Introduction
Acute myeloid leukemia (AML) is a clinically and
biologically heterogeneous clonal hematologic disorder that is
common and lethal in adults (1).
Even with improvements in diagnosis and supportive care, the 5-year
survival rate for adults with AML is only 30%. There have been no
breakthroughs in AML treatment in the last 40 years, although in
the past decade, an increasing number of potential drug targets
have been identified (2).
Heat shock protein 90 (Hsp 90) is a chaperone
protein required for the folding and stabilization of proteins
involved in intracellular signaling such as Akt and the nuclear
factor (NF)-κB signaling pathway component inhibitor of κB kinase
(IKK) α, which regulate cell survival, proliferation and
differentiation (3,4). Hsp90 is overexpressed in many types of
cancer relative to normal tissues and is therefore considered a
potential anticancer drug target (3,5). There
are sixteen different Hsp90 inhibitors that have entered clinical
testing including first generation Hsp90 inhibitors (geldanamycin
and its derivatives) and second generation Hsp90 inhibitors
(NVP-AUY922 and SNX-5422). However, there is no FDA approved Hsp90
inhibitor nor standardized assay to ascertain Hsp90 inhibition. The
most clinically significant off-target toxicity with the
geldanamycin derivatives was hepatotoxicity resulting from the
presence of a benzoquinone moiety. In addition to hepatotoxicity,
ocular and cardiac toxicities also limited further clinical
development of these drugs (6–8).
Therefore, development of an Hsp90 inhibitor with better
pharmacological properties and a safety profile is important.
Most Hsp90 inhibitors exhibit great anti-acute
myeloid leukemia effects. The second-generation Hsp90 inhibitor
NVP-AUY922-AG was demonstrated to be cytotoxic in myeloid cell
lines and primary AML cells (9),
and other Hsp90 inhibitors were tested in phase I clinical trials
and have been well tolerated in patients with advanced AML
(10). SNX-2112 is a novel
inhibitor that competitively binds to the N-terminal ATP-binding
site of Hsp90 and has shown anticancer activity in vitro and
in vivo (3,11).
A hallmark of AML is that leukemic blast cells are
arrested at an early stage of differentiation. It has therefore
been suggested that therapies promoting differentiation may be
effective for AML treatment, a concept known as differentiation
therapy (12).
All-trans-retinoic acid (ATRA) and arsenic trioxide used in
combination with chemotherapy is the standard treatment for acute
promyelocytic leukemia and is a potential paradigm for
differentiation therapy in clinical oncology, but not for other
subtypes of AML (13,14). Studies have shown that isocitrate
dehydrogenase (IDH), glycogen synthase kinase (GSK)-3, and
dihydroorotate dehydrogenase inhibition induces AML cell
differentiation (15–18), but there are no studies on whether
the inhibition of Hsp90 can achieve this effect.
The transcription factor CCAAT/enhancer binding
protein α (C/EBPα) is a critical regulator of myeloid development
that directs granulocyte and monocyte differentiation. Conditional
C/EBPα deficiency in adult mice blocked the transition from common
myeloid progenitor (CMP) to granulocyte-monocyte progenitor (GMP),
resulting in decreased formation of both granulocytes and monocytes
(19). In addition, the suppression
of C/EBPα expression may block differentiation, and also stimulate
proliferation of transformed cells (20). PU.1 is a member of the Ezb
transformation-specific sequence family of transcription factors,
which is expressed in granulocytes, monocytes and B-lymphoid cells
(21), with its level increasing
during differentiation. As such, PU.1 mutation, which has been
identified in a subset of AML patients, has been linked to
leukemogenesis (22). Both C/EBPα
and PU.1 are required for differentiation of the granulocytic
lineage.
Akt is a serine threonine kinase downstream of
phosphoinositide 3-kinase (PI3K) that has many downstream targets
associated with cell survival and cell cycle regulation, and also
inhibits hematopoietic cell apoptosis (23). Nuclear factor (NF)-κB regulates
various biological processes linked to leukemogenesis, including
cell proliferation, differentiation, autophagy and apoptosis, and
is constitutively activated in AML cells (24,25).
The present study investigated the potential of the
Hsp90 inhibitor SNX-2112 to be used for treatment of AML using
human acute leukemia KG-1a cells. We found that SNX-2112 induced
cell cycle arrest at the G2/M phase and apoptosis while promoted
differentiation and suppressed cell growth, effects that involve
modulation of Akt and NF-κB signaling.
Materials and methods
Cell culture and reagents
Human AML KG-1a cells purchased from the Cell Bank
of the Chinese Academy of Sciences (Shanghai, China) were cultured
in Roswell Park Memorial Institute (RPMI)-1640 medium containing
15% fetal bovine serum (FBS) and 100 U/ml penicillin/streptomycin
in a humidified incubator of 5% CO2 at 37°C. SNX-2112
was synthesized as previously described (26) with a purity >98.0%, and 10 mM
SNX-2112 stock solutions were prepared in dimethyl sulfoxide (DMSO)
and stored at 4°C. 17-AAG and bardoxolone methyl (BAR; an NF-κB
pathway inhibitor) were obtained from Selleck Chemicals (Houston,
TX, USA). RPMI-1640 medium and FBS were purchased from Gibco (Grand
Island, NY, USA). Antibodies against glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), caspase-3, Bcl-xl, Bcl-2, Akt, p-Akt
(Thr308), IKKα, IKKβ, p65, p-p65, IκB, and PU.1 were purchased from
Cell Signaling Technology (Beverly, MA, USA), and cluster of
differentiation (CD)11b was purchased from BD Biosciences (Franklin
Lakes, NJ, USA).
Cell proliferation assay
Cell proliferation was evaluated using Cell Counting
Kit-8 (CCK-8). Briefly, 1.0×104 cells/well were seeded
into 96-well plates and treated with SNX-2112 or 17-AAG for 24, 48
or 72 h. A 10-µl volume of CCK-8 working solution was added to each
well for 2 h. The absorbance was assessed at 450 nm on a microplate
reader (Bio-Rad, Hercules, CA, USA) and used to determine the drug
concentration inhibiting growth by 50% (IC50).
