Introduction
Colorectal cancer (CRC) is characterized by the
formation of malignant tumors in the colon or rectum. Recently,
there have been a notable decrease in the incidence rates of CRC,
which can be largely attributed to improvements in screening
techniques, allowing early detection and removal of precancerous
polyps (1). Nevertheless, morbidity
and mortality for CRC is still the 5th most malignant tumor in
China (2) and the 3rd of
cancer-related deaths in the USA (1). Clinical treatment of CRC usually
involves surgery, chemotherapy, radiation therapy and palliative
care, depending on the patient status and tumor characteristics
(3). Such treatments are often
painful and expensive, and are associated with a high risk of
postoperative recurrence and tumor metastasis. Therefore, there is
urgent need to discover safer, cheaper and more effective methods
of treatment for colorectal cancer.
In Western herbal medicine, Prunella vulgaris
L. is a well known herb for self healing. Similarly, in traditional
Chinese medicine (TCM), the spike of Prunella vulgaris L.
termed Spica Prunellae, is often used for the prevention and remedy
of various illnesses (4). The
chemical components of Spica Prunellae contain various bioactive
compounds including triterpenes, flavonoids, phenolic compounds and
carbohydrates (5–8). Among these, oleanolic acid is
associated with anticancer (9–11) and
anti-inflammatory (12) properties,
ursolic acid has shown anti-allergic and anti-inflammatory
activities (11,13), rosmarinic acid has demonstrated
antioxidant (14,15), neuroprotective (16,17)
and anti-inflammatory effects (18), and polysaccharide has anticancer
activity (19). As a whole, Spica
Prunellae has also been reported to exhibit immunosuppressive
activity (20), anti-angiogenic
activity (21), vascular
inflammation properties (22),
anti-estrogenic activity (23),
anti-HIV activity (24,25), and anticancer activity (19, 26).
With regards to the anticancer activity of Spica Prunellae,
research has demonstrated that it can promote cell apoptosis
(9,27,28),
regulate the cell cycle (28,29),
as well as suppress cell invasion and migration (26). Furthermore, this anticancer activity
was mediated by a variety of genes, including Bcl-2, Bax, Bad,
c-Jun N-terminal kinases (JNK), caspase-3 and signal transducer and
activator of transcription 3 (STAT3) (9,26,30).
miRNAs are a cluster of small non-coding RNA
molecules containing 20–25 nucleotides, which play crucial roles
during transcription and post-transcriptional regulation of gene
expression (31). miRNAs can
suppress tumor growth, progression and metastasis by regulating and
stabilizing the metastatic nature of cancers (32). miR-34a is a tumor suppressor of the
miR-34 family, which can regulate Bcl-2, Notch1 and Notch2
expression to promote apoptosis of cancer cells (33–35).
Since Bcl-2 is also mediated by Spica Prunellae (9,30), we
hypothesized that miR-34a must take part in the antitumor activity
for Spica Prunellae.
We previously demonstrated that Spica Prunellae
suppressed cell proliferation with G1/S cell cycle arrest and
promoted mitochondrion-dependent apoptosis in HT-29 colon cancer
cells (27,29). Moreover, it inhibited tumor
angiogenesis in vitro and in vivo (21,26).
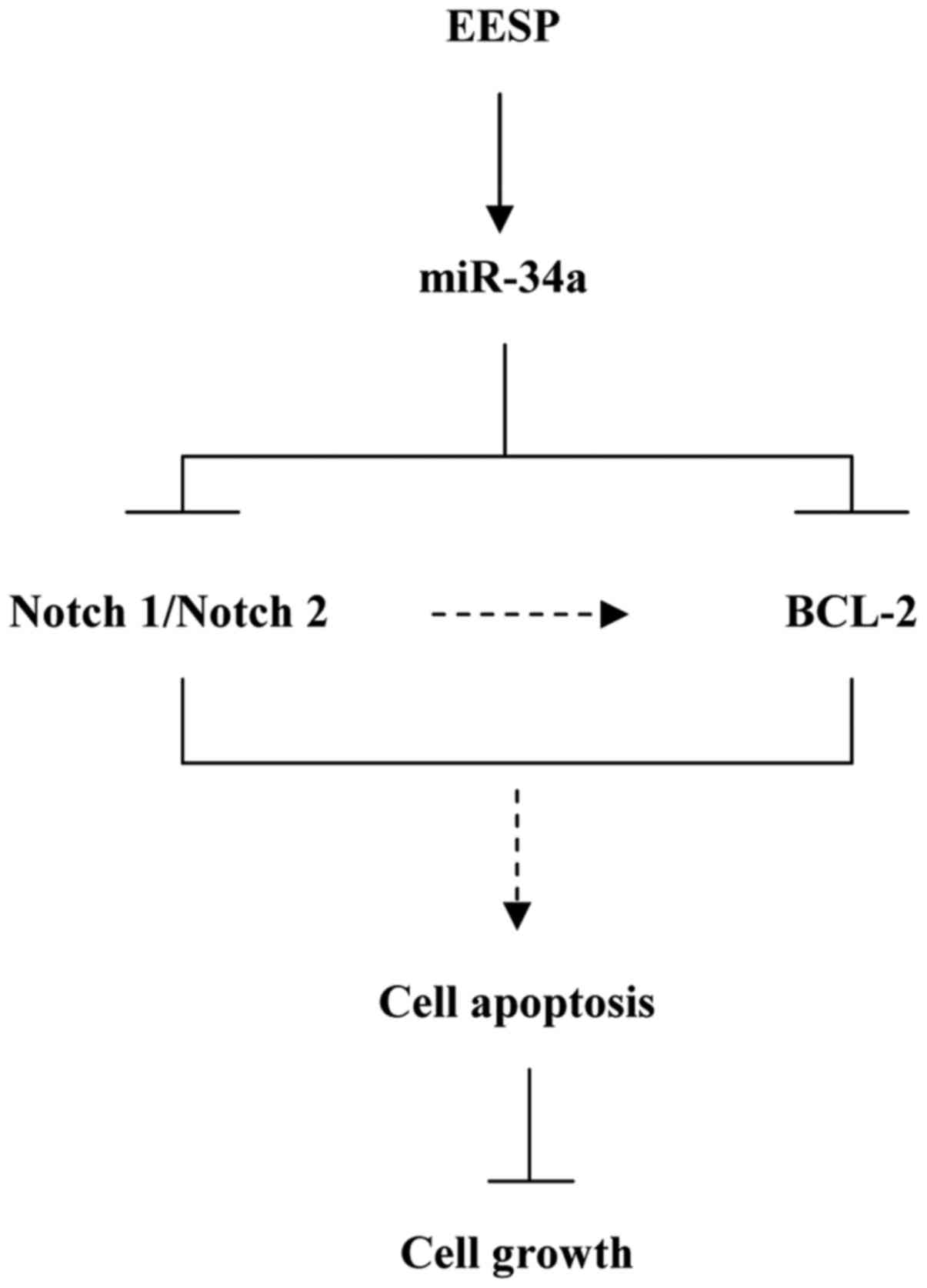
Our present study reveals that EESP can suppress the growth of
HCT-8 colon carcinoma cells and promote cell apoptosis. We propose
a likely mechanism of EESP function, via the activation of miR-34a
pathway and its regulation of Notch1, Notch2 and Bcl-2 expression.
