Introduction
With approximately 13,800 new cases per year in
Germany and 600,000 new cases worldwide according to data from the
Robert Koch Institute, head and neck squamous cell carcinoma
(HNSCC) is one of the most common tumors (1,2).
Despite the new therapeutic and diagnostic procedures, a poor
5-year survival rate of 55–60% has remained nearly unchanged in
recent decades (1,3). Infiltration of locoregional tissue and
lymph node metastasis occur at a high frequency in 66% of HNSCC
patients (4,5). The recently approved immune checkpoint
inhibitors pembrolizumab and nivolumab, which have shown antitumor
efficacy in non-small-cell lung cancer (6–8),
represent the few innovations in the treatment of recurrent or
metastatic HNSCC during the last decades (9). However, tyrosine kinase inhibitors
(TKI) are being investigated in clinical trials, some of which have
shown favorable survival data (10).
Complex relationships exist between the oncogenic
stroma and its surroundings in this heterogeneous disease (11). Cells from the immune system, the
tumor vasculature and lymphatic systems and cancer associated
fibroblasts (CAF) can encourage tumor growth, invasion and
metastasis (12,13). Molecules in this environment, such
as vascular endothelial growth factor (VEGF) and fibroblast growth
factor (FGF), stimulate the neovascularization needed for cancer
growth (13).
The amplification and overexpression of FGFR1-3 have
been observed in HNSCC in several studies (14–16).
Fibroblast growth factors (FGF) -A, -B, -C and -D, which play key
roles in embryonic development, are crucial for angiogenesis and
nerve and cartilage regeneration in adult tissue (17,18).
Schultz-Hector and Haghayegh (19)
demonstrated a correlation between FGF production in tumor cells
and growth rate, making FGF a potential target in HNSCC (20).
Rapid tumor growth causes hypoxia in different areas
of the malignant tissue, which is a feature of most solid tumors
and is associated with reduced radiotherapy and chemotherapeutic
efficacy (21). Oxygen deficiency
releases signaling molecules, including VEGF, to induce
vasculogenesis, which subsequently correlates with faster invasion
and metastasis (22). As
demonstrated by Mărgăritescu and colleagues (23) VEGF is expressed in 87% of HNSCC
specimens, and increased expression levels are noted in neoplastic
compared with dysplastic epithelium (24). Inhibiting the expression of either
the VEGFR or the ligands decreases HNSCC cell proliferation
(25).
As previously mentioned, we focused on investigating
multi-targeted TKIs (pazopanib, dovitinib and nintedanib), which
target VEGFR and FGFR family members. Thus, we conducted the
present study to investigate the efficacy of nintedanib, dovitinib
and pazopanib as HNSCC treatments in vitro. To the best of
our knowledge, this study constitutes the first in vitro
investigation of pazopanib and nintedanib in HNSCC cell lines.
Materials and methods
The Cancer Genome Atlas (TCGA)
analysis
Data of VEGFR1-3 and FGFR1-4 mRNA expression in
HNSCC were retrieved from The Cancer Genome Atlas (TCGA) via
cBioPortal (26,27), and data from 530 cancer samples were
analyzed to assess VEGFR1-3 and FGFR1-4 mutations, amplifications
and gains. Cases with and without alterations were compared in
terms of overall and median (months) survival.
Cell lines
The following cell lines were used: PCI-1, laryngeal
carcinoma of the glottis from a male patient (pT2N0M0G2); PCI-9,
primary carcinoma at the base from the tongue of a male patient
(pT4N3M0G2); PCI-13, male patient who suffered from oral squamous
cell carcinoma of the retromolar triangle (pT4pN1M0G3); PCI-52,
primary carcinoma of the aryepiglottic fold from a male patient
(pT2N0M0G2); and PCI-68, Primary tongue carcinoma from a male
patient (pT4N0M0G1).
The cells were cultured at 37°C and 5%
CO2 in Dulbecco's modified Eagle's medium (DMEM;
low-glucose medium; Invitrogen, Karlsruhe, Germany; 4.5 g/l
D-glucose, 4 mM L-glutamine, 110 mg/l sodium pyruvate, 10% fetal
calf serum (FCS) and 10.000 U/ml penicillin/streptomycin; Life
Technologies, Darmstadt, Germany) and the medium was changed up to
twice a week as previously described (28).
Drugs
Nintedanib (Boehringer Ingelheim Pharma GmbH &
Co. KG, Ingelheim am Rhein, Germany), dovitinib (Novartis Pharma
GmbH, Nürnberg, Germany) and pazopanib (GlaxoSmithKline GmbH &
Co. KG, München, Germany) were purchased from Selleckchem (Houston,
TX, USA). The targets of the drugs are presented in Table I.
 | Table I.The targets of the tyrosine kinase
inhibitors pazopanib, dovitinib and nintedanib. |
Table I.
The targets of the tyrosine kinase
inhibitors pazopanib, dovitinib and nintedanib.
|
| Target |
|---|
|
|
|
|---|
| Drug |
|
Nintedanib | FGFR1–3 | VEGFR1–3 | PDGFRα/β | – | – | – |
|
Dovitinib | FGFR1–3 | VEGFR1–4 | – | cKit | FLT-3 | – |
|
Pazopanib | FGFR1,3 | VEGFR1–3 | PDGFRα/β | cKit | – | cFMS |
Crystal violet assay
After detachment with 0.25% trypsin and 0.53 mM
ethylenediaminetetraacetic acid (EDTA) from the culture flask, the
cell numbers were measured using a Casy cell counter (Roche
Diagnostics GmbH, Penzberg, Germany). A total of 10,000 cells/well
were seeded on a 96-well plate, and after 24 h of incubation, the
cells were exposed to various concentrations (log2 dilutions) of
nintedanib (starting concentration of 100 µM), dovitinib (starting
concentration of 200 µM) and pazopanib (starting concentration of
800 µM). After 72 h of incubation, medium was removed, and the
cells were stained with crystal violet (0.1% crystal violet/20%
MetOH) for 12 min. Afterwards, the cells were washed with distilled
water four times, and the 96-well plates were dried for 24 h in
air. For the photometric determination, 100 µl of MetOH was added
to each well. After 10 min, the absorbance was measured with a
plate reader at 595 nm (Rainbow Spectra; Tecan, Maennedorf,
Switzerland). Each value was measured twice and every experiment
was performed in triplicate. Representative extracts from the data
were obtained for this publication.
