Introduction
Hypopharyngeal squamous cell carcinoma (HSCC) is a
common head and neck malignancy, accounting for 2–6% of all head
and neck cancers (1,2). Due to the lack of evident clinical
symptoms, easy lymph node metastasis and local infiltration in
patients with early-stage HSCC (3,4), make
treatment of HSCC one of the toughest challenges in human
malignancies. Although the curative effect has progressively
improved with the development of science and clinical technology in
recent years, the overall survival has not improved due to the
lymph node metastasis and distant metastasis of HSCC (5,6).
Therefore, studying the molecular mechanisms of HSCC progression
and migration, would improve the diagnosis and prognosis of HSCC,
and help to develop a new therapeutic strategy.
The A disintegrin and metalloprotease (ADAMs) family
which has been reported in more than 30 species, and contains
common structural features, consists of multidomain transmembrane
proteases (7). ADAMs plays an
important role in the degradation of intercellular adhesion,
cell-matrix adhesion and basement membrane. Abberant expression of
ADAMs is closely related to tumor proliferation, differentiation,
adhesion, migration and invasion in tumors (7–11).
ADAM10 is a crucial member of the ADAMs family (12,13).
The chief function of ADAM10 is shedding certain protein molecules
in the extracellular region, thereby activating molecules, such as
Ephrins (14), N-cadherin (15), E-cadherin (16), Notch receptor and its ligand δ 1
(17). Previous research has
revealed that ADAM10 is highly expressed in a variety of human
types of cancer, including liver cancer (18), nasopharyngeal carcinoma (19), lung (20), gastric (21) and bladder cancer (22). Overexpression of ADAM10 promotes
tumorigenesis and the progression, metastasis and invasion of
tumors as well as the poor prognosis for cancer patients, by
participating in a variety of signaling pathways (7–11,23–25).
However, the expression of ADAM10 in HSCC and the role of ADAM10 on
the progression, metastasis and poor prognosis of HSCC have yet to
be elucidated.
In the present study, we first studied the
expression of ADAM10 in HSCC, and the effect of ADAM10 expression
on the proliferation and migration of HSCC. We found that ADAM10
overexpression in HSCC was associated with clinicopathological
characteristics and the poor prognosis of patients with this
disease. Moreover, inhibiting the expression level of ADAM10
decreased the proliferation and migration ability of the FaDu cell
line. Our data indicated that ADAM10 is a potential molecular
target involved in the progression and metastasis mechanism of
HSCC.
Materials and methods
Tissue specimens
All of the HSCC samples were collected from the
Affiliated Hospital of Nantong University and pathologically
diagnosed as HSCC by tissue biopsy. The clinical features of 46
patients with HSCC are listed in Table
I. Fifteen pairs of fresh HSCC tissues and adjacent tissues
were stored in −80°C in a refrigerator. All selected patients had
not undergone preoperative radiotherapy and chemotherapy. Informed
consent was obtained from all individual participants included in
the study. This study was approved by the Ethics Committee of the
Affiliated Hospital of Nantong University and conformed to the
provisions of the Declaration of Helsinki in 1995.
 | Table I.Clinicopathological characteristics
of HSCC patients and IHC staining score for ADAM10. |
Table I.
Clinicopathological characteristics
of HSCC patients and IHC staining score for ADAM10.
| Groups | N | ADAM10 IHC
scorea |
P-valueb |
|---|
| Age (years) |
|
<60 | 20 | 5.80±3.736 | 0.741 |
|
≥60 | 26 | 6.15±3.461 |
|
| Sex |
|
Male | 43 | 6.05±3.605 | 0.740 |
|
Female | 3 | 5.33±3.055 |
|
|
Differentiation |
|
Keratinizing | 20 | 3.90±2.278 |
<0.001c |
|
Non-keratinizing | 26 | 7.62±3.238 |
|
| Tumor size
(cm) |
| ≤2 | 15 | 4.27±3.390 | 0.019c |
|
>2 | 31 | 6.84±3.358 |
|
| Clinical stage |
|
I–II | 18 | 3.67±2.701 |
<0.001c |
|
III–IV | 28 | 7.50±3.226 |
|
| Lymph node
metastasis |
|
Negative | 19 | 3.95±2.896 | 0.001c |
|
Positive | 27 | 7.44±3.274 |
|
| Ki-67
expression |
|
Low | 31 | 4.45±3.064 | 0.001c |
|
High | 15 | 9.20±2.007 |
|
Immunohistochemical staining
Tissue samples were cut into paraffin sections for
immunohistochemical staining. After deparaffinization and
rehydration, the tissue sections were heated with 1X sodium citrate
solution at 100°C for 30 min and washed 3 times. Then they were
soaked in 3% H2O2 for 15 min and subsequently
washed 3 times. Finally the tissue sections were incubated with the
primary antibodies overnight at 4°C. A two-step incubation with a
secondary antibody was performed using an immunohistochemistry
universal kit (ZSGB-BIO, Beijing, China). DAB staining then
followed and microscopic observation. Two pathologists assessed the
staining intensity and percentage of stained cells in the tumor
area, respectively. The expression level of ADAM10 was evaluated
according to a semiquantitative scoring system named ‘H-score
approach’ (26). The staining
intensity was termed category A and assigned scores as strong
(3); moderate (2); weak (1) or negative (0). The proportion of cells
in the tumor area was termed category B and scored from 0 to 4: 0
(0%); 1 (1–25%); 2 (26–50%); 3 (51–75%) and 4 (76–100%). A final
score was calculated by multiplying A by B (minimum 0, maximum 12).
A final score of <6 was regarded as negative or weak expression,
and a score of ≥6 was regarded as high expression.
Cell lines, small interfering RNAs
(siRNAs) and transfection
The FaDu cell line from our laboratory was cultured
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both from Gibco, USA). Control
non-targeted siRNAs and ADAM10-specific siRNAs were purchased from
RiboBio (Guangzhou, China). Transfections were performed using
Lipofectamine 2000 reagent (Invitrogen, USA) following the
manufacturers protocol.
Western blot analysis and
antibodies
Protein was extracted from tissues and FaDu cells
for protein analysis. The samples were boiled at 100°C for 5 min.
