultured in
RPMI-1640 medium or Dulbecco's modified Eagles medium (DMEM) (both
from Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco, Grand Island, NY, USA) in a humidified 5%
carbon dioxide incubator at 37°C.
RNA extraction and real-time
polymerase chain reaction (RT-PCR)
Total RNA was extracted from tissues or cultured
cells using the mirVana miRNA Isolation kit (Ambion, Foster City,
CA, USA) based on the manufacturer's instructions. The poly(A) tail
was added to the RNA using the Poly(A) Tailing kit (Ambion).
Complementary DNA (cDNA) was synthesized using the TaqMan Reverse
Transcription kit (Applied Biosystems, Foster City, CA, USA).
Real-time PCR analyses were performed using the TaqMan Micro-RNA
Assay kit (Applied Biosystems). miRNAs expression was calculated
relative to U6 small nuclear RNA. Changes in the expression were
calculated by the ΔΔCt method (12). The relative expression ratio of
miR-31 was expressed as the fold change normalized to the
endogenous reference (U6snRNA), and also relative to the
non-tumorous controls (adjacent non-tumor tissues and the GES-1
cell line). Therefore, a relative expression ratio of <1.0 was
considered to be a low expression level, and a ratio >1.0 was
considered to be a high expression level. The miRNA primer was
purchased from Ambion. The PCR procedure and data analysis were
performed using an iCycler (Bio-Rad, Hercules, CA, USA). Each
sample was assessed in triplicate. The miR-31 primer sequence was
as follows: 5′-AGGCAAGATGCTGGCATAGCT-3′; and the U6-RNA primer
sequence was, 5′-TGACACGCAAATTCGTGAAG-3′. All protocols were
performed according to the manufacturer's instructions.
RNA oligoribonucleotides and cell
transfection
RNA oligoribonucleotides and their corresponding
normal controls (NCs) were purchased from RiboBio Co., Ltd.
(Guangzhou, China). The cell lines were cultured to 50% confluence
after being transferred into 6-well plates and were then
transfected with a final concentration of 100 nM of RNA mimics or
200 nM of inhibitor and their corresponding NCs using Lipofectamine
2000 (Invitrogen) based on the manufacturer's instructions. The
cells were harvested for further experiments at 48 h
post-transfection.
In vitro migration/invasion assay
The migratory and invasive abilities of the cell
lines were detected using Transwell assay. The Transwells (8-µm
pore size; Corning Costar Corp., Corning, NY, USA) were placed into
new 24-well plates. For the Transwell migration assay,
2.5×104 cells were plated in the top chamber lined with
a non-coated membrane. For the invasion assay, chamber inserts were
coated with 35 µl of Matrigel (1:4 dilution) to form the basement
membrane and were incubated for 4 h at 37°C. Furthermore,
5×104 cells were plated in the top chamber. In both
assays, the cells were suspended in medium without serum in the
lower chamber which was used as a chemoattractant. After incubation
at 37°C with 5% CO2 in an incubator, cotton swabs were
used to wipe off the upper layer of the Matrigel. After being fixed
with 95% absolute alcohol and stained with
46-diamidino-2-phenylindole (DAPI), the number of cells capable of
migrating to the lower chamber was thus calculated by inverted
microscopy (Olympus Corp., Tokyo, Japan) at a magnification of ×200
in over 10 random fields for each well of the plate, and therefore
the mean of the number of cells in each visual field represented
the invasion ability of the cells. Each experiment was conducted in
triplicate.
Scratch wound-healing assays
The cells were cultured into 6-well plates at a
density of 3×105 cells/well. After being cultured for 24
h at 37°C in an incubator with 5% CO2, the cells were
then transfected with miR-31 mimics and NC. A straight-line scratch
was made on the bottom of the cell culture plate using a sterile
200-µl yellow pipette tip 5 h post-transfection. Fresh and complete
media were added, and the wound healing ability was observed for 24
h. Images were obtained every 8 h.
In vivo metastasis assay
After transfection with the miR-31 mimics or stable
NC using trypsin, SGC-7901 cell lines were harvested from tissue
culture flasks and were washed three times with phosphate-buffered
saline. Next, 5×105 cells/l were suspended in 0.2 ml of
serum-free RPMI-1640 medium and were injected into the lateral tail
vein of each mouse (six in each group, female BALB/c, 6–8 weeks of
age). The mice were sacrificed five weeks post-injection. The
number of visible tumor lesions on the lung surface was counted.
The lung tissues were then cut into serial sections, fixed with
phosphate-buffered neutral formalin, stained with hematoxylin and
eosin and then examined histologically. Nude mice were manipulated
and cared for according to the NIH Animal Care and Committee
guidelines of the Experimental Animal Center of the Chinese PLA
General Hospital.
Bioinformatics analysis
The miRNA target predictions were obtained from
PicTar (http://pictar.mdc-berlin.de/),
TargetScan 7.0 (http://www.targetscan.org/vert-70/) and miRDB
(http://mirdb.org/miRDB). The overlapping targets
were further studied using the Expression Analysis Systematic
Explorer (EASE) based on the Gene Ontology database.