Semi-quantitative reverse
transcription polymerase chain reaction (sqRT-PCR)
RNA from cell pellets was isolated using the
RNeasy® Mini kit (Qiagen®, Venlo, the
Netherlands) following the manufacturers instructions. The RNA
concentration was determined photometrically using NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at
260/280 nm. cDNA synthesis was performed with 1 µg of RNA/probe
using the QuantiTect® reverse transcription kit (Qiagen)
following the manufacturers instructions. Semi-quantitative gene
expression levels were assessed via real-time polymerase chain
reaction (sqRT-PCR) with the CFX96 Real-Time PCR Detection system
(Bio-Rad Laboratories GmbH, München, Germany). The amplification
was performed with QuantiTect® SYBR-Green PCR kit
(Qiagen) in a total volume of 25 µl/probe following the
manufacturers instruction, and 1.5 µl of gene-specific QuantiTect
primers (Qiagen, Hilden, Germany) was added to each probe. The
thermal profile was as follows: 1x 95°C for 15 min, 40x 15 sec at
95°C, 30 sec at 55°C and 30 sec at 72°C). The primers used in the
present study are listed in Table
II. Each value was measured twice and every experiment was
performed in duplicate.
 | Table II.The primers used for sqRT-PCR. |
Table II.
The primers used for sqRT-PCR.
| Primer no. | Receptor | Order code |
|---|
| 1 | FGFR1_1 | QT00102837 |
| 2 | FGFR2_1 | QT00098560 |
| 3 | FGFR3_1 | QT01000685 |
| 4 | FGFR4_1 | QT00027636 |
| 5 | PDGFRA_1 | QT00012719 |
| 6 | PDGFRB_1 | QT00082327 |
| 7 | FLT1_1 | QT00073640 |
| 8 | KDR_1 | QT00069818 |
| 9 | FLT4_1 | QT00063637 |
| 10 | CSF1R_1 | QT00073276 |
| 11 | KIT_1 | QT00080409 |
| 12 | FLT3_1 | QT00071316 |
The comparative ΔCT method was used for
normalization of the PCR data (29). Using β-actin as a standard gene
(assuming an expression level of 100%), we quantified the
expression of each gene relative to that of β-actin. The relative
expression levels were classified into four different groups: very
strong expression (≥0.1%); strong expression (0.01–0.09%);
intermediate expression (0.001–0.009%); and low expression
(≤0.0009%).
Statistical analysis
Statistical analysis was performed using Microsoft
Excel 2010 (Microsoft Corp., Redmond, WA, USA) and Prism 6.05
(GraphPad, Inc., La Jolla, CA, USA). Different experimental aspects
