Introduction
In the past few years, the prognosis for
hepatocellular carcinoma patients has improved through treatment
with a combination of chemotherapy and aggressive surgical
resection. However, patients with local relapse or distant
metastasis have a poor prognosis (1–3).
Hence, searching for brand-new approaches to treat relapsing and/or
metastatic hepatocellular carcinoma has become essential. Recently,
more and more studies have reported that microRNAs (miRNAs) are
closely associated with cancer. miRNAs are a recently discovered
series of non-coding small RNAs that exert their functions via
regulation of diverse gene expression. Mature miRNAs achieve their
functions via merging with an RNA-inducing silencing complex (RISC)
and binding to respective complementary sites within the 3
untranslated region (3′UTR) of the mRNA of the specific target
genes, thus hindering translation or directly inducing degradation
(4–7).
miRNA expression significantly varies with various
types of cancers. In addition, it could potentially be a notable
diagnostic and/or prognostic tool (8). It is important to clarify the effect
of miRNAs on the pathogenic mechanisms and progression of tumors as
a result of miRNAs feasible regulation of various critical
biological processes, including the differentiation, progression,
apoptosis, proliferation and sensitivity to treatment of tumor
cells (9). However, the expression
and dysregulation of miRNAs in hepatocellular carcinoma are still
unclear and hepatocellular carcinoma exhibits a poor prognosis and
high possibility of tumor proliferation and migration. Although
results from numerous studies have identified proliferation and
migration as the causes of death from solid tumors, not much was
known concerning the molecular mechanism underlying them.
Bioinformatic algorithms estimate that up to 30% of
human genes are modulated by all the human miRNAs, which may affect
most genetic pathways (10).
Numerous studies have clarified specific miRNA expression profiles
of a variety of cancer tissues compared to those of normal adjacent
tissues. miRNAs may have either a suppressive or promotive effect
on tumors via the cellular microenvironment and targeting the genes
that they regulate (11,12). According to previous studies,
miR-138 was confirmed to be significantly downregulated in various
types of cancer and play a key role as a tumor suppressor among
these functional miRNAs (13). For
example, miR-138 suppresses nasopharyngeal carcinoma proliferation
and migration (14). miR-138
inhibits tumor growth through suppression of EZH2 in non-small cell
lung cancer (15). miR-138 inhibits
the proliferation of non-small cell lung cancer cells by targeting
3-phosphoinositide-dependent protein kinase-1 (16). Moreover, one study found that
miR-138 was downregulated in 77.8% (14/18) of HCC tissues compared
with adjacent non-tumor tissues, which demonstrated the involvement
of miR-138 in hepatocellular carcinoma proliferation and metastasis
(17). However, the biological role
and mechanism of miR-138 in hepatocellular carcinoma have yet to be
reported. These aforementioned studies inspired us to investigate
the detailed functions and mechanism of miR-138, a well-established
tumor-suppressor miRNA, in HCC.
Sirtuins are a series of class III histone
deacetylases that are dependent on nicotinamide adenine
dinucleotide (NAD+) and are conserved across species.
There are seven members in the mammalian sirtuin family, including
sirtuin type 1 (SIRT1) to SIRT7. They are characterized by a
conserved 275-amino acid catalytic core and specifically added
N-terminal and/or C-terminal sequences of diverse lengths (18). Among all the members, SIRT1 is the
most studied sirtuin. Studies have reported that it has >10
substrates, including Ku70, p53, NF-κB and forkhead transcription
factors (FOXOs). Through cooperation with different substrates,
SIRT1 responds to stress and DNA damage, and also affects cellular
responses to DNA damage (19).
In the present study, we revealed the regulatory
association between miR-138, known as a tumor suppressor, and
Sirt1, known as an oncogene. We demonstrated that miR-138
suppressed the proliferation and migration of hepatocellular
carcinoma cells, most likely by targeting Sirt1.
Materials and methods
Tissue samples, cell lines and cell
transfection
We obtained a total of 37 pairs of primary
hepatocellular carcinoma and their matched adjacent normal tissues
from patients who underwent surgical resections at Xiangya Hospital
of Central South University (Changsha, China). All samples were
snap-frozen in liquid nitrogen, and then stored at −80°C until
further use. This project was approved by the Ethics Committee of
Xiangya Hospital of Central South University. All patients informed
consents were obtained.
We purchased the human hepatocellular cell line L02
and four human hepatocellular carcinoma cell lines, including
HepG2, SMMC7721, Bel7404 and HCCM3 from the American Type Culture
Collection (ATCC; Manassas, VA, USA). We routinely cultured cells
in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (Gibco, CA, USA) and incubation
followed at 37°C in a humidified atmosphere of 5% CO2.
By transfection with miR-138 mimics we achieved ectopic expression
of miR-138 in cells (GenePharma, Shanghai, China) using
Lipofectamine 2000 (Invitrogen). We performed overexpression of
Sirt1 using Sirt1 ORF expression clone (GeneCopoecia, Guangzhou,
China). Then, we plated cells in 6-well clusters or 96-well plates
and transfected them for 24 or 48 h. Transfected cells were used in
further assays or RNA/protein extraction.
RNA extraction and SYBR-Green
quantitative PCR analysis
We extracted total RNA from cells using TRIzol
reagent (Invitrogen), and detected mature miR-138 expression in
cells using a Hairpin-it™ miRNAs qPCR Quantitation kit
(GenePharma). We used the expression of RNU6B and L02 cells as
endogenous controls and used SYBR-Green qPCR assay (Takara, Dalian,
China) to assess the expression of Sirt1. The 2−ΔΔCt
method was used to process the data.
