Introduction
Melanoma is the most dangerous type of skin tumor,
although it accounts for less than 5% of all skin tumors. It is
responsible for over 80% of all skin cancer-related deaths. In
2012, 232,000 new cases of melanoma and 55,000 melanoma-related
deaths were reported worldwide (1).
Moreover, the incidence of melanoma is increasing at a rate faster
than that of any other solid tumor, and is thought to be the
highest in white-skinned people living at low latitudes (2). In its advanced stages, melanoma is
highly malignant, owing to its potential for distant metastasis
(3), and an extremely low 5-year
survival rate (5–16%) (4).
Unfortunately, melanoma is refractory to conventional
chemotherapeutics, thus, the treatment options for patients with
advanced disease are limited (5).
Understanding the molecular mechanisms of melanoma may help to
improve the current therapeutic strategies.
The mitogen-activated protein kinase (MAPK)
signaling pathway is a key regulator of cellular growth and
proliferation, and has been found to play crucial roles in the
pathogenesis of melanoma. Most melanomas exhibit constitutive
activation of the MAPK pathway (6).
Mutations in the rapidly accelerated fibrosarcoma isoform B (BRAF)
lead to constitutive activation of the MAPK pathway and are
associated with poor outcome in melanoma (7,8). It
has been reported that more than half of the melanoma cases contain
BRAF mutations (9). Thus, a
distinct approach may be to use BRAF inhibitors to extend survival
in patients with metastatic melanoma (10). However, preexisting or acquired
resistance to these agents appears soon following a transient
response (11). In addition,
various studies have found that BRAF inhibitors may cause
acanthopapilloma, keratoacanthoma or cutaneous squamous-cell
carcinoma in the early stages of treatment (11,12).
Therefore, identification of additional core members of key
molecular pathways implicated in the pathogenesis of melanoma is
crucial for the design of novel therapies.
The RAF family of serine/threonine kinases is
comprised of three members: CRAF (RAF-1), BRAF and ARAF (13,14).
As aforementioned, BRAF is reportedly involved in the pathogenesis
of melanoma via the MAPK pathway. Notably, RAF-1 was also found to
play an important role in the activation of the MAPK pathway in
melanoma (15,16). RAF-1 dysregulation represents a
prominent resistance mechanism in melanoma (14,17).
Furthermore, RAF-1 can bind to serine/threonine kinase 3 (STK3),
also known as MST-2, thus influencing apoptosis (18,19).
Notably, MST-2 is one of the core components of the Hippo pathway
in mammals, which is involved in cell proliferation, growth, and
apoptosis (17,20). Alterations in the Hippo pathway have
been found to be associated with tumorigenesis, including melanoma
development (21,22). However MST-2, yes-associated protein
(YAP) and tafazzin (TAZ) are also major effectors of the Hippo
pathway, and have been suggested to contribute to the metastatic
and invasive capacities of melanoma cells (23). Moreover, it has been demonstrated
that YAP can regulate the response of cancer cells to MAPK pathway
inhibitors (24). Tumorigenesis is
a consequence of the combined action of many factors and intricate
pathways. In light of the role of the MAPK and Hippo pathways and
their effectors (RAF-1, MST-2, YAP and TAZ) in melanoma, we
speculated that there may exist some links among these pathways via
their effectors in melanoma tumorigenesis.
In the present study, we assessed the expression
levels of RAF-1, MST-2, YAP and TAZ proteins of the MAPK and Hippo
pathways in four melanoma cell lines and found that RAF-1 formed a
complex with MST-2. We further investigated the effects of
RAF-1/MST-2 interaction on melanoma cell proliferation, migration
and invasion, as well as on the cell cycle and apoptosis of
melanoma cells. The present study, expounds on the interactions
between the MAPK and Hippo pathway members elucidating the
molecular mechanisms of melanoma tumorigenesis.
Materials and methods
Cell culture
Four melanoma cell lines, including C32, HS695T,
SK-MEL-28 and A375, were used in the present study. C32, HS695T and
SK-MEL-28 were cultured in minimum essential medium (MEM). A375 was
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
100 U/ml penicillin, 10% fetal bovine serum (FBS), and 100 µg/ml
streptomycin. The cell lines were maintained in a humid atmosphere
at 37°C and 5% CO2.
Western blot analysis
After being washed with ice-cold phosphate-buffered
saline (PBS), the four cell lines were lysed in whole-cell
extraction buffer. Proteins (20 µl) were resolved using 10% sodium
dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE),
and transferred to polyvinylidene fluoride (PVDF) membranes. The
membranes were blocked with 5% skim milk and incubated overnight at
4°C with primary antibodies specific to human RAF-1, YAP and TAZ
(sc-227, sc-15407 and sc-17130, respectively; 1:1,000; Santa Cruz
Biotechnology, Santa Cruz, CA, USA); MST-2 (#3952; 1:1,000; Cell
Signaling Technology, Boston, MA, USA); and glyceraldehyde
3-phosphate dehydrogenase (GAPDH; 1:1,000; Santa Cruz
Biotechnology). Then, the membranes were incubated with the
appropriate secondary antibodies for 2 h at 25°C. The membranes
were washed and the protein bands were visualized using
3,3′-diaminobenzidine (DAB).
Immunoprecipitation
Cells were seeded in 6-well plates and lysed with
ice-cold radioimmunoprecipitation assay (RIPA) buffer. Homogenates
were precleared with 20 µl of Protein A-Agarose (Santa Cruz
Biotechnology) at 4°C for 1 h with rocking. RAF-1 proteins were
added as bait and immunoprecipitated with MST-2 and incubated
overnight at 4°C with rocking. The bound proteins were then
immunoprecipitated with Protein A-Agarose for 2 h at 4°C. After
being washed, the immune complexes were released in 15 µl of 2X SDS
loading buffer by boiling at 100°C for 5 min and resolved by
SDS-PAGE. The immune complexes were then transferred to PVDF
membranes and subjected to immunoblot analysis.
RAF-1 knockdown
Cells were seeded in plates 24 h before
transfection. RAF-1-siRNA and negative control siRNAs (Santa Cruz
Biotechnology) were transfected using Lipofectamine 2000 according
to the manufacturer's protocol. The efficacy and specificity of
RAF-1 knockdown were ascertained by western blot analysis.