were investigated [data derived from TCGA database (26,27)].
First, we examined the potency of nintedanib, dovitinib and
pazopanib in single agent therapy against HNSCC in vitro.
Afterwards, we compared single drug efficacy between the drugs.
Following at least three experimental replicates, data were
evaluated with a non-parametric Mann-Whitney test, and the
significance level was set at P≤0.05. A correlation analysis was
performed for the variables ‘receptor expression’ and ‘fraction of
viable cells’ at a specific drug concentration (6 µM) (Tables I–III). The statistical analysis was based
on several repeated and representative experiments.
 | Table III.The effects of different nintedanib
concentrations on the cell lines PCI-1, PCI-9, PCI-13, PCI-52 and
PCI-68 by Mann-Whitney test. |
Table III.
The effects of different nintedanib
concentrations on the cell lines PCI-1, PCI-9, PCI-13, PCI-52 and
PCI-68 by Mann-Whitney test.
|
| Concentration
(µM) |
|---|
|
|
|
|---|
|
| 0 vs. 6 | 0 vs. 13 | 0 vs. 25 | 0 vs. 50 | 0 vs. 100 |
|---|
| Cell line |
|
PCI-1 | 0.0022b | 0.0022b | 0.0022b | 0.0022b | 0.0022b |
|
PCI-9 | 0.0022b | 0.0022b | 0.0022b | 0.0022b | 0.0022b |
|
PCI-13 | 0.6753 | 0.0022b | 0.0022b | 0.0022b | 0.0022b |
|
PCI-52 | 0.0043a | 0.0022b | 0.0022b | 0.0022b | 0.0022b |
|
PCI-68 | 0.0043a | 0.6753 | 0.0411a | 0.0022b | 0.0022b |
Results
TCGA analysis
Data on VEGFR and FGFR mRNA expression in HNSCC were
retrieved from TCGA. In 10% of the cases, a mutation, gain or
amplification was detected for VEGFR1. VEGFR2 alterations were
noted in 16% of the cases, whereas anomalies in VEGFR3 were
detected in 9% of the cases. Thus, based on the collection of
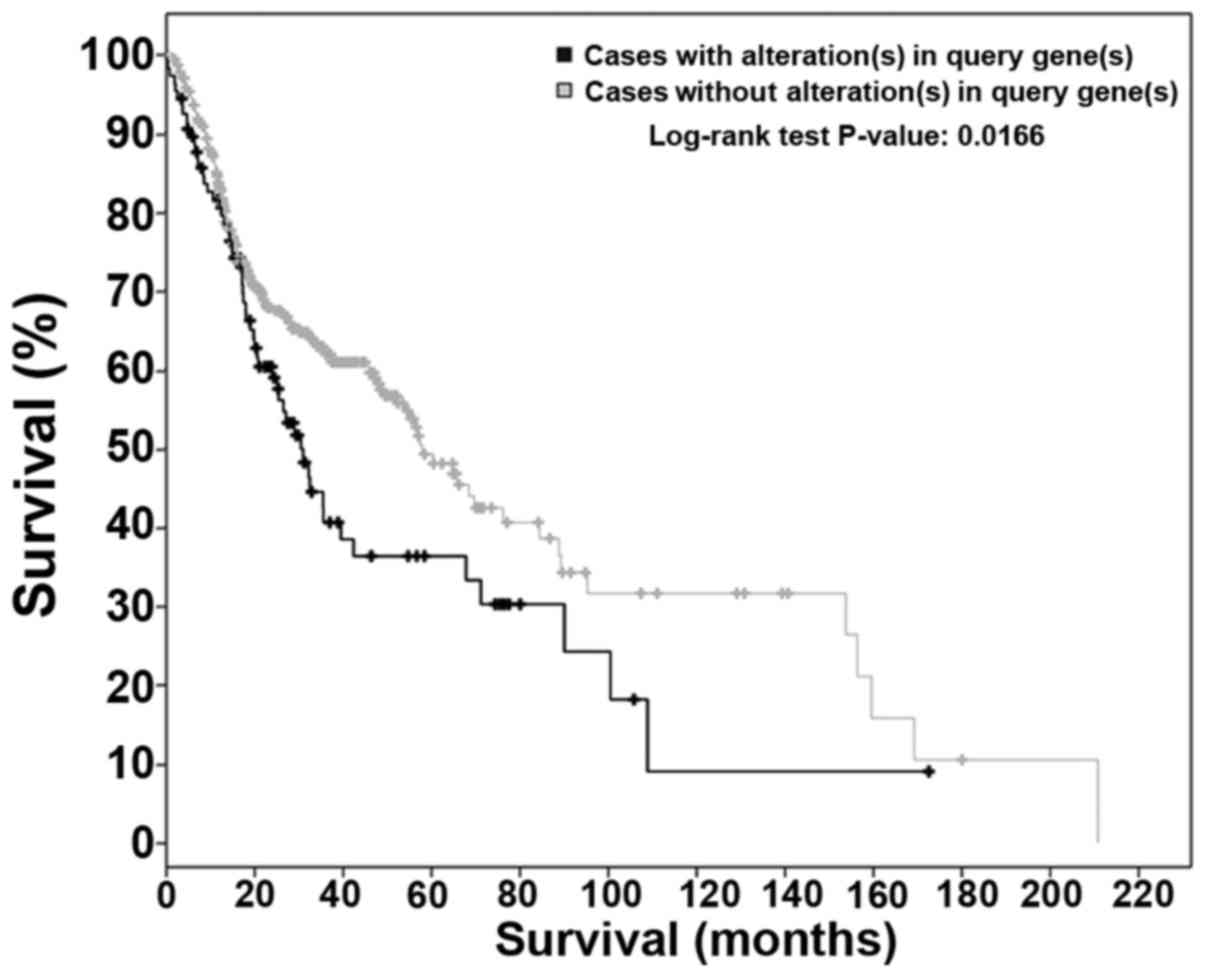
mutations, 30% overall among the cases showed alterations. VEGFR1+3
mutations were significantly (P=0.0166) decreased with increased
overall survival (median survival, 57.88 vs. 30.45 months)
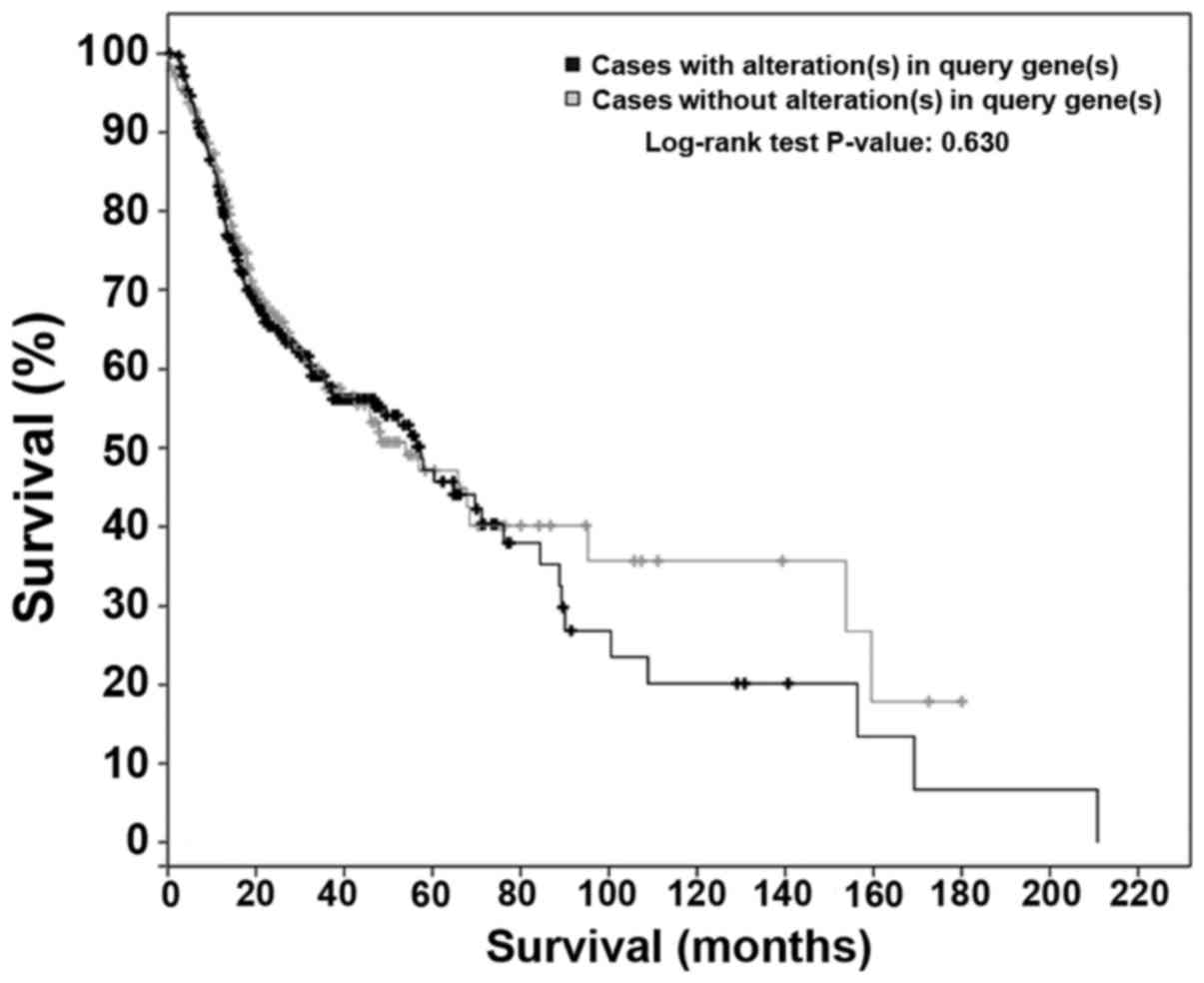
(Fig. 1). Changes to FGFR family
members were evident in 46% of the cases, whereas FGFR1
amplifications and gains were noted in 31% of patients. In
addition, FGFR3 amplification, gain or mutation was noted in 12% of
the cases. FGFR2 and FGFR4 mutations were detected in 9 and 8% of
the cases, respectively (26,27).