An equal amount of protein samples was separated by SDS-PAGE and
transferred to PVDF membranes (Millipore, USA). The membranes were
incubated with primary antibodies overnight at 4°C. The antibodies
used were as follows: anti-ADAM10 (1:300, polyclonal, rabbit
anti-human; BBI Life Sciences Corporation, Shanghai, China),
anti-Ki-67 (1:1,000; polyclonal, rabbit anti-human),
anti-E-cadherin (1:1,000), anti-N-cadherin (1:1,000, monoclonal,
mouse anti-human), anti-vimentin (1:1,000, monoclonal, mouse
anti-human), and anti-β-actin polyclonal antibody (1:2,500,
monoclonal, mouse anti-human) (all from Santa Cruz Biotechnology,
USA). Immunoreactive protein bands were detected using an ECL
detection system (Cell Signaling Technology, USA).
Cell proliferation assay
The CCK-8 assay was used to detect cell
proliferation. The cells were seeded in 96-well plates (10,000
cells/well), incubated overnight, and then transfected with
siRNA-ADAM10 or control non-targeted siRNAs and cultured for 12,
24, 36, 48, 60 and 72 h. The CCK-8 kit reagent (10 µl/well; BBI
Life Sciences Corporation) was added and incubation followed in the
dark in an incubator for 2 h. Subsequently the OD was assessed at
450 nm.
Cell cycle analysis
Flow cytometry was used to analyze the effect of
ADAM10 on cell cycle progression. Briefly, FaDu cells were cultured
in 6-well plates. The medium was replaced with 10% FBS at 6, 12, 24
and 36 h and after serum starvation at 72 h, or siRNA-ADAM10
interference for 48 h. The adherent cells were collected by trypsin
digestion and centrifuged at 95 × g for 5 min. The cells were then
washed twice with phosphate-buffered saline (PBS), and fixed with
70% alcohol. The cells were then stored at −20°C for 24 h, washed
with PBS 2 times and resuspended in 1 ml of 1X PBS containing 0.1%
Triton X-100, 40 µg/ml RNase and 20 µg/ml propidium iodide (PI)
followed by a 30-min incubation at room temperature. Finally, the
samples were analyzed using the FACSCalibur flow cytometer (BD
Biosciences, USA) and BD CellQuest software.
Migration assay
A wound healing assay was performed for cell
migration. The cells were seeded in 6-well plates and incubated
overnight, then transfected with siRNA-ADAM10 or control
non-targeted siRNAs and a scratch wound was made using a 200-µl
yellow tip when the cells reached ~90% density. Subsequently the
cells were cultured with serum-free DMEM medium for 36 h. The
percentage of migration was calculated based on the measured
cell-free area. Similarly, Transwell migration assays were
performed for cell migration. FaDu cells were transfected with
siRNA-ADAM10 or control non-targeted siRNAs and seeded to the upper
chamber containing serum-free DMEM with a non-coated membrane
(24-well insert, 8-µm pore size; Millipore) and DMEM containing 10%
serum was added to the lower chamber. After 24 h, the cells from
the upper chamber were removed and the cells that had migrated to
the lower chamber were fixed with formaldehyde, and then stained
with 0.1% crystal violet. The cells were then counted using an IX70
inverted microscope.
Statistical analysis
Statistical software (IBM SPSS Statistics 20; IBM
SPSS, Armonk, NY, USA) was used for statistical analysis. The
Students t-test was used to determine the statistical differences
between groups. P<0.05 was considered statistically
significant.
Results
ADAM10 protein overexpression in HSCC
specimens
It was reported that ADAM10 is aberrant in tumors
(18–22). Here, we first detected the
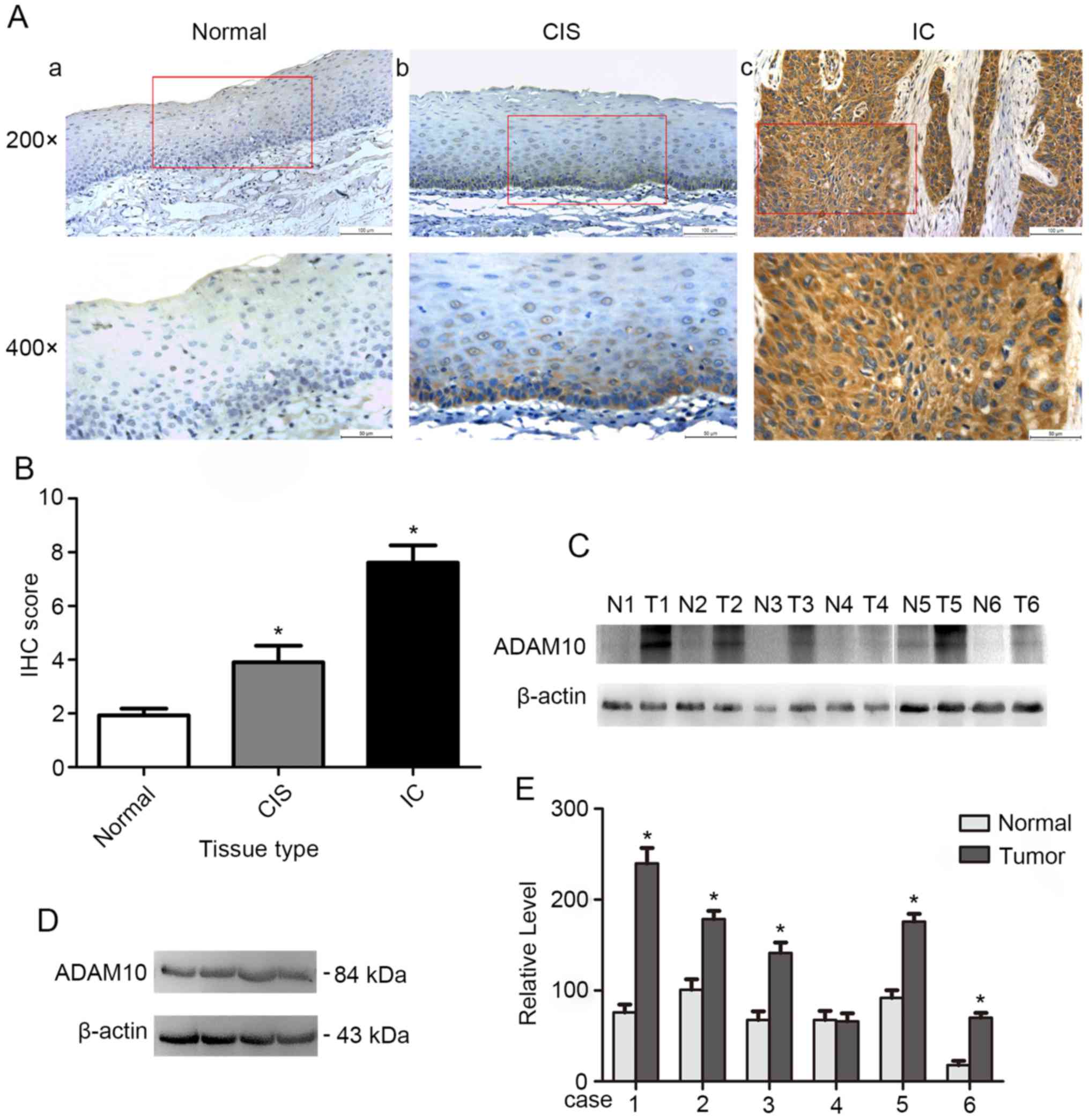
expression of ADAM10 in HSCC. Immunohistochemical analysis of
ADAM10 expression in hypopharyngeal normal tissues and tumor
tissues revealed that ADAM10 was weakly expressed in normal
tissues, moderately expressed in cancer in situ (CIS) and
highly expressed in invasive cancer (IC) (Fig. 1A). The IHC score of ADAM10 was
significantly decreased in normal tissues (1.93±0.88) compared with
that in CIS (3.90±2.79, P<0.05) or IC (7.62±3.24, P<0.05)
(Fig. 1B). In addition, there was a
statistical difference in the IHC score of ADAM10 between CIS and
IC tissues (P<0.05). Subsequently, we examined the expression of
ADAM10 in 15 pairs of HSCC and precancerous tissues by western blot
analysis, which revealed that the expression level of ADAM10 in
cancer tissues was significantly higher than that in adjacent
tissues (Fig. 1C and E). Finally,
we also detected the expression of ADAM10 in the HSCC cell line
FaDu (Fig. 1D). The results
revealed that ADAM10 was overexpressed and may promote
tumorigenesis and progression in HSCC.