These findings provide important insight into understanding the
role of miR-34a in regulating the antitumor activity for Spica
Prunellae, and establish a theoretical basis for the prevention and
clinical treatment of CRC.
Materials and methods
Preparation of EESP
Prunella vulgaris L. plants were purchased
from Guo Yi Tang Chinese Herbal Medicine store (Fujian, China). The
herb was collected from Hunan Province in China. The extraction and
the chemical profile of EESP were prepared by Lin et al
(26). EESP powder was then
dissolved with DMSO to a stock concentration of 500 mg/ml. The
final concentration of DMSO in working solution for all experiments
was ≤0.4%.
Cell culture
Human carcinoma HCT-8 cells were obtained from
Nanjing Kaiji Biological Technology Development Co., Ltd. (Nanjing,
China). RPMI-1640 medium, fetal bovine serum (FBS), trypsin-EDTA
and penicillin-streptomycin were obtained from Hyclone (Carlsbad,
CA, USA). The cells were cultured in RPMI-1640 medium supplemented
with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin
(Hyclone), in a 37°C humidified incubator with 5%
CO2.
Cell viability evaluation and
IC50 determinations
HCT-8 cells were seeded with a density of
0.5×104 cells/ml in 96-well plates. Cell culture medium
was replaced with different concentrations (0, 0.25, 0.50, 0.75,
1.00, 1.25 and 1.50 mg/ml) of EESP for 48 h at 80–90% cell
confluency. Cell viability was determined at 570 nm using
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
colorimetric assay with ELX800 (BioTek, Winooski, VT, USA).
Subsequent cell survival rates and IC50 of EESP were
calculated and analyzed using SPSS 16.0 software.
Colony formation
HCT-8 cells were seeded with a density of
2×105 cells/ml into 6-well plates. After treatment with
different concentrations (0, 0.25, 0.50, 0.75 and 1.00 mg/ml) of
EESP for 48 h, cells were collected and reseeded with a density of
1×103 cells/well in new 6-well plates. Cells were
maintained in RPMI-1640 medium supplemented with 10% FBS,
penicillin and streptomycin for 2 weeks. The resulting cell
colonies were fixed using methanol, stained with 0.01% crystal
violet and counted.
Apoptosis detection by flow cytometry
analysis with Annexin V/PI staining
After treated with different concentrations (0,
0.25, 0.50 and 1.00 mg/ml) of EESP for 48 h, HCT-8 cells were
operated with Annexin V FITC/PI Apoptosis Detection kit from
Nanjing Kaiji Biological Technology Development Co., Ltd. Finally,
cell apoptosis was detected by fluorescence-activated cell sorting
(FACS) (Becton-Dickinson, San Jose, CA, USA).
Transfection
HCT-8 cells were seeded with a density of
2×105 cells/ml in 6-well plates and transfected with
miR-34a inhibitor (RiboBio Co. Ltd., Guangzhou, China) or negative
control with Lipofectamine 2000 (Life Technologies Corp., Shanghai,
China) according to the manufacturer's instructions when cells are
at 50–60% confluency. Transfections were performed in triplicate
independent experiments. After 24 h, culture medium was replaced by
the IC50 volume of EESP for 48 h.
RNA preparation
Total RNA of cells was extracted using TRIzol
reagent (Life Technologies, Grand Island, NY, USA) according to the
manufacturer's instructions. Nanodrop spectrophotometer (Nanodrop
Technologies, Oxfordshire, UK) was used to assessed the quantity
and quality of RNA.
RT-qPCR
cDNA synthesis was carried out following the
instruction of PrimeScript™ RT reagent kit with gDNA Eraser:
Perfect Real-time (Takara, Dalian, China). The RT-primers of
miR-34a were designed based on Chen et al (36). Primers for U6 was designed by Primer
Premier 5.0 (37), primers for
miR-34a was provided by Yang et al (38) and other primers were obtained from
RTPrimerDB or Primer Bank (data not shown). qPCR was performed
using DyNAmo ColorFlash SYBR Green qPCR kit (Thermo Fisher
Scientific Inc., Waltham, MA, USA) and Applied
Biosystems® 7500 Real-time PCR System (Life
Technologies).
Western blotting
Proteins were extracted by RIPA buffer (Thermo
Fisher Scientific Inc.) and separated in 7.5% SDS-PAGE and then
transferred to NC membranes (Millipore, Billerica, MA, USA) by
semi-dry electrotransfer. After incubation with antibody against
Notch1, Notch2 [AI Bo (Shanghai) Trading Co., Ltd., Shanghai,
China], Bcl-2 and GAPDH (Thermo Fisher Scientific Inc.) and
anti-rabbit IgG, HRP-linked antibody (Cell Signaling Technology,
Inc., Danvers, MA, USA), the bands were detected with SuperSignal™
West Pico Chemiluminescent Substrate or SuperSignal™ West Femto
Maximum Sensitivity Substrate (Thermo Fisher Scientific Inc.). Band
intensity of western blots was obtained by Image Lab Software
(Bio-Rad, Hercules, CA, USA) and normalized to GADPH.