No significant (P=0.63) degree of correlation could be identified
between FGFR alterations and overall survival (Fig. 2).
Expression of receptor tyrosine
kinases in HNSCC cell lines
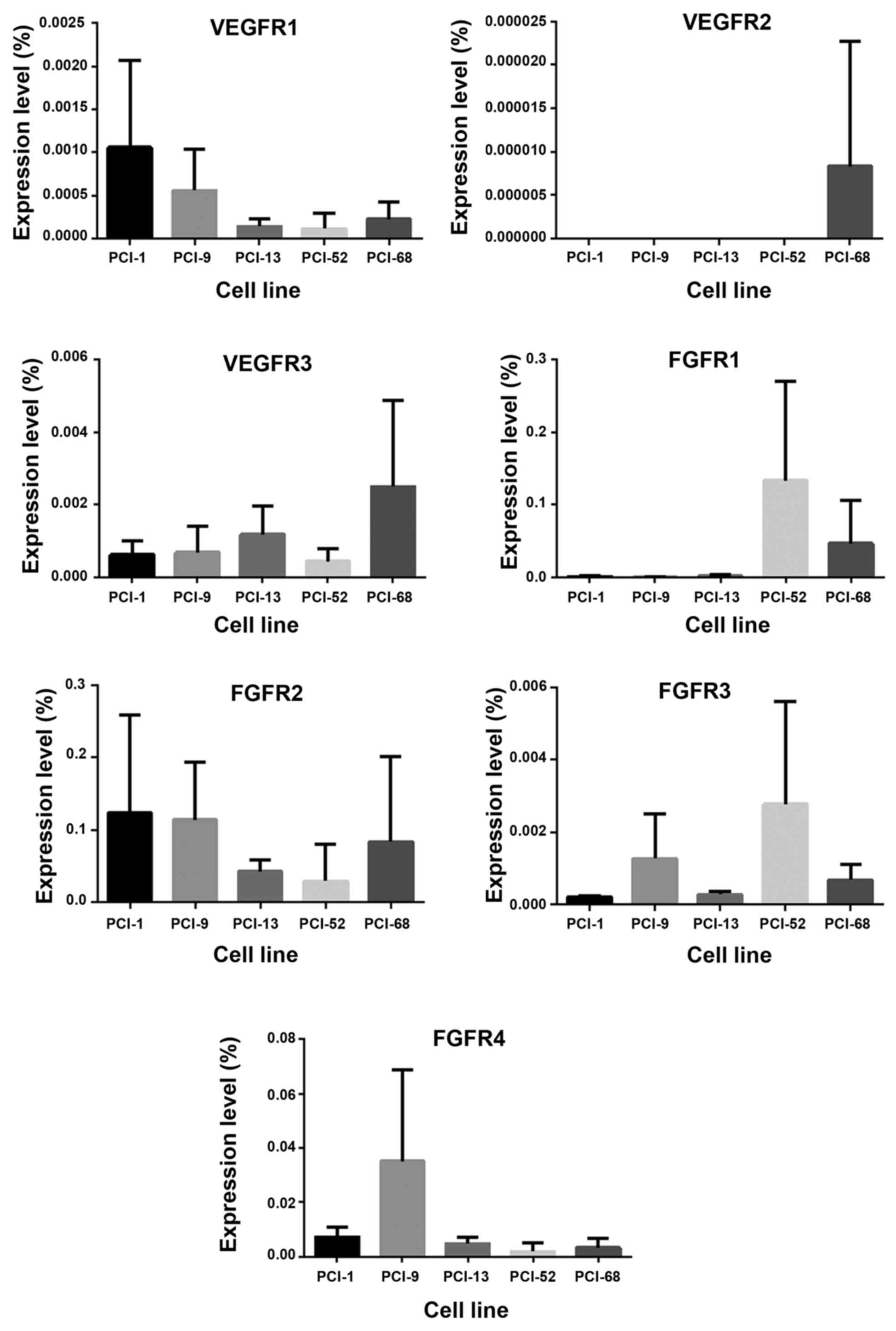
VEGFR1-3 and FGFR1-4 expression in five cell lines
(PCI-1, PCI-9, PCI-13, PCI-52 and PCI-68) was analyzed via RT-PCR.
VEGFR1+3 exhibited low and intermediate expression levels in all of
the cell lines, whereas VEGFR-2 was only detected at very low
levels in PCI-68. FGFR1 was highly expressed in the PCI-52 and
PCI-68 cell lines and exhibited low or intermediate expression in
the remaining cell lines. Four of the five cell lines (PCI-1,
PCI-9, PCI-52 and PCI-68) expressed high levels of FGFR-2, whereas,
PCI-13 expressed an intermediate level (Fig. 3).