CCK-8 cell proliferation assay
Cell Counting Kit-8 (CCK-8) (Beyotime, Hangzhou,
China) was used to assess cell proliferation rates. We seeded
0.5×104 cells/well in a 96-well plate for 24 h,
transfected them with the indicated miRNA or siRNA, and further
incubated the cells for 24, 48, 72 and 96 h, respectively. One hour
before the endpoint of incubation we added 10 µl of CCK-8 reagent
to each well. A microplate reader was used to determine the
OD450nm value in each well.
Western blot analysis
The expression of Sirt1 and epidermal growth factor
receptor (EGFR) in hepatocellular carcinoma cell lines was detected
by performing immunoblotting. We lysed, cultured or transfected
cells in RIPA buffer with 1% phenylmethylsulfonyl fluoride (PMSF)
and loaded protein onto an SDS-PAGE minigel and then transferred
the protein onto a polyvinylidene fluoride (PVDF) membrane. We
probed the blots with 1:1,000 diluted rabbit polyclonal Sirt1
antibody (Abcam, Cambridge, MA, USA) at 4°C overnight, and then
incubated them with an HRP-conjugated secondary antibody (1:5,000).
Subsequently, enhanced chemiluminescence (ECL) substrates
(Millipore, Billerica, MA, USA) were used to visualize the signals.
We used β-actin as an endogenous protein for normalization.
Luciferase reporter assay
PCR was performed to amplify a fragment of the 3′UTR
of Sirt1 (1,089 bp) containing the putative miR-138 binding site
(1510–1517) using the following primers: wt-Sirt1 (forward)
5′-CCGCTCGAGCACCAGTAAAACAAGGAACTTG-3′ and wt-Sirt1 (reverse)
5′-GAATGCGGCCGCTTTACAGAAACAAATGCAATGTTAC-3′. Then, we subcloned the
PCR product into a psiCHECK-2 vector (Promega, Madison, WI, USA)
immediately downstream to the luciferase gene sequence. We also
synthesized a psiCHECK-2 construct containing the 3′UTR of Sirt1