Cell cycle analysis
KG-1a cells were seeded at 1.0×105
cells/ml and treated with SNX-2112 for 48 h. The cells were
collected by centrifugation at 500 × g for 5 min; 70% ethanol was
then added, followed by 2 washes with phosphate-buffered saline
(PBS). Cells were resuspended in 1 ml PBS containing 2.5 µg/ml
ribonuclease and 50 µg/ml propidium iodide (PI) (Beyotime Institute
of Biotechnology, Shanghai, China) and incubated in the dark for 30
min at room temperature before analysis by flow cytometry (BD
FACSCalibur, Lake Franklin, NJ, USA; BD Biosciences).
Cell differentiation assay
Cells were seeded at 1.0×105 cells/ml and
incubated with SNX-2112 for 48 h. The cells were collected by
centrifugation at 500 × g for 5 min and washed with PBS twice.
Then, a 20-µl volume of CD11b was added for 20 min in the dark and
analyzed by flow cytometry.
Cell apoptosis assay
Cells were seeded at 1.0×105 cells/ml and
incubated with SNX-2112/Bar for 48 h. Samples were prepared
according to the instructions provided with the Annexin
V-fluorescein isothiocyanate/PI staining kit (Beyotime Institute of
Biotechnology) and analyzed by flow cytometry.
Quantitative real-time (qRT)-PCR
KG-1a cells were seeded at 1.0×105
cells/ml, and incubated with DMSO or SNX-2112 for 48 h, and total
RNA was immediately extracted using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's instructions.
For analysis of PU.1 and C/EBPα, 2 µg of total RNA was used to
synthesize first-strand DNA with reverse transcriptase (Promega,
Madison, WI, USA). qRT-PCR was then carried out using Green PCR
Master Mix (Shine Co., Shanghai, China), 1 µl of cDNA,
gene-specific primers (Table I),
and the Hot Start Fluo-PCR Mix in a 20-µl reaction under the
following cycling conditions: 95°C for 5 min, followed by 35 cycles
at 95°C for 10 sec, 57°C for 15 sec, and 72°C for 20 sec. Each
sample was prepared in triplicate and transcript levels were
quantified using the 2−ΔΔCt method (27).
 | Table I.Primers used for qRT-PCR. |
Table I.
Primers used for qRT-PCR.
| Gene name |
| Sequence
(5′→3′) |
|---|
| GAPDH | F |
CGTCTTCACCACCATGGAGA |
|
| R |
CGGCCATCACGCCACAGTTT |
| PU.1 | F |
GTGCCCTATGACACGGATCT |
|
| R |
GAAGCTCTCGAACTCGCTGT |
| C/EBPα | F |
TGGACAAGAACAGCAACGAG |
|
| R |
TTGTCACTGGTCAGCTCCAG |
Immunofluorescence analysis
KG-1a cells cultured in 6-well plates were treated
with DMSO or SNX-2112 (0, 0.25, 0.5 or 1 µM) for 48 h. The cells
were washed 3 times with ice-cold PBS and fixed with 4%
paraformaldehyde. The cell membrane was permeabilized by adding
0.1% Triton X-100, and the cells were blocked with 5% bovine serum
albumin (HyClone, Logan, UT, USA) and incubated for 2 h at room
temperature with an antibody against NF-κB p65 (1:400) (Cell
Signaling Technology), followed by incubation with a
fluorophore-conjugated anti-rabbit antibody for 2 h at room
temperature. The cells were then stained with PI (Beyotime
Institute of Biotechnology) for 15 min, and subsequently washed and
examined with an epifluorescence microscope (Zeiss, Jena,
Germany).
Western blotting
KG-1a cells were washed twice in ice-cold PBS, lysed
in radioimmunoprecipitation buffer for 30 min on ice at 4°C, and
centrifuged at 12,000 × g for 15 min. The protein content in the
supernatant was determined with the bicinchoninic acid assay.
Equivalent amounts (30–50 µg) of protein were denatured in sodium
dodecyl sulfate (SDS) sample buffer and resolved by 10–15%
SDS-polyacrylamide gel electrophoresis, and then transferred to an
Immobilon polyvinylidene difluoride membrane, which was blocked in
5% skimmed milk in Tris-buffered saline containing 0.1% Tween-20
(TBST) at room temperature for 1 h and then probed with primary
antibodies (1:1,000) overnight at 4°C. The membrane was washed 3
times for 10 min in TBST, and incubated with a secondary antibody
(1:6,000–1:8,000) in TBST with 5% skimmed milk at room temperature
for 1 h. After washing, immunoreactivity was detected with an
enhanced chemiluminescence kit (Pierce, Rockford, IL, USA). GAPDH
served as a loading control.
Clone formation assay
Cells were treated with SNX-2112 or 17-AAG for 2
days, and then washed twice with PBS. The cells were seeded
(1×103 cells/dish) in 1.5 ml of 0.30–0.35% soft agar
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 20% FBS and
cultured for 12 days at 37°C and 5% CO2. After 12 days,
the colonies were counted under a light microscope.
Statistical analysis
All data were confirmed by at least 3 independent
experiments. Data are expressed as the mean ± SD. Statistical
significance was determined by one-way analysis of variance (ANOVA)
and covariance calculated using GraphPad 6.0 (GraphPad Software,
Inc., La Jolla, CA, USA). Differences between 2 groups were
detected using two-tailed Student's t-test. P<0.05 and P<0.01
were considered to indicate a statistically significant result.