Correlation of ADAM10 expression level
with clinicopathological features
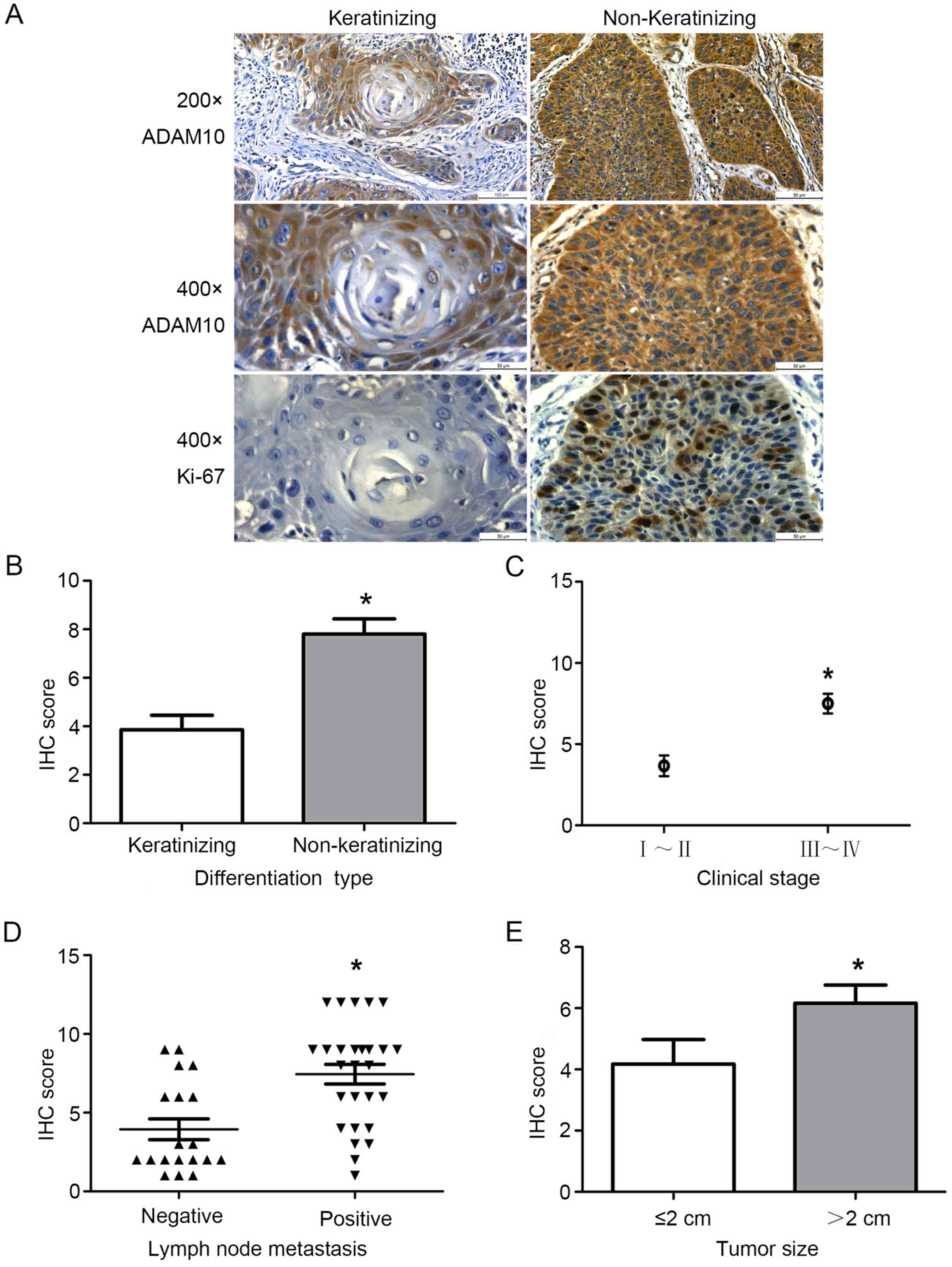
We further assessed the correlation of the
expression level of ADAM10 with clinicopathological characteristics
in HSCC. As shown in Table I and
Fig. 2, high expression of ADAM10
in HSCC was significantly correlated with the degree of tumor
differentiation (p<0.001). Squamous cell carcinoma is divided
into keratinizing and non-keratinizing squamous cell carcinoma
(27). In addition, overexpression
of ADAM10 in HSCC was also associated with tumor size (p=0.019),
lymph node metastasis (p=0.001) and clinical stage (p<0.001).