Cell viability assay
The cell viability after RAF-1 knockdown was
determined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Transfected cells were cultured in a 96-well plate for 48 h
and 20 µl of MTT was added to each well. After incubation for 4 h,
150 µl of dimethyl sulfoxide (DMSO) was added and the optical
density (OD) at 490 nm was assessed. The inhibitory rate (IR) was
calculated as follows:
Inhibitory rate (IR) (%) = [1 -
(ODtransfection/ODcontrol)] × 100%
Cell migration assay
Wound-healing assay was used to detect the effects
of RAF-1-siRNA on the migration ability of cells. Cells in three
groups (RAF-1-siRNA, control-siRNA and no-siRNA control) were
cultured in 6-well plates. After the cells reached 90% confluence,
a wound track, ~5 mm in size, was scored in each dish. After 48 h,
the cells that migrated into the wounded area were visualized and
photographed. The healing rate (HR) was calculated as follows:
Healing rate (HR) (%) = [1 - (scratch area at each
time-point/scratch area at time 0)] × 100%
Cell invasion assay
The invasion assay was performed using Transwell
cell culture chambers as previously described (25). After 48 h of transfection, the cells
were harvested with 0.02% ethylenediaminetetraacetic acid (EDTA),
and suspended in serum-free MEM. Then, 100 µl of cell suspension at
a density of 2×105 cells/ml was added to the upper
chamber and MEM containing 20% FBS was added to the lower
compartment of the Transwell. The chambers were incubated at 37°C
for 4 h, and the cells on the upper surface of the filter, which
had not migrated, were removed with cotton swabs. The migrated
cells on the lower surface of the filter were collected, fixed with
methanol, and stained with hematoxylin. Cells in >10 random
fields of view were counted at a magnification of ×200, under an
inverted microscope.
Cell cycle assay
The cell cycle analysis of transfected cells was
performed using flow cytometry. Transfected cells were seeded into
a 6-cm dish at a density of 2×105 cells/ml, and cultured
in a 37°C incubator for 40 h. After being washed with ice-cold PBS,
the cells were fixed with ice-cold 70% ethanol for 30 min at 4°C,
and then resuspended in PBS. The suspension was filtered and
stained with propidium iodide (PI; BD Biosciences, San Jose, CA,
USA) for 30 min at 4°C in the dark. The DNA content was analyzed
using flow cytometry. Three independent experiments were
performed.
Cell apoptosis assay
The transfected cells were seeded in a culture dish
at a density of 2×105 cells/ml, and cultured at 37°C in
an incubator for 40 h. After being washed twice with ice-cold PBS,
the cells were resuspended in 1X binding buffer (BD Biosciences).
Apoptosis of the transfected cells was quantified by staining with
5 µl of Annexin-fluorescein isothiocyanate (FITC; BD Biosciences)
and 2.5 µl of PI. The cells were incubated on ice for 10 min in the
dark and 400 µl of 1X binding buffer was added. The apoptosis rates
were analyzed using flow cytometry.
Statistical analysis
Statistical analyses were performed using SPSS 15.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation (SD) from at least three independent
experiments. Data were analyzed by Student's t-test (for two
groups) or one-way analysis of variance (ANOVA; for three or more
groups). P<0.05 was considered to indicate a statistically
significant result.
Results
Expression of MAPK and Hippo pathway
members in human melanoma cell lines
The expression levels of the MAPK and Hippo pathway
effector proteins, RAF-1, MST-2, YAP and TAZ, in four human
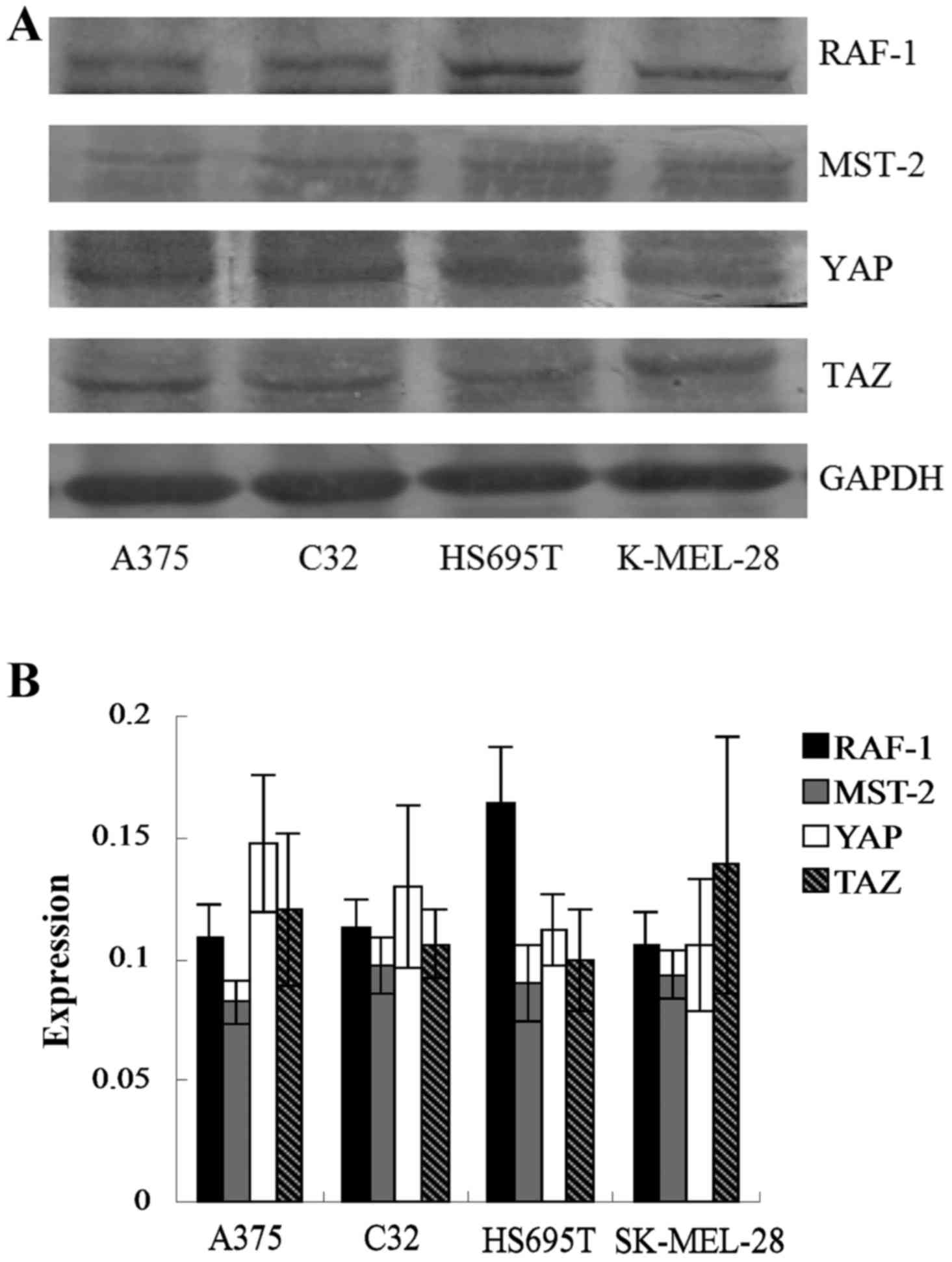
melanoma cell lines were assessed by western blotting. As shown in
Fig. 1A and B, the expression
levels of these proteins were variable across the panel. HS695T
cells expressed the highest levels of RAF-1. The expression levels
were not significantly different among the proteins and the four
cell types (P>0.05).
