Introduction
Cervical cancer (CC), one the most common
gynecological malignant tumors, is the second cause of
cancer-related death in women. In addition, there is no fully
effective treatment for CC at present (1). The currently available traditional
radiotherapies and chemotherapies for the treatment of CC have not
been able to reduce the rate of mortality, whereas the inevitable
side-effects of these mentioned remedies greatly reduce the life
quality of CC patients (2,3). To date, persistent human papilloma
virus (HPV) infection is considered as the major pathogen of CC.
Furthermore, considering the complexity of antigen and virus
subtype diversification, the research for a CC vaccine has
increasingly become difficult as well (4). Therefore, it is urgent to discover new
treatments to prolong the life of CC patients and to improve their
life quality.
Lactobacillus (lactic acid bacteria), a group
of normal bacteria in the woman's vagina, plays a vital role in
maintaining normal ecological balance in the vagina (5). Currently, several studies have
indicated that CC is closely associated with vaginal
micro-ecological balance disorders (6). Previous studies have revealed that a
decrease in lactobacillus could lead to the mass propagation of
vaginal Gardner or mixed anaerobic bacteria, resulting in
production of harmful substances from these two pathogenic
bacterial. These harmful substances mentioned above may combine
with other carcinogenic factors in the cervix (such as HPV) to
accelerate the development of CC (7). Previous studies suggest that
epithelial/interstitial transformation (EMT) is not only the basis
of the embryonic development process in multicellular organism and
organs, but also plays an important role in tumorigenesis, invasion
and metastasis, and the occurrence of a variety of chronic diseases
(8,9). E-cadherin, one of the most important
factors in EMT, promotes cell connection. In addition, it is also
demonstrated that downregulation of E-cadherin could be an
important factor of CC metastasis (10,11).
The present study aimed to investigate whether or
not lactobacillus intervention affects the cell migration of CC
cells, and to explore the corresponding pharmacological mechanisms,
which could be beneficial for identifying a new strategy for future
prevention or treatment of CC in the clinic.
Materials and methods
Cell lines and bacterial strain
Human carcinoma of the uterine cervix cell lines,
HeLa and U14, were supplied by the Department of Immunology, Hebei
Medical University (Shijiazhuang, China). Lactobacillus DM8909
(Lactobacillus debrueckii subsp. lactis) was kept in
our laboratory and cultured in magnetic resonance spectroscopy
(MRS) medium.
Animals
Female BALB/c mice (age, 4–6 weeks old; weight, 20±2
g) were purchased from the Experimental Animal Centre of Hebei
Medical University. They were kept at 21±1°C under a 12-h
light/dark cycle with free access to food and water. All animal
protocols were approved by the Animal Care and Use Committee of
Hebei Medical University.
Chemicals and reagents
Dulbeccos modified Eagles medium (DMEM), dimethyl
sulfoxide (DMSO) and trypsin were purchased from Gibco Co.
(Carlsbad, CA, USA). Fetal bovine serum (FBS) was purchased from
Hangzhou Sijiqing Biotech (Hangzhou, China). Penicillin and
streptomycin were purchased from North China Pharmaceutical Group
Corp. (Shijiazhuang, China). Primary antibodies for E-cadherin,
vimentin and FOXO4 were purchased from Bioworld Technology, Inc.
(St. Louis Park, MN, USA). Primary antibodies for Snail, Twist and
β-catenin were purchased from Abcam Co. (Cambridge, UK). Primary
antibody for GAPDH and HRP-conjugated secondary antibody were
purchased from SAB Co. (Linhai, China). BSA and PMA kits were
purchased from Sigma-Aldrich Co. (Shanghai, China). Cell lysis
buffer for western blotting and IP kit and hematoxylin and eosin
(H&E) staining kit were purchased from Beyotime Biotech
(Haimen, China). Biotin-labeled secondary antibody and DAB kit were
purchased from ZSGB-BIO Co. (Beijing, China). Cell Counting Kit-8
(CCK-8) kit was purchased from Promega Corporation (Madison, WI,
USA).
CCK-8 assay
HeLa and U14 cells were cultured in DMEM containing
10% (v/v) FBS, 100 U/ml streptomycin and 100 U/ml penicillin at
37°C with 5% CO2. Cells (5×103/100 µl) were
seeded in 96-well plates and cultured for 8 h at 37°C.
Subsequently, lactobacilli at different MOIs (10:1, 100:1 and
1,000:1) were added to the well and incubated for 24, 48 and 72 h,
respectively. Then, the plates were carefully washed with culture
medium to remove the lactobacilli and subsequently 10 µl CCK-8
reagent with culture medium was added to each well, and the cells
were cultured for another 2.5 h at 37°C with 5% CO2.
Optical density (OD) values were determined at 450 nm by an HT2
microplate reader (Thermo Fisher Scientific Co., Carlsbad, CA,
USA).
Wound healing assay
After the CCK-8 determination, a wound healing assay
was carried out according to the methods previously reported with
minor modifications (12). Briefly,
HeLa cells were cultured in 6-well plates for 24 h, and then a 10
µl sterile pipette was applied to scratch the cell layer to form a
linear wound. Then, the cells were washed 3 times with cold
phosphate-buffered saline (PBS) buffer, and incubated with
lactobacilli for another 24, 48 and 72 h, respectively. After being
washed with PBS buffer 3 times, migration of the cells in each
group was measured under an inverted microscope (LH50A; Olympus,
Tokyo, Japan).
Western blot assay
E-cadherin expression in the CC cells and CC tumor
tissues was examined by western blotting. Briefly, total proteins
of CC cells or tissues were harvested after treatment with cell
lysis buffer for western blotting and IP kit. Then, the protein
contents were determined and equal amounts of protein (40 µg) were
separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and subseqently blotted on
polyvinylidene difluoride (PVDF) membranes. Then, the protein bands
were probed with primary antibodies for the corresponding detecting
proteins, respectively and subsequently with goat anti-rabbit
horseradish peroxidase (HRP). Finally, the protein bands were
visualized and detected by a chemiluminescence method. In addition,
GAPDH was used as the internal reference to normalize the protein
loading, and the target protein expression levels were expressed as
a relative value to that of GAPDH.