There was no correlation with the sex and age of the patients and
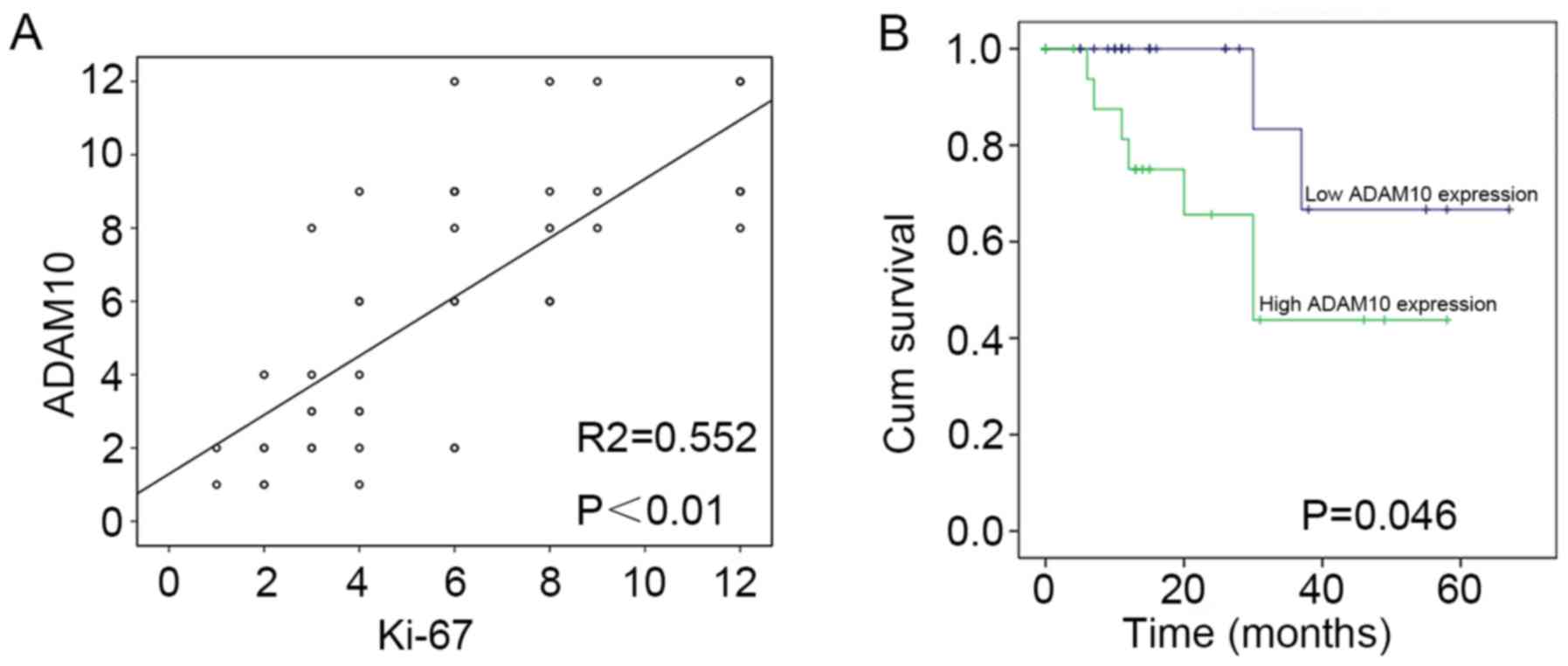
HSCC. Moreover, we also analyzed the correlation of the expression
level of ADAM10 with Ki-67 expression (p=0.001). The result
revealed that there was a significant positive correlation in the
expression level of ADAM10 with the expression of Ki-67 (Fig. 3A). The data revealed that the
expression of ADAM10 had a significant correlation with the
clinicopathological characteristics of HSCC. Thus, high expression
of ADAM10 may promote proliferation and migration.
High expression of ADAM10 is
associated with the poor prognosis of HSCC patients
Similarly, we analyzed the relationship between the
expression of ADAM10 and the prognosis of 46 patients with HSCC by
statistical analyses. The result revealed that overall survival was
significantly decreased in patients with high expression of ADAM10
than in those with low expression of ADAM10 (Fig. 3B) (p<0.046). It is therefore
implied that overexpression of ADAM10 led to the poor prognosis of
patients with HSCC.
Expression of ADAM10 in proliferating
HSCC cells
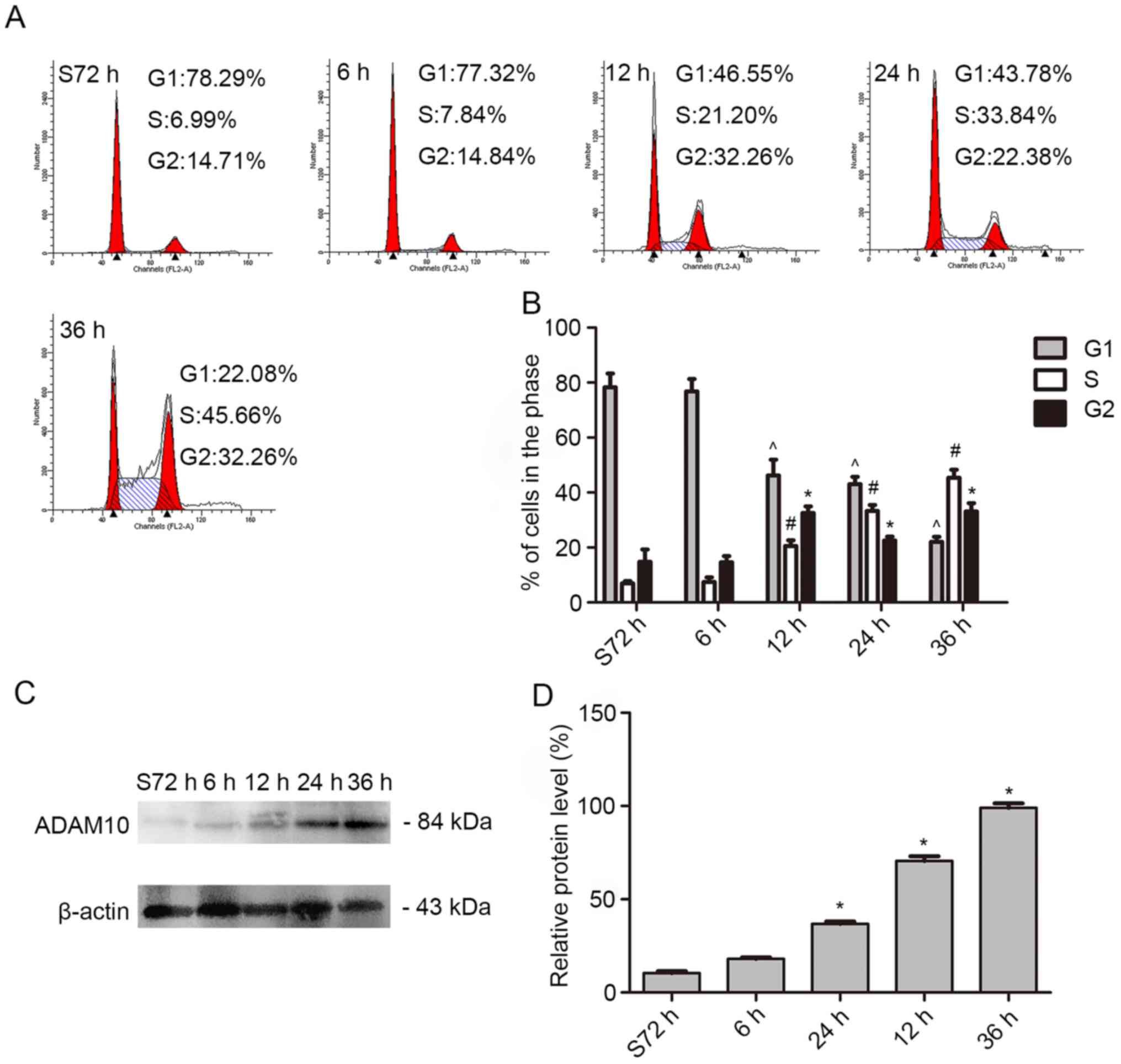
In order to further explore the possible biological