Relationship between Hippo and MAPK
pathway members in melanoma
The HS695T cells expressed the highest levels of
RAF-1, thus, the subsequent experiments were carried out in these
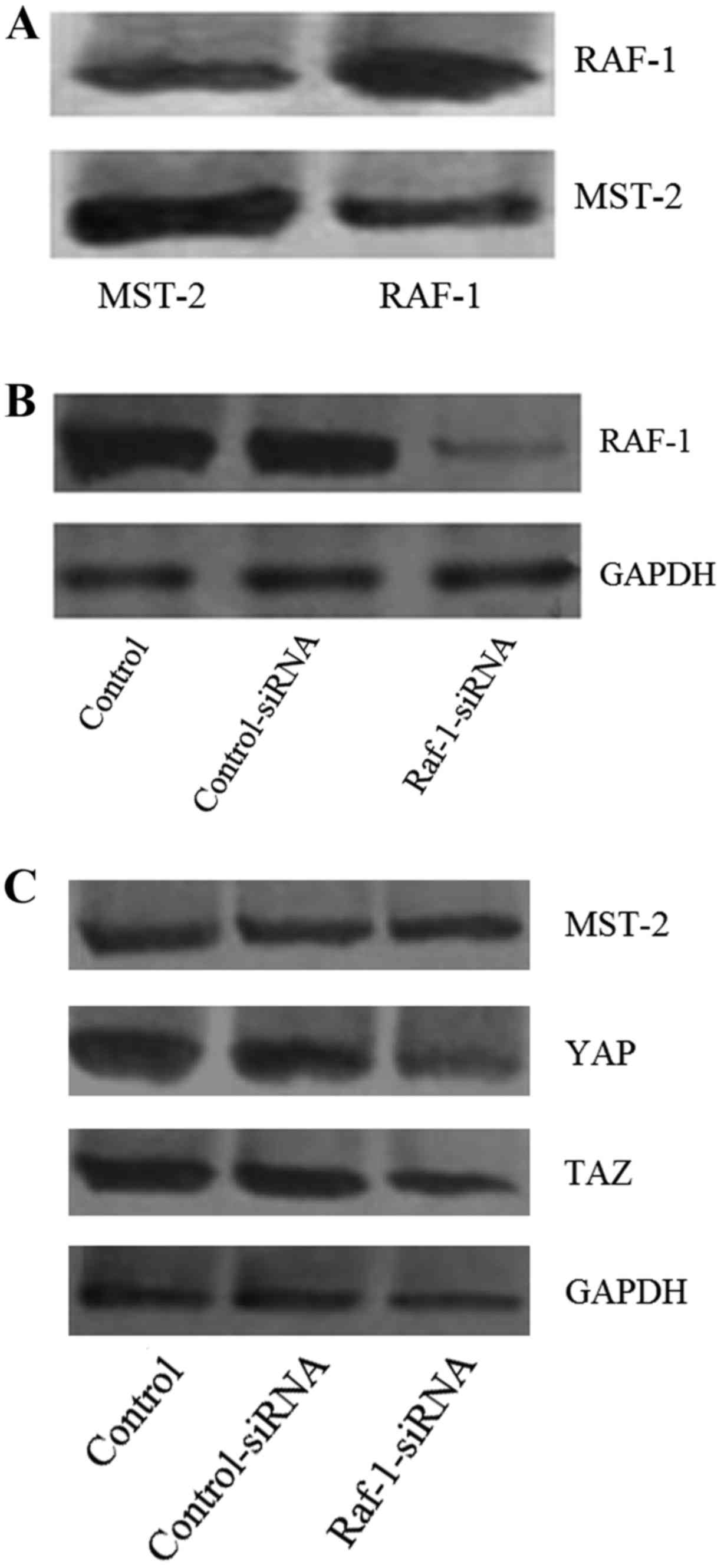
cells. To determine whether there was a causal relationship between
the Hippo and MAPK pathways, we performed immunoprecipitation in
HS695T cells using RAF-1 as bait. The immunoprecipitation
experiments revealed that RAF-1 was highly enriched with MST-2,
indicating that RAF-1 bound to MST-2 in melanoma cells (Fig. 2A). Therefore, the interaction
between RAF-1 and MST-2 may provide a link between the MAPK and
Hippo pathways in these cells. Furthermore, we performed
siRNA-mediated RAF-1 knockdown in melanoma cells. As shown in
Fig. 2B, the expression of RAF-1
decreased significantly after siRNA transfection. Additionally, as
presented in Fig. 2C, the
expression levels of YAP and TAZ also decreased significantly after
RAF-1 knockdown. However, the expression of MST-2 did not
significantly change, which further suggests that RAF-1 bound to
MST-2 (Fig. 2C).