with a mutant seed sequence of miR-138 using the following primers:
mut-Sirt1 (forward) 5′-TTAAAATTTCCTACTTGTGTATAGAAATGGAAAG-3 and
mut-Sirt1 (reverse) 5′-ACAAGTAGGAAATTTTAATACAGTGGTTCTC-3′.
DNA sequencing was used to verify all
constructs
HepG2 and SMMC7721 cells were plated into 96-well
clusters, then co-transfected with 100 ng of constructs with or
without miR-138 precursors. At 48 h after transfection, a
Dual-Luciferase Reporter Assay System (Promega) was used to detect
luciferase activity. In addition, luciferase activity was
normalized to Renilla activity.
RNA immunoprecipitation
RNA immunoprecipitation assays were performed using
the Imprint RNA Immunoprecipitation kit (Sigma-Aldrich, St. Louis,
MO, USA) along with the AGO2 antibody (Cell Signaling, Rockford,
IL, USA). The AGO2 antibody was then recovered by protein A/G
beads. Sirt1 and miR-138 RNA levels in the immunoprecipitates were
assessed by qRT-PCR.
Statistical analysis
All data were obtained from three independent
experiments and are expressed as the mean ± SD and processed using
SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA). We
compared the expression of miR-138 in hepatocellular carcinoma
tissues and their matched adjacent normal bone and myeloid tissues
by Wilcoxon's-paired test, and estimated the differences among the
groups in the migration and invasion assays using Student's t-test
or one-way ANOVA. A P-value of <0.05 was considered to indicate
a statistically significant result.
Results
miR-138 is significantly downregulated
in hepatocellular carcinoma tissues and cell lines, and inhibits
the proliferation and invasion of hepatocellular carcinoma cell
lines
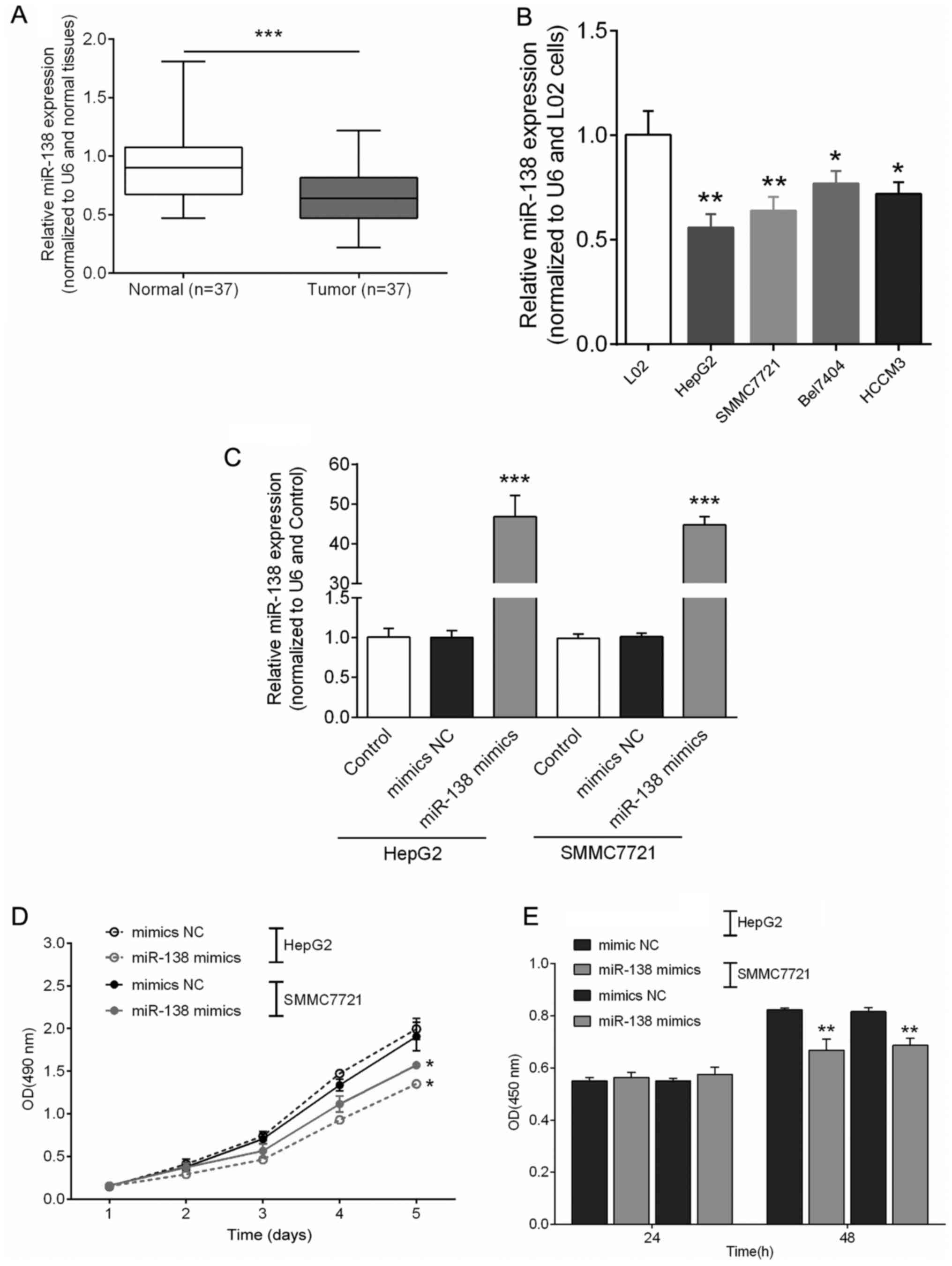
The expression levels of miR-138 in hepatocellular