Results
SNX-2112 inhibits KG-1a cells
proliferation
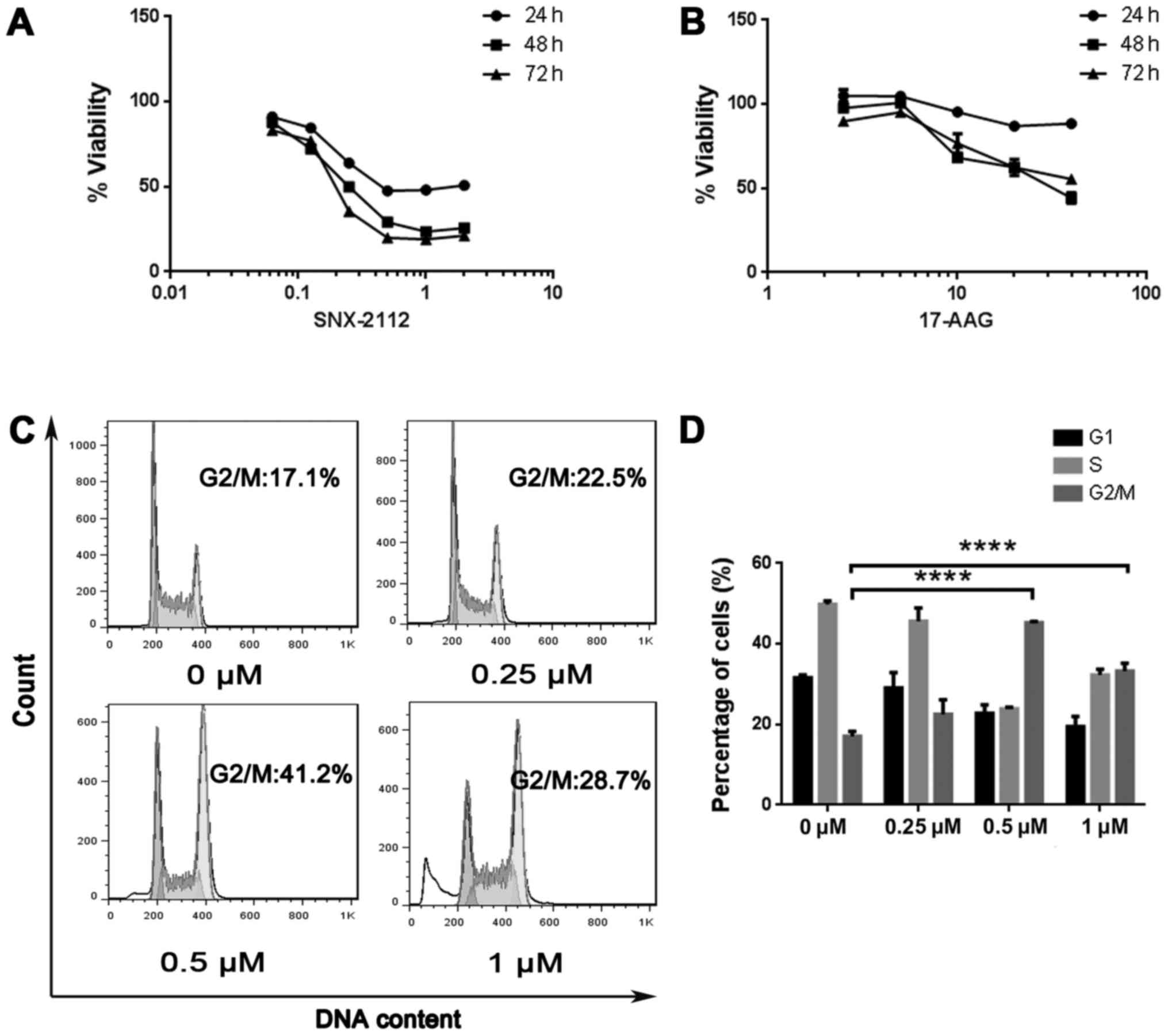
The effects of SNX-2112 and 17-AAG on the viability
of KG-1a cells were evaluated using CCK-8 assay. Both compounds
showed dose- and time-dependent inhibition of KG-1a cells
proliferation after 24, 48 and 72 h. The IC50 values of
SNX-2112 were 1.02, 0.29 and 0.22 µM, respectively. These were
lower than the IC50 values of 17-AAG, which were 209.6,
30.27 and 44.75 µM, respectively (Fig.
1A and B), indicating that SNX-2112 inhibits cell growth to a
greater degree.
SNX-2112 induces cells cycle arrest at
the G2/M phase
To determine whether cell growth inhibition by
SNX-2112 is associated with cell cycle arrest, the cell cycle
distribution was analyzed using flow cytometry. KG-1a cells treated
with SNX-2112 were arrested in the G2/M phase after 48 h (Fig. 1C); the proportion of arrested cells
was 22.5, 41.2 and 28.7% at concentrations of 0.25, 0.5 and 1 µM,
respectively, as compared to 17% in the control group (Fig. 1D).