Effect of RAF-1 knockdown on melanoma
cell viability, migration and invasion
To determine whether the RAF-1/MST-2 complex
contributed to the tumorigenicity of melanoma, we performed MTT,
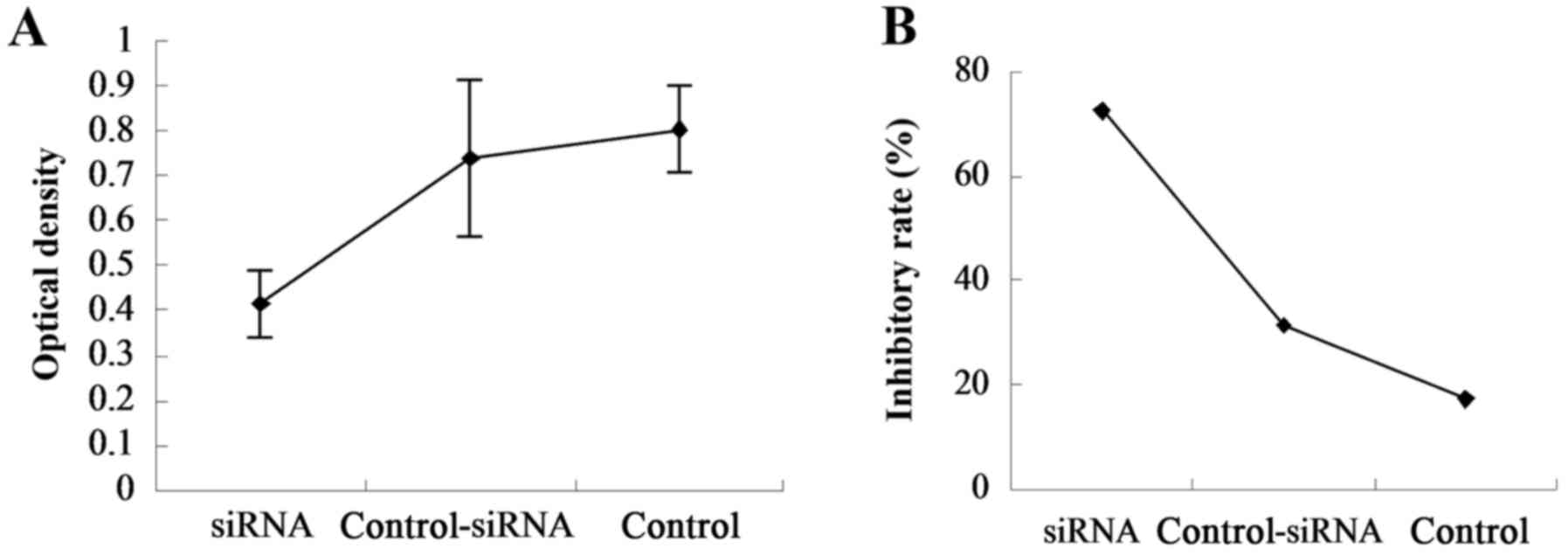
wound-healing and cell invasion assays. The MTT assay was used to
determine cell viability after 48 h of RAF-1 knockdown. The ODs of
the RAF-1-siRNA, control-siRNA and no-siRNA control groups were
0.4157±0.0763, 0.7407±0.1734 and 0.8053±0.0991, respectively
(Fig. 3A), and their IRs were
72.56, 31.42 and 17.23%, respectively (Fig. 3B). Significant differences were
found among the three groups (P=0.000), indicating that RAF-1
knockdown significantly inhibited cell viability.
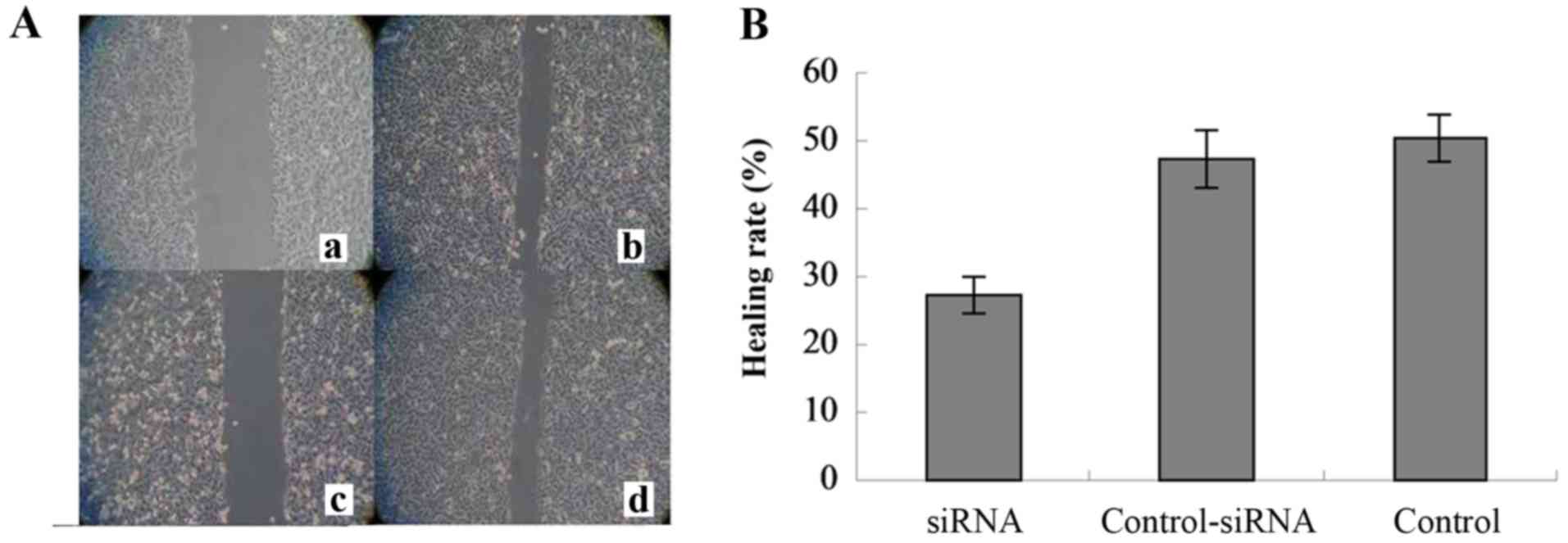
Additionally, wound-healing assay was carried out to
determine changes in cell migration after RAF-1 knockdown. As shown
in Fig. 4A, the number of migrated
cells was significantly decreased in the RAF-1-siRNA group, but
significantly increased in the control-siRNA and no-siRNA control
groups. The HRs of the RAF-1-siRNA, control-siRNA, and no-siRNA
control groups were 27.33±2.80, 47.30±4.26 and 50.47±3.38%,
respectively (Fig. 4B), and were
significantly different (P=0.000). These results indicate that
disruption of the RAF-1/MST-2 interaction inhibited cell
migration.