Xenograft assay in vivo
The effects of lactobacilli were determined using an
xenograft animal model on female BALB/c mice according to a
previous study (13). Briefly, a
total of 25 mice were randomly divided into 5 groups (n=5): i)
PBS-treated mice; ii and iii) inactivated lactobacilli-treated mice
(2.25 and 5.0×106/mice/day); iv and v)
lactobacilli-treated mice (2.25 and 5.0×106/mice/day).
U14 cells (2.0×106/0.2 ml/mouse) were subcutaneously
injected into the right flank of each mouse. After tumors grew to
~2–3 mm in diameter, the mice were intraperitoneally injected
(i.p.) with PBS, inactivated lactobacilli (2.25 and
5.0×106/mice/day) or lactobacilli (2.25 and
5.0×106/mice/day) for a continuous 20 days. Tumor sizes
were measured every 4 days. Tumor volume (V) was calculated using a
reported formula (14): V =
(width2 × length)/2.
All mice were sacrificed after 21 days of treatment,
and the tumors were separated and weighed. Then, the tumor tissues
were collected for the following western blot and
immunohistochemistry assays.
Immunohistochemistry assay
Tumor tissue was collected and fixed in 10% formalin
and then tissue sections were prepared according to normal and
standard protocols. Then, tissue sections were stained with a
primary antibody for E-cadherin and subsequently stained with
biotin-labeled secondary antibody. Furthermore, quantitative
analysis of the results was performed using an image analysis
system (Motic Med 6.0; Motic China Group Co., Ltd., Tokyo,
Japan).
Statistical analysis
Data are presented as means ± SD. Differences
between groups were evaluated using multiple comparisons of the
data as performed by ANOVA with Dunnett's test. P-values <0.05
were considered significant.
Results
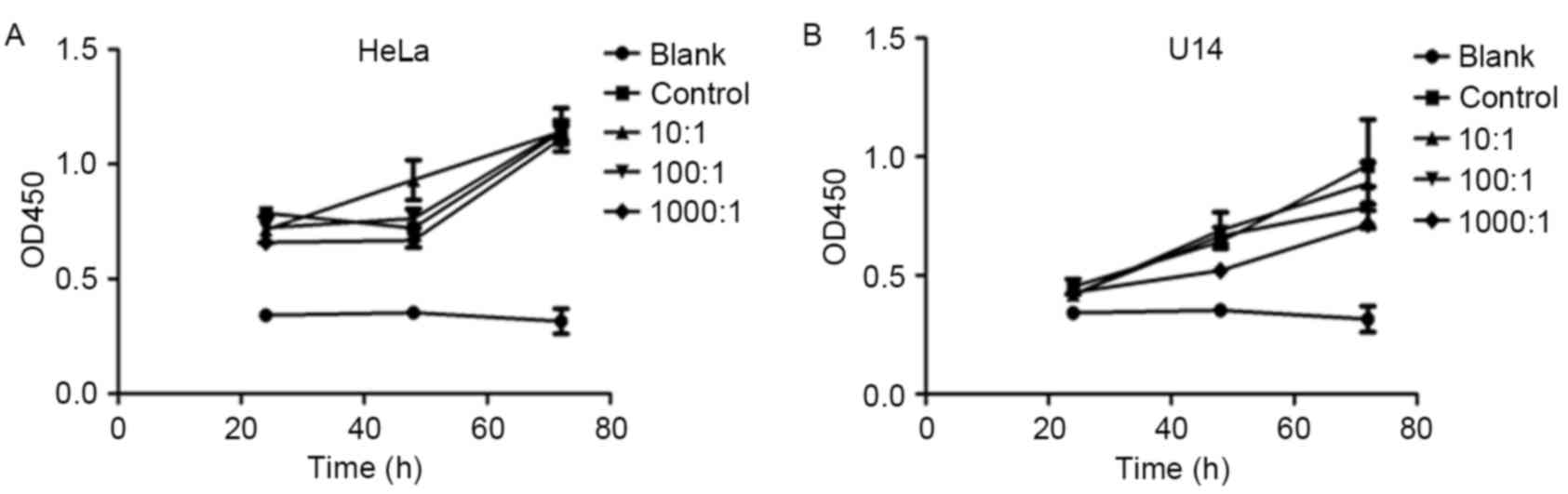
Lactobacilli at MOIs of 10:1, 100:1
and 1,000:1 had no effect on the proliferation of CC cell
lines
The cytotoxic effects of lactobacilli against CC
cell lines were determined using CCK-8 assays. As shown in Fig. 1, after incubation with lactobacilli
under the multiplicity of infection (MOI) of 10:1, 100:1 and
1,000:1 for 2, 48 and 72 h, the cell proliferation of both the HeLa
and U14 cell lines was not significantly affected (p>0.05,
p>0.05). These results showed that the MOIs selected did not
affect the cell viability of the CC cell lines, and were suitable
for further investigation of the inhibitory effect of lactobacilli
on cell migration.