roles of ADAM10 in HSCC we examined the expression level of ADAM10
in proliferating FaDu cells. First, we cultured FaDu cells in
serum-free medium for 72 h to stop the cell cycle in the G1 phase.
Then, we collected the cells after being cultured with 10% FBS at
0, 6, 12, 24, and 36 h. Flow cytometric analysis revealed that the
percentage of cells in the S phase of the cell cycle increased from
6.99 to 45.66% (Fig. 4A and B).
Similarly, the expression level of ADAM10 in the cells was
obviously increased as detected by western blot analysis (Fig. 4C and D). The result revealed that
ADAM10 was involved in the process of cell proliferation.
The proliferation of FaDu cells was
attenuated by knockdown of ADAM10
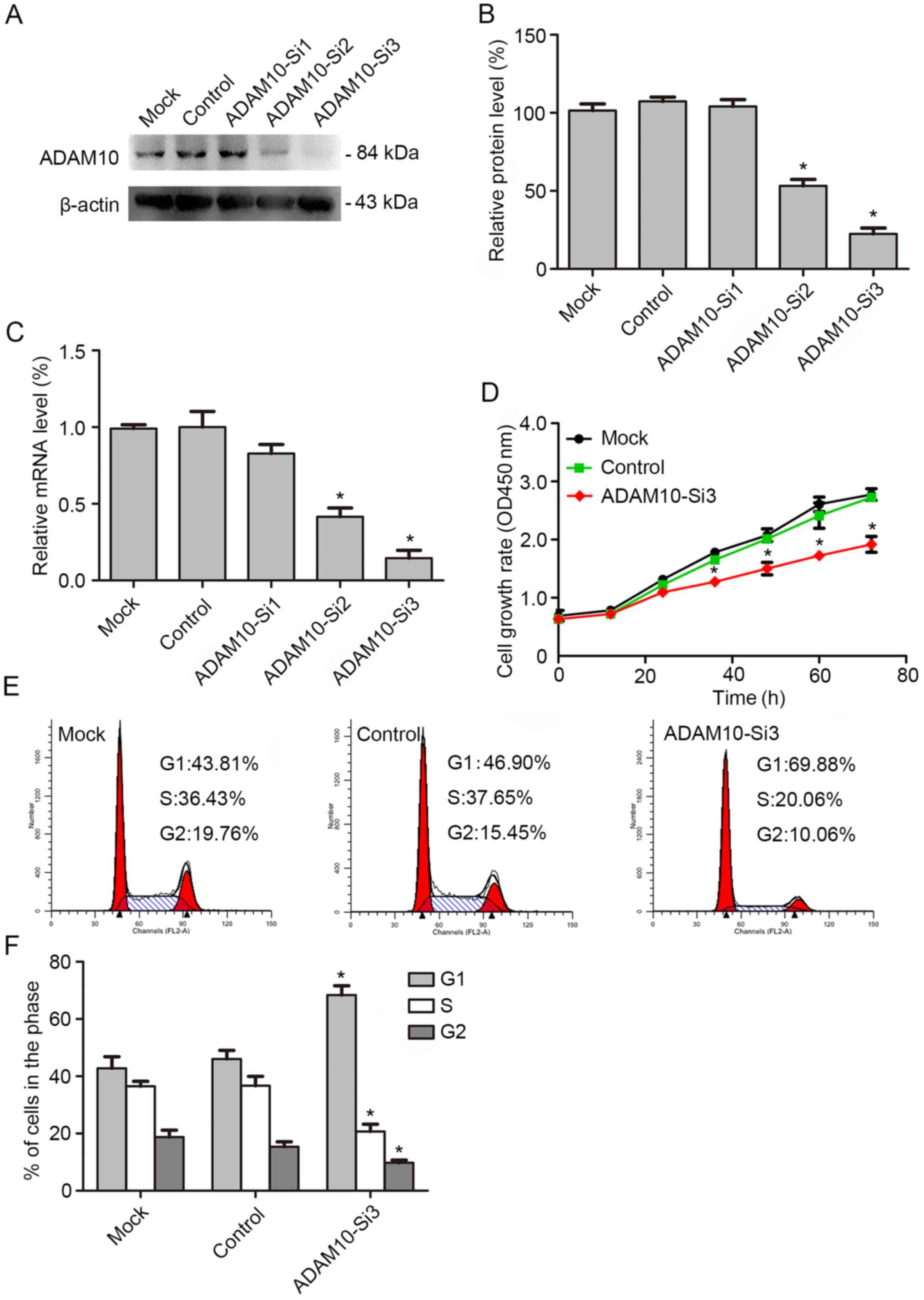
The above studies revealed that ADAM10 could promote
the proliferation of HSCC. Therefore, FaDu cells were transfected
with ADAM10 siRNAs to decrease the expression of ADAM10 in order to
detect the function of ADAM10 in HSCC. First, we assessed the
knockdown efficiency by western blotting and real-time PCR. The
results revealed that the expression level of ADAM10 in the FaDu
cell line was significantly decreased after knockdown, especially
in ADAM10-siRNA3 (Fig. 5A-C).