Impact of nintedanib treatment on
HNSCC cell lines
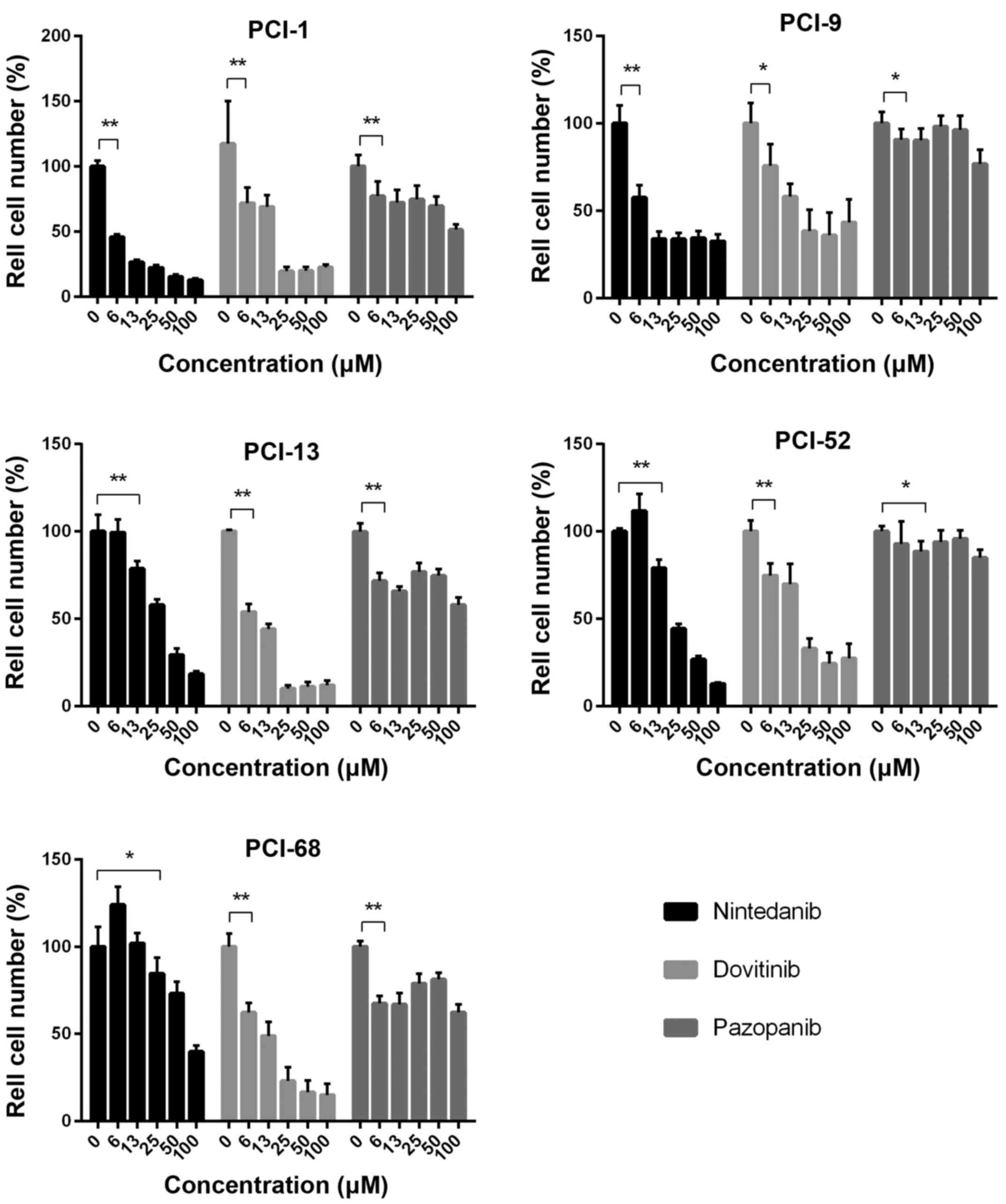
Each cell line exhibited a concentration-dependent
response toward different concentrations of nintedanib (log2
dilution) as a monotherapy. The untreated control for each cell
line was set to 100%. In PCI-1, the lowest concentration of 6 µM
caused a cell reduction of 45% (SD±2.01%). Maximum cell reduction
was observed upon the addition of 100 µM nintedanib, 12.78%
(SD±1.01%). The comparison of the respective concentration
increases revealed highly significant differences (P=0.0022). In
PCI-9, a viable fraction of 57.6% (SD±6.66%) was noted for 6 µM
nintedanib, which was highly significant compared with the control
(P<0.0022). The viable fraction obtained with the concentration
of 13 µM was 33.81% (SD±4.07%), which was also significantly
decreased (P=0.0022). Maximum cell reduction was detected with the
highest concentration of 100 µM, which resulted in a 32.59%
(SD±3.84%) viability decrease. Similar results were observed for
cell line PCI-13. Although the viable fraction of 99.39% (SD±7.17%)
at 6 µM nintedanib did not exhibit significant differences compared
with the control group, we found highly significant differences
with the concentration increases (P=0.0022) and detected the lowest
viable fraction of 18.37% (SD±1.61%) with the highest nintedanib
concentration (100 µM). In the PCI-52 cell line, we detected the
first significant (P=0.0022) decrease in the viable fraction of
79.01% (SD±4.7%) with 13 µM nintedanib. A viable fraction of 12.77%
(SD±0.69%) was obtained with the highest nintedanib concentration
of 100 µM. PCI-68 cells showed varied responsiveness to
single-agent therapy with log2 dilutions of nintedanib. All
concentrations >13 µM exhibited significant
(P<0.05/P<0.01) cell count decreases compared with the
control samples, and a maximum cell reduction of 39.79% (SD±3.39%)
was achieved with the highest nintedanib concentration of 100 µM
(Fig. 4 and Table III).
Impact of dovitinib treatment on HNSCC
cell lines
Each cell line exhibited varying responsiveness
toward dovitinib monotherapy. The untreated control was set to
100%. In the PCI-1 cell line, a high significant (P=0.0022) cell
reduction was achieved with 6 µM dovitinib. The highest cell
reduction of (19.45% (SD±3.37) was achieved with a concentration of
25 µM dovitinib. No additional cell reduction was achieved with
subsequent concentration increases to 50 and 100 µM. In the PCI-9
cell line, we observed a similar effect to the applied drug. A
viable fraction of 75.74% (SD±11.77%) was detected with 6 µM
dovitinib, and a further significant (P=0.0022) cell reduction to
58.09% (SD±7.05%) was observed with the next concentration increase
to 13 µM. In addition, 25 µM dovitinib decreased the viable
fraction to 38.31% (SD±11.58%). As previously demonstrated in
PCI-1, no significant further cell reductions were observed with
higher drug concentrations. Similar results were observed for the
PCI-13 cell line. We observed significant (P=0.0022) cell reduction
for the first three increases in concentration (6, 13 and 25 µM) to
viable fractions of 53.98 (SD±4.19%), 44.01 (SD±3.0%) and 10.08%
(SD±1.68%), respectively. As previously noted with the PCI-1 and
PCI-9 cell lines, further dovitinib concentration increases to 50
and 100 µM did not lead to additional cell count reductions. A
viable fraction of 74.81% (SD±6.67%) was detected with 6 µM
dovitinib in PCI-52. Viable fractions of 69.86 (SD±11.05%) and
32.9% (5.52%) were obtained with 13 and 25 µM dovitinib. As
previously noted for PCI-1, further significant cell viability
decreases were not observed with increased dovitinib concentrations
of 50 and 100 µM in PCI-9 and PCI-13 cells. In PCI-68, varied
responses were observed with the different dovitinib concentrations
applied. A high significant (P=0.0022) cell reduction of 62.35%
(SD±5.24%) was noted for 6 µM dovitinib, and a viable fraction of
48.89% (SD±7.83%) was detected with 13 µM dovitinib. With a
concentration increase to 25 µM, a cell reduction to 23.02%
(SD±7.68%) was observed. Further cell reduction to 14.82%
(SD±6.35%) was achieved with 100 µM dovitinib, but this effect was
not significant (Fig. 4 and
Table IV).
 | Table IV.The effects of different dovitinib
concentrations on the cell lines PCI-1, PCI-9, PCI-13, PCI-52 and
PCI-68 by Mann-Whitney test. |
Table IV.