Vector construction and luciferase
reporter assay
Luciferase reporters were constructed based on the
firefly luciferase-expressing vector pGL3-control (Promega,
Madison, WI, USA). To construct the pGL3-RhoA-3 untranslated region
(3′UTR), a partial 3′UTR of the RhoA segment of human RhoA mRNA
(GenBank accession no. NM_001664) containing the putative miR-31
binding sites was thus amplified and cloned into the pGL3-control
vector. The following primers were used for the amplification of
RhoA: forward, 5′-GGCTGCCATCCGGAAGAAA-3′; and reverse,
5′-CACAAGACAAGGCACCCAGA-3′. In addition, we constructed a
luciferase reporter that had DNA segments with scrambled target
sites to miR-31 as a positive control.
SGC-7901 cells were transfected using Lipofectamine
2000 in 24-well plates based on the manufacturer's instructions,
with 0.8 µg of the firefly luciferase reporter vector and 0.08 µg
of the control vector containing Renilla luciferase, pRL-TK
(Promega). miR-31 (40 nM) or NC was used for each well. Firefly and
Renilla luciferase activities were detected using a
Dual-Luciferase Reporter Assay (Promega) 24 h post-transfection
using the Centro LB 960 system (Berthold Technologies, Bad Wildbad,
Germany).
Western blotting
Total protein was extracted from the cultured cells
using the total protein extraction kit based on the manufacturer's
instructions (KeyGen Biotech Co., Ltd., Nanjing, China). Proteins
were separated by 8% SDS polyacrylamide gels and were then
transferred to polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA) electrophoretically. The membranes were blocked
with 5% non-fat milk in TBS with 0.05% Tween-20 (TBST) at 37°C for
2 h. Antibodies targeting against RhoA (1:200; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and β-actin (1:4,000;
Sigma-Aldrich, St. Louis, MO, USA) were used. The proteins were
visualized using an enhanced chemiluminescence (ECL) kit (Pierce,
Rockford, IL, USA) and MF-Chemi BIS 3.2 Pro (Micro Photonics,
Allentown, PA, USA) with GelCapture software (DNR Bio-Imaging
Systems, Ltd., Jerusalem, Israel). The intensity of protein
fragments was quantified by FluorChem 2.01 software (Alpha
Innotech, San Leandro, CA, USA).
Statistical analysis
Data are presented as the means ± standard deviation
(SD) based on at least three separate experiments. Statistical
analysis was performed using Student's t-test, a non-parametric
test (Mann-Whitney U test between two groups and Kruskal-Wallis
test for three or more groups). The correlations between the
expression of miR-31 and RhoA protein were calculated by
Chi-squared test and Spearman's rank correlation. Differences were
considered to be statistically significant for a P-value <0.05.
Statistical analysis was performed using SPSS 16 (SPSS, Inc.,
Chicago, IL, USA).
Results
miR-31 expression and its correlation
with the clinicopathological characteristics of GC
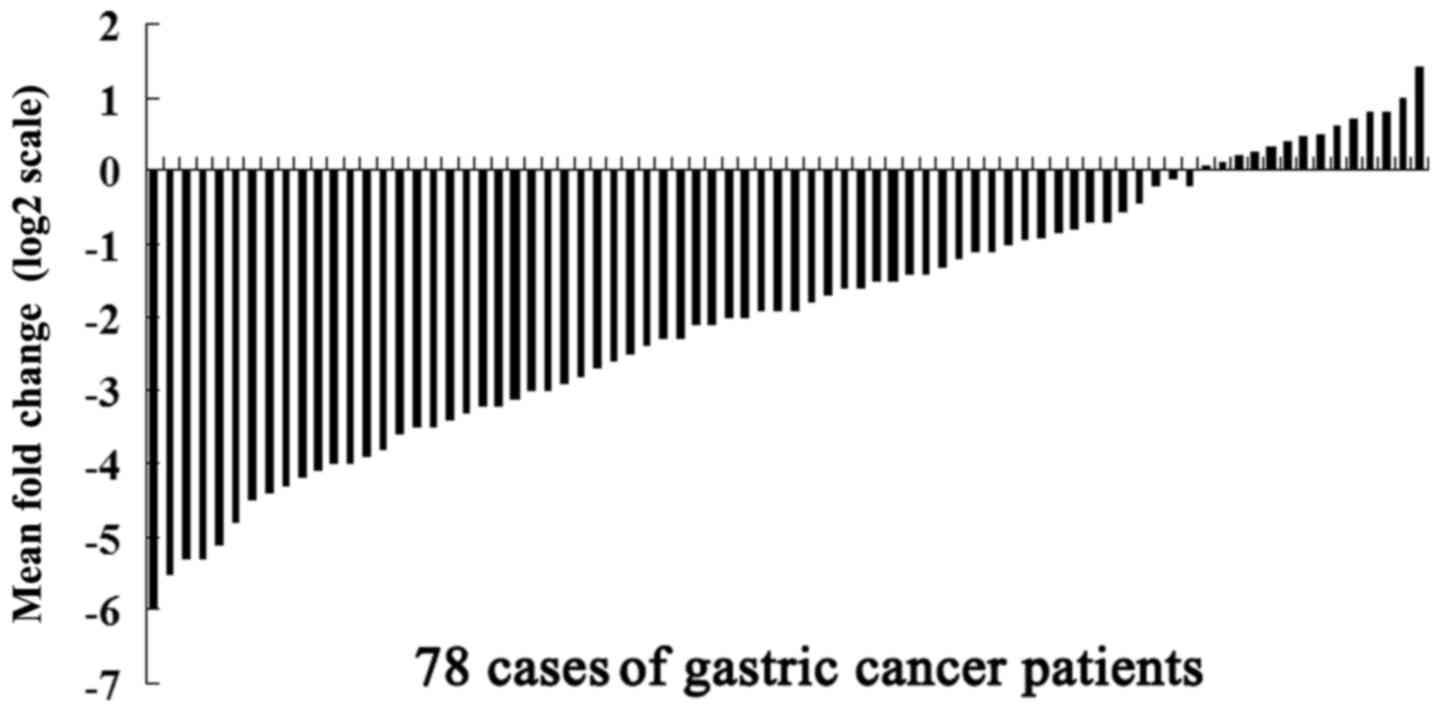
miR-31 was detected in all 78 pairs of GC tissues,
and their matched non-tumor adjacent tissues using quantitative
reverse transcription real-time PCR (qRT-PCR) analysis. Among the
78 patients with GC, 53 (67.95%) cases showed a >50% decrease in
the miR-31 expression level relative to their matched adjacent
non-tumor tissues (Fig. 1). We then
studied the correlation between miR-31 expression and the
clinicopathological characteristics of GC. The Mann-Whitney U test
revealed that lower expression levels of miR-31 were associated
with higher lymph node metastasis, poorer pT and pN stage (Table I).