Subsequently, we examined cell proliferation by CCK-8 assay. The
result revealed that the cell proliferation ability was
significantly decreased after knockdown with ADAM10-siRNA3
(Fig. 5D). The cell cycle was
investigated by flow cytometric analysis. The results revealed that
the percentage of cells in the G0/G1 phase increased significantly
and the percentage of cells in the S phase decreased significantly
after knockdown of ADAM10 (Fig. 5E and
F). Thus, it was concluded that ADAM10 can promote the growth
of HSCC.
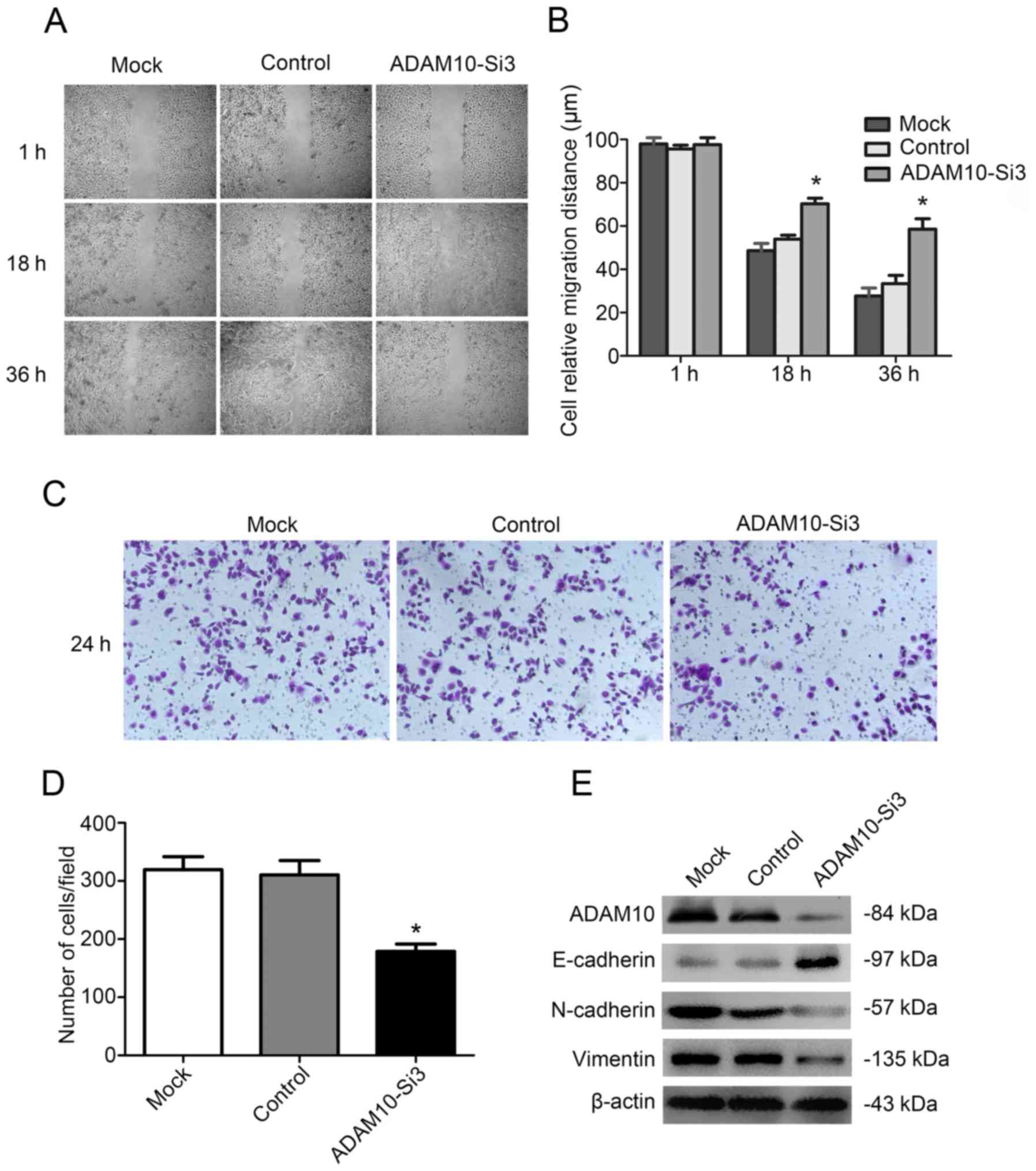
The migration of FaDu cells was
inhibited by knockdown of ADAM10
We then examined the migration ability of FaDu cells
with ADAM10-siRNA3 knockdown by wound-healing and Transwell assay.
The study revealed that wound healing was significantly decreased
by ADAM10-siRNA3 compared with the control group after 36 h
(Fig. 6A and B). Similarly,
Transwell assay revealed that the number of migratory cells were
significantly lower in the ADAM10-siRNA3 group than those in the
control group (Fig. 6C and D). In
conclusion, the migration ability of FaDu cells was significantly
decreased after knockdown of ADAM10.
In the process of tumor migration,
epithelial-mesenchymal transition (EMT) plays a decisive role
(28). It could decrease cell
adhesion, eliminate cell polarity, empower cell motility and
invasive ability, thus leading to the metastasis of tumor cells
from the original tumor location to adjacent organs and distant
organs (29). The EMT process is
usually accompanied with decreased expression of epithelial markers
(E-cadherin, cytokeratin, and β-catenin) and increased expression
of mesenchymal phenotypic markers (vimentin, N-cadherin and
fibronectin), which play important roles in cell adhesion (29,30).
Therefore, we detected the expression of N-cadherin, vimentin and
E-cadherin in FaDu cells after knockdown with ADAM10-siRNA3. The
result revealed that the expression of E-cadherin was increased and
N-cadherin and vimentin were decreased in the FaDu cells after
treatment with ADAM10-siRNA3 (Fig.
6E). The result demonstrated that ADAM10 promoted tumor
migration by affecting EMT.