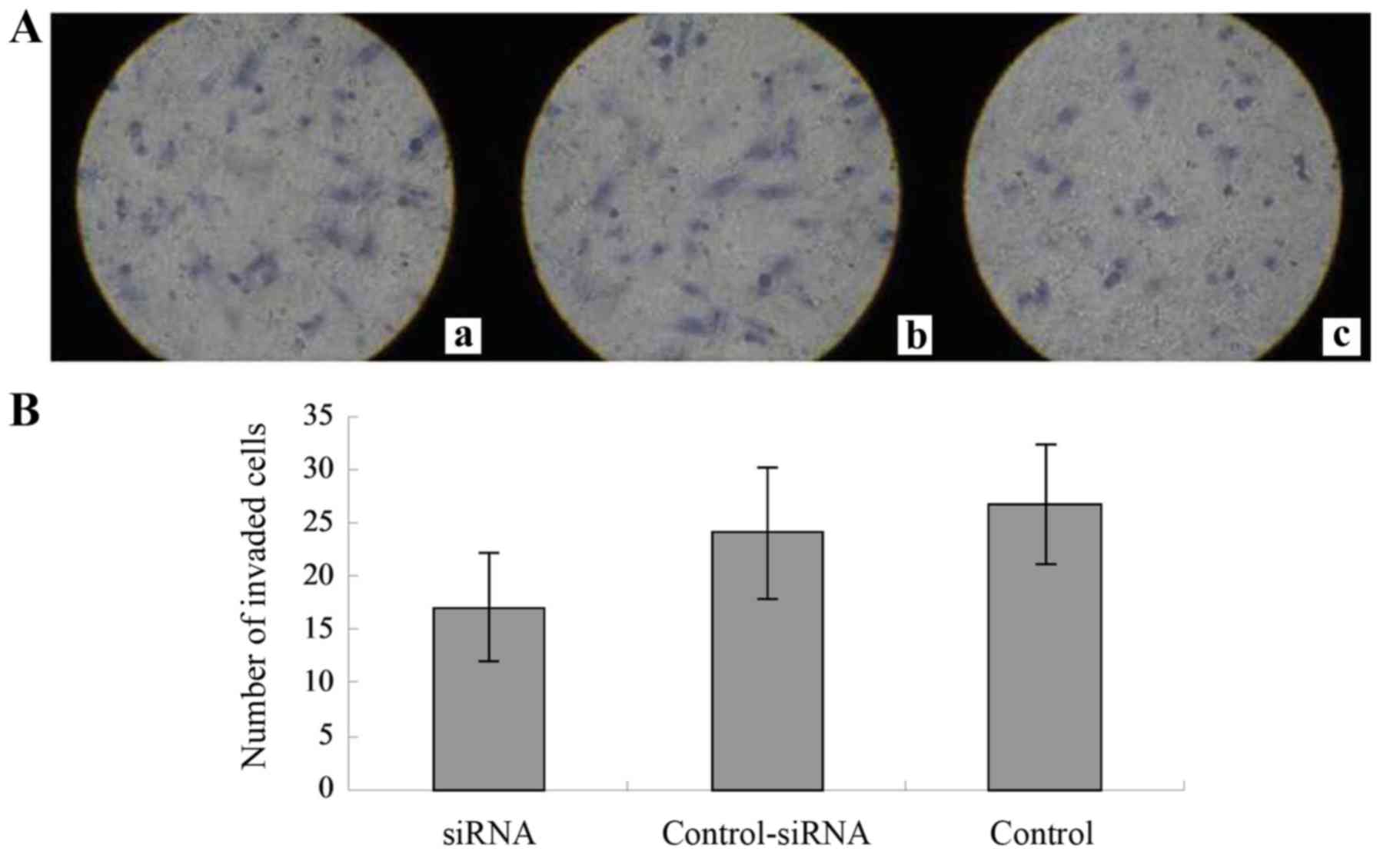
The Transwell cell culture chambers were used to
determine the effects of RAF-1 knockdown on cell invasion (Fig. 5A). The number of invaded cells for
the RAF-1-siRNA, control-siRNA, and no-siRNA control groups were
17.1±5.1, 24.1±6.2 and 26.8±5.6, respectively (Fig. 5B). There was a significant
difference between the RAF-1-siRNA and control groups (P=0.001).
These findings indicate that RAF-1 knockdown significantly
inhibited cell invasion.