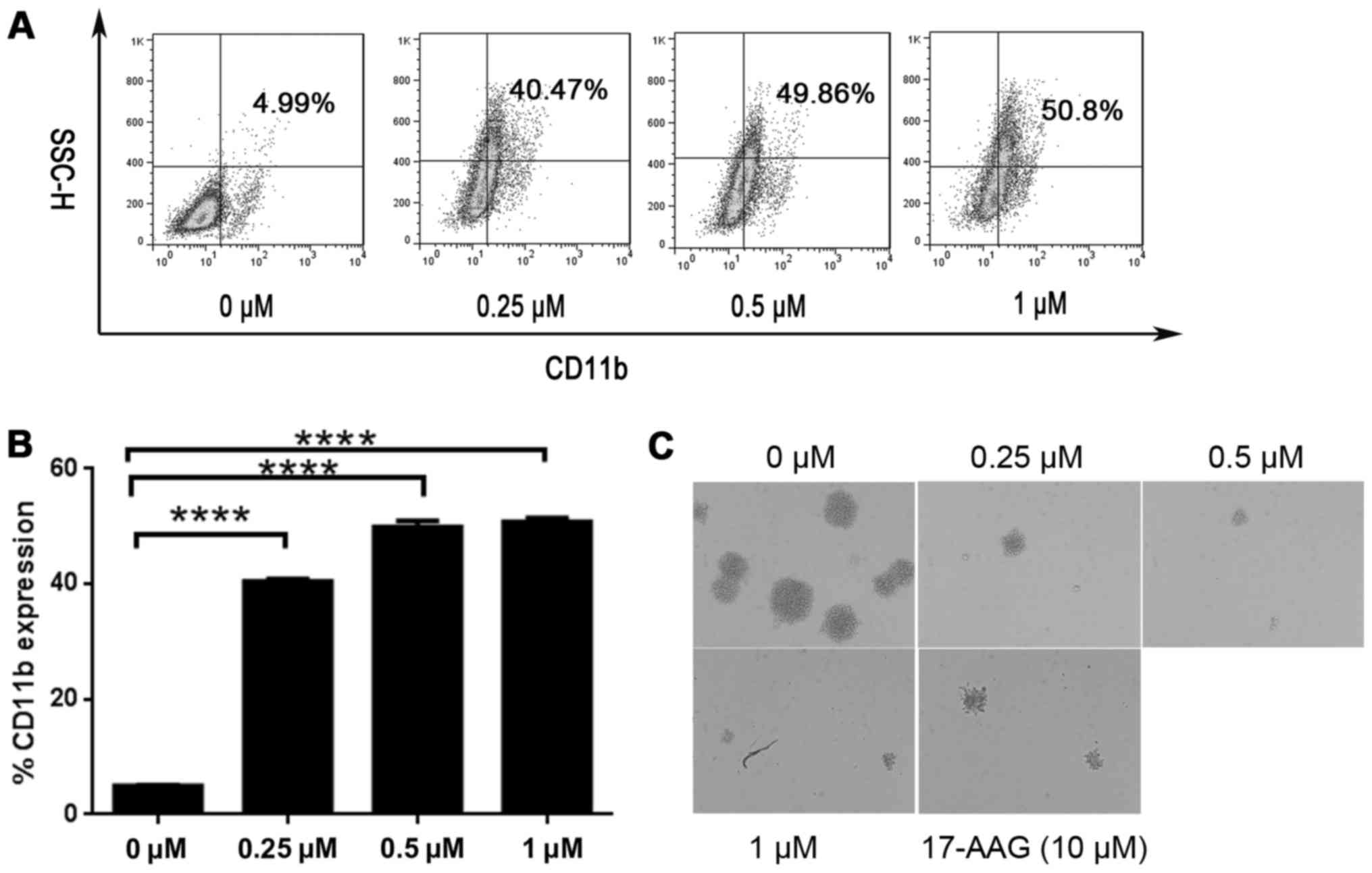
SNX-2112 induces differentiation and
decreases colony formation
To determine whether KG-1a cells differentiation was
affected by SNX-2112 treatment, we analyzed the expression of the
differentiation marker CD11b by flow cytometry at 24 and 48 h.
CD11b expression was increased by treatment with SNX-2112 from 3.32
to 19.18% at 24 h (data not shown), and from 4.99 to 50.8% at 48 h
(Fig. 2A and B).
Since the aim of AML differentiation therapy is to
suppress the growth of AML cells, we performed a colony formation
assay to determine whether SNX-2112 induced irreversible growth
arrest. KG-1a cells were exposed to the drug for 2 days, and after
washing, an equal number of viable cells were plated on soft agar.
A marked decrease in the number of colonies formed was observed
upon treatment with SNX-2112 (Fig.
2C), indicating that SNX-2112 can cause KG-1a cells growth
arrest, and that SNX-2112 was more potent than 17-AAG.
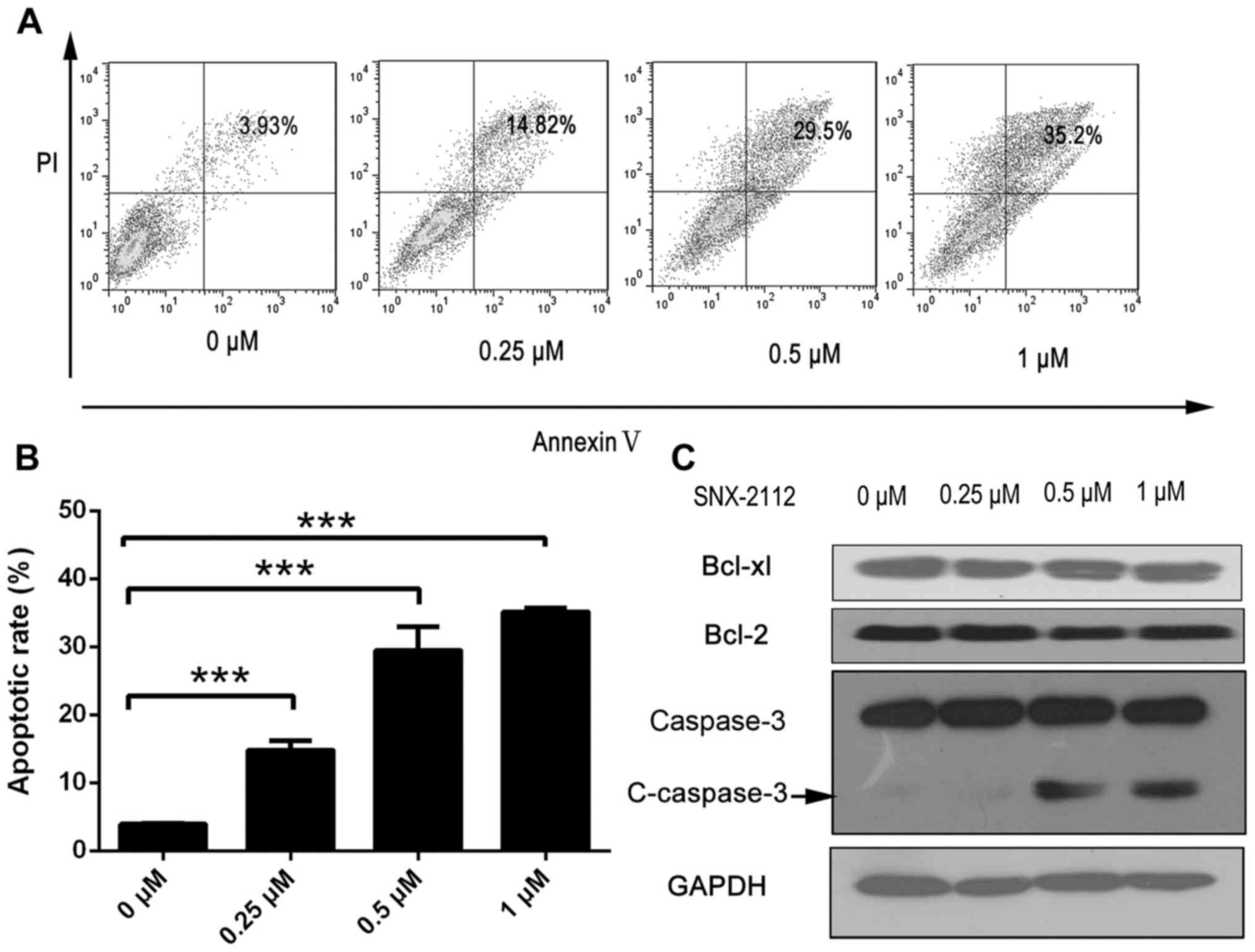
SNX-2112 induces KG-1a cells
apoptosis
To determine whether SNX-2112 inhibits KG-1a cells
growth by inducing apoptosis, the treated cells were labeled with
Annexin V/PI and analyzed by flow cytometry. Annexin
V+/PI− and Annexin
V+/PI+ cells were designated as early
apoptotic and necrotic cells, respectively. The number of apoptotic
cells was increased from 3.93% in the control cells to 14.82, 29.5
and 35.2% by treatment with 0.25, 0.5 and 1 µM SNX-2112,
respectively, for 48 h (Fig. 3A and
B). Moreover, the expression of the apoptosis-related protein
cleaved caspase-3 was increased in the presence of SNX-2112,
although that of B cell lymphoma (Bcl)-2 and Bcl-2-associated X
protein were unaltered (Fig.