Discussion
In our present study, we investigated whether ADAM10
had a potential effect on HSCC. To clarify the effect of ADAM10 on
HSCC, we performed immunohistochemical and western blot analysis to
detect the expression of ADAM10 and its relationship with
clinicopathological characteristics. In addition, we observed the
effect of the expression of ADAM10 on the proliferation and
migration ability of the FaDu cell line. Collectively, our results
revealed that ADAM10 was highly expressed and promoted tumor
progression, migration and the poor prognosis of patients with
HSCC.
ADAM10, a member of the ADAMs family, is a
multinomial transmembrane protease, which is widely expressed in
the body (31–34). The main function of ADAM10 is
ectodomain shedding on a variety of transmembrane receptors,
cytokines and adhesion molecules, such as TNF-α, EGF, E-cadherins,
N-cadherins and Notch (15,16,31–35).
High expression of ADAM10 has been reported in a variety of
malignancies and is involved in the development and migration of
tumors (7–11,16,23–25).
However, the role of ADAM10 in HSCC has not been reported yet.
In this study, we found that ADAM10 was highly
expressed in CIS and IC of HSCC compared with normal tissues,
likewise, the expression level of IC was also higher than that of
CIS (Fig. 1). Moreover, we found
that the expression level of ADAM10 was correlated with tumor size,
differentiation, clinical stage and lymph node metastasis in HSCC
(Fig. 2). It is suggested that
ADAM10 may be a potential oncogene and play an important role in
the tumorigenesis and progression of HSCC. The aberrant expression
of ADAM10 may be involved in tumor cell proliferation and
migration-related signaling pathways, thus promoting tumor growth,
lymph node metastasis and distant metastasis. These results were
similar with those of You et al (19) that reported the effect of ADAM10 in
nasopharyngeal carcinoma and Liu et al (36) that reported the effect of ADAM10 in
hepatocellular carcinoma.
The studies revealed that the prognosis of HSCC was
mainly related to the clinical stage, malignancy and lymph node
metastasis and distant metastasis (5,6). Thus,
we analyzed the effect of ADAM10 on the prognosis of HSCC. The
results revealed that overexpression of ADAM10 significantly
decreased the time of survival. Overall survival was significantly
decreased in HSCC patients with high expression of ADAM10 compared
to those with low expression (Fig.
3B). Combined with the above results (Figs. 1 and 2) it is implied that high expression of
ADAM10 may promote migration and invasion, increase the difficulty
of treatment and enhance the recurrence rate in HSCC, thus
decreasing the overall survival rate of HSCC patients.
To further test our hypothesis, we used the HSCC
cell line FaDu for validation. First, we detected the expression
level of ADAM10 in proliferating FaDu cells, which revealed that
the percentage of cells in the S phase increased with the increase
of ADAM10 expression (Fig. 4).
Subsequently, we knocked down the expression of ADAM10 in FaDu
cells, The results revealed that the proliferation ability of FaDu
cells (Fig. 5D) and the percentage
of S phase cells (Fig. 5E and F)
were significantly decreased after knockdown of ADAM10 expression.
It was further demonstrated that the expression of ADAM10 promoted
the abnormal proliferation of cells in HSCC. Furthermore, we
investigated the effect of ADAM10 on the migration of FaDu cells.
It was revealed that the ability of scratches to heal (Fig. 6A and B) and the number of cells that
passed through the membrane (Fig. 6C
and D) were markedly decreased after knockdown of ADAM10
expression in FaDu cells. These results indicated that
downregulation of ADAM10 expression can significantly inhibit the
movement and migration of FaDu cells. In other words, high
expression of ADAM10 may promote the proliferation and migration of
tumors in HSCC.
Tumor invasion and metastasis are very complex
molecular regulation processess. The current study determined that
EMT plays a decisive role in these processes (28). EMT is mainly characterized by the
decrease of cell epithelial characteristics, the increase of
interstitial characteristics, remodeling of the cytoskeleton and
disappearance of cell matrix adhesion; and then a decrease of cell
adhesion, elimination of cell polarity, empowerement of cell
motility and invasive ability (29). This process is usually accompanied
by decreased expression of epithelial phenotypic markers (e.g.,
E-cadherin and β-catenin) and increased expression of interstitial
phenotypic markers (e.g., vimentin, and N-cadherin) (29,30).
We employed proteomic analysis and found that the expression of
E-cadherin was significantly increased, and the expression of
vimentin and N-cadherin were significantly decreased after
downregulation of ADAM10 expression in FaDu cells (Fig. 6E). The results suggested that high
expression of ADAM10 may increase EMT regulation, and promote tumor
cell migration and infiltration in HSCC.
In conclusion, complex biological processes are
involved in the unrestricted tumor growth and metastasis of HSCC.
Therefore, it is very important to understand the biological
mechanism of the proliferation and metastasis of HSCC, which could
detect the potential genes that promote the proliferation and
metastasis of HSCC. In this study, our data clearly revealed that
ADAM10 was highly expressed in HSCC and promoted the proliferation,
migration and the poor prognosis of patients with HSCC. Thus,
ADAM10 may be an important molecular target of HSCC proliferation
and metastasis, with potentially important therapeutic
implications.
Acknowledgements
We would like to thank Zhifeng Gu for the
experimental help, and the Jiangsu Province Key Laboratory of
Neuroregeneration of Nantong University for their support. This
study was supported by the People's Livelihood Scientific and
Technological Innovation and Demonstration Promotion, Nantong,
Jiangsu (MS32016015).
References
|
1
|
Ligier K, Belot A, Launoy G, Velten M,
Bossard N, Iwaz J, Righini CA, Delafosse P and Guizard AV: network
Francim: Descriptive epidemiology of upper aerodigestive tract
cancers in France: Incidence over 1980–2005 and projection to 2010.
Oral Oncol. 47:302–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takes RP, Strojan P, Silver CE, Bradley PJ
Jr, Haigentz M Jr, Wolf GT, Shaha AR, Hartl DM, Olofsson J,
Langendijk JA, et al: International Head and Neck Scientific Group:
Current trends in initial management of hypopharyngeal cancer: The
declining use of open surgery. Head Neck. 34:270–281. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoffman HT, Karnell LH, Shah JP, Ariyan S,
Brown GS, Fee WE, Glass AG, Goepfert H, Ossoff RH and Fremgen AM:
Hypopharyngeal cancer patient care evaluation. Laryngoscope.