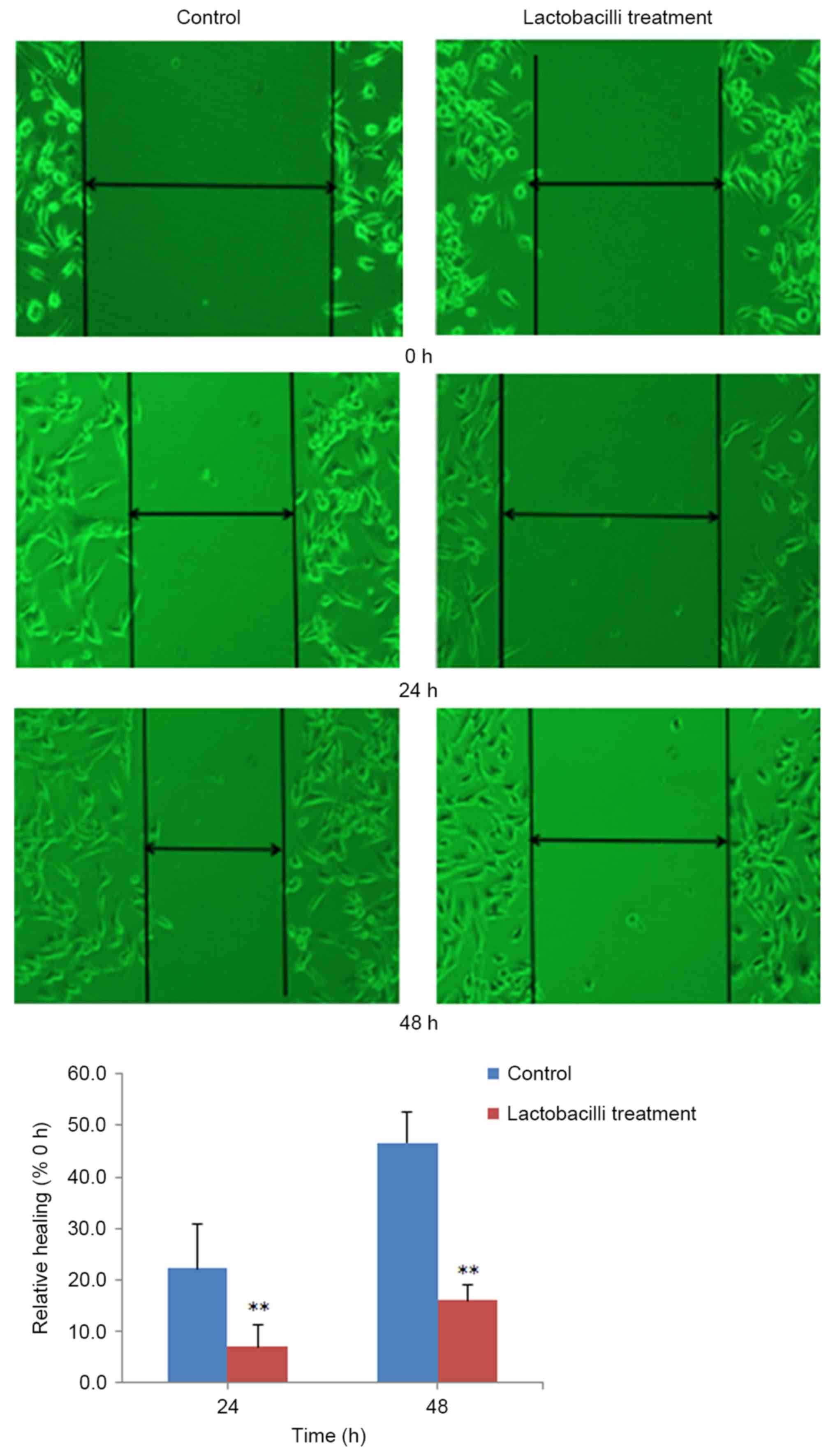
Lactobacilli significantly inhibit
cell migration of HeLa cells
After selecting suitable MOIs of lactobacilli, we
further investigated the effects of lactobacilli on the migration
of HeLa cells using wound healing assay. From the results shown in
Fig. 2, we concluded that
lactobacilli at the MOI of 1,000:1 obvious inhibited the migration
of the HeLa cells after incubation for 24 h (p<0.01) and 48 h
(p<0.01).
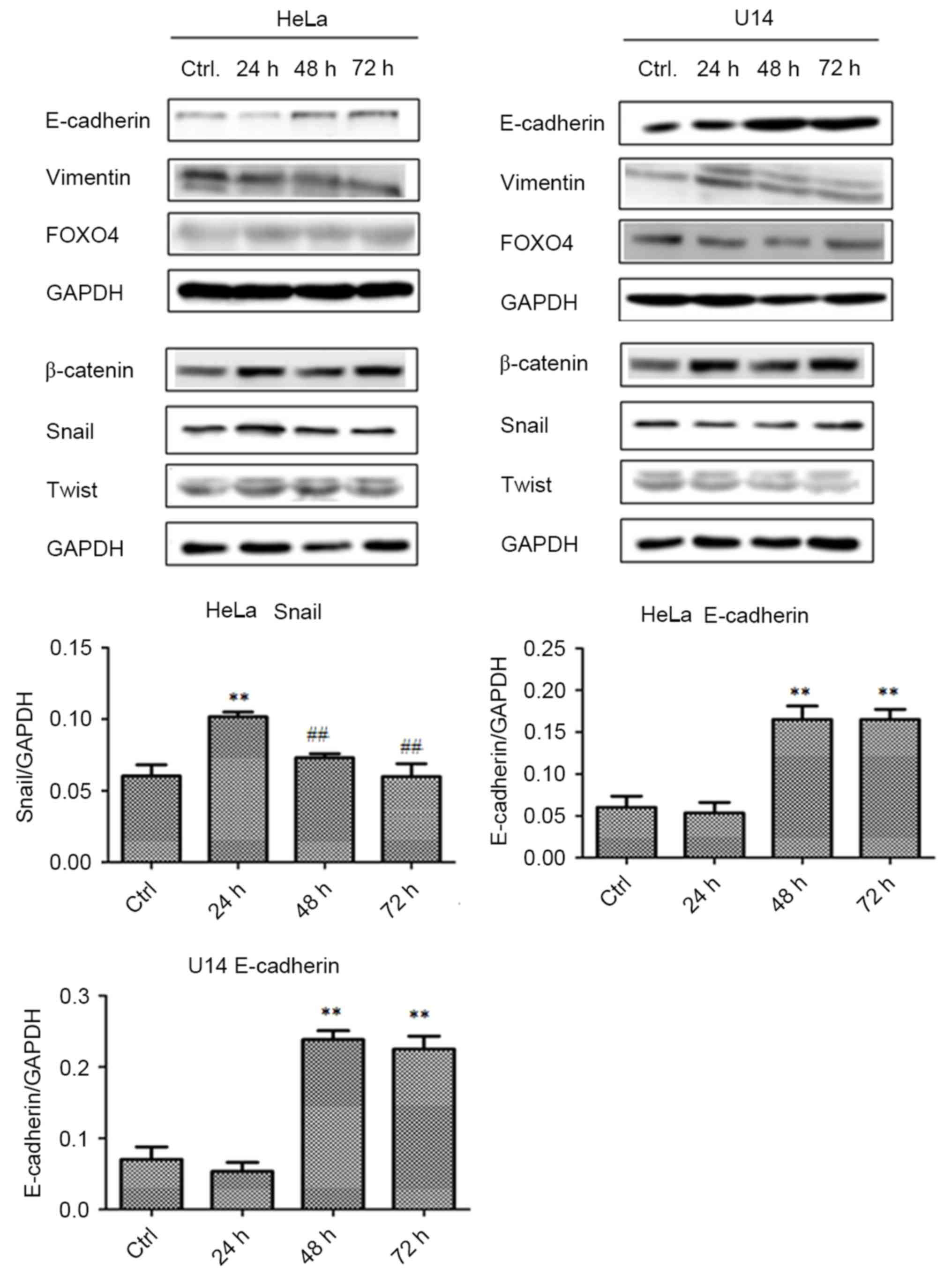
Lactobacilli upregulate E-cadherin and
Snail expression in the CC cell lines
Furthermore, we explored the potential
pharmacological mechanisms of lactobacilli by western blot assays.