Statistics analysis
Data are presented as mean ± SD. Statistical
significance of differences was assessed by one way ANOVA or
Independent-Samples t-test using SPSS 16.0 software. A P-value of
<0.05 was considered to indicate a statistically significant
difference. Data for RT-qPCR were analyzed by ∆∆Cq method (39).
Results
EESP suppresses the cell growth of
HCT-8 cells
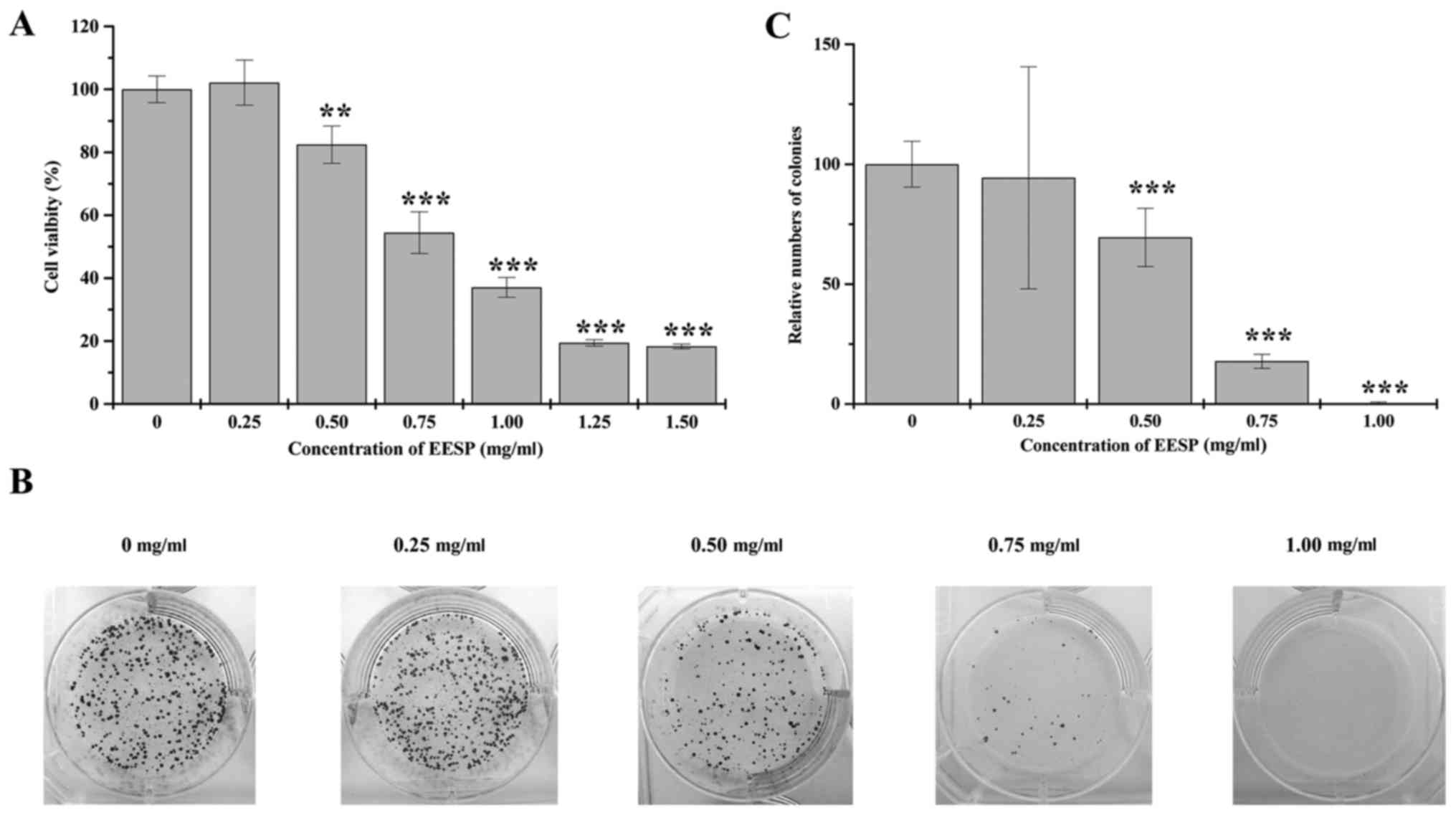
We examined the cell survival rate of HCT-8 cells
following EESP treatment using MTT assay. Cell viability decreased
significantly by 82.46–18.31% following 0.5–1.5 mg/ml of EESP
treatment for 48 h, compared to untreated control cells. Based on
these data, we calculated the IC50 value of EESP to be
0.77 mg/ml (Fig. 1A). In addition,
we performed colony formation assays to determine the cell
proliferation of HCT-8 cells following EESP treatment. There was a
dose-dependent reduction in the number of cell colonies by
94.34–0.43% following EESP treatment, compared to untreated control
cells (Fig. 1B and C). These
results demonstrated that EESP suppressed HCT-8 cell growth in a
dose-dependent manner.