carcinoma tissues and cell lines were detected by performing
SYBR-Green quantitative PCR analysis. Among the 37 cases of primary
hepatocellular carcinoma and their adjacent normal bone and myeloid
tissues, the results revealed that miR-138 expression was at a
significant lower expression level in 27 (73%) hepatocellular
carcinoma tissues compared with the adjacent normal tissues
(Fig. 1A). Moreover, miR-138
expression was attenuated in all of the four hepatocellular
carcinoma cell lines compared to that in the L02 cell line
(Fig. 1B). The HepG2 and SMMC7721
cell lines were transfected with miR-138 mimics, and the expression
of miR-138 was analyzed by real-time PCR. The endogenous miR-138
expression in both HepG2 and SMMC7721 cell lines was induced by
miR-138 mimics (Fig. 1C). The
effects of miR-138 on cell proliferation were determined using
CCK-8 assay and BrdU assay, respectively (P<0.05, P<0.01).
The results revealed that cell proliferation was notably inhibited
in both the HepG2 and SMMC7721 cell lines (Fig. 1D and E).
miR-138 efficiently suppresses
Sirt1
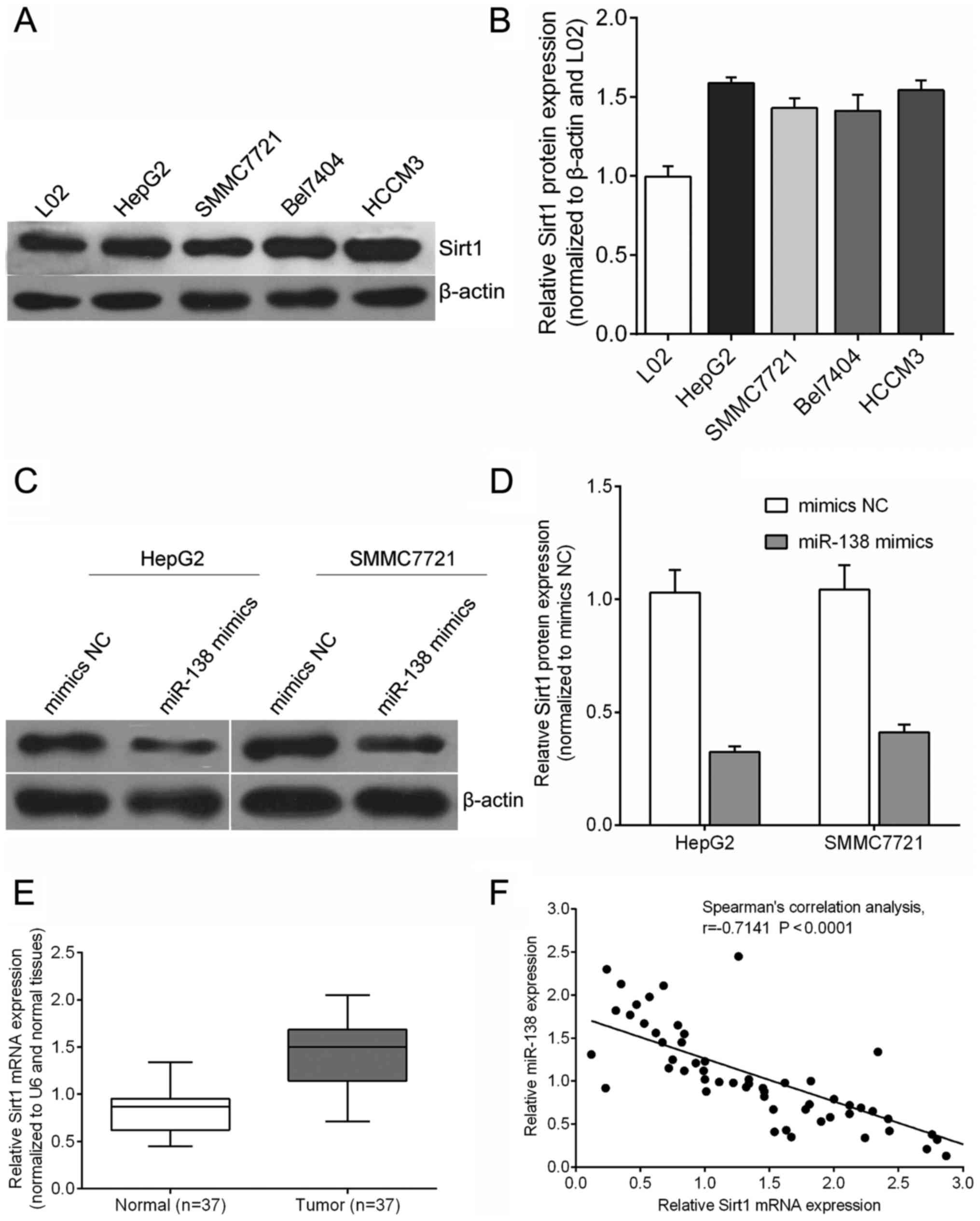
In hepatocellular carcinoma cells including HepG2,
SMMC7721, Bel7404 and HCCM3, the Sirt1 protein was at a higher
expression level compared with that in the human normal hepatic
cell line L02 (Fig. 2A and B). To
further explore the biological effect of miR-138 in hepatocellular
carcinoma tumor progression, we infected a lentivirus carrying
miR-SCR or miR-138 mimics into HepG2 and SMMC7721 cells.
Quantitative RT-PCR revealed that, at 72 h after infection, the
expression of the Sirt1 protein was downregulated in the miR-138
mimic infected cells as compared with the miR-SCR (mimics NC)
infected cells (Fig. 2C and D).
Moreover, Sirt1 mRNA expression was upregulated in tumor tissues