3C).
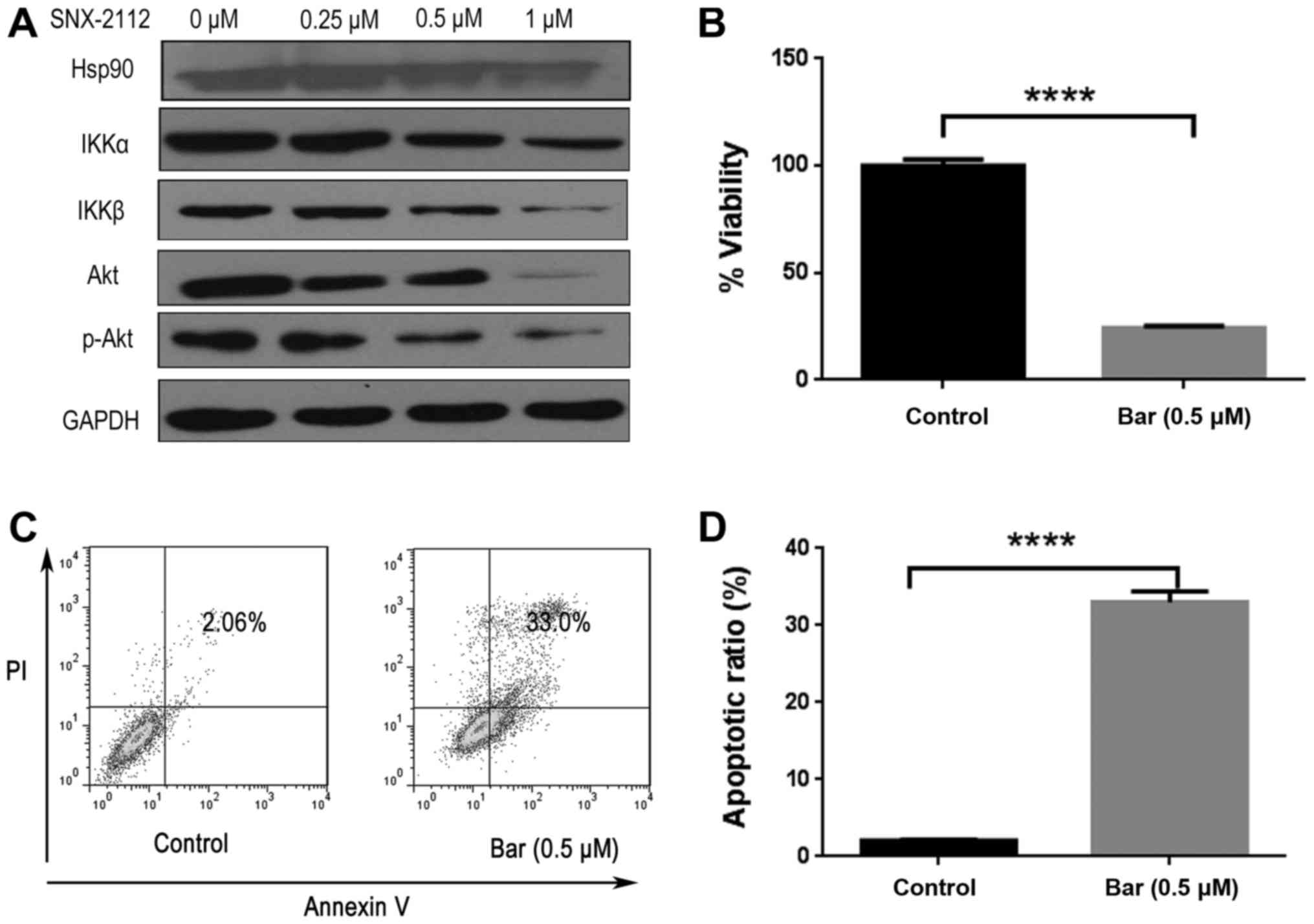
SNX-2112 inhibits Akt and NF-κB
signaling
Hsp90 inhibition has been demonstrated to block PI3K
and IKK signaling pathways (9). We
therefore investigated whether SNX-2112 treatment affected the
expression of Akt and IKK by western blotting. Previous studies
revealed that Hsp90 was overexpressed in many types of cancer
(3,5). Our results revealed that Hsp90 was
highly expressed in KG-1a cells, and Hsp90 client proteins IKKα,
IKKβ and Akt expression levels were downregulated upon treatment
with SNX-2112 for 48 h (Fig. 4A).