The effects of different dovitinib
concentrations on the cell lines PCI-1, PCI-9, PCI-13, PCI-52 and
PCI-68 by Mann-Whitney test.
|
| Concentration
(µM) |
|---|
|
|
|
|---|
|
| 0 vs. 6 | 0 vs. 13 | 0 vs. 25 | 0 vs. 50 | 0 vs. 100 |
|---|
| Cell line |
| PCI-1 | 0.0022b | 0.0022b | 0.0022b | 0.0022b | 0.0022b |
| PCI-9 | 0.0152a | 0.0022b | 0.0022b | 0.0022b | 0.0022b |
| PCI-13 | 0.0022b | 0.0022b | 0.0022b | 0.0022b | 0.0022b |
| PCI-52 | 0.0022b | 0.0022b | 0.0022b | 0.0022b | 0.0022b |
| PCI-68 | 0.0022b | 0.0022b | 0.0022b | 0.0022b | 0.0022b |
Impact of pazopanib treatment on HNSCC
cell lines
The cell lines exhibited a minimal response to
varying concentrations of pazopanib monotherapy. In summary, we
demonstrated significant differences in the lower and upper
concentration ranges for each cell line. The control was set to
100%. In PCI-1, the viable fraction at 6 µM pazopanib was 77.05%
(SD±10.63%), which was significantly different (P=0.0043) from the
control. Similar results were noted in PCI-9, in which we detected
viable fractions ranging from 90.63 (SD±5.74%) to 76.86%
(SD±7.57%). A significant difference (P=0.0411) was noted
incubating the cells with a concentration of 6 µM pazopanib.
Comparing the concentrations 25 and 50 µM with the control no
significant differences were noted. PCI-13 exhibited varied
reactions toward different pazopanib concentrations. A viable
fraction of 71.73% (SD±4.22%) was detected with 6 µM pazopanib, and
this effect was highly significant (P=0.0022) compared with the
control. Incubating PCI-52 cells with a concentration of 13 µM
pazopanib we detected the first significant (P=0.0043) decrease of
the cell number. No other significant differences could be detected
in this cell line. In PCI-68, we detected viable fractions ranging
from 81.2 (SD±3.58%) to 62.46% (SD±4.33%). Highly significant
differences (P=0.0022) were noted for all concentrations applied by
comparing with the control (Fig. 4
and Table V).
 | Table V.The effects of different pazopanib
concentrations on the cell lines PCI-1, PCI-9, PCI-13, PCI-52 and
PCI-68 by Mann-Whitney test. |
Table V.
The effects of different pazopanib
concentrations on the cell lines PCI-1, PCI-9, PCI-13, PCI-52 and
PCI-68 by Mann-Whitney test.
|
| Concentration
(µM) |
|---|
|
|
|
|---|
|
| 0 vs. 6 | 0 vs. 13 | 0 vs. 25 | 0 vs. 50 | 0 vs. 100 |
|---|
| Cell line |
| PCI-1 | 0.0043a | 0.0022b | 0.0043a | 0.0022b | 0.0022b |
| PCI-9 | 0.0411a | 0.0411a | 0.5714 | 0.5714 | 0.0022b |
| PCI-13 | 0.0022b | 0.0022b | 0.0022b | 0.0022b | 0.0022a |
| PCI-52 | 0.0931 | 0.0043b | 0.0649 | 0.1797 | 0.0022b |
| PCI-68 | 0.0022b | 0.0022b | 0.0022b | 0.0022b | 0.0022b |
Correlation analysis
A correlation analysis was performed for the
variables ‘receptor expression’ and ‘fraction of viable cells’ at a
specific drug concentration (6 µM) to quantify the degree of
relationship between receptor expression and TKI efficacy. No
significant degree of association was observed between receptor
expression and TKI efficacy (data not shown).
Discussion
Squamous cell carcinoma of the head and neck is one
of the most common tumor presentations worldwide (1). Therapy, which consists of surgery,
radiation and systemic chemotherapy, has changed minimally in
recent years and provides a devastating 5-year survival rate of
55–60%. Locoregional recurrence and lymph nodal metastasis are the
most common mortality patterns for HNSCC and impair function and
quality of life as well as overall survival (1,3). The
literature suggests that neoangiogenesis is one of the main reasons
for this poor progress (30).
Pentheroudakis et al (31)
demonstrated high VEGFR1+3 transcriptional activity driven by
pro-angiogenic factors at the time of relapse. Similarly, these
mutations occur in relevant quantities in HNSCC and result in a
significant (P=0.0166) decrease of overall survival (57.88 vs.
30.45 median months survival) (26,27).
The role of the FGFR family remains unclear. Ipenburg et al
(32) demonstrated the prognostic
value of FGFR1 expression in CAFs and an association with poor
survival, although the literature examining the role of other
family members is both limited and conflicting. Although
alterations in FGFR1-4 were detected in 46% of the cases, a TCGA
analysis did not reveal a significant (P=0.630) correlation between
FGFR1-4 alterations and overall survival (26,27).
Given that angiogenesis plays a critical role in tumor growth in
solid tumors (33) and that
VEGFR-targeted therapies, such as bevacizumab, do not generate the
desired effect, multi-targeted TKI might enhance therapeutic
strategies for HNSCC by inhibiting several downstream targets
(34).
In view of the above facts, our cell line model
exhibited receptor expression patterns comparable to those detailed
in the literature, and TCGA appears to be a useful method for
investigating the efficacies of nintedanib, dovitinib and pazopanib
for HNSCC treatment in vitro. These agents are potent VEGFR
and FGFR inhibitors that exhibited different effects on different
HNSCC cell lines.