(Fig. 2E). As shown in Fig. 2F, the miR-138 expression was
significantly inversely correlated with Sirt1 mRNA expression
levels according to Spearman's correlation test in hepatocellular
carcinoma tissues, R2=0.518 (P<0.0001), confirming
that decreased miR-138 expression had a significant association
with increased Sirt1 mRNA expression in the same set of
hepatocellular carcinoma tissues (Fig.
2F).
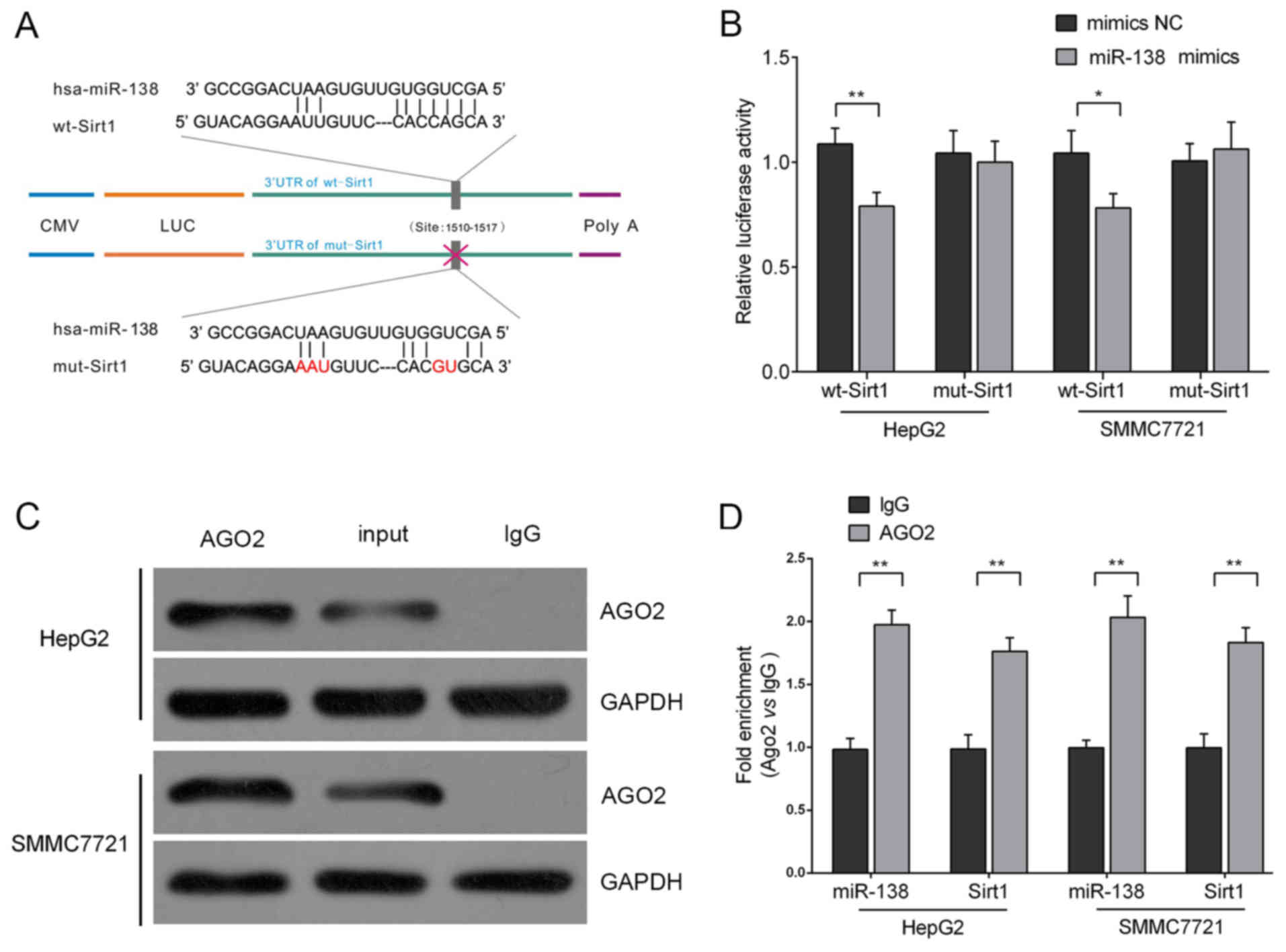
miR-138 directly targets Sirt1
We used TargetScan (www.targetsan.org) and microRNA.org
(www.microRNA.org) to predict the combining sites
of the Sirt1 3′UTR with miR-138, and its position was 403–409 of
the Sirt1 3′UTR. Then, we performed a sequential substitution of a
five-base pair region to produce a mutant vector. The 3′UTR of
Sirt1 (wt-Sirt1) was downstream cloned to a luciferase reporter
gene to further explore whether the forecasted binding site of
miR-138 to the 3′UTR of Sirt1 responded to this modulation. Its
mutant version (mut-Sirt1) by mutation at the binding site was also
constructed (Fig. 3A). The wt-Sirt1
vector and the miR-138 mimics or scrambled control were
co-transfected into HepG2 and SMMC7721 cells. The results revealed
that the luciferase activity of the miR-138 mimic transfected cells
were markedly attenuated compared to the scrambled control cells
(mimics NC). In addition, the mutant putative binding site
abolished the suppression of the luciferase activity mediated by
miR-138 (Fig. 3B). These data
revealed the possible existence of an RNA-induced silencing complex
(RISC complex) in both miR-138 and Sirt1. Argonaute2 (AGO2)
promotes the target mRNA degradation or inhibits its protein
translation; it has been regarded as the core component of RISC
(20). In the present study, we