As shown in Fig. 3, in both the
HeLa and U14 cell lines, treatment with lactobacilli significantly
upregulated the expression of E-cadherin after 48 (p<0.01) and
72 h (p<0.01) of incubation, compared with the control (Ctrl)
group. In addition, Snail expession in HeLa cells was also
upregulated at 24 h (p<0.01) after lactobacillus treatment,
compared with that noted in the Ctrl group, and sharply decreased
after 48 h, compared to the 24 h cells.
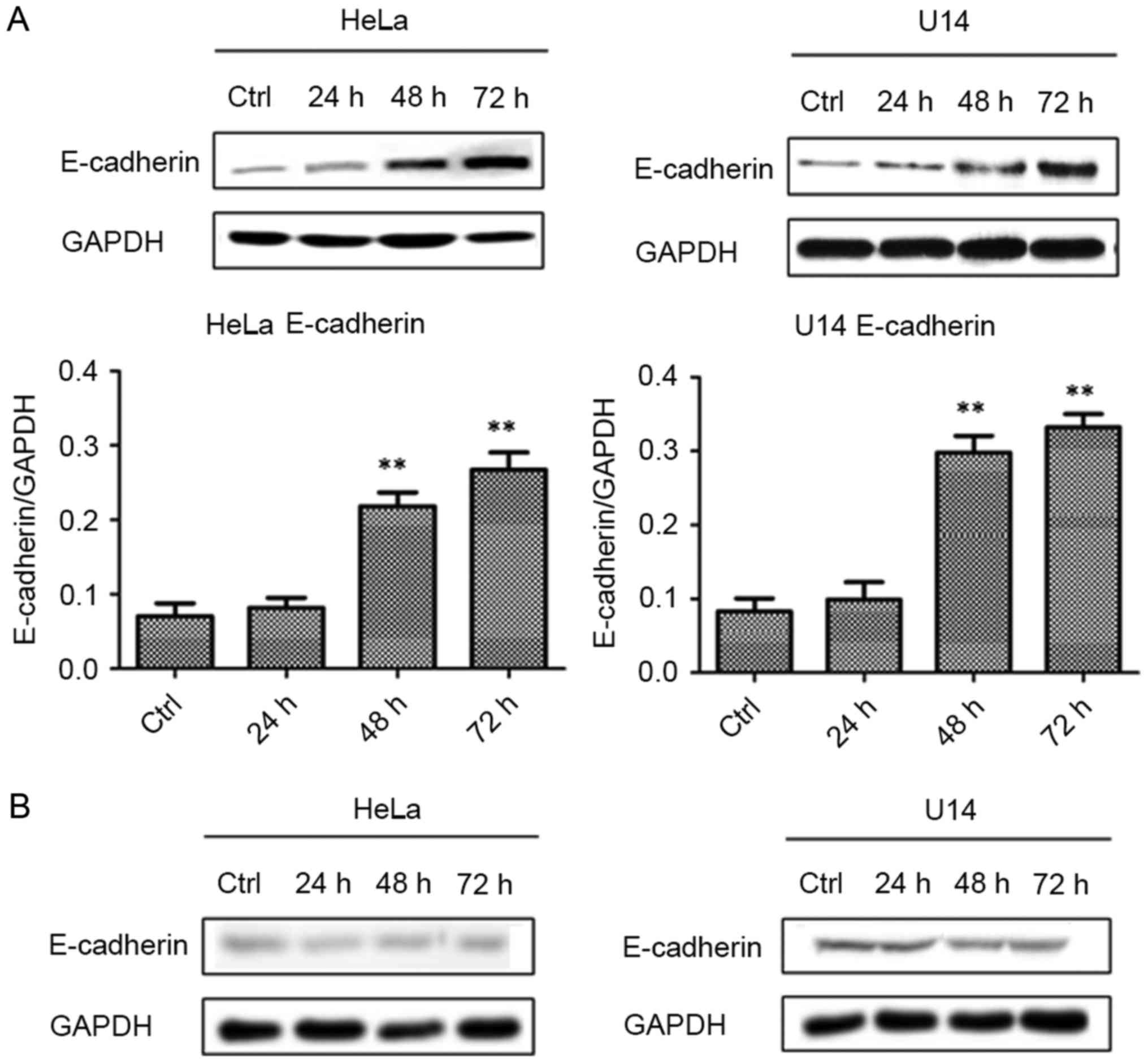
Lactobacilli upregulate E-cadherin via
its secreted constituents
As shown in Fig. 4,
active lactobacilli significantly upregulated the E-cadherin
protein levels in the HeLa cells after 48 (p<0.01) and 72 h
(p<0.01) of culture, compared to the level in the Ctrl cells.
Similar to HeLa cells, treatment with lactobacilli obviously
increased the E-cadherin expressions after 24 (p<0.01), 48
(p<0.01) and 72 h (p<0.01) of culture in the U14 cells,
compared with that in the Ctrl cells. On the contrary, inactivated
lactobacilli could not affect the E-cadherin expression in both
HeLa and U14 cell lines, compared with levels in the Ctrl cells
(p>0.05). These results as mentioned above indicate that live
lactobacilli upregulated the E-cadherin expression in CC cells
instead of inactivated lactobacilli; therefore, the potential
mechanisms may be related to various secreted constituents of live
lactobacilli.