 | Table I.Expression levels of miR-31 according
to the clinicopathological features of patients with gastric
cancer. |
Table I.
Expression levels of miR-31 according
to the clinicopathological features of patients with gastric
cancer.
|
| N | miR-31a | P-value |
|---|
| Age (years) |
|
| 0.373 |
| ≤50 | 30 | 0.41 (0.08–0.79) |
|
|
>50 | 48 | 0.45 (0.14–1.20) |
|
| Sex |
|
| 0.500 |
| Male | 51 | 0.43 (0.10–0.89) |
|
|
Female | 27 | 0.39 (0.09–1.21) |
|
| Tumor size (cm) |
|
| 0.621 |
|
≤3.5 | 35 | 0.44
(0.12–1.25) |
|
|
>3.5 | 43 | 0.42
(0.15–1.19) |
|
| Histological
grade |
|
| 0.500 |
|
Well | 25 | 0.41
(0.15–0.98) |
|
|
Moderate and poor | 53 | 0.44
(0.06–1.34) |
|
| pT stage |
|
| 0.01a |
|
T1+T2 | 36 | 0.53
(0.18–1.07) |
|
|
T3+T4 | 42 | 0.37
(0.05–0.96) |
|
| pN stage |
|
| 0.03a |
| N0 | 24 | 0.73
(0.19–1.38) |
|
| N1 | 20 | 0.36
(0.11–0.94) |
|
| N2 | 19 | 0.29
(0.05–0.66) |
|
| N3 | 15 | 0.24
(0.03–0.71) |
|
| Invasion into
lymphatic vessels |
|
| 0.000a |
|
Negative | 33 | 0.63
(0.19–1.34) |
|
|
Positive | 45 | 0.25
(0.07–0.67) |
|
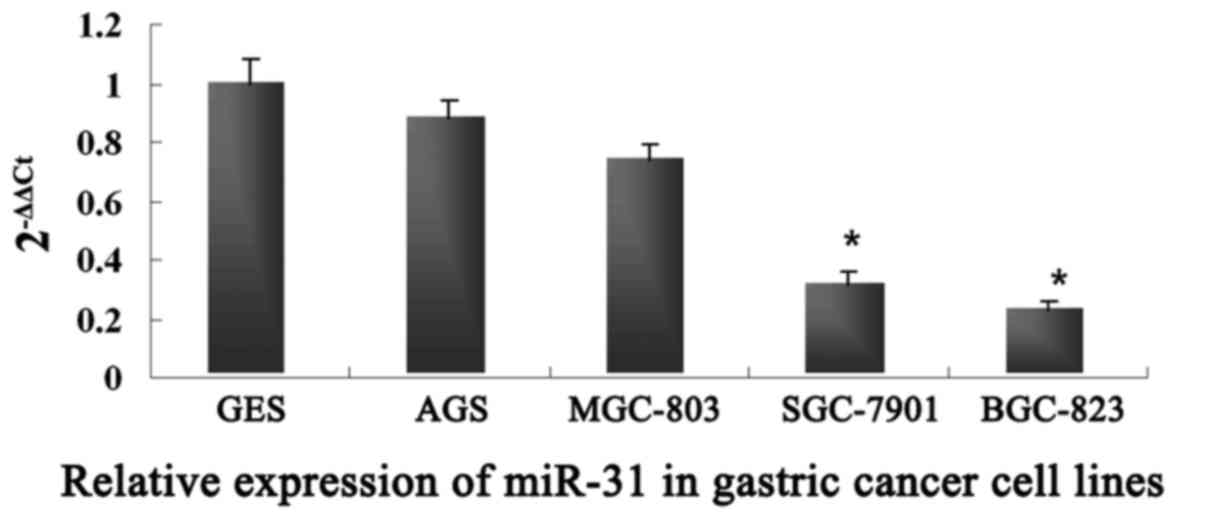
To further validate the role of miR-31 in GC cell
metastasis, we examined the mRNA expression levels of miR-31 in
four human GC cell lines: BGC-823, SGC-7901, MGC-803 and AGS. As
shown in Fig. 2, the expression of
miR-31 was lower in the BGC-823 and SGC-7901 cells, which had a
relatively high metastatic potential, while miR-31 was highly
expressed in the MGC-803 and AGS cells with a relatively low
metastatic potential. These results revealed that the expression of
miR-31 was negatively correlated with GC metastasis and may play an
important role in GC metastasis.