EESP induces apoptosis of HCT-8
cells
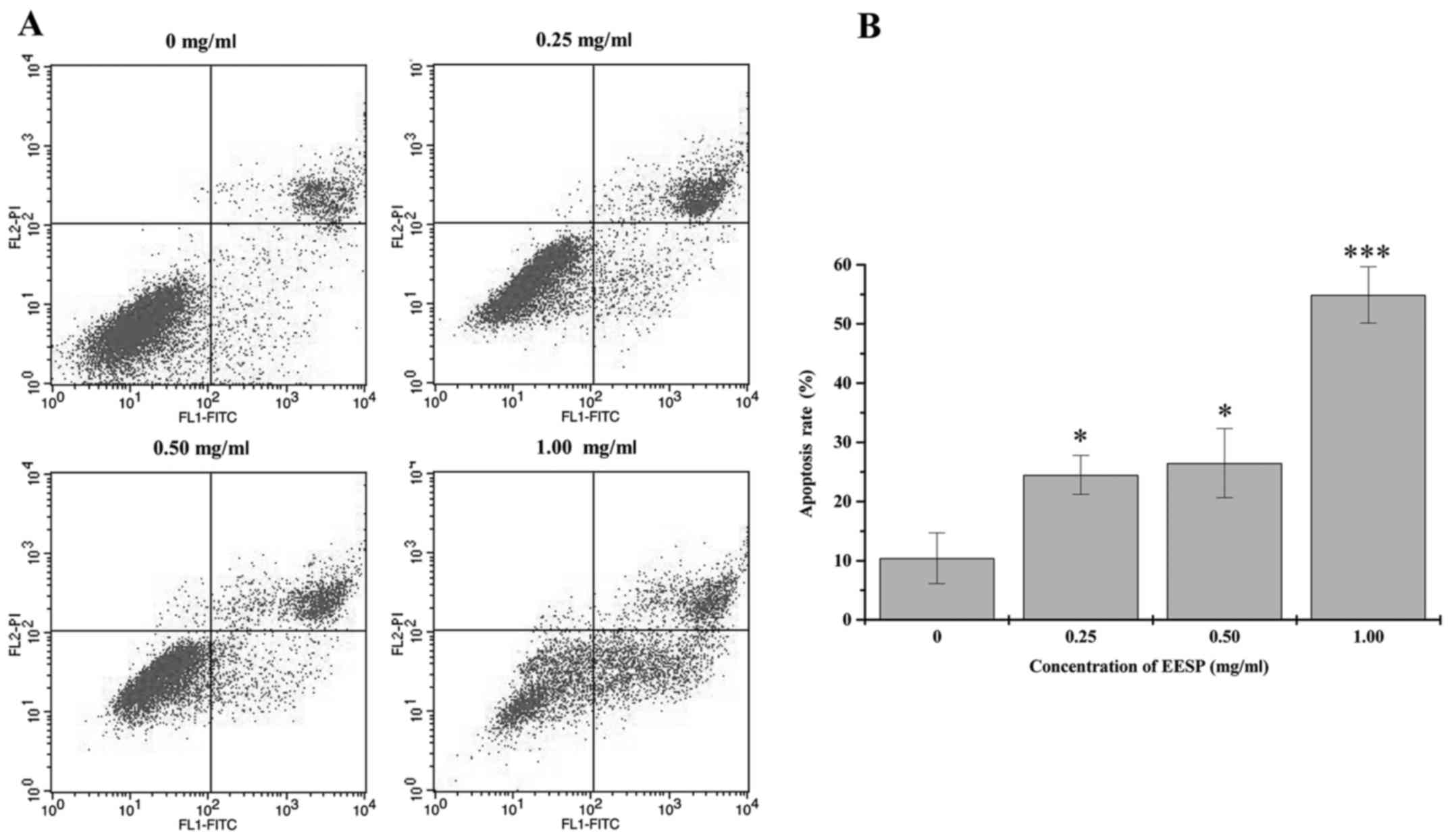
We determined whether the suppression of HCT-8 cell
growth following EESP treatment was due to apoptosis. There was a
significant dose-dependent increase in the percentage of apoptosis
which consisted of early and late apoptotic in HCT-8 cells
(Fig. 2A and B). This indicated
that EESP could induce apoptosis of HCT-8 cells.
EESP upregulates endogenous miR-34a
expression while downregulating its target genes in HCT-8
cells
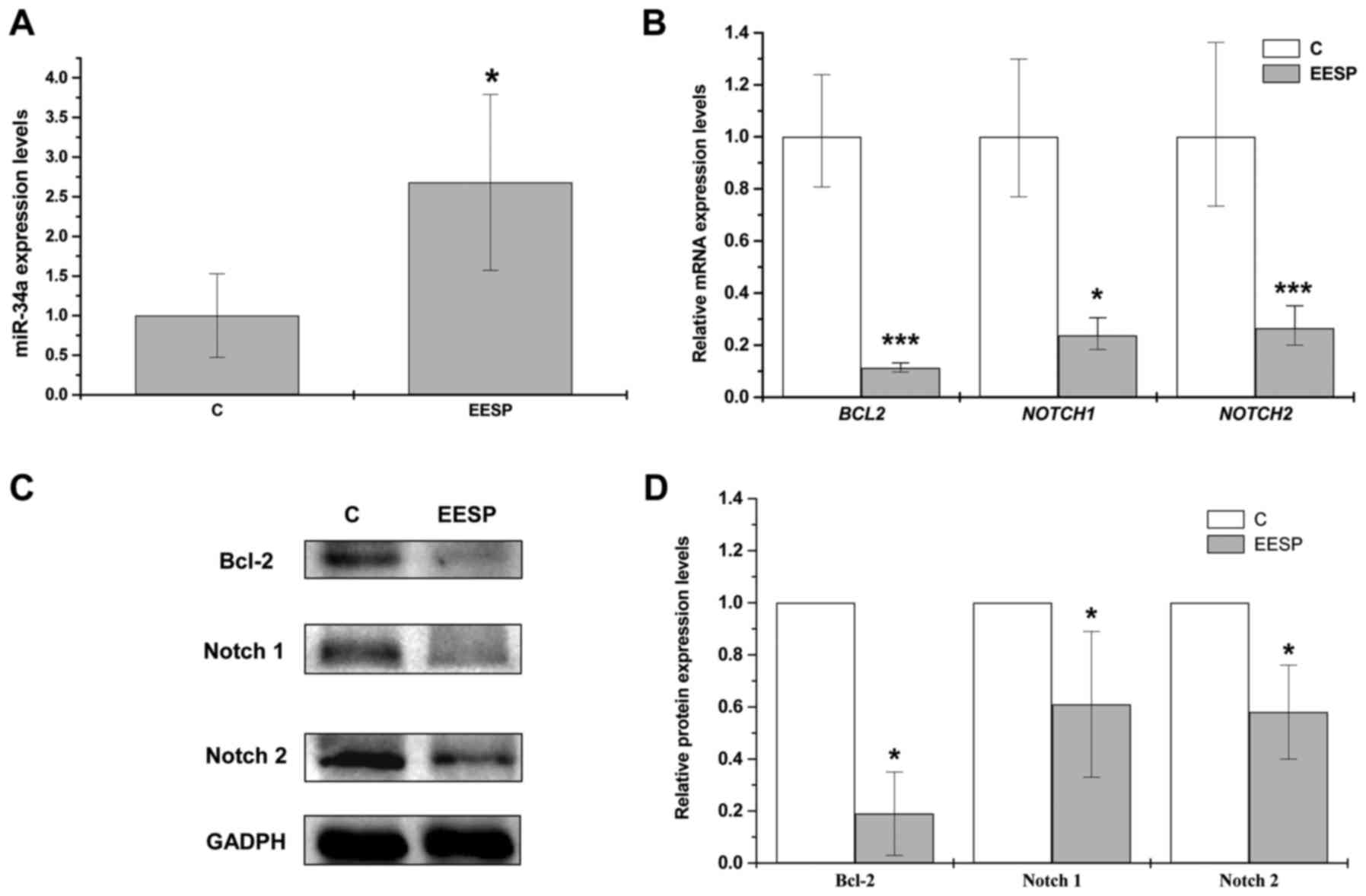
Previous studies have found that miR-34a can promote
cell apoptosis by regulating Bcl-2, Notch1 and Notch2 (33–35).