further investigated the interaction between Sirt1 and miR-138 in
HepG2 and SMMC7721 cells using RNA immunoprecipitation assays with
the AGO2 antibody. As exhibited using western blot assays, the AGO2
protein could be precipitated from the cellular extract (Fig. 3C). In RNA extracted from the
precipitated AGO2 protein, we detected both miR-138 and Sirt1 with
a >1.8~2-fold enrichment compared to IgG (Fig. 3D), indicating that RISC existed in
both miR-138 and Sirt1.
miR-138 overexpression decreases the
protein level of Sirt1
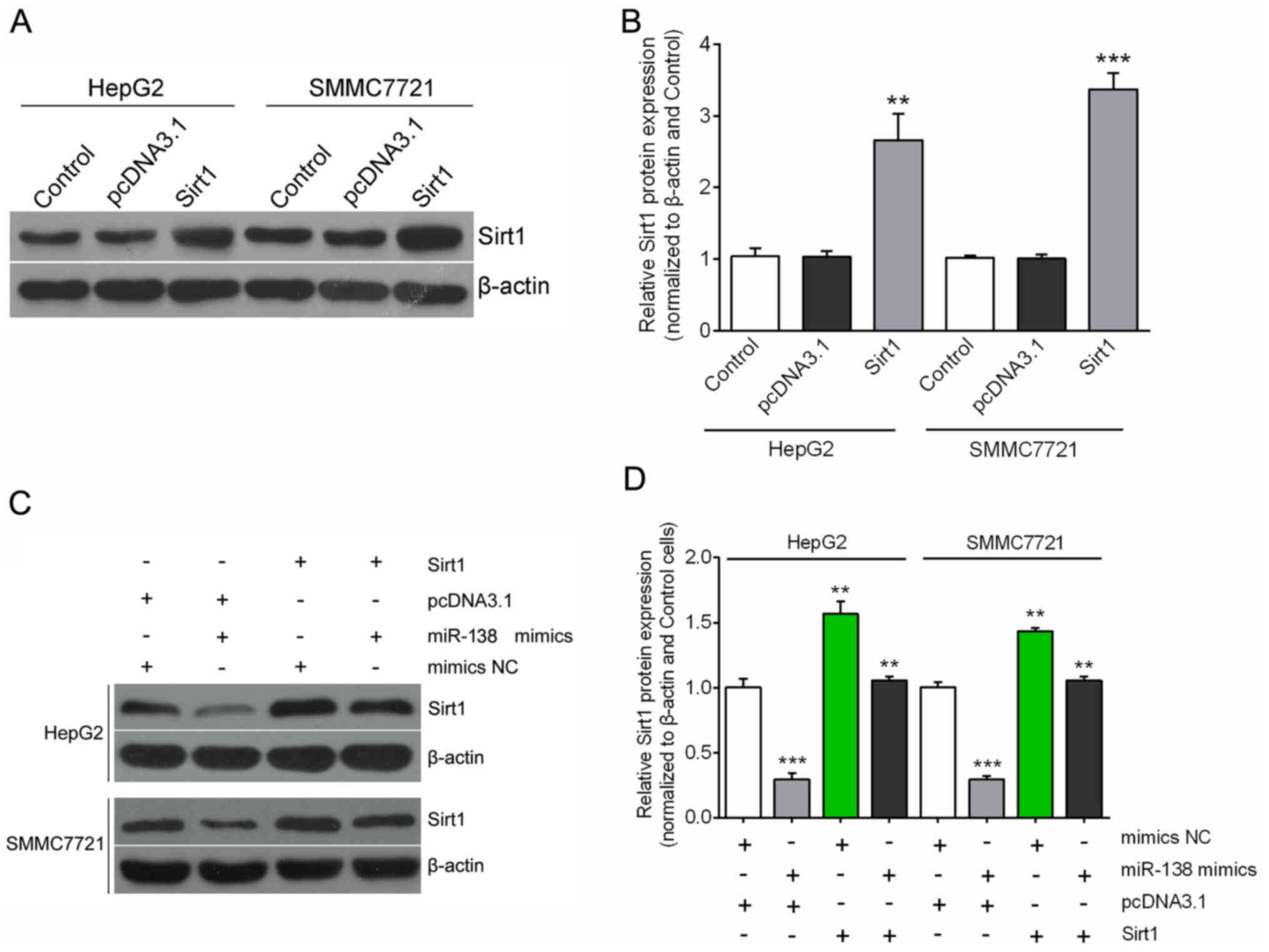
To further confirm the potential relationship
between miR-138 and its downstream gene Sirt1, we assessed the cell
growth and motility with overexpression of Sirt1. The expression of
Sirt1 was effectively upregulated by the Sirt1-ORF clone in both
HepG2 and SMMC7721 cell lines (Fig. 4A
and B). We transfected Sirt1 ORF-expressing plasmid or pcDNA3.1
into cells treated with miR-138 mimics or mimics NC. As shown in
Fig. 4C and D, the forced Sirt1
expression restored significant suppression on Sirt1 expression by
miR-138 (Fig. 4C and D).
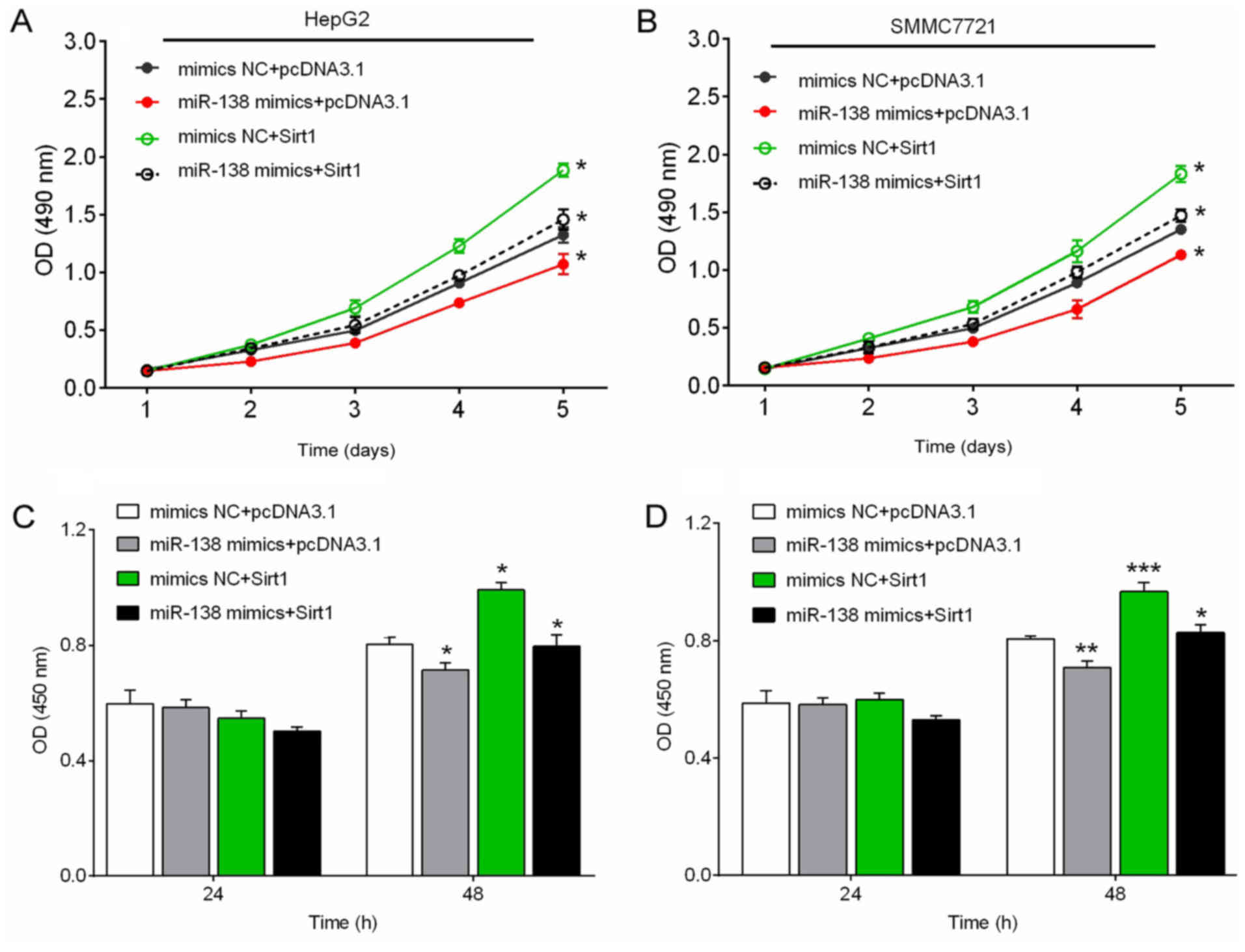
Forced expression of Sirt1 increases
hepatocellular carcinoma cell growth
Next, we investigated the effect of miR-138/Sirt1 on
the viability and proliferation of hepatocellular carcinoma cell
using CCK-8 and BrdU assays. The results revealed that, miR-138
mimic transfection significantly suppressed the cell viability and
proliferation of hepatocellular carcinoma cells, while forced Sirt1
expression promoted the cell viability and proliferation of
hepatocellular carcinoma cells. The suppressive effect of miR-138
on the cell viability and proliferation of hepatocellular carcinoma
cells could be partially abolished by forced Sirt1 expression
(Fig. 5A-D).