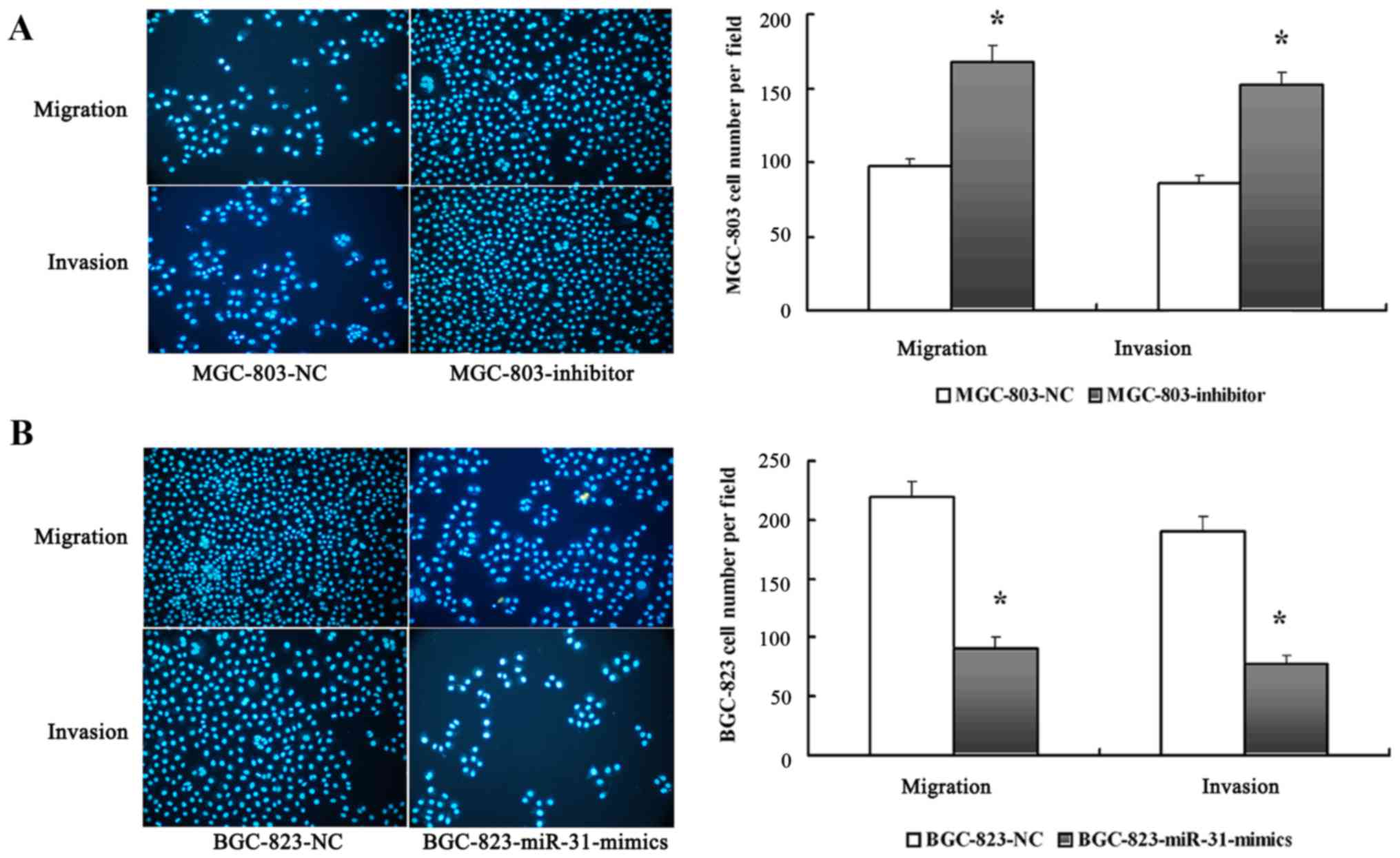
miR-31 inhibits the invasion and
migration of GC cells in vitro
The significant low expression of the miR-31 in GC
cell lines with high metastatic potential prompted us to further
explore the potential biological significance of miR-31 in GC
metastasis. We investigated the effects of miR-31 expression on the
metastatic abilities of GC cells with different metastatic
potential in vitro. An inhibitor and an NC oligonucleotide
(MGC-803-inhibitor and MGC-803-NC, respectively) were thus,
introduced into MGC-803 cells to perform metastasis assays in
vitro. In addition, miR-31 mimics and an NC oligonucleotide
(BGC-823-miR-31-mimics and BGC-823-NC) were constructed and
introduced into BGC-823 cells. The results revealed that the
depletion of miR-31 significantly enhanced the invasion and
migration abilities of MGC-803 cells as determined by Transwell
assay (Fig. 3A). Conversely, the
increased expression of miR-31 significantly inhibited the invasion
and migration abilities of BGC-823 cells (Fig. 3B).
Wound scratch assay results revealed that the cell
migration distances in the BGC-823-miR-31-mimics and BGC-823-NC
cells were 537±22 and 210±14 µm, respectively at 24 h after the
wound scratch. There was a significant difference in the cell
migration distance between the BGC-823-miR-31-mimics and the
BGC-823-NC. In addition, the results indicated that the
miR-31-mimics significantly inhibited the migration ability of the
BGC-823 cells while the miR-131 inhibitor promoted the migration
ability of the SGC-7901 cells.