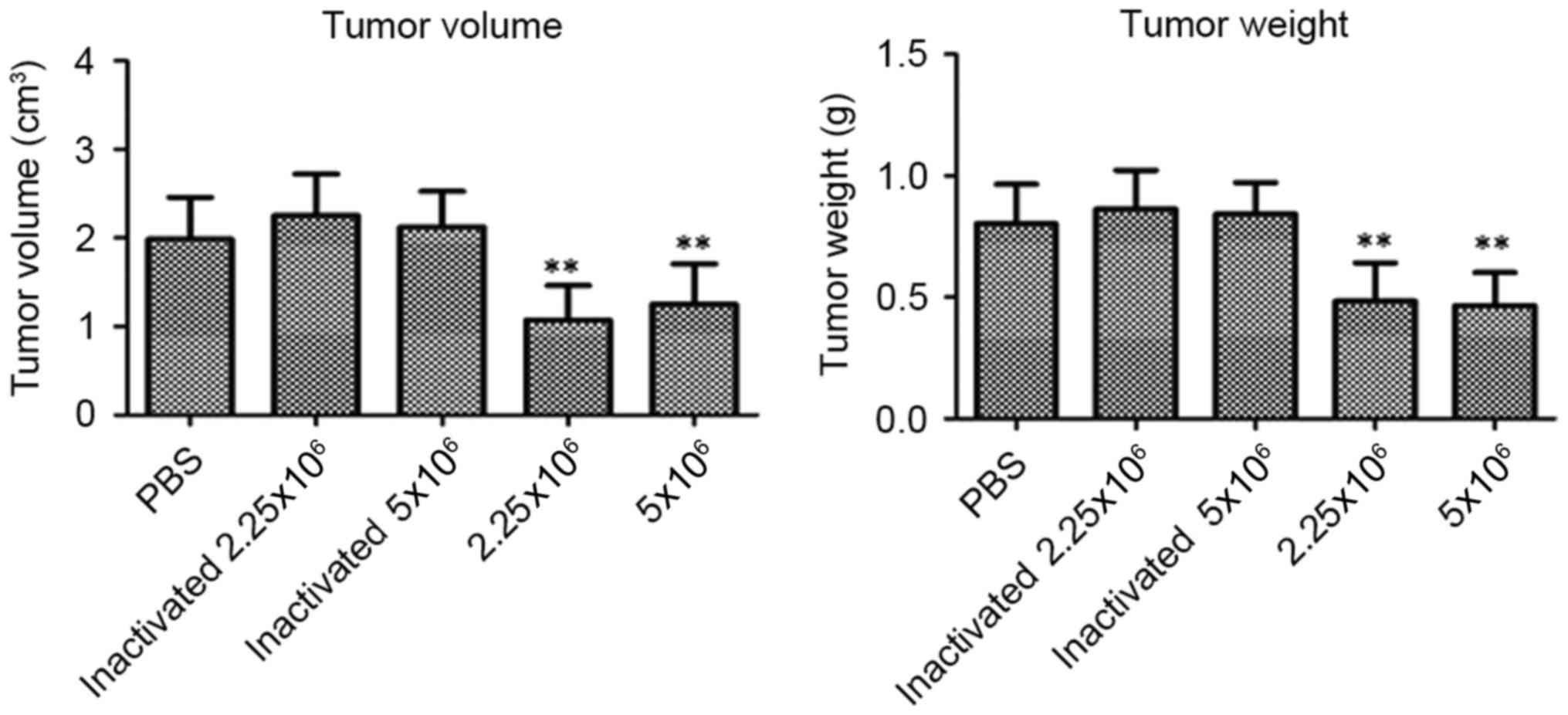
Lactobacilli decrease tumor weight and
volume in the tumor-burdened mice
From the results shown in Fig. 5, lactobacillus treatments (2.25 and
5.0×106/mice) significantly decreased the volume
(p<0.01, p<0.01) and weight (p<0.01, p<0.01) of the
tumors in the mice, compared with the PBS-treated mice. In
addition, the inactivated lactobacilli did not affect the tumor
growth in the U14 tumor-burdened mice (p>0.05).
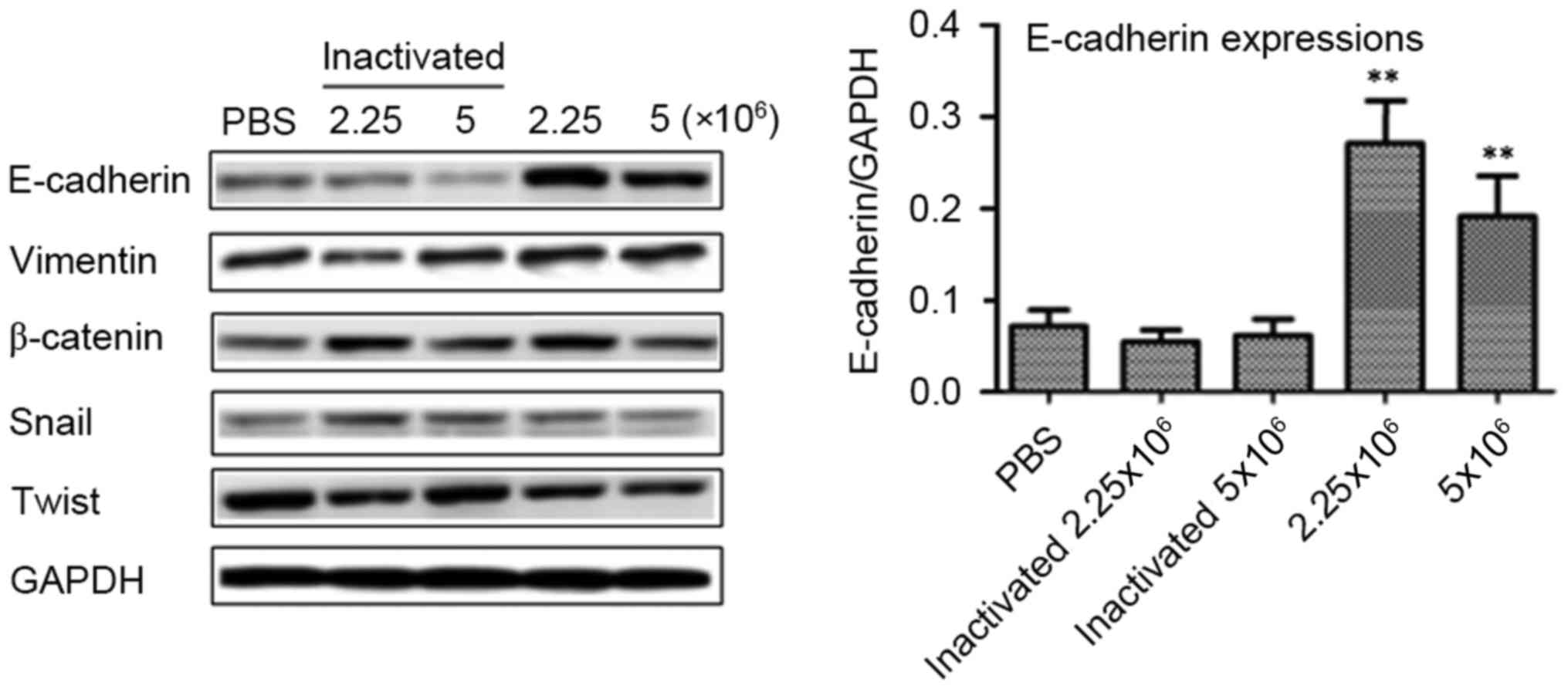
Lactobacilli upregulate E-cadherin in
the tumor-burdened mice
Furthermore, we determined the protein expression
levels of E-cadherin, vimentin, β-catenin, Snail and Twist in the
tumor tissues of the tumor-burdened mice at 21 days by western blot
assays. As shown in Fig. 6, no
obvious difference was observed between the PBS-treated mice and
the inactivated/activated lactobacillus-treated mice for the
proteins of vimentin (p>0.05), β-catenin (p>0.05), Snail
(p>0.05) and Twist (p>0.05). Importantly and notably,
compared with the PBS-treated mice, the lactobacilli significantly