107:1005–1017. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Godballe C, Jørgensen K, Hansen O and
Bastholt L: Hypopharyngeal cancer: Results of treatment based on
radiation therapy and salvage surgery. Laryngoscope. 112:834–838.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee MS, Ho HC, Hsiao SH, Hwang JH, Lee CC
and Hung SK: Treatment results and prognostic factors in locally
advanced hypopharyngeal cancer. Acta Otolaryngol. 128:103–109.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung EJ, Lee SH, Baek SH, Park IS, Cho SJ
and Rho YS: Pattern of cervical lymph node metastasis in medial
wall pyriform sinus carcinoma. Laryngoscope. 124:882–887. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seals DF and Courtneidge SA: The ADAMs
family of metalloproteases: Multidomain proteins with multiple
functions. Genes Dev. 17:7–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mochizuki S and Okada Y: ADAMs in cancer
cell proliferation and progression. Cancer Sci. 98:621–628. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu X, Lu D, Scully M and Kakkar V: ADAM
proteins - therapeutic potential in cancer. Curr Cancer Drug
Targets. 8:720–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murphy G: The ADAMs: Signalling scissors
in the tumour microenvironment. Nat Rev Cancer. 8:929–941. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klein T and Bischoff R: Active
metalloproteases of the A disintegrin and metalloprotease (ADAM)
family: Biological function and structure. J Proteome Res.
10:17–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saftig P and Lichtenthaler SF: The alpha
secretase ADAM10: A metalloprotease with multiple functions in the
brain. Prog Neurobiol. 135:1–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vincent B: Regulation of the α-secretase
ADAM10 at transcriptional, translational and post-translational
levels. Brain Res Bull. 126:154–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janes PW, Saha N, Barton WA, Kolev MV,
Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen JP, Lackmann M
and Nikolov DB: Adam meets Eph: An ADAM substrate recognition
module acts as a molecular switch for ephrin cleavage in trans.
Cell. 123:291–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reiss K, Maretzky T, Ludwig A, Tousseyn T,
de Strooper B, Hartmann D and Saftig P: ADAM10 cleavage of
N-cadherin and regulation of cell-cell adhesion and beta-catenin
nuclear signalling. EMBO J. 24:742–752. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maretzky T, Reiss K, Ludwig A, Buchholz J,
Scholz F, Proksch E, de Strooper B, Hartmann D and Saftig P: ADAM10
mediates E-cadherin shedding and regulates epithelial cell-cell
adhesion, migration, and beta-catenin translocation. Proc Natl Acad
Sci USA. 102:9182–9187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moss ML, Stoeck A, Yan W and Dempsey PJ:
ADAM10 as a target for anti-cancer therapy. Curr Pharm Biotechnol.
9:2–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang W, Liu S, Liu K, Wang Y, Ji B, Zhang
X and Liu Y: A disintegrin and metalloprotease (ADAM)10 is highly
expressed in hepatocellular carcinoma and is associated with tumour
progression. J Int Med Res. 42:611–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
You B, Shan Y, Shi S, Li X and You Y:
Effects of ADAM10 upregulation on progression, migration, and
prognosis of nasopharyngeal carcinoma. Cancer Sci. 106:1506–1514.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo J, He L, Yuan P, Wang P, Lu Y, Tong F,
Wang Y, Yin Y, Tian J and Sun J: ADAM10 overexpression in human
non-small cell lung cancer correlates with cell migration and
invasion through the activation of the Notch1 signaling pathway.
Oncol Rep. 28:1709–1718. 2012.PubMed/NCBI
|
|
21
|
Wang YY, Ye ZY, Li L, Zhao ZS, Shao QS and
Tao HQ: ADAM 10 is associated with gastric cancer progression and
prognosis of patients. J Surg Oncol. 103:116–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu L, Liu N, Han Y, Xie C, Li Q and Wang
E: ADAM10 regulates proliferation, invasion, and chemoresistance of
bladder cancer cells. Tumour Biol. 35:9263–9268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jing P, Sa N and Xu W: miR-140-5p affects
the migration and invasion of hypopharyngeal carcinoma cells by
downregulating ADAM10 expression. Zhonghua Er Bi Yan Hou Tou Jing
Wai Ke Za Zhi. 51:189–196. 2016.(In Chinese). PubMed/NCBI
|
|
24
|
Siney EJ, Holden A, Casselden E, Bulstrode
H, Thomas GJ and Willaime-Morawek S: Metalloproteinases ADAM10 and
ADAM17 mediate migration and differentiation in glioblastoma
sphere-forming cells. Mol Neurobiol. Aug 19–2016.(Epub ahead of
print). PubMed/NCBI
|
|
25
|
Zepeda-Nuño JS, Guerrero-Velázquez C, Del
Toro-Arreola S, Vega-Magaña N, Ángeles-Sánchez J, Haramati J,
Pereira-Suárez AL and Bueno-Topete MR: Expression of ADAM10, Fas,
FasL and soluble FasL in patients with oral squamous cell carcinoma
(OSCC) and their association with clinical-pathological parameters.
Pathol Oncol Res. 23:345–353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Detre S, Jotti Saclani G and Dowsett M: A
‘quickscore’ method for immunohistochemical semiquantitation:
Validation for oestrogen receptor in breast carcinomas. J Clin
Pathol. 48:876–878. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Graham JK, Kunze E and Hammerstedt RH:
Analysis of sperm cell viability, acrosomal integrity, and
mitochondrial function using flow cytometry. Biol Reprod. 43:55–64.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Edwards DR, Handsley MM and Pennington CJ:
The ADAM metalloproteinases. Mol Aspects Med. 29:258–289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reiss K and Saftig P: The ‘a disintegrin
and metalloprotease’ (ADAM) family of sheddases: Physiological and
cellular functions. Semin Cell Dev Biol. 20:126–137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weber S and Saftig P: Ectodomain shedding
and ADAMs in development. Development. 139:3693–3709. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sahin U, Weskamp G, Kelly K, Zhou HM,
Higashiyama S, Peschon J, Hartmann D, Saftig P and Blobel CP:
Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six
EGFR ligands. J Cell Biol. 164:769–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Tetering G, van Diest P, Verlaan I,
van der Wall E, Kopan R and Vooijs M: Metalloprotease ADAM10 is
required for Notch1 site 2 cleavage. J Biol Chem. 284:31018–31027.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu S, Zhang W, Liu K, Ji B and Wang G:
Silencing ADAM10 inhibits the in vitro and in vivo
growth of hepatocellular carcinoma cancer cells. Mol Med Rep.
11:597–602. 2015.PubMed/NCBI
|