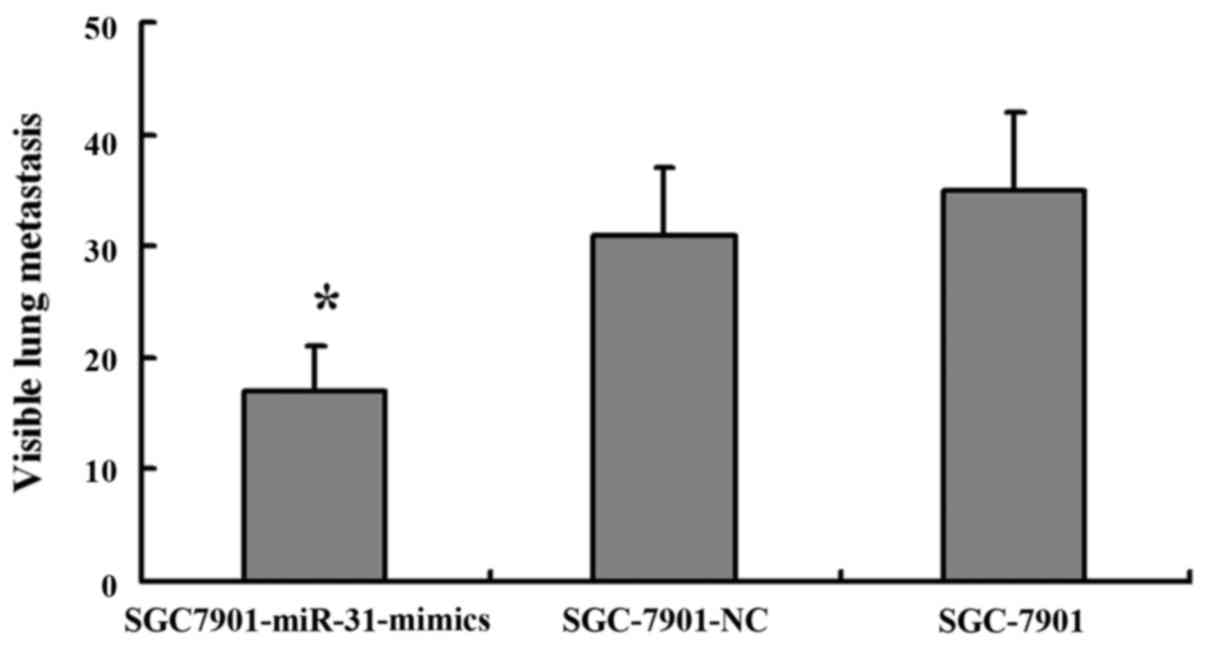
miR-31 suppresses GC metastasis in a
nude mouse xenograft model
To further confirm the aforementioned findings, we
performed an in vivo study using a nude mouse xenograft
model. SGC-7901 cells transfected with the miR-31 mimics or NC were
injected into the lateral tail vein of nude mice, and the mice were
sacrificed five weeks after inoculation. The number of lung
metastatic lesions were markedly decreased in the nude mice
injected with the miR-31 mimic-transfected cells compared with the
negative control-injected ones (Fig.
5). These findings provide strong evidence that miR-31 also
inhibited tumor metastasis in vivo.
RhoA may be a functional target of
miR-31 in the process of GC metastasis
To investigate how the low expression of miR-31
contributes to the enhanced metastatic ability of GC, we explored
potential regulatory targets of miR-31 using the combination of
prediction tools, including PicTar, TargetScan and miRBase target.
Although hundreds of different targets could be predicted, the
genes involved in the migration or invasion process may be the real
relevant targets related to the biological functions of miR-31. We
next performed functional classification of the predicted targets
using the Database for Annotation, Visualization and Integrated
Discovery (DAVID) program (http://david.abcc.ncifcrf.gov/). Among these genes,
RhoA attracted our attention and may possibly contribute to the
metastasis of GC.