Effect of RAF-1 knockdown on the cell
cycle and apoptosis of melanoma cells
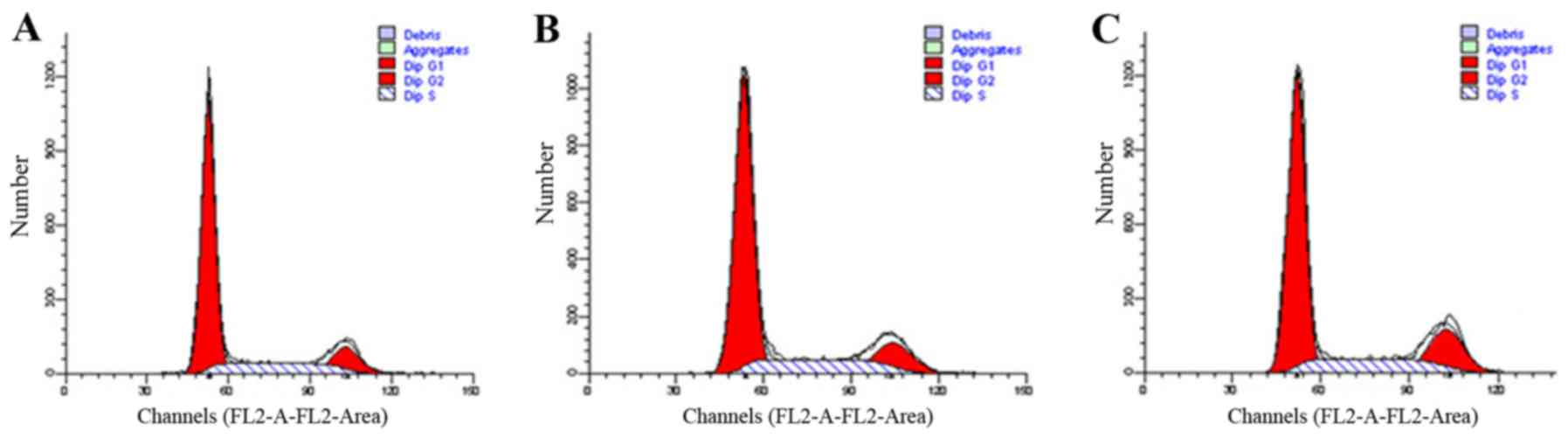
To determine whether RAF-1 knockdown affected the
cell cycle in melanoma cells, we performed flow cytometry after 48
h of RAF-1 knockdown. The results are shown in Fig. 6 and Table I. The percentages of cells in the G1
phase in the RAF-1-siRNA, control-siRNA, and no-siRNA control
groups were 64.31±4.50, 65.90±2.64 and 65.82±1.02%, respectively,
and the difference was not statistically significant (P=0.783).
Similarly, for cells in the G2 and S phases, no significant
difference was found among the three groups.
 | Table I.Cell cycle of HS695T cells after 48 h
of RAF-1 knockdown. |
Table I.
Cell cycle of HS695T cells after 48 h
of RAF-1 knockdown.
| Cell cycle | Control group
(%) | Control-siRNA
(%) | RAF-1-siRNA
(%) | F-value | P-value |
|---|
| G1 | 65.82±1.02 | 65.90±2.64 | 64.31±4.50 | 0.256 | 0.783 |
| G2 | 14.30±1.18 | 14.59±2.84 | 15.56±4.40 | 0.135 | 0.877 |
| S | 19.85±0.50 | 19.50±0.41 | 20.13±0.13 | 2.000 | 0.216 |
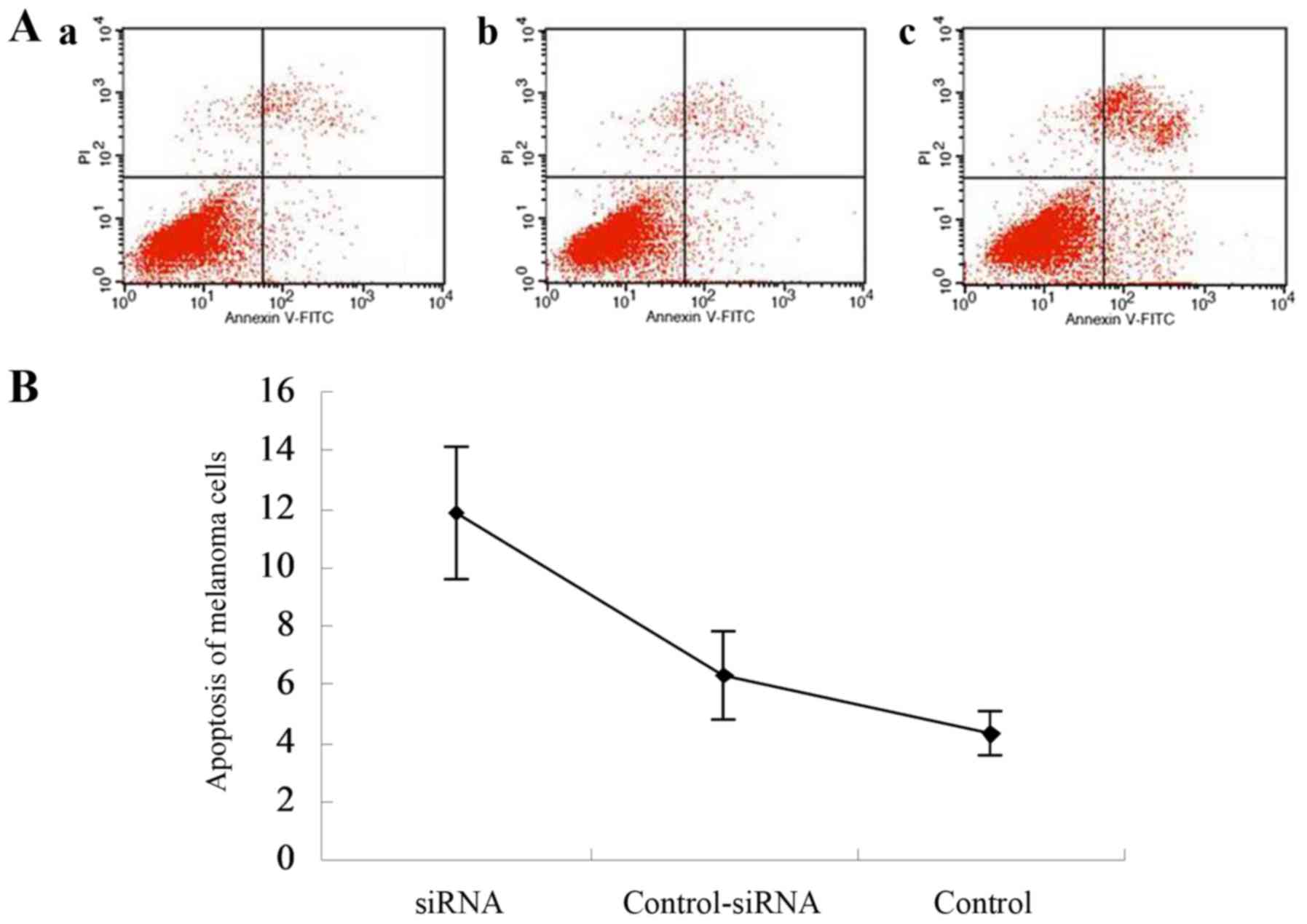
The apoptosis of melanoma cells 48 h after siRNA
transfection was also detected using flow cytometry. As shown in
Fig. 7A and B, the apoptosis rates
of melanoma cells were 11.88±2.25, 6.32±1.54 and 4.30±0.75 for the
RAF-1-siRNA, control-siRNA, and no-siRNA control groups,
respectively. Significant differences were found between the
control-siRNA and RAF-1-siRNA groups (P=0.006), and between the
no-siRNA control and RAF-1-siRNA groups (P=0.003). These findings
indicate that RAF-1 knockdown significantly increased the apoptosis
rate of melanoma cells.