Discussion
Complications arising from metastasis cause most
cancer-related deaths. In view of this, treatment for metastatic
disease is a vital approach to defeat cancer. Previous studies on
tumor invasion and metastasis determined the key role of miRNAs in
these processes via the mechanism by which miRNA could regulate
various genes which are pivotal to proliferation, invasion or
metastasis (21,22). Recently, some miRNAs have been
confirmed to have a promotive (23–25) or
suppressive (26–28) effect on tumor invasion or
metastasis, and provide potential therapeutic targets to defeat
metastasis.
In hepatocellular carcinoma, miR-199a-3p expression
played a significant role in hepatocellular carcinoma cell growth
in vitro. Overexpression of miR-199a-3p by transfection
significantly attenuated hepatocellular carcinoma cell growth and
migration (29). Moreover,
miR-199a-3p was also demonstrated to regulate mTOR and Met to
influence doxorubicin sensitivity in liver cancer cells (30). In the present study, we proposed the
hypothesis that miR-138 may contribute to the hepatocellular
carcinoma metastatic process. Moreover, we confirmed the
relationship between miR-138 and Sirt1, which has been identified
as a positive tumor metastasis-related gene, and found that miR-138
inhibited hepatocellular carcinoma cell invasion and migration by
directly targeting Sirt1. Quantitative RT-PCR results ascertained
that miR-138 expression was commonly suppressed in hepatocellular
carcinoma cell lines and in 27 out of 37 (73.0%) enrolled
hepatocellular carcinoma patients, consistent to previous studies.
Subsequently, we restored the expression of miR-138 in HepG2 and
SMMC7721 cells and found that miR-138 suppressed cell proliferation
and invasion. Collectively, it was determined that miR-138
contributed to the processes of metastasis. Furthermore, the
expression levels of miR-138 had a reverse correlation with Sirt1
mRNA levels in hepatocellular carcinoma tissues. Sirt1 has been
identified as an independent prognostic indicator of metastasis
formation and metastasis-free survival. The present study,
ascertained a crucial molecular relationship between miR-138 and
Sirt1. We revealed that, upregulation of miR-138 expression in
HepG2 and SMMC7721 cells effectively downregulated Sirt1 expression
at both the mRNA and protein levels, while forced expression of
Sirt1 reversed the expression of Sirt1. A potential inverse
regulatory trend of miR-138 and Sirt1 was noted in hepatocellular
carcinoma cells, and the main effect of Sirt1 on the cells was an
autocrine effect, due to the downregulation of the level at the
cellular Sirt1 mRNA and protein by miR-138.
Furthermore, by performing a luciferase reporter
assay we confirmed that miR-138 directly targets the Sirt1 gene
through binding to the unique complementary site within its 3′UTR.
This result ascertained the key role of miR-138 in cellular
proliferation, migration and invasion via direct inhibition of the
expression of Sirt1. The aforementioned results confirmed the
inhibitory effect of miR-138 on Sirt1, and in addition in part
elucidated a potential molecular mechanism by which miR-138
participated in hepatocellular carcinoma invasion. Recently, Hurst
et al (31) suggested a new
series of cancer-related miRNAs known as metasta-miRs that are
observably involved in the metastatic processes. For example,
miR-21 is an inducer of metastasis that promotes cell survival,
migration, invasion and metastasis (32–34),
while the miR-200 family plays an essential role in tumor
suppresion and its deficiency contributes to the EMT phenotype
(35–37). miR-204, whose expression was
downregulated in different cancer cell lines, has currently been
identified as a direct post-transcriptional suppressor of Snai1
mRNA and consistent with its predicted tumor-suppressive role.
Suppressed expression of miR-204 led to loss of adhesion between
cells supporting the EMT-related properties of Snai1 (38). These metasta-miRs represent
potential candidate cancer prognostic markers and therapeutic
targets for metastatic cancers. In the present study, we revealed
that miR-138 functioned as a metasta-miR via targeting of Sirt1.
Moreover, miR-138 could potentially be a significant diagnostic and
prognostic tool to hepatocellular carcinoma.
In conclusion, we newly described the link between
miR-138 and Sirt1 and elucidated a potential mechanism in which
Sirt1 is regulated by miR-138 and contributes to the inhibition of
hepatocellular carcinoma cell proliferation and invasion. Moreover,
restoration of miR-138 expression was markedly implicated in the
clinical management of hepatocellular carcinoma.
Acknowledgements
The present study was supported by the Science and
Technology Plan Fund in Hunan Province, China (2011FJ3188).
References
|
1
|
Yao DF and Dong ZZ: Hepatocellular-related
gamma-glutamyl transferase in laboratory or clinical diagnosis of
hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 6:9–11.
2007.PubMed/NCBI
|
|
2
|
Gao J, Chen M and Ren H: [Clinical effects
of dendritic cells pulsed with autologous hepatocellular cell
lysates on the postoperative recurrence and metastasis of
hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi.