To confirm that RhoA was the direct target of
miR-31, we constructed the luciferase reporter pGL3-RhoA-3′UTR. The
scrambled target site pGL3-RhoA-MUT was also constructed as a
negative control. All reporters were transfected into SGC-7901
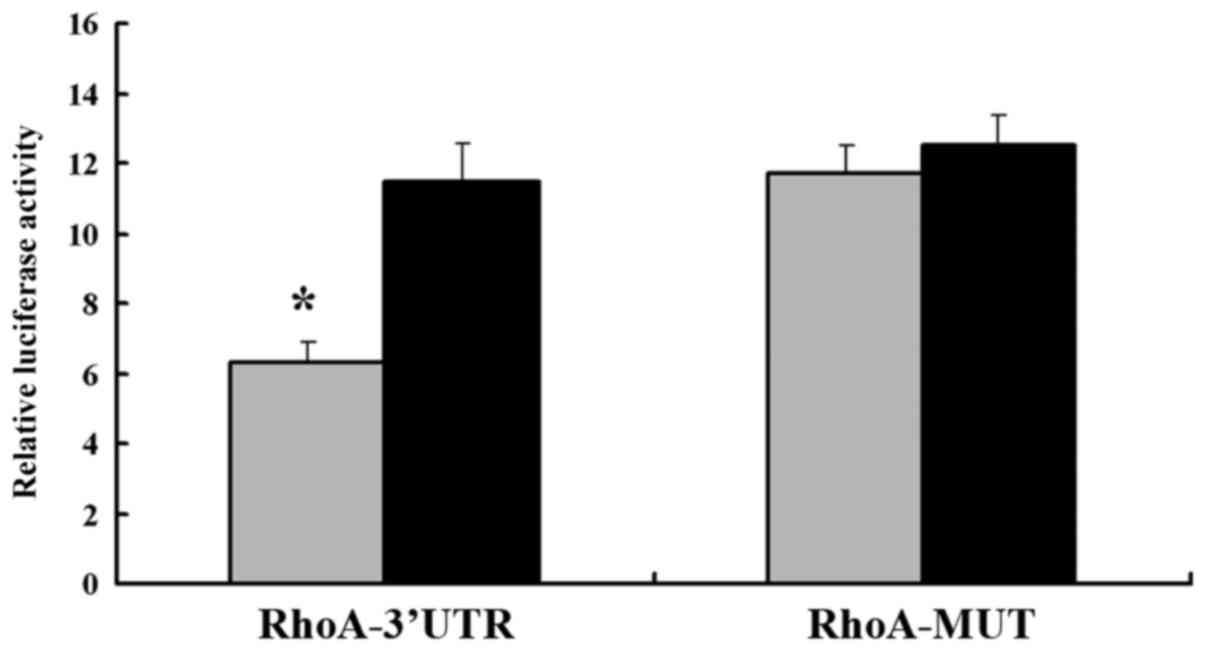
cells. The luciferase activity of the pGL3-RhoA-3′UTR was
significantly suppressed in the SGC-7901-miR-31-mimic cells
compared with that in SGC-7901-NC cells when normalized to the
control vector pRL-TK, containing Renilla luciferase.
(Fig. 6). However, no significant
difference in the relative luciferase activity of the pGL3-RhoA-MUT
reporters was observed in the SGC-7901-miR-31-mimics compared with
that in the SGC-7901-NC. These results revealed that RhoA proteins
were negatively and directly regulated by miR-31 in GC cells, and
that RhoA may serve as a target of miR-31.
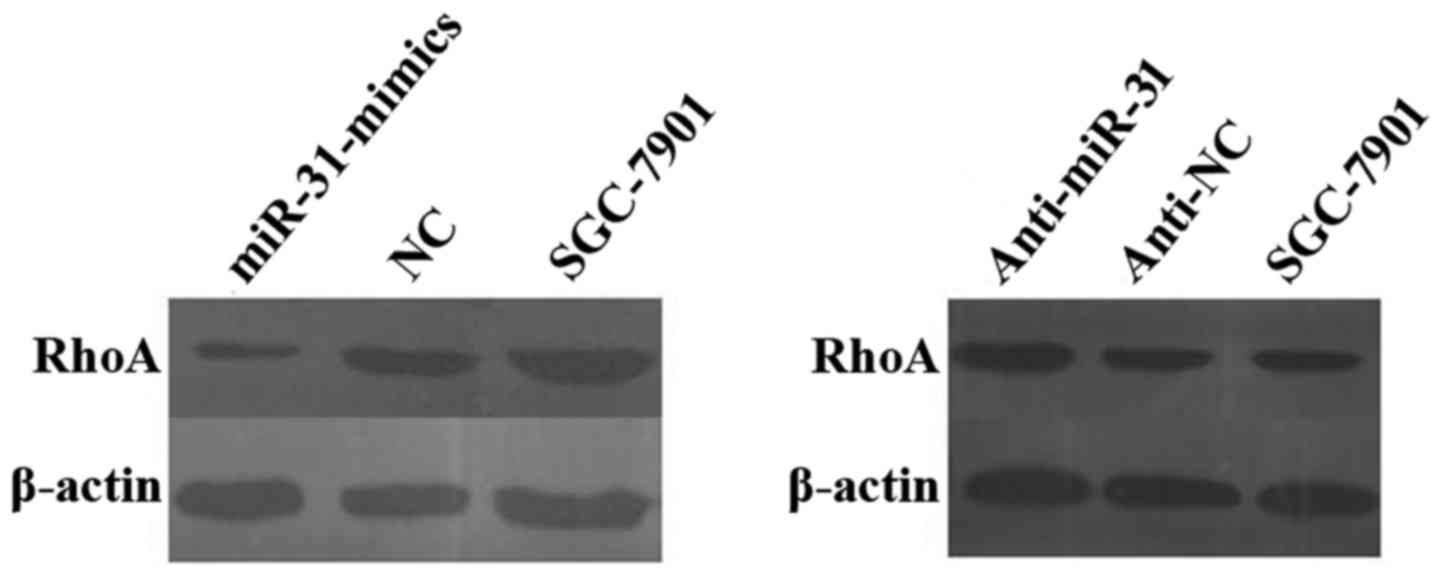
To further confirm these findings, we examined RhoA
protein levels in both miR-31-transfected and
anti-miR-31-transfected cells, as well as in their corresponding NC
and parental SGC-7901 cells, using western blotting and real-time
qRT-PCR analysis. We observed a significant decrease in the level
of the endogenous RhoA proteins in SGC-7901-miR-31-mimic cells than
that in the SGC-7901-NC cells when normalized to the endogenous
reference β-actin protein. Overexpression of RhoA proteins was also
observed in the anti-miR-31-transfected SGC-7901 cells compared
with that in the anti-NC-transfected and parental SGC-7901 cells
(Fig. 7). These results
demonstrated that miR-31 may target and regulate RhoA in GC.