Therefore, we wished to determine the potential role of miR-34a
pathway in Spica Prunellae, due to the ability of EESP to induce
HCT-8 cell apoptosis as demonstrated above. There was a significant
upregulation in miR-34a expression following treatment of EESP at
IC50 for 48 h, whereas the relative mRNA expression
levels and protein levels of its target genes (Bcl-2, Notch1 and
Notch2) were significantly downregulated (Fig. 3A-D).
EESP can rescue miR-34a-induced
downregulation of Bcl-2, Notch1 and Notch2 expression
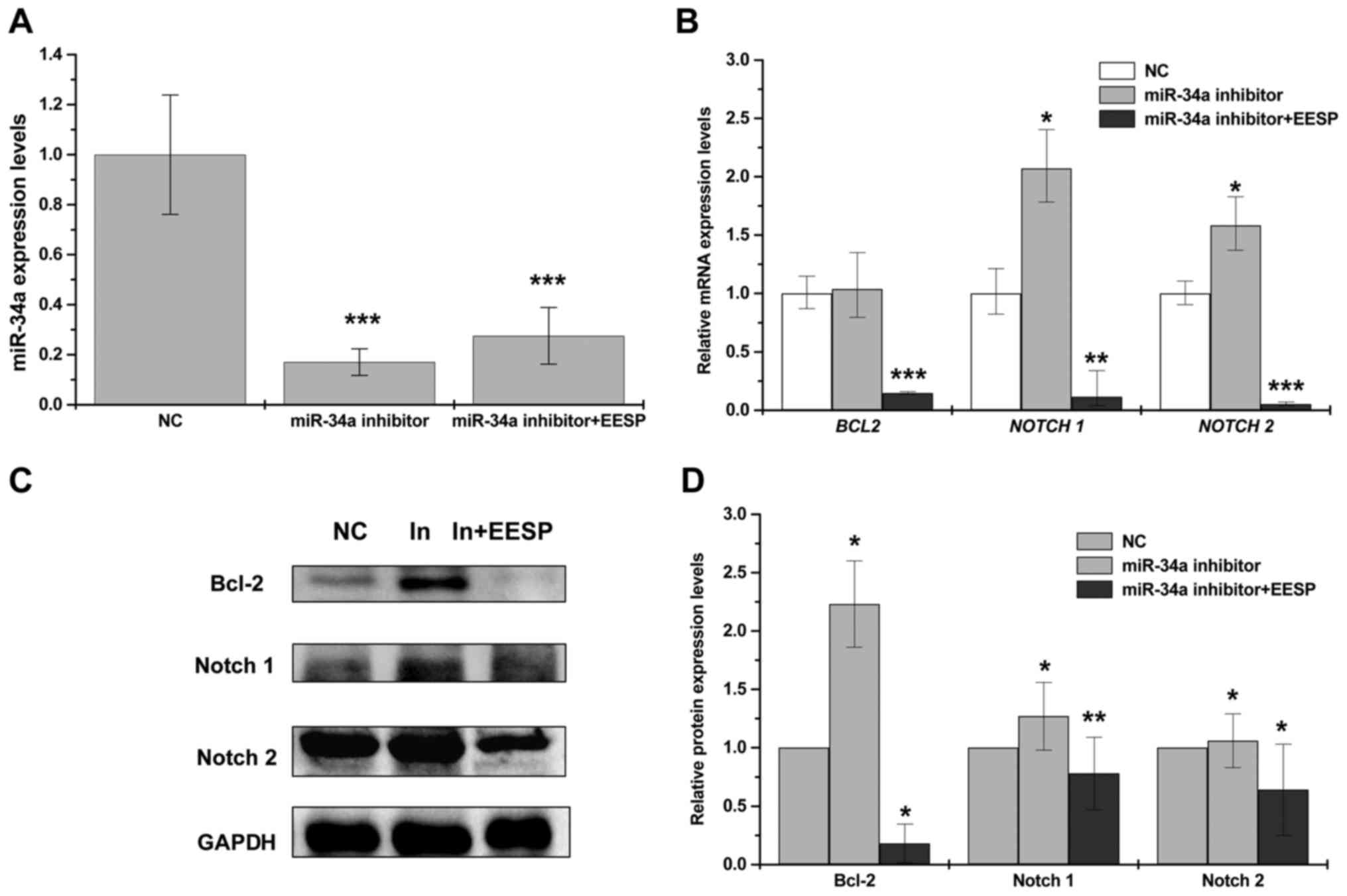
In order to verify that the effect of EESP was via
activation of miR-34a pathway, we transfected miR-34a inhibitors
into HCT-8 cells following EESP treatment to observe its
antagonistic effects. With transfection of miR-34a inhibitors, the
level of endogenous miR-34a was significantly decreased whereas its
target genes Bcl-2, Notch1 and Notch2 were all increased
significantly (Fig. 4A-D). In
contrast, following EESP treatment, transfection of miR-34a
inhibitors resulted in a significant downregulation of miR-34a
target genes (Bcl-2, Notch1 and Notch2), which was consistent with
our previous results (Fig. 3A-D).
These results indicated that EESP can antagonize the effect of
miR-34a inhibitors by increasing endogenous miR-34a expression.
Furthermore, its regulation of miR-34a was most likely by targeting
multiple oncogenes. Taken together, we revealed that EESP can
rescue miR-34a-induced downregulation of Bcl-2, Notch1 and Notch2
expression.