Consistent with previous studies (28), NF-κB pathway inhibitor Bar,
inhibited KG-1a cell proliferation and induced apoptosis (Fig. 4B-D). Bar is an IKKβ-inhibitor which
inhibited the expression of downstream protein p65 but did not
directly interfere with the expression of IKK after treatment with
Bar (0.5 µM) for 48 h (Fig. 4E), as
Bar could possibly block the upstream kinases that have been
implicated in the activation of IKK (29). SNX-2112 treatment resulted in the
upregulation of NF-κB inhibitors (i.e., IκB) and downregulation of
p65 and p-p65 after 48 h (Fig. 4F and
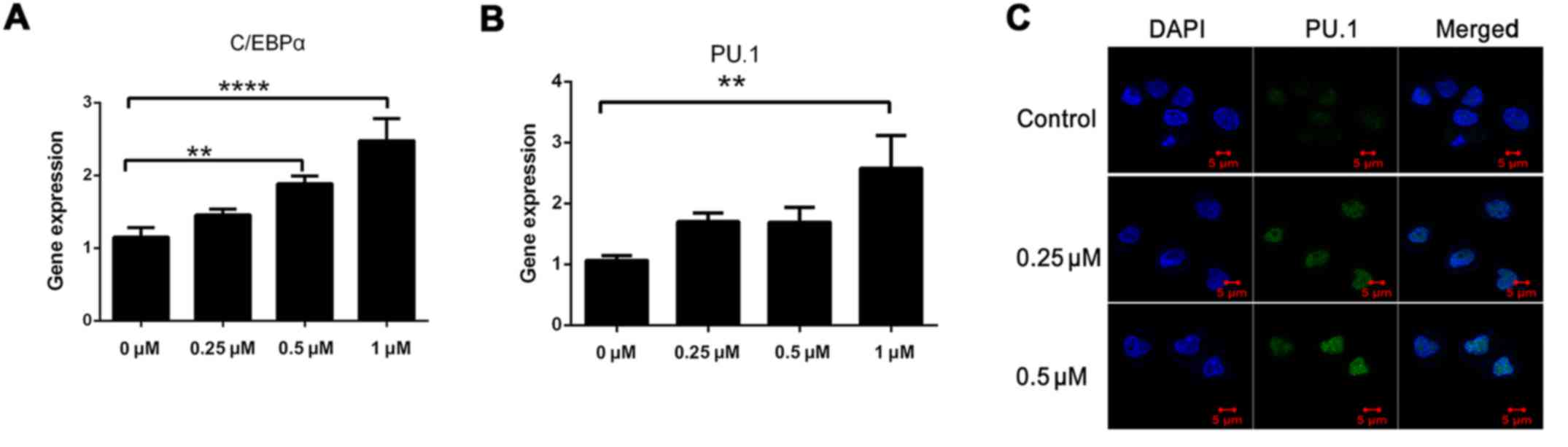
G). Since C/EBPα and PU.1 play key roles in myeloid
differentiation and NF-κB has been demonstrated to regulate PU.1
(30–32), we evaluated their expression after
48 h by qRT-PCR. The levels of both transcripts were increased by
>2-fold in the presence of SNX-2112 (Fig. 5A and B). The increase in PU.1
expression was confirmed by immunofluorescence analysis (Fig. 5C). These results indicate that
inhibition of Hsp90 by SNX-2112 suppresses the expression of Akt
and IKK and induces that of C/EBPα and PU.1 via inhibition of NF-κB
signaling in KG-1a cells.
Discussion
Hsp90 is overexpressed in most cancers;
pharmacological inhibition of this protein can induce apoptosis in
hematologic and other types of tumors (9). In the present study, we demonstrated
that the Hsp90 inhibitor SNX-2112 suppressed the proliferation of
KG-1a cells and induced their arrest in the G2/M phase and their
differentiation and apoptosis in a time- and dose-dependent manner.
Previous studies have revealed that SNX-2112 affects the activity
and expression of Hsp90 client proteins (11), and our results revealed that
SNX-2112 treatment resulted in the downregulation of the
Hsp90-associated proteins Akt and IKKα as well as cleavage of
caspase-3. We previously demonstrated that SNX-2112 suppressed K562
cell proliferation (33). Other
studies have reported that it also inhibits the growth of multiple
myeloma and other hematologic tumors (34). In the present study SNX-2112 had
more potent effects than the classical Hsp90 inhibitor 17-AAG in
KG-1a human acute leukemia cells.
AML is characterized by uncontrolled proliferation
of myeloid progenitors that have decreased capacity for
differentiation into mature granulocytes or macrophages (35). Differentiation therapy is a
promising approach for the treatment of AML. Small-molecule
inhibitors of mutant IDH2 (15) or
IDH1 (16) can induce tumor cell
differentiation in a subset (15%) of AML patients with
IDH1/2 mutations; GSK-3 inhibition sensitizes AML cells to
differentiation induced by an ATRA analog (17), with inhibition of dihydroorotate
dehydrogenase having a similar effect (18). It has been reported that
co-treatment with tubacin and 17-AAG decreased the viability of
primary AML cells (36), whereas
17-AAG suppressed the growth of lymphoma stem cells (37). However, few studies have
investigated the effect of HSP90 inhibition on AML. In the present
study, we found that the Hsp90 inhibitor SNX-2112 promoted the
differentiation of KG-1a cells.
Aberrant expression of C/EBPα and PU.1 contributes
to the development of AML. PU.1 is a critical transcription factor
during early granulopoiesis; PU.1 deficiency impairs hematopoietic
development and can lead to leukemia (38). But PU.1 knockdown inhibited the
proliferation of human AML U937 cells (39). In contrast, C/EBPα expression is
required for the differentiation of myeloid lineage cells, and
C/EBPα overexpression in primary human CD34+ cells led
to granulocytic differentiation (40). In the present study, we found that
C/EBPα and PU.1 were upregulated by SNX-2112 treatment, indicating
that this drug induces AML cell differentiation by increasing
C/EBPα and PU.1 levels.