upregulated the E-cadherin expression in the tumor tissues at the
doses of 2.25×106/mice (p<0.01) and
5.0×106/mice (p<0.01). On the contrary, the
inactivated lactobacilli did not affect the E-cadherin expression
compared with that in the PBS-treated mice (p>0.05).
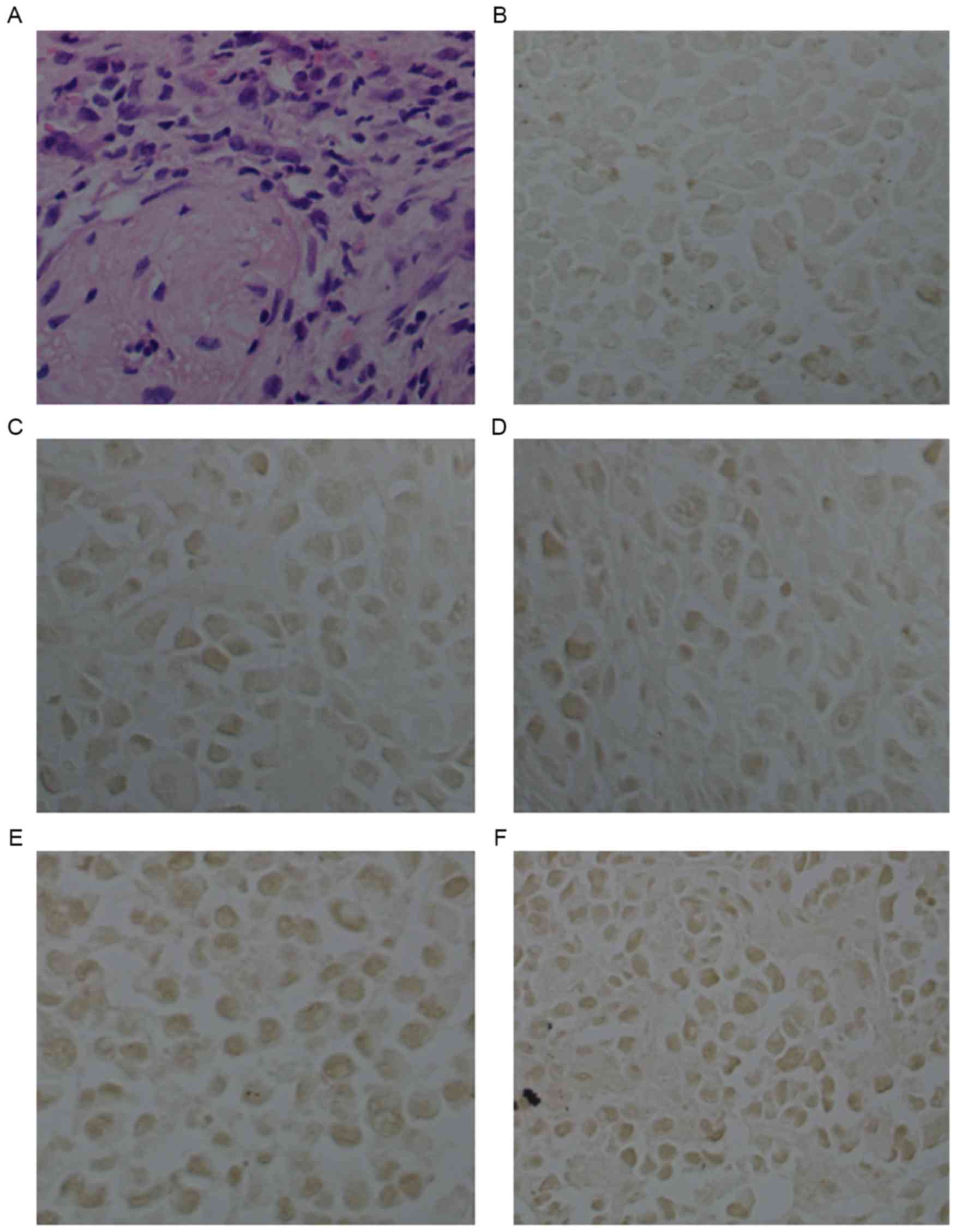
E-cadherin expression in U14 tumor
tissues
In addition, we further determined the protein
expression level of E-cadherin in tumor tissues using
immunohistochemical assay with E-cadherin antibody straining. As
shown in Fig. 7 and Table I, in the tumor tissues of the
PBS-treated mice, the expression level of E-cadherin was low
(Fig. 7B; Table I). For the inactivated
lactobacillus-treated mice (2.25 and 5.0×106/mice), the
E-cadherin expression levels were still low (Fig. 7C and D; Table I), and did not show obvious
differences compared to the PBS-treated mice (p>0.05,
p>0.05). Notably, the lactobacillus treatments (2.25 and
5.0×106/mice) significantly upregulated the protein
expression level of E-cadherin in the tumor tissues (p<0.05,
p<0.05), compared with that in the PBS-treated mice (Fig. 7E and F; Table I).