Discussion
The microRNA-31 (miR-31) gene is located at 9p21.3.
Numerous studies have shown that miR-31 has different expression
patterns in different types of cancer, for example, it was
downregulated in urothelial carcinoma of the bladder (13), breast (9) and serous ovarian cancer (14), while it was upregulated in
colorectal cancer (CRC) (15,16),
head and neck squamous cell carcinoma (HNSCC) (17), hepatocellular carcinoma (18) and lung cancer (19). Although numerous studies have
investigated the different expression levels of miR-31 in different
types of cancer, the function of miR-31 still remained unclear.
In the present study, we demonstrated that miR-31
expression was markedly downregulated in both GC tissues and cell
lines, suggesting that the low expression of miR-31 may be
associated with GC development. Furthermore, we demonstrated that
the low expression of miR-31 was significantly associated with a
higher rate of lymph node metastasis, poorer pT and pN stage, and
invasion into lymphatic vessels. Lymph node metastasis, pT and pN
stage were independent prognostic factors for the overall survival
rates of GC patients, and lymphatic vessel invasion was identified
as an independent prognostic factor predicting lymph node
metastasis. GC patients with lymph node metastasis, poor pT and pN
stage and invasion into lymphatic vessels tended to have a lower
survival rate (20,21). This suggested that the expression of
miR-31 may act as an independent prognostic factor for the overall
survival rates of GC patients.
Recent studies revealed that miR-31 expression was
specifically attenuated in metastatic breast cancer cell lines, and
miR-31 could inhibit breast cancer metastasis by targeting multiple
genes (9). In the present study, we
found that overexpression of miR-31 suppressed GC cell invasion and
metastatic abilities. These results were consistent with a previous
study, which revealed that blockade of miR-31 expression
significantly decreased the invasion and migration abilities of the
HNSCC cell line (17). The
development of GC metastasis is characterized by multiple genetic
alterations. Previous studies have investigated the effects of
specific miRNAs on the pattern of GC metastasis. Zheng et al
found that miRNA-145 inhibited the metastasis and angiogenesis
abilities of GC cells by targeting the 3′UTR of Ets1 (22). The expression of let-7f was
decreased in gastric tumors compared with that in normal gastric
tissue and inhibited tumor metastasis by targeting MYH9 (23). Tie et al found that decreased
miR-218 expression was associated with advanced clinical stage
lymph node metastasis and poor prognosis in GC patients, and that
the overexpression of miR-218 in metastatic cells inhibited
migration, invasion, and metastasis formation as shown both by
in vitro and in vivo experiments (24). Although numerous studies have
investigated the role of miRNA in GC metastasis, the underlying
mechanisms remain unclear. Notably, to the best of our knowledge,
few studies have investigated whether GC metastasis is regulated by
miRNA-31.
To examine the molecular mechanism by which miR-31
functioned as a metastasis suppressor in GC, we used the luciferase
reporter assay and western blotting to confirm that RhoA was a
potential target of miR-31 in GC cells. RhoA, with a molecular mass
of 21 kDa, is the most widely studied member of the Rho GTPase
family, and belongs to the Ras superfamily of small G proteins. The
Rho GTPase family consists of at least 11 members sharing >50%
sequence identity, with one of the most well known members being
RhoA. RhoA acts as a molecular switch in cells, regulating signal
transduction from cell surface receptors to intracellular target
molecules, and is involved in various biological process, including
cell morphology (25), motility
(26), cytokinesis (27,28),
smooth muscle contraction (29,30)
and tumor progression (31,32).
In conclusion, the present study demonstrated that
miR-31 could suppress the metastasis of GC by directly binding to
the 3′UTR of RhoA. Although there is still a lot to learn
concerning the role of miR-31 in GC tumorigenesis, miR-31 may serve
as a promising potential target for GC treatment.
Acknowledgements
The present study was supported by the National
Ministry of Technology Foundation of China (2009BAI86B05). The
authors would like to thank Professor Yao He for his technical
assistance.
References
|
1
|
Compare D, Rocco A and Nardone G: Risk
factors in gastric cancer. Eur Rev Med Pharmacol Sci. 14:302–308.
2010.PubMed/NCBI
|
|
2
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma L and Weinberg RA: Micromanagers of
malignancy: Role of microRNAs in regulating metastasis. Trends
Genet. 24:448–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zamore PD and Haley B: Ribo-gnome: The big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valastyan S, Reinhardt F, Benaich N,
Calogrias D, Szász AM, Wang ZC, Brock JE, Richardson AL and
Weinberg RA: A pleiotropically acting microRNA, miR-31, inhibits
breast cancer metastasis. Cell. 137:1032–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang CJ, Stratmann J, Zhou ZG and Sun XF:
Suppression of microRNA-31 increases sensitivity to 5-FU at an
early stage, and affects cell migration and invasion in HCT-116
colon cancer cells. BMC Cancer. 10:6162010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang WH, Gui JH, Wang CZ, Chang Q, Xu SP,
Cai CH, Li YN, Tian YP, Yan L and Wu B: The identification of
miR-375 as a potential biomarker in distal gastric adenocarcinoma.