Discussion
Conventional cancer chemotherapy is presented with
various shortcomings, including increased drug resistance and
adverse effects. Therefore, the use of natural products for
treatment has received increased interest due to the potential for
fewer side-effects. Currently, the use of TCM is the most common
form of natural treatment, often used clinically for cancer therapy
(40,41). Spica Prunellae is a well-known
ingredient in TCM which is used for the clinical treatment of many
cancers (42,43). Previous research into the anticancer
mechanisms of Spica Prunellae was mainly focused on it protein
expression, and currently very little is known on the basis of its
miRNA expression. Our present study focuses on the miRNA expression
of Spica Prunellae, and provides crucial insight into the function
and involvement of miRNAs for its tumoricidal activity.
miR-34a is a member of the miR-34 family, which can
suppress tumor growth and metastasis by repressing cell cycle,
epithelial-to-mesenchymal transition (EMT) and metastasis, while
promoting apoptosis and senescence (44). In our study, EESP inhibited growth
of human colon carcinoma HCT-8 cells and induced cell apoptosis.
With miR-34a upregulation, its target genes Notch1, Notch2 and
Bcl-2 were repressed following EESP treatment. Since these target
genes were oncogenes and had apoptosis-targeting therapeutic
potential in cancers (45–47), the results suggested that EESP might
suppress growth of human colon carcinoma cells through promoting
cell apoptosis by targeting Notch1, Notch2 and Bcl-2 via activating
miR-34a (Fig. 5). Moreover, if
miR-34a was the only downstream target for EESP, with miR-34a
suppression by its inhibitor, the anticancer effect of EESP should
be weakened or even disappeared in theory. However, compared with
levels of cells transfected with miR-34a inhibitor only, the
expression levels of Notch1, Notch2 and Bcl-2 were all
downregulated significantly following the treatment of EESP with
miR-34a inhibitor (Fig. 4). With
these results, we believed that EESP could suppress expression of
Notch1, Notch2 and Bcl-2 not only via miR-34a. In addition,
previous work indicates that miR-34a suppressed tumors by the
induction of cell apoptosis or cell cycle arrest (48). The process was mediated by the
inhibitory effects of miR-34a on CDK4, CDK6, E2F3, cyclin D1,
cyclin E2, c-MET, c-MYC and SIRT1 (48–50).
We believed that the inhibition of cancer cells growth by miR-34a
following EESP treatment must be achieved via targeting of multiple
oncogenes besides the targets we studied above. As it consists of
various compounds, EESP is involved in multiple pathways and
targeted in various genes (51).
Our results provide the possibility that EESP also suppress tumors
or cancer cells through other pathways. The addition pathways await
further investigation.
Notch signaling pathway is important for various
developmental processes controlling cell fate decisions (52,53).
It is triggered through the binding of a ligand like
Jagged/Delta/Delta-like/Serrate on the membrane of one cell to a
receptor such as Notch1/Notch2/Notch3/Notch4 on the membrane of the
contacting cell (54). After
activation, the cleaved Notch would release the cytoplasmic tail of
NOTCH (NICD). With NICD translocated into the nucleus, it would
associate with transcriptional factors and then regulate its target
genes and modulate cell fate (55).
In Notch pathway, some targets of miR-34a including Notch ligands
such as Delta-like1 (Dll1), Jagged1 (JAG1) and transcription factor
hairy and enhancer of split-1 (HES1) (56–58)
were reported, it does not rule out the possibility that they also
take part in the mechanism as targets of miR-34a following EESP
treatment. It is noteworty that Notch1 and Notch2 were found to
induce apoptosis through Bcl-2 (59,60),
with the fact that Notch1, Notch2 and Bcl-2 were downregulated
following EESP treatment, we speculated that the repression of
Bcl-2 following EESP treatment may be a consequence of regulation
of both miR-34a and Notch1/Notch2 (Fig.
5). Future studies should address this hypothesis.
In summary, our study revealed that EESP suppresses
the growth of HCT-8 cells by targeting multiple oncogenes via
activation of miR-34a pathway. It helps us to understand the effect
for the anticancer activity of EESP better. We propose that the
regulation of miR-34a is the likely mechanism for the anticancer
activity of Spica Prunellae, although whether it can also induce
cell apoptosis by directly regulating the expression of miR-34a
target genes still requires further investigation.
Acknowledgements
We thank Jin Lin for help with the colony formation
experiment. We appreciate Yiyi Jin for help with western blot
experiment. Project 81603413 supported by National Natural Science
Foundation of China, Natural Science Foundation of Fujian province
of China (grant no. 2014J01360) and the Research Fund of the
Education bureau of Fujian Province (grant no. JAT160244).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stein A, Atanackovic D and Bokemeyer C:
Current standards and new trends in the primary treatment of
colorectal cancer. Eur J Cancer. 47:(Suppl 3). S312–S314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoo BH, Lee BH, Kim JS, Kim NJ, Kim SH and
Ryu KW: Effects of Shikunshito-Kamiho on fecal enzymes and
formation of aberrant crypt foci induced by 1,2-dimethylhydrazine.
Biol Pharm Bull. 24:638–642. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee MK, Ahn YM, Lee KR, Jung JH, Jung O-S
and Hong J: Development of a validated liquid chromatographic
method for the quality control of Prunellae Spica: Determination of
triterpenic acids. Anal Chim Acta. 633:271–277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Psotová J, Kolár M, Soušek J, Švagera Z,
Vičar J and Ulrichová J: Biological activities of Prunella
vulgaris extract. Phytother Res. 17:1082–1087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheung H-Y and Zhang Q-F: Enhanced
analysis of triterpenes, flavonoids and phenolic compounds in
Prunella vulgaris L. by capillary zone electrophoresis with
the addition of running buffer modifiers. J Chromatogr A.
1213:231–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu X, Li Y, Mu J and Zhang Y: Chemical
constituents of Prunella vulgaris. J Environ Sci (China).
25:(Suppl 1). S161–S163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng L, Au-Yeung W, Xu Y-H, Wang S-S, Zhu
Q and Xiang P: Oleanolic acid from Prunella vulgaris L.
induces SPC-A-1 cell line apoptosis via regulation of Bax, Bad and
Bcl-2 expression. Asian Pac J Cancer Prev. 12:403–408.