 | Table I.E-cadherin expression in U14 tumor
tissues. |
Table I.
E-cadherin expression in U14 tumor
tissues.
|
| Inactivated
lactobacilli | Lactobacilli |
|---|
|
|
|
|
|---|
| PBS |
2.25×106/mice |
5×106/mice |
2.25×106/mice |
5×106/mice |
|---|
| 0.137±0.004 | 0.140±0.003 | 0.138±0.005 |
0.477±0.006a |
0.467±0.003a |
Discussion
Previous research has demonstrated that tumor cell
invasion and migration to the extracellular matrix (ECM) is the
crucial step in tumor metastasis (15). In the present study, the wound
healing assay results indicated that lactobacilli could
significantly inhibit the migration of cervical cancer (CC) cells
in vitro. In addition, CCK-8 assay results demonstrated that
the inhibitory effects of lactobacilli on the migration of CC cells
are not due to its cytotoxicity. These results as mentioned above
suggest that lactobacilli have potential inhibitory effects on CC
cell migration ability. Epithelial-mesenchymal transition (EMT)
plays an important role in the development, invasion and metastasis
of tumors, and invasion and metastasis are two crucial hallmarks of
malignancy which commonly results in the loss of E-cadherin. In
addition, previous studies have revealed that E-cadherin loss is
the most important event in the development of EMT (10,11).
E-cadherin, a 12-kDa calcium-dependent membrane glycoprotein,
possesses important physiological activities regarding the
maintenance of cell-cell adhesion, epithelial tissue polarity and
structural integrity (15–17). Increasing evidence has demonstrated
that levels of E-cadherin are commonly downregulated in various
human malignancies, including lung and skin cancer, oral squamous
carcinoma, as well as CC. Thus, low E-cadherin expression has been
considered as an indicator of the poor prognosis of tumors
(18,19). Our present research showed that
lactobacilli notably upregulated the E-cadherin expression in CC
tumor cells in vitro and in vivo, indicating that
this effect may be a key factor attributed to its inhibitory
effects on CC cell migration ability.
The Wnt/β-catenin signaling pathway also plays an
important role in the development of cancers, and previous studies
have indicated that β-catenin takes part in the process of ETM in
the invasion of colorectal cancer (20–22).
Previous research indicates that overexpression of WIF1 (an
inhibitor of Wnt) in PC3 cells effectively inhibited the expression
of transcription factors of Slug and Twist, leading to upregulation
of E-cadherin whereas loss of vimentin is a hallmark of mesenchymal
cells (22,23). Our present investigation revealed
that lactobacilli induced upregulation of E-cadherin, but showed no
effect on expression of vimentin, suggesting that the inhibitory
effects of lactobacilli on CC cell migration may possess no
relation with vimentin. Furthermore, Snail, a DNA-binding protein,
recognizes and binds to the E-box sequence in the promoter of the
E-cadherin gene, subsequently resulting in low expression of
E-cadherin, leading to the development of EMT. Thus, Snail could
promote the invasive and metastatic abilities of tumors, such as
gastric cancer (24,25). However, there is no evidence for the
Snail expression in CC. In our present results, we found that after
culture with lactobacilli for 24 h, the Snail expression in HeLa
cells was upregulated; however this effect was not detected in U14
cells in vitro and tumor tissues in vivo. Thus, the
inhibitory effects of lactobacilli may be not related to Snail.
Twist 1 binds to β-catenin, leading to promotion of the development
of tumors. In addition, Yang et al reported that Twist 1
inhibits the expression of E-cadherin in human mammary epithelial
cells (26,27). FOXO transcription factors play
important roles in the proliferation, differentiation and apoptosis
of cells. Yang et al revealed that FOXO4 inhibited tumor
cell proliferation. Tang et al indicated that FOXO4
upregulated Bcl-6 which could bind to the promoter of Bcl-2,
leading to the downregulation of Bcl-2 (28,29).
However, our present results did not provide an obvious evidence
for demonstrating that the upregulation of E-cadherin by
lactobacilli is related to the regulation of Twist 1 and FOXO4. In
addition, we also investigated whether or not the inhibitory
effects of lactobacilli on the migration of CC cells were due to
the surface proteins of lactobacilli. Our results showed that
inactivated lactobacilli had no obvious effects on tumor growth and
E-cadherin expression. Thus, we deduced that the active components
of lactobacilli may be related to various secreted constituents of
live lactobacilli.
In conclusion, our results indicate that live
lactobacilli have potential activities for inhibiting the migration
of CC cells, and the possible pharmacological mechanism may be
closely related to the upregulation of E-cadherin.
Acknowledgements
The present study was supported by funding from the
Hebei Provincial Health and Family Planning Commission (no.
20150630).
References
|
1
|
Vaccarella S, Franceschi S, Zaridze D,
Poljak M, Veerus P, Plummer M and Bray F: Preventable fractions of
cervical cancer via effective screening in six Baltic, central, and
eastern European countries 2017–40: A population-based study.
Lancet Oncol. 17:1445–1452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee SJ, Yang A, Wu TC and Hung CF:
Immunotherapy for human papillomavirus-associated disease and
cervical cancer: Review of clinical and translational research. J
Gynecol Oncol. 27:e512016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ:
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group: Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dochez C, Bogers JJ, Verhelst R and Rees
H: HPV vaccines to prevent cervical cancer and genital warts: An
update. Vaccine. 32:1595–1601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao BB and Liao QP: Research progress of
vaginal micro-ecology. J Int Obstet Gynecol. 38:479–482. 2011.
|
|
6
|
Lu FG, Hu JZ, Wu CR, Hu ZG and Zhao WN:
Study of biological antagonism on lactobacilli in human vaginae.