Discussion
The present study, investigated the interaction
between MAPK and Hippo signaling pathway members (RAF-1, MST-2, YAP
and TAZ) in four malignant melanoma cell lines. The results
revealed that RAF-1, MST-2, YAP and TAZ were expressed in all the
melanoma cells examined. However, RAF-1 and MST-2 were found to
interact in HS695T cells. Furthermore, preventing the formation of
the RAF-1/MST-2 complex (through knockdown of RAF-1) inhibited
melanoma cell viability, migration and invasion, and promoted
apoptosis. Our findings suggest that in malignant melanoma, the
RAF-1/MST-2 interaction may provide a functional link between the
MAPK and Hippo signaling pathways.
Activation of the MAPK signaling pathway, mainly
owing to oncogenic mutations in proto-oncogenes such as RAF-1,
plays a critical role in the promotion of melanoma cell
proliferation (26). The main
function of RAF-1 is to activate mitogen-activated
protein/extracellular signal-regulated kinase (ERK) kinase (MEK, a
component of the MAPK cascade) by direct phosphorylation (27). Additionally, disruption of RAF-1
leads to widespread apoptosis (28). Notably, this additional function
(i.e., controlling apoptosis) of RAF-1 is independent of MAPK and
is achieved by binding to MST-2.
MST-2 has been found to interact with both wild-type
and kinase-inactive RAF-1 by mass spectrometry analysis (29). It has been demonstrated that RAF-1
controls MST-2 activity by (29):
interfering with MST-2 dimerization, thus, permitting the
transphosphorylation of critical residues required for MST-2
activation (31), in addition to
recruiting a phosphatase to dephosphorylate these residues. The
MST-2 interaction domain is located between amino acids 150 and 303
in the RAF-1 regulatory domain (32). Dissociation of the RAF-1/MST-2
complex is associated with MST-2 activation, and subsequent
apoptosis. In a review study by Kyriakis (28), it has been suggested that MST-2
promotes death signaling, rather than inhibiting survival
signaling, which is not consistent with the present study. In the
present study, RAF-1 was found to bind MST-2 in melanoma cells. The
suppression of RAF-1 and subsequent prevention of the RAF-1/MST-2
complex formation not only promoted apoptosis, but also inhibited
melanoma cell proliferation significantly, which is in accordance
with the findings of a recent study by Romano et al
(30).
Given that RAF-1 is an effector of the MAPK
signaling pathway and MST-2 is a core component of the Hippo
pathway, we propose that the RAF-1/MST-2 interaction may provide a
link between these two pathways. Notably, Romano et al
(33) have reported that RAF-1
mutation stimulates both the Hippo and MAPK pathways,
simultaneously driving apoptosis and proliferation, whereas
concomitant MST-2 downregulation switches signaling to cell
proliferation, transformation and survival.
The Hippo pathway plays a tumor-suppressor role and
is mutated in a variety of cancers (21). This pathway is comprised of a
cascade of kinases, including MST-2, YAP and TAZ. MST-2 is an
upstream regulator of this pathway, while YAP and its paralog TAZ
are the downstream effectors (23).
A recent study found that YAP and TAZ contribute to the metastatic
and invasive capacities of melanoma cells (23). Lamar et al (34) have demonstrated that YAP
overexpression, with exclusive nuclear localization, improves the
metastatic potential of melanoma cells. Based on the results of the
present study, we postulate that RAF-1 binding may inactivate
MST-2, consequently inactivating the Hippo pathway, and resulting
in the accumulation of YAP and TAZ. The accumulation of YAP and TAZ
may subsequently activate the target genes involved in cell
proliferation and invasion. Upon knockdown of RAF-1, MST-2 could be
released and activated, leading to the activation of the Hippo
pathway. Yu et al (35)
recently found that the Hippo pathway inhibited the expression of
YAP and TAZ to regulate cell proliferation, apoptosis and
differentiation, thus, modulating tissue growth and homeostasis.
We, therefore, speculate that the downregulation of YAP and TAZ in
the present study may be attributed to the activation of the Hippo
pathway. In turn, the downregulation of YAP and TAZ may be
associated with the inhibition of melanoma cell invasion and
metastasis. Collectively, our data provides novel evidence
suggesting that endogenous RAF-1/MST-2 interaction contributes to
the deregulation of the Hippo pathway and results in the metastatic
behavior of melanoma cells. Moreover, the results further reveal an
intricate connection between the MAPK and Hippo pathway effector
molecules.
In conclusion, our results indicate that the
RAF-1/MST-2 interaction may be a novel link between the MAPK and
Hippo pathways. Targeting this interaction may serve as a novel
approach in the treatment of melanoma. However, further studies are
needed to elucidate the mechanisms underlying the crosstalk between
the two pathways.
Acknowledgements
The present study was supported by a special fund
for medical service from the Jilin Finance Department
(SCZSY201507).
References
|
1
|
Stewart BW and Wild C: International
Agency for Research on Cancer. World Health Organization (ed) Lyon,
France: International Agency for Research on Cancer; pp.