Oncol Res. 20:139–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaefer A, Jung M, Mollenkopf HJ, Wagner
I, Stephan C, Jentzmik F, Miller K, Lein M, Kristiansen G and Jung
K: Diagnostic and prognostic implications of microRNA profiling in
prostate carcinoma. Int J Cancer. 126:1166–1176. 2010.PubMed/NCBI
|
|
14
|
Creighton CJ, Fountain MD, Yu Z, Nagaraja
AK, Zhu H, Khan M, Olokpa E, Zariff A, Gunaratne PH, Matzuk MM, et
al: Molecular profiling uncovers a p53-associated role for
microRNA-31 in inhibiting the proliferation of serous ovarian
carcinomas and other cancers. Cancer Res. 70:1906–1915. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang CJ, Zhou ZG, Wang L, Yang L, Zhou B,
Gu J, Chen HY and Sun XF: Clinicopathological significance of
microRNA-31, −143 and −145 expression in colorectal cancer. Dis
Markers. 26:27–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motoyama K, Inoue H, Takatsuno Y, Tanaka
F, Mimori K, Uetake H, Sugihara K and Mori M: Over- and
under-expressed microRNAs in human colorectal cancer. Int J Oncol.
34:1069–1075. 2009.PubMed/NCBI
|
|
17
|
Liu CJ, Tsai MM, Hung PS, Kao SY, Liu TY,
Wu KJ, Chiou SH, Lin SC and Chang KW: miR-31 ablates
expression of the HIF regulatory factor FIH to activate the HIF
pathway in head and neck carcinoma. Cancer Res. 70:1635–1644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong QW, Lung RW, Law PT, Lai PB, Chan KY,
To KF and Wong N: MicroRNA-223 is commonly repressed in
hepatocellular carcinoma and potentiates expression of
Stathmin1. Gastroenterology. 135:257–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Sempere LF, Ouyang H, Memoli VA,
Andrew AS, Luo Y, Demidenko E, Korc M, Shi W, Preis M, et al:
MicroRNA-31 functions as an oncogenic microRNA in mouse and human
lung cancer cells by repressing specific tumor suppressors. J Clin
Invest. 120:1298–1309. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Z, Li DM, Wang ZN, Huang BJ, Xu Y, Li
K and Xu HM: Prognostic significance of microscopic positive
margins for gastric cancer patients with potentially curative
resection. Ann Surg Oncol. 16:3028–3037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang CG, Wang ZN, Sun Z, Liu FN, Yu M and
Xu HM: Clinicopathologic characteristics and prognosis of gastric
cancer invading the subserosa. J Surg Oncol. 102:737–741. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng L, Pu J, Qi T, Qi M, Li D, Xiang X,
Huang K and Tong Q: miRNA-145 targets v-ets erythroblastosis virus
E26 oncogene homolog 1 to suppress the invasion, metastasis, and
angiogenesis of gastric cancer cells. Mol Cancer Res. 11:182–193.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang S, He L, Zhao X, Miao Y, Gu Y, Guo
C, Xue Z, Dou W, Hu F, Wu K, et al: MicroRNA let-7f inhibits tumor
invasion and metastasis by targeting MYH9 in human gastric cancer.
PLoS One. 6:e184092011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S,
Guo X, Wang B, Gang Y, Zhang Y, et al: MiR-218 inhibits invasion
and metastasis of gastric cancer by targeting the Robo1 receptor.
PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paterson HF, Self AJ, Garrett MD, Just I,
Aktories K and Hall A: Microinjection of recombinant p21rho induces
rapid changes in cell morphology. J Cell Biol. 111:1001–1007. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takaishi K, Kikuchi A, Kuroda S, Kotani K,
Sasaki T and Takai Y: Involvement of rho p21 and its
inhibitory GDP/GTP exchange protein (rho GDI) in cell
motility. Mol Cell Biol. 13:72–79. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kishi K, Sasaki T, Kuroda S, Itoh T and
Takai Y: Regulation of cytoplasmic division of Xenopus embryo by
rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI). J
Cell Biol. 120:1187–1195. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jantsch-Plunger V, Gönczy P, Romano A,
Schnabel H, Hamill D, Schnabel R, Hyman AA and Glotzer M: CYK-4: A
Rho family gtpase activating protein (GAP) required for central
spindle formation and cytokinesis. J Cell Biol. 149:1391–1404.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirata K, Kikuchi A, Sasaki T, Kuroda S,
Kaibuchi K, Matsuura Y, Seki H, Saida K and Takai Y: Involvement of
rho p21 in the GTP-enhanced calcium ion sensitivity of
smooth muscle contraction. J Biol Chem. 267:8719–8722.
1992.PubMed/NCBI
|
|
30
|
Gong MC, Iizuka K, Nixon G, Browne JP,
Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV and Somlyo
AP: Role of guanine nucleotide-binding proteins - ras-family or
trimeric proteins or both - in Ca2+ sensitization of
smooth muscle. Proc Natl Acad Sci USA. 93:1340–1345. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perona R, Esteve P, Jiménez B, Ballestero
RP, Cajal Ramóny S and Lacal JC: Tumorigenic activity of rho genes
from Aplysia californica. Oncogene. 8:1285–1292.
1993.PubMed/NCBI
|
|
32
|
Prendergast GC, Khosravi-Far R, Solski PA,
Kurzawa H, Lebowitz PF and Der CJ: Critical role of Rho in cell
transformation by oncogenic Ras. Oncogene. 10:2289–2296.
1995.PubMed/NCBI
|