13:432–435. 2005.(In Chinese). PubMed/NCBI
|
|
3
|
Wang S and Fang W: Increased expression of
hepatocellular-derived growth factor correlates with poor prognosis
in human nasopharyngeal carcinoma. Histopathology. 58:217–224.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Filipowicz W: RNAi: The nuts and bolts of
the RISC machine. Cell. 122:17–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mishra PJ and Merlino G: MicroRNA
reexpression as differentiation therapy in cancer. J Clin Invest.
119:2119–2123. 2009.PubMed/NCBI
|
|
9
|
Ryan BM, Robles AI and Harris CC: Genetic
variation in microRNA networks: The implications for cancer
research. Nat Rev Cancer. 10:389–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu R, Zeng G, Gao J, Ren Y, Zhang Z, Zhang
Q, Zhao J, Tao H and Li D: miR-138 suppresses the proliferation of
oral squamous cell carcinoma cells by targeting Yes-associated
protein 1. Oncol Rep. 34:2171–2178. 2015.PubMed/NCBI
|
|
14
|
Liu X, Lv XB, Wang XP, Sang Y, Xu S, Hu K,
Wu M, Liang Y, Liu P, Tang J, et al: MiR-138 suppressed
nasopharyngeal carcinoma growth and tumorigenesis by targeting the
CCND1 oncogene. Cell Cycle. 11:2495–2506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Zhang H, Zhao M, Lv Z, Zhang X,
Qin X, Wang H, Wang S, Su J, Lv X, et al: MiR-138 inhibits tumor
growth through repression of EZH2 in non-small cell lung cancer.
Cell Physiol Biochem. 31:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye XW, Yu H, Jin YK, Jing XT, Xu M, Wan ZF
and Zhang XY: miR-138 inhibits proliferation by targeting
3-phosphoinositide-dependent protein kinase-1 in non-small cell
lung cancer cells. Clin Respir J. 9:27–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Zhao LJ, Tan YX, Ren H and Qi ZT:
MiR-138 induces cell cycle arrest by targeting cyclin D3 in
hepatocellular carcinoma. Carcinogenesis. 33:1113–1120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maiese K, Chong ZZ, Shang YC and Hou J:
Novel avenues of drug discovery and biomarkers for diabetes
mellitus. J Clin Pharmacol. 51:128–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knight JR and Milner J: SIRT1, metabolism
and cancer. Curr Opin Oncol. 24:68–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikeda K, Satoh M, Pauley KM, Fritzler MJ,
Reeves WH and Chan EK: Detection of the argonaute protein Ago2 and
microRNAs in the RNA induced silencing complex (RISC) using a
monoclonal antibody. J Immunol Methods. 317:38–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dalmay T and Edwards DR: MicroRNAs and the
hallmarks of cancer. Oncogene. 25:6170–6175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gaziel-Sovran A, Segura MF, Di Micco R,
Collins MK, Hanniford D, de Vega-Saenz Miera E, Rakus JF, Dankert
JF, Shang S, Kerbel RS, et al: miR-30b/30d regulation of
GalNAc transferases enhances invasion and immunosuppression during
metastasis. Cancer Cell. 20:104–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang CH, Yue J, Pfeffer SR, Handorf CR and
Pfeffer LM: MicroRNA miR-21 regulates the metastatic behavior of
B16 melanoma cells. J Biol Chem. 286:39172–39178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oneyama C, Morii E, Okuzaki D, Takahashi
Y, Ikeda J, Wakabayashi N, Akamatsu H, Tsujimoto M, Nishida T,
Aozasa K, et al: MicroRNA-mediated upregulation of integrin-linked
kinase promotes Src-induced tumor progression. Oncogene.
31:1623–1635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y and
Zheng X: miR-34a inhibits migration and invasion by down-regulation
of c-Met expression in human hepatocellular carcinoma cells. Cancer
Lett. 275:44–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang JH, Zhou HC, Zeng C, Yang J, Liu Y,
Huang X, Zhang JP, Guan XY and Zhuang SM: MicroRNA-29b suppresses
tumor angiogenesis, invasion, and metastasis by regulating matrix
metalloproteinase 2 expression. Hepatology. 54:1729–1740. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang
X, Jiang L, Sun Z, Miao Z and Xu H: MicroRNA-335 acts as a
metastasis suppressor in gastric cancer by targeting Bcl-w and
specificity protein 1. Oncogene. 31:1398–1407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang Y, Chen HC, Chiang CW, Yeh CT, Chen
SJ and Chou CK: Identification of a two-layer regulatory network of
proliferation-related microRNAs in hepatocellular cells. Nucleic
Acids Res. 40:10478–10493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fornari F, Milazzo M, Chieco P, Negrini M,
Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L and Gramantieri
L: MiR-199a-3p regulates mTOR and c-Met to influence the
doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res.
70:5184–5193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hurst DR, Edmonds MD and Welch DR:
Metastamir: The field of metastasis-regulatory microRNA is
spreading. Cancer Res. 69:7495–7498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang P, Zou F, Zhang X, Li H, Dulak A,
Tomko RJ Jr, Lazo JS, Wang Z, Zhang L and Yu J: microRNA-21
negatively regulates Cdc25A and cell cycle progression in colon
cancer cells. Cancer Res. 69:8157–8165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol
Chem. 283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang FE, Zhang C, Maminishkis A, Dong L,
Zhi C, Li R, Zhao J, Majerciak V, Gaur AB, Chen S, et al:
MicroRNA-204/211 alters epithelial physiology. FASEB J.
24:1552–1571. 2010. View Article : Google Scholar : PubMed/NCBI
|