6302014
|
|
2
|
Bataille V, Winnett A, Sasieni P, Bishop
Newton JA and Cuzick J: Exposure to the sun and sunbeds and the
risk of cutaneous melanoma in the UK: A case-control study. Eur J
Cancer. 40:429–435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gray-Schopfer V, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
La Porta CA: Drug resistance in melanoma:
New perspectives. Curr Med Chem. 14:387–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Todd JR, Scurr LL, Becker TM, Kefford RF
and Rizos H: The MAPK pathway functions as a redundant survival
signal that reinforces the PI3K cascade in c-Kit mutant melanoma.
Oncogene. 33:236–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Greaves WO, Verma S, Patel KP, Davies MA,
Barkoh BA, Galbincea JM, Yao H, Lazar AJ, Aldape KD, Medeiros LJ,
et al: Frequency and spectrum of BRAF mutations in a
retrospective, single-institution study of 1112 cases of melanoma.
J Mol Diagn. 15:220–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flaherty KT, Infante JR, Daud A, Gonzalez
R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N,
et al: Combined BRAF and MEK inhibition in melanoma with BRAF V600
mutations. N Engl J Med. 367:1694–1703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Viola JR, Rafael DF, Wagner E, Besch R and
Ogris M: Gene therapy for advanced melanoma: Selective targeting
and therapeutic nucleic acids. J Drug Deliv. 2013:897348. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su F, Viros A, Milagre C, Trunzer K,
Bollag G, Spleiss O, Reis-Filho JS, Kong X, Koya RC, Flaherty KT,
et al: RAS mutations in cutaneous squamous-cell carcinomas
in patients treated with BRAF inhibitors. N Engl J Med.
366:207–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oberholzer PA, Kee D, Dziunycz P, Sucker
A, Kamsukom N, Jones R, Roden C, Chalk CJ, Ardlie K, Palescandolo
E, et al: RAS mutations are associated with the development
of cutaneous squamous cell tumors in patients treated with RAF
inhibitors. J Clin Oncol. 30:316–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baccarini M: An old kinase on a new path:
Raf and apoptosis. Cell Death Differ. 9:783–785. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antony R, Emery CM, Sawyer AM and Garraway
LA: C-RAF mutations confer resistance to RAF inhibitors. Cancer
Res. 73:4840–4851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Avruch J, Khokhlatchev A, Kyriakis JM, Luo
Z, Tzivion G, Vavvas D and Zhang XF: Ras activation of the Raf
kinase: Tyrosine kinase recruitment of the MAP kinase cascade.
Recent Prog Horm Res. 56:127–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hüser M, Luckett J, Chiloeches A, Mercer
K, Iwobi M, Giblett S, Sun XM, Brown J, Marais R and Pritchard C:
MEK kinase activity is not necessary for Raf-1 function. EMBO J.
20:1940–1951. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Avruch J, Zhou D, Fitamant J, Bardeesy N,
Mou F and Barrufet LR: Protein kinases of the Hippo pathway:
Regulation and substrates. Semin Cell Dev Biol. 23:770–784. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
ONeill E, Rushworth L, Baccarini M and
Kolch W: Role of the kinase MST2 in suppression of apoptosis by the
proto-oncogene product Raf-1. Science. 306:2267–2270. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas NE, Edmiston SN, Alexander A,
Millikan RC, Groben PA, Hao H, Tolbert D, Berwick M, Busam K, Begg
CB, et al: Number of nevi and early-life ambient UV exposure are
associated with BRAF-mutant melanoma. Cancer Epidemiol
Biomarkers Prev. 16:991–997. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Praskova M, Xia F and Avruch J:
MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell
proliferation. Curr Biol. 18:311–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pollock PM, Harper UL, Hansen KS, Yudt LM,
Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J,
et al: High frequency of BRAF mutations in nevi. Nat Genet.
33:19–20. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nallet-Staub F, Marsaud V, Li L, Gilbert
C, Dodier S, Bataille V, Sudol M, Herlyn M and Mauviel A:
Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in
cutaneous melanoma. J Invest Dermatol. 134:123–132. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin L and Bivona TG: The Hippo effector
YAP regulates the response of cancer cells to MAPK pathway
inhibitors. Mol Cell Oncol. 3:e10214412015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng HX, Wu LN, Xiao H, Du Q and Liang
JF: Inhibitory effects of dobutamine on human gastric
adenocarcinoma. World J Gastroenterol. 20:17092–17099. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chin L, Garraway LA and Fisher DE:
Malignant melanoma: Genetics and therapeutics in the genomic era.
Genes Dev. 20:2149–2182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hüser M, Luckett J, Chiloeches A, Mercer
K, Iwobi M, Giblett S, Sun XM, Brown J, Marais R and Pritchard C:
MEK kinase activity is not necessary for Raf-1 function. EMBO J.
20:1940–1951. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kyriakis JM: The integration of signaling
by multiprotein complexes containing Raf kinases. Biochim Biophys
Acta. 1773:1238–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Neill E, Rushworth L, Baccarini M and
Kolch W: Role of the kinase MST2 in suppression of apoptosis by the
proto-oncogene product Raf-1. Science. 306:2267–2270. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Romano D, Matallanas D, Weitsman G,
Preisinger C, Ng T and Kolch W: Proapoptotic kinase MST2
coordinates signaling crosstalk between RASSF1A, Raf-1, and Akt.
Cancer Res. 70:1195–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hay BA and Guo M: Coupling cell growth,
proliferation, and death. Hippo weighs in. Dev Cell. 5:361–363.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Avruch J, Zhou D, Fitamant J, Bardeesy N,
Mou F and Barrufet LR: Protein kinases of the Hippo pathway:
Regulation and substrates. Semin Cell Dev Biol. 23:770–784. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Romano D, Nguyen LK, Matallanas D, Halasz
M, Doherty C, Kholodenko BN and Kolch W: Protein interaction
switches coordinate Raf-1 and MST2/Hippo signalling. Nat Cell Biol.
16:673–684. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lamar JM, Stern P, Liu H, Schindler JW,
Jiang ZG and Hynes RO: The Hippo pathway target, YAP, promotes
metastasis through its TEAD-interaction domain. Proc Natl Acad Sci
USA. 109:E2441–E2450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu FX, Meng Z, Plouffe SW and Guan KL:
Hippo pathway regulation of gastrointestinal tissues. Annu Rev
Physiol. 77:201–227. 2015. View Article : Google Scholar : PubMed/NCBI
|