Nintedanib showed a concentration-dependent efficacy
in all cell lines, and a significant reduction in the viable cell
fraction was observed even at the lower concentration ranges. In
contrast, Kutluk Cenik et al (35) did not observe any anti-proliferative
effects in their in vitro investigation of lung and
pancreatic cancers. However, in their in vivo models, tumor
growth was inhibited in all cases (35), indicating that the tumor
microenvironment might have a large impact on drug efficacy. Kudo
et al (36) suggested that
VEGFR2 is a feasible pharmacodynamic biomarker for hepatocellular
carcinoma (HCC) in vivo, whereas the cell lines used in the
present study did not show high VEGFR2 expression levels, even
though significant cell count reductions were observed. A different
pharmacodynamic effect might occur when treating different tumor
types and cannot be excluded as a possibility, although the tumor
microenvironment likely plays a crucial role during multi-targeted
inhibitor therapy due to interactions with CAF. In contrast to
VEGFR1+3, our TCGA data analysis did not reveal a significant
correlation between VEGFR2 alteration and overall survival.
A significant decrease in the viable cell fraction
was observed in response to treatment with dovitinib, even at lower
concentration ranges. Because other receptors targeted by the drug
are not expressed at appreciable levels, the effect is assumed to
be mainly mediated through VEGFR and FGFR. Sweeny et al
(11) demonstrated that dovitinib
yielded significant cell count reductions in their HNSCC in
vitro models. Konecny et al (37) demonstrated significant cell count
reductions in endometrial cancer cell lines harboring activating
FGFR2 mutations, and FGFR2 was also highly expressed in all of our
cell lines. An in vivo investigation revealed significant
tumor regression and growth inhibition after dovitinib treatment
(38). Although our correlation
analysis did not obtain a significant relationship between receptor
expression and drug efficacy, the expression strength appears to
play a decisive role. Furthermore, Ku (39) reported a strong interaction between
dovitinib and VEGFR3, which is supported by our data.
In the tested concentration range, pazopanib showed
a lack of efficacy. Although significant cell count reductions were
noted for the lower concentration ranges, no further cytotoxic
effects were observed. Hamberg et al (33) noted that the main target of
pazopanib is VEGFR2, which was minimally expressed in our cell
lines. Similar results were demonstrated by Canter et al
(40) in their in vitro
investigation of renal cell carcinoma (RCC) cell lines. By
investigating the efficacy of two different TKIs, a cytostatic
effect was noted exclusively with pazopanib. Kim et al
(41) reported potent anti-tumoral
activity in gastric cancer cell lines harboring FGFR2
amplification. Although FGFR2 mutations appear in only 9% of HNSCC
cases, they have no significant impact on overall survival
(26,27). However, this result does not exclude
potential efficacy in a clinical situation by affecting the tumor
microenvironment. Importantly, a phase I clinical trial
investigating the efficacies of pazopanib and cetuximab for the
treatment of incurable HNSCC patients is currently under way
(NCT01716416) and aims to show additive/synergistic effects in the
treatment of recurrent/metastatic HNSCC. A phase II study
evaluating axitinib, a multi-targeted inhibitor that mainly targets
VEGFR1-3, in patients with unresectable, recurrent or metastatic
head and neck cancer revealed a favorable median overall survival
compared with standard therapies (10).
In conclusion, the present study demonstrated a
significant decrease in cell proliferation after the in
vitro treatment of HNSCC with nintedanib and dovitinib. In
contrast, the cell lines appeared to be resistant to pazopanib,
which could be explained by their lack of VEGFR2 expression. The
results should be critically considered due to the fact that the
cell lines were treated outside their normal environment resulting
in a lack of interaction with surrounding tissue, blood flow or a
missing supply of nutrients. Further in vivo investigation
is required to determine a specific role for TKIs in HNSCC
treatment.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta S, Kong W, Peng Y, Miao Q and
Mackillop WJ: Temporal trends in the incidence and survival of
cancers of the upper aerodigestive tract in Ontario and the United
States. Int J Cancer. 125:2159–2165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlito A, Shaha AR, Silver CE, Rinaldo A
and Mondin V: Incidence and sites of distant metastases from head
and neck cancer. ORL J Otorhinolaryngol Relat Spec. 63:202–207.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozdek A, Sarac S, Akyol MU, Unal OF and
Sungur A: Histopathological predictors of occult lymph node
metastases in supraglottic squamous cell carcinomas. Eur Arch
Otorhinolaryngol. 257:389–392. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: KEYNOTE-024 Investigators: Pembrolizumab versus
chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl
J Med. 375:1823–1833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seiwert TY, Zuo Z, Keck MK, Khattri A,
Pedamallu CS, Stricker T, Brown C, Pugh TJ, Stojanov P, Cho J, et
al: Integrative and comparative genomic analysis of HPV-positive
and HPV-negative head and neck squamous cell carcinomas. Clin
Cancer Res. 21:632–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swiecicki PL, Zhao L, Belile E, Sacco AG,
Chepeha DB, Dobrosotskaya I, Spector M, Shuman A, Malloy K, Moyer
J, et al: A phase II study evaluating axitinib in patients with
unresectable, recurrent or metastatic head and neck cancer. Invest
New Drugs. 33:1248–1256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sweeny L, Zimmermann TM, Liu Z and
Rosenthal EL: Evaluation of tyrosine receptor kinases in the
interactions of head and neck squamous cell carcinoma cells and
fibroblasts. Oral Oncol. 48:1242–1249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balkwill FR, Capasso M and Hagemann T: The
tumor microenvironment at a glance. J Cell Sci. 125:5591–5596.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freier K, Schwaenen C, Sticht C,
Flechtenmacher C, Mühling J, Hofele C, Radlwimmer B, Lichter P and
Joos S: Recurrent FGFR1 amplification and high FGFR1 protein
expression in oral squamous cell carcinoma (OSCC). Oral Oncol.
43:60–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Henson BJ and Gollin SM: Overexpression of
KLF13 and FGFR3 in oral cancer cells. Cytogenet Genome Res.
128:192–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wheeler SE, Shi H, Lin F, Dasari S,
Bednash J, Thorne S, Watkins S, Joshi R and Thomas SM: Enhancement
of head and neck squamous cell carcinoma proliferation, invasion,
and metastasis by tumor-associated fibroblasts in preclinical
models. Head Neck. 36:385–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ornitz DM and Marie PJ: Fibroblast growth
factor signaling in skeletal development and disease. Genes Dev.