2011.PubMed/NCBI
|
|
10
|
Chu R, Zhao X, Griffin C, Staub RE,
Shoemaker M, Climent J, Leitman D, Cohen I, Shtivelman E and Fong
S: Selective concomitant inhibition of mTORC1 and mTORC2 activity
in estrogen receptor negative breast cancer cells by BN107 and
oleanolic acid. Int J Cancer. 127:1209–1219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan SL, Huang CY, Wu ST and Yin MC:
Oleanolic acid and ursolic acid induce apoptosis in four human
liver cancer cell lines. Toxicol In Vitro. 24:842–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nelson AT, Camelio AM, Claussen KR, Cho J,
Tremmel L, DiGiovanni J and Siegel D: Synthesis of oxygenated
oleanolic and ursolic acid derivatives with anti-inflammatory
properties. Bioorg Med Chem Lett. 25:4342–4346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryu SY, Oak M-H, Yoon S-K, Cho DI, Yoo GS,
Kim TS and Kim KM: Anti-allergic and anti-inflammatory triterpenes
from the herb of Prunella vulgaris. Planta Med. 66:358–360.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Popov AM, Osipov AN, Korepanova EA,
Krivoshapko ON and Artiukov AA: Study of antioxidant and membrane
activity of rosmarinic acid using different model systems.
Biofizika. 58:775–785. 2013.(In Russian). PubMed/NCBI
|
|
15
|
Maheswarappa N Basappa, Subbaiah V,
Muthupalani M, Yamagani PK, Mohan K, Keshapaga UR, Asokan S
Vaikkathukattil and Kalappurakkal RC: Antioxidant activity of
carnosic acid and rosmarinic acid in raw and cooked ground chicken
patties. J Sci Food Agric. 94:273–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fallarini S, Miglio G, Paoletti T, Minassi
A, Amoruso A, Bardelli C, Brunelleschi S and Lombardi G: Clovamide
and rosmarinic acid induce neuroprotective effects in in vitro
models of neuronal death. Br J Pharmacol. 157:1072–1084. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khamse S, Sadr SS, Roghani M, Hasanzadeh G
and Mohammadian M: Rosmarinic acid exerts a neuroprotective effect
in the kainate rat model of temporal lobe epilepsy: Underlying
mechanisms. Pharm Biol. 53:1818–1825. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Han S, Lei K, Chang X, Wang K, Li Z
and Liu J: Anti-Warburg effect of rosmarinic acid via miR-155 in
colorectal carcinoma cells. Eur J Cancer Prev. 25:481–489. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hao J, Ding XL, Yang X and Wu XZ:
Prunella vulgaris polysaccharide inhibits growth and
migration of breast carcinoma-associated fibroblasts by suppressing
expression of basic fibroblast growth factor. Chin J Integr Med.
Sep 1–2016.(Epub ahead of print). View Article : Google Scholar
|
|
20
|
Sun H-X, Qin F and Pan Y-J: In vitro and
in vivo immunosuppressive activity of Spica Prunellae ethanol
extract on the immune responses in mice. J Ethnopharmacol.
101:31–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin W, Zheng L, Zhao J, Zhuang Q, Hong Z,
Xu W, Chen Y, Sferra JT and Peng J: Anti-angiogenic effect of Spica
Prunellae extract in vivo and in vitro. Afr J Pharm Pharmacol.
5:2647–2654. 2011.http://www.academicjournals.org/article/article1380896211_Lin%20et%20al%20%202.pdf
|
|
22
|
Hwang SM, Lee YJ, Yoon JJ, Lee SM, Kim JS,
Kang DG and Lee HS: Prunella vulgaris suppresses HG-induced
vascular inflammation via Nrf2/HO-1/eNOS activation. Int J Mol Sci.
13:1258–1268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Collins NH, Lessey EC, DuSell CD,
McDonnell DP, Fowler L, Palomino WA, Illera MJ, Yu X, Mo B, Houwing
AM, et al: Characterization of antiestrogenic activity of the
Chinese herb, Prunella vulgaris, using in vitro and in vivo
(Mouse Xenograft) models. Biol Reprod. 80:375–383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng L, Wang L, Ma YY, Li M and Zhao GQ: A
potential in vitro and in vivo anti-HIV drug screening system for
Chinese herbal medicines. Phytother Res. 26:899–907. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh C, Price J, Brindley MA, Widrlechner
MP, Qu L, McCoy JA, Murphy P, Hauck C and Maury W: Inhibition of
HIV-1 infection by aqueous extracts of Prunella vulgaris L.
Virol J. 8:1882011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin W, Zheng L, Zhuang Q, Zhao J, Cao Z,
Zeng J, Lin S, Xu W and Peng J: Spica prunellae promotes cancer
cell apoptosis, inhibits cell proliferation and tumor angiogenesis
in a mouse model of colorectal cancer via suppression of stat3
pathway. BMC Complement Altern Med. 13:1442013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng L, Chen Y, Lin W, Zhuang Q, Chen X,
Xu W, Liu X, Peng J and Sferra TJ: Spica Prunellae extract promotes
mitochondriondependent apoptosis in a human colon carcinoma cell
line. Afr J Pharm Pharmacol. 5:327–335. 2011.http://www.academicjournals.org/article/article1380789242_Zheng%20et%20al.pdf
View Article : Google Scholar
|
|
28
|
Feng L, Jia X, Zhu M, Chen Y and Shi F:
Chemoprevention by Prunella vulgaris L. extract of non-small
cell lung cancer via promoting apoptosis and regulating the cell
cycle. Asian Pac J Cancer Prev. 11:1355–1358. 2010.PubMed/NCBI
|
|
29
|
Lin W, Zheng L, Zhuang Q, Shen A, Liu L,
Chen Y, Sferra TJ and Peng J: Spica Prunellae extract inhibits the
proliferation of human colon carcinoma cells via the regulation of
the cell cycle. Oncol Lett. 6:1123–1127. 2013.PubMed/NCBI
|
|
30
|
Kim S-H, Huang C-Y, Tsai C-Y, Lu S-Y, Chiu
C-C and Fang K: The aqueous extract of Prunella vulgaris
suppresses cell invasion and migration in human liver cancer cells
by attenuating matrix metalloproteinases. Am J Chin Med.