The Hsp90 inhibitor NVP-AUY922-AG has been reported
to induce apoptosis of AML cells by inhibiting the PI3K and IKK
signaling pathways (9). Our
findings indicate that Bar blocks KG-1a cell proliferation and
induces apoptosis. Since p65 is constitutively active in AML
(25), we speculated that IKK
signaling is activated in KG-1a cells. SNX-2112 treatment decreased
IKK expression as well as p65 expression, phosphorylation, and
nuclear translocation. It has been previously shown that p65
regulates PU.1 expression to induce myeloid differentiation
(32). Additionally, PU.1
expression is suppressed as a result of C/EBPα inhibition (20). We propose that SNX-2112 exerts its
effects on KG-1a cells by inhibiting NF-κB signaling and modulating
the expression of PU.1 and C/EBPα, although additional studies are
warranted to clarify the detailed molecular mechanisms.
Nonetheless, our results suggest that SNX-2112 is a promising
therapeutic agent for the treatment of AML.
Acknowledgements
The present study was supported by the Postdoctoral
Science Foundation of China (2015M582472), the Natural Science
Foundation of Guangdong Province (2014A030310434), and the National
Natural Science Foundation of China (81500227)
Glossary
Abbreviations
Abbreviations:
|
AML
|
acute myeloid leukemia
|
|
GM
|
geldanamycin
|
|
17-AAG
|
17-(2-dimethylaminoethyl)amino-17-demethoxygeldanamycin
|
|
ATRA
|
all-trans-retinoic acid
|
|
CMP
|
common myeloid progenitor
|
|
GMP
|
granulocyte-monocyte progenitor
|
|
Bar
|
bardoxolone methyl
|
References
|
1
|
De Kouchkovsky I and Abdul-Hay M: Acute
myeloid leukemia: A comprehensive review and 2016 update. Blood
Cancer J. 6:e4412016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kadia TM, Ravandi F, Cortes J and
Kantarjian H: New drugs in acute myeloid leukemia. Ann Oncol.
27:770–778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brandt GE and Blagg BS: Alternate
strategies of Hsp90 modulation for the treatment of cancer and
other diseases. Curr Top Med Chem. 9:1447–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patki JM and Pawar SS: HSP90:
Chaperone-me-not. Pathol Oncol Res. 19:631–640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegelin MD: Inhibition of the
mitochondrial Hsp90 chaperone network: A novel, efficient treatment
strategy for cancer? Cancer Lett. 333:133–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jhaveri K and Modi S: Ganetespib: Research
and clinical development. Onco Targets Ther. 8:1849–1858.
2015.PubMed/NCBI
|
|
7
|
Jhaveri K, Taldone T, Modi S and Chiosis
G: Advances in the clinical development of heat shock protein 90
(Hsp90) inhibitors in cancers. Biochim Biophys Acta. 1823:742–755.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gartner EM, Silverman P, Simon M, Flaherty
L, Abrams J, Ivy P and Lorusso PM: A phase II study of
17-allylamino-17-demethoxygeldanamycin in metastatic or locally
advanced, unresectable breast cancer. Breast Cancer Res Treat.
131:933–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walsby EJ, Lazenby M, Pepper CJ, Knapper S
and Burnett AK: The HSP90 inhibitor NVP-AUY922-AG inhibits the PI3K
and IKK signalling pathways and synergizes with cytarabine in acute
myeloid leukaemia cells. Br J Haematol. 161:57–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lancet JE, Gojo I, Burton M, Quinn M,
Tighe SM, Kersey K, Zhong Z, Albitar MX, Bhalla K, Hannah AL, et
al: Phase I study of the heat shock protein 90 inhibitor
alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice
weekly to patients with acute myeloid leukemia. Leukemia.
24:699–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chandarlapaty S, Sawai A, Ye Q, Scott A,
Silinski M, Huang K, Fadden P, Partdrige J, Hall S, Steed P, et al:
SNX2112, a synthetic heat shock protein 90 inhibitor, has potent
antitumor activity against HER kinase-dependent cancers. Clin
Cancer Res. 14:240–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eitan Fibach, Makoto Hayashi and Sachs L:
Control of normal differentiation of myeloid leukemic cells. Proc
Natl Acad Sci USA. 70:343–346. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haanen C and Vermes I: Arsenic trioxide, a
new drug for the treatment of acute promyelocytic leukemia
resistant to tretinoine. Ned Tijdschr Geneeskd. 143:1738–1741.
1999.(In Dutch). PubMed/NCBI
|
|
14
|
Huang ME, Ye YC, Chen SR, Chai JR, Lu JX,
Zhoa L, Gu LJ and Wang ZY: Use of all-trans retinoic acid in the
treatment of acute promyelocytic leukemia. Blood. 72:567–572.
1988.PubMed/NCBI
|
|
15
|
Wang F, Travins J, DeLaBarre B,
Penard-Lacronique V, Schalm S, Hansen E, Straley K, Kernytsky A,
Liu W, Gliser C, et al: Targeted inhibition of mutant IDH2 in
leukemia cells induces cellular differentiation. Science.
340:622–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okoye-Okafor UC, Bartholdy B, Cartier J,
Gao EN, Pietrak B, Rendina AR, Rominger C, Quinn C, Smallwood A,
Wiggall KJ, et al: New IDH1 mutant inhibitors for treatment of
acute myeloid leukemia. Nat Chem Biol. 11:878–886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gupta K, Stefan T, Ignatz-Hoover J,
Moreton S, Parizher G, Saunthararajah Y and Wald DN: GSK-3
inhibition sensitizes acute myeloid leukemia cells to
1,25D-mediated differentiation. Cancer Res. 76:2743–2753. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sykes DB, Kfoury YS, Mercier FE, Wawer MJ,
Law JM, Haynes MK, Lewis TA, Schajnovitz A, Jain E, Lee D, et al:
Inhibition of dihydroorotate dehydrogenase overcomes
differentiation blockade in acute myeloid leukemia. Cell.