29:1463–1486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y and Adjei AA: Targeting
angiogenesis in cancer therapy: moving beyond vascular endothelial
growth factor. Oncologist. 20:660–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schultz-Hector S and Haghayegh S:
Beta-fibroblast growth factor expression in human and murine
squamous cell carcinomas and its relationship to regional
endothelial cell proliferation. Cancer Res. 53:1444–1449.
1993.PubMed/NCBI
|
|
20
|
Koole K, Brunen D, van Kempen PM, Noorlag
R, de Bree R, Lieftink C, van Es RJ, Bernards R and Willems SM:
FGFR1 is a potential prognostic biomarker and therapeutic target in
head and neck squamous cell carcinoma. Clin Cancer Res.
22:3884–3893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris BH, Barberis A, West CM and Buffa
FM: Gene expression signatures as biomarkers of tumour hypoxia.
Clin Oncol (R Coll Radiol). 27:547–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu HW, Wall NR, Hsueh CT, Kim S, Ferris
RL, Chen CS and Mirshahidi S: Combination antiangiogenic therapy
and radiation in head and neck cancers. Oral Oncol. 50:19–26. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mărgăritescu C, Pirici D, Stîngă A,
Simionescu C, Raica M, Mogoantă L, Stepan A and Ribatti D: VEGF
expression and angiogenesis in oral squamous cell carcinoma: An
immunohistochemical and morphometric study. Clin Exp Med.
10:209–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denhart BC, Guidi AJ, Tognazzi K, Dvorak
HF and Brown LF: Vascular permeability factor/vascular endothelial
growth factor and its receptors in oral and laryngeal squamous cell
carcinoma and dysplasia. Lab Invest. 77:659–664. 1997.PubMed/NCBI
|
|
25
|
Tong M, Lloyd B, Pei P and Mallery SR:
Human head and neck squamous cell carcinoma cells are both targets
and effectors for the angiogenic cytokine, VEGF. J Cell Biochem.
105:1202–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brands RC, Herbst F, Hartmann S, Seher A,
Linz C, Kübler AC and Müller-Richter UD: Cytotoxic effects of
SMAC-mimetic compound LCL161 in head and neck cancer cell lines.
Clin Oral Investig. 20:2325–2332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kramer B, Hock C, Birk R, Sauter A, Stuck
BA, Hörmann K, Schultz JD and Aderhold C: Targeted therapies in
HPV-positive and -negative HNSCC - alteration of EGFR and VEGFR-2
expression in vitro. Anticancer Res. 36:2799–2807. 2016.PubMed/NCBI
|
|
31
|
Pentheroudakis G, Angouridakis N, Wirtz R,
Nikolaou A, Kalogeras KT, Pavlidis N and Fountzilas G:
Transcriptional activity of human epidermal growth factor receptor
family and angiogenesis effectors in locoregionally recurrent head
and neck squamous cell carcinoma and correlation with patient
outcome. J Oncol. 2009:8541272009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ipenburg NA, Koole K, Liem KS, van Kempen
PM, Koole R, van Diest PJ, van Es RJ and Willems SM: Fibroblast
growth factor receptor family members as prognostic biomarkers in
head and neck squamous cell carcinoma: A Systematic Review. Target
Oncol. 11:17–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamberg P, Verweij J and Sleijfer S:
(Pre-)clinical pharmacology and activity of pazopanib, a novel
multikinase angiogenesis inhibitor. Oncologist. 15:539–547. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cohen EE, Davis DW, Karrison TG, Seiwert
TY, Wong SJ, Nattam S, Kozloff MF, Clark JI, Yan DH, Liu W, et al:
Erlotinib and bevacizumab in patients with recurrent or metastatic
squamous-cell carcinoma of the head and neck: A phase I/II study.
Lancet Oncol. 10:247–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cenik B Kutluk, Ostapoff KT, Gerber DE and
Brekken RA: BIBF 1120 (nintedanib), a triple angiokinase inhibitor,
induces hypoxia but not EMT and blocks progression of preclinical
models of lung and pancreatic cancer. Mol Cancer Ther. 12:992–1001.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kudo K, Arao T, Tanaka K, Nagai T, Furuta
K, Sakai K, Kaneda H, Matsumoto K, Tamura D, Aomatsu K, et al:
Antitumor activity of BIBF 1120, a triple angiokinase inhibitor,
and use of VEGFR2+pTyr+ peripheral blood
leukocytes as a pharmacodynamic biomarker in vivo. Clin Cancer Res.
17:1373–1381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Konecny GE, Finkler N, Garcia AA, Lorusso
D, Lee PS, Rocconi RP, Fong PC, Squires M, Mishra K, Upalawanna A,
et al: Second-line dovitinib (TKI258) in patients with
FGFR2-mutated or FGFR2-non-mutated advanced or metastatic
endometrial cancer: A non-randomised, open-label, two-group,
two-stage, phase 2 study. Lancet Oncol. 16:686–694. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee SH, de Lopes Menezes D, Vora J, Harris
A, Ye H, Nordahl L, Garrett E, Samara E, Aukerman SL, Gelb AB, et
al: In vivo target modulation and biological activity of CHIR-258,
a multitargeted growth factor receptor kinase inhibitor, in colon
cancer models. Clin Cancer Res. 11:3633–3641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ku X: Development and application of small
molecule probes for kinase affinity purification and quantitative
chemical proteomics (Thesis)Fakultät Wissenschaftszentrum
Weihenstephan für Ernährung. Landnutzung und Umwelt Technische
Universität München; München: pp. 1292014, http://docplayer.net/6560816-Technische-universitat-munchen-lehrstuhl-fur-proteomik-und-bioanalytik-xin-ki.html
|
|
40
|
Canter D, Kutikov A, Golovine K, Makhov P,
Simhan J, Uzzo RG and Kolenko VM: Are all multi-targeted tyrosine
kinase inhibitors created equal? An in vitro study of sunitinib and
pazopanib in renal cell carcinoma cell lines. Can J Urol.
18:5819–5825. 2011.PubMed/NCBI
|
|
41
|
Kim ST, Jang HL, Lee SJ, Lee J, Choi YL,
Kim KM, Cho J, Park SH, Park YS, Lim HY, et al: Pazopanib, a novel
multitargeted kinase inhibitor, shows potent in vitro antitumor
activity in gastric cancer cell lines with FGFR2 amplification. Mol
Cancer Ther. 13:2527–2536. 2014. View Article : Google Scholar : PubMed/NCBI
|