Pract Prev Med. 8:19–20. 2001.
|
|
7
|
Voravuthikunchai SP, Bilasoi S and
Supamala O: Antagonistic activity against pathogenic bacteria by
human vaginal lactobacilli. Anaerobe. 12:221–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mlcochova H, Machackova T, Rabien A,
Radova L, Fabian P, Iliev R, Slaba K, Poprach A, Kilic E, Stanik M,
et al: Epithelial- mesenchymal transition-associated microRNA/mRNA
signature is linked to metastasis and prognosis in clear-cell renal
cell carcinoma. Sci Rep. 6:318522016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Chen H, Xu C, Song L, Huang L,
Lai Y, Wang Y, Chen H, Gu D, Ren L, et al: Curcumin inhibits tumor
epithelial- mesenchymal transition by downregulating the Wnt
signaling pathway and upregulating NKD2 expression in colon cancer
cells. Oncol Rep. 35:2615–2623. 2016.PubMed/NCBI
|
|
10
|
Braga V: Spatial integration of E-cadherin
adhesion, signalling and the epithelial cytoskeleton. Curr Opin
Cell Biol. 42:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carvalho S, Oliveira T, Bartels MF,
Miyoshi E, Pierce M, Taniguchi N, Carneiro F, Seruca R, Reis CA,
Strahl S, et al: O-mannosylation and N-glycosylation:
Two coordinated mechanisms regulating the tumour suppressor
functions of E-cadherin in cancer. Oncotarget. 7:65231–65246.
2016.PubMed/NCBI
|
|
12
|
Yang XK, Yang YD and Tang SQ: Inhibitory
effect of polysaccharides from Scutellaria barbata D. Don on
invasion and metastasis of 95-D cells lines via regulation of C-MET
and E-CAD expressions. Trop J Pharm Res. 12:517–522. 2013.
|
|
13
|
Peng W, Wu JG, Jiang YB, Liu YJ, Sun T, Wu
N and Wu CJ: Antitumor activity of
4-O-(2″-O-acetyl-6″-O-p-coumaroyl-β-D-glucopyranosyl)-p-coumaric
acid against lung cancers via mitochondrial-mediated apoptosis.
Chem Biol Interact. 233:8–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou T, Li G, Cao B, Liu L, Cheng Q, Kong
H, Shan C, Huang X, Chen J and Gao N: Downregulation of Mcl-1
through inhibition of translation contributes to benzyl
isothiocyanate-induced cell cycle arrest and apoptosis in human
leukemia cells. Cell Death Dis. 4:e5152013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martínez A, Spencer ML, Borlando J, Flores
M and Rojas IG: E-cadherin and c-Met expression in actinic cheilits
and lip squamous cell carcinoma. Rehabil Oral. 4:122–125. 2011.
|
|
17
|
Yao Z and Shulan Z: Inhibition effect of
Guizhi-Fuling-decoction on the invasion of human cervical cancer. J
Ethnopharmacol. 120:25–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dohadwala M, Yang SC, Luo J, Sharma S,
Batra RK, Huang M, Lin Y, Goodglick L, Krysan K, Fishbein MC, et
al: Cyclooxygenase-2-dependent regulation of E-cadherin:
Prostaglandin E2 induces transcriptional repressors ZEB1
and snail in non-small cell lung cancer. Cancer Res. 66:5338–5345.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valizadeh A, Karayiannakis AJ, el-Hariry
I, Kmiot W and Pignatelli M: Expression of E-cadherin-associated
molecules (alpha-, beta-, and gamma-catenins and p120) in
colorectal polyps. Am J Pathol. 150:1977–1984. 1997.PubMed/NCBI
|
|
20
|
Hlubek F, Spaderna S, Schmalhofer O, Jung
A, Kirchner T and Brabletz T: Wnt/FZD signaling and colorectal
cancer morphogenesis. Front Biosci. 12:458–470. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rattner A, Hsieh JC, Smallwood PM, Gilbert
DJ, Copeland NG, Jenkins NA and Nathans J: A family of secreted
proteins contains homology to the cysteine-rich ligand-binding
domain of frizzled receptors. Proc Natl Acad Sci USA. 94:2859–2863.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wissmann C, Wild PJ, Kaiser S, Roepcke S,
Stoehr R, Woenckhaus M, Kristiansen G, Hsieh JC, Hofstaedter F,
Hartmann A, et al: WIF1, a component of the Wnt pathway, is
down-regulated in prostate, breast, lung, and bladder cancer. J
Pathol. 201:204–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yee DS, Tang Y, Li X, Liu Z, Guo Y,
Ghaffar S, McQueen P, Atreya D, Xie J, Simoneau AR, et al: The Wnt
inhibitory factor 1 restoration in prostate cancer cells was
associated with reduced tumor growth, decreased capacity of cell
migration and invasion and a reversal of epithelial to mesenchymal
transition. Mol Cancer. 9:1622010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galván JA, González MV, Crespo G,
Folgueras MV and Astudillo A: Snail nuclear expression parallels
higher malignancy potential in neuroendocrine lung tumors. Lung
Cancer. 69:289–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SH, Kim JH and Lee YS: M1958
regulation of GLI1 and snail in Helicobacter pylori infected
gastric epithelial cells. Gastroenterology. 138:447–448.
2010.PubMed/NCBI
|
|
26
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang TT and Lasky LA: The forkhead
transcription factor FOXO4 induces the down-regulation of
hypoxia-inducible factor 1 alpha by a von Hippel-Lindau
protein-independent mechanism. J Biol Chem. 278:30125–30135. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang H, Zhao R, Yang HY and Lee MH:
Constitutively active FOXO4 inhibits Akt activity, regulates p27
Kip1 stability, and suppresses HER2-mediated tumorigenicity.
Oncogene. 24:1924–1935. 2005. View Article : Google Scholar : PubMed/NCBI
|