40:643–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwakawa HO and Tomari Y: The functions of
microRNAs: mRNA decay and translational repression. Trends Cell
Biol. 25:651–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li L, Yuan L, Luo J, Gao J, Guo J and Xie
X: MiR-34a inhibits proliferation and migration of breast cancer
through down-regulation of Bcl-2 and SIRT1. Clin Exp Med.
13:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Guessous F, Zhang Y, Dipierro C,
Kefas B, Johnson E, Marcinkiewicz L, Jiang J, Yang Y, Schmittgen
TD, et al: MicroRNA-34a inhibits glioblastoma growth by targeting
multiple oncogenes. Cancer Res. 69:7569–7576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pang RTK, Leung CON, Ye TM, Liu W, Chiu
PC, Lam KK, Lee KF and Yeung WS: MicroRNA-34a suppresses invasion
through downregulation of Notch1 and Jagged1 in cervical carcinoma
and choriocarcinoma cells. Carcinogenesis. 31:1037–1044. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fang Y, Feng Y, Wu T, Srinivas S, Yang W,
Fan J, Yang C and Wang S: Aflatoxin B1 negatively regulates
Wnt/β-catenin signaling pathway through activating miR-33a. PLoS
One. 8:e730042013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang W, Lian J, Feng Y, Srinivas S, Guo Z,
Zhong H, Zhuang Z and Wang S: Genome-wide miRNA-profiling of
aflatoxin B1-induced hepatic injury using deep sequencing. Toxicol
Lett. 226:140–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−∆∆C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McPherson L, Cochrane S and Zhu X: Current
usage of traditional Chinese medicine in the management of breast
cancer: A practitioner's perspective. Integr Cancer Ther.
15:335–342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liao YH, Lin CC, Lai HC, Chiang JH, Lin JG
and Li TC: Adjunctive traditional Chinese medicine therapy improves
survival of liver cancer patients. Liver Int. 35:2595–2602. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo Q, Li J and Lin H: Effect and
molecular mechanisms of traditional chinese medicine on regulating
tumor immunosuppressive microenvironment. BioMed Res Int.
2015:2616202015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Ethyl acetate extraction
from a Chinese herbal formula, Jiedu Xiaozheng Yin, inhibits the
proliferation of hepatocellular carcinoma cells via induction of
G0/G1 phase arrest in vivo and in vitro. Int J Oncol.
42:202–210. 2013.PubMed/NCBI
|
|
44
|
Vermeulen K, Berneman ZN and Van
Bockstaele DR: Cell cycle and apoptosis. Cell Prolif. 36:165–175.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Duechler M, Shehata M, Schwarzmeier JD,
Hoelbl A, Hilgarth M and Hubmann R: Induction of apoptosis by
proteasome inhibitors in B-CLL cells is associated with
downregulation of CD23 and inactivation of Notch2. Leukemia.
19:260–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brzozowa-Zasada M, Piecuch A, Dittfeld A,
Mielańczyk Ł, Michalski M, Wyrobiec G, Harabin-Słowińska M, Kurek J
and Wojnicz R: Notch signalling pathway as an oncogenic factor
involved in cancer development. Contemp Oncol (Pozn). 20:267–272.
2016.PubMed/NCBI
|
|
47
|
Gu Y, Masiero M and Banham AH: Notch
signaling: Its roles and therapeutic potential in hematological
malignancies. Oncotarget. 7:29804–29823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Misso G, Di Martino MT, De Rosa G, Farooqi
AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P,
Tassone P, et al: Mir-34: A new weapon against cancer? Mol Ther
Nucleic Acids. 3:e1942014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun F, Fu H, Liu Q, Tie Y, Zhu J, Xing R,
Sun Z and Zheng X: Downregulation of CCND1 and CDK6 by miR-34a
induces cell cycle arrest. FEBS Lett. 582:1564–1568. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mao X, Wang G, Zhang W and Li S: A study
on inhibitory effect of Spica prunellae extract on T lymphoma cell
EL-4 tumour. Afr J Tradit Complement Altern Med. 10:318–324.
2013.PubMed/NCBI
|
|
52
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lai EC: Notch signaling: Control of cell
communication and cell fate. Development. 131:965–973. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sikandar SS, Pate KT, Anderson S, Dizon D,
Edwards RA, Waterman ML and Lipkin SM: NOTCH signaling is required
for formation and self-renewal of tumor-initiating cells and for
repression of secretory cell differentiation in colon cancer.
Cancer Res. 70:1469–1478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Z, Zhang Y, Banerjee S, Li Y and
Sarkar FH: Notch-1 down-regulation by curcumin is associated with
the inhibition of cell growth and the induction of apoptosis in
pancreatic cancer cells. Cancer. 106:2503–2513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pang B, Pang Q, Pang H and Song G:
Clinical effect of Jiutengzhuyu tablets on promoting blood
circulation in women with oviducal obstruction. J Tradit Chin Med.
32:549–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
de Antonellis P, Medaglia C, Cusanelli E,
Andolfo I, Liguori L, De Vita G, Carotenuto M, Bello A, Formiggini
F, Galeone A, et al: MiR-34a targeting of Notch ligand delta-like 1
impairs CD15+/CD133+ tumor-propagating cells
and supports neural differentiation in medulloblastoma. PLoS One.
6:e245842011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun F, Wan M, Xu X, Gao B, Zhou Y, Sun J,
Cheng L, Klein OD, Zhou X and Zheng L: Crosstalk between miR-34a
and Notch Signaling Promotes Differentiation in Apical Papilla Stem
Cells (SCAPs). J Dent Res. 93:589–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ
and He LY: Silencing Notch-1 induces apoptosis and increases the
chemosensitivity of prostate cancer cells to docetaxel through
Bcl-2 and Bax. Oncol Lett. 3:879–884. 2012.PubMed/NCBI
|
|
60
|
Gao F, Yao M, Shi Y, Hao J, Ren Y, Liu Q,
Wang X and Duan H: Notch pathway is involved in high
glucose-induced apoptosis in podocytes via Bcl-2 and p53 pathways.
J Cell Biochem. 114:1029–1038. 2013. View Article : Google Scholar : PubMed/NCBI
|