167:171–186.e15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang P, Iwasaki-Arai J, Iwasaki H, Fenyus
ML, Dayaram T, Owens BM, Shigematsu H, Levantini E, Huettner CS,
Lekstrom-Himes JA, et al: Enhancement of hematopoietic stem cell
repopulating capacity and self-renewal in the absence of the
transcription factor C/EBP alpha. Immunity. 21:853–863. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng R, Friedman AD, Levis M, Li L, Weir
EG and Small D: Internal tandem duplication mutation of FLT3 blocks
myeloid differentiation through suppression of C/EBPalpha
expression. Blood. 103:1883–1890. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen HM, Zhang P, Voso MT, Hohaus S,
Gonzalez DA, Glass CK, Zhang DE and Tenen DG: Neutrophils and
monocytes express high levels of PU.1 (Spi-1) but not Spi-B. Blood.
85:2918–2928. 1995.PubMed/NCBI
|
|
22
|
Renneville A, Roumier C, Biggio V,
Nibourel O, Boissel N, Fenaux P and Preudhomme C: Cooperating gene
mutations in acute myeloid leukemia: A review of the literature.
Leukemia. 22:915–931. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bueso-Ramos CE, Rocha FC, Shishodia S,
Medeiros LJ, Kantarjian HM, Vadhan-Raj S, Estrov Z, Smith TL,
Nguyen MH and Aggarwal BB: Expression of constitutively active
nuclear-κB RelA transcription factor in blasts of acute myeloid
leukemia. Hum Pathol. 35:246–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guzman ML, Neering SJ, Upchurch D, Grimes
B, Howard DS, Rizzieri DA, Luger SM and Jordan CT: Nuclear
factor-κB is constitutively activated in primitive human acute
myelogenous leukemia cells. Blood. 98:2301–2307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barta TE, Veal JM, Rice JW, Partridge JM,
Fadden RP, Ma W, Jenks M, Geng L, Hanson GJ, Huang KH, et al:
Discovery of benzamide tetrahydro-4H-carbazol-4-ones as
novel small molecule inhibitors of Hsp90. Bioorg Med Chem Lett.
18:3517–3521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Liu ZH, Xia J, Li XP, Li KQ, Xiong
W, Li J and Chen DL: 20(S)-ginsenoside Rh2 inhibits the
proliferation and induces the apoptosis of KG-1a cells through the
Wnt/β-catenin signaling pathway. Oncol Rep. 36:137–146.
2016.PubMed/NCBI
|
|
28
|
Konopleva M, Tsao T, Ruvolo P, Stiouf I,
Estrov Z, Leysath CE, Zhao S, Harris D, Chang S, Jackson CE, et al:
Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and
differentiation in acute myelogenous leukemia. Blood. 99:326–335.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shishodia S, Sethi G, Konopleva M,
Andreeff M and Aggarwal BB: A synthetic triterpenoid, CDDO-Me,
inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF
and chemotherapeutic agents through down-regulation of expression
of nuclear factor kappaB-regulated gene products in human leukemic
cells. Clin Cancer Res. 15:1828–1838. 2006. View Article : Google Scholar
|
|
30
|
Scott LM, Civin CI, Rorth P and Friedman
AD: A novel temporal expression pattern of three C/EBP family
members in differentiating myelomonocytic cells. Blood.
80:1725–1735. 1992.PubMed/NCBI
|
|
31
|
Fisher RC and Scott EW: Role of PU.1 in
hematopoiesis. Stem Cells. 16:25–37. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerloff D, Grundler R, Wurm AA,
Bräuer-Hartmann D, Katzerke C, Hartmann JU, Madan V, Müller-Tidow
C, Duyster J, Tenen DG, et al: NF-κB/STAT5/miR-155 network targets
PU.1 in FLT3-ITD-driven acute myeloid leukemia. Leukemia.
29:535–547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jin L, Xiao CL, Lu CH, Xia M, Xing GW,
Xiong S, Liu QY, Liu H, Li YC, Ge F, et al: Transcriptomic and
proteomic approach to studying SNX-2112-induced K562 cells
apoptosis and anti-leukemia activity in K562-NOD/SCID mice. FEBS
Lett. 583:1859–1866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okawa Y, Hideshima T, Steed P, Vallet S,
Hall S, Huang K, Rice J, Barabasz A, Foley B, Ikeda H, et al:
SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell
growth, angiogenesis, and osteoclastogenesis in multiple myeloma
and other hematologic tumors by abrogating signaling via Akt and
ERK. Blood. 113:846–855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roe JS and Vakoc CR: C/EBPα: Critical at
the origin of leukemic transformation. J Exp Med. 211:1–4. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rao R, Fiskus W, Yang Y, Lee P, Joshi R,
Fernandez P, Mandawat A, Atadja P, Bradner JE and Bhalla K: HDAC6
inhibition enhances 17-AAG - mediated abrogation of hsp90 chaperone
function in human leukemia cells. Blood. 112:1886–1893. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Newman B, Liu Y, Lee HF, Sun D and Wang Y:
HSP90 inhibitor 17-AAG selectively eradicates lymphoma stem cells.
Cancer Res. 72:4551–4561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iwasaki H, Somoza C, Shigematsu H, Duprez
EA, Iwasaki-Arai J, Mizuno S, Arinobu Y, Geary K, Zhang P, Dayaram
T, et al: Distinctive and indispensable roles of PU.1 in
maintenance of hematopoietic stem cells and their differentiation.
Blood. 106:1590–1600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou J, Zhang X, Wang Y and Guan Y: PU.1
affects proliferation of the human acute myeloid leukemia U937 cell
line by directly regulating MEIS1. Oncol Lett. 10:1912–1918.
2015.PubMed/NCBI
|
|
40
|
Cammenga J, Mulloy JC, Berguido FJ,
MacGrogan D, Viale A and Nimer SD: Induction of C/EBPalpha activity
alters gene expression and differentiation of human
CD34+ cells. Blood. 101:2206–2214. 2003. View Article : Google Scholar : PubMed/NCBI
|